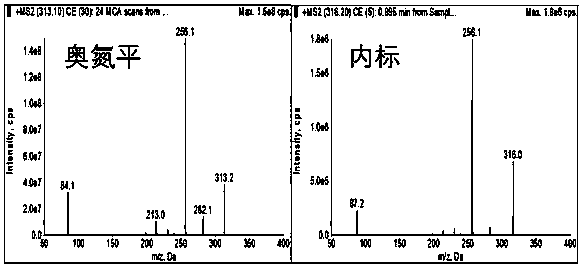
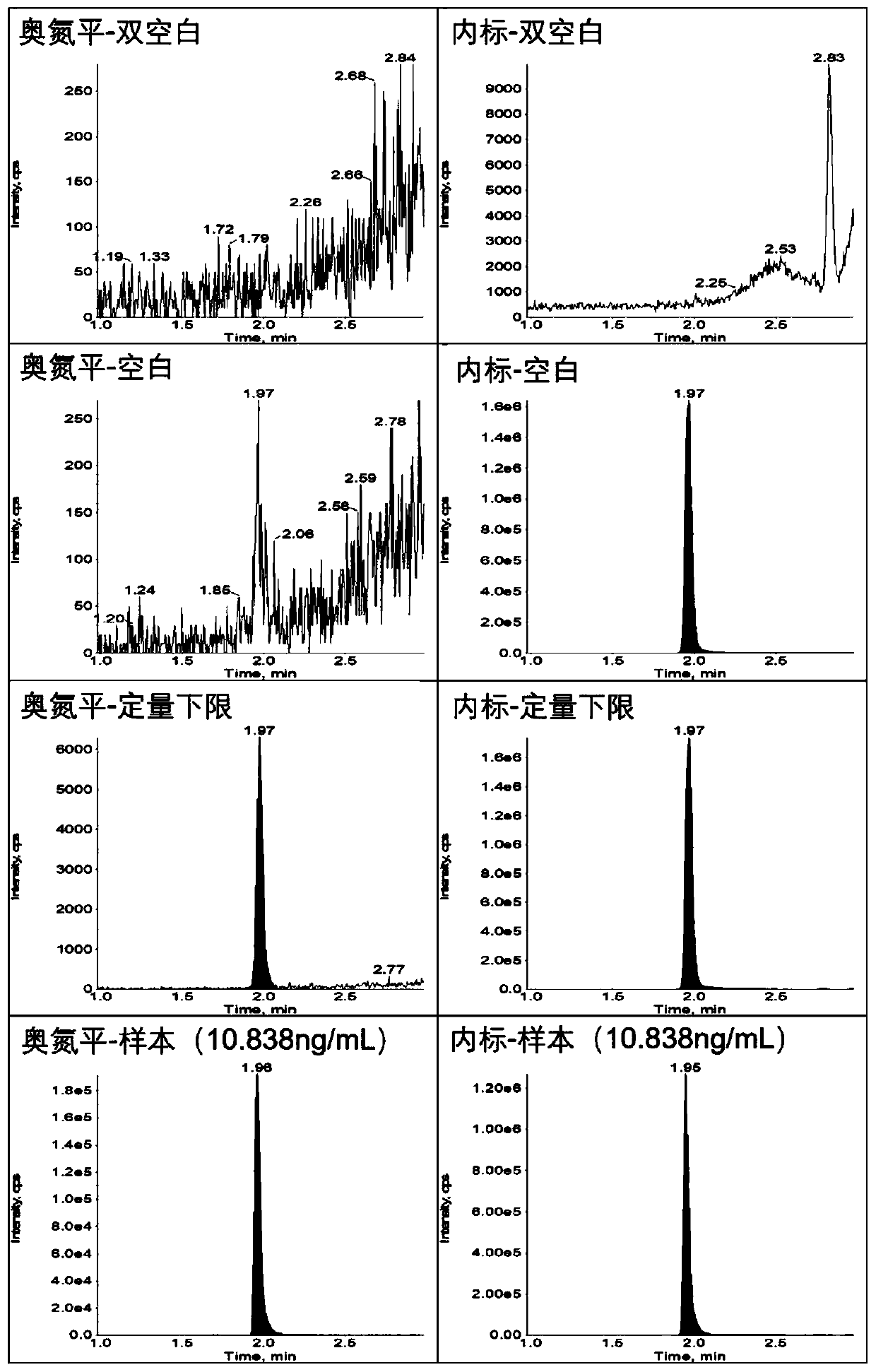
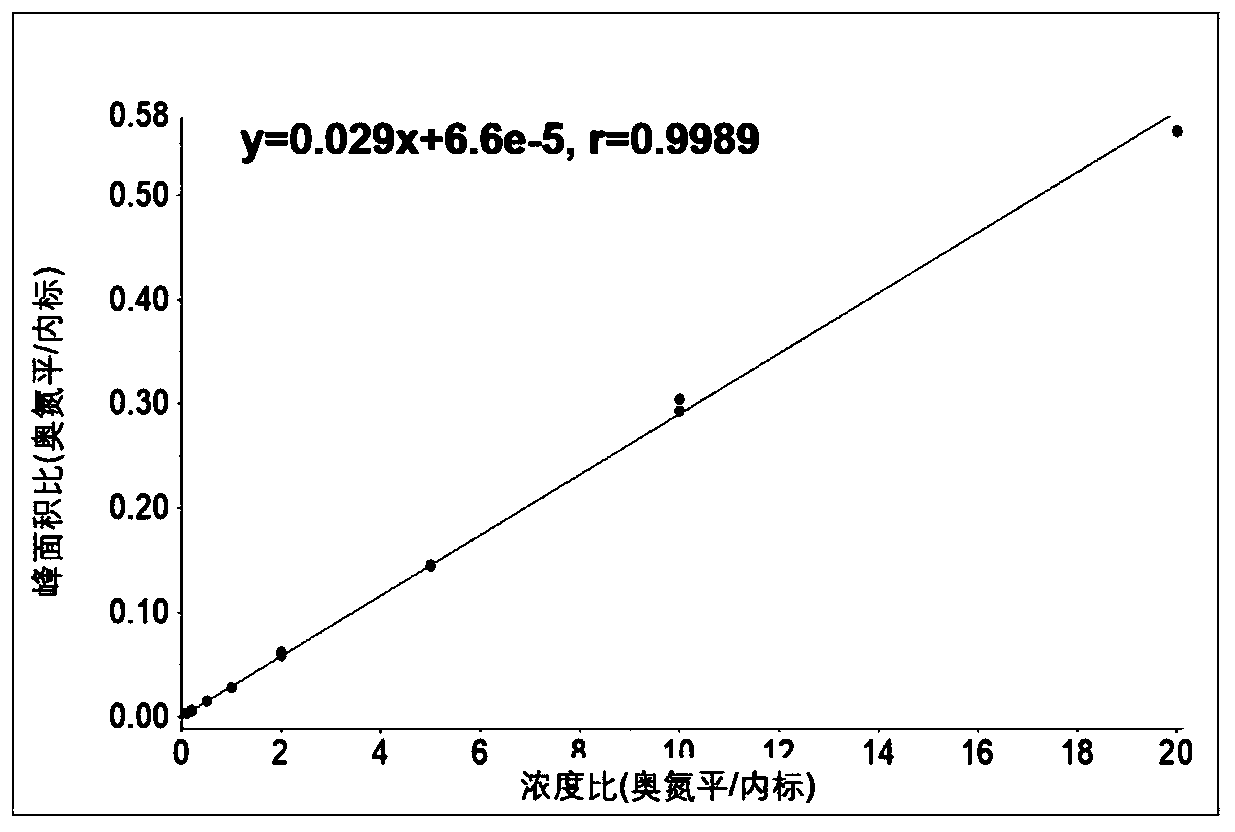
Method for determining concentration of olanzapine in blood plasma through high-performance liquid chromatography-tandem mass spectrometry
A high-performance liquid chromatography and tandem mass spectrometry technology, which is applied in the field of high-performance liquid chromatography-tandem mass spectrometry for determining the concentration of olanzapine in plasma, can solve the problem of not meeting the requirements of detection limit, quality control and sample processing efficiency, reducing the efficiency of biological samples, Problems such as large sample volume, to achieve the effect of eliminating residual effect, suitable retention time, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] 1 solution preparation
[0034] The volumes and weights used to prepare solutions can be adjusted proportionally, and all solutions are stored at room temperature unless otherwise stated.
[0035] 1.1 Preparation of mobile phase solution
[0036] Organic phase (A1): The organic phase used in the experiment was chromatographically pure methanol, which was fixed to volume with a 1L volumetric flask, filtered through a G6 vertical fused glass funnel with a 0.22 μm organic filter membrane, placed in a mobile phase bottle, and ultrasonically degassed. Ready to use after labeling.
[0037]Water phase (B1): Use a graduated cylinder to measure an appropriate amount of pure water and put it in a beaker, add 10mL of 1M ammonium formate solution and 50μL of heptafluorobutyric acid, mix well, place in a volumetric flask and set the volume to 1L, and prepare a solution containing 10mM formic acid The aqueous phase of ammonium and 0.005% heptafluorobutyric acid was filtered through...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com