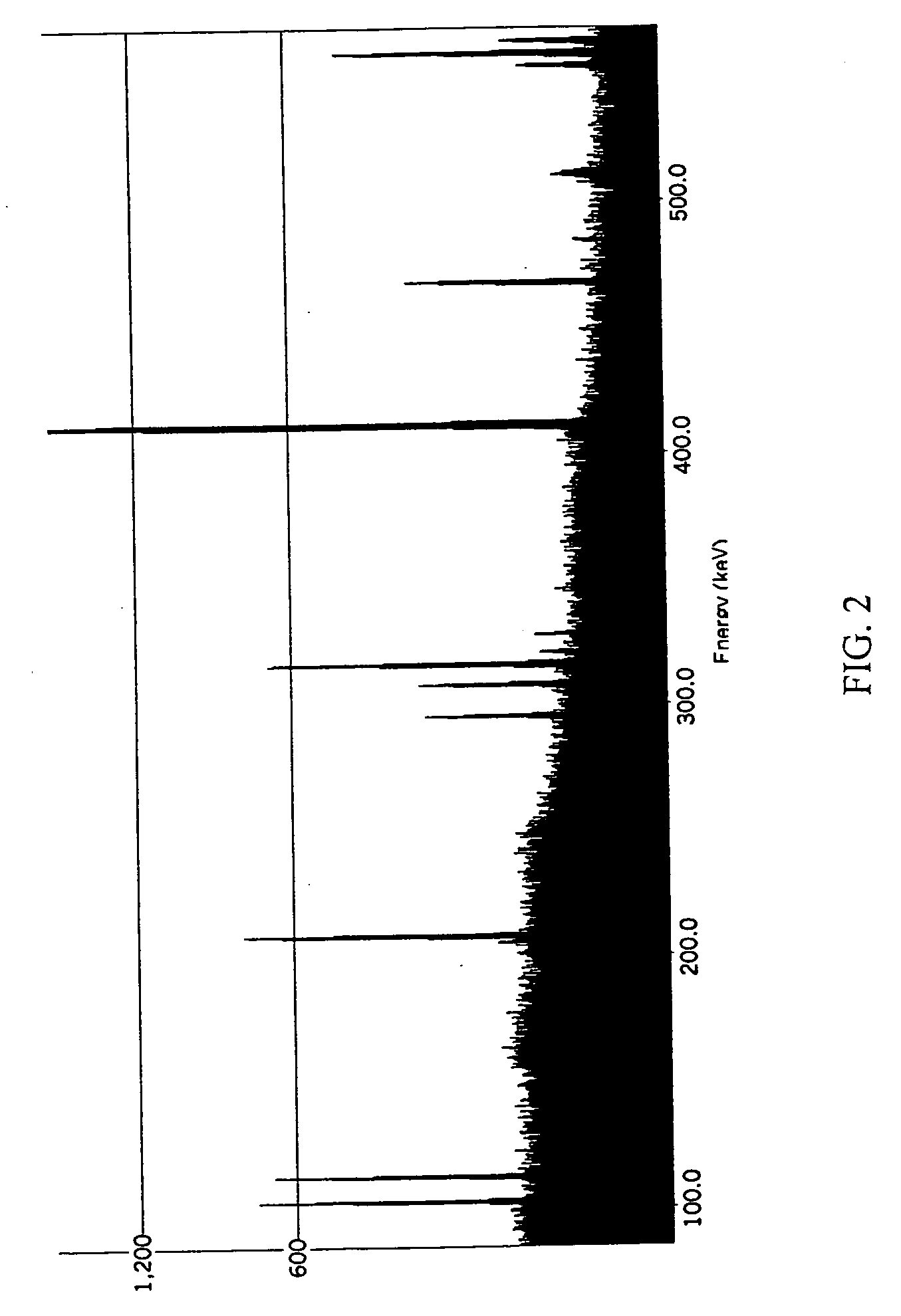
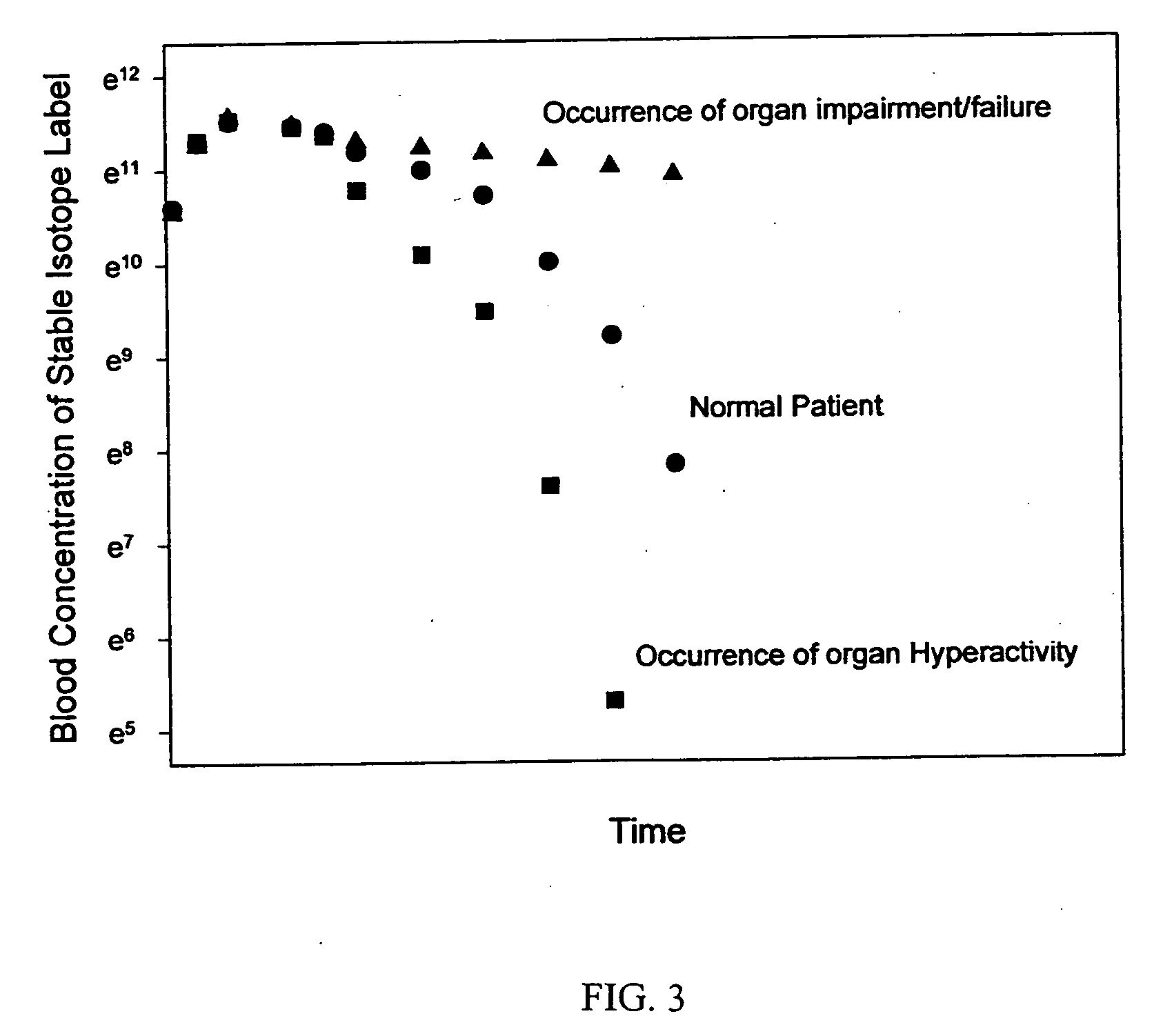
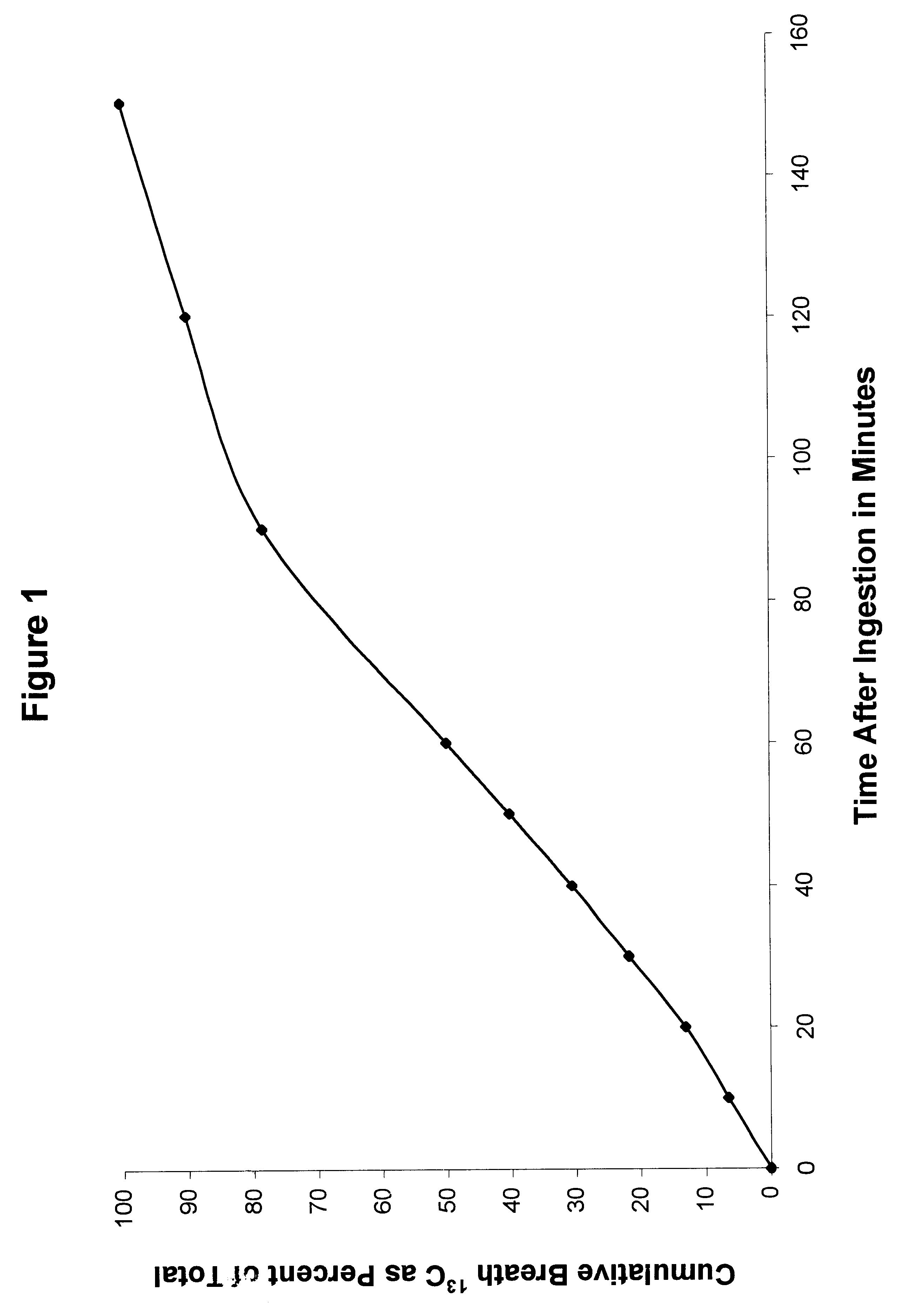
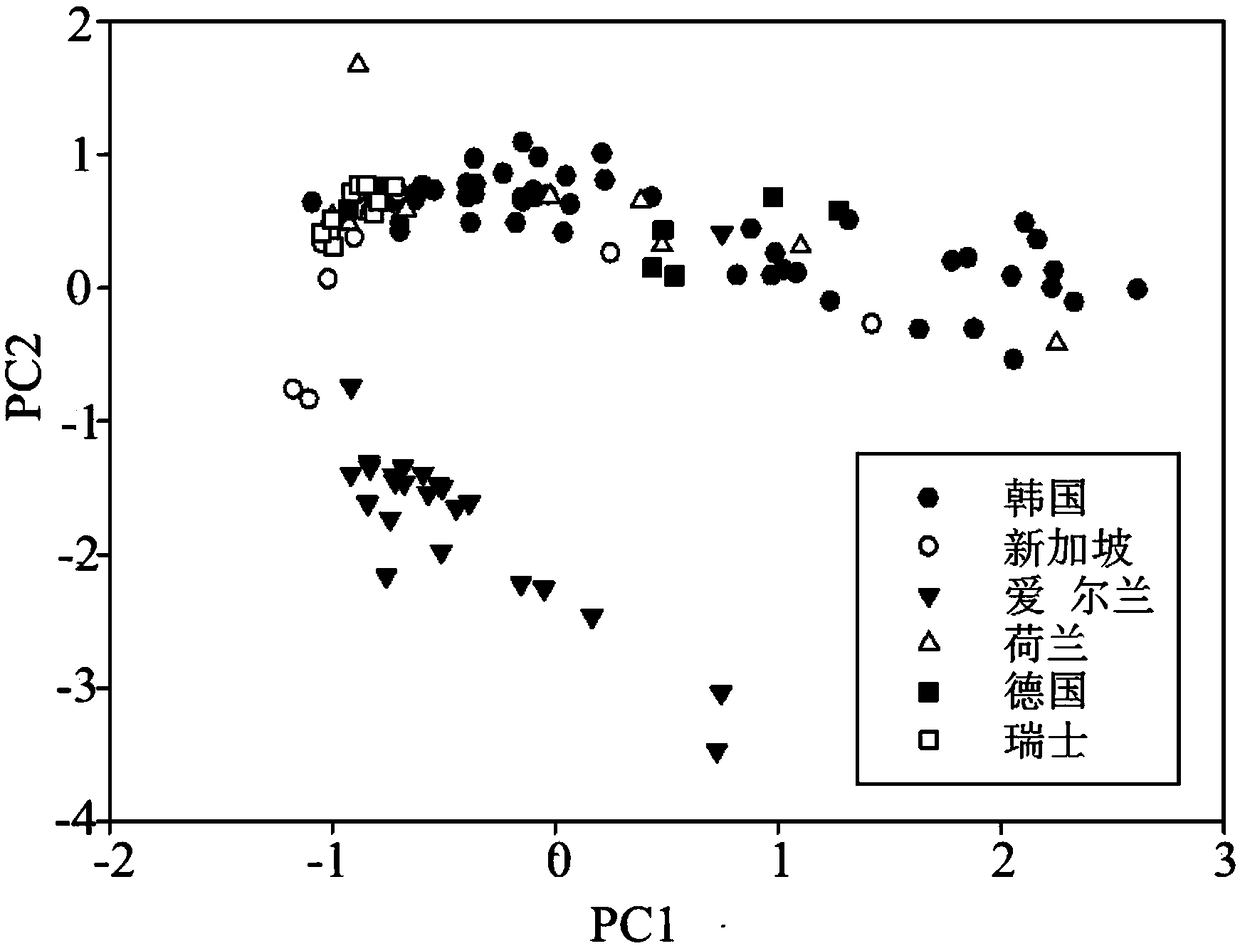
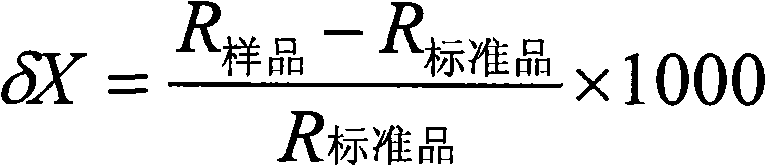Patents
Literature
424 results about "Stable nuclide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
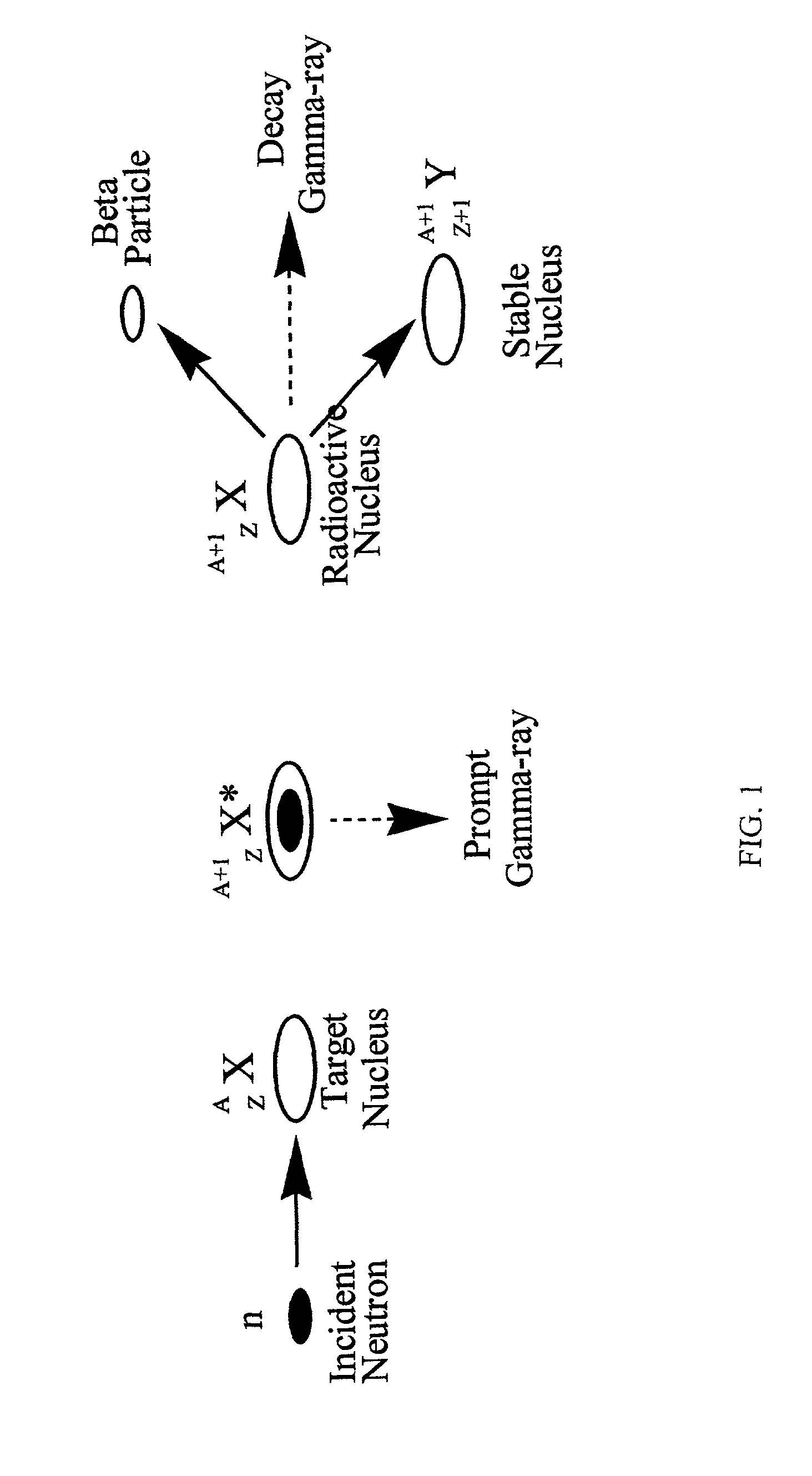
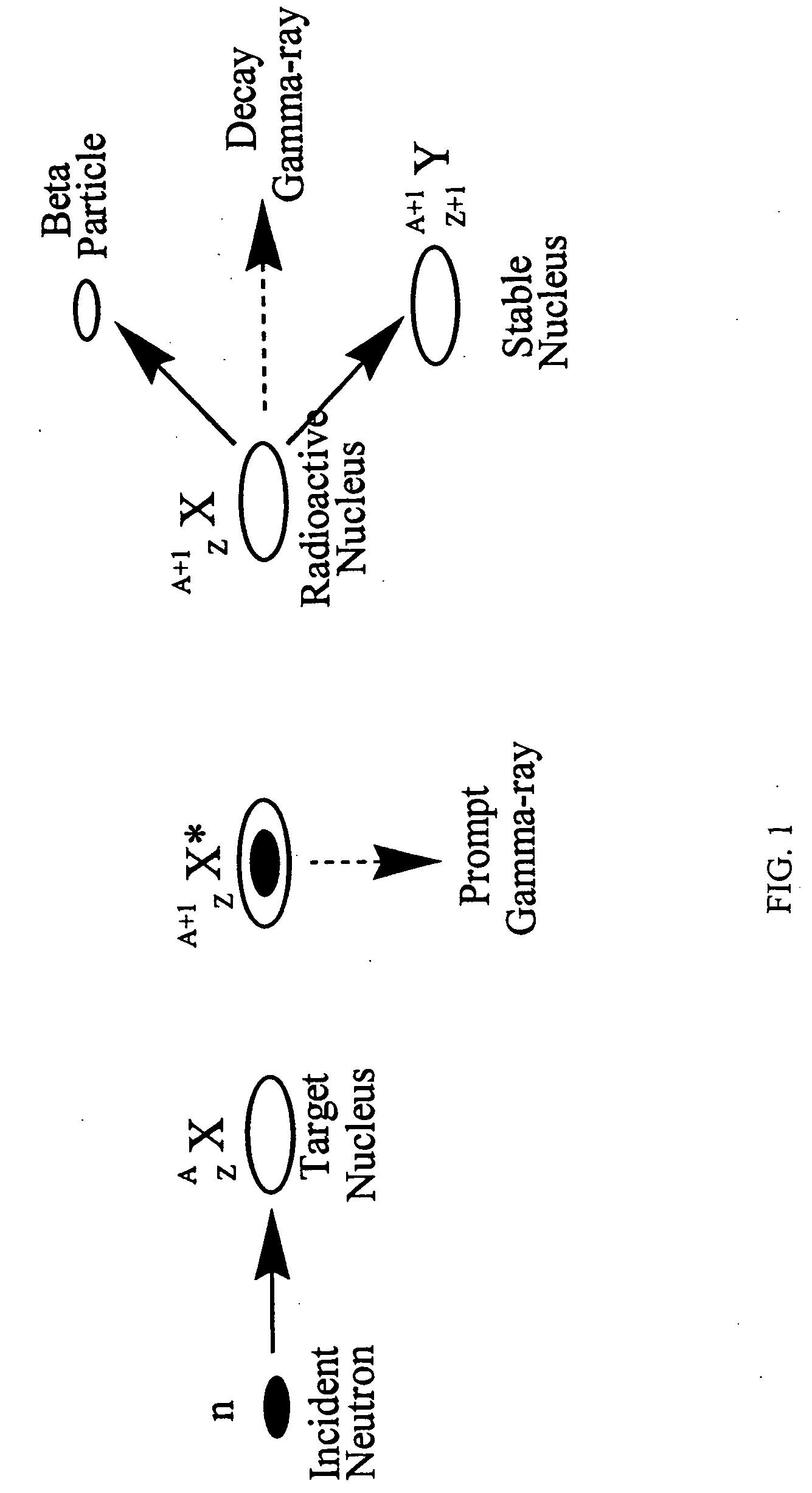
Stable nuclides are nuclides that are not radioactive and so (unlike radionuclides) do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes.
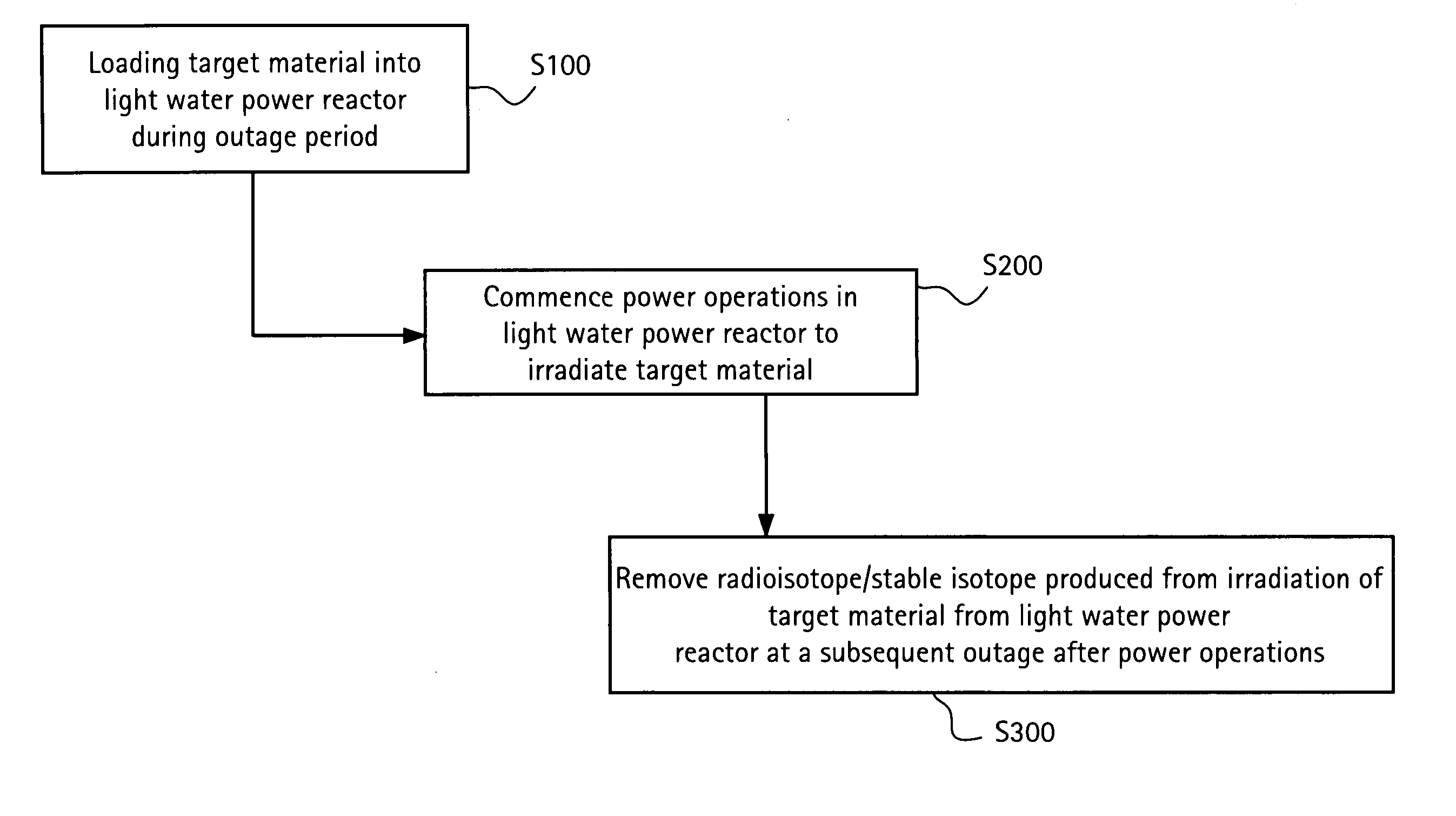
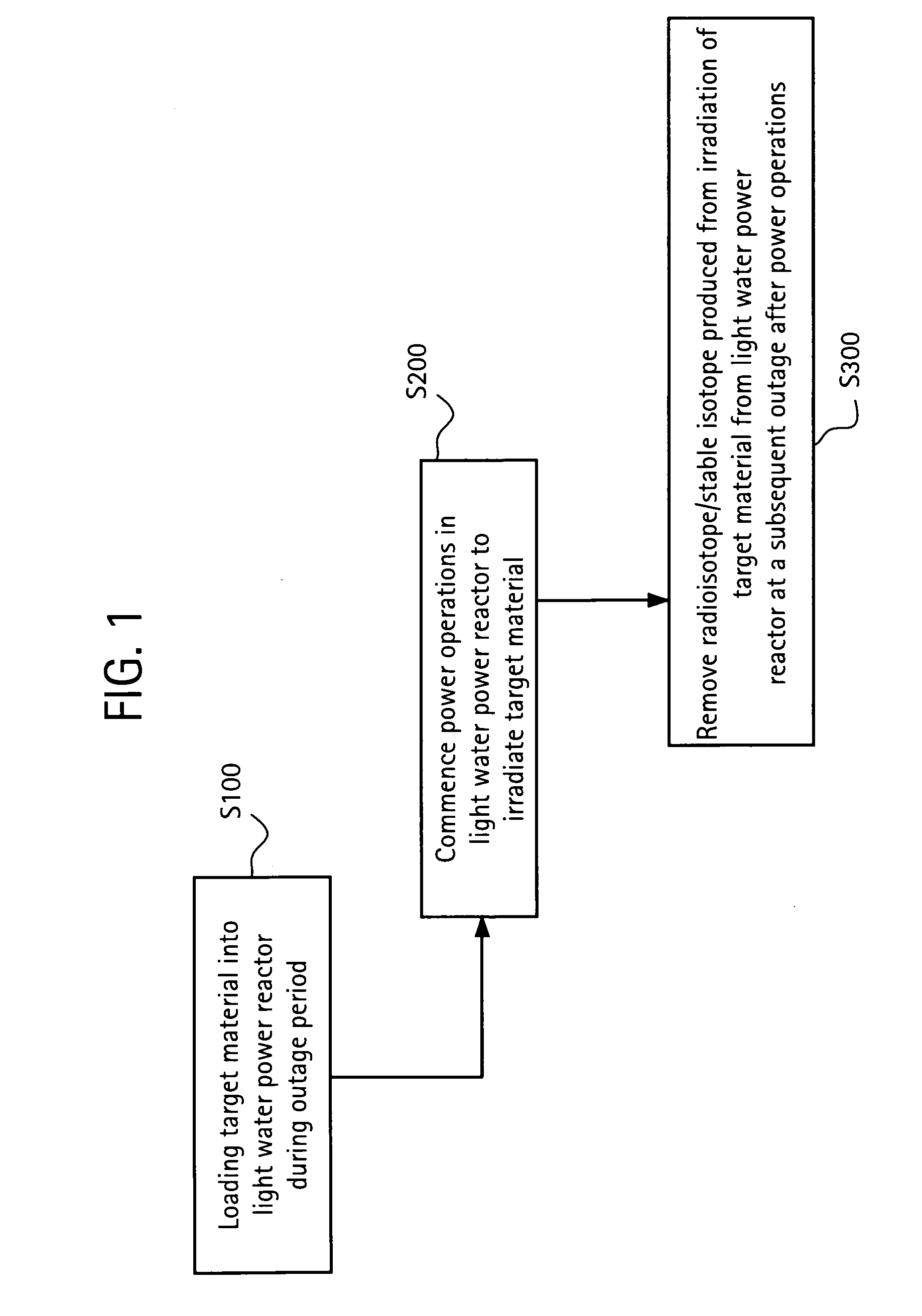
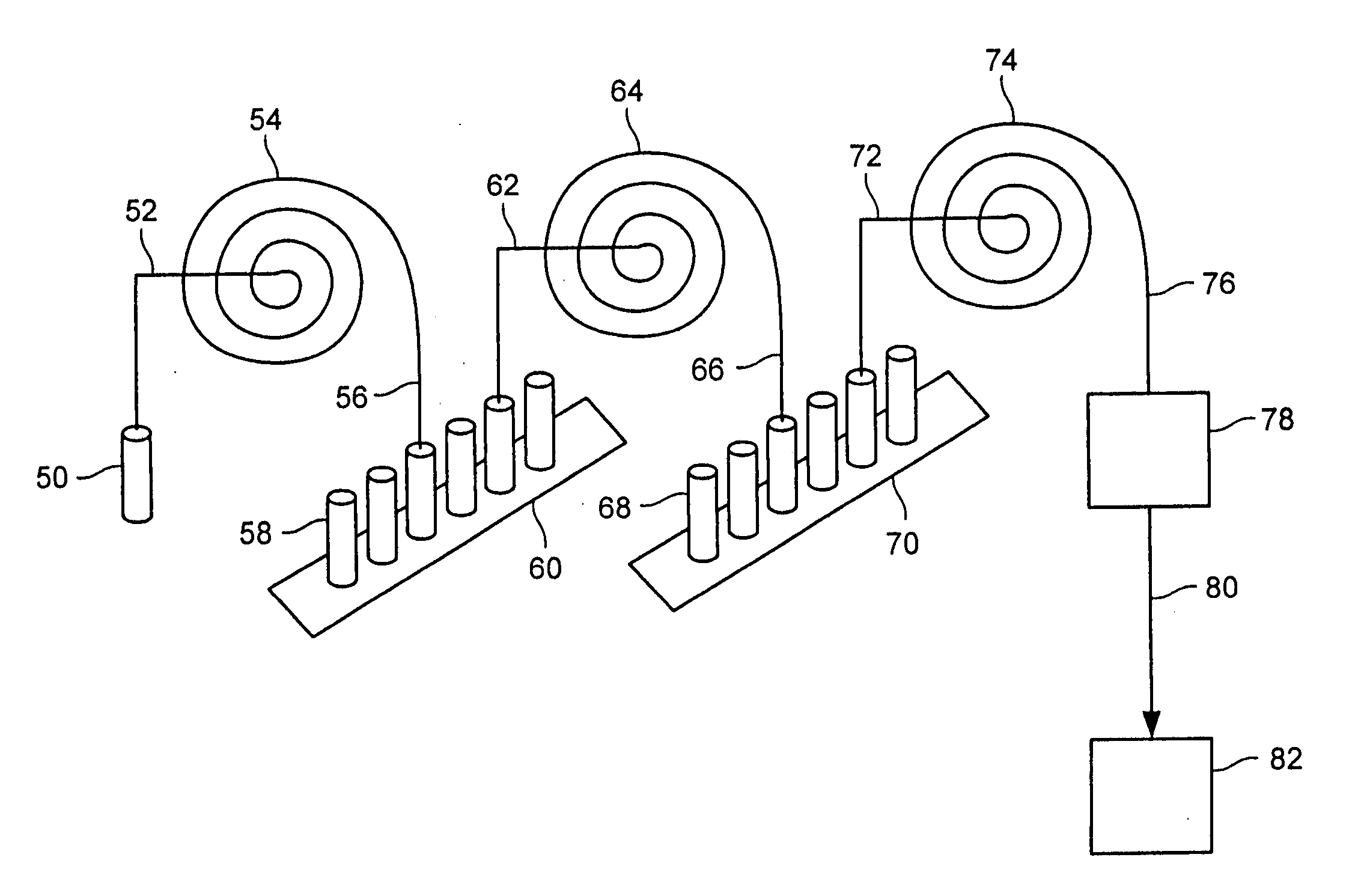
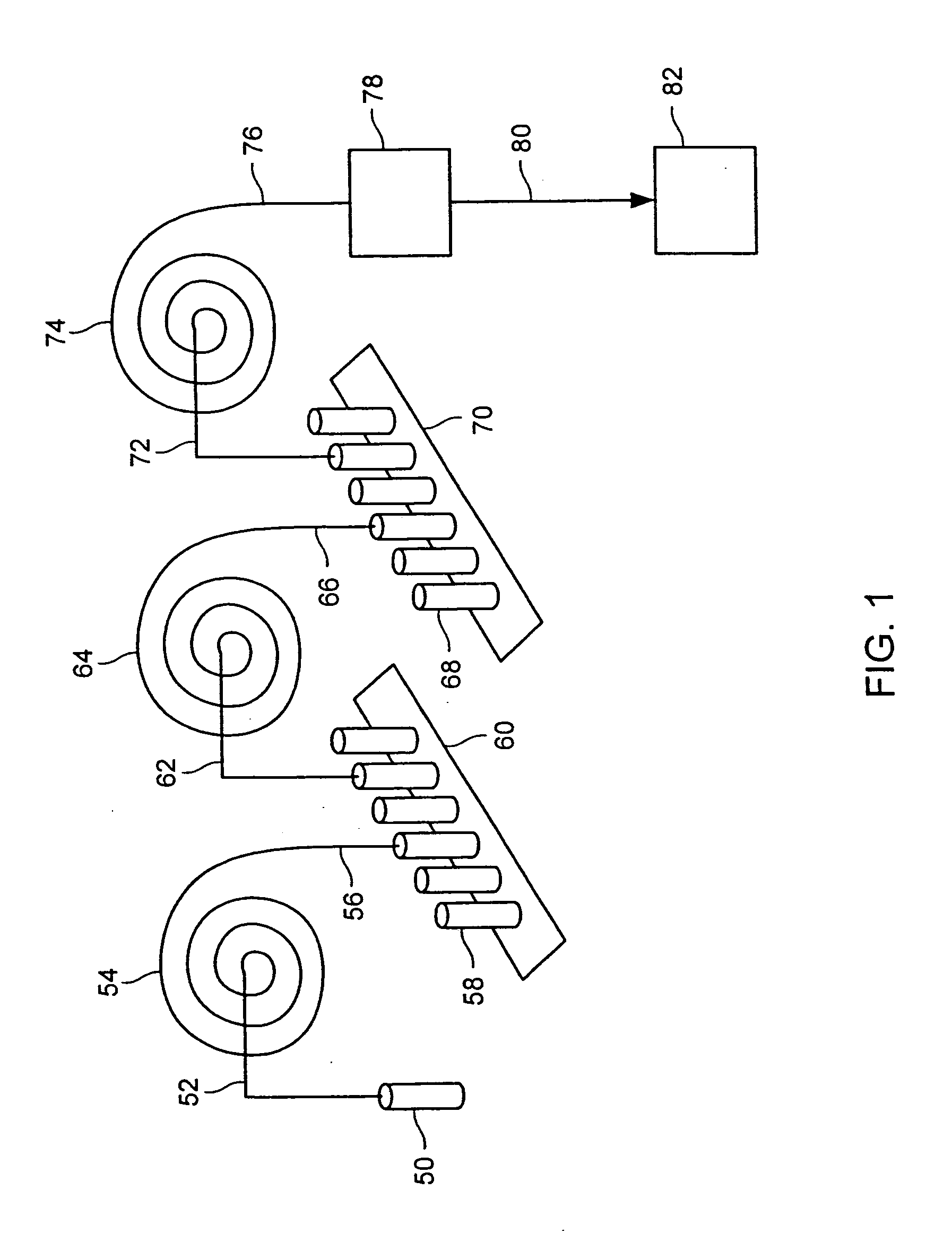
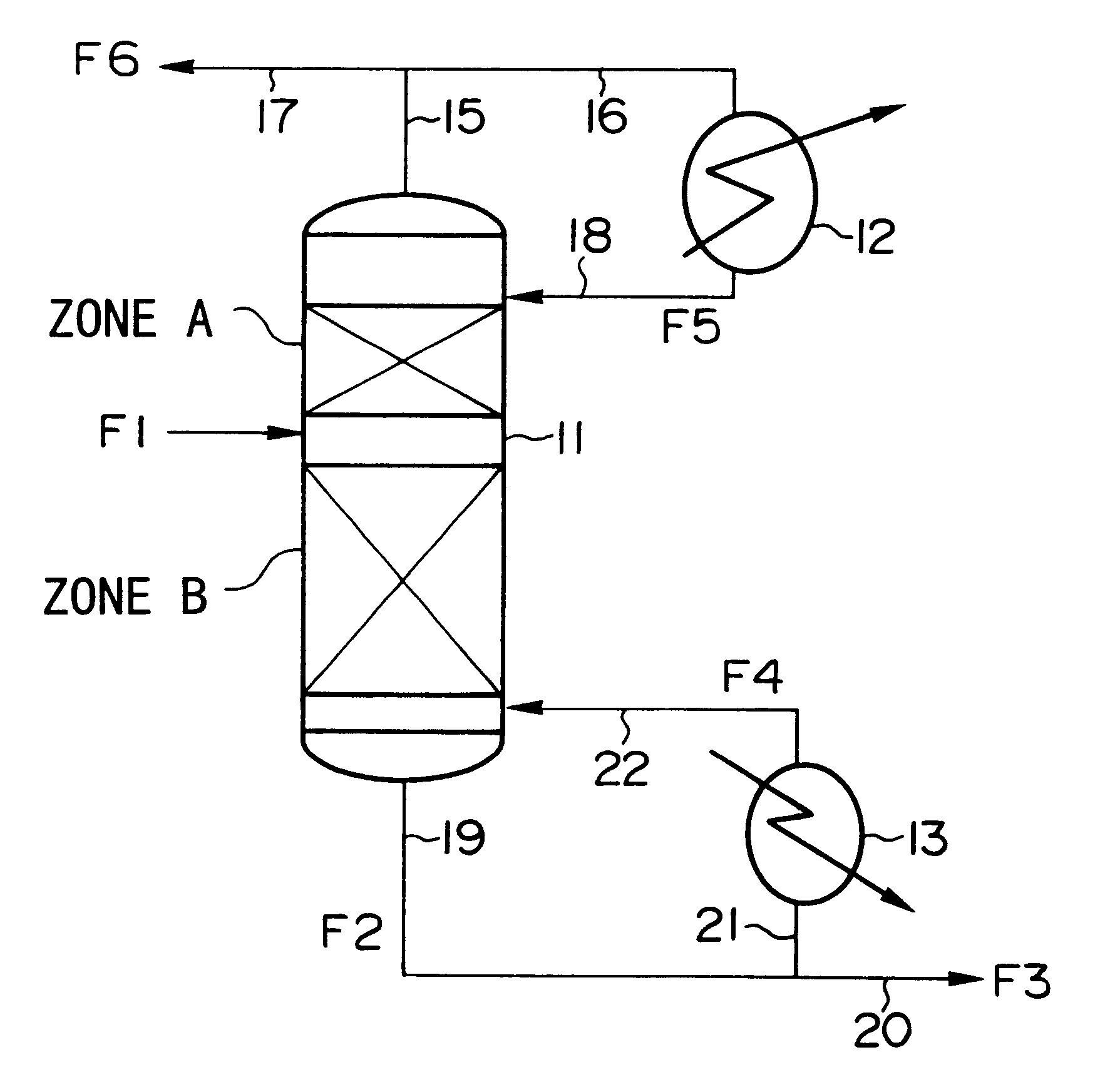
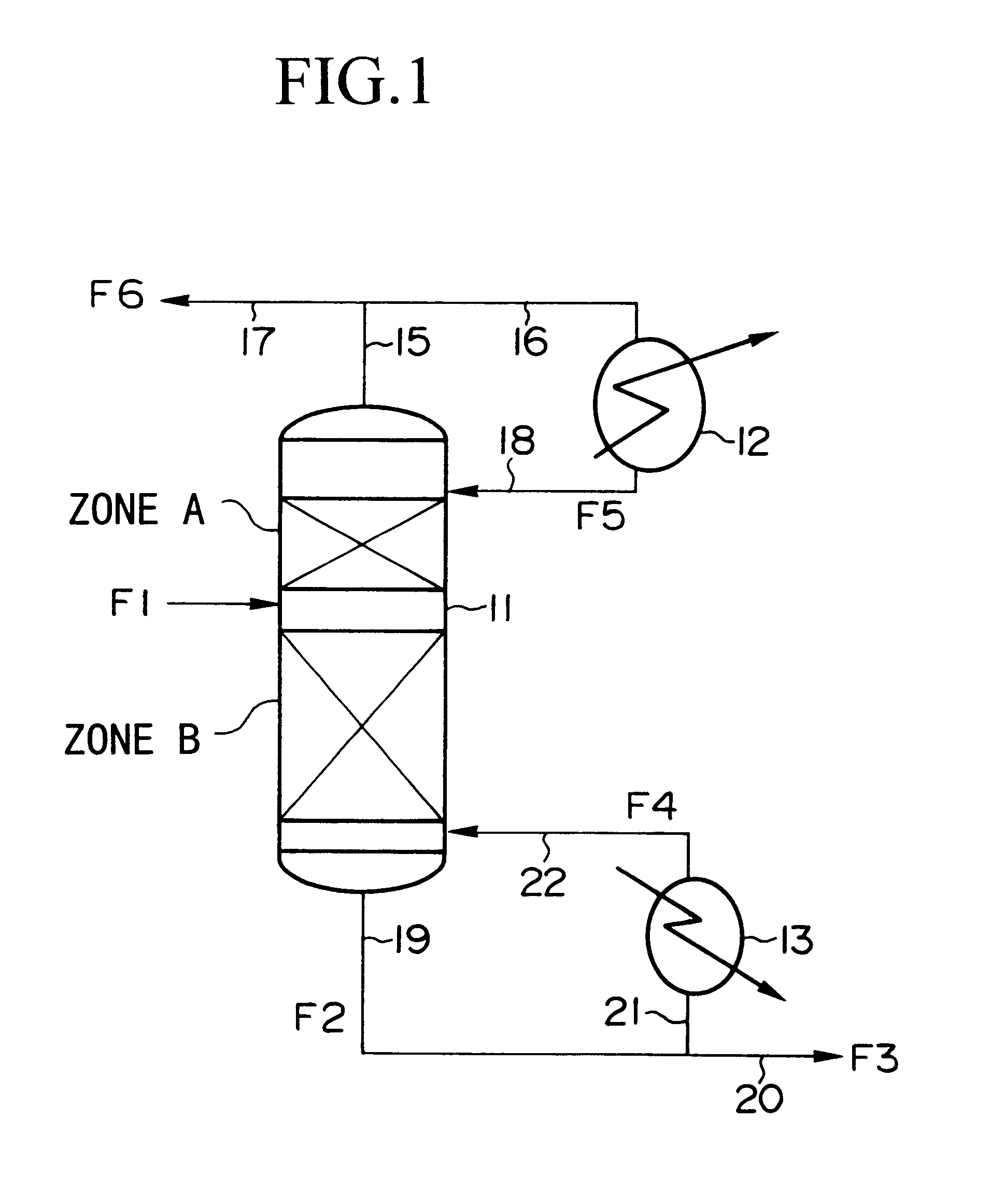
Method of producing isotopes in power nuclear reactors
ActiveUS20070133731A1Nuclear energy generationConversion in nuclear reactorNuclear reactorNeutron flux
In a method of producing isotopes in a light water power reactor, one or more targets within the reactor may be irradiated under a neutron flux to produce one or more isotopes. The targets may be assembled into one or more fuel bundles that are to be loaded in a core of the reactor at a given outage. Power operations in the reactor irradiate the fuel bundles so as to generate desired isotopes, such as one or more radioisotopes at a desired specific activity or stable isotopes at a desired concentration.
Owner:NORDION (CANADA) INC
Methods for conducting metabolic analyses
InactiveUS6849396B2Electrolysis componentsVolume/mass flow measurementStable Isotope LabelingAnalyte
The present invention provides methods and apparatus for purifying metabolites of interest and conducting metabolic analyses. The methods generally involve determining metabolic flux values for a plurality of target analytes by monitoring the relative isotope abundance of a stable isotope in a substrate labeled with the stable isotope and / or one or more target metabolites formed through metabolism of the labeled substrate. Certain methods utilize multiple electrophoretic methods to separate the target analytes from other components within the sample being analyzed. The methods can be used in a variety of applications including screens to identify metabolites that are correlated with certain diseases and diagnostic screens for identifying individuals having, or susceptible to, a disease.
Owner:TARGET DISCOVERY
Methods for measuring cellular proliferation and destruction rates in vitro and in vivo
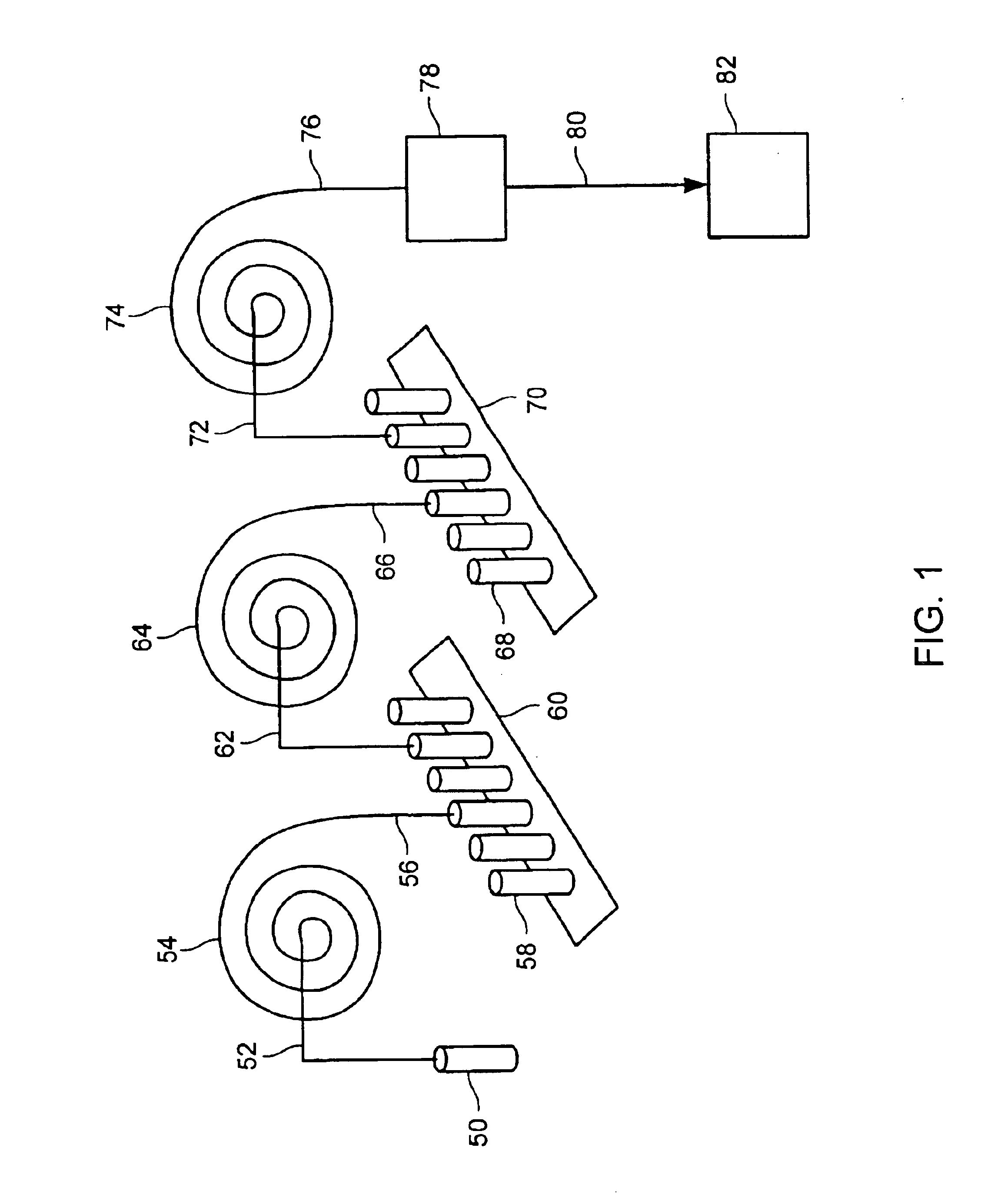
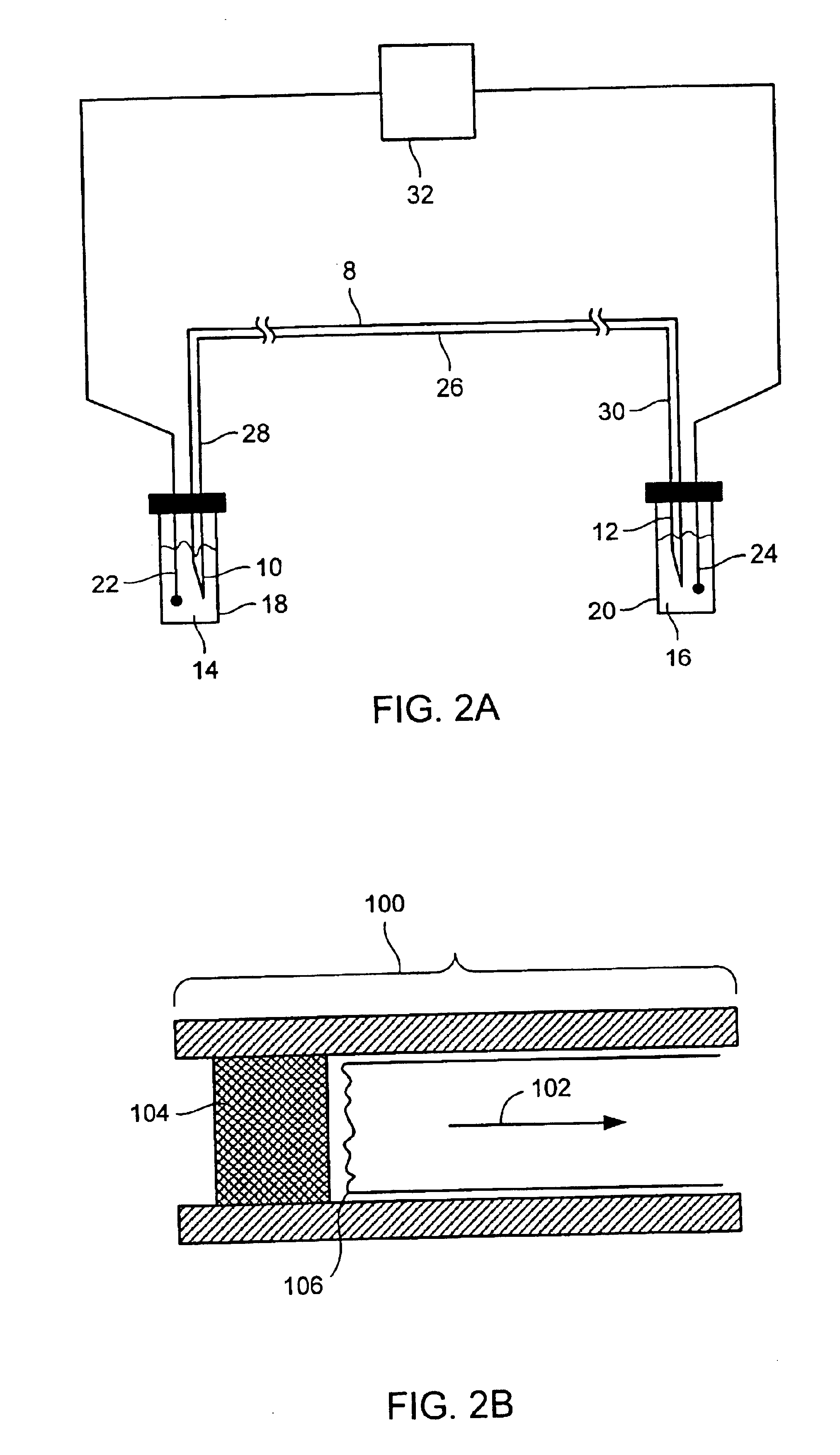
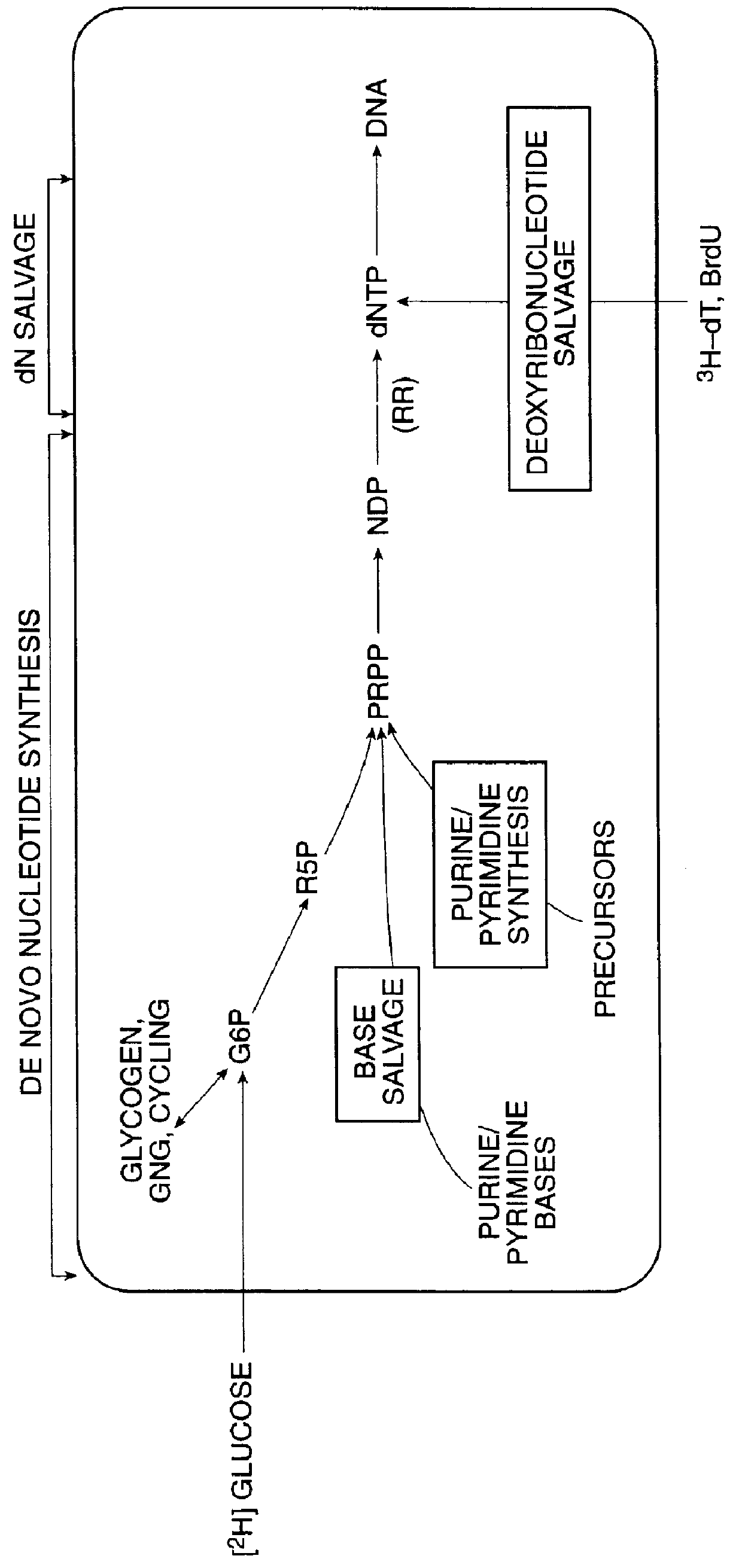
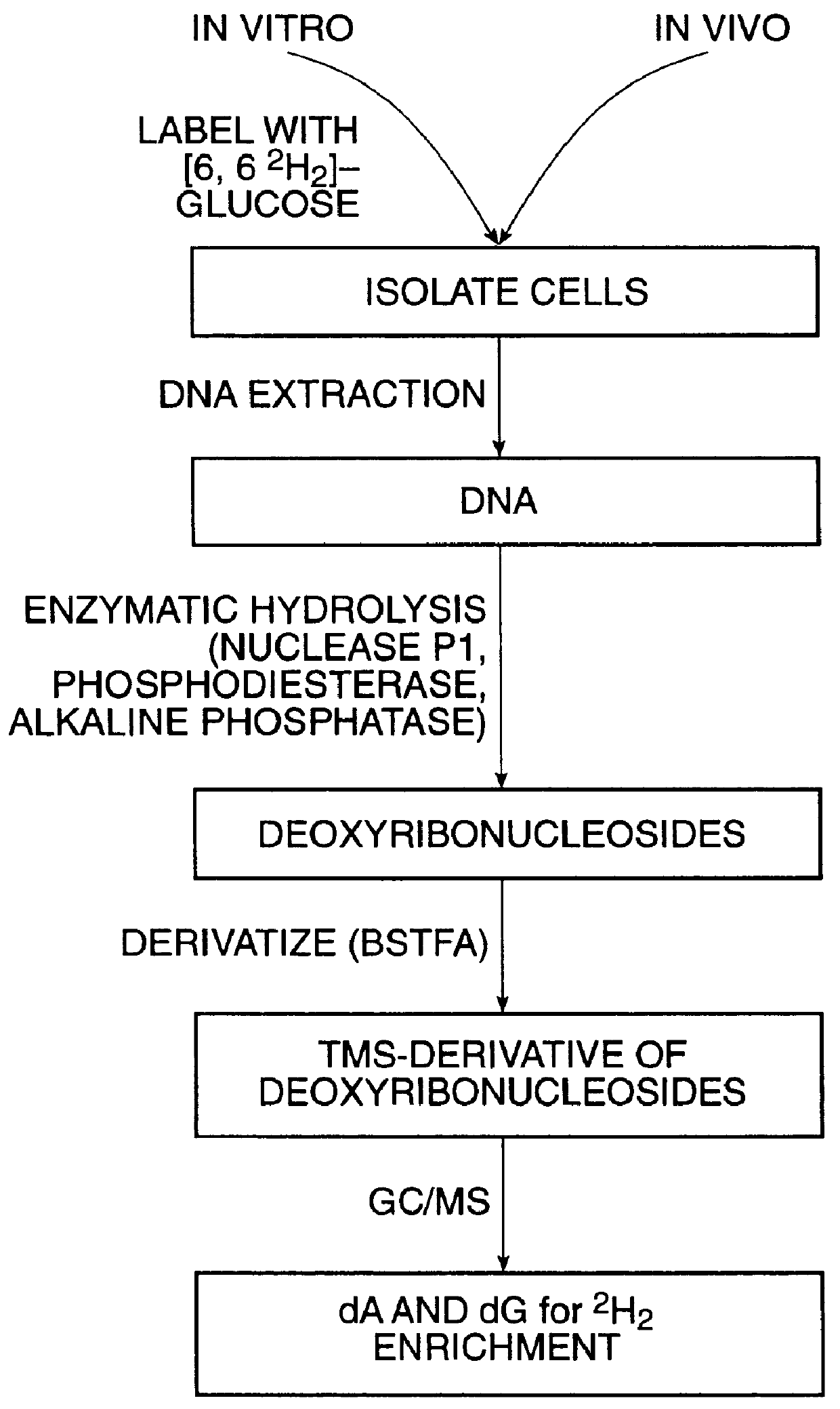
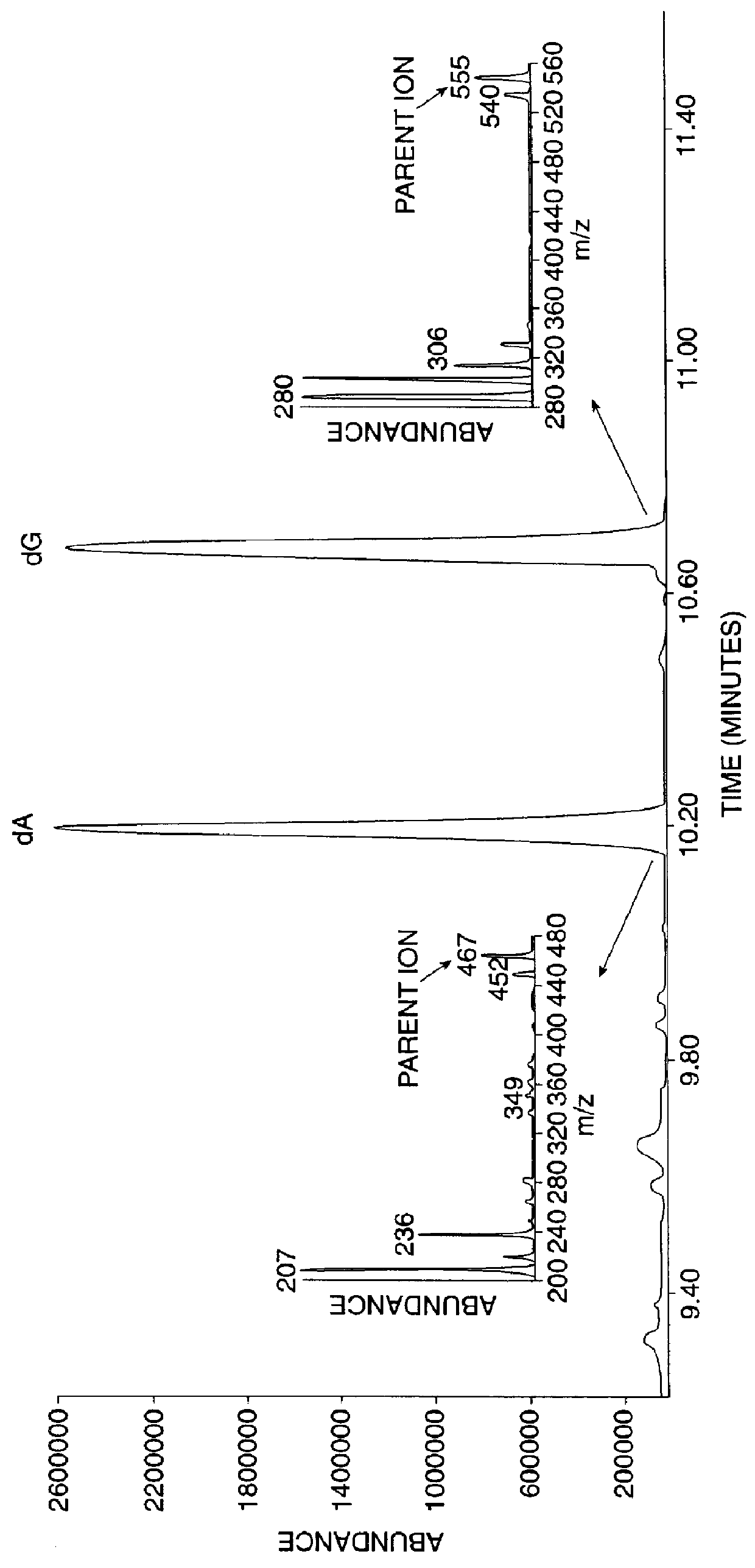
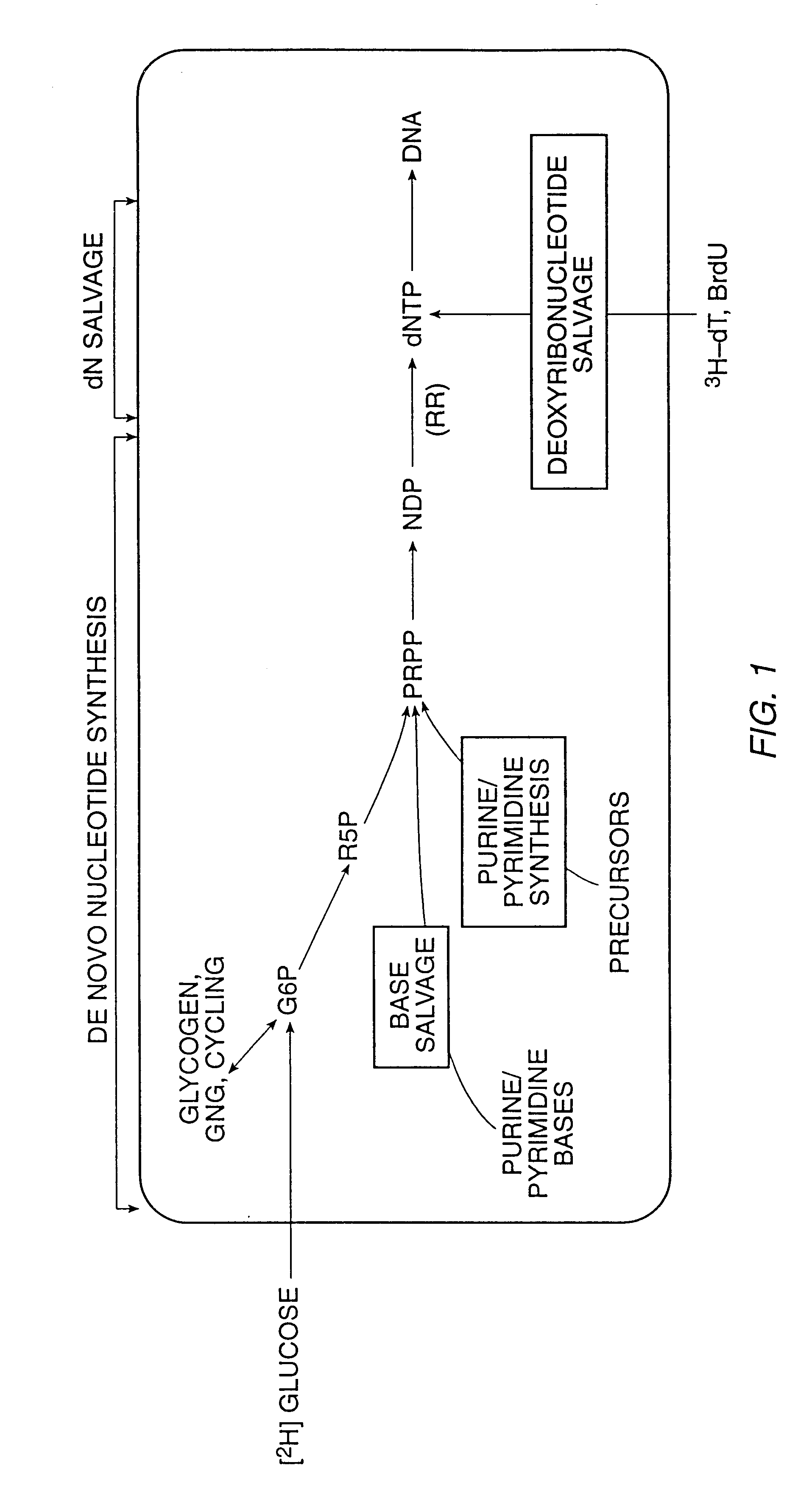
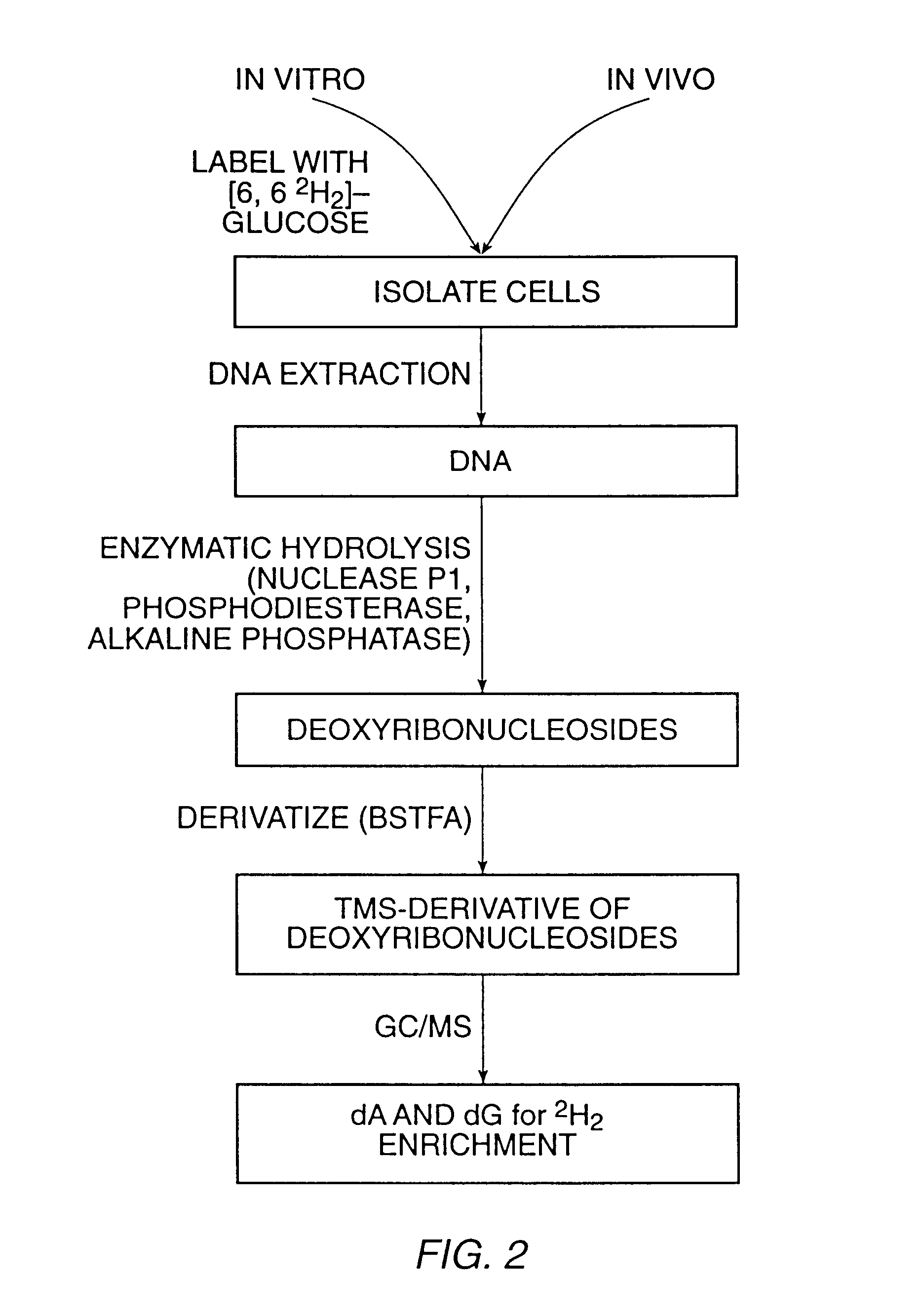
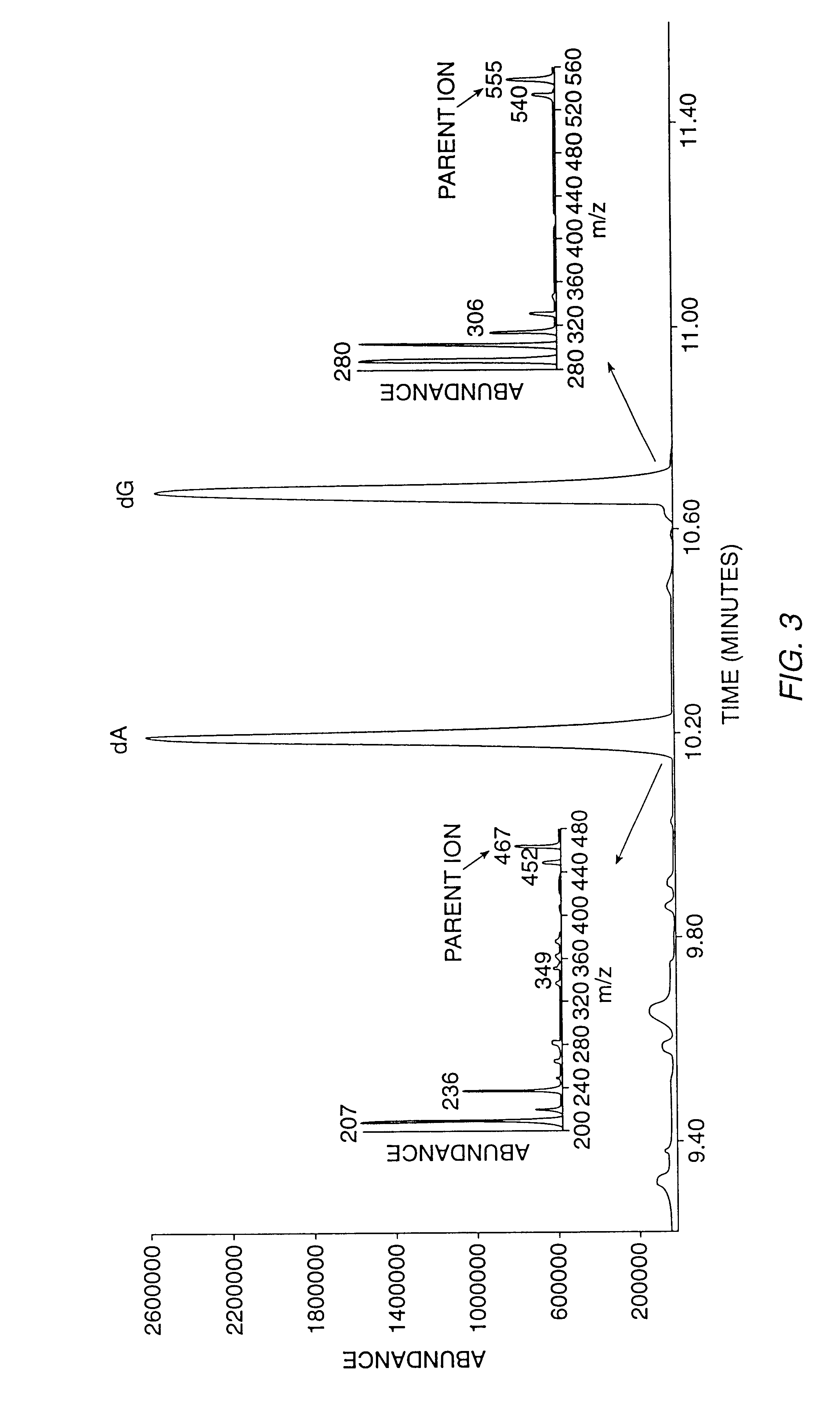
InactiveUS6010846AEasy to detectOrganic active ingredientsIn-vivo radioactive preparationsPopulationStable nuclide
The present invention relates to methods for measuring the proliferation and destruction rates of cells by measuring deoxyribonucleic acid (DNA) synthesis and / or destruction. In particular, the methods utilize non-radioactive stable isotope labels to endogenously label DNA synthesized through the de novo nucleotide synthesis pathway in a cell. The amount of label incorporated in the DNA is measured as an indication of cellular proliferation. The decay of labeled DNA over time is measured as an indication of cellular destruction. Such methods do not involve radioactivity or potentially toxic metabolites, and are suitable for use both in vitro and in vivo. Therefore, the invention is useful for measuring cellular proliferation or cellular destruction rates in humans for the diagnosis, prevention, or management of a variety of disease conditions in which cellular proliferation or cellular destruction is involved. The invention also provides methods for measuring proliferation or destruction of T cells in a subject infected with human immunodeficiency virus (HIV) and methods of screening an agent for a capacity to induce or inhibit cellular proliferation or destruction. In addition, the invention provides methods for measuring cellular proliferation in a proliferating population which utilize both radioactive isotope labels and stable isotopes to endogenously label DNA through the de novo nucleotide synthesis pathway.
Owner:RGT UNIV OF CALIFORNIA
Methods for measuring cellular proliferation and destruction rates in vitro and in vivo
InactiveUS6461806B1Organic active ingredientsIn-vivo radioactive preparationsStable Isotope LabelingDisease
The present invention relates to methods for measuring the proliferation and destruction rates of cells by measuring deoxyribonucleic acid (DNA) synthesis and / or destruction. In particular, the methods utilize non-radioactive stable isotope labels to endogenously label DNA synthesized through the de novo nucleotide synthesis pathway in a cell. The amount of label incorporated in the DNA is measured as an indication of cellular proliferation. The decay of labeled DNA over time is measured as an indication of cellular destruction. Such methods do not involve radioactivity or potentially toxic metabolites, and are suitable for use both in vitro and in vivo. Therefore, the invention is useful for measuring cellular proliferation or cellular destruction rates in humans for the diagnosis, prevention, or management of a variety of disease conditions in which cellular proliferation or cellular destruction is involved. The invention also provides methods for measuring proliferation or destruction of T cells in a subject infected with human immunodeficiency virus (HIV) and methods of screening an agent for a capacity to induce or inhibit cellular proliferation or destruction. In addition, the invention provides methods for measuring cellular proliferation in a proliferating population which utilize both radioactive isotope labels and stable isotopes to endogenously label DNA through the de novo nucleotide synthesis pathway.
Owner:RGT UNIV OF CALIFORNIA
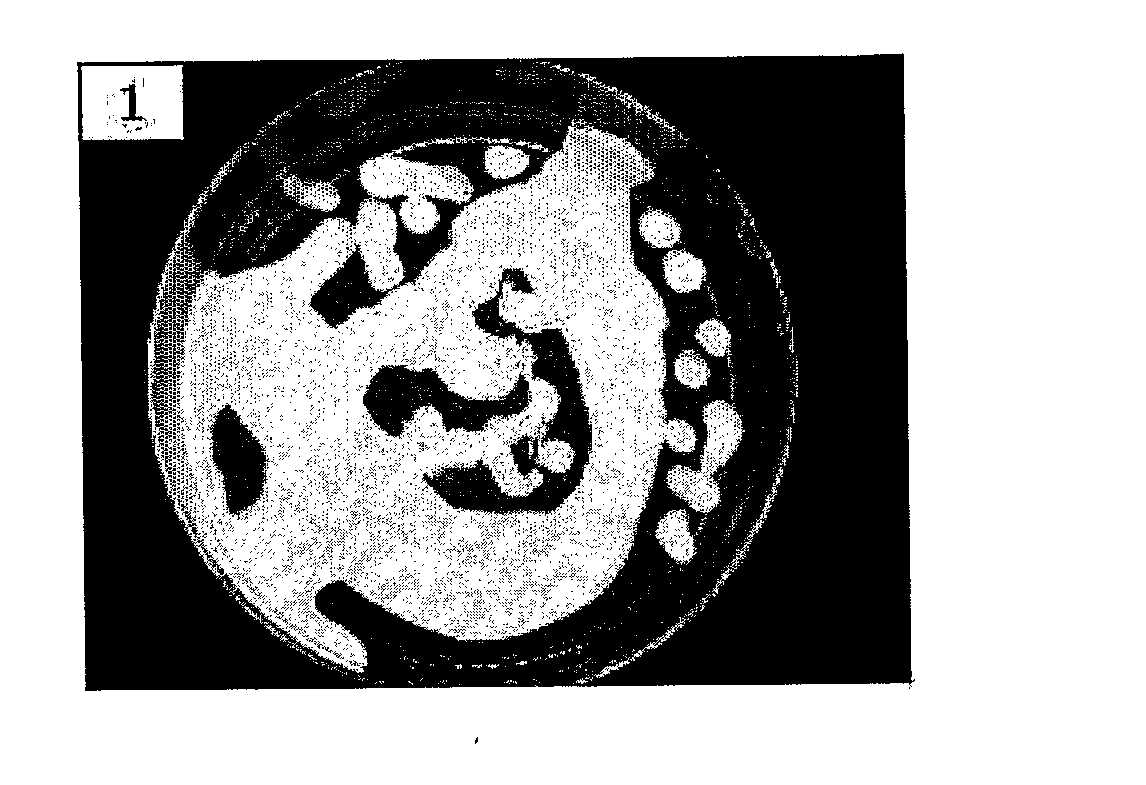
Method For Preparing Radioactive Film
InactiveUS20080031811A1Uniform thickness distributionLow amount of residual solventOther chemical processesRadioactive preparation formsNuclear reactorNeutron irradiation
The present invention relates to a method for preparing a radioactive film for local radioactive treatment. More particularly, the present invention relates to a method for preparing a radioactive film comprising the steps of; dissolving 0.1˜14.5 weight % of a stable nuclide and 13˜32.5 weight % of a film-forming base for the total amount of a solvent in the solvent; applying a stable nuclide solution on a release paper by a coater and drying; and irradiating a stable nuclide film with neutrons in a nuclear reactor. A method for preparing a radioactive film according to the present invention provides a radioactive film having a uniform distribution of radionuclides and an even thickness. Therefore, the therapeutic efficacy of the radioactive film for selective treatment of a lesion may be maximized by attaching the radioactive film on a patient's skin or a mucous membrane and by direct radioactive radiation.
Owner:DONG WHA PHARM CO LTD +1
Fake-proof marking of a composition
InactiveUS20060035380A1Ensure reliabilitySmall possible amountCosmetic preparationsHair cosmeticsAtomic elementRadiochemistry
The invention relates to a process for the fake-proof marking of a product, by introducing at least one non-radioactive stable isotope of at least one atomic element, such that the content of the isotope in the product is higher than its natural content, the isotope being chosen from natural isotopes that have a natural isotopic content of less than 1.2%, and better still less than 1%.
Owner:LOREAL SA
Methods for conducting metabolic analyses
InactiveUS20050153346A1In-vivo radioactive preparationsMicrobiological testing/measurementStable Isotope LabelingMetabolite
The present invention provides methods and apparatus for purifying metabolites of interest and conducting metabolic analyses. The methods generally involve determining metabolic flux values for a plurality of target analytes by monitoring the relative isotope abundance of a stable isotope in a substrate labeled with the stable isotope and / or one or more target metabolites formed through metabolism of the labeled substrate. Certain methods utilize multiple electrophoretic methods to separate the target analytes from other components within the sample being analyzed. The methods can be used in a variety of applications including screens to identify metabolites that are correlated with certain diseases and diagnostic screens for identifying individuals having, or susceptible to, a disease.
Owner:TARGET DISCOVERY
Synthesis, compositions and methods for the measurement of the concentration of stable-isotope labeled compounds in life forms and life form excretory products
Stable isotope labeling and neutron activation to measure biological functions are provided, as are the use and method of adding a chemical monitor to correct for neutron flux to sample vials prior to the addition of sample is presented, and the use of stable isotopes as a chemical bar code for vials and other items. Methods are provided also for measuring glomerular filtration rate and glomerular sieving function in a subject, and for measuring other physiological functions.
Owner:BIOPAL
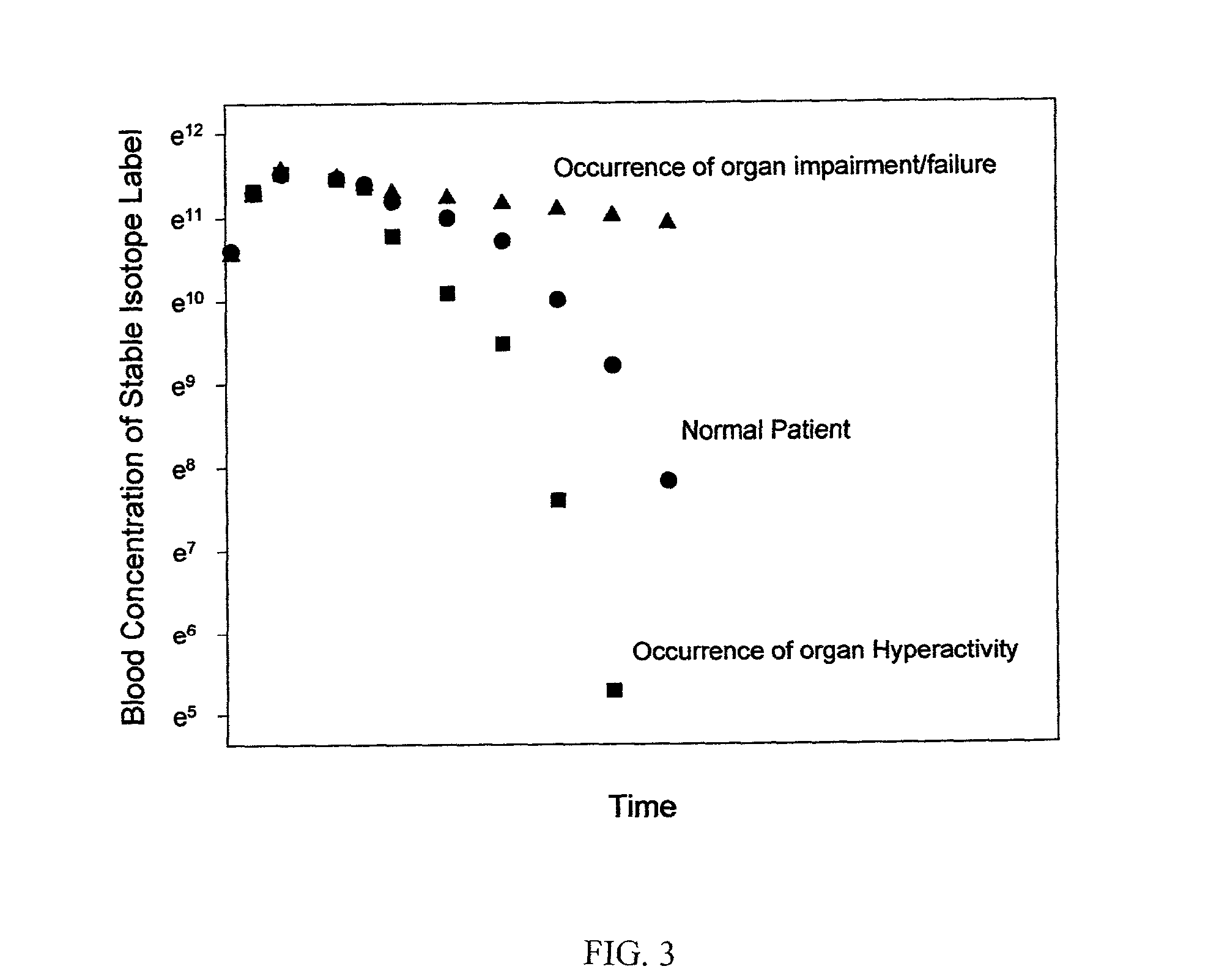
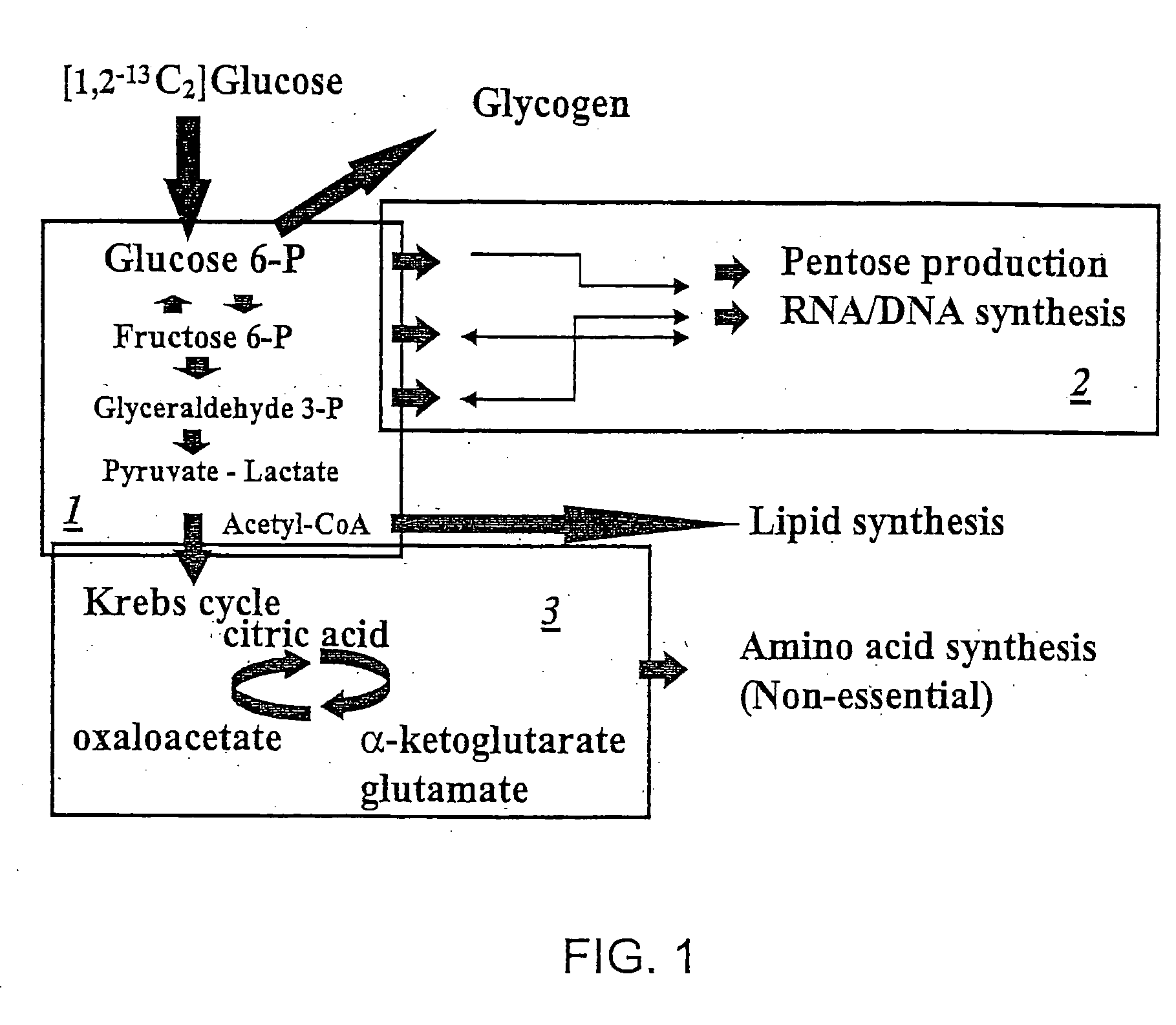
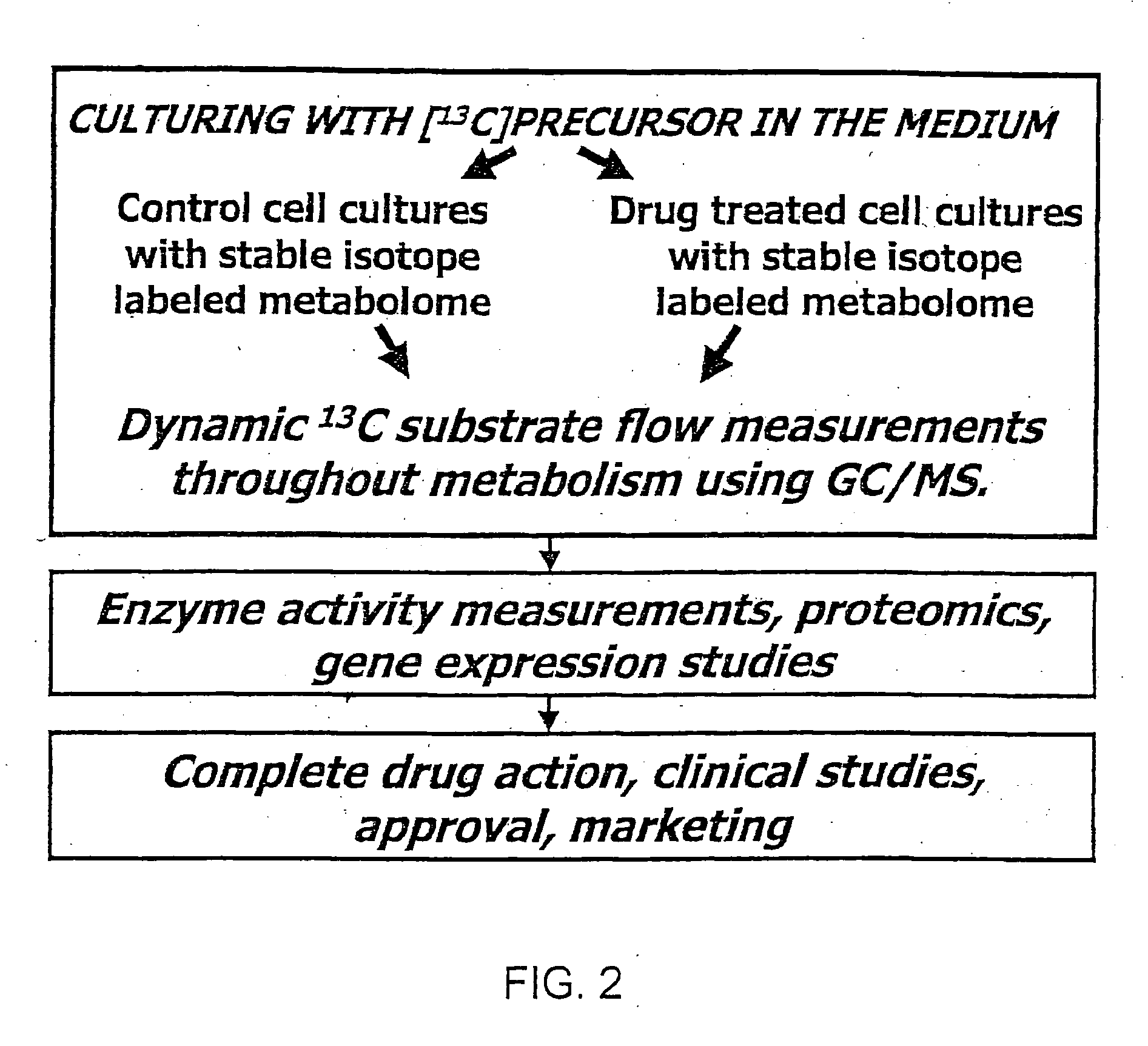
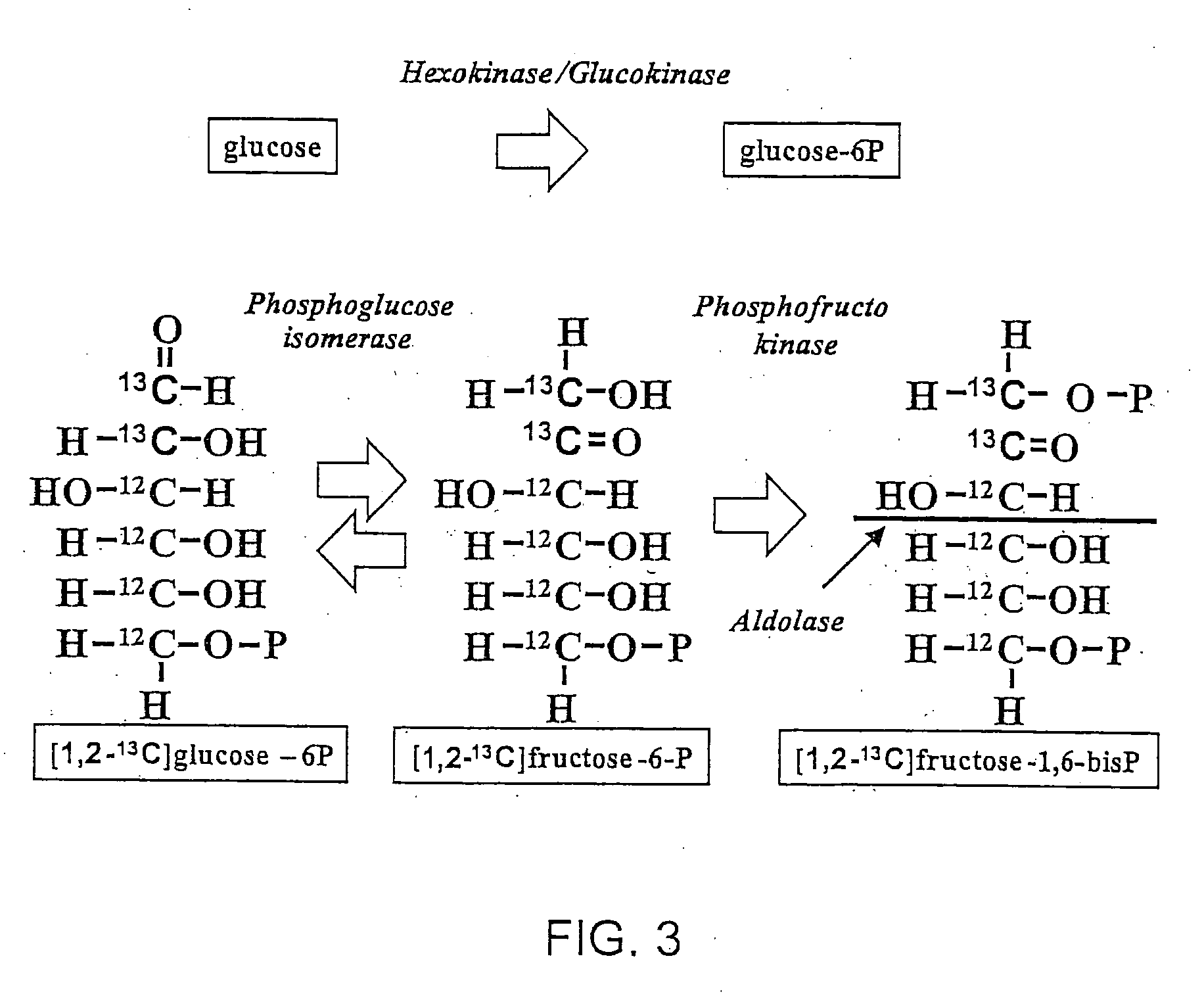
Stable isotope based dynamic metabolic profiling of living organisms for characterization of metabolic diseases, drug testing and drug development
The metabolic processes involved in the formation of any glucose-based metabolite of a metabolic network are determined. A precursor molecule is labeled with a stable carbon (13C) isotope at specific positions. The label is allowed to distribute and rearrange in the system. Metabolites are recovered and analyzed against a control system to determine a set of metabolic pathway substrate fluxes caused by changes to the test system relative to the control system such as the addition of compound being tested as a potential drug.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Method of analyzing metabolism for animal, method of producing labeled animals, labeled animals and method of measuring NMR for animals
InactiveUS20060035382A1Magnetic measurementsVertebrate cellsStable Isotope LabelingNMR - Nuclear magnetic resonance
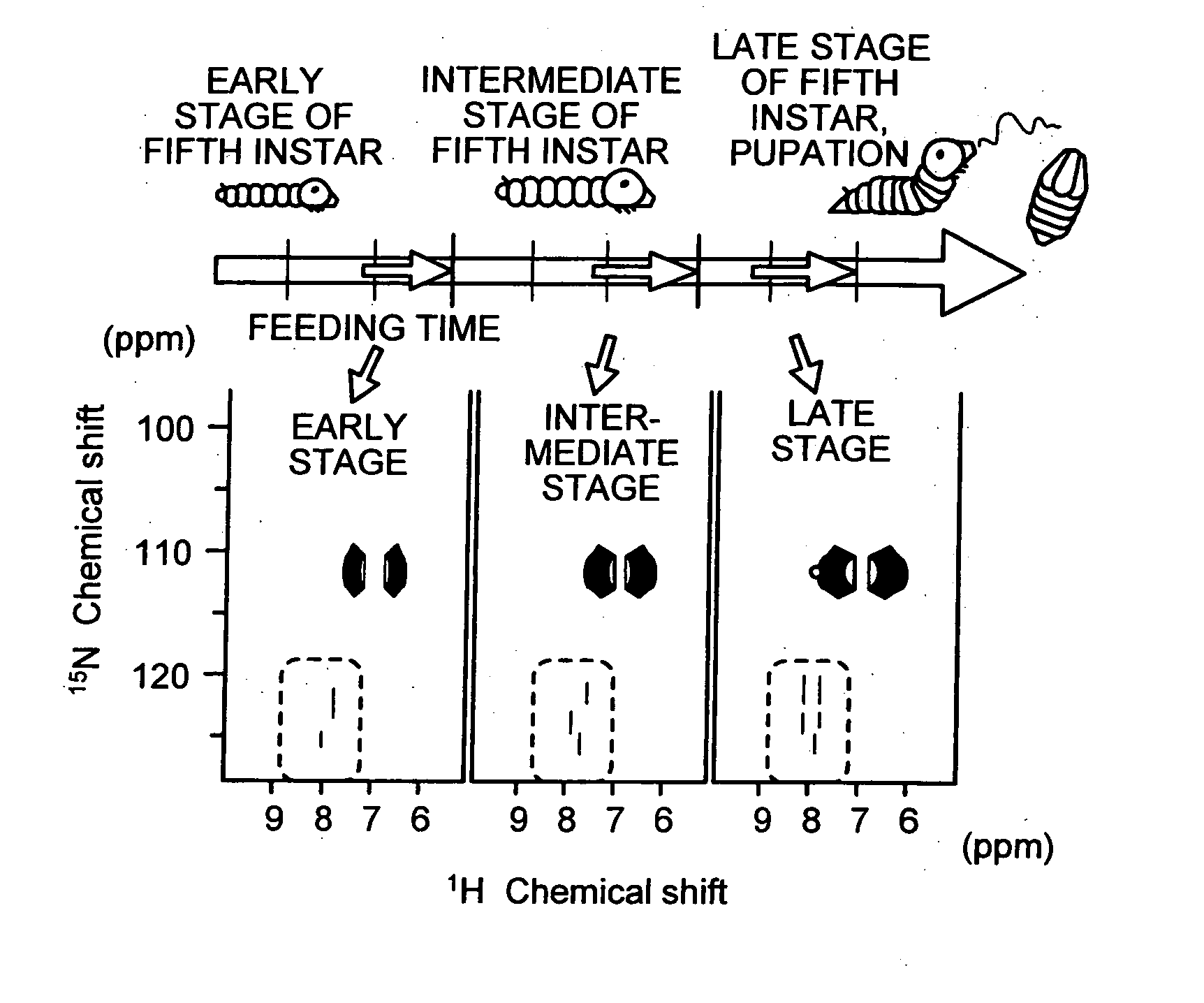
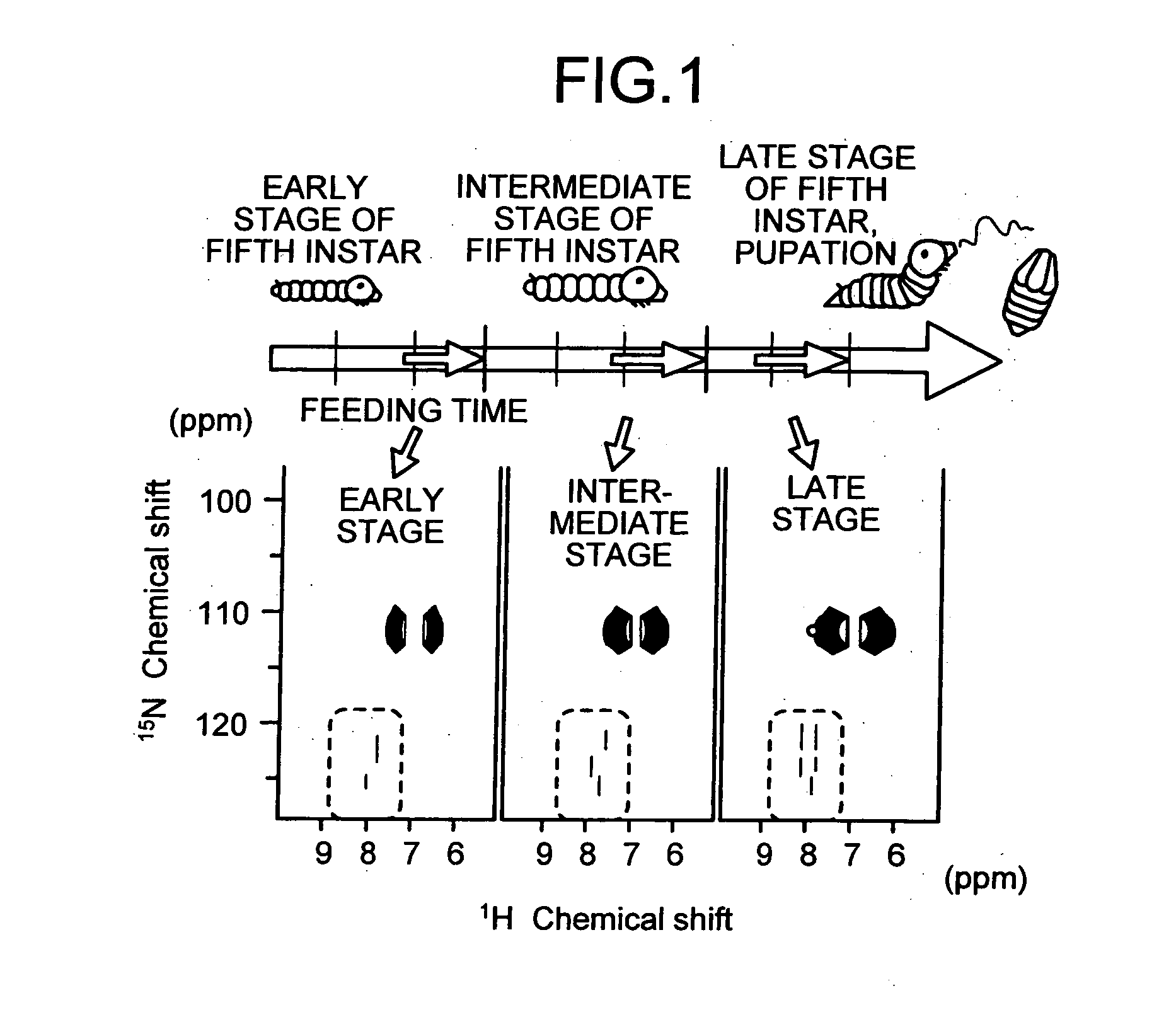
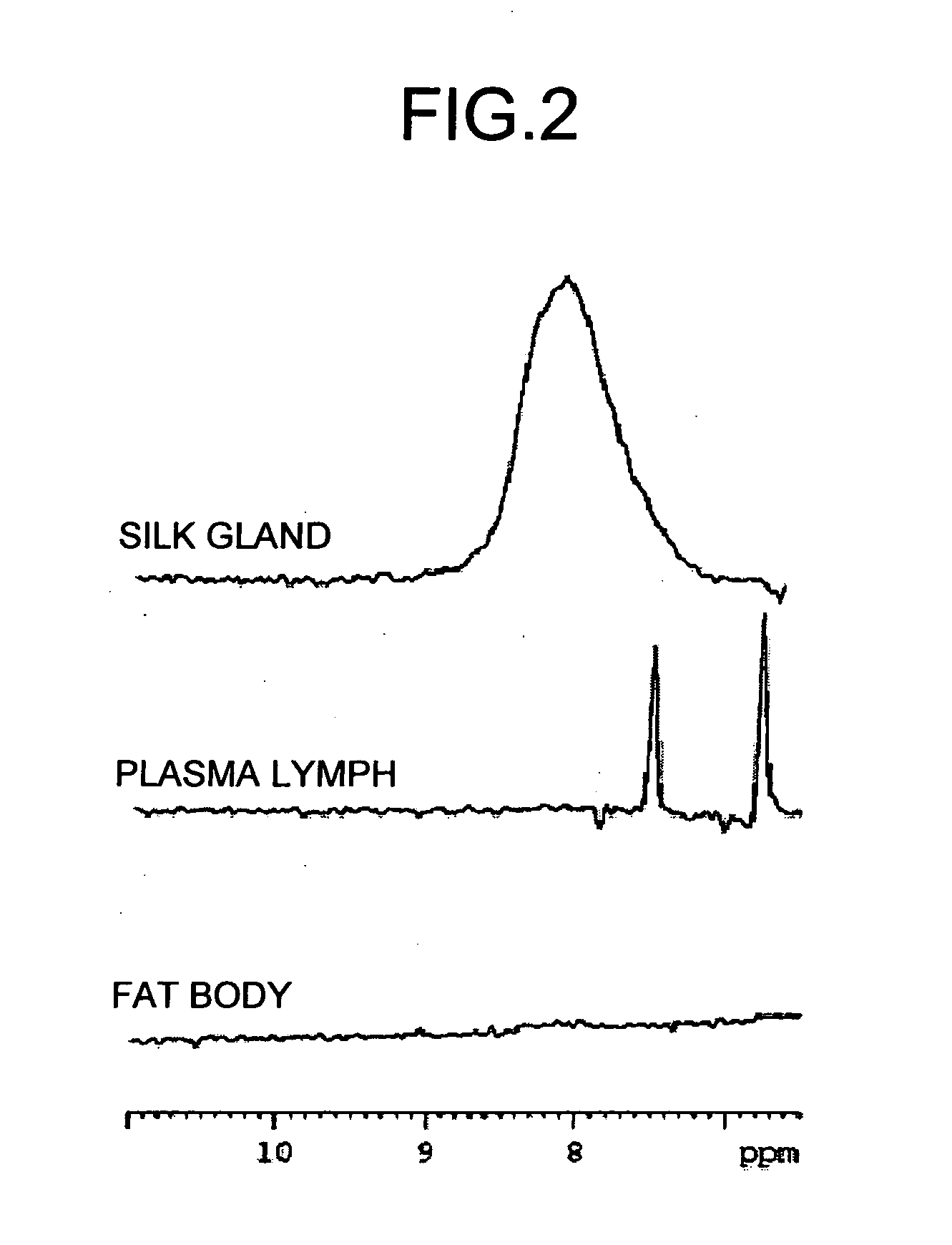
The present invention provides a novel NMR metabolomics method for observing an animal by labeling the animal itself. A labeled animal labeled with a stable isotope is produced by feeding an animal a labeled food labeled with the stable isotope. Metabolism of a biological substance in the animal is analyzed based on nuclear magnetic resonance data of the biological substance containing the stable isotope. The nuclear magnetic resonance data is acquired by performing NMR measurement on a body of the labeled animal, a portion of the body, or an extract.
Owner:RIKEN
Compositions and method for stable isotope labelling of biological compounds
InactiveUS20070082399A1Meaningful NMR spectralEnhanced and good resolvedMicroorganism lysisDepsipeptidesStable Isotope LabelingSpectroscopy
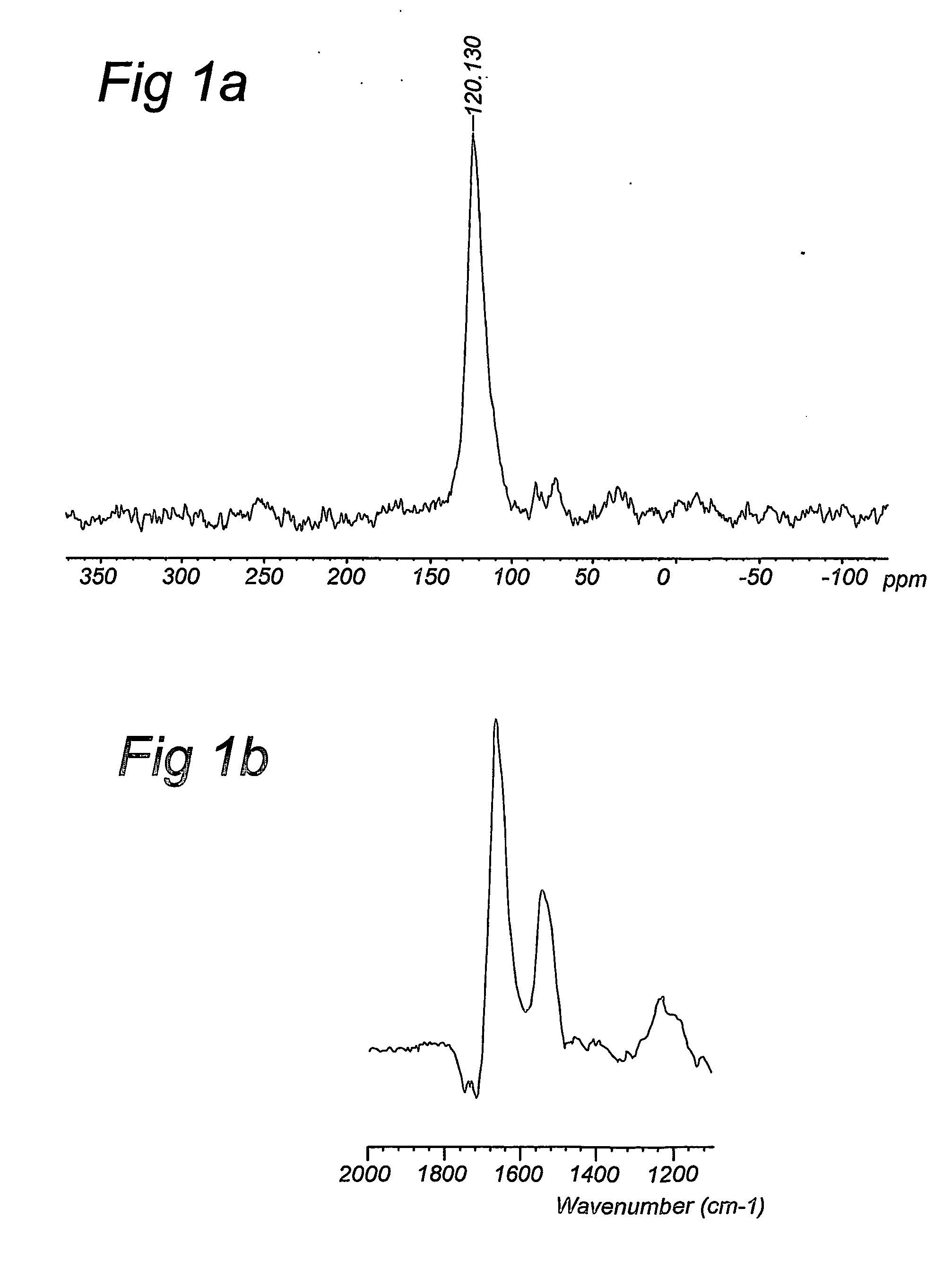
The present invention is concerned the labelling of biological compounds with stable isotopes such that the three-dimensional structure of the biological compounds may be analysed by e.g. NMR spectroscopy. The invention employs microorganisms that are grown on mineral media comprising carbon and nitrogen sources that contain stable isotopes to produce biomass that is uniformly labelled with stable isotopes. The biomass may be autolysed to produce an autolysate. The biomass may further be extracted with organic solvent to produce lipids. The (delipidised) biomass is hydrolysed to produce labelled amino acids and other nutrients, which are used together with the autolysate, extracted lipids and further components to compose a culture medium for a mammalian or insect host cells for the production of biological compounds that are uniformly labelled with stable isotopes. The biological compound preferably is a biological acromolecule, such as e.g. a mammalian membrane protein. Data supplied from the esp@cenet database—Worldwide
Owner:TATIANA A EGOROVA ZACHERNYUK
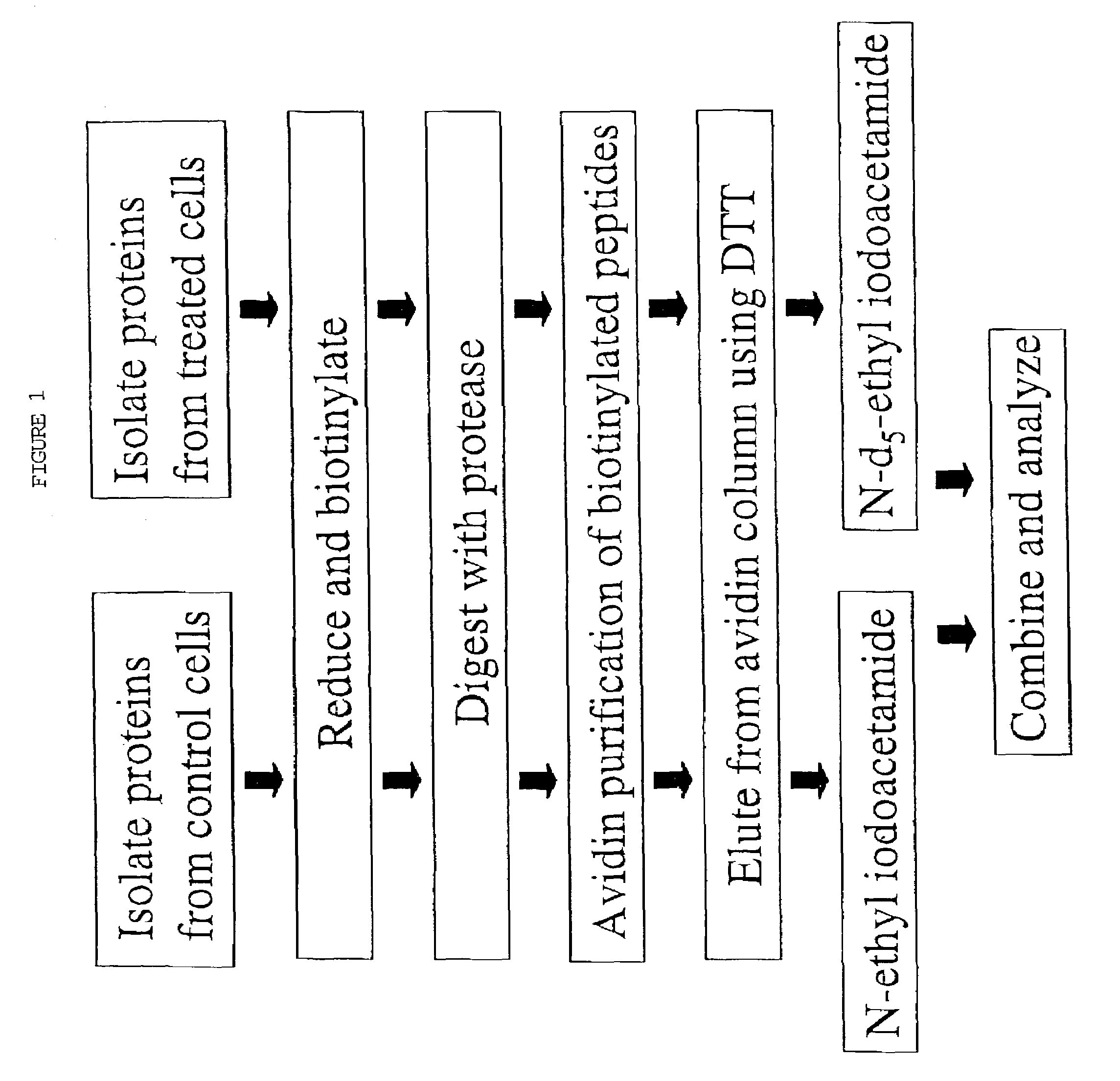
Rapid quantitative analysis of proteins or protein function in complex mixtures
InactiveUS7544518B2Facilitates quantitative determinationFacilitates quantitative determination of the absolute amountsComponent separationMaterial analysis by electric/magnetic meansIsotopic labelingProtein expression profile
Analytical reagents and mass spectrometry-based methods using these reagents for the rapid, and quantitative analysis of proteins or protein function in mixtures of proteins. The methods employ affinity labeled protein reactive reagents having three portions: an affinity label (A) covalently linked to a protein reactive group (PRG) through a linker group (L). The linker may be differentially isotopically labeled, e.g., by substitution of one or more atoms in the linker with a stable isotope thereof. These reagents allow for the selective isolation of peptide fragments or the products of reaction with a given protein (e.g., products of enzymatic reaction) from complex mixtures. The isolated peptide fragments or reaction products are characteristic of the presence of a protein or the presence of a protein function in those mixtures. Isolated peptides or reaction products are characterized by mass spectrometric (MS) techniques. The reagents also provide for differential isotopic labeling of the isolated peptides or reaction products which facilitates quantitative determination by mass spectrometry of the relative amounts of proteins in different samples. The methods of this invention can be used for qualitative and quantitative analysis of global protein expression profiles in cells and tissues, to screen for and identify proteins whose expression level in cells, tissue or biological fluids is affected by a stimulus or by a change in condition or state of the cell, tissue or organism from which the sample originated.
Owner:UNIV OF WASHINGTON
Method for resolving collinearity problem in origin parse for particles in air
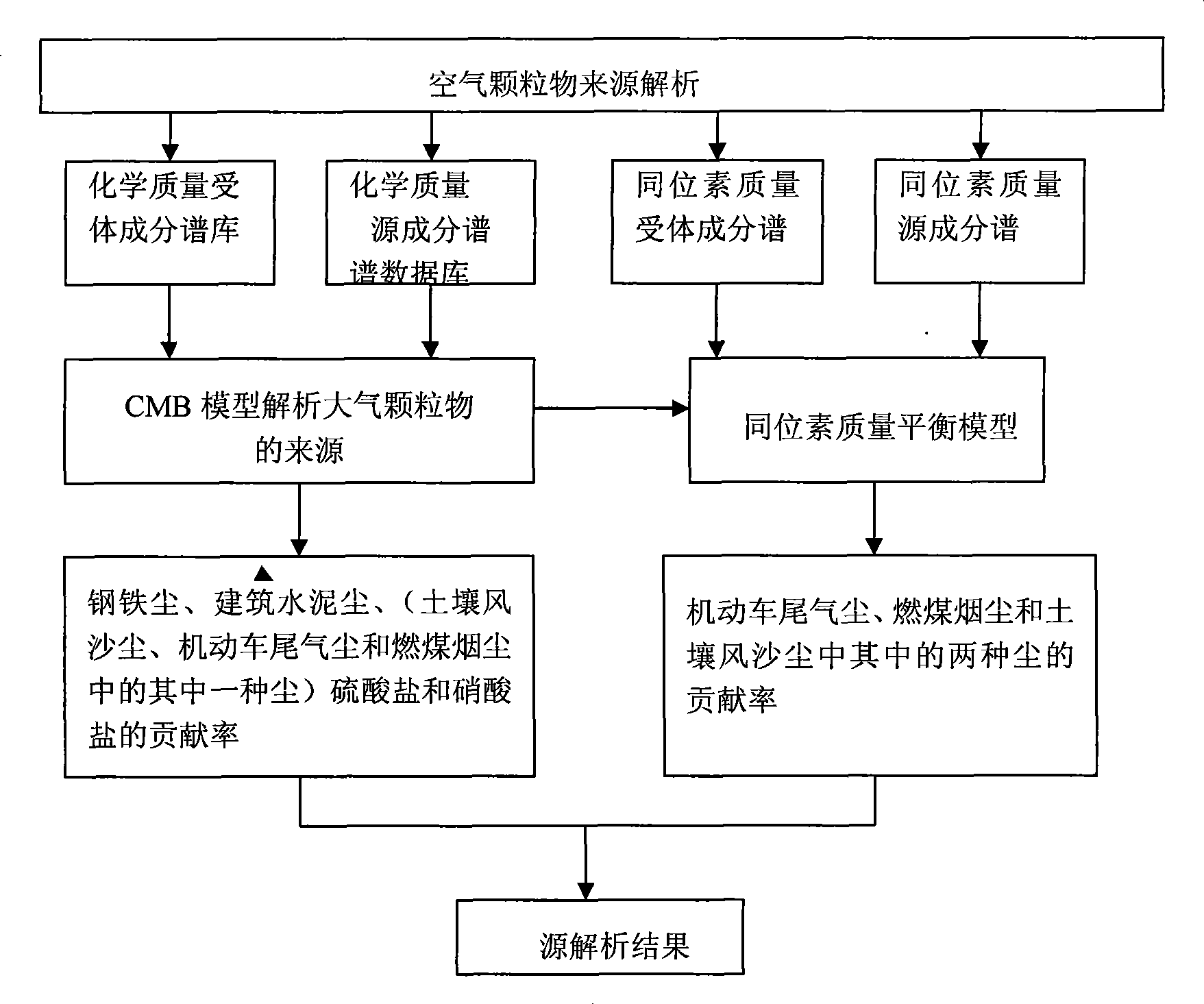
InactiveCN101419208AImprove accuracyImprove stabilityAir quality improvementMaterial analysisSootAmbient air
The invention provides a method for solving the collinearity problem in the process of analyzing the source of air particulate matters, which comprises the following steps: firstly, collecting an ambient air particulate matter sample and a pollution source sample, and performing stable carbon isotope analysis on the collected samples; secondly, reconstructing a stable carbon isotope mass balance model of three pollution sources including automotive vehicle tail gas and dust, coal soot, and soil wind dust and sand which have collinearity through analyzing the component characteristics of stable isotopes of a total carbon in the pollution sources and the ambient air particulate matters; and thirdly, analyzing the contribution of the three pollution sources to the ambient air particulate matters. The isotope mass balance model is the development of a chemical mass balance receptor model, and solves the difficult problem of one set of data with various results encountered by singly using a chemical mass balance to analyze the source of the air particulate matters.
Owner:TAIYUAN UNIV OF TECH
Synthesis, compositions and methods for the measurement of the concentration of stable-isotope labeled compounds in life forms and life form excretory products
InactiveUS20060067881A1Powder deliveryHeavy metal active ingredientsStable Isotope LabelingChemical compound
Stable isotope labeling and neutron activation to measure biological functions are provided, as are the use and method of adding a chemical monitor to correct for neutron flux to sample vials prior to the addition of sample is presented, and the use of stable isotopes as a chemical bar code for vials and other items. Methods are provided also for measuring glomerular filtration rate and glomerular sieving function in a subject, and for measuring other physiological functions.
Owner:BIOPHYSICS ASSAY LAB
Measurement of gastric emptying using stable isotopes
The invention provides a diagnostic test for gastrointestinal disorders by providing a safe, convenient, reliable method for measuring gastric emptying. In this test, a tracer amount of 13C-glutamine or 13C-glutamic acid is added to a liquid meal, or 13C-glutamic acid added to a solid meal. After digestion in the stomach, the meal is emptied into the small bowel, where the labeled 13C-tracer is immediately taken up and metabolized by the cells lining the small bowel. The released 13CO2 is detected in the breath. The measurement of rise in the level of 13CO2 as a function of time is correlated to the rate of gastric emptying.
Owner:THE NEMOURS FOUND
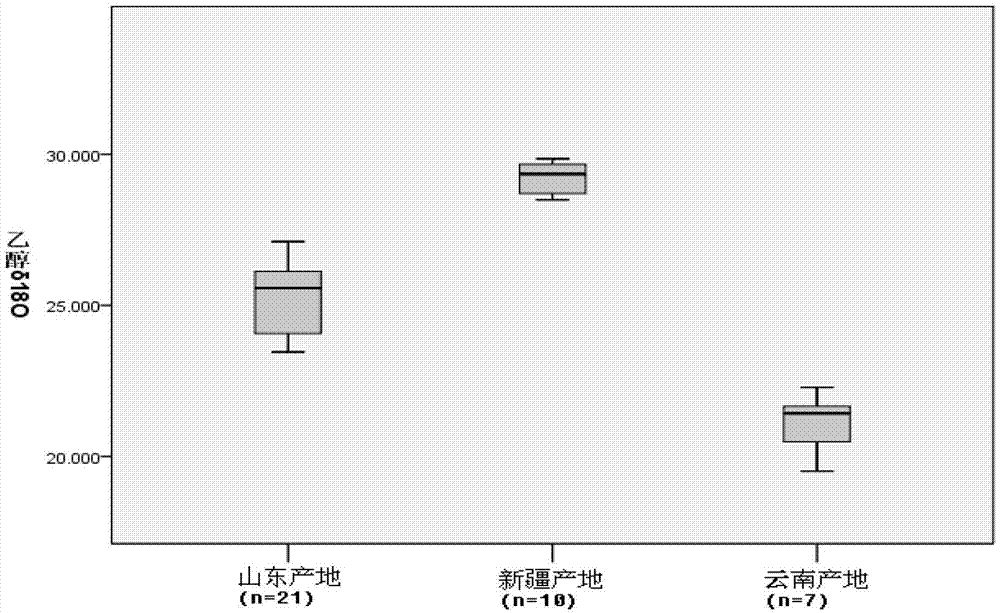
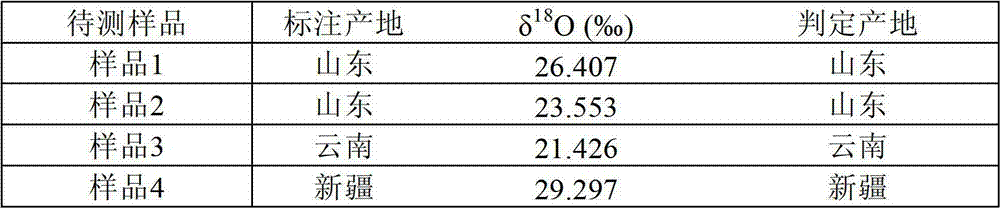
Method for distinguishing milk powder original production place through elemental analysis-stable isotope mass spectrometry
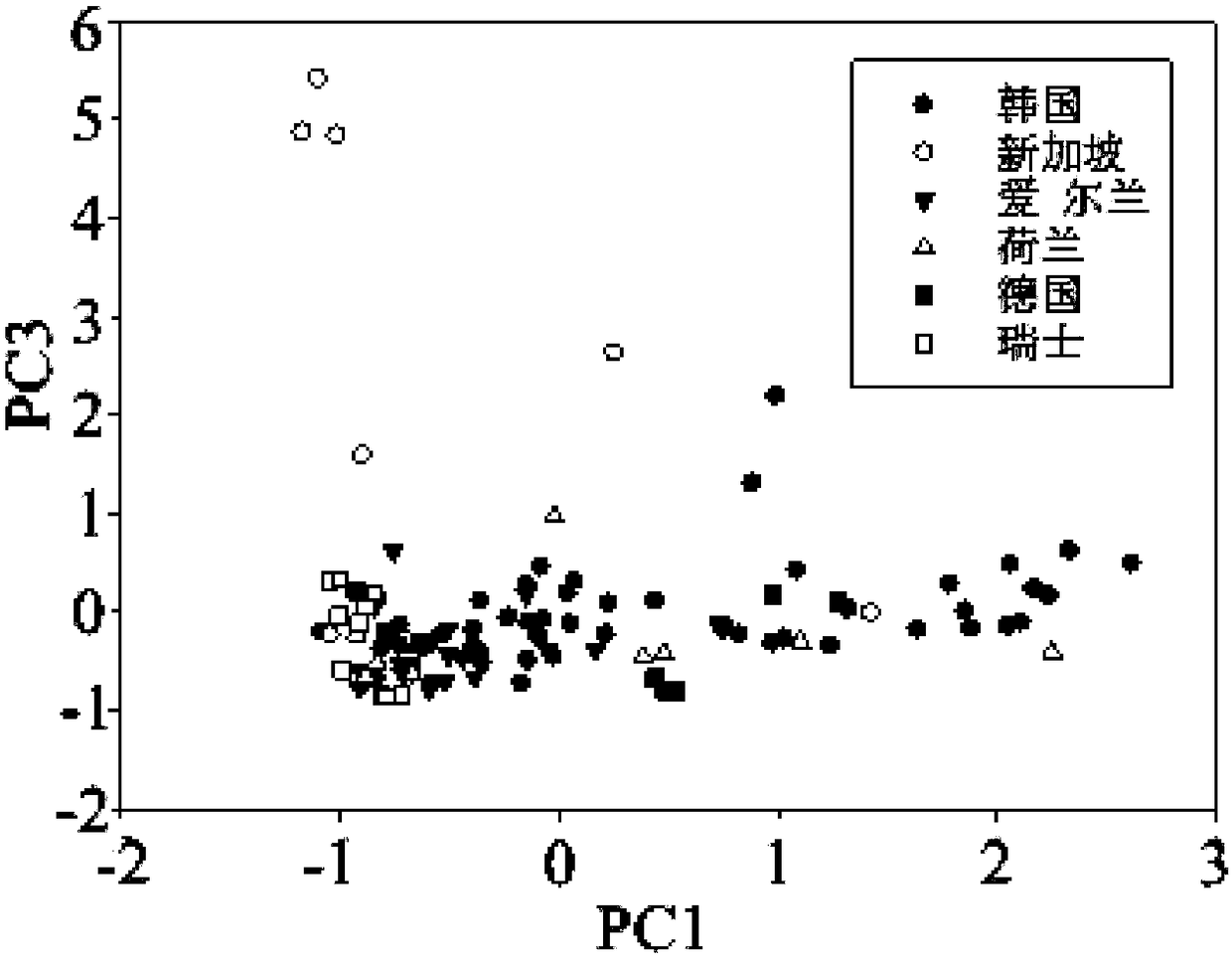
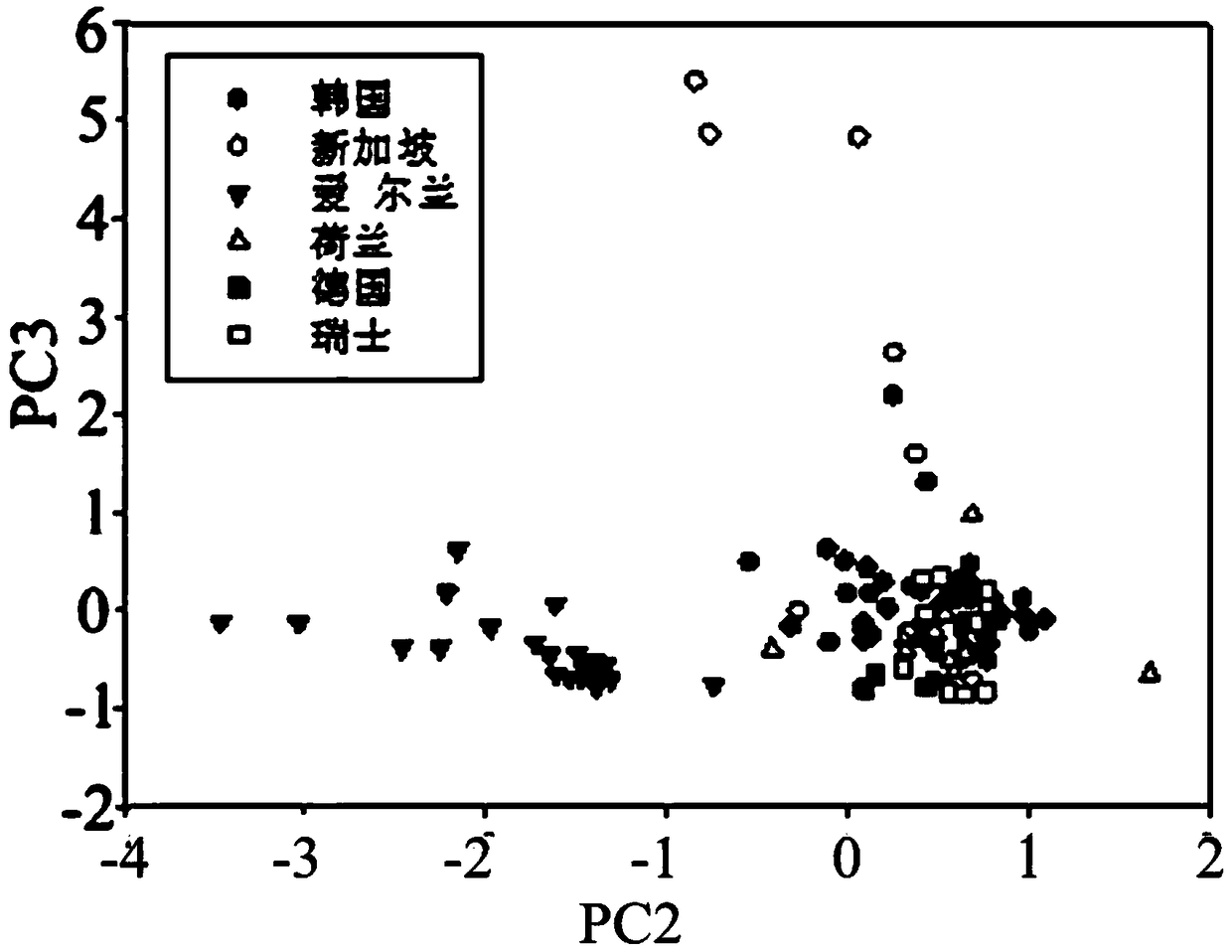
ActiveCN108982692ALow accuracy rateHigh Yield Discrimination Error RateComponent separationPreparing sample for investigationAnalysis dataPrincipal component analysis
The invention relates to the field of milk powder quality detection and more specifically relates to a method for distinguishing milk powder original production places through an elemental analysis-stable isotope mass spectrometry. The method is characterized by comprising the following steps: S1, obtaining samples of milk powder imported from a plurality of Countries; S2, respectively performingstable carbon and nitrogen isotope detection on a plurality of samples, performing stable hydrogen and oxygen isotope detection and performing analysis; S3, performing microelement analysis on the plurality of samples; S4, utilizing a principle component analysis method for performing principle component analysis on elements in the plurality of the samples and primarily distinguishing the samplesof the milk powder from different original production places; S5, utilizing original production place information of the plurality of samples and analysis data in the S3 and S4 and discriminant analysis to perform statistical modeling to obtain an original production place discrimination model coefficient matrix and corresponding original production place discrimination prediction accuracy; S6, bringing unknown samples into the model to perform original production place discrimination and finally judging original production place attributes. By means of the method, original production place traceability and discrimination can be primarily performed on the milk powder; thus, convenience is brought to checking of milk powder quality and fighting of counterfeit and shoddy products.
Owner:FOOD INSPECTION CENT OF CIQ SHENZHEN +1
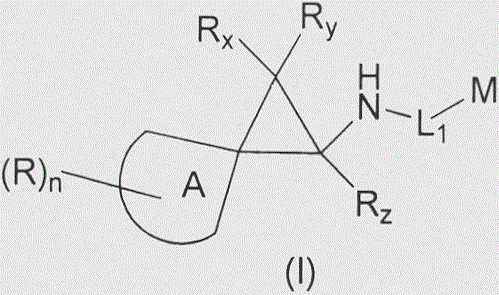
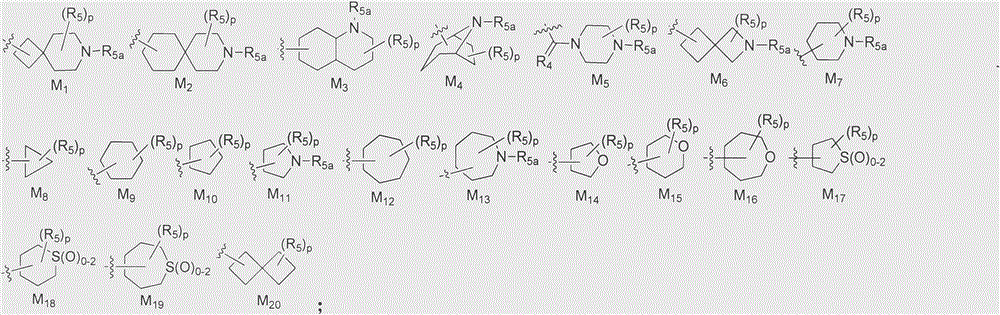
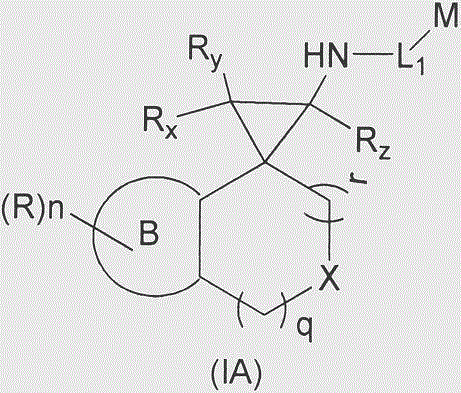
Cyclopropylamine spiro(hetero)cyclic compound, and pharmaceutical composition and application thereof
The invention relates to a preparation method for a cyclopropylamine spiro(hetero)cyclic compound and a composition containing the same, and application of the compound and the composition as an inhibitor for human lysine-specific demethylase (LSD1). The inhibitor is the cyclopropylamine spiro(hetero)cyclic compound as shown in a formula (I) which is described in the specification, or a pharmaceutically acceptable salt, predrug, solvate, polymorphic crystal or stable isotope derivative thereof. The compound can be used for treating or preventing diseases related to human lysine-specific demethylase, e.g., cancers and nerve diseases.
Owner:SHANGHAI DE NOVO PHARMA
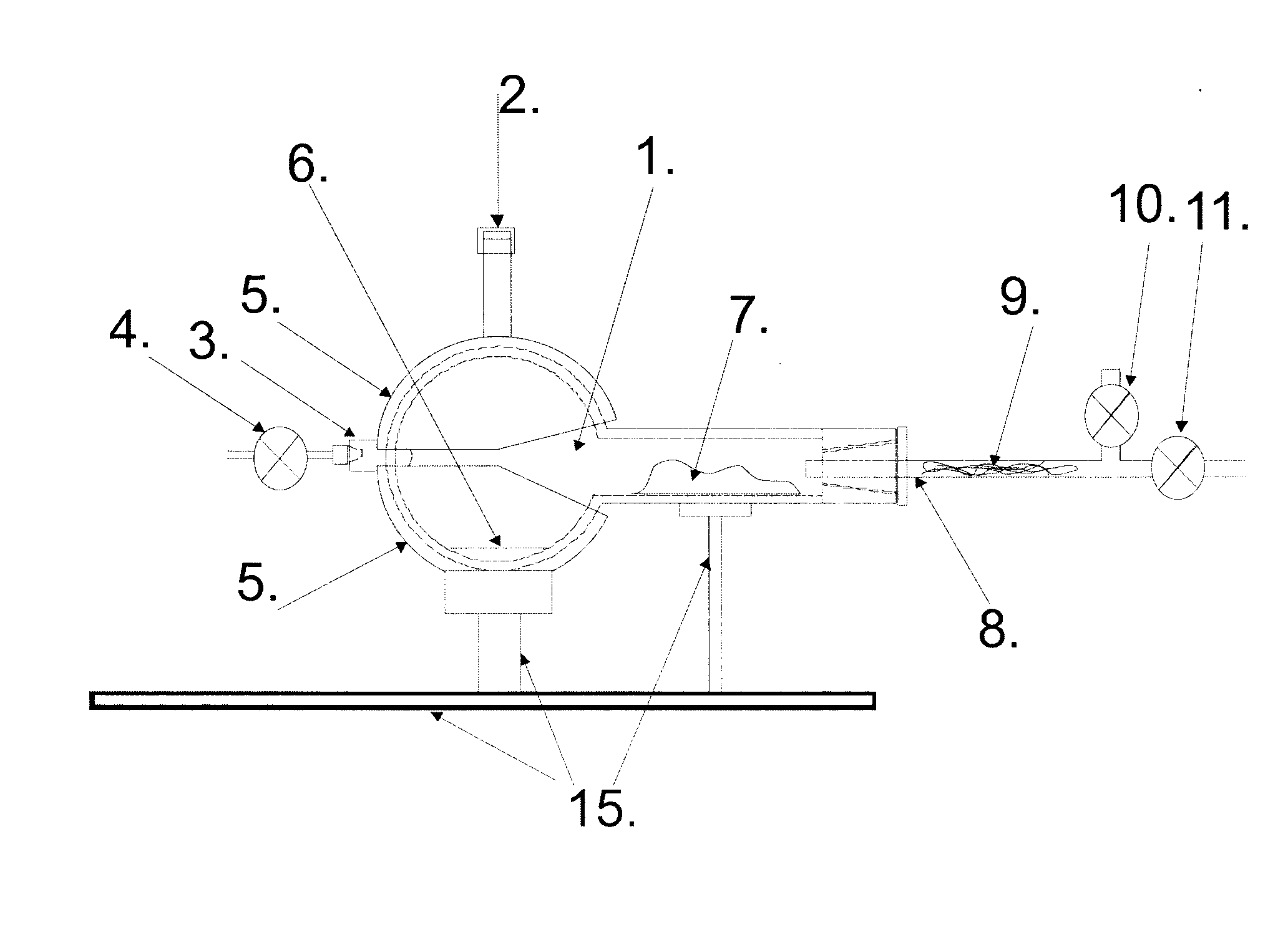
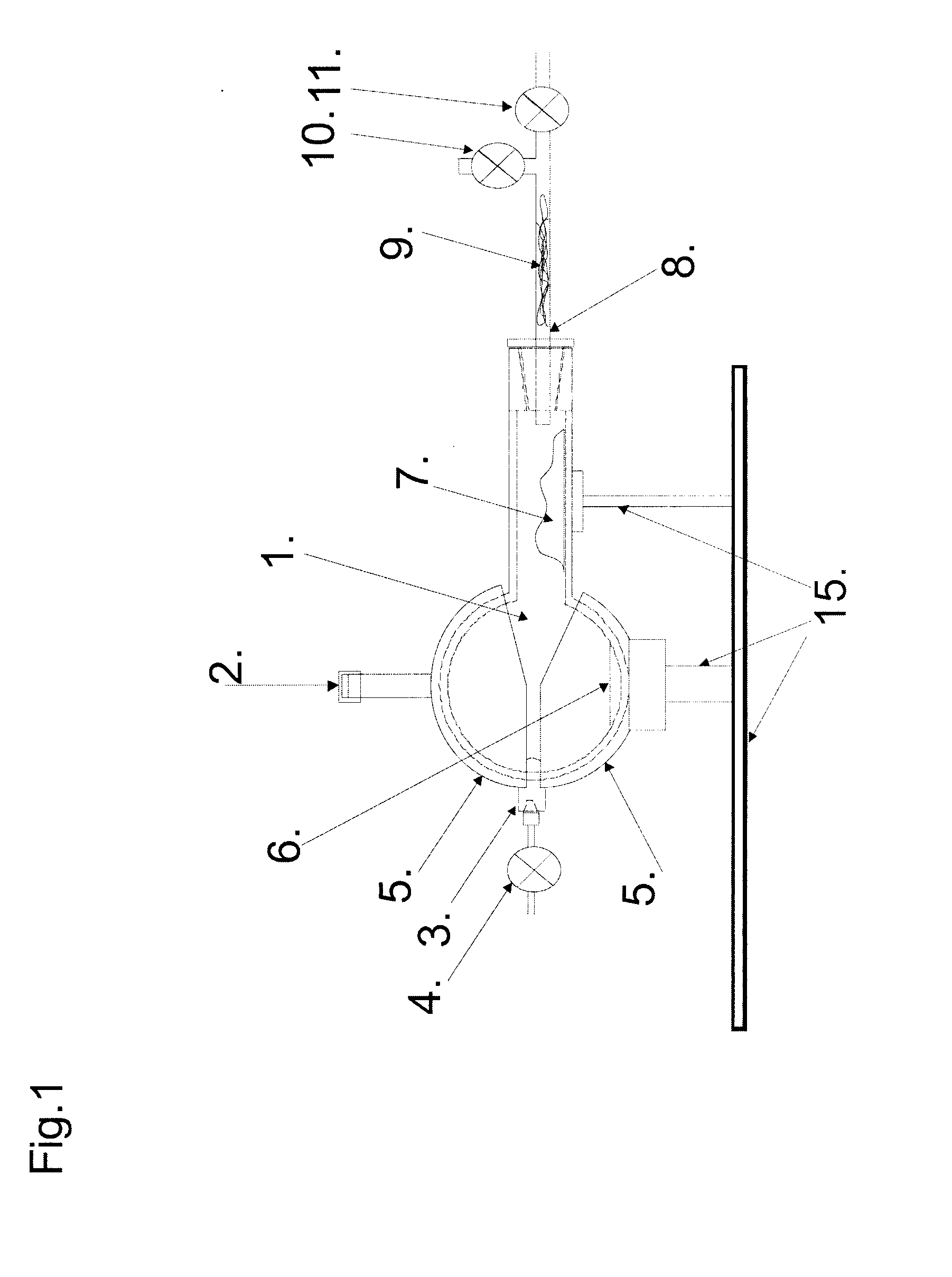
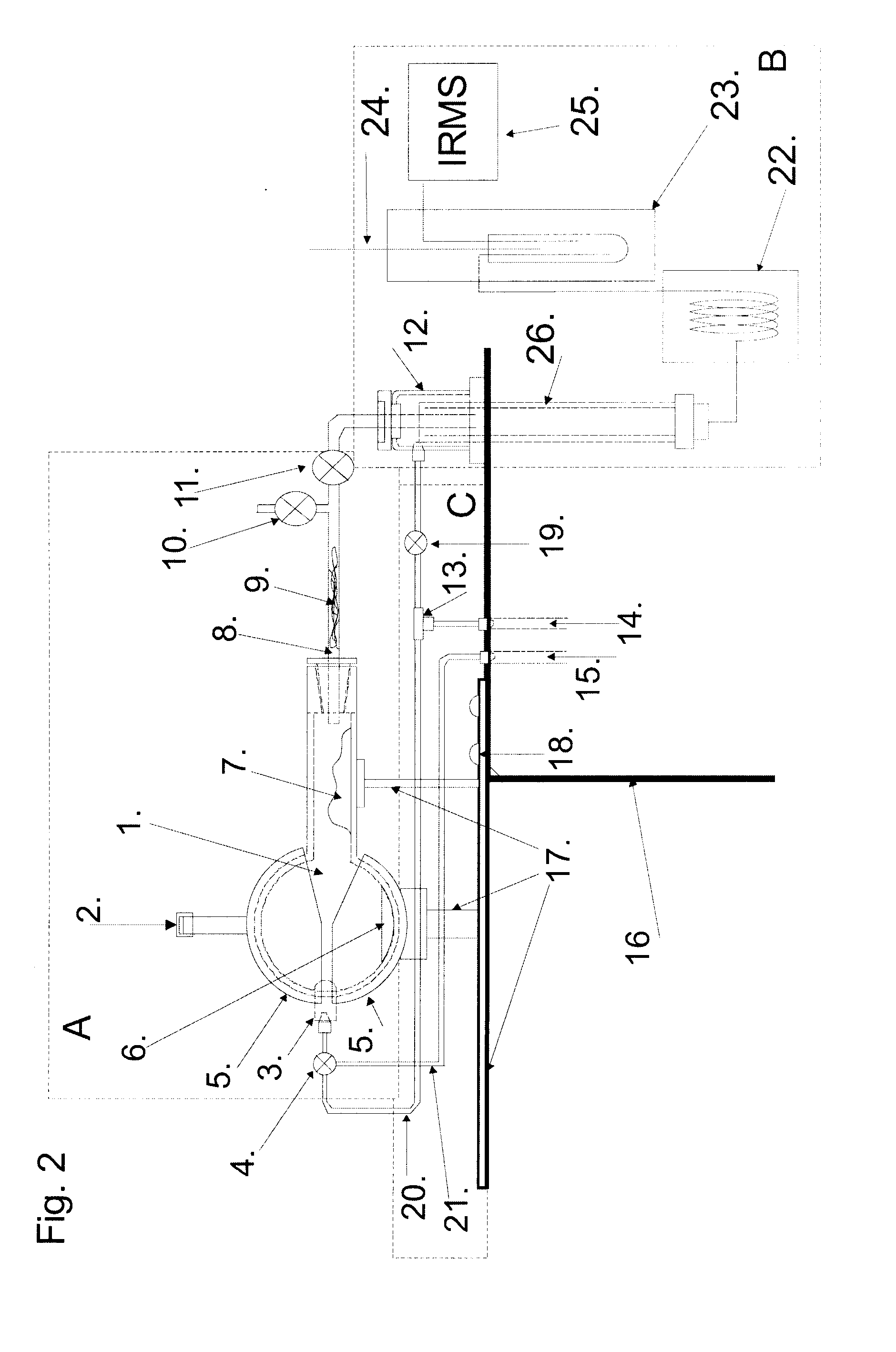
Alcohol thermal dehydratation chamber, apparatus and method for determination of isotopic composition of non-exchangeable hydrogen and deuterium atoms in ethanol samples
The invention is related to the instrumental analytical chemistry, further more to the stable isotope determination in food products. The present invention is referred to the alcohol thermal dehydration chamber, apparatus for “online” determination of isotopic composition of non-exchangeable hydrogen stable isotopes in ethanol samples, which comprises of: A) Alcohol thermal dehydration chamber, B) Detection device, which contains pyrolysis reactor and the Continuous Flow Isotope Spectrometer, and it is connected to the alcohol thermal dehydration chamber over C) System of valves, connectors and capillary tubes which are used for transfer of analyzed sample as for the purging of the alcohol thermal dehydration chamber; procedure for “online” determination of isotopic composition of non-exchangeable hydrogen stable isotopes in ethanol samples and procedure for “offline” preparation of ethene (ethylene) gas by means of alcohol thermal dehydration chamber and for the purpose of authenticity and geographical origin determination of wines, strong spirits, beer, fruit juices, honey and other food product which contain alcohol and / or fermentable sugars.
Owner:SMAJLOVIC IVAN
Low temperature rectification cascade system for rectifying CO to produce stable isotope 13C
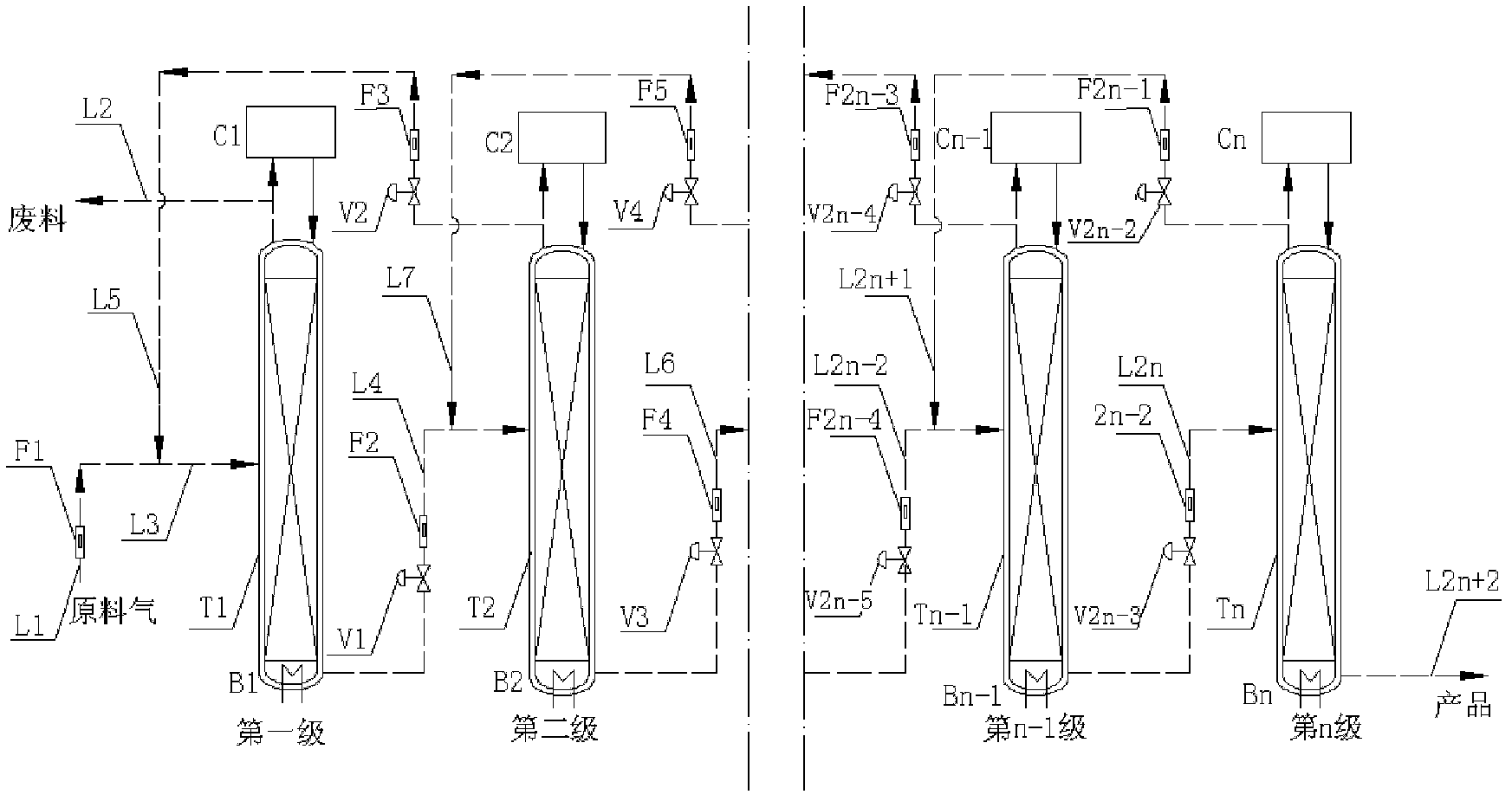
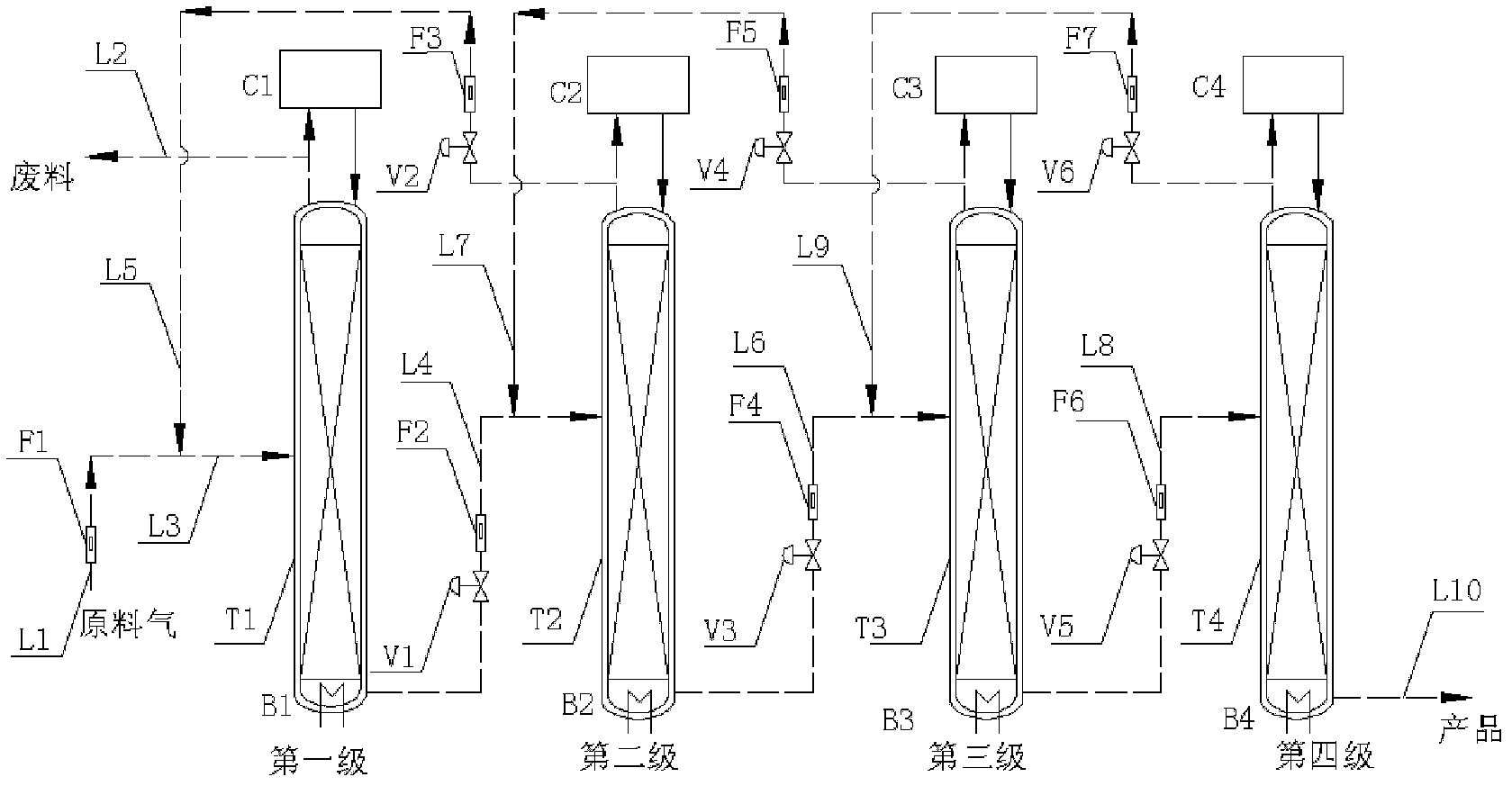
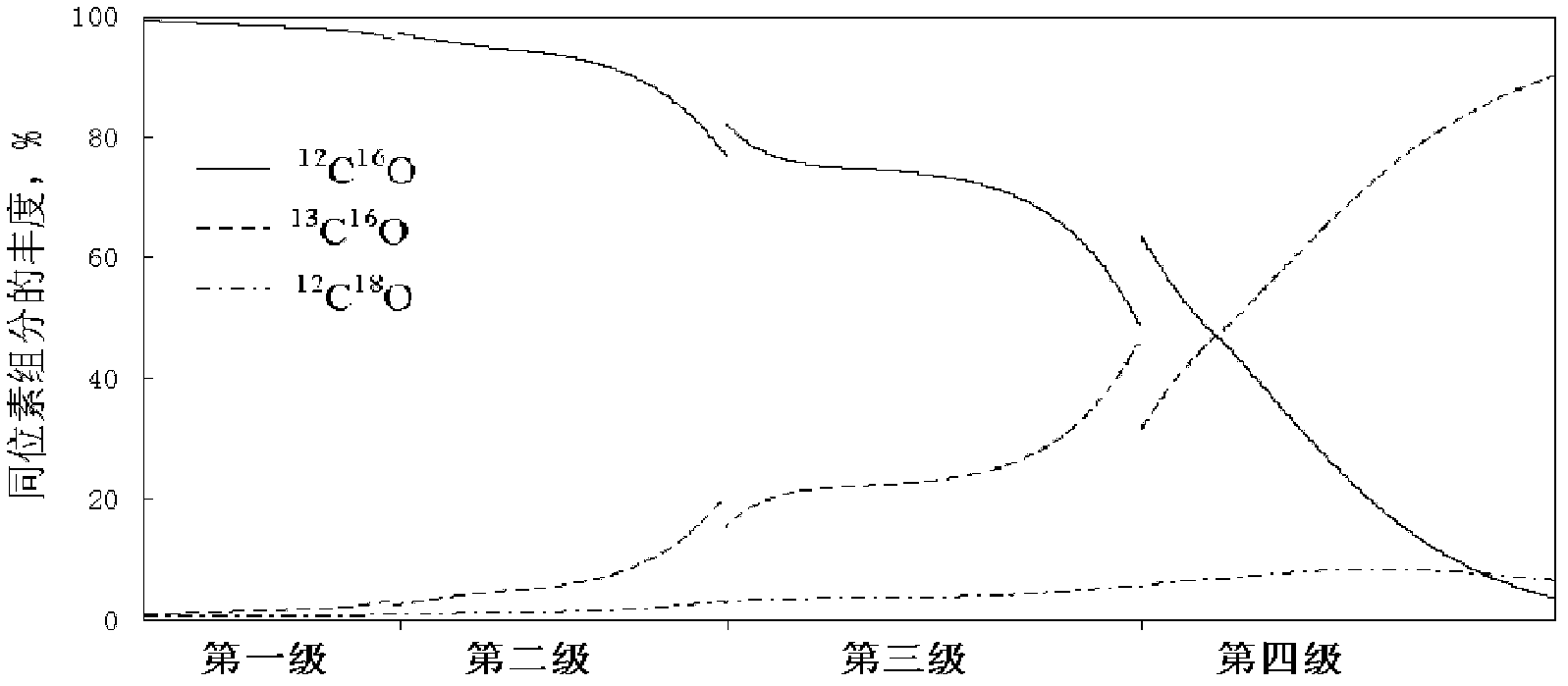
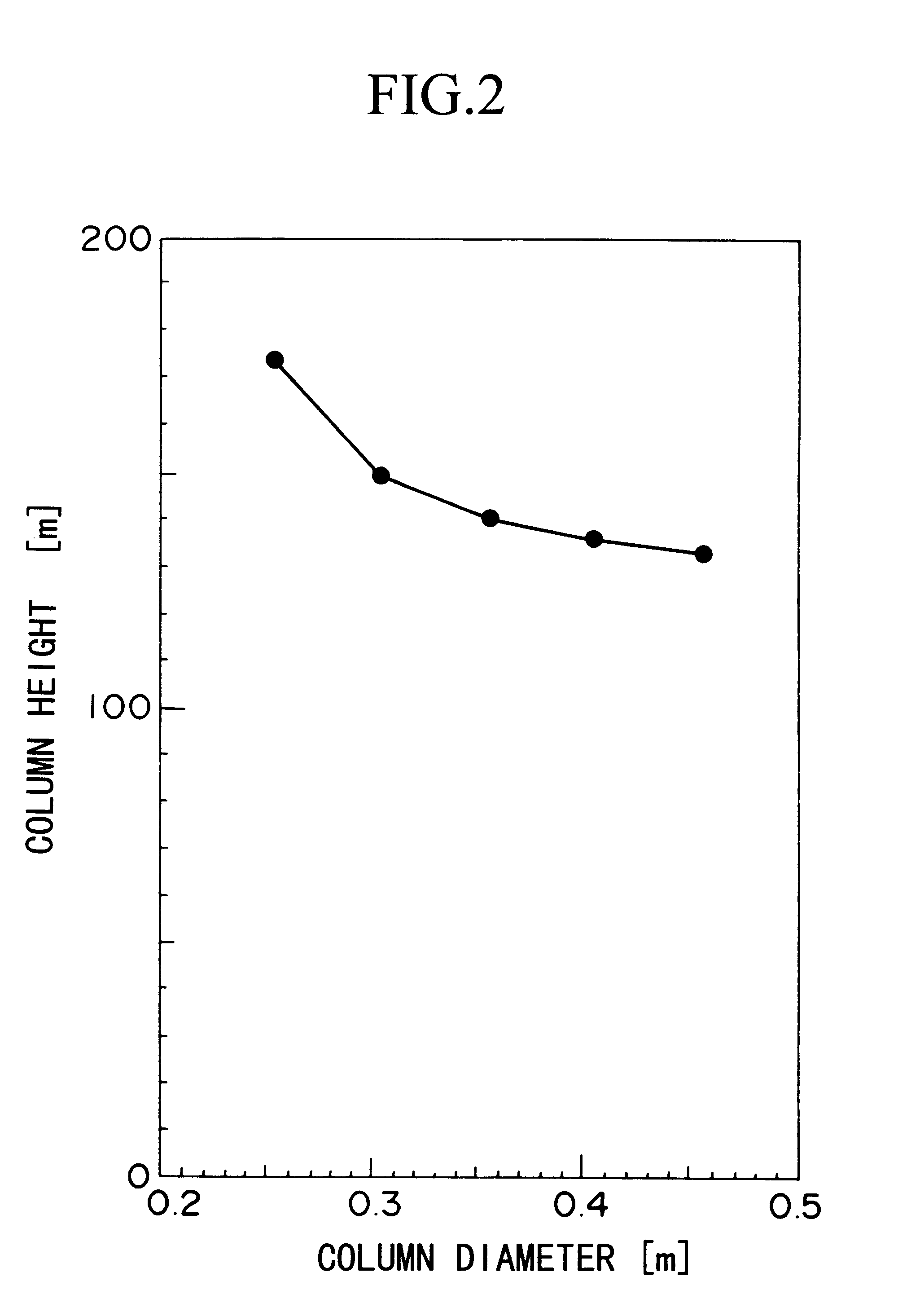
ActiveCN102380315AReduce delivery flowRealize automatic flowIsotope separationReboilerStable nuclide
The invention relates to a low temperature rectification cascade system for rectifying CO to produce stable isotope 13C. The low temperature rectification cascade system is a cascade unit comprising n stage rectifying towers that are arranged horizontally; the rectifying towers comprise overhead condensers, tower bottom reboilers and rectifying columns; and the rectifying towers are connected through pipelines. Compared with the prior art, extraction sections are arranged in the horizontally cascaded rectifying towers so as to reduce the material conveying flow rate between cascaded rectifying towers, the automatic material flow between the rectifying towers can be achieved at the same time by changing the pressure distribution of the horizontal cascade system, the material is conveyed among the intermediate rectifying towers without power conveying equipment, and the cascade unit can be ensured to operate stably and continuously for a long time.
Owner:SHANGHAI RES INST OF CHEM IND
Method for detecting reactive metabolites using stable-isotope trapping and mass spectroscopy
ActiveUS20050287623A1Material analysis using wave/particle radiationMicrobiological testing/measurementMetaboliteTrapping
The invention is directed to a method for detecting reactive metabolites using isotope trapping and mass spectroscopy.
Owner:JANSSEN PHARMA NV
Analytical method of tilapia mossambica food source
InactiveCN101603944APrecise positioningAccurate measurementMaterial analysis by electric/magnetic meansTilapiaData treatment
The invention discloses an analytical method of tilapia mossambica food source, which adopts stable isotope technology to analyze the tilapia mossambica food source and comprises the following steps: (1) collecting tilapia mossambica samples, collecting baits which tilapia mossambica probably ingests and processing the collected tilapia mossambica samples and the baits which are probably ingested; (2) carrying out the stable isotope determination on the processed tilapia mossambica samples and the baits which are probably ingested; and (3) data processing the stable isotope determination results, calculating and comparing the stable isotope numerical value of the tilapia mossambica samples with that of the baits which are probably ingested to obtain tilapia mossambica food source. The invention combines stable isotope technology and traditional stomach contents technology, in favor of improving analytic accuracy and precisely determining the nutrient source of tilapia mossambica, thus locating accurately the interrelation between the tilapia mossambica and other creature types and the flow of energy of the system.
Owner:SUN YAT SEN UNIV
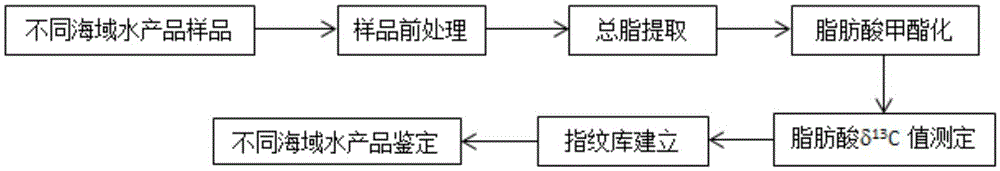
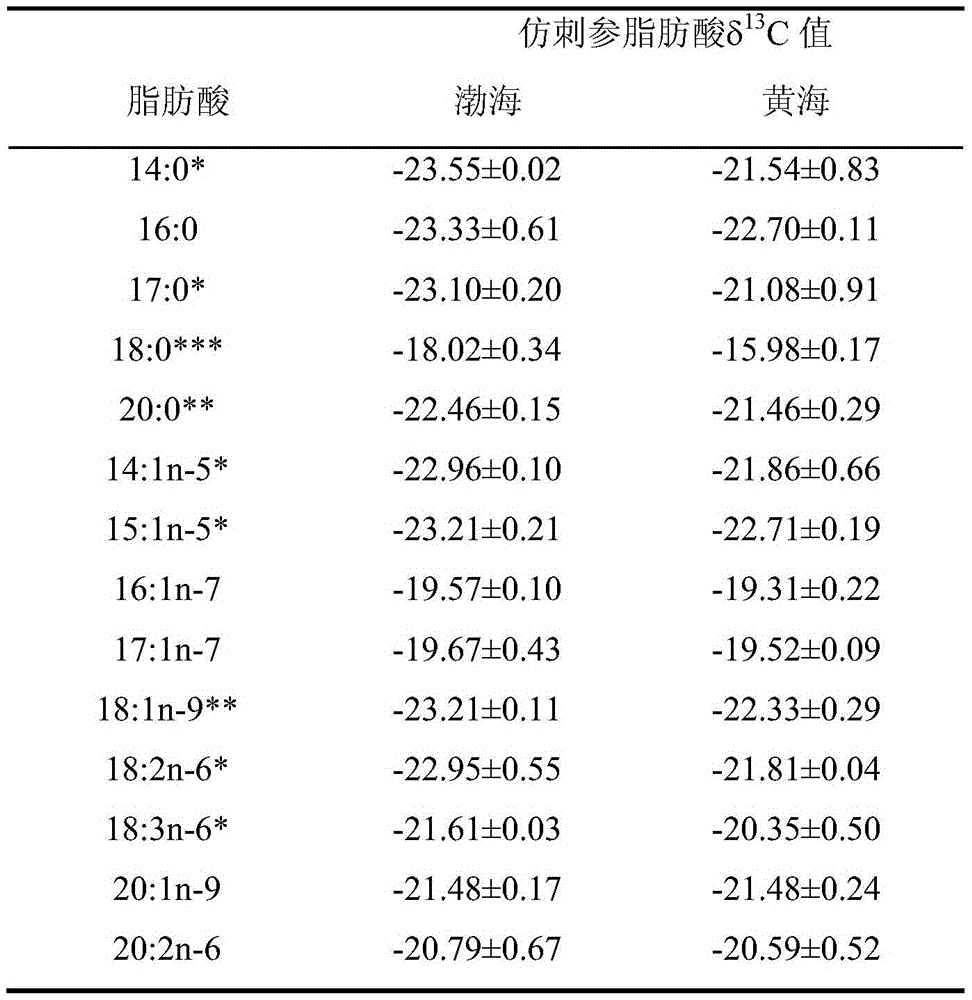
Aquatic product producing area tracing method based on fatty acid carbon stable isotopes
InactiveCN105606750AThe identification result is accurateAccurate and reliable identification resultsComponent separationLipid formationWater baths
The invention relates to an aquatic product producing area tracing method based on fatty acid carbon stable isotopes. The method includes the steps that A, preprocessing is performed, wherein collected aquatic products are processed, completely frozen, dried and ground into powder; B, total lipid extraction is performed, wherein 5.0 g of samples are taken, a chloroform and methanol solution is added, digestion stays overnight after oscillating extraction, filtering is performed, an extraction solution is washed with a sodium chloride solution, standing is performed so that layering can be achieved, a chloroform layer is collected, and after drying performed through anhydrous sodium sulfate, sample total lipid is acquired by means of concentration of a nitrogen blowing method; C, methyl esterification of fatty acid is performed, wherein a sulfuric acid-methyl alcohol methyl esterification method is adopted, a sulfuric acid-methyl alcohol solution is added into the total lipid, methyl esterification is performed in a water bath, n-hexane is added for oscillation after cooling, standing is performed so that layering can be achieved, supernatant is taken and placed in a bottle to be refrigerated and stored; D, the fatty acid C stable isotopes are measured, wherein fatty acid methyl ester samples are analyzed and identified through a gas chromatograph mass spectrometer; E, a result is analyzed, wherein by comparing fatty acid in different sea area samples, aquatic products in different sea areas and different varieties are identified.
Owner:DALIAN MARITIME UNIVERSITY
Method for identifying wine producing area based on stable isotope ratio
ActiveCN102967668ASolve the technical problems of authenticity identification of originPromote healthy developmentComponent separationAlcohol contentGas phase
The invention relates to a method for identifying original wines based on stable isotope ratio, and belongs to the technical field of trueness identification of wines. The method mainly comprises the following steps of: (1) measuring the delta 180 of alcohol contained in a true original wine sample by adopting a gas phase chromatography / stable isotope mass spectrometry coupling technology, and establishing a delta 180 database of the true original wine sample; (2) measuring the delta 180 of the alcohol contained in a wine sample to be measured; (3) analyzing the significant difference of the delta 180 of an alcohol content contained in the wine sample to be measured and the delta 180 of the alcohol contained in the true original wines, wherein if no significant difference exists, the wine sample to be measured and the true sample are indicated to be from a same producing area, and if the significant difference exists, the wine sample to be measured and the true sample are indicated to be from different producing area. The method capable of accurately identifying the original wines, which is disclosed by the invention, is established by utilizing a stable isotope mass spectrometry technology, can be used for solving the technical problem of national original wines on market supervision and is conductive to specifying the market behaviors, protecting the benefits of consumers and legal enterprises and promoting the healthy and orderly development of a wine industry.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Process and apparatus for separation of stable isotope compound
Stable isotope atoms present in the form of stable isotope compounds, for example, 13C present in the form of 13CO, are separated by distillation using a distillation column packed orderly with a formed packing, and preferably by distillation using a distillation column packed with a "promoting-fluid-dispersion type" structured packing.
Owner:NIPPON SANSO CORP
Wuyi rock tea production place identification method through combination of four detection technologies
InactiveCN106560692ADiscriminate objectivelyAccurate discriminationComponent separationMaterial analysis by electric/magnetic meansMetrologyAmino acid
The invention relates to a Wuyi rock tea production place identification method through combination of four detection technologies, and belongs to the technical field of geographical indication product authenticity recognition. In the prior art, the single detection data cannot represent all production place traceability key information, the data matching problem exists when different types of the detection data are subjected to combined use in the metrology method, and other problems exist. A purpose of the present invention is to solve the problems in the prior art. According to the present invention, based on the partial least square discrimination model, the near infrared characteristic spectrum data, the stable isotope data, the trace element data and the amino acid data of the rock teas (produced inside and outside the geographical indication production place) from different production places are integrally fused, the analysis model is established, the sample is extracted, and the rock tea production place is objectively and accurately determined by using the model, wherein the recognition rate is high, achieves 100.0%, and is higher than the PLSDA determining result of the single data, and the recognition rate of the blind sample achieves 100%; and the method has the good application prospect, and can be used as the Wuyi rock tea production place traceability recognition technical method.
Owner:CHINA JILIANG UNIV
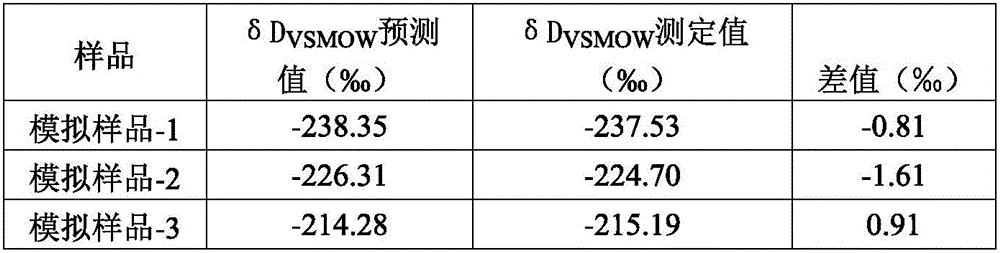
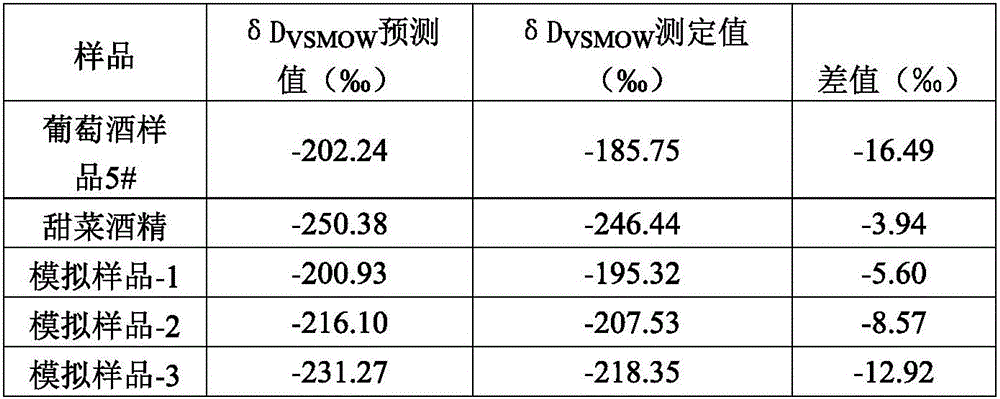
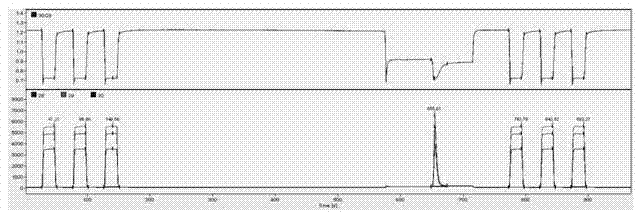
Method for measuring hydrogen isotope ratio of ethanol in grape wine
InactiveCN105866312AAvoid damageConvenient direct autosampling technologyComponent separationGas phaseGrape wine
The invention relates to a method for measuring the hydrogen isotope ratio of ethanol in grape wine and belongs to the technical field of stable isotope analysis. The method comprises the steps that (1) hydrogen isotope is used for forming a known to-be-tested water treatment sample; (2) acetone is used for diluting a fermentation ethanol sample treated by water, and the mixture is mixed for use; (3) a liquid sample injection needle is used for sample injection, and gas chromatography equipped with a molecular sieve capillary chromatographic column is used for separating ethanol, acetone, water and other hydrogen compounds; (4) an on-line cracking device is used for converting ethanol into hydrogen; (5) CF-IMRS is used for measuring the hydrogen delta D produced by the ethanol; (6) correction computation of delta DSMOW of sample ethanol is performed according to the delta D measured value of an ethanol isotopic reference material. According to the method for measuring the hydrogen isotope ratio of ethanol in grape wine, GC-TC-IRMS is used for measuring the hydrogen isotopic compositions of the fermentation ethanol, and the technical difficulty of series of pretreatment of ethanol purification before measurement of fermentation ethanol hydrogen isotope in the past is solved; advance of the ethanol hydrogen isotope measurement technology can be promoted, and a technical method is provided for source identification of fermentation ethanol, sugar mixing detection of a plant source product and study of metabolic pathways in future.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
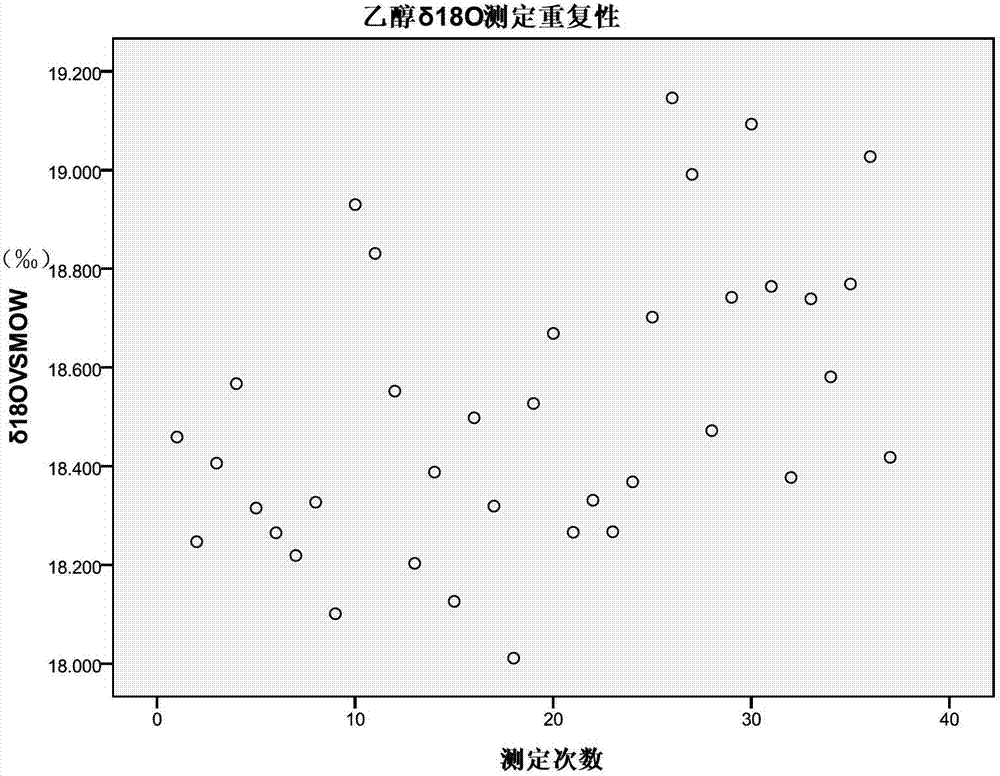
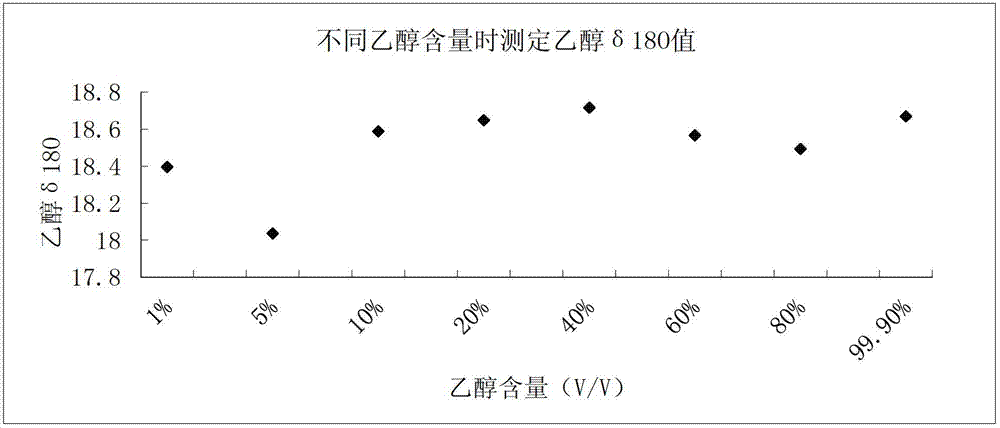
Rapid determination method for oxygen stable isotope of ethanol in alcoholic beverage
The invention relates to a rapid determination method for oxygen stable isotope of ethanol in an alcoholic beverage, and belongs to the technical field of stable isotope analysis. The method comprises the following steps: 1) using acetone to dilute an alcoholic beverage, and mixing for stand-by; 2) injecting a sample by a liquid injection needle, and using a GC with molecular sieve chromatographic capillary column to separate ethanol, acetone, water and other oxygen-containing compounds; 3) converting ethanol into CO with an on-line pyrolysis device; 4) determining delta 18O in CO produced by ethanol by CF-IRMS; and 5) based on a measured delta 18 O value of an isotopic reference material, correcting and calculating delta 18OVSMOW of the ethanol sample. The invention utilizes GC-TC-IRMS to realize rapid determination of oxygen stable isotope of ethanol in alcoholic beverage, and solves series of existing technical problems in pretreatment, such as ethanol purification before determination of oxygen stable isotope of alcohol in alcoholic beverage. The project can promote technological progress of ethanol oxygen isotope determination, and also provides technical methods for the future research on authentication and metabolic path of alcoholic beverage or biofuel.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
Method for judging place of production of traditional Chinese medicinal pulsatilla by using stable isotope ratio
InactiveCN103675083AImprove accuracyEmbodying characteristicsMaterial analysis by electric/magnetic meansMedicineElemental analysis
The invention discloses a method for judging the place of production of traditional Chinese medicinal pulsatilla by using a stable isotope ratio. According to the method, the values of N%, C%, sigma 15N and sigma 13C in a pulsatilla sample are measured by an elemental analysis-stable isotope mass spectrometry technology. The method mainly comprises the following steps of constructing a database including N%, C%, sigma 15N and sigma 13C of the pulsatilla sample in the real place of production by measuring the values of N%, C%, sigma 15N and sigma 13C in the pulsatilla samples in different places of production; measuring the values of N%, C%, sigma 15N and sigma 13C in an unknown place of production; performing Fisher judgment analysis by statistical software, and running a program to judge the real place of production of the pulsatilla sample in the unknown place of production. According to the method disclosed by the invention, the method for accurately judging the place of production of the pulsatilla can be constructed by the elemental analysis-stable isotope mass spectrometry technology, so that the technical difficulty in tracing of the place of production of traditional Chinese medicines in China can be solved; the benefit of a customer can be protected, and the traditional Chinese medicine industry can be developed in a healthy and ordered way.
Owner:SHANGHAI JIAO TONG UNIV
Polypeptide analyses using stable isotope labeling
InactiveUS7052916B2Material analysis using wave/particle radiationComponent separationStable Isotope LabelingProtein level
Methods for incorporating a stable isotope into a protein or peptide fragment are disclosed. The methods include providing isolated isotope-labeled protein or peptide fragments and analyzing the isolated labeld fragments. In another aspect of the invention, methods for quantitatively comparing peptides in two protein or peptide fragment mixtures are provided. In these methods, protein levels are measured using stable-isotope coded protein or peptide fragments.
Owner:IMMUNEX CORP
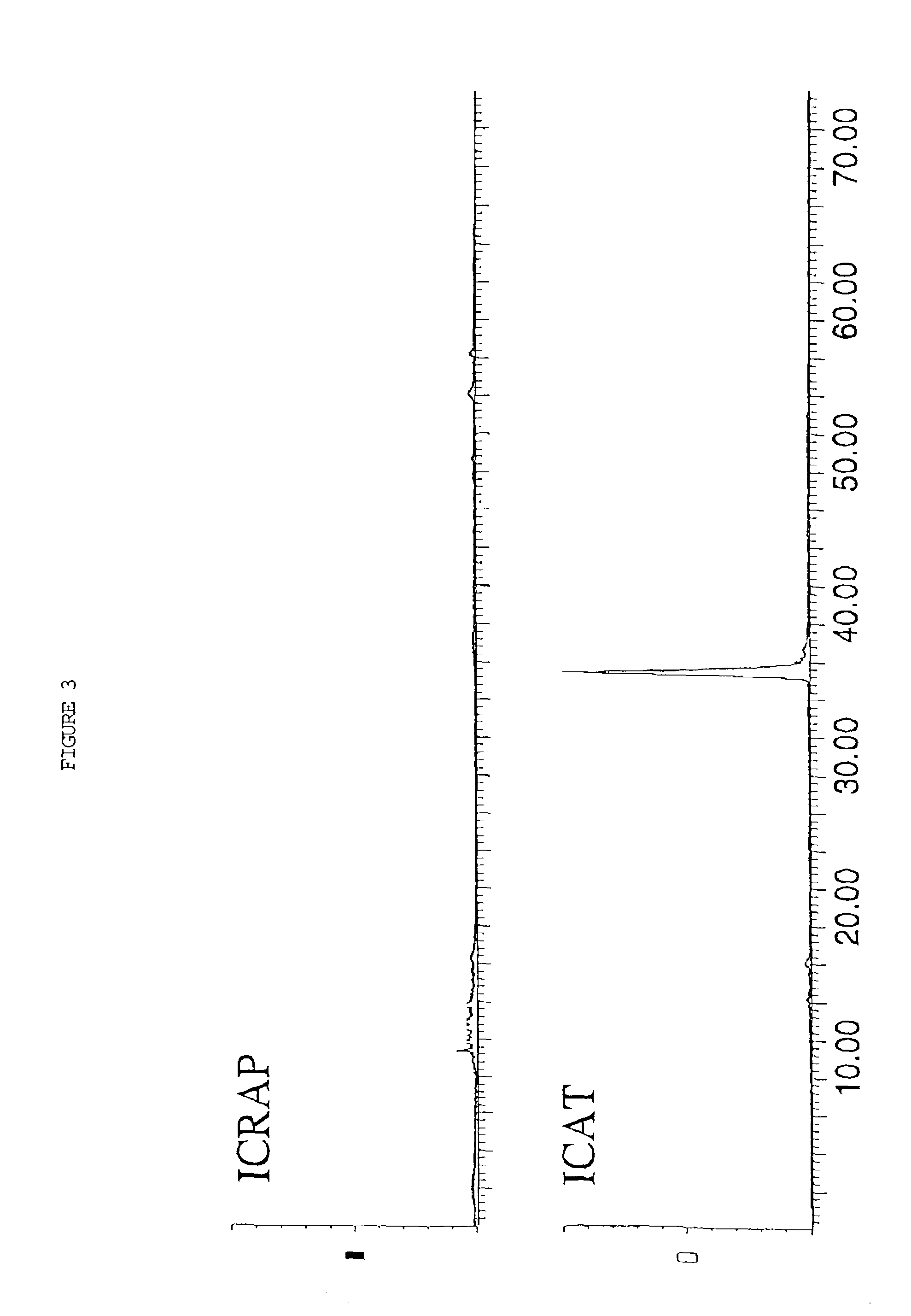
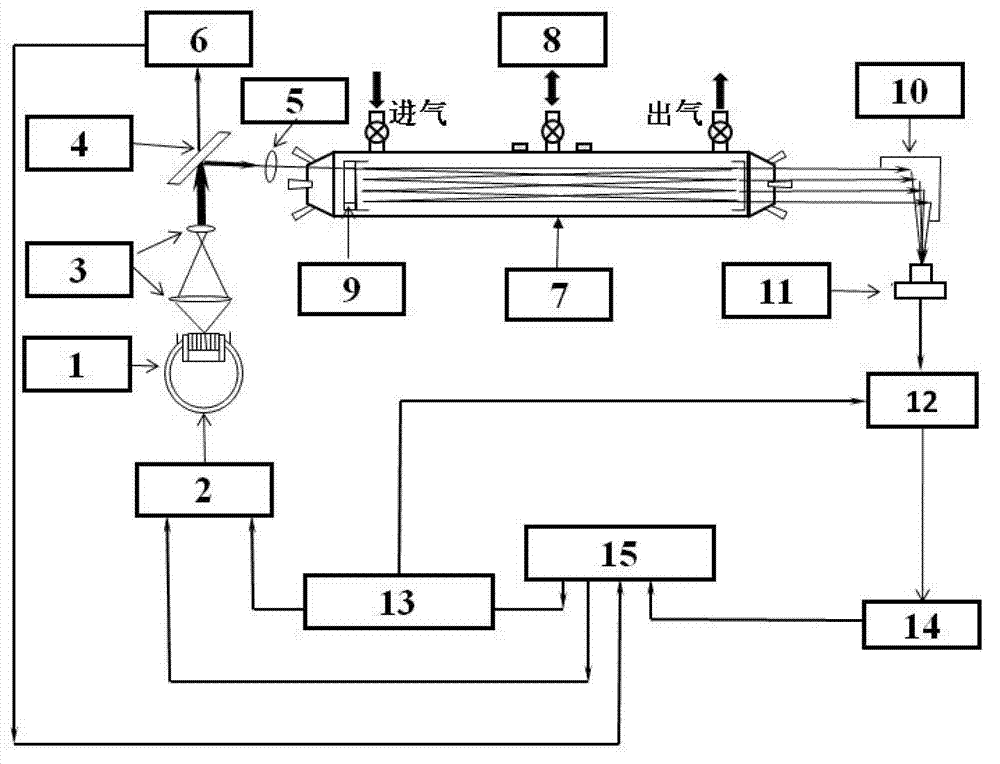
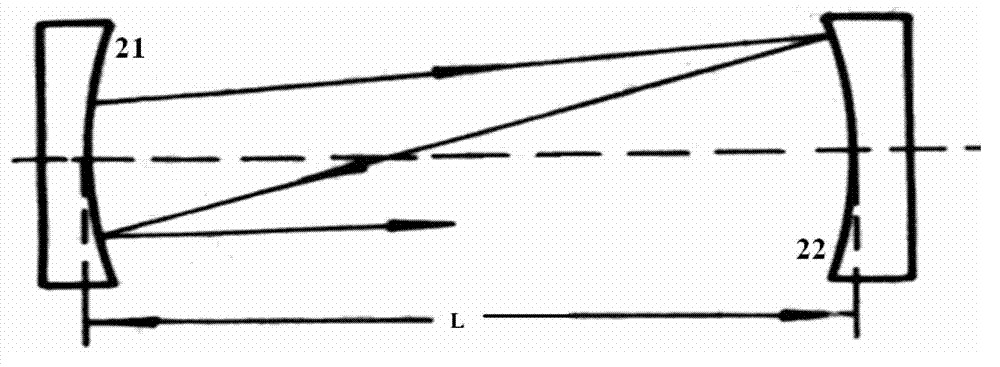
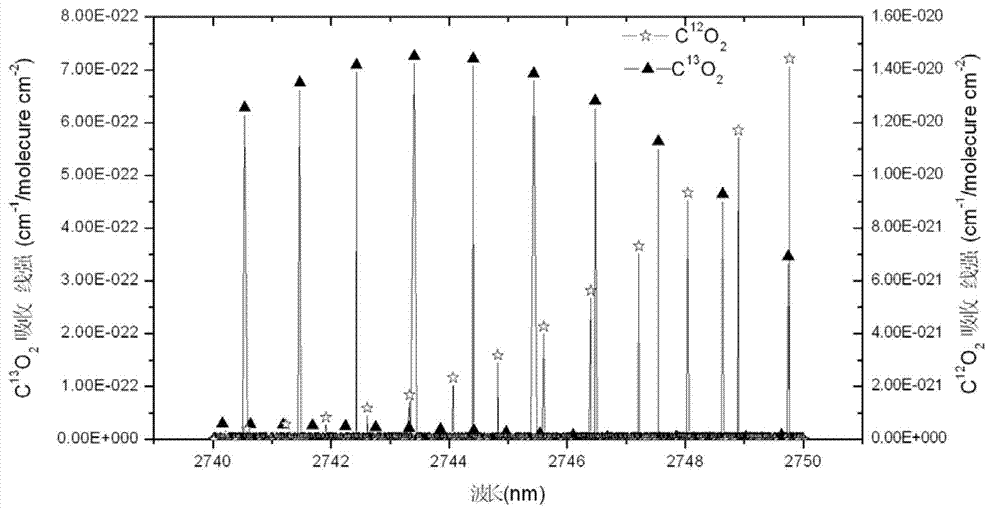
Stable isotopic abundance ratio real-time online monitoring device and method
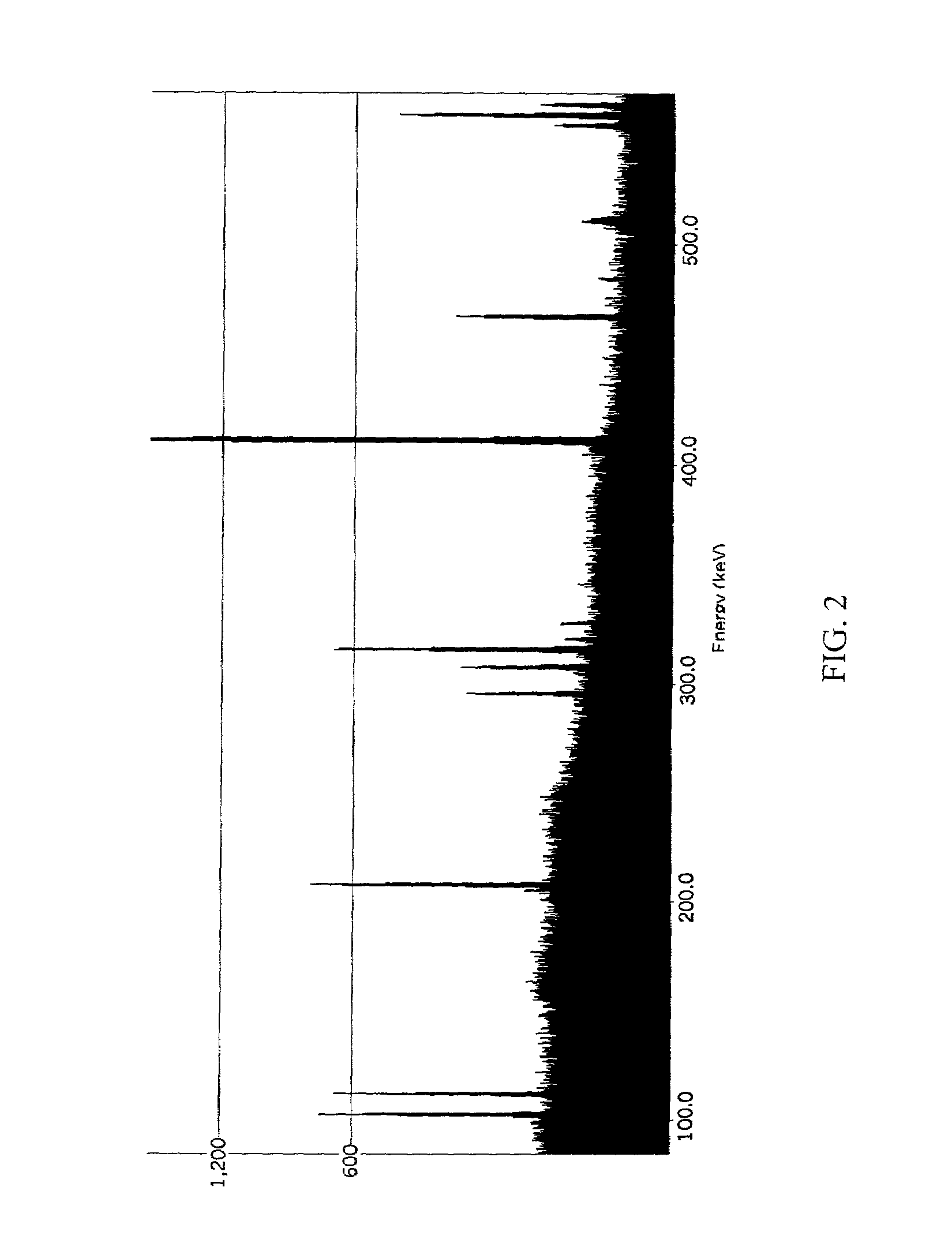
ActiveCN103115894AAccurate measurementEasy to measureColor/spectral properties measurementsField experimentRatio measurement
The invention discloses a stable isotopic abundance ratio real-time online monitoring device and a stable isotopic abundance ratio real-time online monitoring method. A wavelength scanning off-axis integrated cavity spectrum technology is utilized for performing precision measurement on isotopic abundance ratio of an object element, and the device comprises a laser device, a laser controller, a signal generator, an angle adjustable prism, a wavemeter, an off-axis integrated cavity, a temperature and pressure controller, a piezoelectric ceramic controller, an off-axis parabolic mirror, a photoelectric detector, a lock-in amplifier and a signal processing system for acquiring A / D (analog / digital) and terminal signals. The method disclosed by the invention is performed on the basis of the off-axis integrated cavity laser absorption spectrum technology and is further combined with wavelength scanning, cavity regulation, temperature, pressure and wavelength precision control technology, so that the stable isotopic abundance ratio real-time online monitoring can be realized, and trace gas concentration and isotopic abundance ratio measurement can be simultaneously performed; furthermore, the preparation of a sample is not required, so that the measurement frequency is greatly improved; and the device has the advantages of simplicity in operation, no need of calibration, almost no drift during a long period of time, good stability, low power, convenience in carrying, mounting and field experiments and large dynamic range.
Owner:ANHUI CASZT PHOTOELECTRIC MEASUREMENT & CONTROL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com