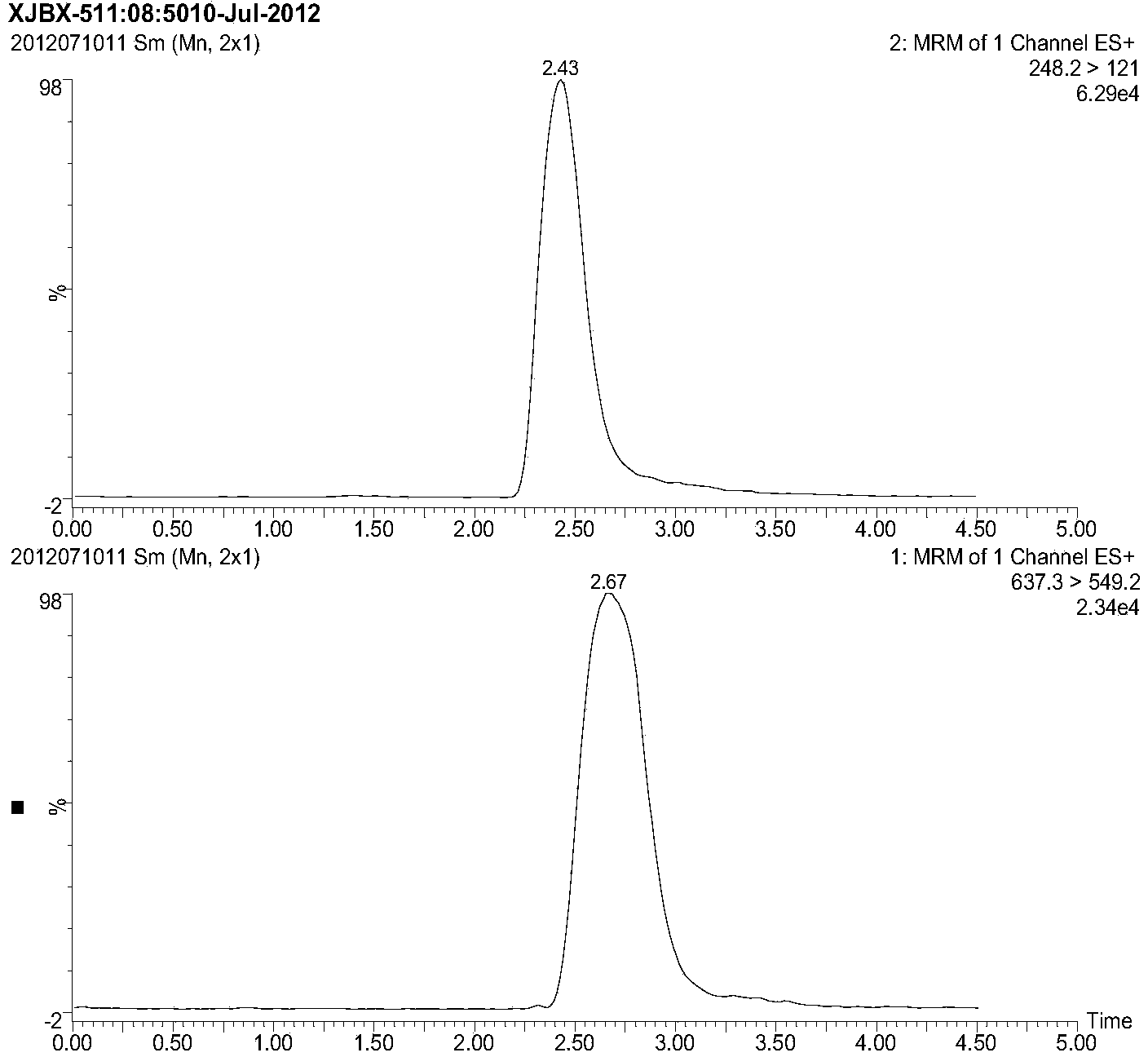
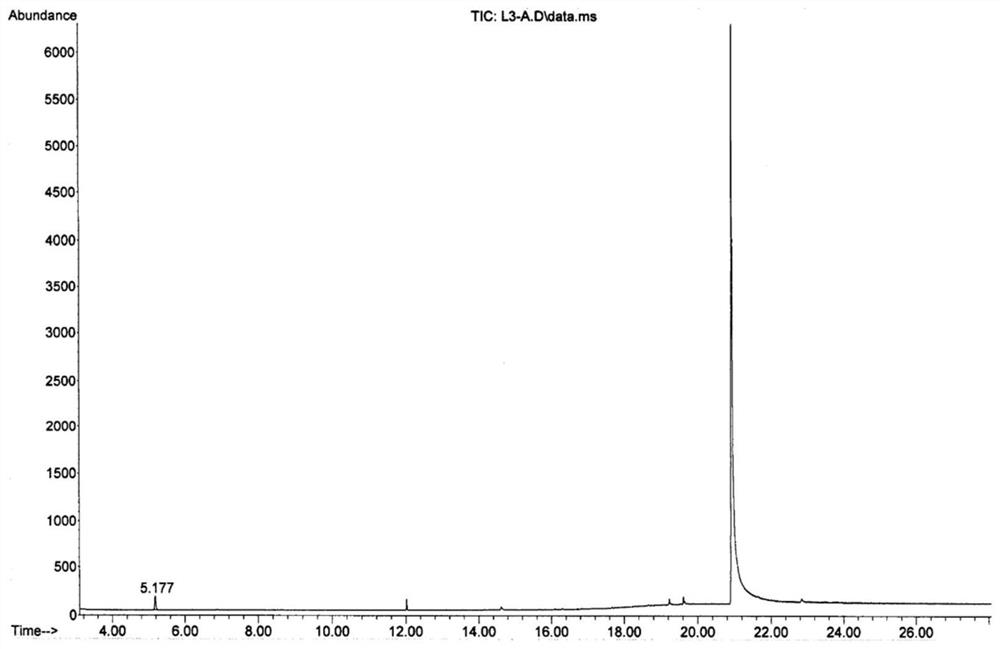
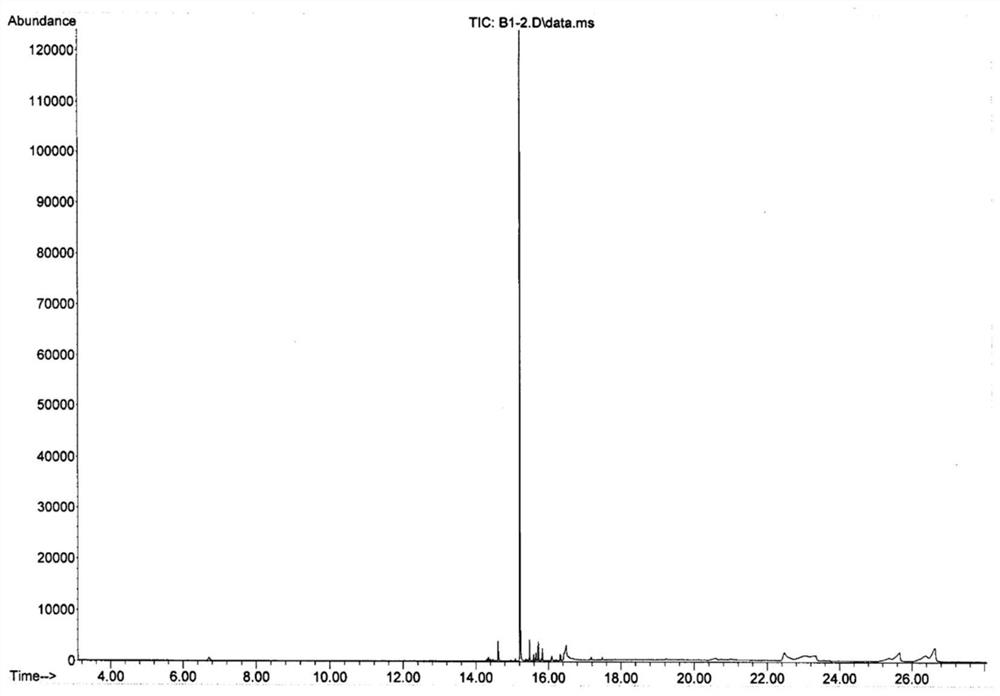
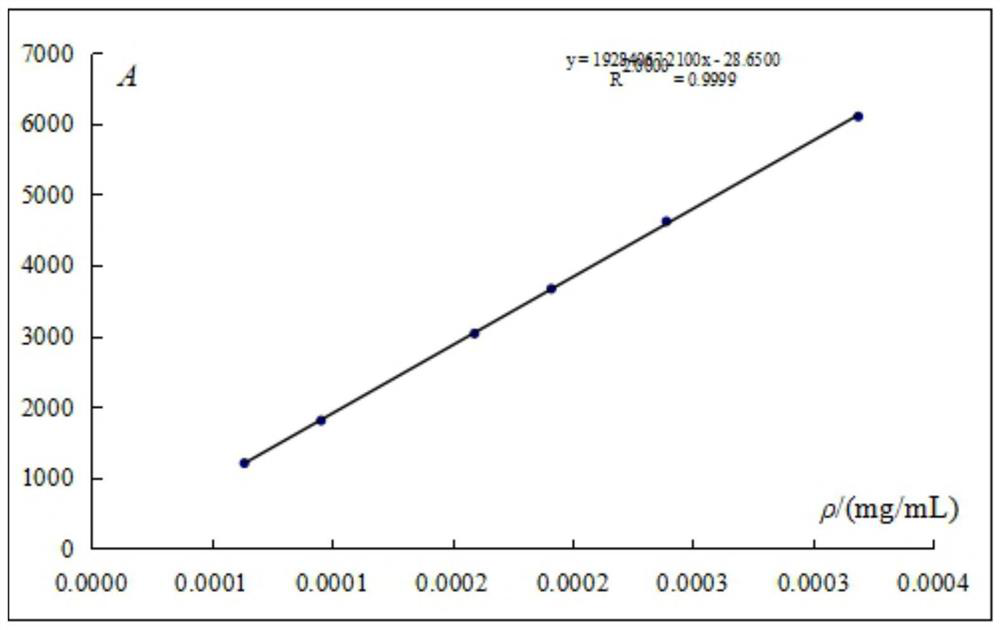
Patents
Literature
42results about How to "Suitable for routine determination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
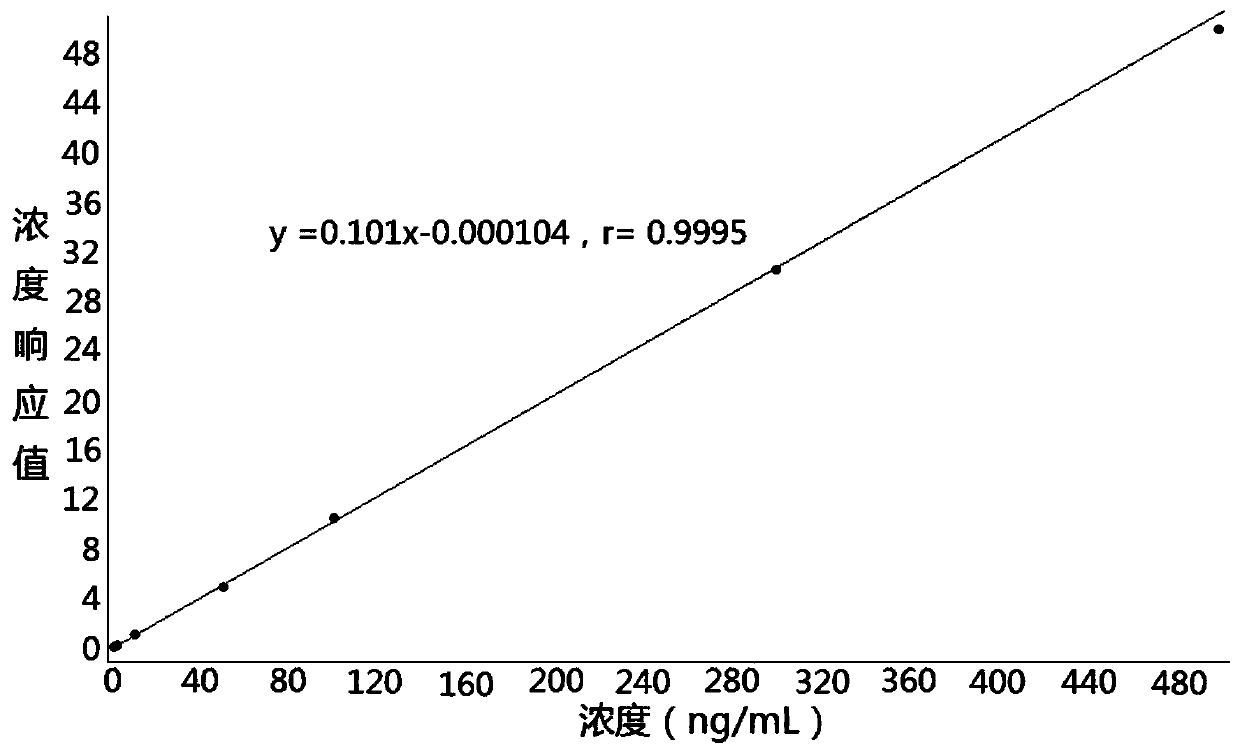
Method for determining concentration of tadalafil in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927307AThe pretreatment method is simpleGood peak shapeComponent separationChemistryChromatography column
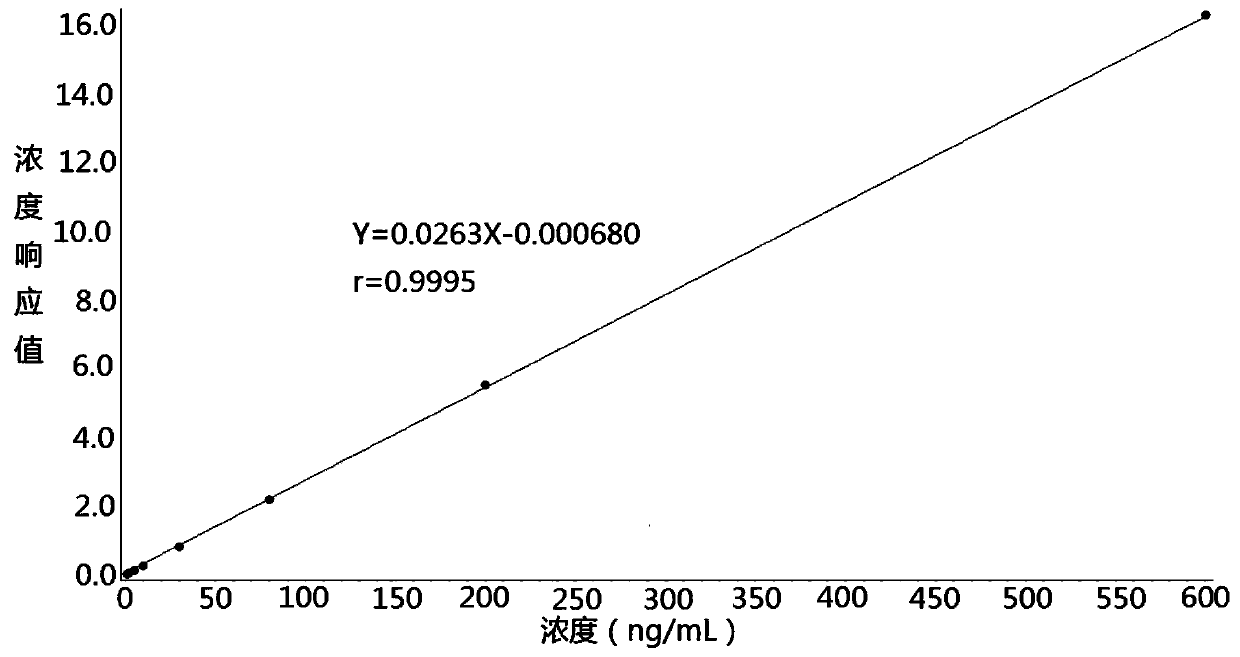
The invention discloses a method for determining the concentration of tadalafil in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by usinga chromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of tadalafil; according to the method, the linear range of a plasma standard curve is 1-600ng / mL, the intra-batch precision RSD and theinter-batch precision RSD are both less than + / -15%, and the method is suitable for measuring the concentration of tadalafil in plasma.
Owner:徐州立兴佳正医药科技有限公司
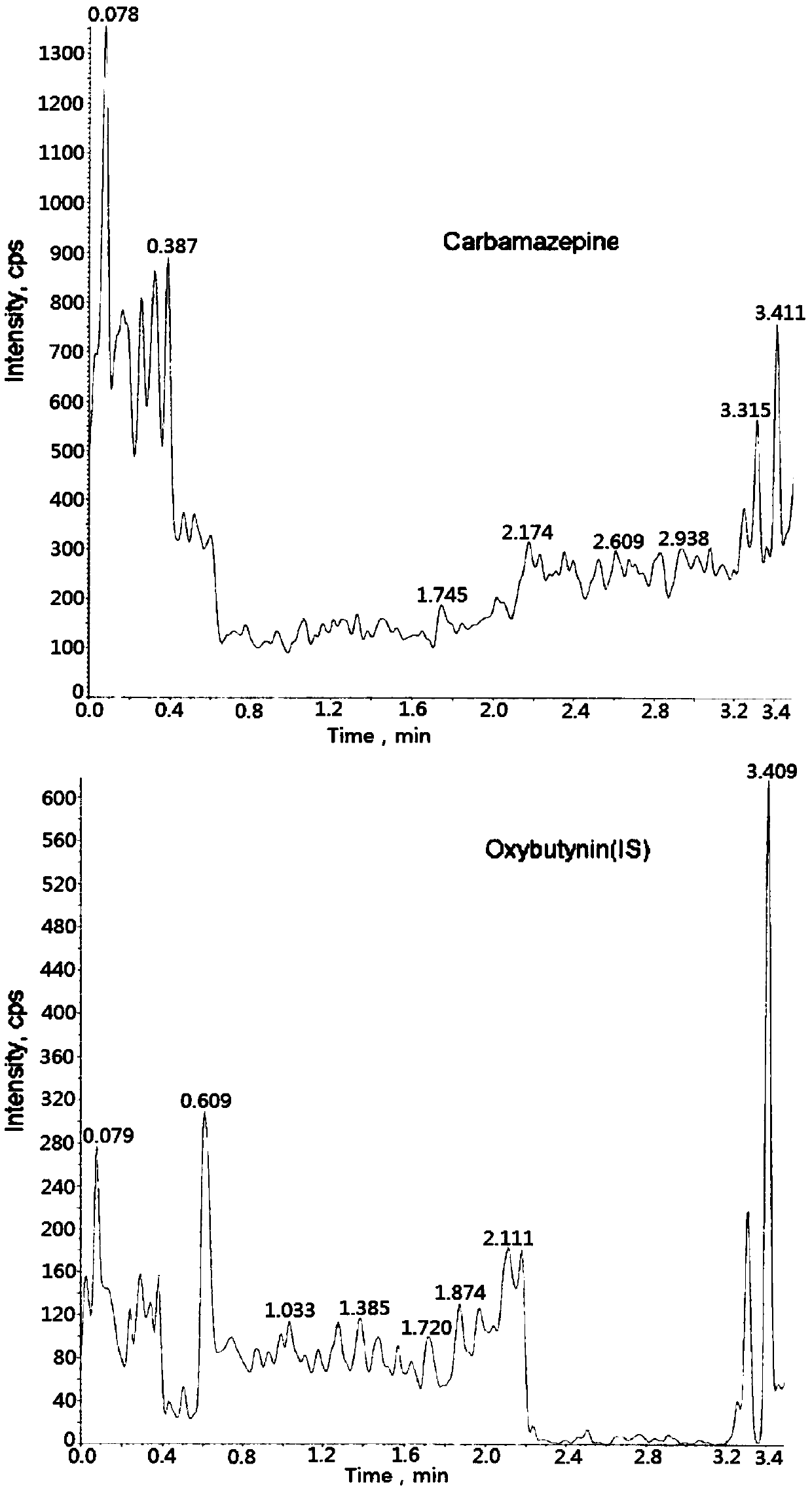
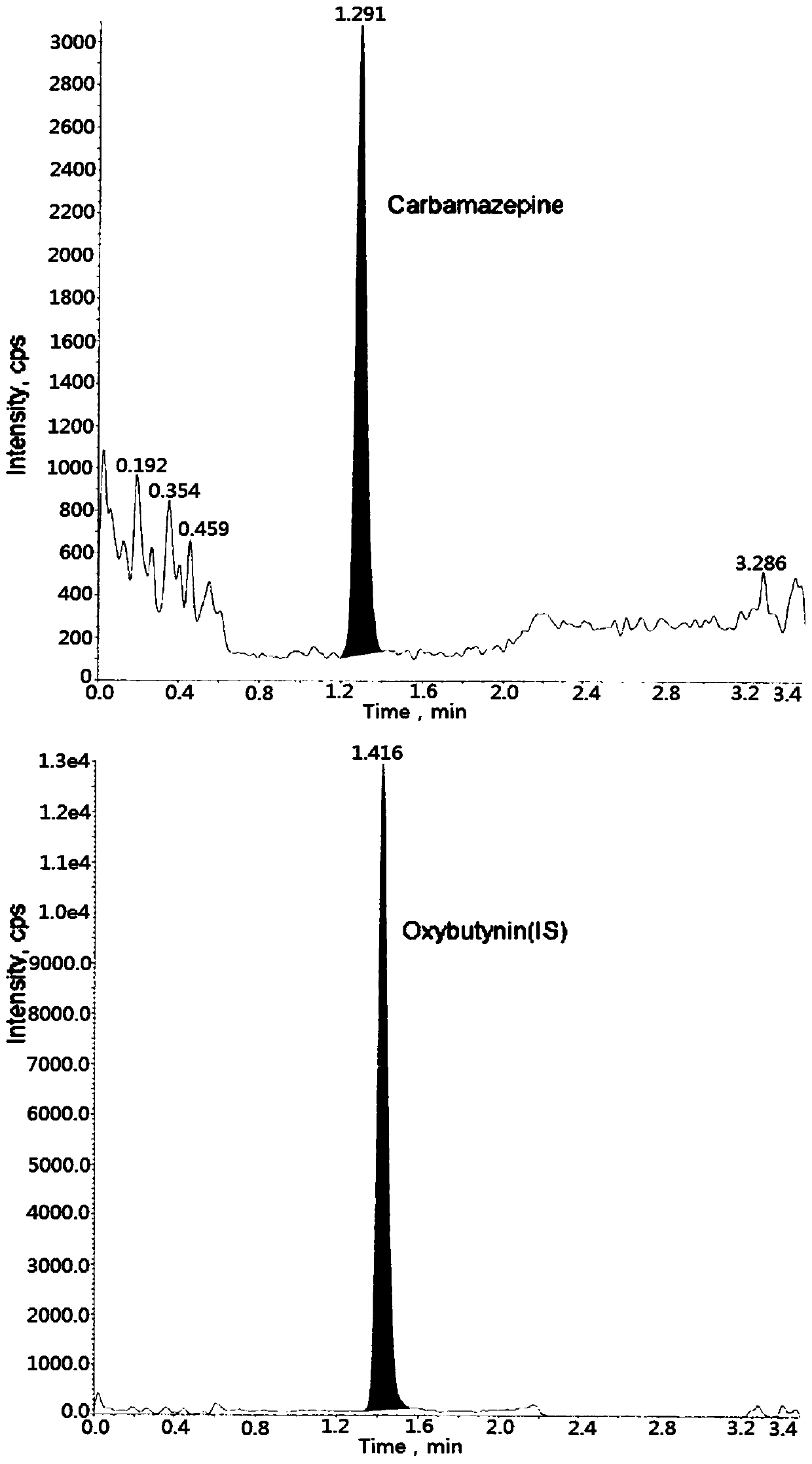
Method for determining concentration of carbamazepine in plasma by liquid chromatography mass spectrometry
InactiveCN109541107AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventGas chromatography–mass spectrometry
The invention discloses a method for determining concentration of carbamazepine in plasma by liquid chromatography mass spectrometry. A liquid chromatography mass spectrometry system is used for determining. A sample to be determined is added with a certain amount of mixed organic solvent to extract twice, is separated through a chromatographic column after pretreatment, and is detected by a massspectrometer. The method is rapid, accurate, high in sensitivity, and simple and convenient to operate. The method provides a basis for the blood drug concentration determination of carbamazepine. Thelinear range of a plasma standard curve in the method is 5 to 1000 ng / mL. The intra-batch and inter-batch precision RSD are less than + / -15%. The method is suitable for determining the concentrationof carbamazepine in the plasma.
Owner:徐州立顺康达医药科技有限公司
Method for determining concentration of 5'-methoxyl-3',4'-methylenedioxyphenyl cinnamic acid isobutyl amide in plasma
InactiveCN104076116AThe pretreatment method is simpleHigh sensitivityComponent separationChemistryChromatography column
The invention discloses a met / hod for determining the concentration of 5'-methoxyl-3',4'-methylenedioxyphenyl cinnamic acid isobutyl amide in plasma. A liquid chromatography-mass system is adopted to determine. The method comprises the following steps: taking a to-be-determined sample first, adding a certain quantity of organic solvent for extraction, separating through a chromatographic column after pretreatment, and determining by using a mass spectrometry detector. The method disclosed by the invention has the advantages of rapidity, accuracy, high sensitivity, simplicity and convenience in operation and is suitable for determining the concentration of 5'-methoxyl-3',4'-methylenedioxyphenyl cinnamic acid isobutyl amide in plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
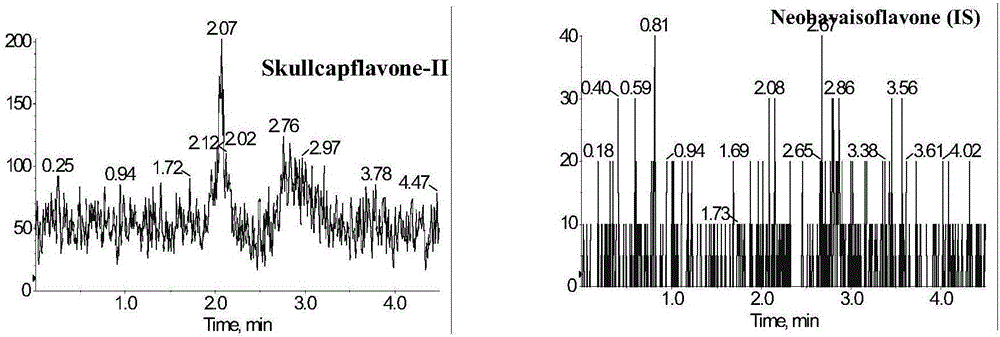
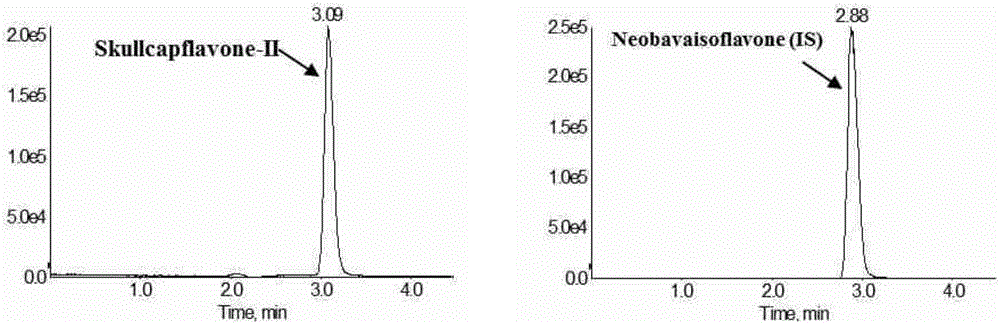
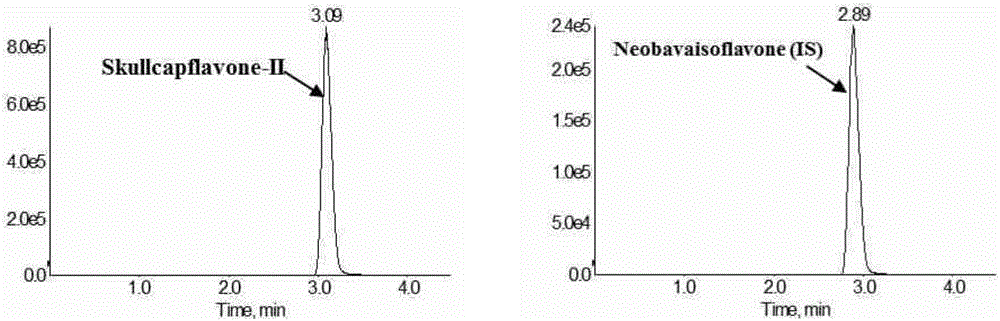
Method for measuring concentration of skullcapflavone II in plasma
InactiveCN106018580AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventPhysical chemistry
The invention discloses a method for measuring concentration of 5,2'-dyhydroxyl,6,7,8,5'-tetramethoxyflavone (skullcapflavone II) in plasma. A liquid chromatography-mass spectrometry system is used for carrying out measuring, a sample to be measured is firstly taken, a certain amount of organic solvent is added for medicine liquid-liquid extraction, after pretreatment, chromatographic column separation is carried out, and a mass spectrometry detector is used for carrying out detection. According to the method, quickness and accuracy are achieved, sensitivity is high, and operation is easy and convenient; the method is suitable for measuring the concentration of skullcapflavone II in the plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Method for detecting chaetoglobosin concentration in blood plasma
InactiveCN104101665AThe pretreatment method is simpleSuitable for routine determinationComponent separationMass spectrometry detectorBlood plasma
The invention discloses a method for detecting chaetoglobosin concentration in blood plasma. The method adopts a liquid chromatography tandem mass spectrometry system to perform detection, a sample to be detected is firstly taken, a certain amount of organic menstruum protein precipitant is added to perform protein precipitation, and detection is performed through chromatographic column separation and a mass spectrum detector after pretreatment. The method is quick, accurate, high in sensitivity, simple and convenient to operate and suitable for concentration detection in the blood plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Analysis method for measuring dimethyl sulfate in pyraclostrobin by gas chromatography-mass spectrometry
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to an analysis method for determining dimethyl sulfate in pyraclostrobin by a gas chromatography-mass spectrometry method. The invention discloses an analysis method for measuring dimethyl sulfate in pyraclostrobin by gas chromatography-mass spectrometry. The analysis method comprises the following steps: (1) preparing a standard solution; (2) drawing a standard curve; and (3) determining the content of dimethyl sulfate in the sample. Compared with the prior art, the method disclosed by the invention has the advantages of simplicity and convenience in sample preparation, small sample dosage and suitability for conventional determination; the sensitivity is high, and the lowest detection limit of dimethyl sulfate is 0.20 mg / kg. The method is simple in test process, high in sensitivity and good in accuracy.
Owner:SUZHOU GUOCHEN BIOTEK CO LTD
Method for determining concentration of omeprazole in plasma by liquid chromatography-mass spectrometry
InactiveCN112782323AThe method is simpleStrong specificityComponent separationChromatography columnOmeprazole
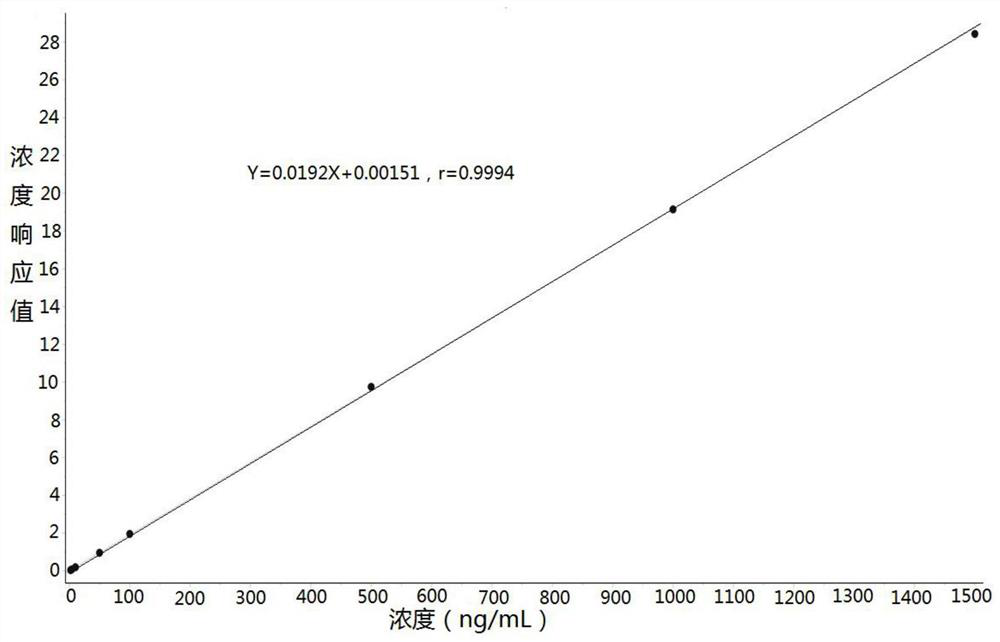
The invention discloses a method for determining the concentration of omeprazole in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination and comprises the steps of firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of omeprazole; the linear range of a plasma standard curve of the method is 2 to 1500ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for determining the concentration of omeprazole in plasma.
Owner:徐州立顺康达医药科技有限公司
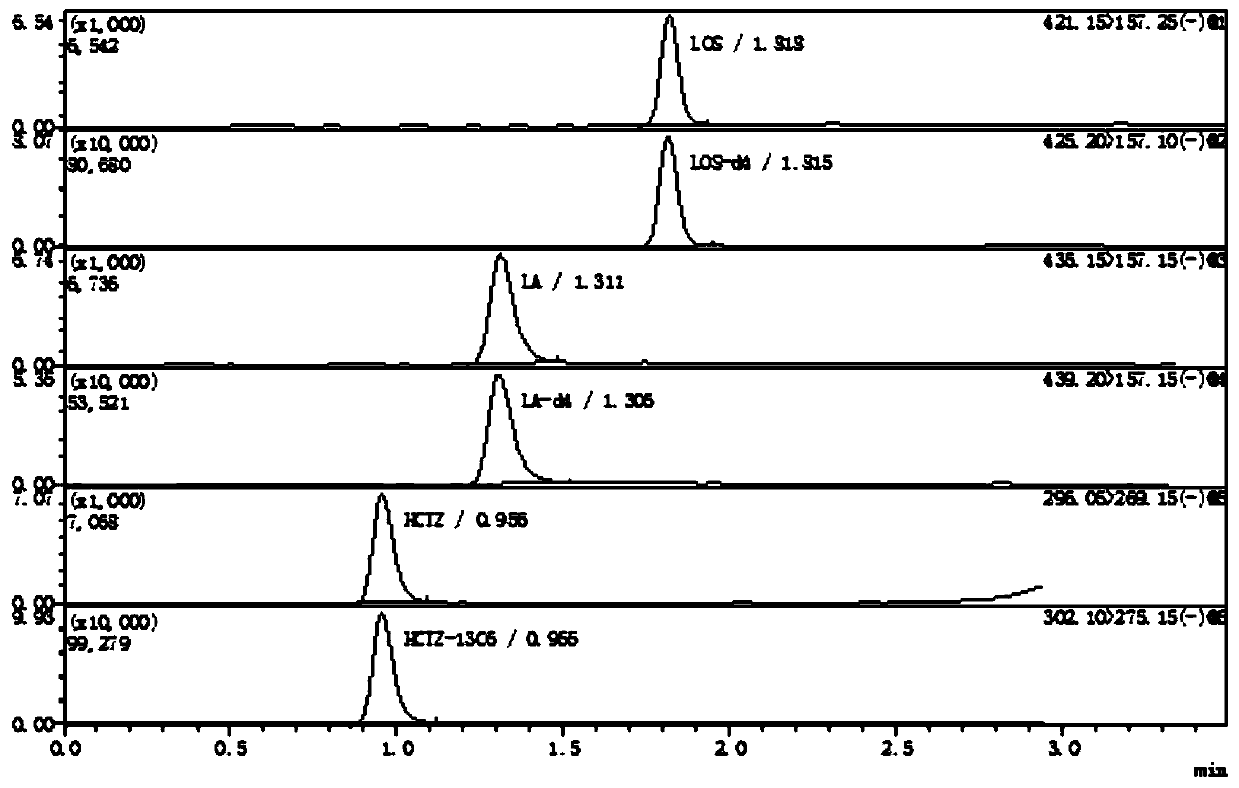
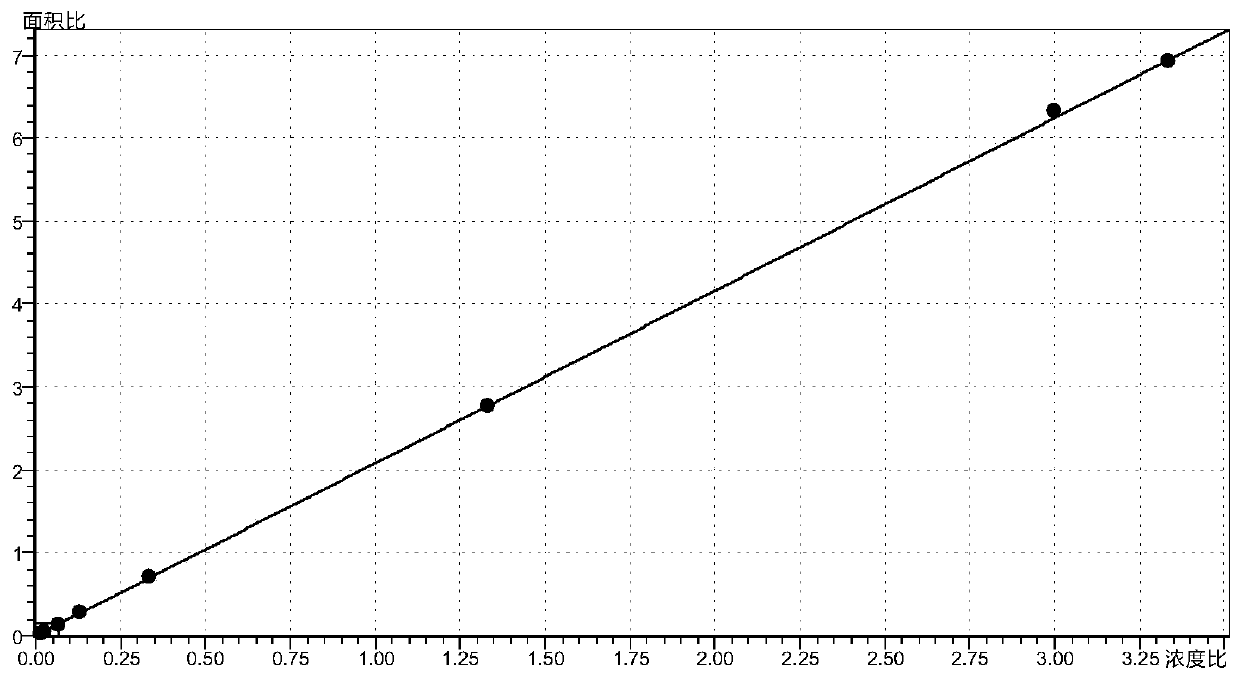
Method for determining concentration of hydrochlorothiazide, losartan and 5-carboxylic acid losartan in plasma by liquid chromatography-mass spectrometry
InactiveCN111443135AThe preprocessing method is simpleSuitable for routine determinationComponent separationMetaboliteLiquid chromatography mass spectroscopy
The invention provides a method for determining the concentration of hydrochlorothiazide, losartan and active hydroxy acid metabolite 5-carboxylic acid losartan in plasma by liquid chromatography-massspectrometry. The method comprises the following steps of: (1) pretreating a plasma sample; (2) detecting by liquid chromatography-mass spectrometry; (3) preparing a standard curve; and (4) determining the concentration of hydrochlorothiazide, losartan and active hydroxy acid metabolite 5-carboxylic acid losartan in plasma. The method provided by the invention is rapid, accurate, high in sensitivity and simple to operate, and serves clinical application of medicines.
Owner:SUZHOU GUOCHEN BIOTEK CO LTD
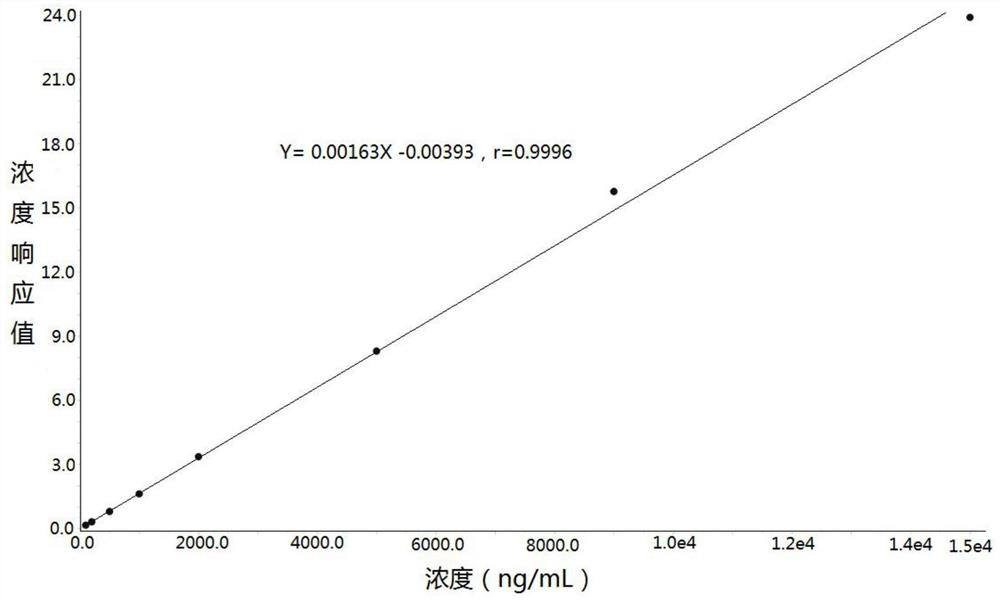
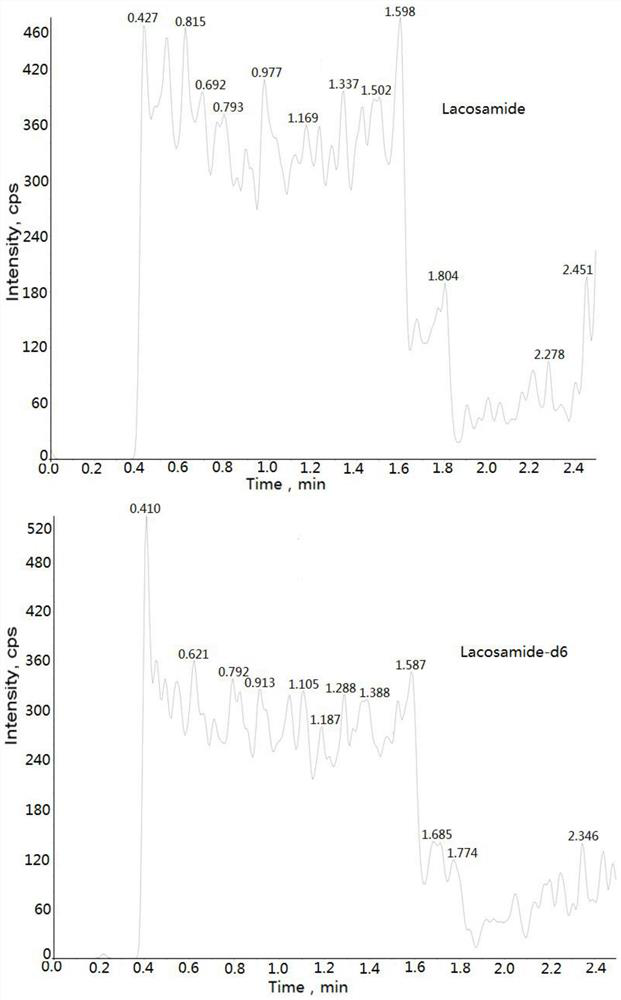
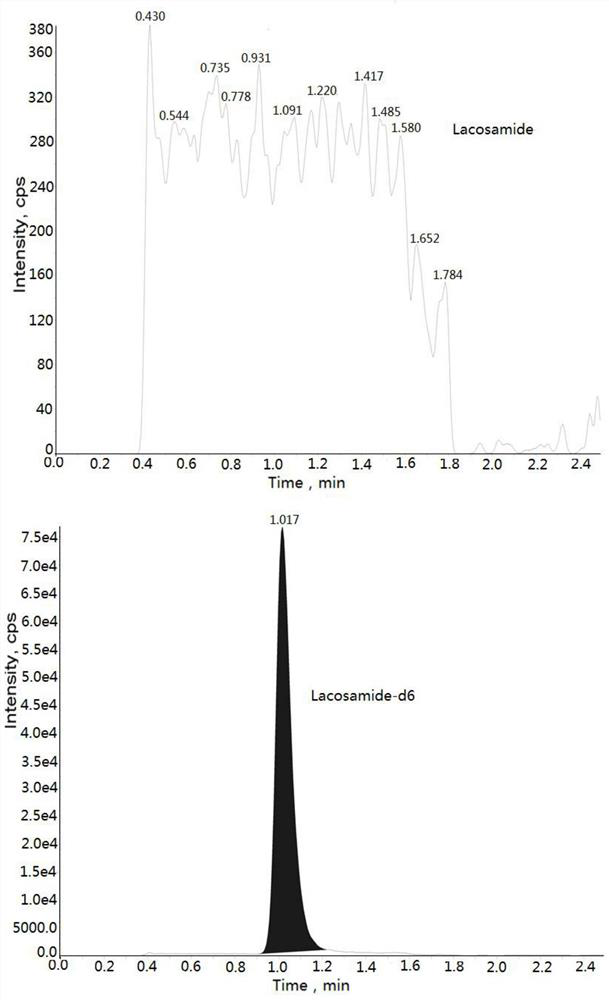
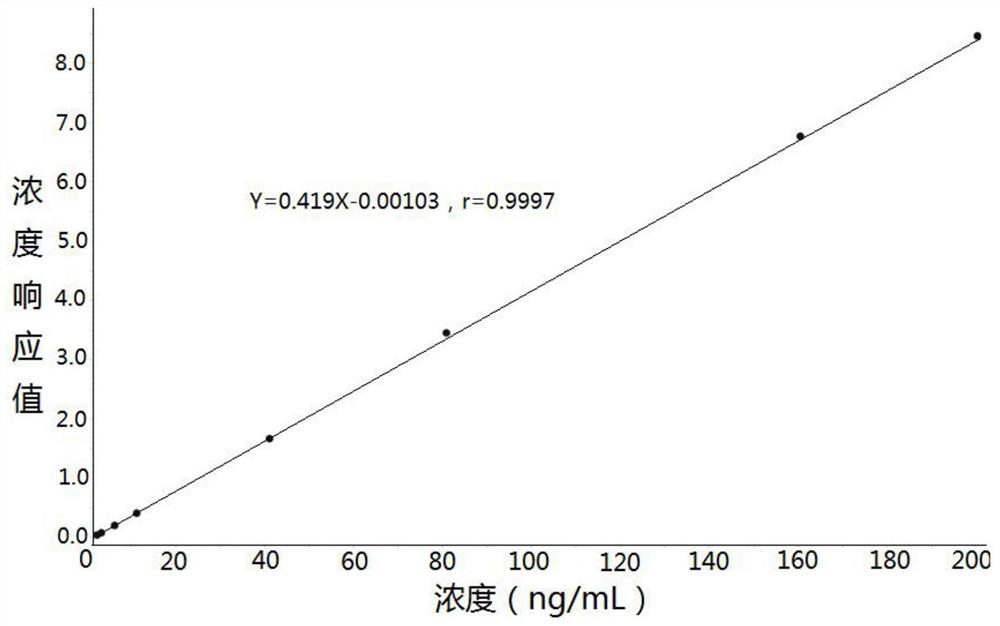
Method for determining concentration of lacosamide in blood plasma by liquid chromatography-mass spectrometry
InactiveCN112630352AThe pretreatment method is simpleGood peak shapeComponent separationChromatography columnBlood plasma
The invention discloses a method for determining the concentration of lacosamide in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for determining the blood concentration of lacosamide; and according to the method, the linear range of the plasma standard curve is 100-15000 ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of lacosamide in plasma.
Owner:徐州立顺康达医药科技有限公司
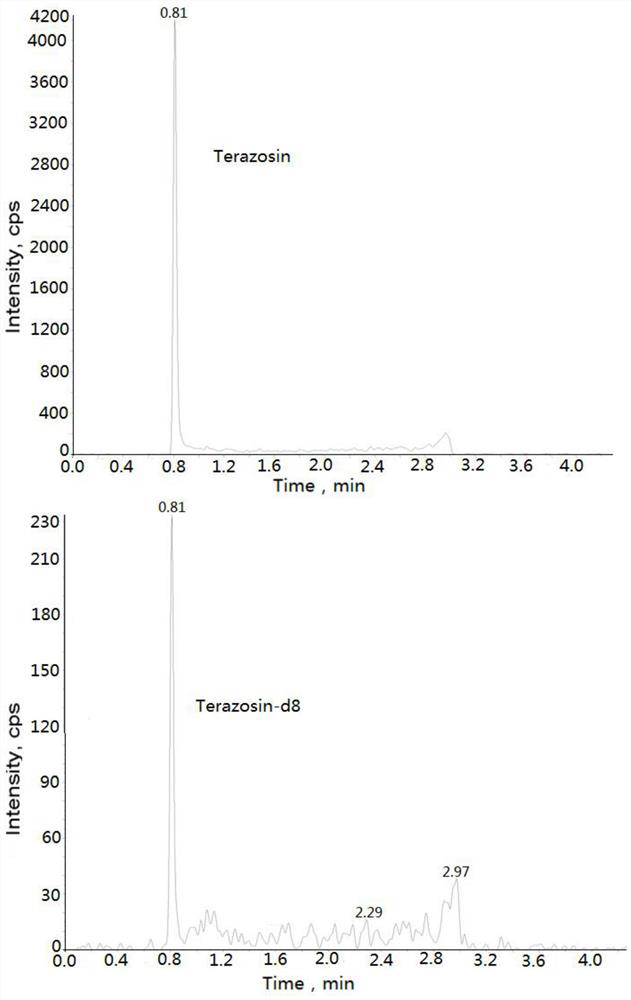
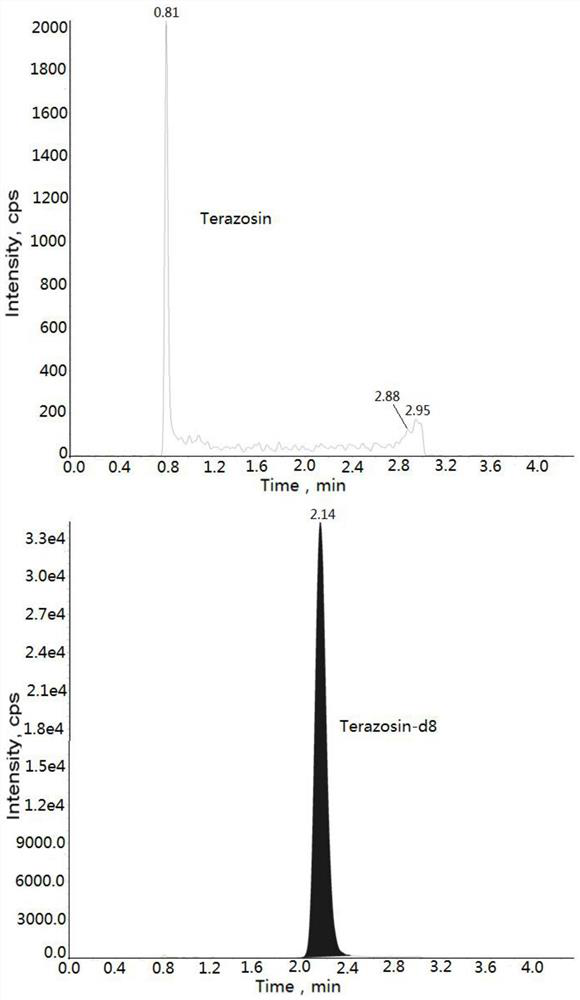
Method for measuring concentration of terazosin in plasma by liquid chromatography-mass spectrometry
InactiveCN112748205AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventTerazosin
The invention discloses a method for measuring the concentration of terazosin in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for determining the plasma concentration of terazosin; and the linear range of a plasma standard curve of the method is 1-200 ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for measuring the concentration of terazosin in plasma.
Owner:徐州立顺康达医药科技有限公司
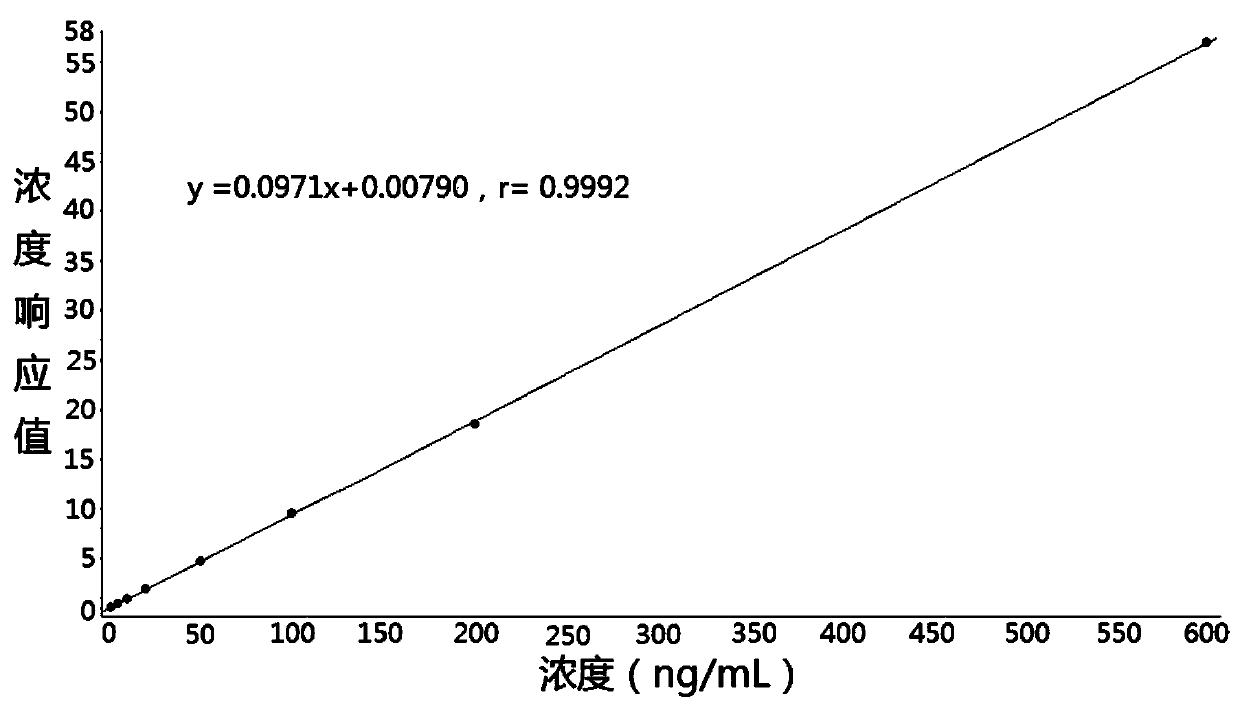
Method for determining concentration of cetirizine in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927308AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
The invention discloses a method for determining the concentration of cetirizine in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by using a chromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of cetirizine; according to the method, the linear range of the plasma standard curve is 1-600ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both less than + / -15%, and the method is suitable for measuring the concentration of cetirizine in plasma.
Owner:徐州立兴佳正医药科技有限公司
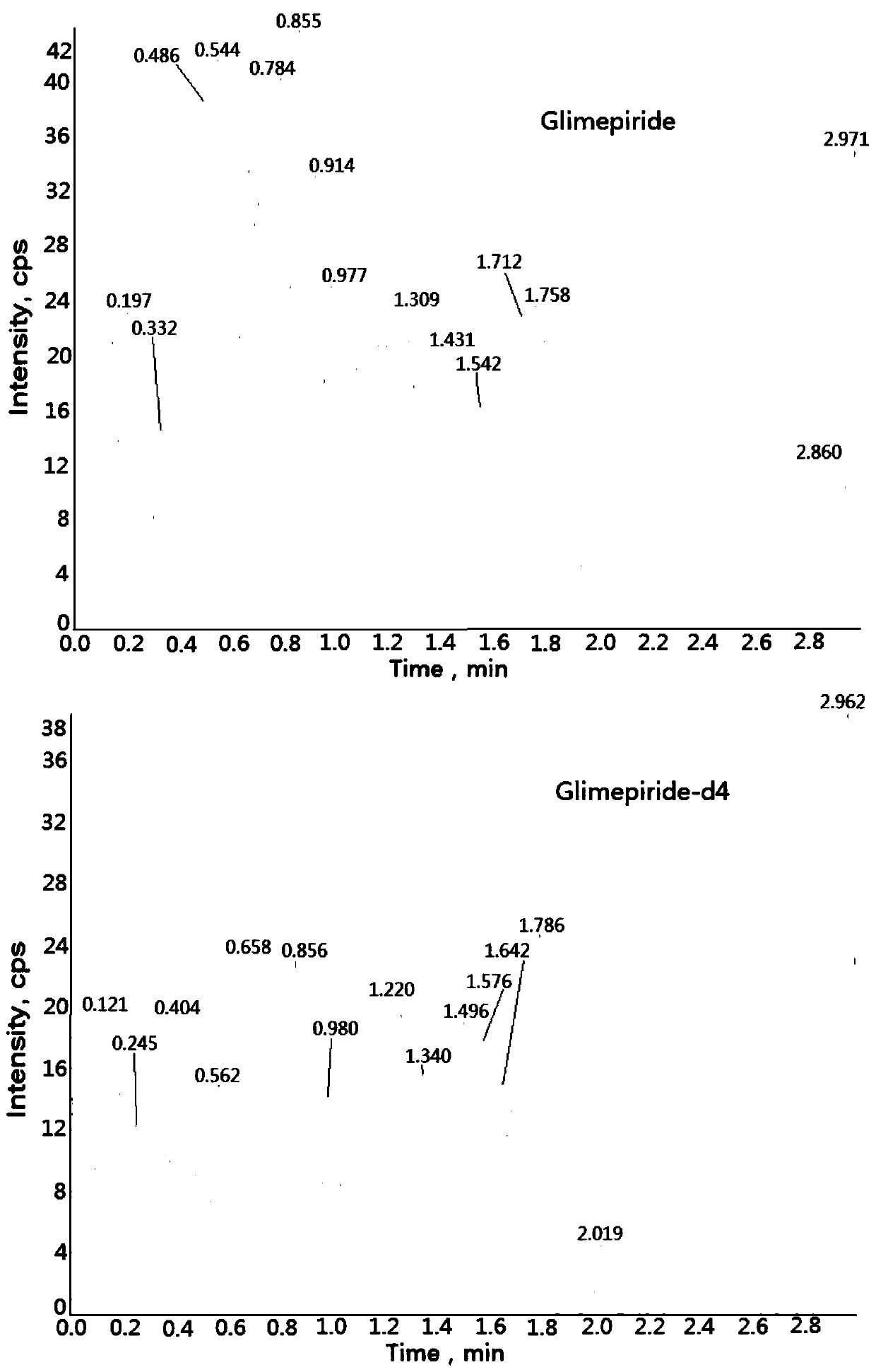
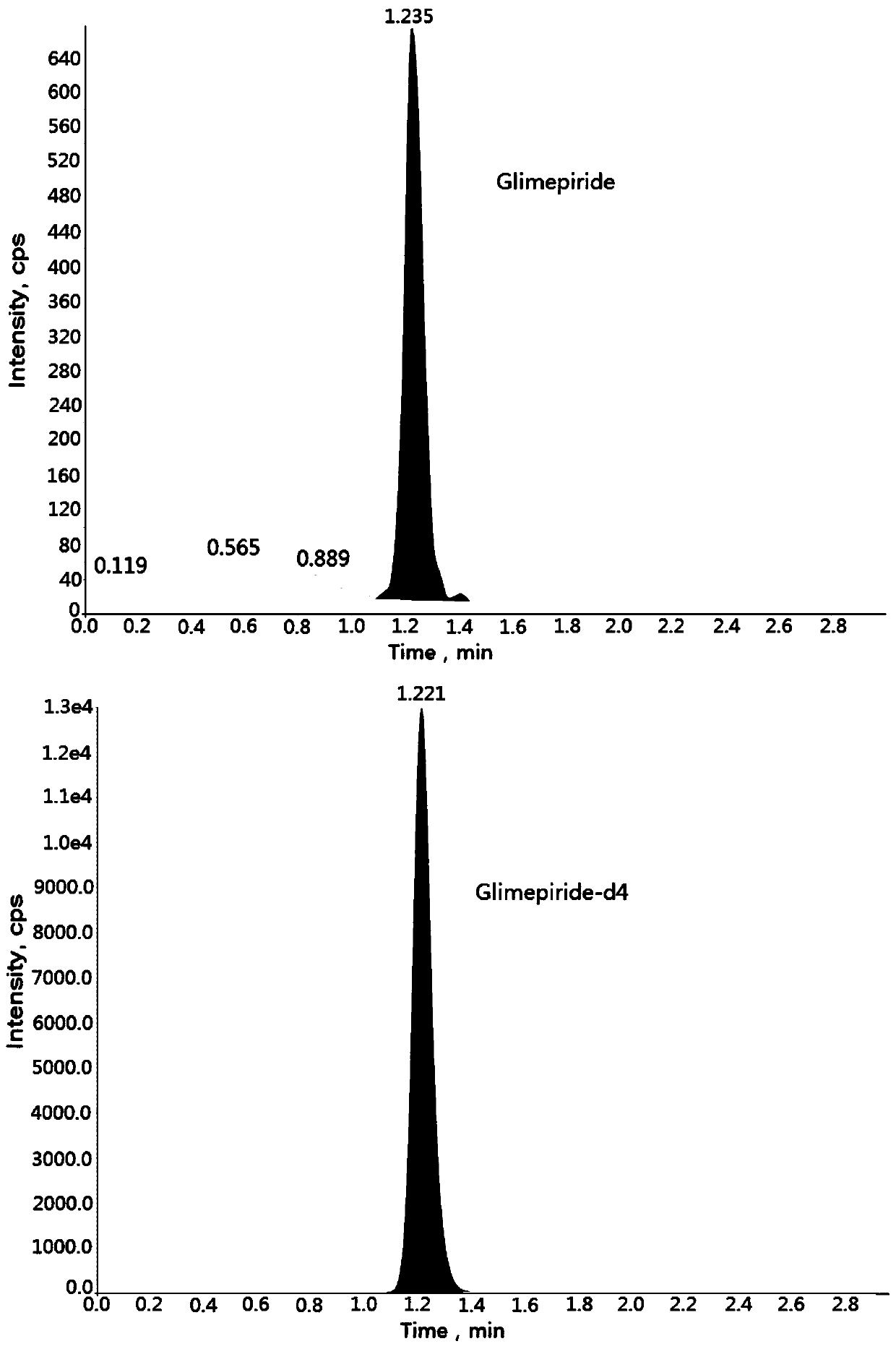
Method for determining concentration of glimepiride in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927304AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
The invention discloses a method for determining the concentration of glimepiride in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be determined, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by achromatographic column, and performing detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of glimepiride; the linear range of a plasma standard curve of the method is 0.5-500 ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of glimepiride in plasma.
Owner:徐州立兴佳正医药科技有限公司
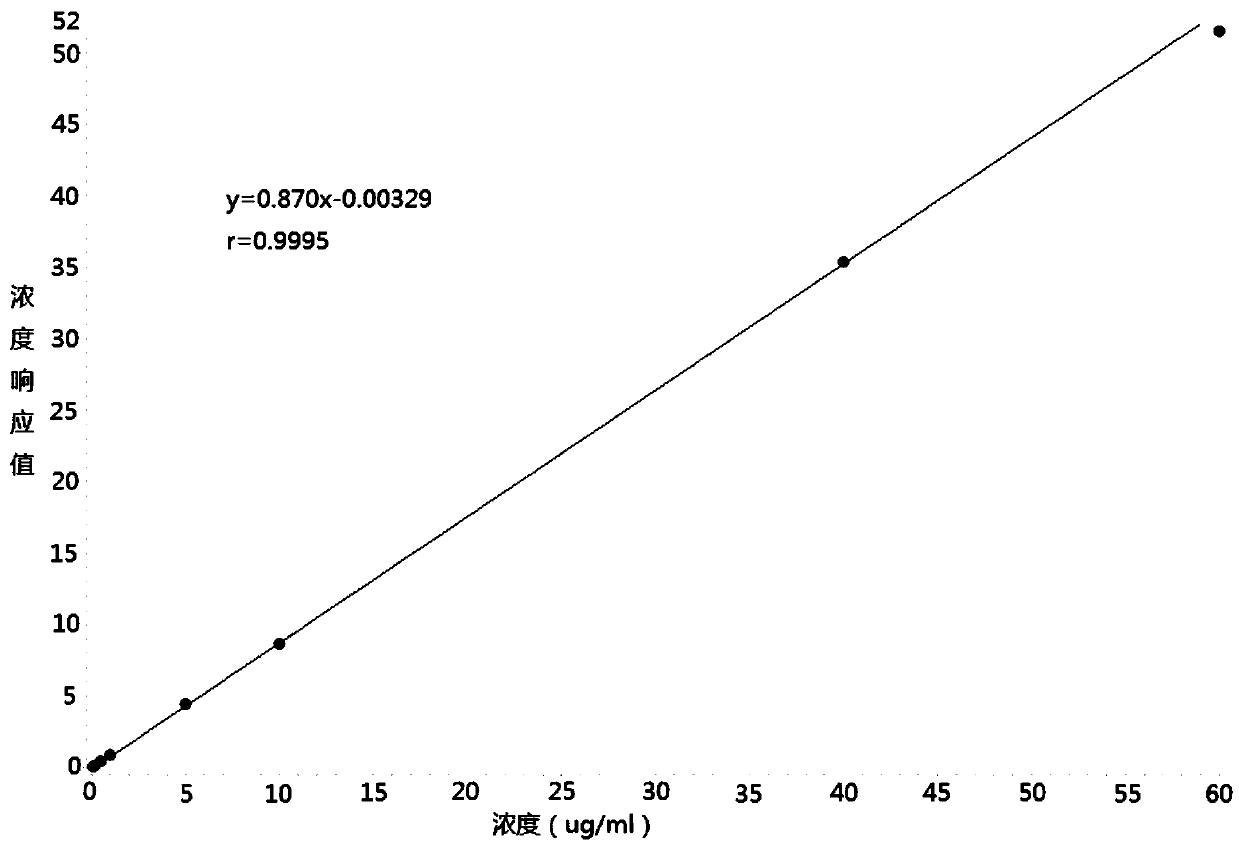
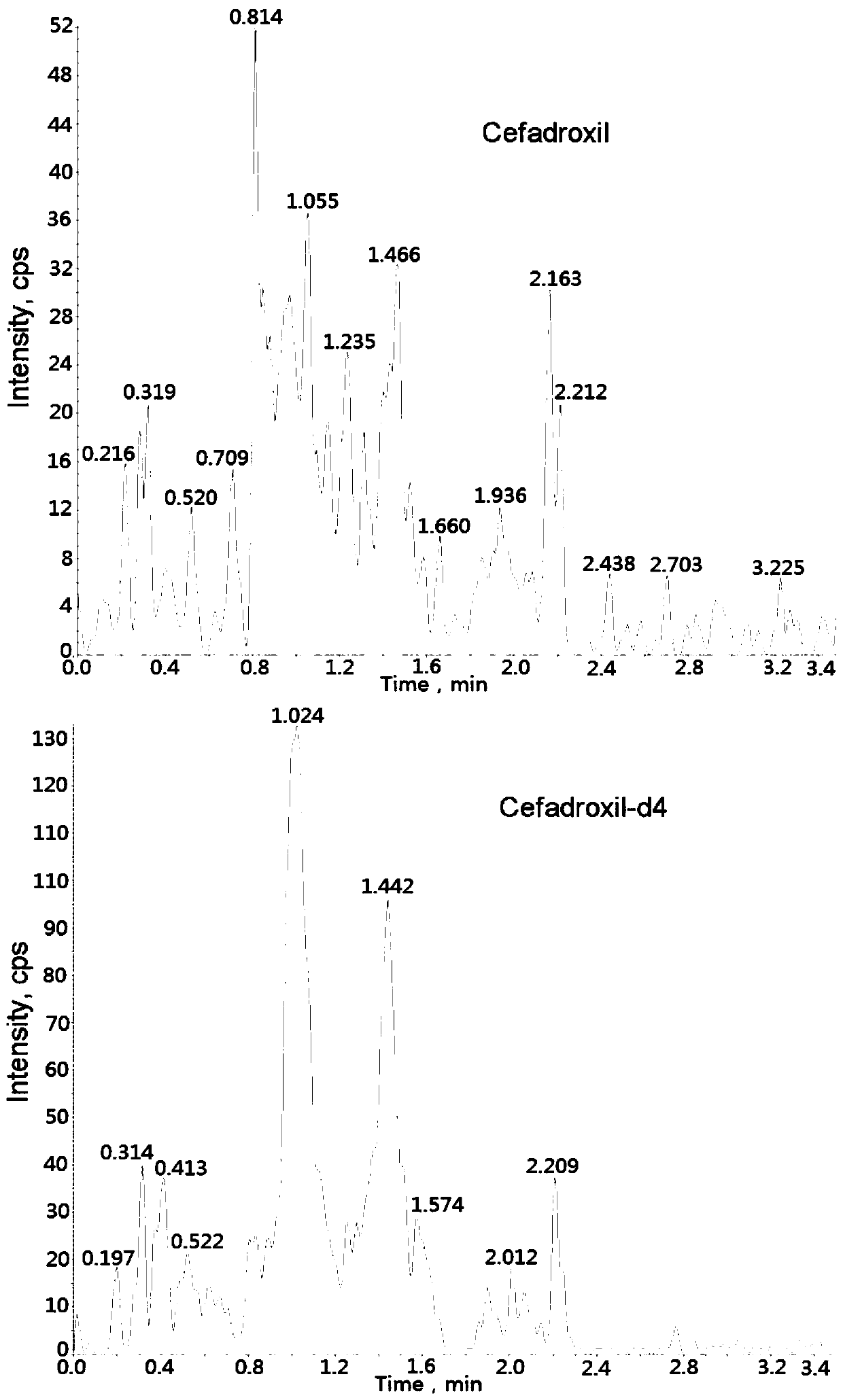
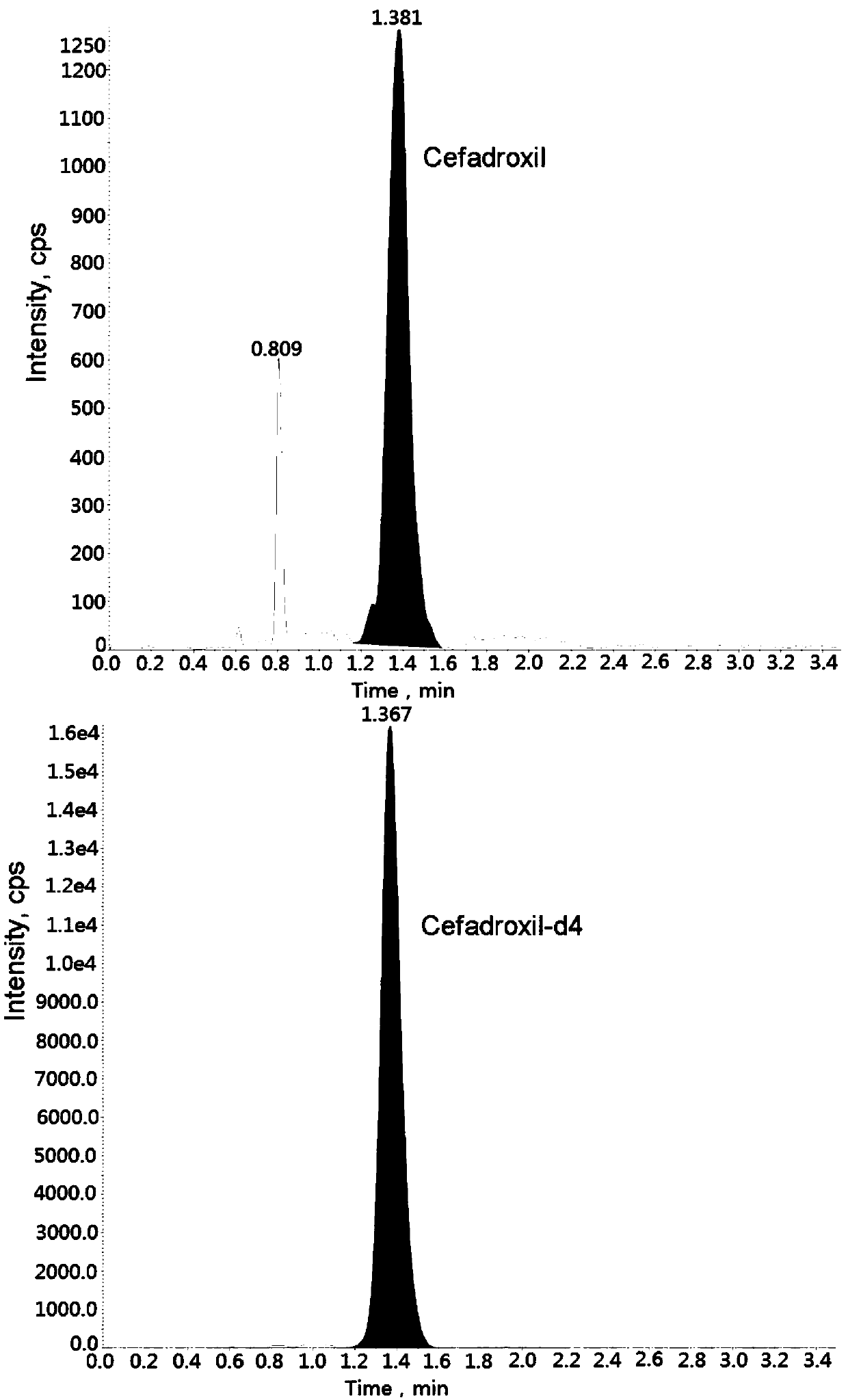
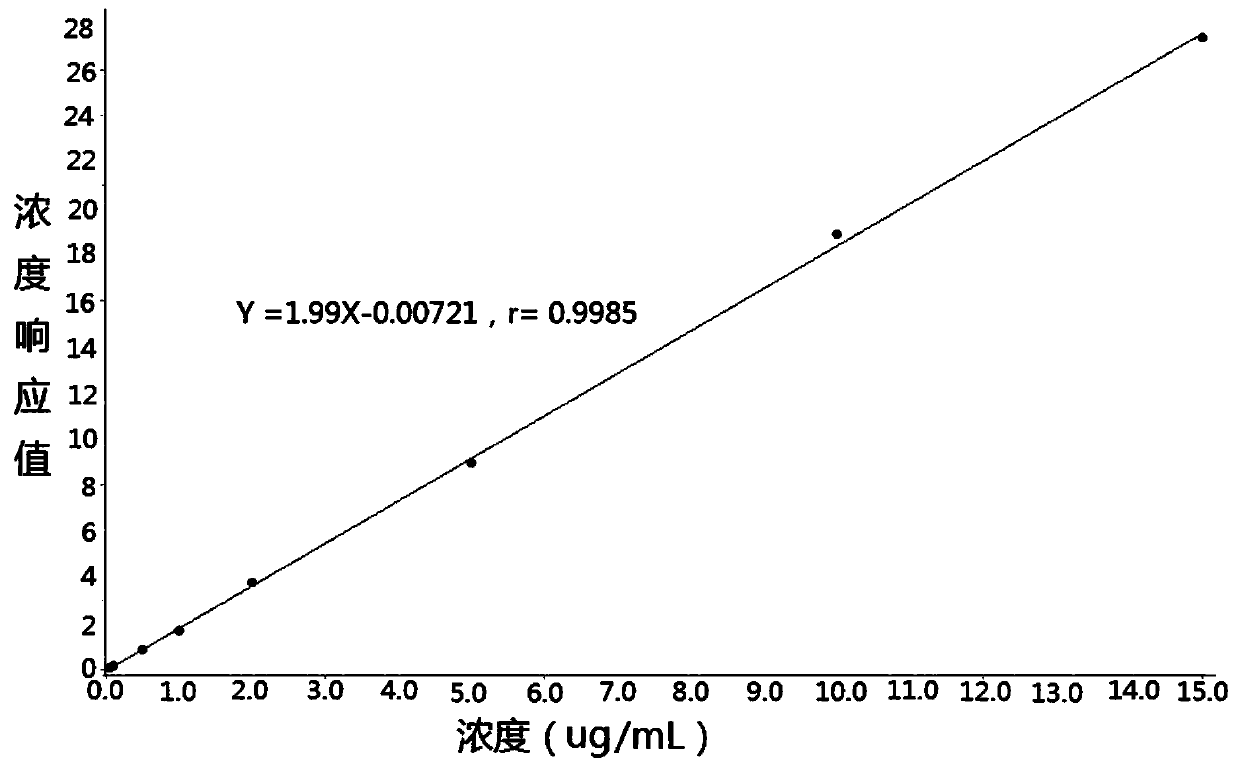
Method for measuring cefadroxil concentration in plasma through hygroplasm combination
InactiveCN109682913AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for measuring cefadroxil concentration in plasma through hygroplasm combination. A hygroplasm combination system is adopted for measurement. The method comprises the steps that firstly, a to-be-measured sample is taken, a certain quantity of mixed organic solvent is added for conducting extraction twice, and after pretreatment, through chromatographic column separation, a mass spectrometry detector is used for detection. The method is quick to use, accurate, high in sensitivity and easy and convenient to operate, and the basis is provided for plasma concentration measurement of cefadroxil. The linear range of a plasma standard curve is within 0.1-60 mug / mL, the within-run precision and between-run precision RSD are smaller than + / -15%, and the method is suitable for measurement of cefadroxil concentration in plasma.
Owner:徐州立兴佳正医药科技有限公司
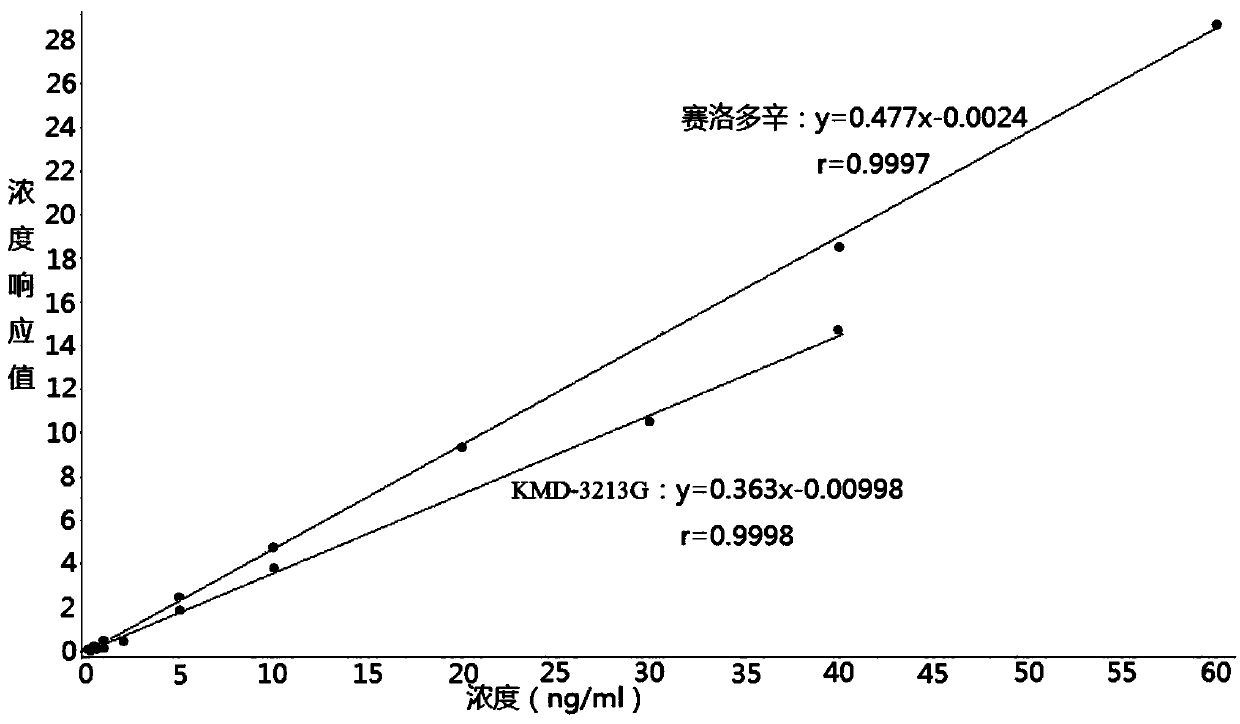
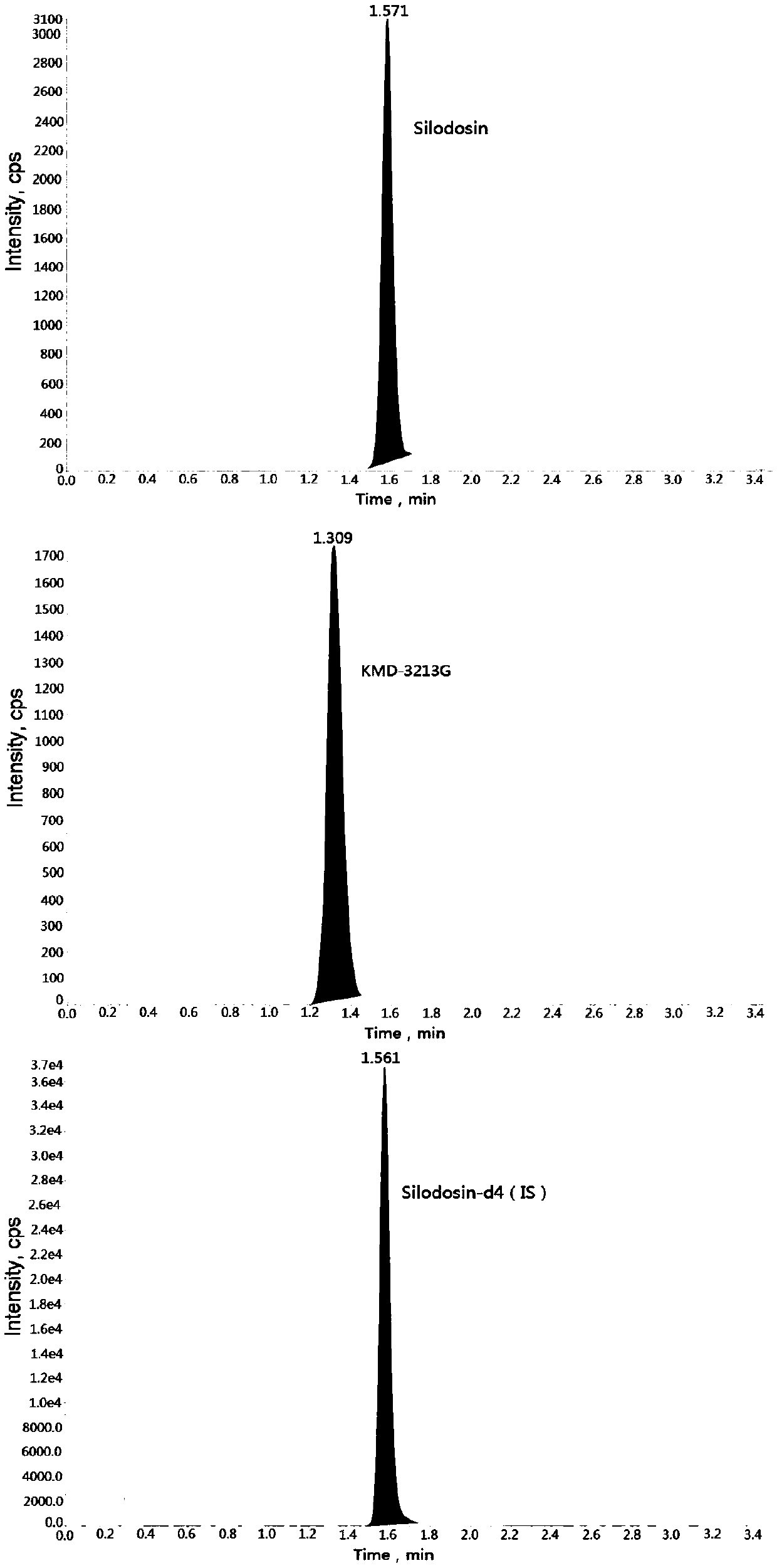
Method for determining concentration of serodosin and KMD-3213G in plasma by using liquid chromatography-mass spectrometry
InactiveCN109541109AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventBlood concentration
The invention discloses a method for determining the concentration of serodosin and KMD-3213G in plasma by using liquid chromatography-mass spectrometry. The concentration of serodosin and KMD-3213G in the plasma is determined by using a liquid chromatography-mass spectrometry system; taking a sample to be tested first, and adding a certain amount of mixed organic solvent for extraction twice; after pretreatment, performing chromatographic column separation, and then detecting by using a mass spectrometry detector. The method for determining the concentration of serodosin and KMD-3213G in theplasma by using the liquid chromatography-mass spectrometry disclosed by the invention has the advantages of rapid, accurate, high sensitivity and simple operation, and provides a basis for determining the blood concentration of serodosin and KMD-3213G. According to the plasma standard curve of the method, the quantitative range of serodosin is 0.2-60 ng / mL, the quantitative range of KMD-3213G is0.2-40 ng / mL, and the intra-assay and inter-assay precisions RSDs are less than + / -15%, which is suitable for determining the concentration of serodosin and KMD-3213G in the plasma.
Owner:徐州立顺康达医药科技有限公司
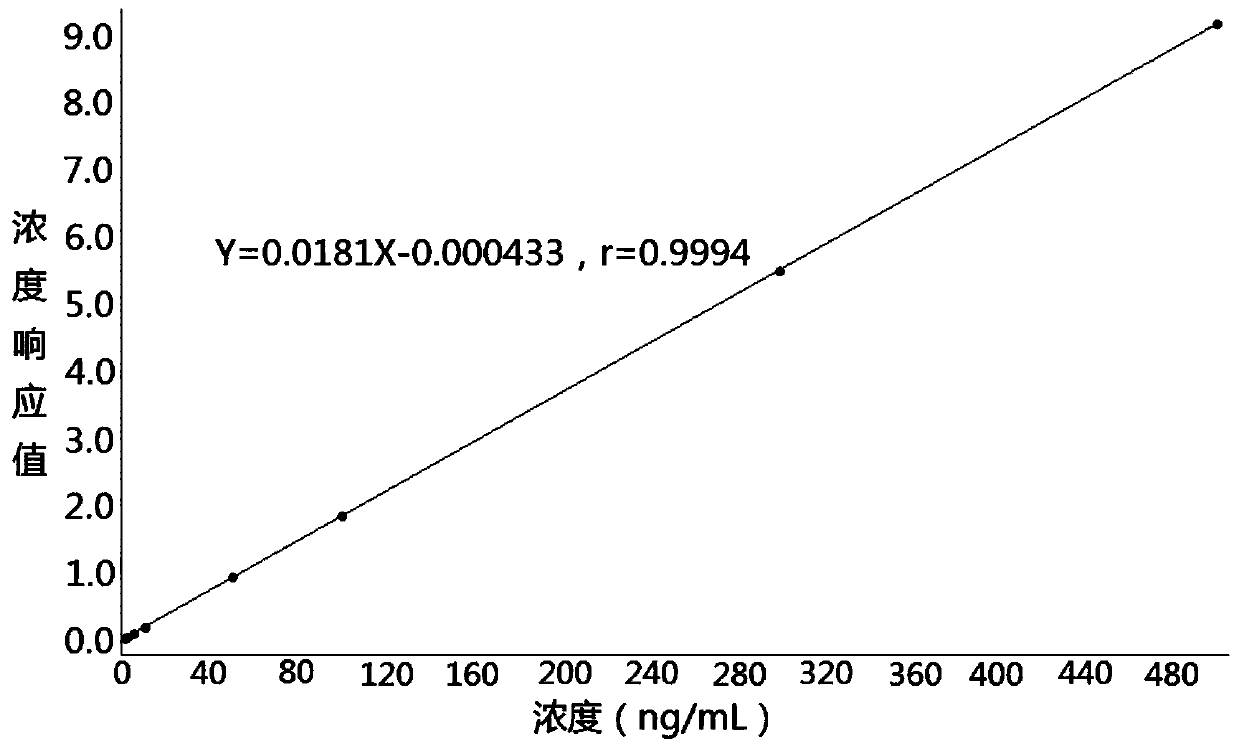
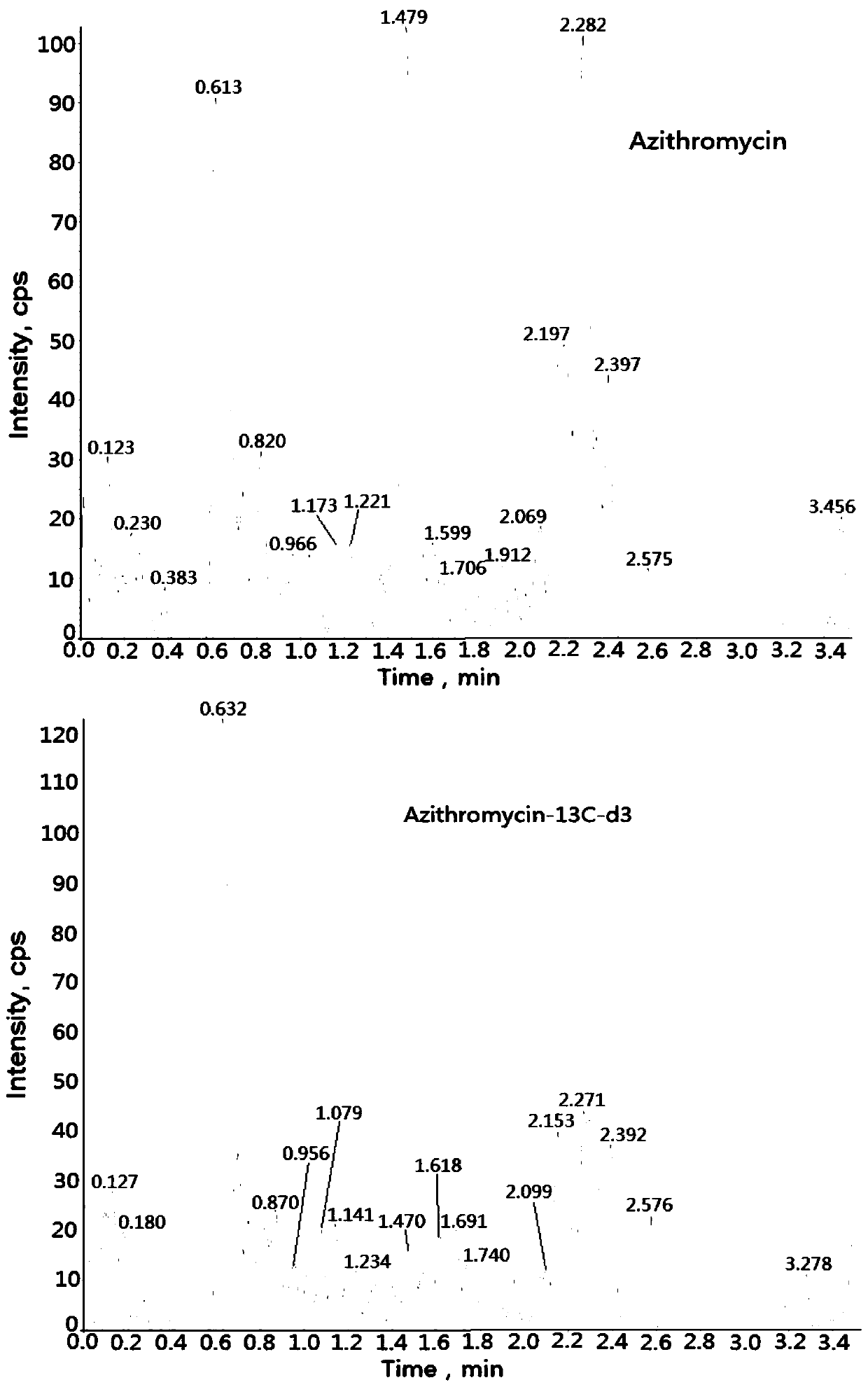
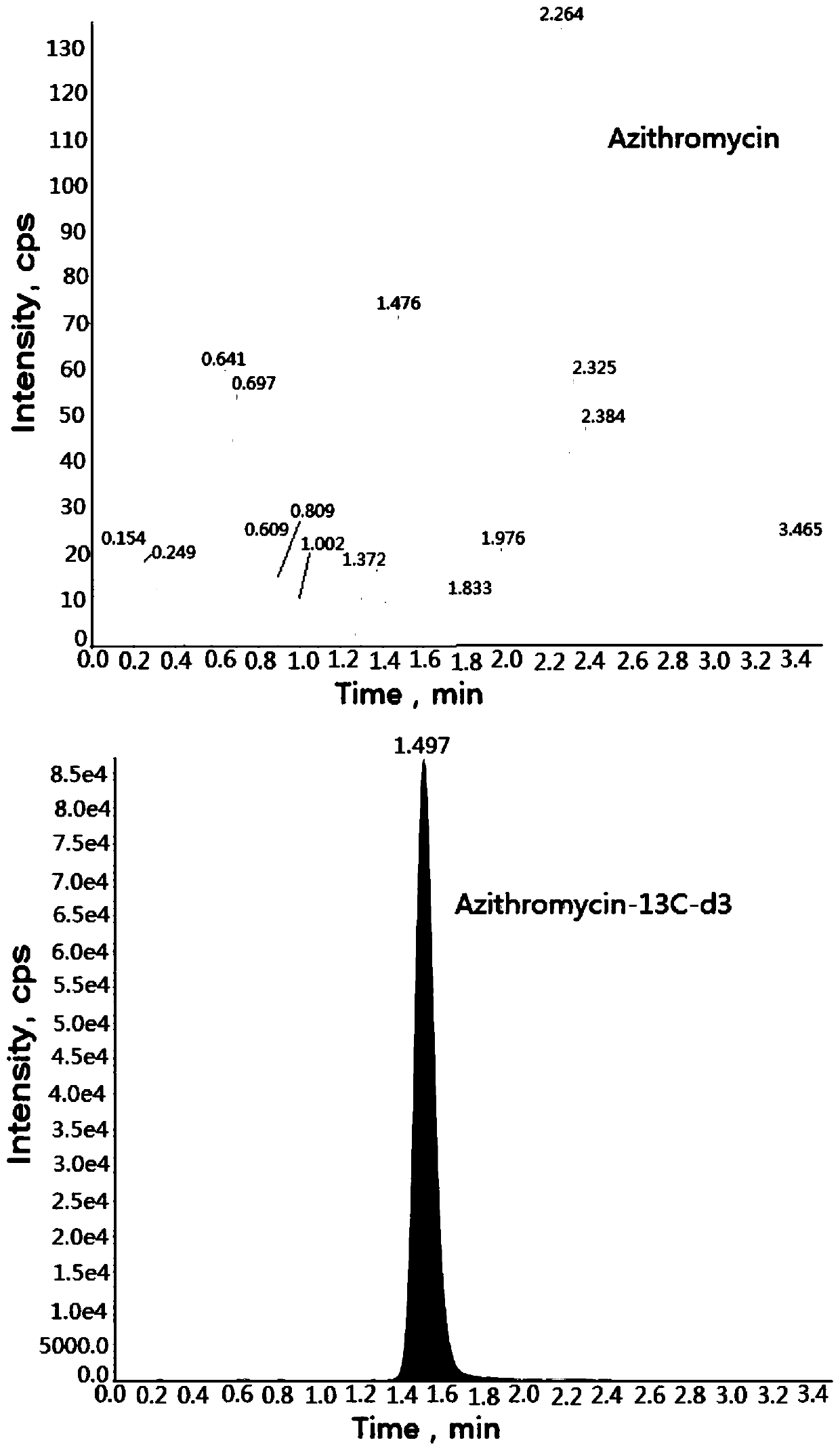
Method for determining concentration of azithromycin in plasma by liquid chromatography-mass spectrometry
InactiveCN111044659AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for determining the concentration of azithromycin in plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination and comprises the steps of taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction, separating by a chromatographic column after pretreatment,and detecting by a mass spectrum detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the plasma concentration of azithromycin. According to the method, the linear range of a plasma standard curve is 1-500ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both less than + / -15%, and the method is suitable for determining the concentration of azithromycin in plasma.
Owner:南京立顺康达医药科技有限公司
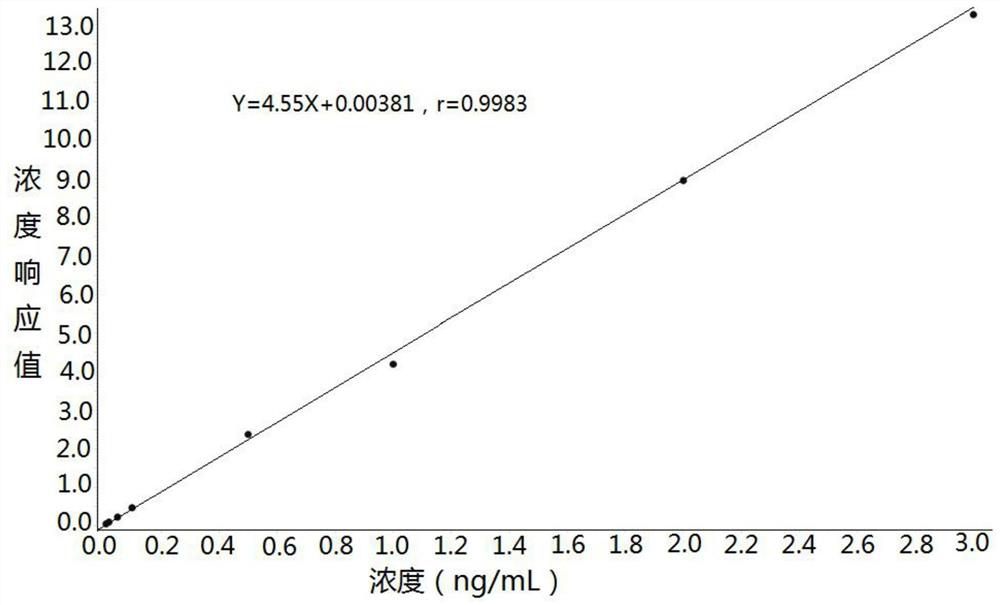
Method for detecting concentration of hydrocortisone in plasma by liquid chromatography-mass spectrometry
InactiveCN112763619AThe pretreatment method is simpleSuitable for routine determinationComponent separationFludrocortisoneBlood concentration
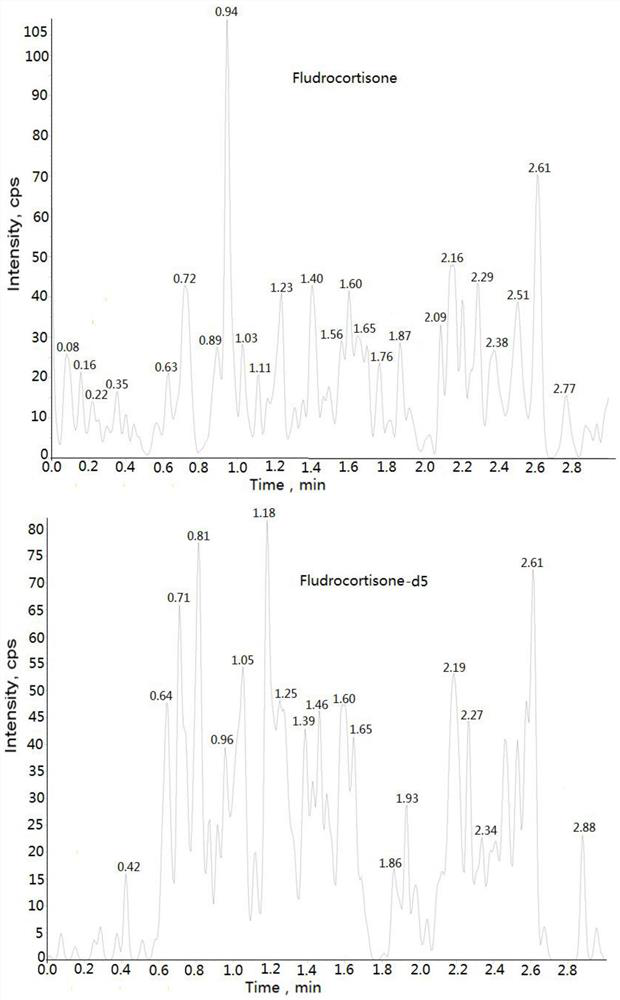
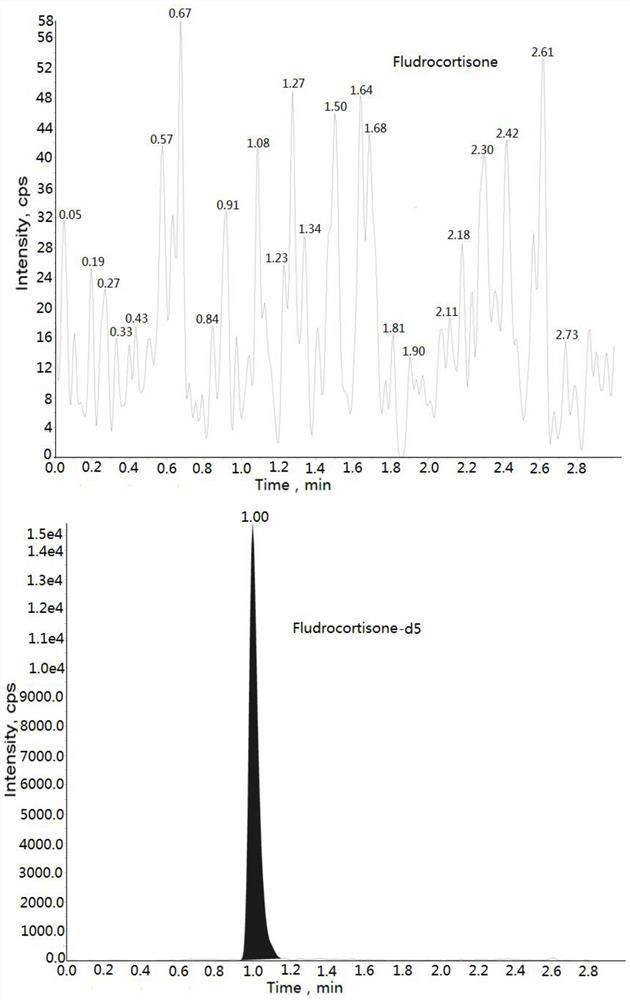
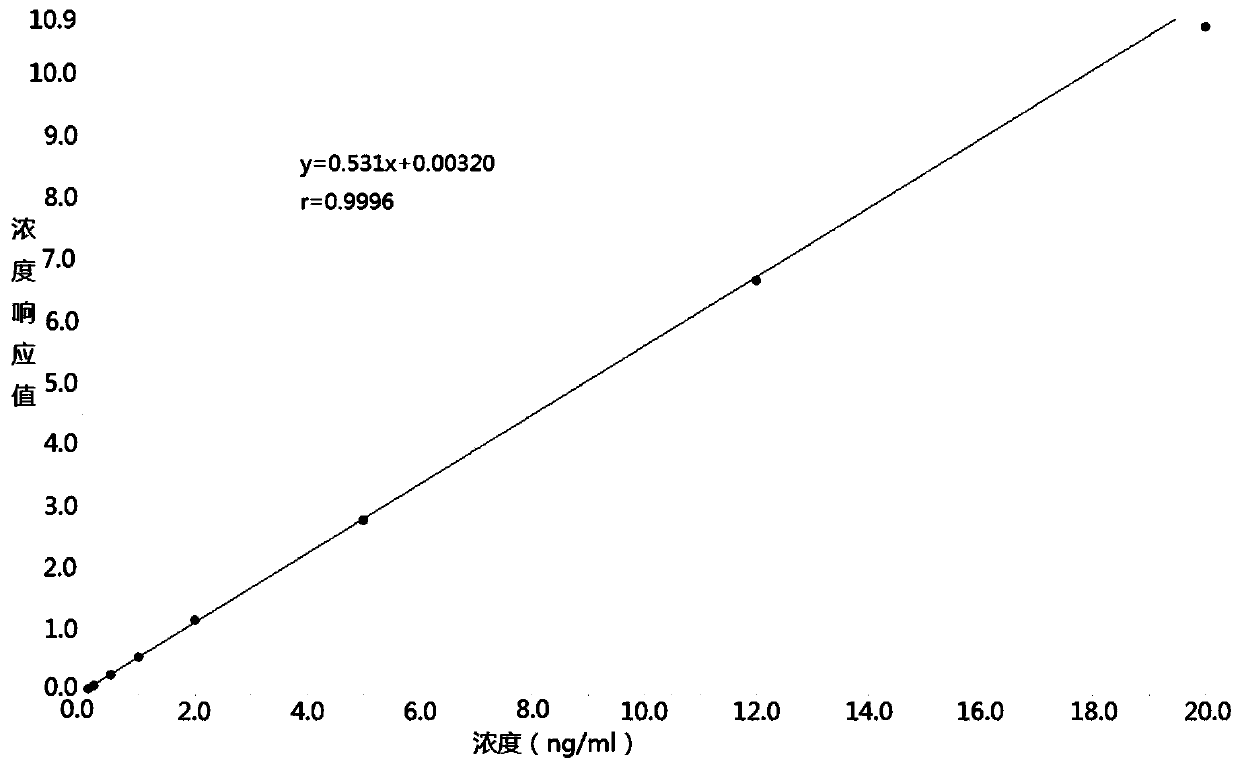
The invention discloses a method for detecting the concentration of hydrocortisone in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction, pretreating, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for determining the blood concentration of the hydrocortisone; and the linear range of the plasma standard curve of the method is 0.01-3 ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for measuring the concentration of the hydrocortisone in the plasma.
Owner:徐州立兴佳正医药科技有限公司
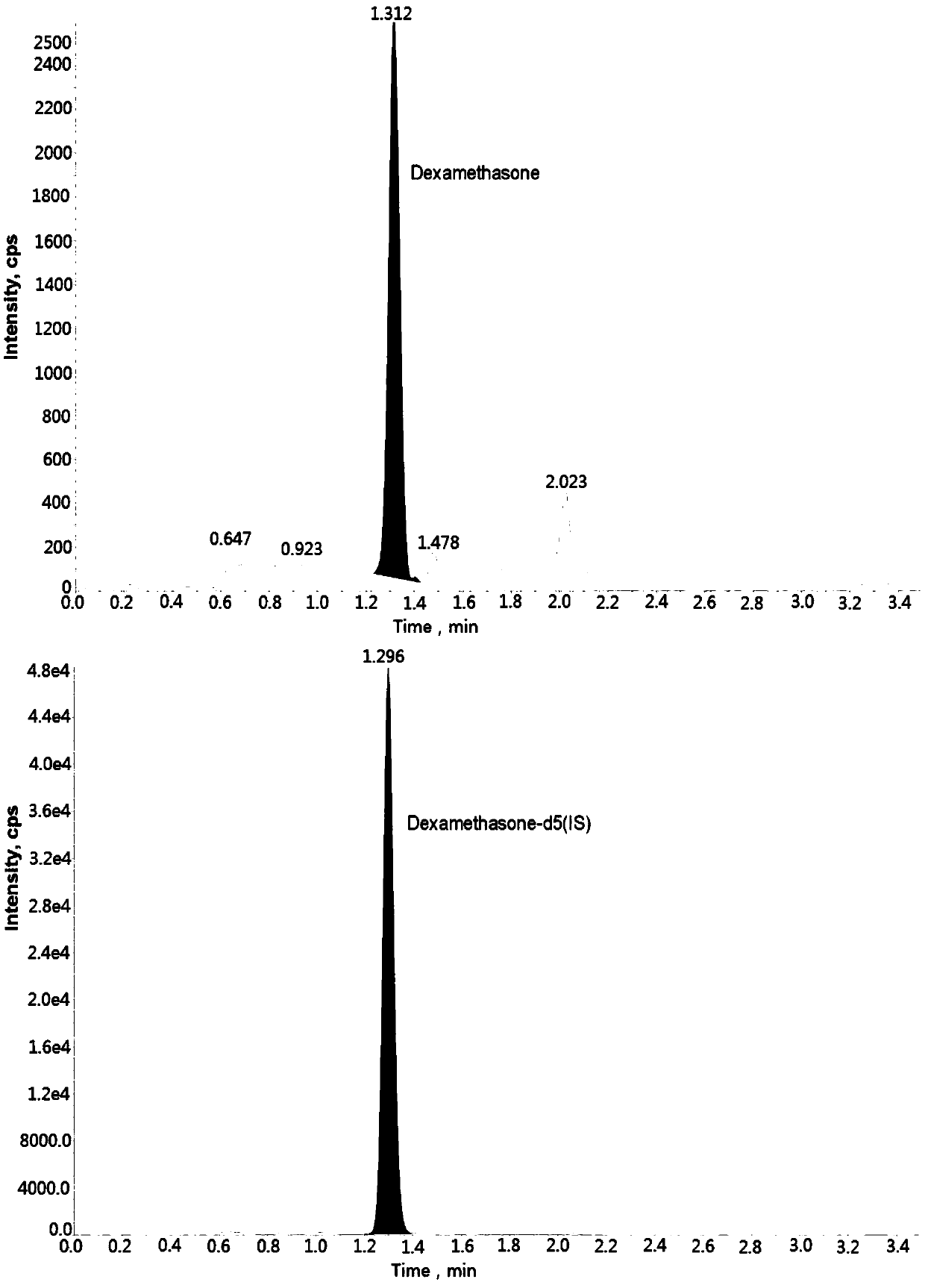
Method for determining concentration of carbamazepine in plasma by liquid chromatography mass spectrometry
InactiveCN109541108AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventGas chromatography–mass spectrometry
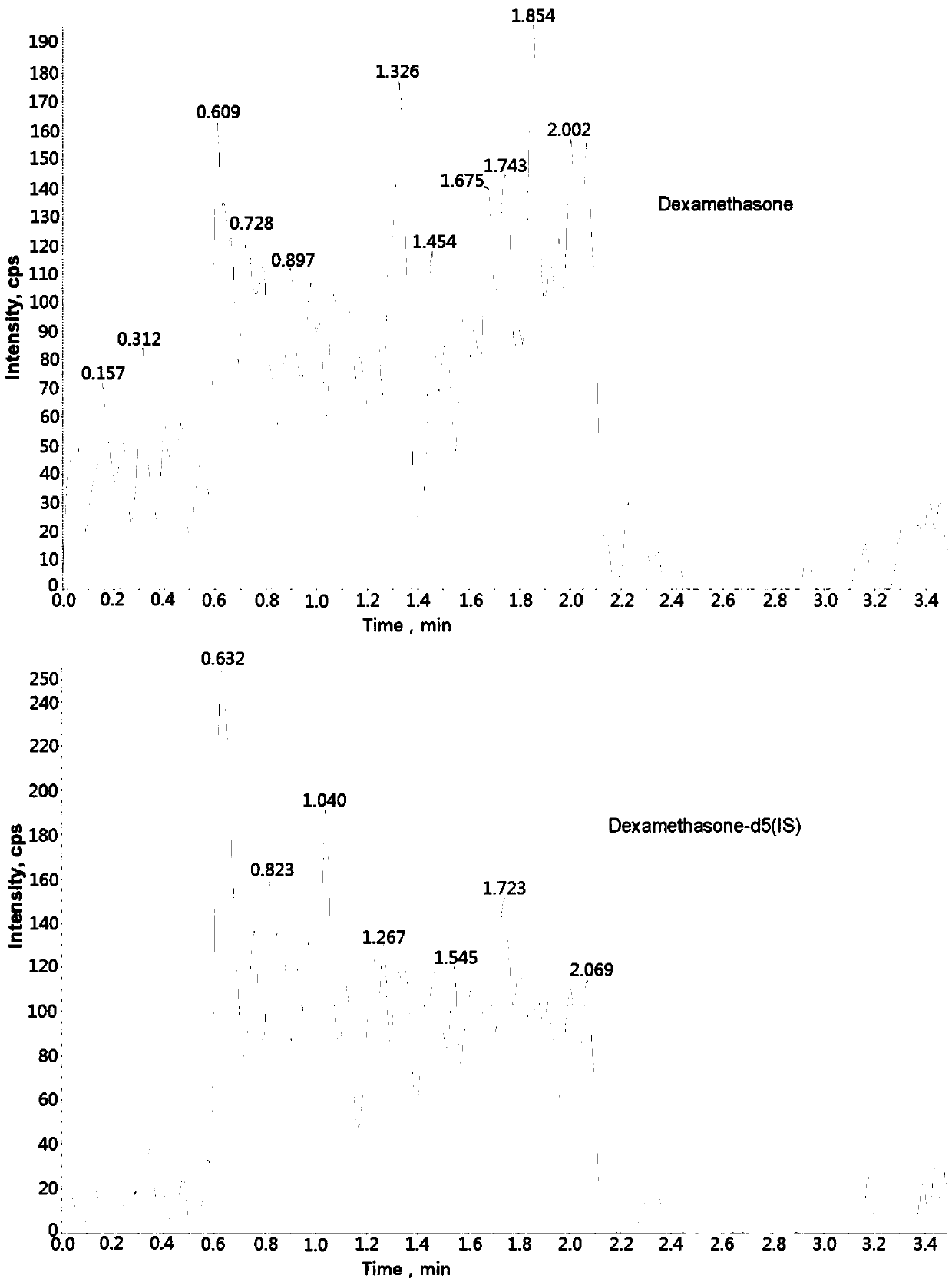
The invention discloses a method for determining concentration of dexamethason in plasma by liquid chromatography mass spectrometry. A liquid chromatography mass spectrometry system is used for determining. A sample to be determined is added with a certain amount of mixed organic solvent to extract twice, is separated by a chromatographic column after pretreatment, and is detected by a mass spectrometer. The method is rapid, accurate, high in sensitivity, and simple and convenient to operate. The method provides a basis for the blood drug concentration determination of dexamethason. The linearrange of a plasma standard curve in the method is 0.1 to 20 ng / mL. The intra-batch and inter-batch precision RSD are less than + / -15%. The method is suitable for determining the concentration of dexamethason in the plasma.
Owner:徐州立顺康达医药科技有限公司
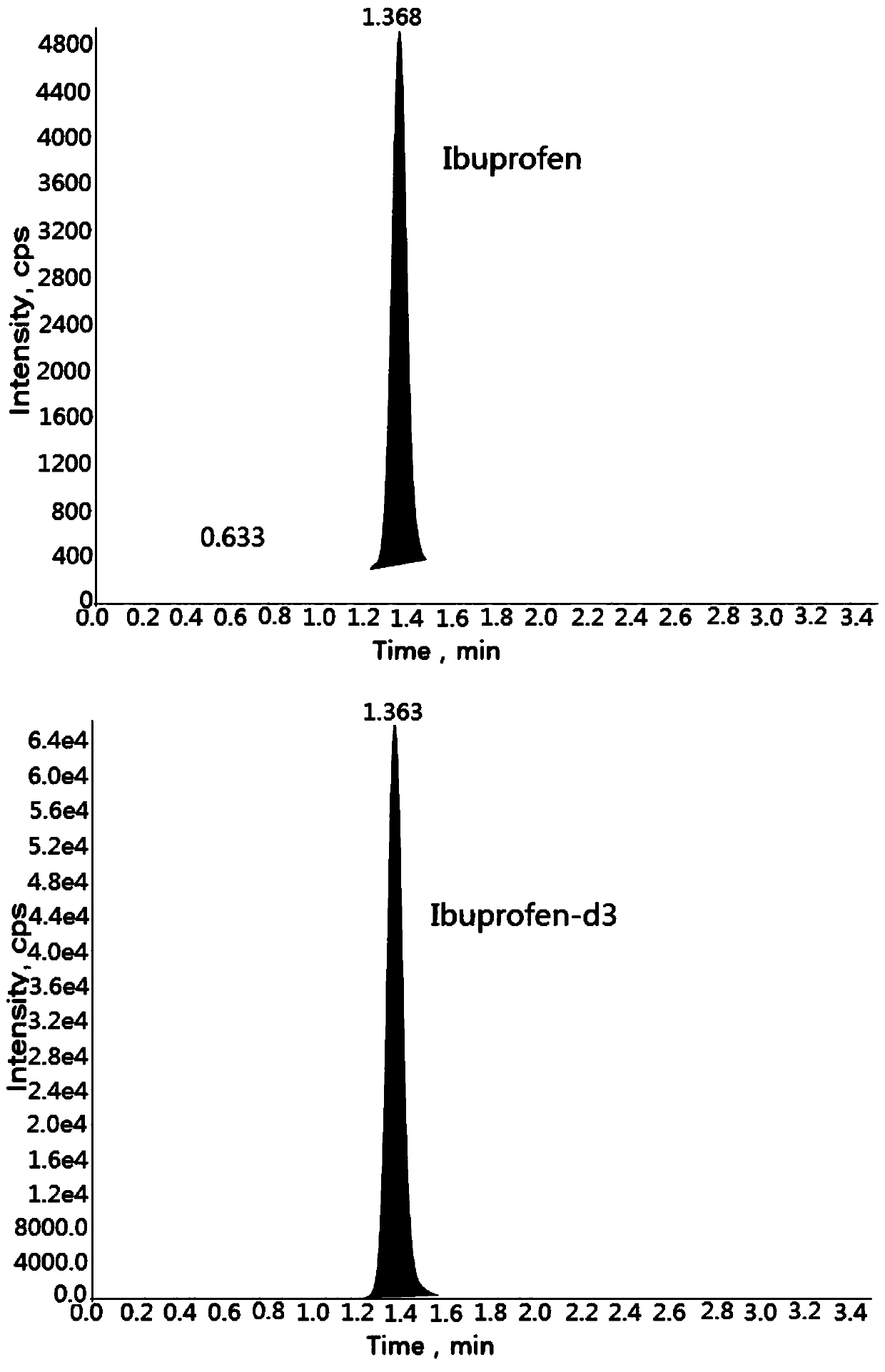
Method for determining concentration of ibuprofen in blood plasma by liquid chromatography-mass spectrometry
InactiveCN110927306AThe pretreatment method is simpleSuitable for routine determinationComponent separationBlood concentrationOrganic solvent
The invention discloses a method for determining the concentration of ibuprofen in blood plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system for determination, and comprises the following steps: taking a sample to be determined, adding a certain amount of mixed organic solvent for extraction and pretreatment, performing separating by using a chromatographic column, and performing detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of ibuprofen; according to the method, the linear range of the plasma standard curve is 0.1-40 mu g / mL, the intra-batch precision RSD andthe inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of ibuprofen in plasma.
Owner:徐州立兴佳正医药科技有限公司
Method for determining concentration of 5'-methoxyl-3',4'-methylenedioxyphenyl cinnamic acid isobutyl amide in plasma
InactiveCN104076116BThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventPhysical chemistry
The invention discloses a met / hod for determining the concentration of 5'-methoxyl-3',4'-methylenedioxyphenyl cinnamic acid isobutyl amide in plasma. A liquid chromatography-mass system is adopted to determine. The method comprises the following steps: taking a to-be-determined sample first, adding a certain quantity of organic solvent for extraction, separating through a chromatographic column after pretreatment, and determining by using a mass spectrometry detector. The method disclosed by the invention has the advantages of rapidity, accuracy, high sensitivity, simplicity and convenience in operation and is suitable for determining the concentration of 5'-methoxyl-3',4'-methylenedioxyphenyl cinnamic acid isobutyl amide in plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
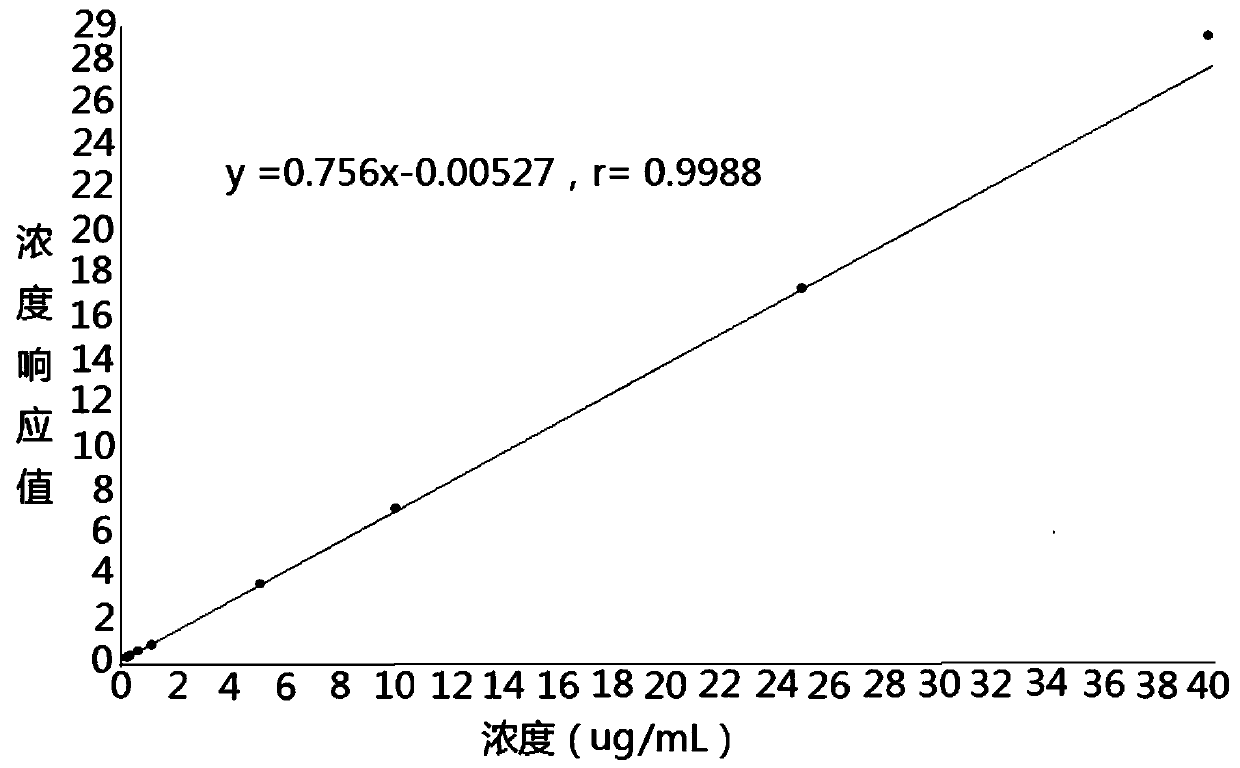
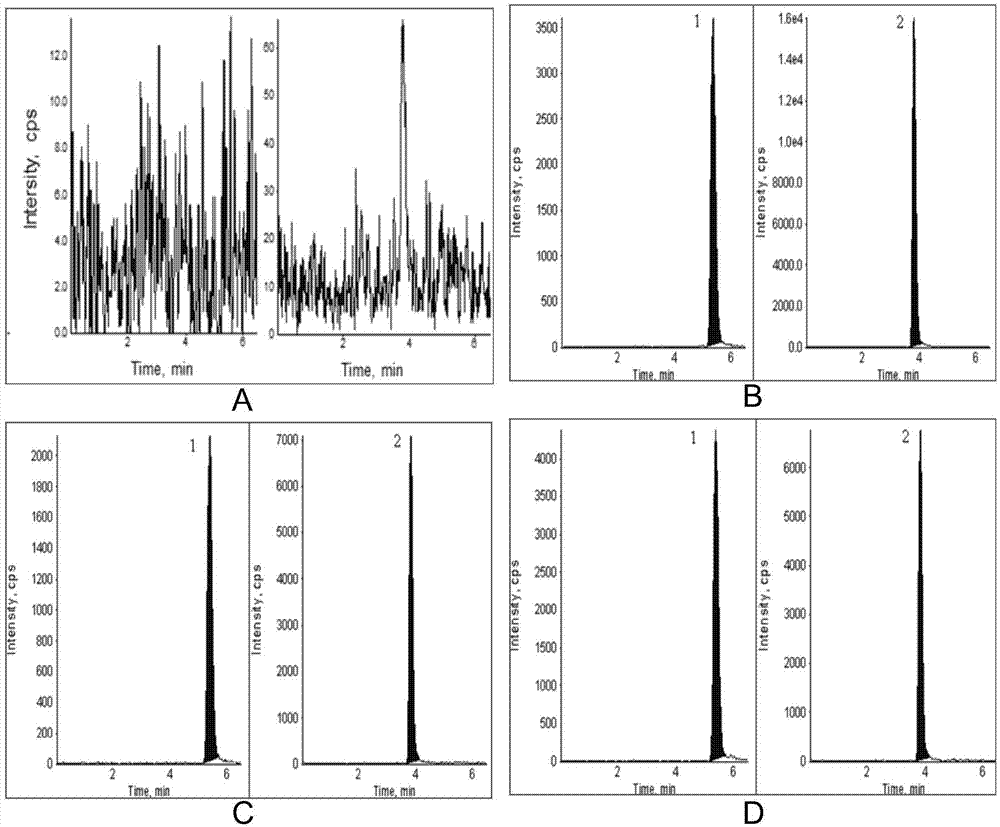
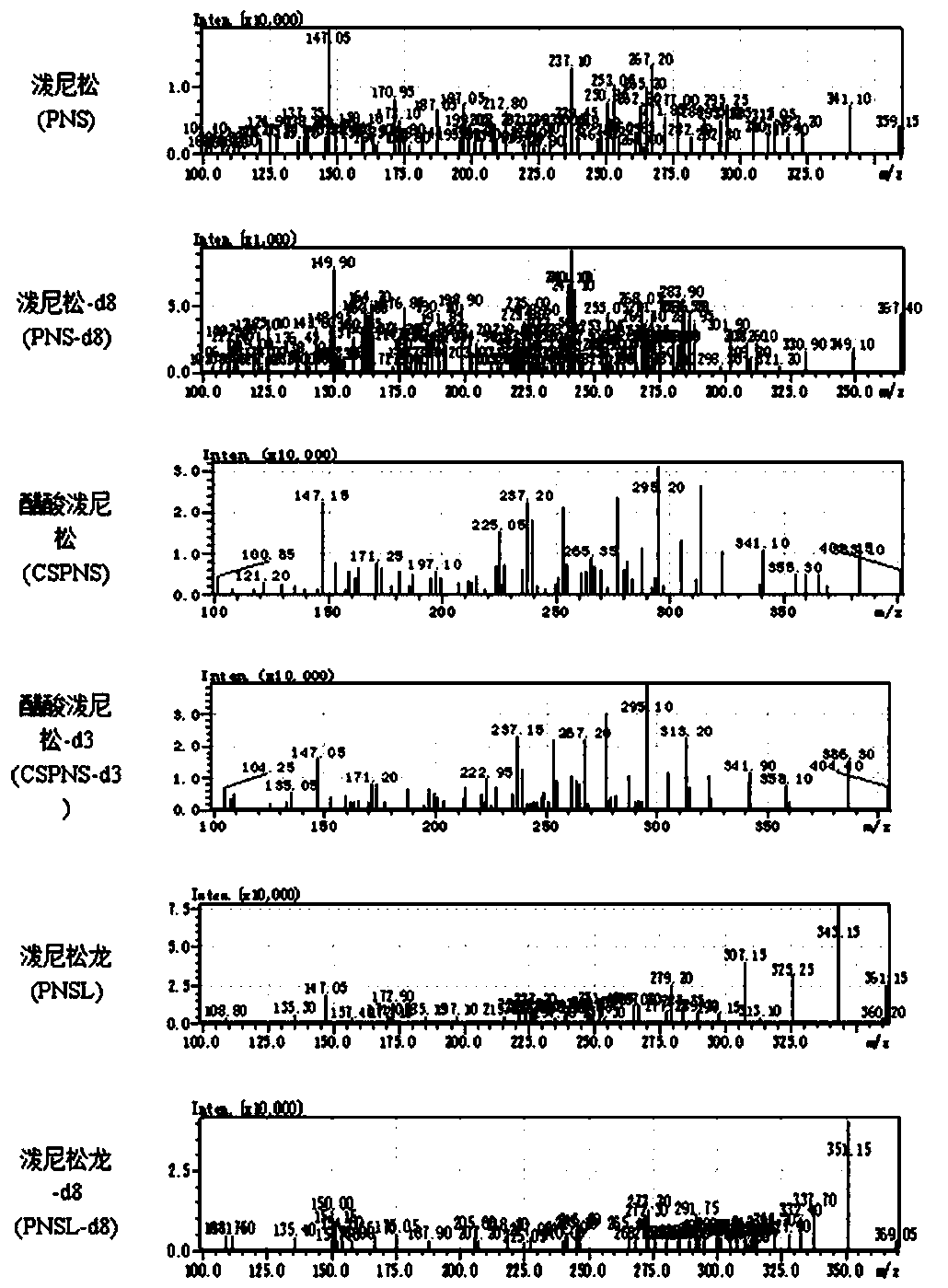
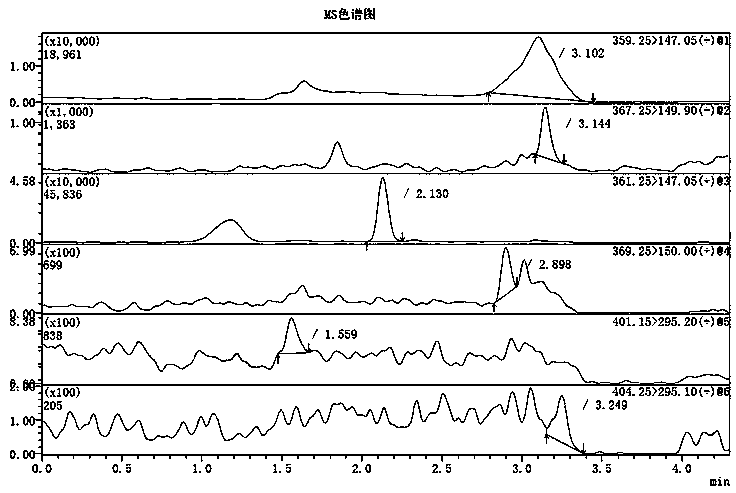
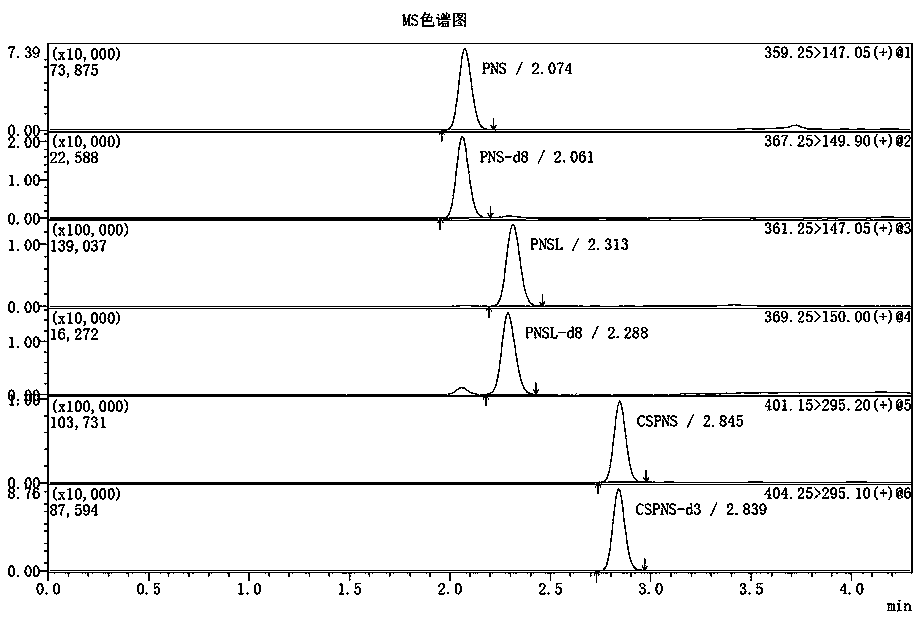
Method for determining concentrations of prednisone, prednisone acetate and active metabolite prednisolone thereof in blood plasma by means of liquid chromatography-mass spectrometry
ActiveCN111060612AThe preprocessing method is simpleSuitable for routine determinationComponent separationPrednisoloneLiquid chromatography mass spectroscopy
The invention provides a method for determining concentrations of prednisone, prednisone acetate and active metabolite prednisolone thereof in blood plasma by means of liquid chromatography-mass spectrometry. The method comprises the steps of: (1) pretreating a blood plasma sample; (2) detecting the blood plasma sample by adopting liquid chromatography-mass spectrometry; (3) preparing a standard curve; and (4) determining the concentrations of prednisone, prednisone acetate and active metabolite prednisolone thereof in the blood plasma. The pretreatment method disclosed by the invention is simple, high in specificity and high in sensitivity, and the method is rapid, precise, high in sensitivity, simple in operation and suitable for clinical application of medicines.
Owner:SUZHOU GUOCHEN BIOTEK CO LTD
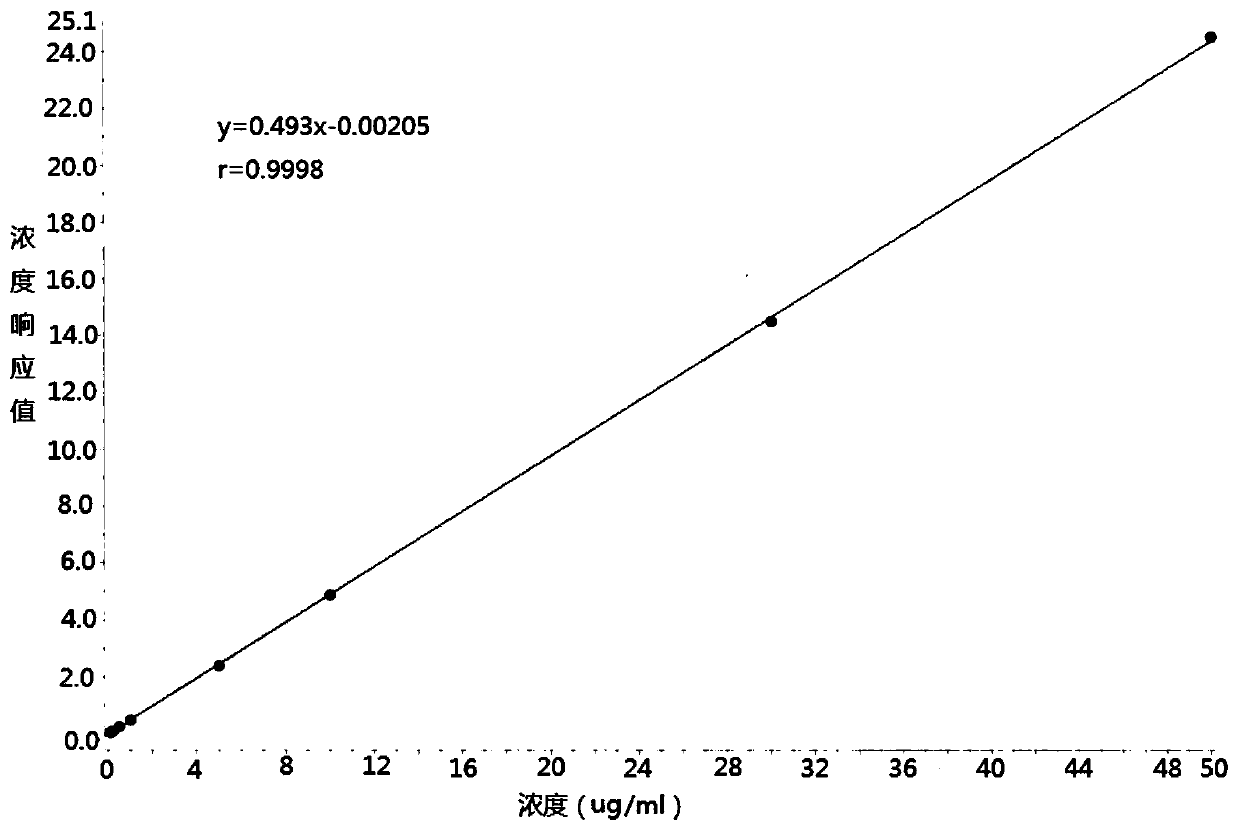
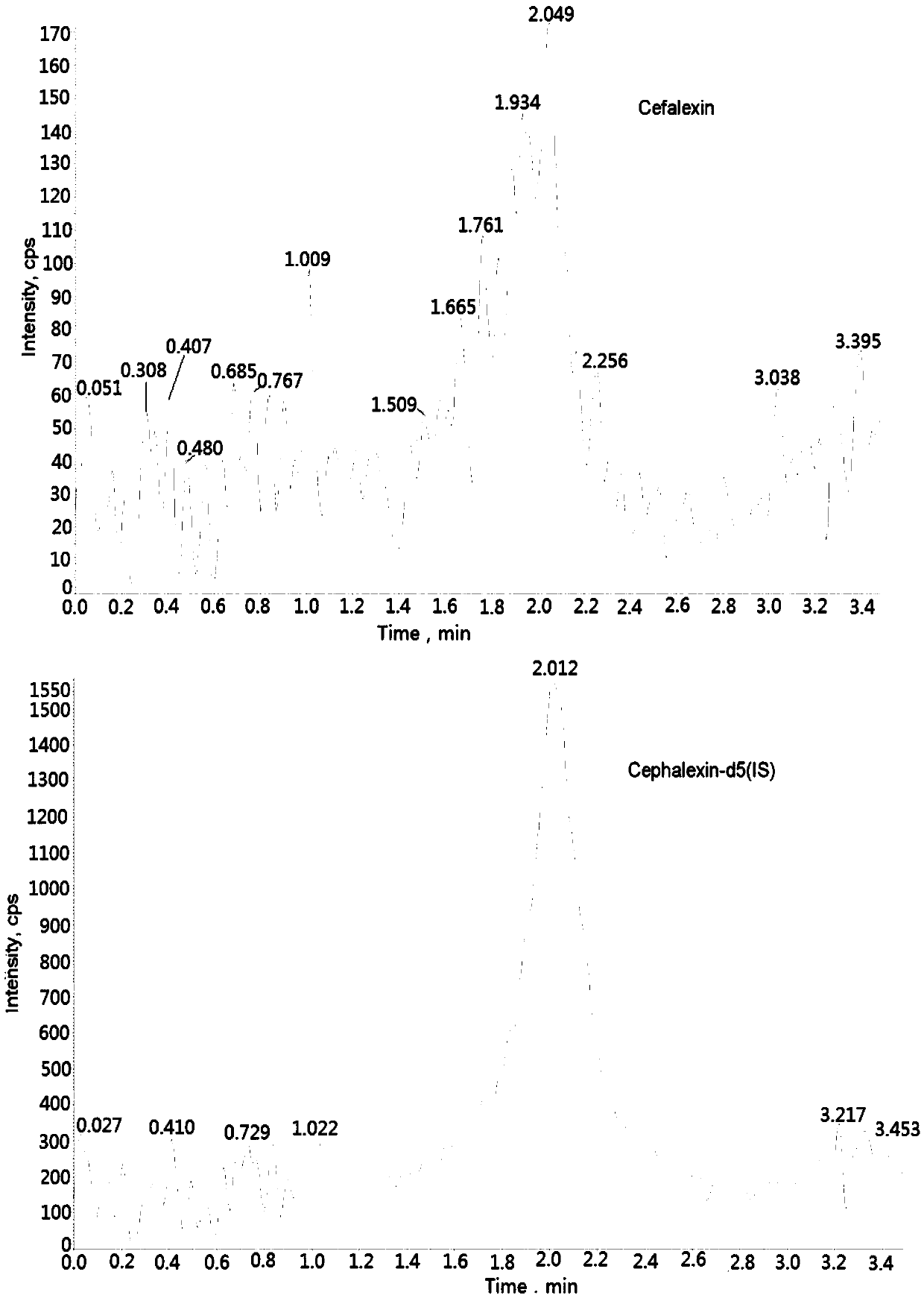
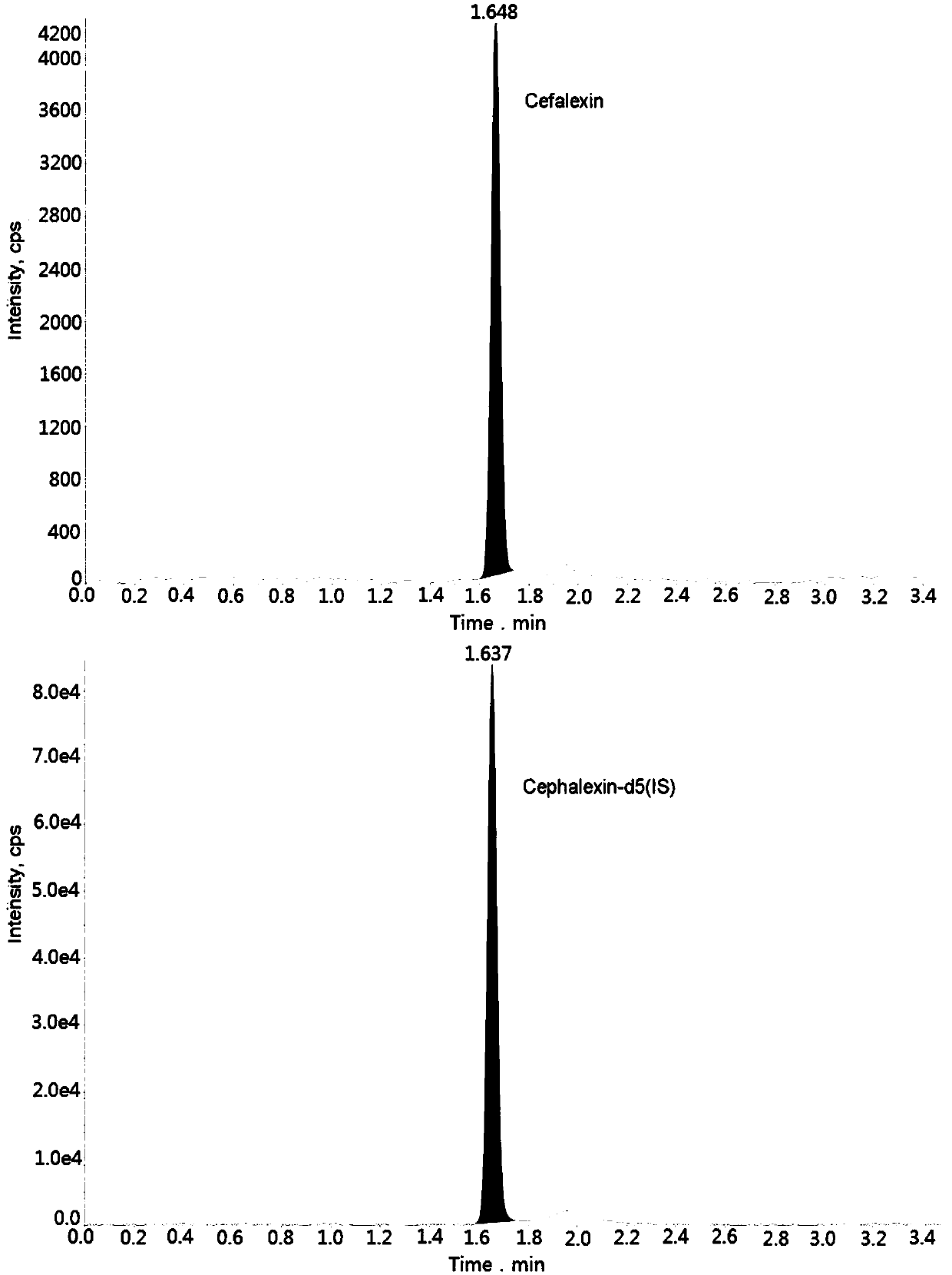
Method for measuring cefalexin concentration in plasma through hygroplasm combination
InactiveCN109682916AThe pretreatment method is simpleSuitable for routine determinationComponent separationCefalexinOrganic solvent
The invention discloses a method for measuring cefalexin concentration in plasma through hygroplasm combination. A hygroplasm combination system is adopted for measurement. The method comprises the steps that firstly, a to-be-measured sample is taken, a certain quantity of mixed organic solvent is added for conducting extraction twice, and after pretreatment, through chromatographic column separation, a mass spectrometry detector is used for detection. The method is quick to use, accurate, high in sensitivity and easy and convenient to operate, and the basis is provided for plasma concentration measurement of cefalexin. The linear range of a plasma standard curve is within 0.1-50 mug / mL, the within-run precision and between-run precision RSD are smaller than + / -15%, and the method is suitable for measurement of cefalexin concentration in plasma.
Owner:徐州立兴佳正医药科技有限公司
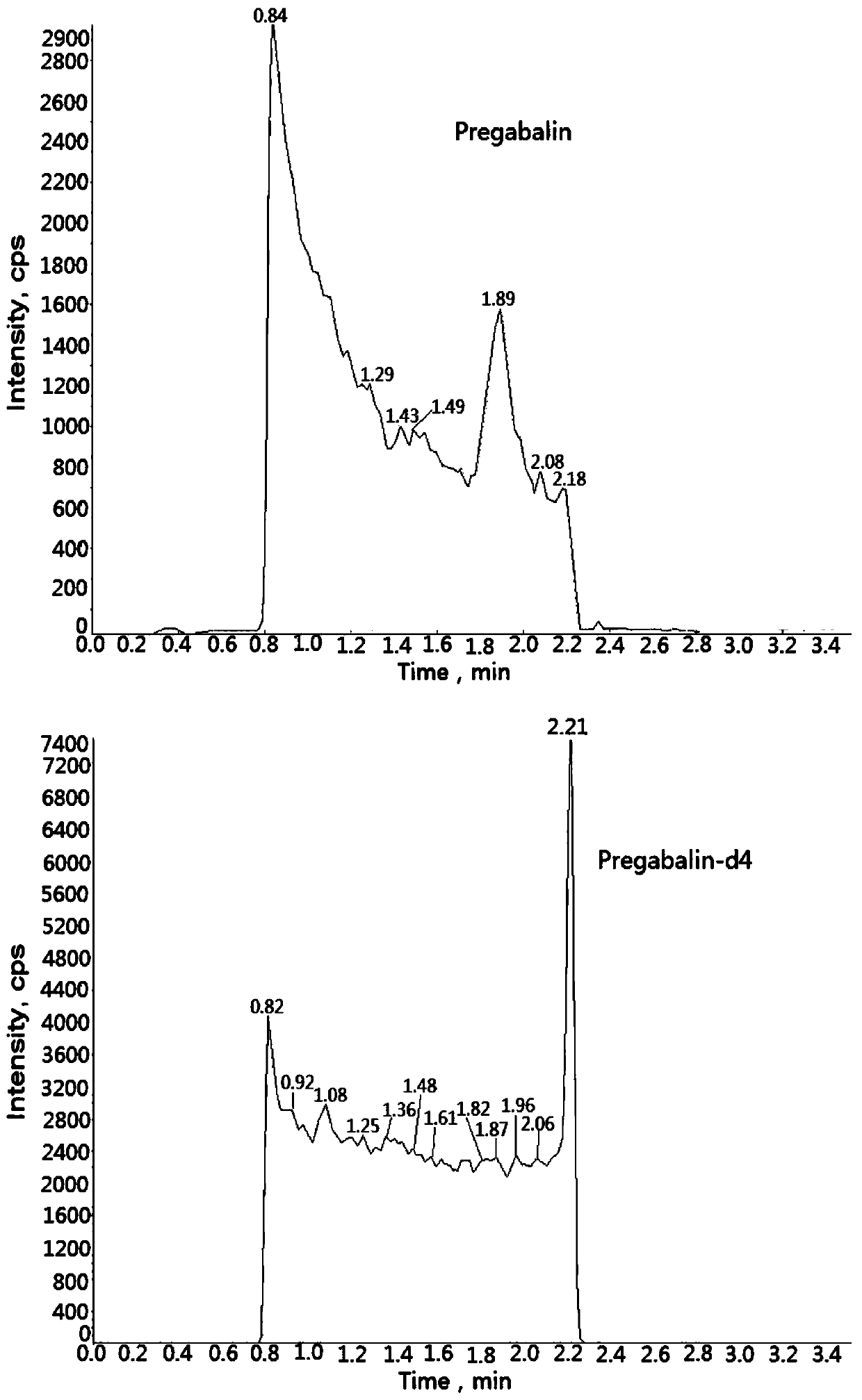
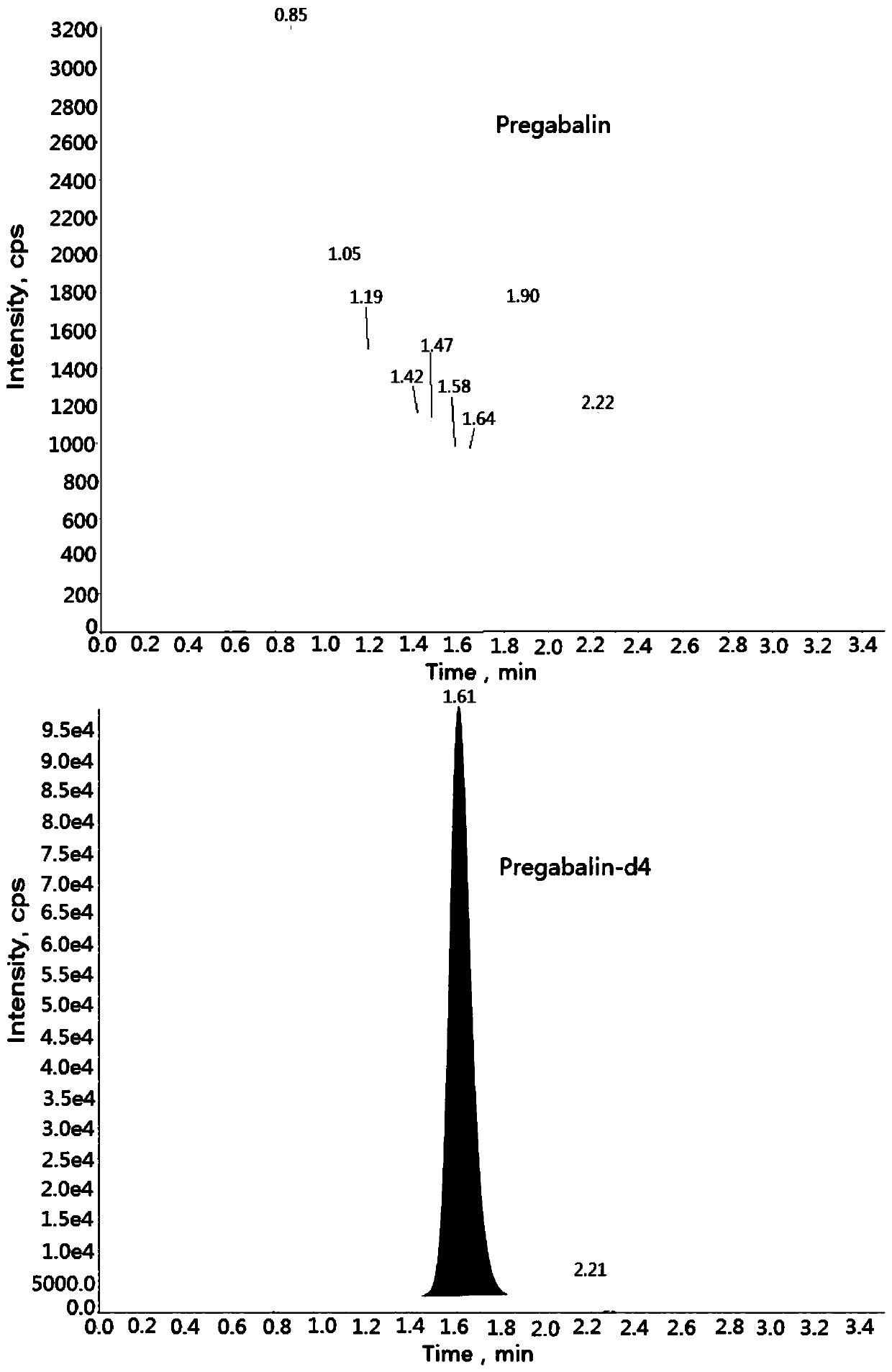
Method for determining concentration of pregabalin in plasma by liquid chromatography-mass spectrometry
InactiveCN111044662AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventPregabalin
The invention discloses a method for determining the concentration of pregabalin in plasma by liquid chromatography-mass spectrometry, which adopts a liquid chromatography-mass spectrometry system fordetermination and comprises the steps of taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction, separating by using a chromatographic column after pretreatment, and detecting by using a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the plasma concentration of pregabalin; and the linear range of a plasma standard curve of the method is 0.05-15[mu]g / mL, the intra-batch precision RSD and the inter-batch precision RSD are both less than + / -15%, and the method is suitable for measuring the concentration of pregabalin in plasma.
Owner:南京立顺康达医药科技有限公司
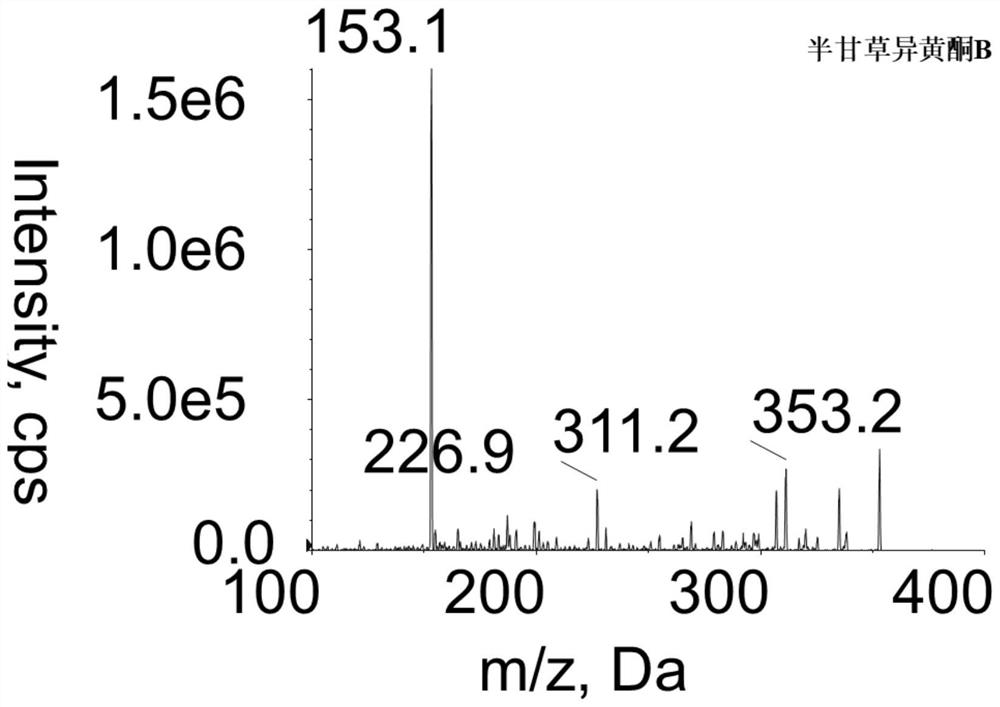
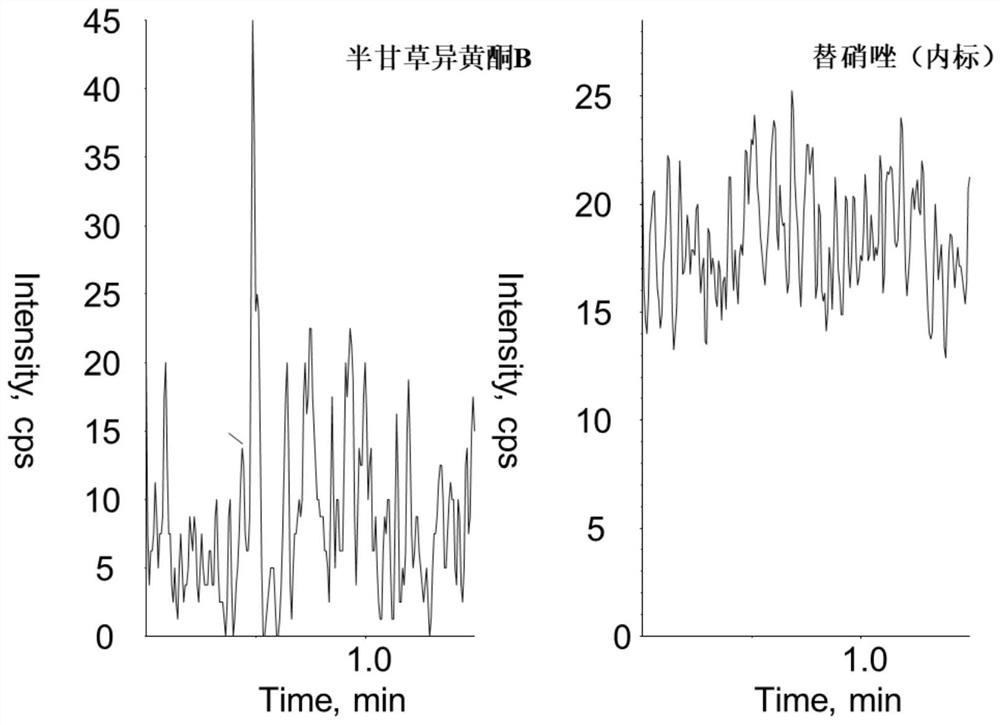
A method for measuring the concentration of semi-licorice isoflavone b in blood plasma
ActiveCN110568097BThe pretreatment method is simpleSuitable for routine determinationComponent separationGlycyrrhiza lepidotaPhysical chemistry
The invention discloses a method for measuring the concentration of semiglycyrrhizin isoflavone B in blood plasma. The liquid-mass spectrometry system is used for the measurement. Firstly, the sample to be tested is taken, and a certain amount of organic solvent protein precipitation agent is added to carry out protein precipitation. After pretreatment, the method is chromatographically Column separation and detection by a mass spectrometer, the method of the invention is fast, accurate, high in sensitivity and easy to operate, and is suitable for determining the concentration of the semiglycyrrhizin isoflavone B in blood plasma.
Owner:ZHANGJIAGANG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
A method for measuring the concentration of flutrimazole in blood plasma
InactiveCN106018581BThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for measuring the concentration of flutrimazole in blood plasma. The liquid-mass spectrometry system is used for the measurement. The sample to be tested is firstly taken, and a certain amount of organic solvent liquid-liquid extraction agent is added for liquid-liquid extraction. After pretreatment, the Separation by chromatographic column and detection by a mass spectrometer, the method of the invention is fast, accurate, high in sensitivity and easy to operate, and is suitable for determining the concentration of flutrimazole in blood plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
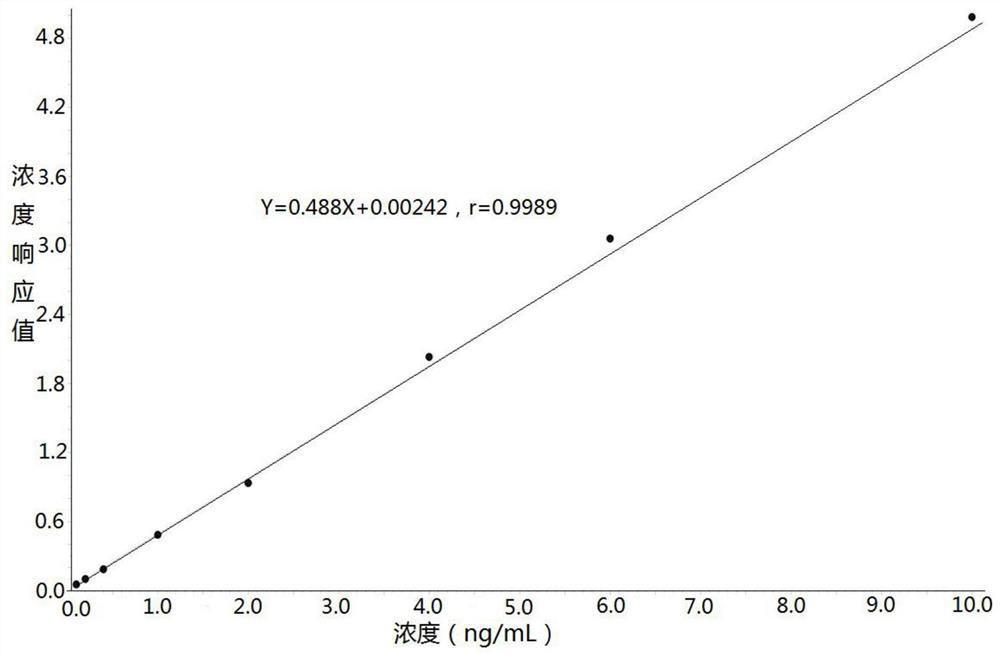
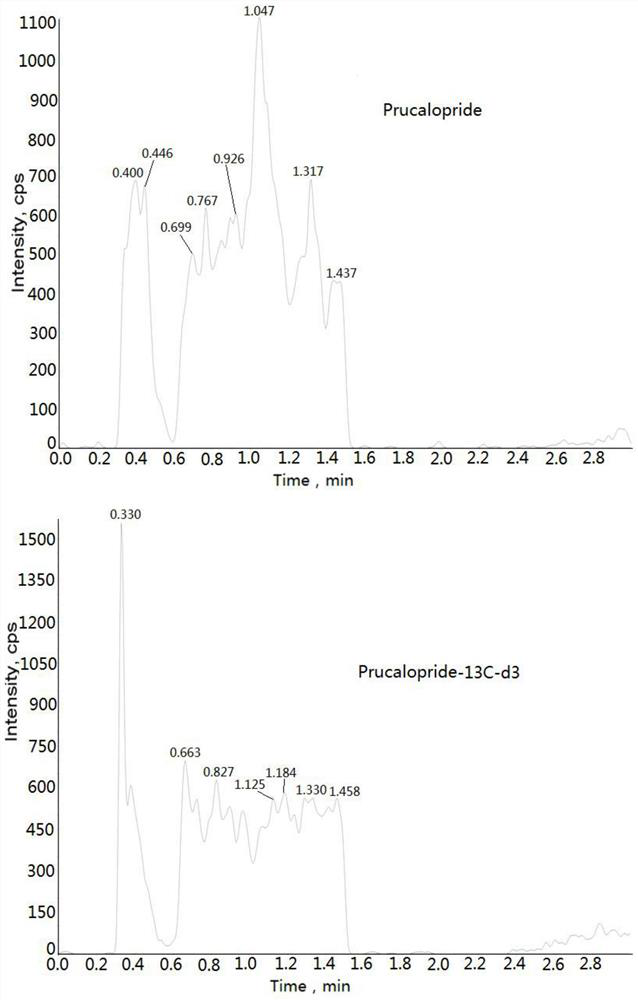
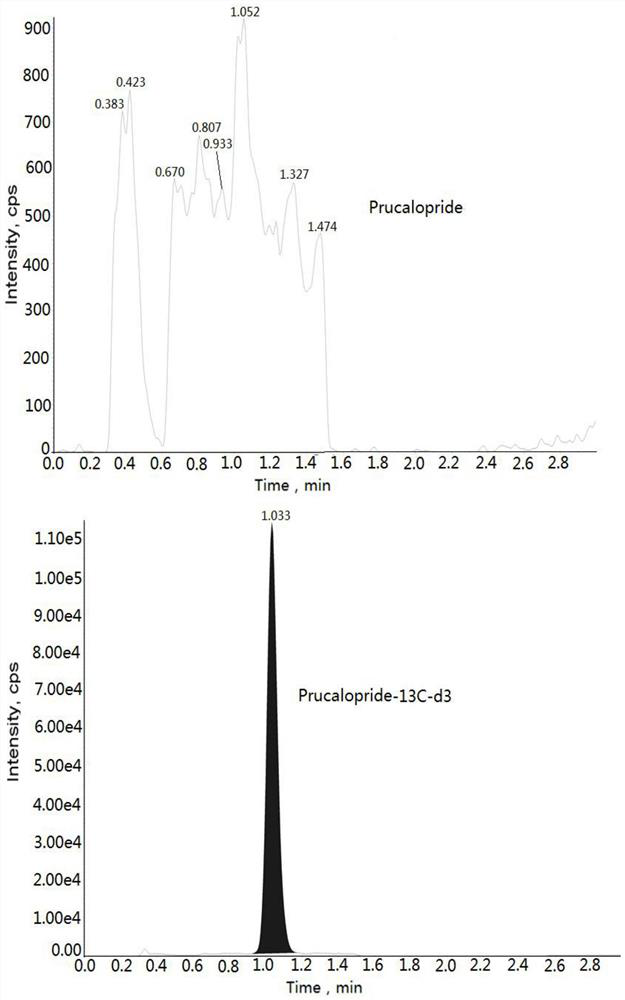
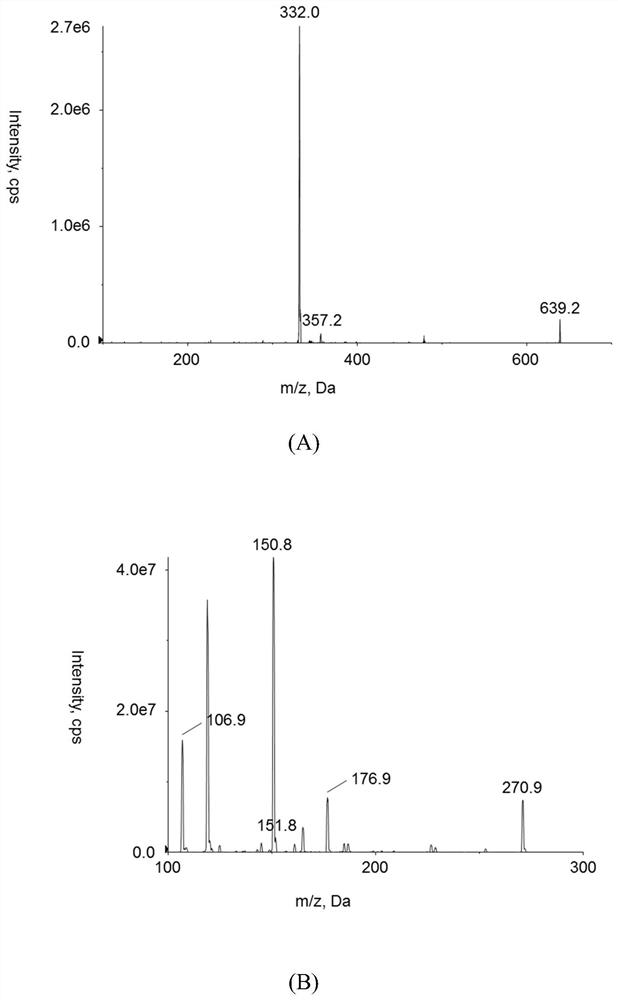
Method for determining concentration of prucalopride in blood plasma by liquid chromatography-mass spectrometry
InactiveCN112630351AThe pretreatment method is simpleGood peak shapeComponent separationChromatography columnBlood plasma
Owner:徐州立顺康达医药科技有限公司
Method for determining concentration of chartreusin in blood plasma by liquid chromatography-tandem mass spectrometry
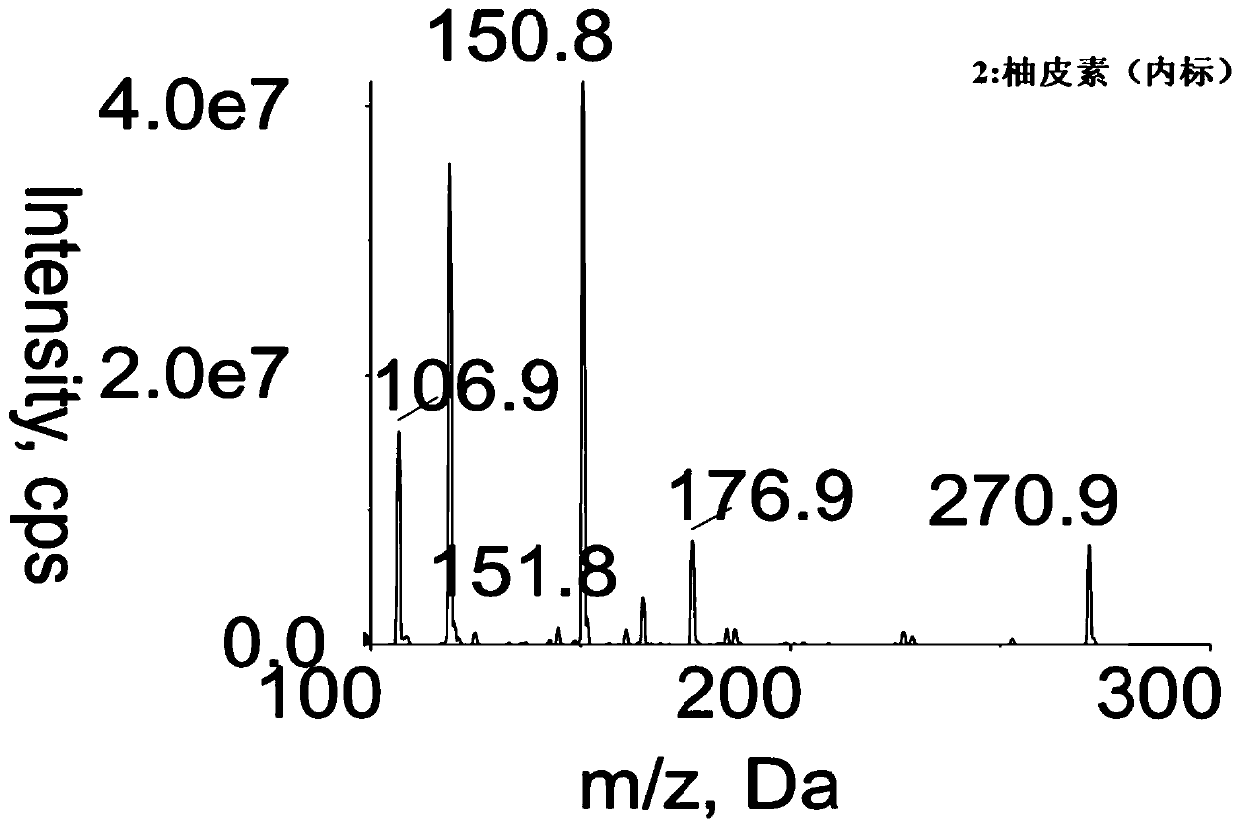
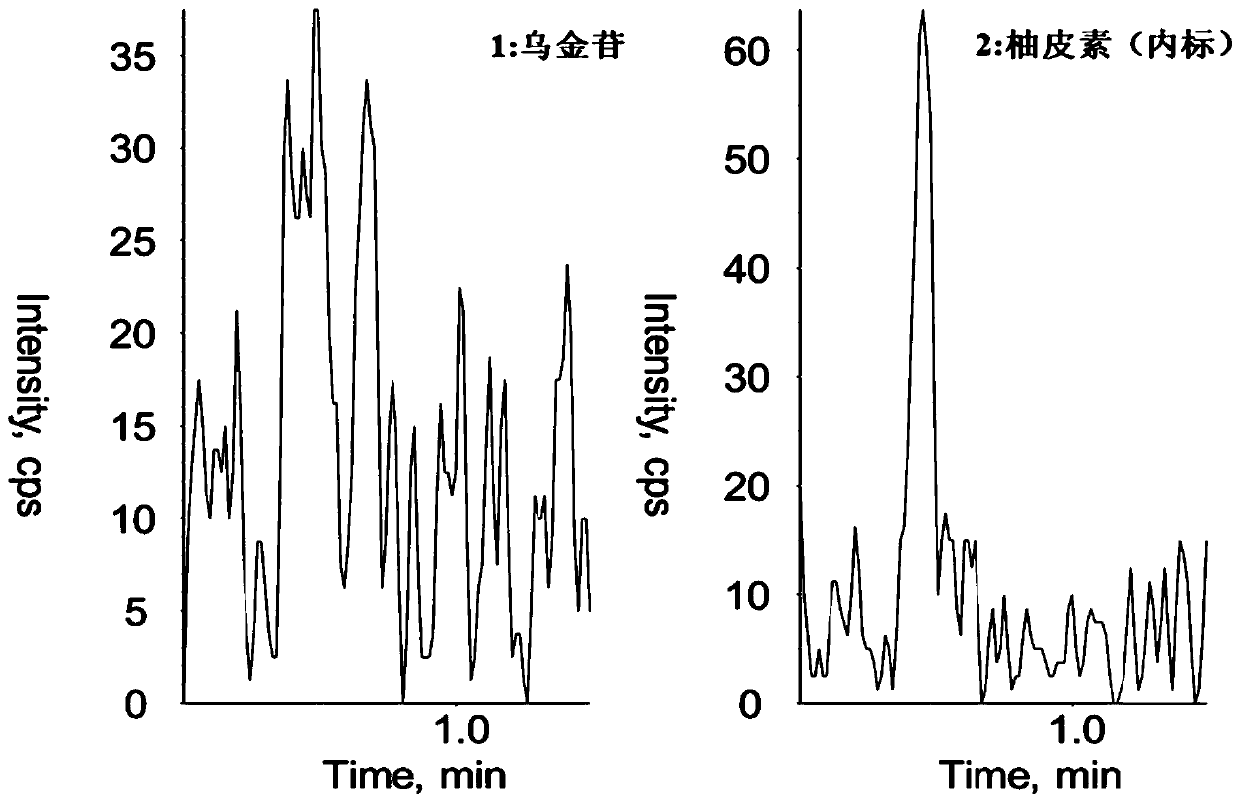
ActiveCN111751469AThe pretreatment method is simpleSuitable for routine determinationComponent separationFluid phaseInternal standard
The invention discloses a method for determining the concentration of chartreusin in blood plasma through liquid chromatography-tandem mass spectrometry. The method comprises the following steps: separating chartreusin from a blood plasma sample by liquid chromatography, then establishing a standard curve by taking the concentration of chartreusin in a calibration standard sample as an X axis andthe peak area ratio of chartreusin to an internal standard substance in the calibration standard sample as a Y axis by utilizing a mass spectrum internal standard quantitative method, and calculatingthe concentration of chartreusin in the blood plasma. The method has the following advantages of: (1) strong specificity: the retention time of the chartreusin and the internal standard naringenin isabout 0.6min, and endogenous substances do not interfere with the determination of the chartreusin and the internal standard naringenin; (2) short analysis time: the detection time of each sample is 1min; (3) high sensitivity: the minimum quantification limit is 1ng.ML<-1>; and (4) the linear range of the blood plasma standard curve of the method is 1-1000ng.ML<-1>, and the intra-batch precision CV and the inter-batch precision CV are both less than 10%.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
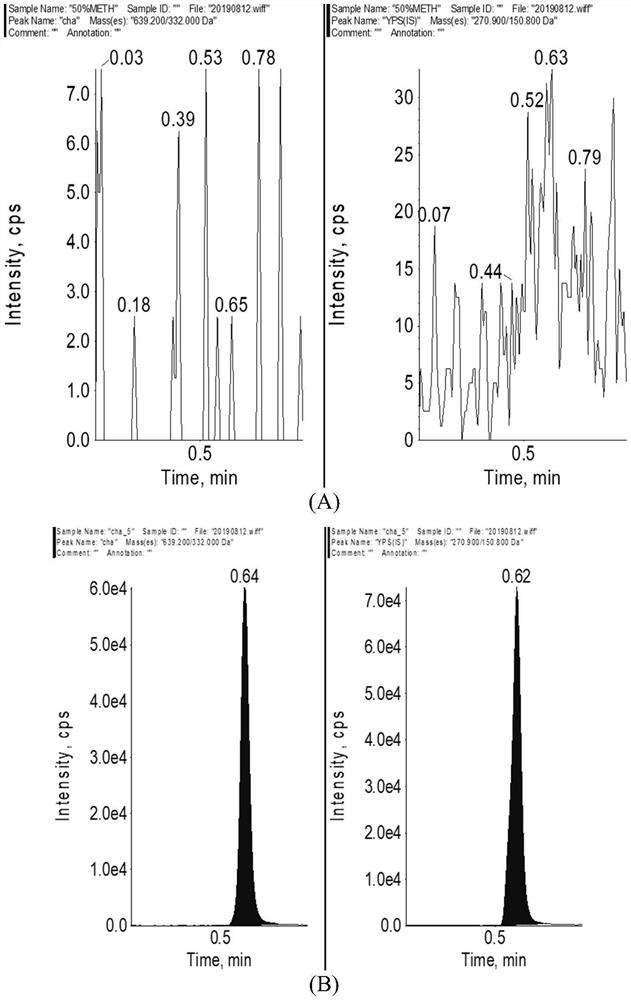
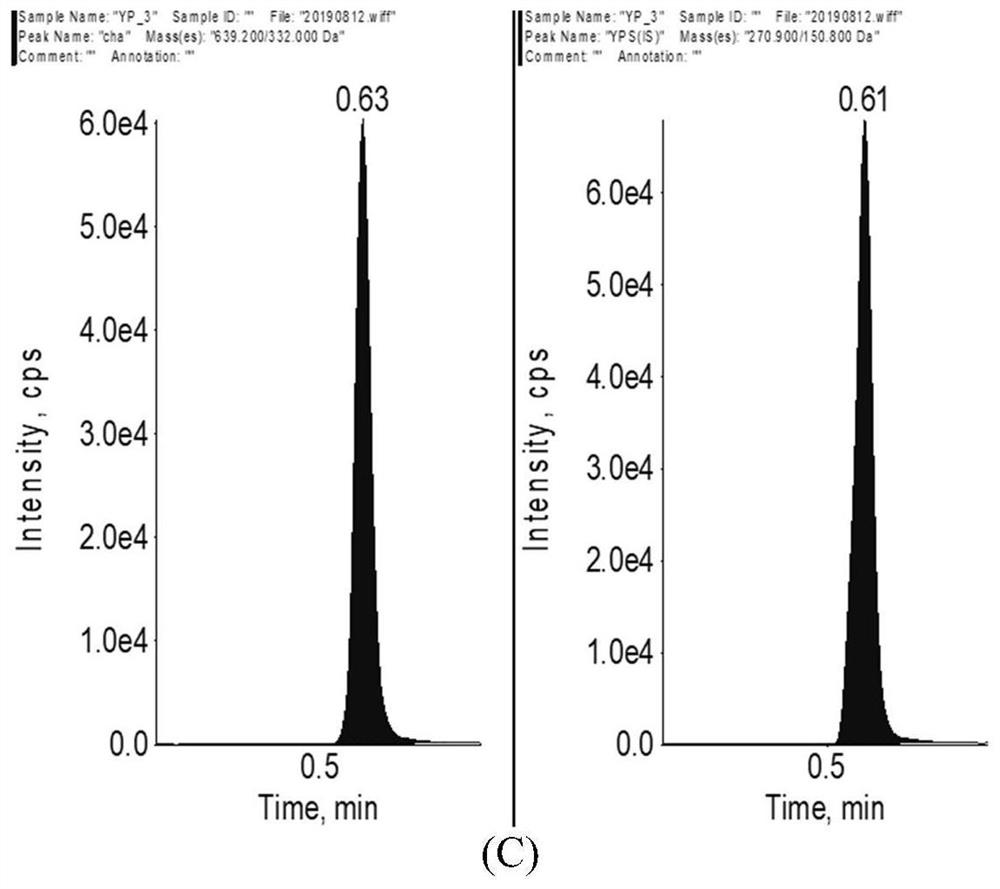
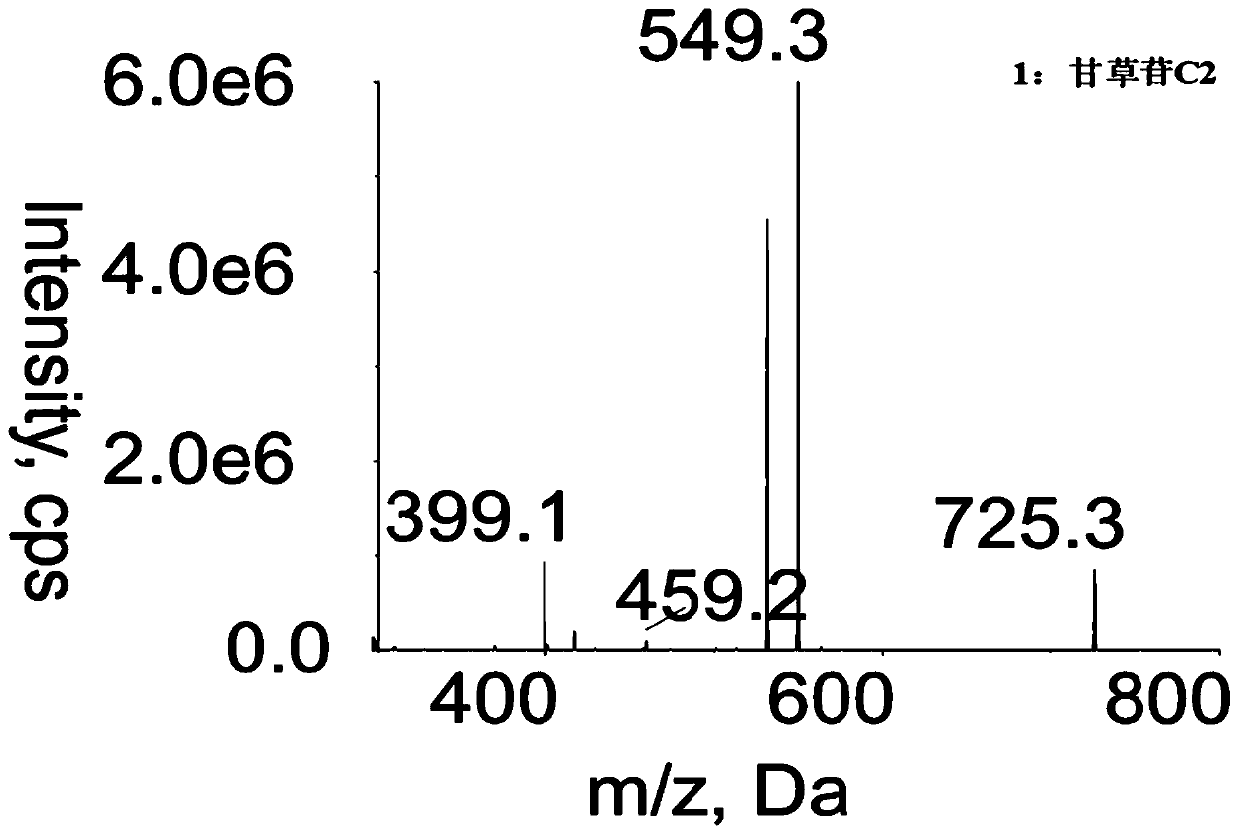
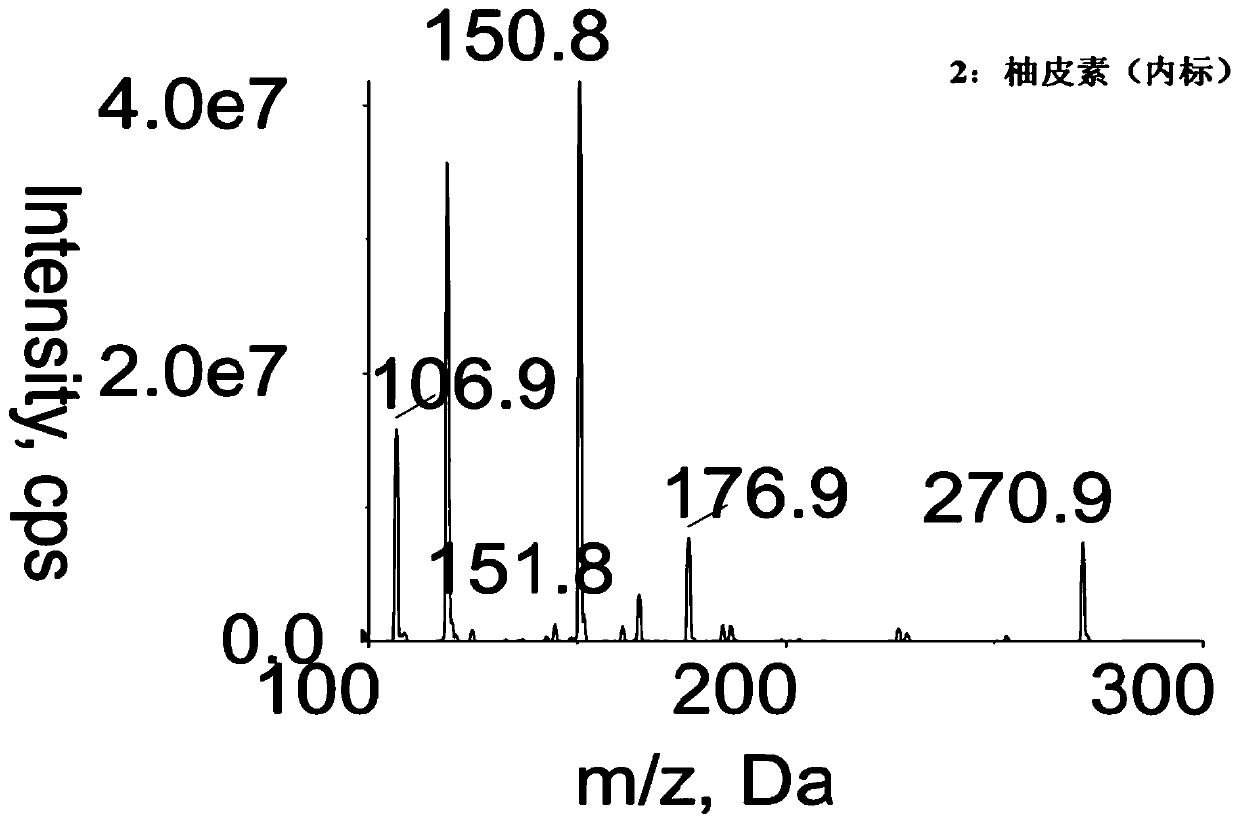
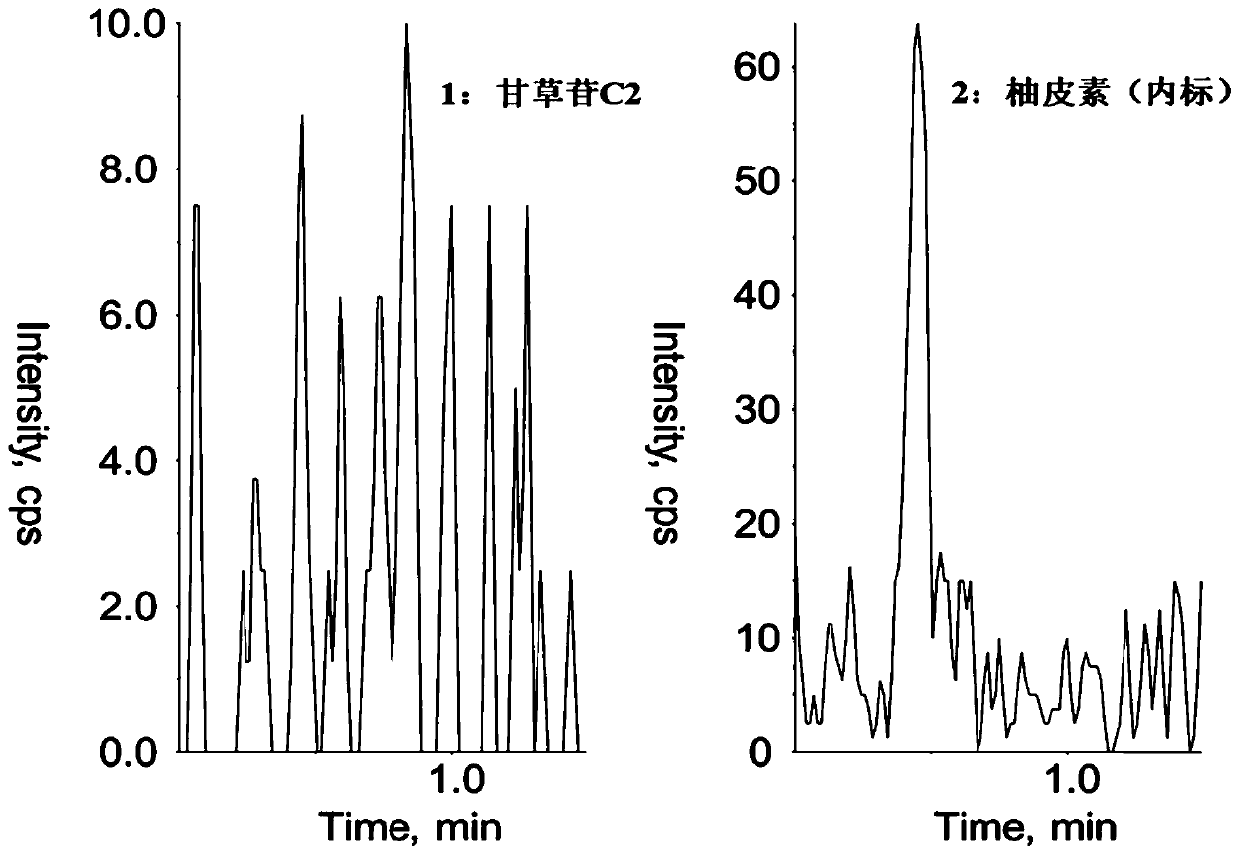
Method for determining concentration of licorice glycoside C2 in blood plasma
InactiveCN110455968ALower limit of quantitationNo distractionComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for determining concentration of licorice glycoside C2 in blood plasma. A liquid chromatography-mass spectrometry system is adopted for determination, firstly, a to-be-determined sample is taken, a certain quantity of organic solvent protein precipitation solutions are added, a chromatographic column is used for separation after pretreatment, and a mass spectrometry detector is used for detection. The method is rapid, accurate, high in sensitivity, simple and convenient to operate and suitable for determining the concentration of licorice glycoside C2 in the blood plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
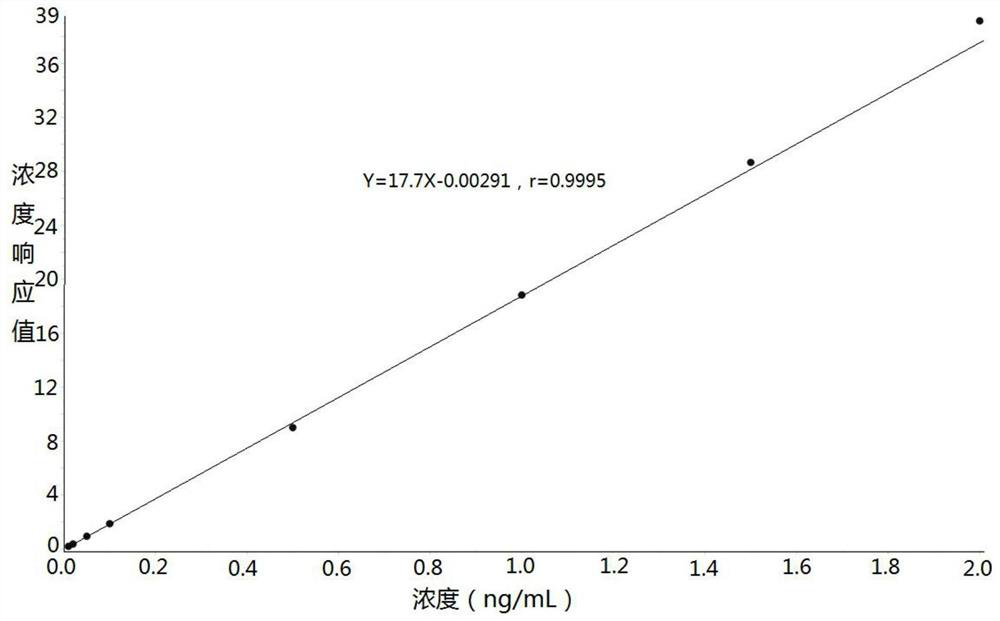
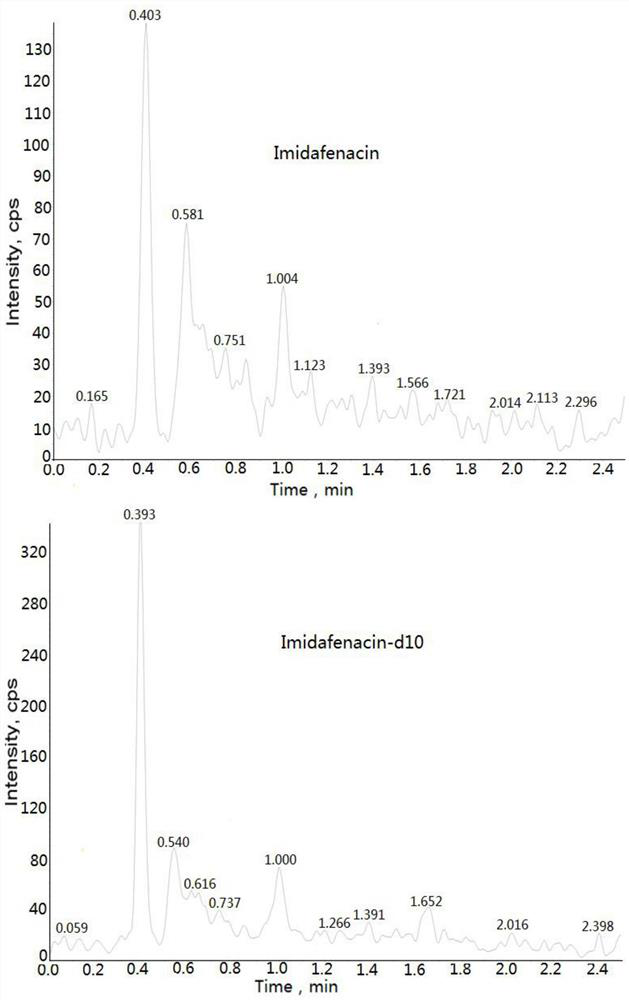
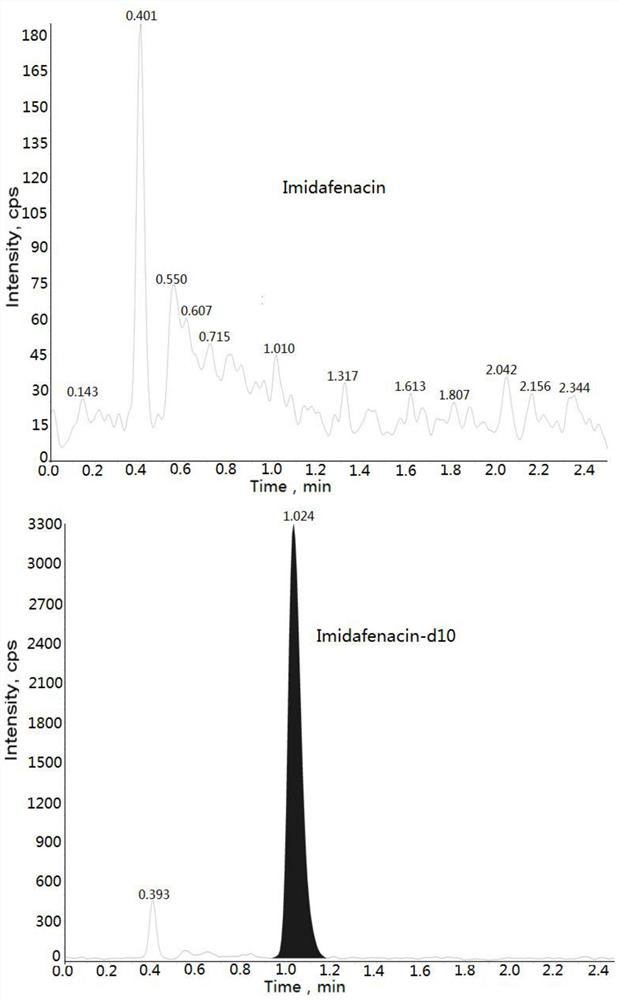
Method for determining concentration of imidafenacin in blood plasma by liquid chromatography-mass spectrometry
InactiveCN112730701AThe pretreatment method is simpleSuitable for routine determinationComponent separationBlood concentrationOrganic solvent
The invention discloses a method for determining the concentration of imidafenacin in blood plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by using a chromatographic column, and detecting by using a mass spectrum detector. The method is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of imidafenacin; the linear range of the plasma standard curve of the method is 0.01-2ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of imidafenacin in blood plasma.
Owner:徐州立顺康达医药科技有限公司
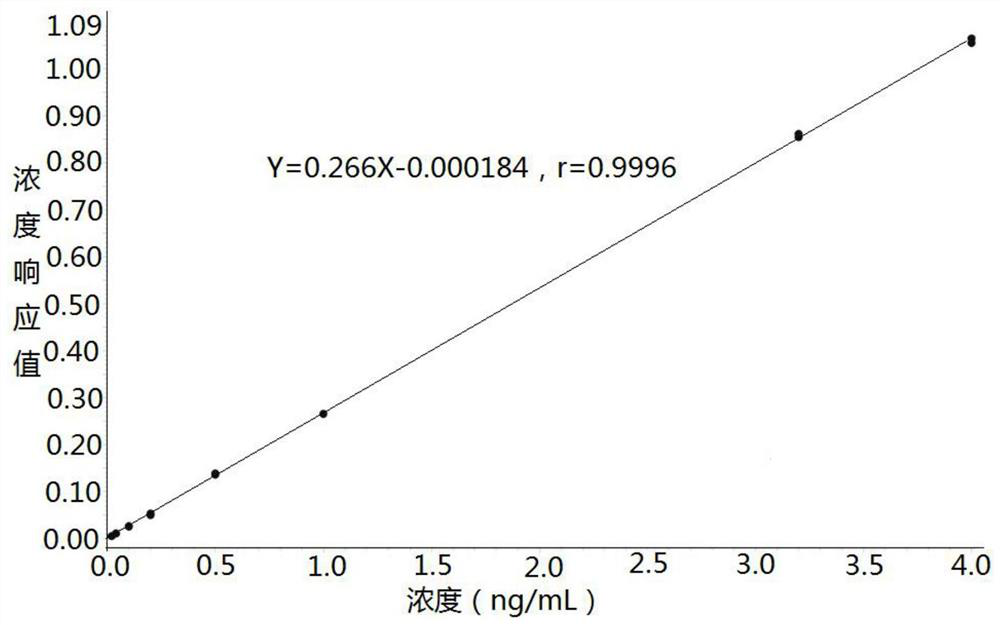
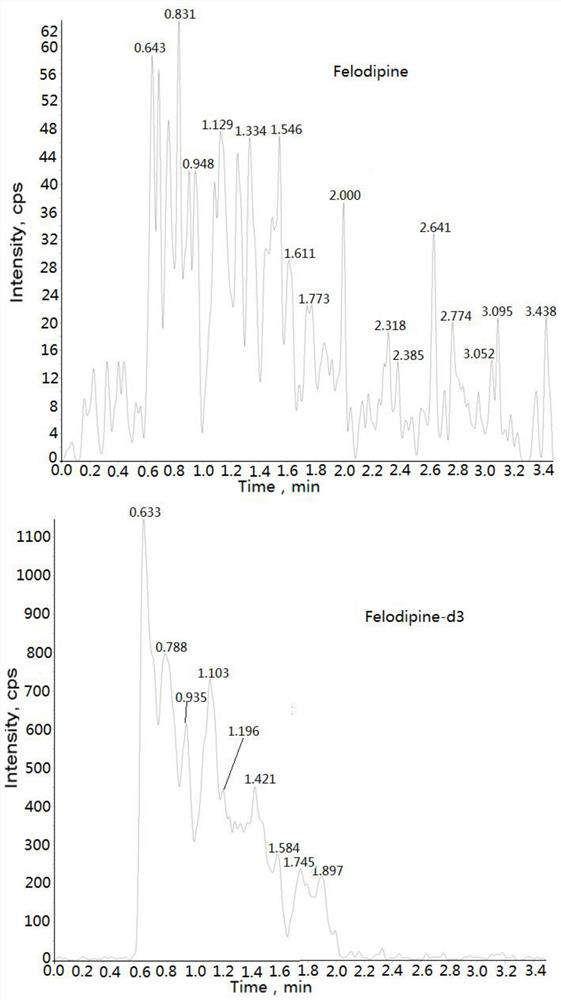
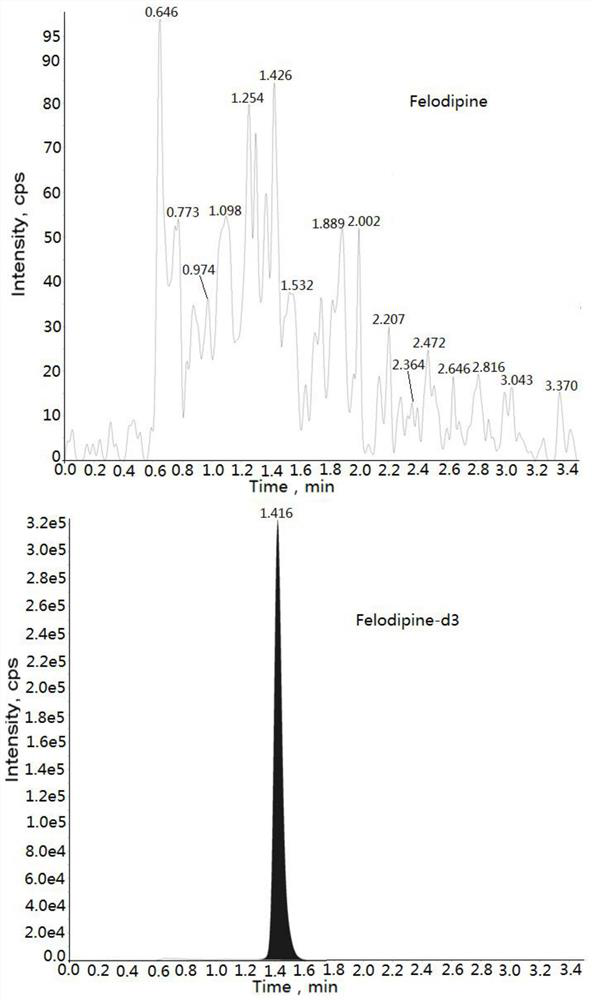
Method for measuring concentration of felodipine in plasma by liquid chromatography-mass spectrometry
InactiveCN112782310AThe pretreatment method is simpleSuitable for routine determinationComponent separationBlood concentrationPhysical chemistry
The invention discloses a method for measuring the concentration of felodipine in plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for measurement, and comprises the following steps: firstly taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by a chromatographic column, and detecting by a mass spectrometry detector. The method disclosed by the invention is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of felodipine; and the linear range of the plasma standard curve of the method is 0.02-4 ng / mL, the intra-batch and inter-batch precision RSD is less than + / -15%, and the method is suitable for measuring the concentration of felodipine in the plasma.
Owner:徐州立兴佳正医药科技有限公司
Method for determining concentration of robustaside B in blood plasma
InactiveCN110455967AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventRobustaside B
The invention discloses a method for determining concentration of robustaside B in blood plasma. A liquid chromatography-mass spectrometry system is adopted for determination, firstly, a to-be-determined sample is taken, a certain quantity of organic solvent protein precipitation solutions are added, a chromatographic column is used for separation after pretreatment, and a mass spectrometry detector is used for detection. The method is rapid, accurate, high in sensitivity, simple and convenient to operate and suitable for determining the concentration of robustaside B in the blood plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com