Blood-production preparation quality test method and construction method of standard fingerprint spectrum thereof
A standard fingerprint and quality detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that the production process and product quality cannot be effectively controlled, and the clinical efficacy cannot be better guaranteed, so as to ensure the whole process of quality control , The pretreatment method is simple, the process is stable and uniform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
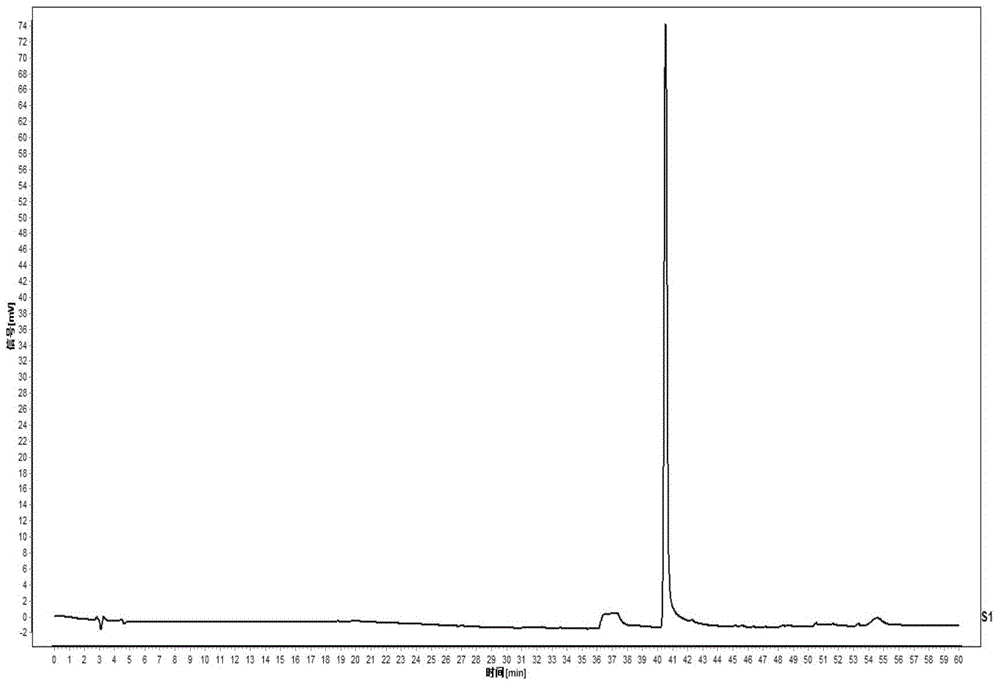
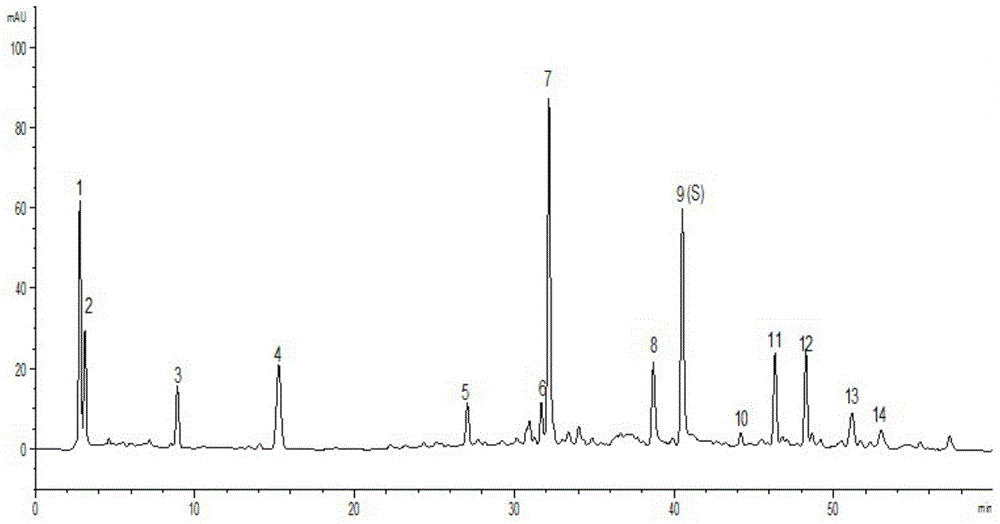
[0053] Example 1 Establishment of fingerprint of Shengxuebao preparation. (Take Shengxuebao Granules as an example)
[0054] (1) Instruments and reagents
[0055] Agilent 1260 HPLC, including online vacuum degasser (G-1311C), binary pump (G-1311C), standard autosampler (G-1329B), intelligent column oven (G-1316A) , DAD detector (G-1314B), Agilent1260Infinity chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sartorius Scientific Instruments (Beijing) Co., Ltd.).
[0056] (2) Selection of chromatographic conditions
[0057] ①Selection of mobile phase
[0058] Select acetonitrile-water, acetonitrile-0.1% phosphoric acid solution, acetonitrile-0.2% phosphoric acid solution, acetonitrile-0.1% formic acid solution, methanol-0.1% phosphoric acid solution, methanol-0.1% formic acid solution, methanol-water, etc. to carry out the t...
Embodiment 2
[0091] Embodiment 2 The establishment of the fingerprint of Shengxuebao preparation intermediate
[0092] (1) Instruments and reagents
[0093] Agilent 1260 HPLC, including online vacuum degasser (G-1311C), binary pump (G-1311C), standard autosampler (G-1329B), intelligent column oven (G-1316A) , DAD detector (G-1314B), Agilent1260Infinity chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sartorius Scientific Instruments (Beijing) Co., Ltd.).
[0094] (2) Chromatographic conditions
[0095] The column is Agilent ZORBAX XDB-C 18 (5μm, 4.6×250mm) reverse phase chromatographic column; column temperature is 25°C; detection wavelength is 200nm; mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid solution, the total flow rate is 1.0ml / min; analysis time is 60 minutes ; Elution gradient (see Table 4):
[0096]...
Embodiment 3
[0117] Example 3 Establishment of fingerprints of Shengxuebao preparations (taking Shengxuebao capsules as an example).
[0118] (1) Instruments and reagents
[0119] Agilent 1260 HPLC, including online vacuum degasser (G-1311C), binary pump (G-1311C), standard autosampler (G-1329B), intelligent column oven (G-1316A) , DAD detector (G-1314B), Agilent1260Infinity chromatographic workstation (Agilent Technologies Co., Ltd.); SB-5200D ultrasonic cleaner (Ningbo Xinzhi Biotechnology Co., Ltd.); FAZ004B analytical balance (Shanghai Youke); BT125D electronic balance (Sartorius Scientific Instruments (Beijing) Co., Ltd.).
[0120] (2) Chromatographic conditions
[0121] The column is Thermo Syncronis-C 18 (5μm, 4.6×250mm) reverse-phase chromatographic column; column temperature is 30°C; detection wavelength is 210nm; mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid solution, the total flow rate is 0.8ml / min; analysis time is 60 minutes ; Elution gradient (se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com