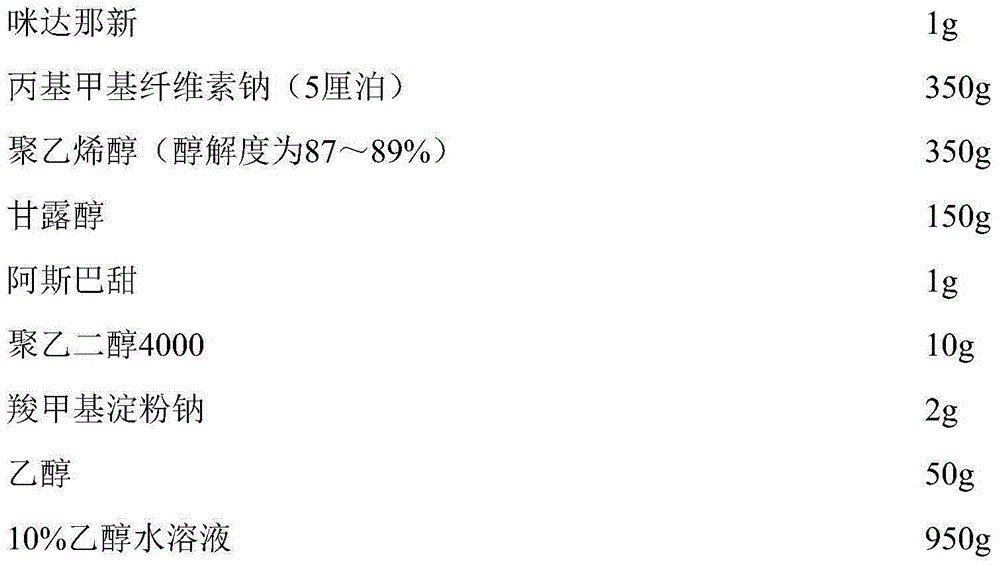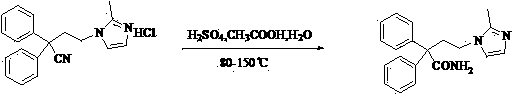Patents
Literature
40 results about "Imidafenacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
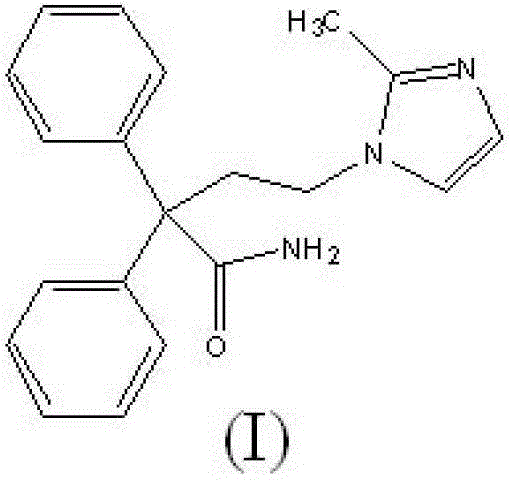
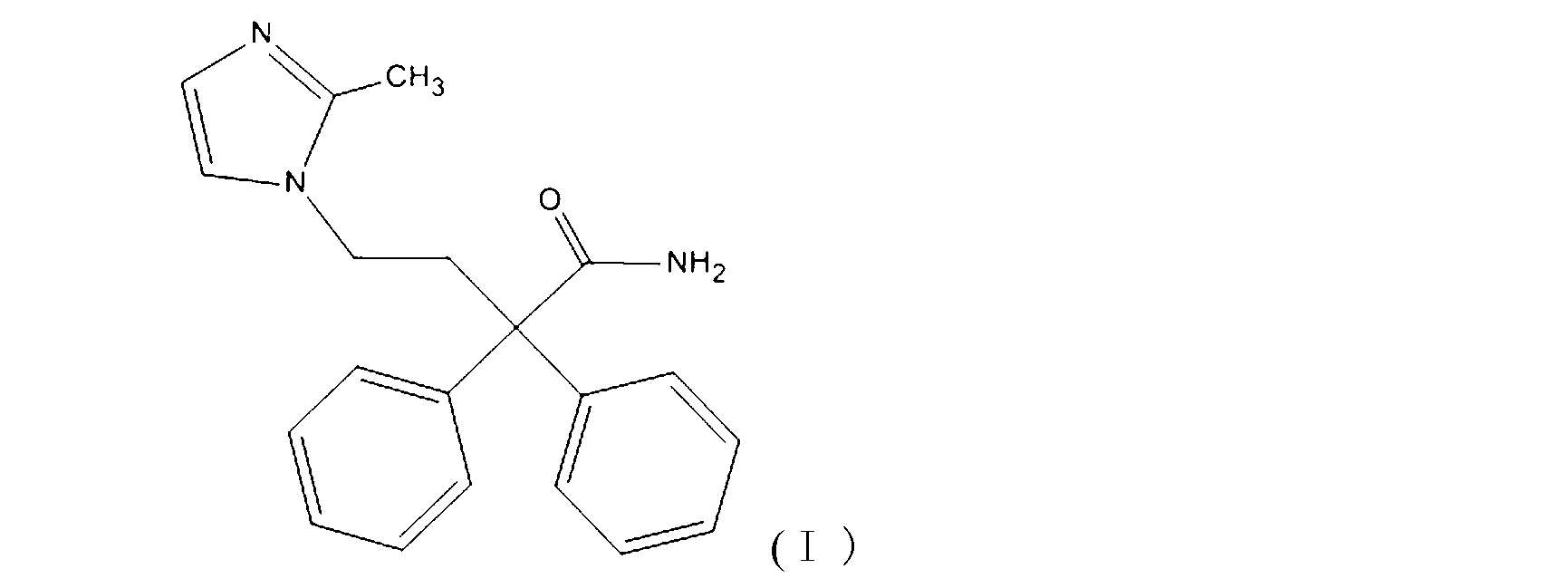
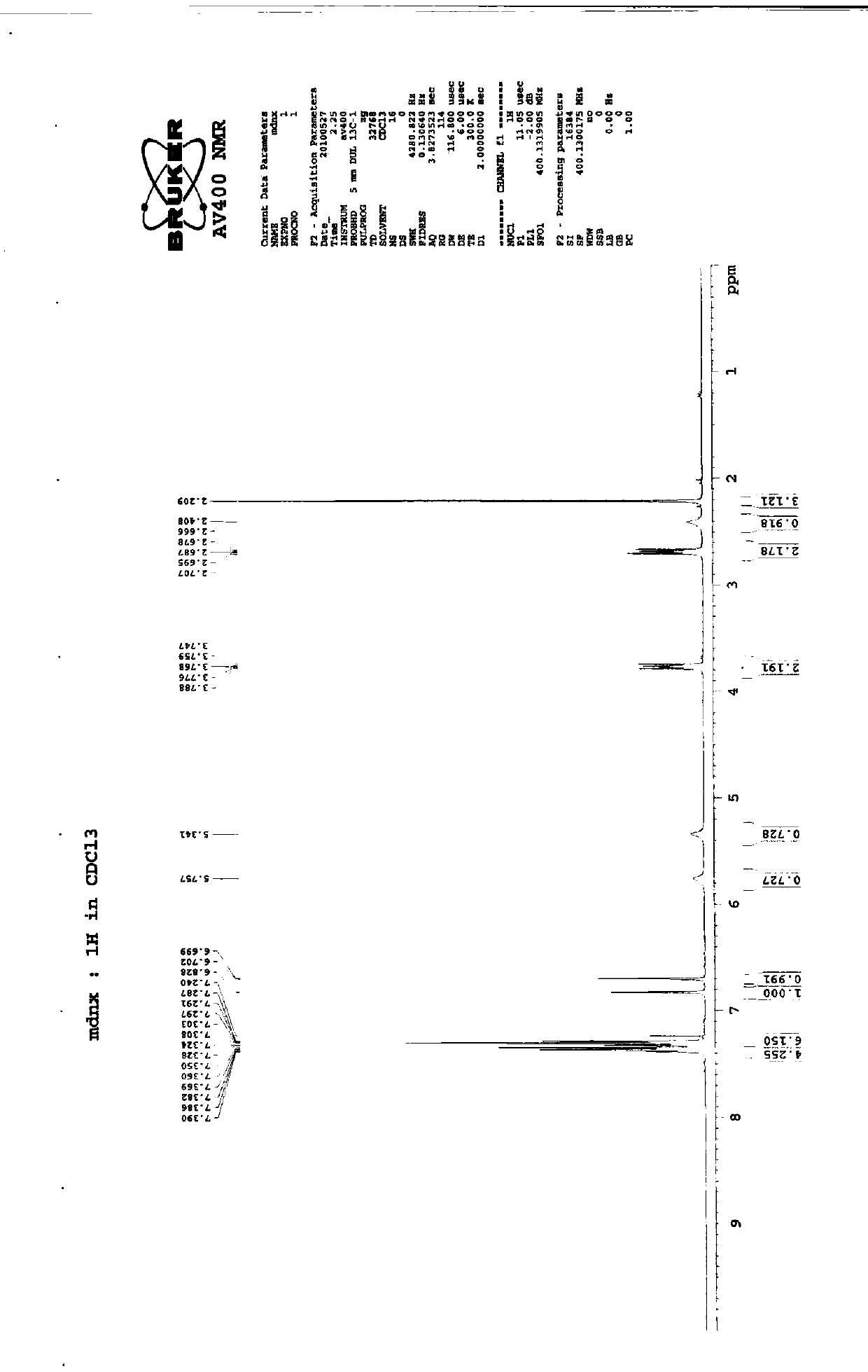
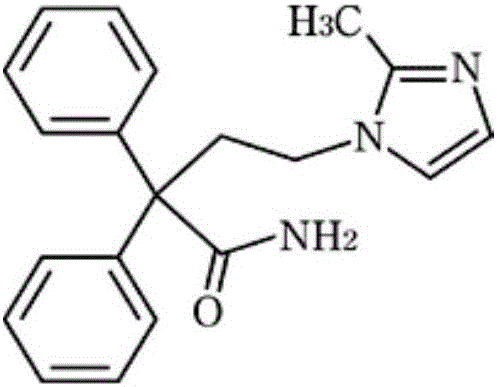
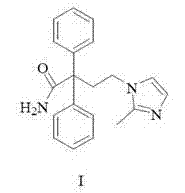
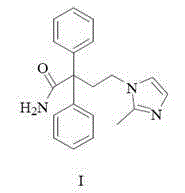
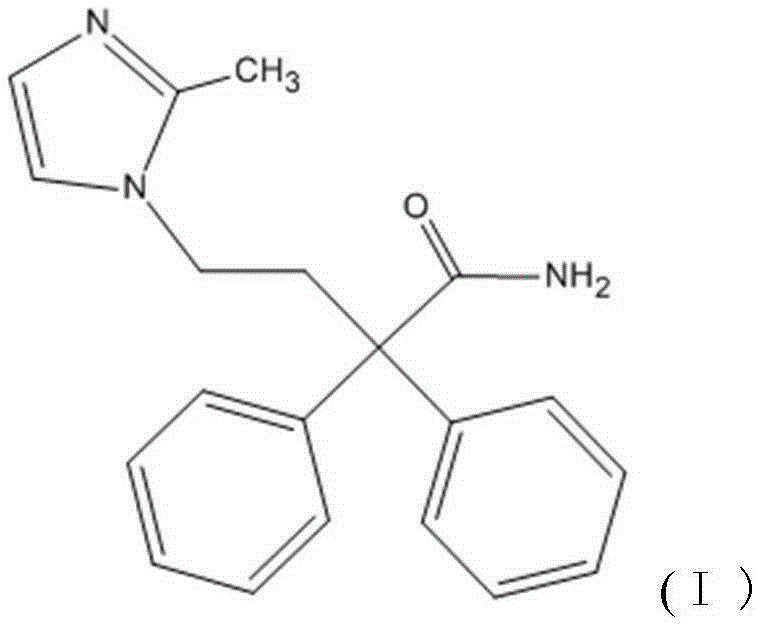
Imidafenacin (INN) is a urinary antispasmodic of the anticholinergic class.
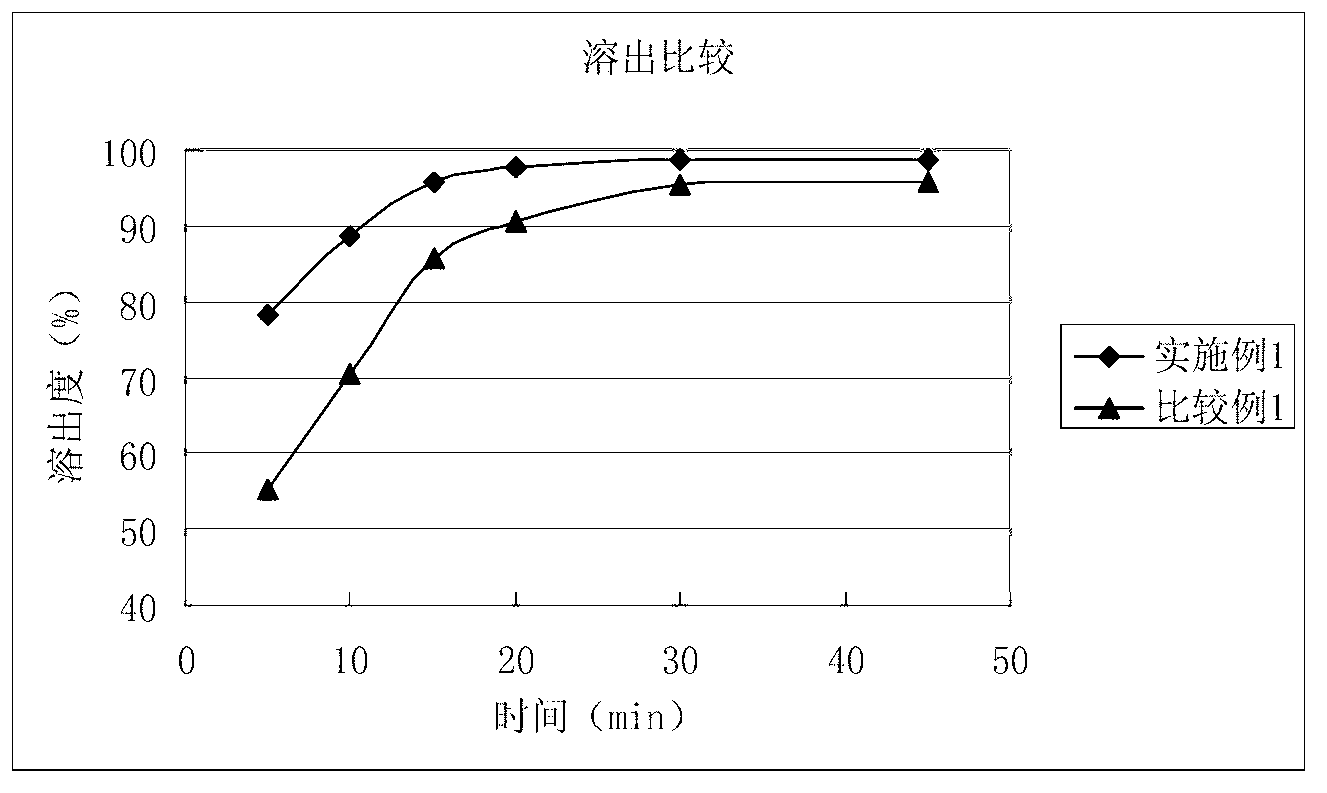
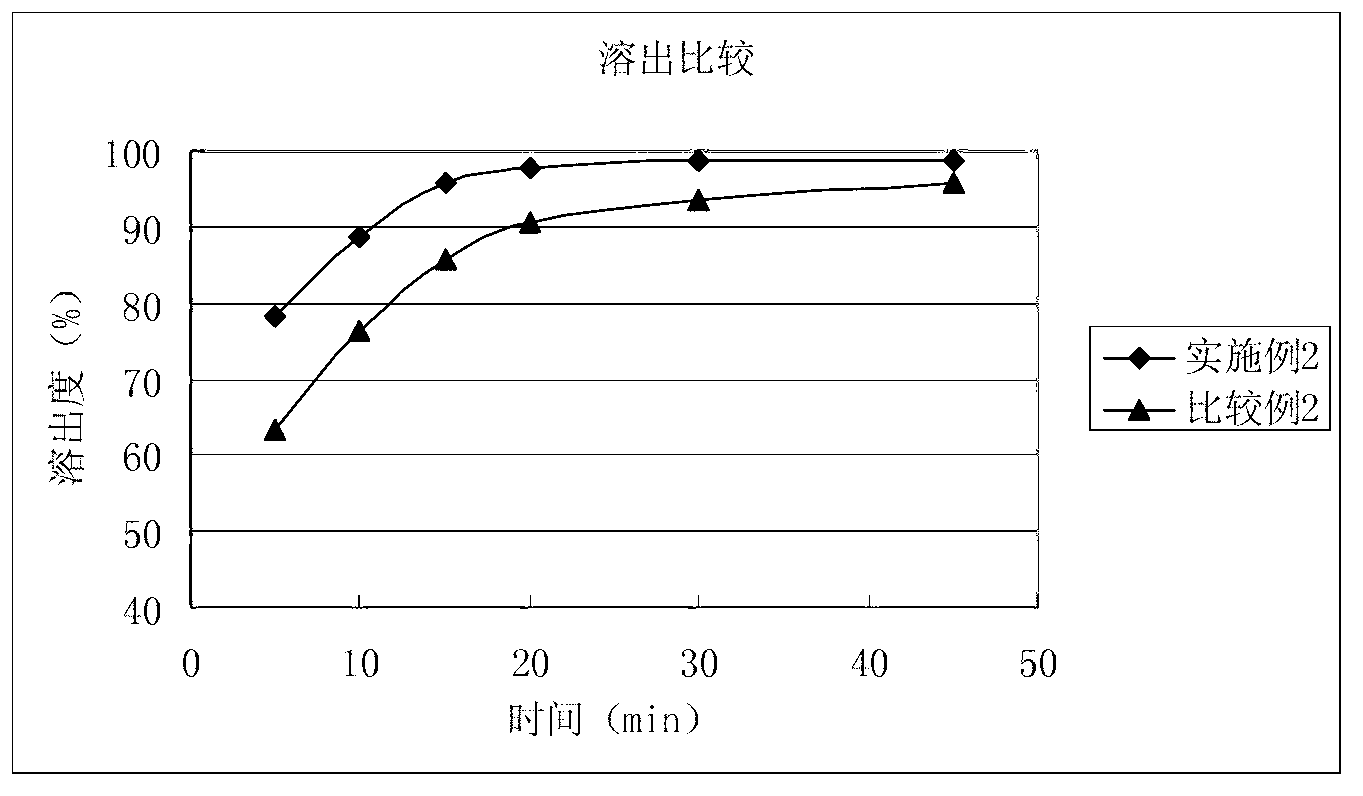
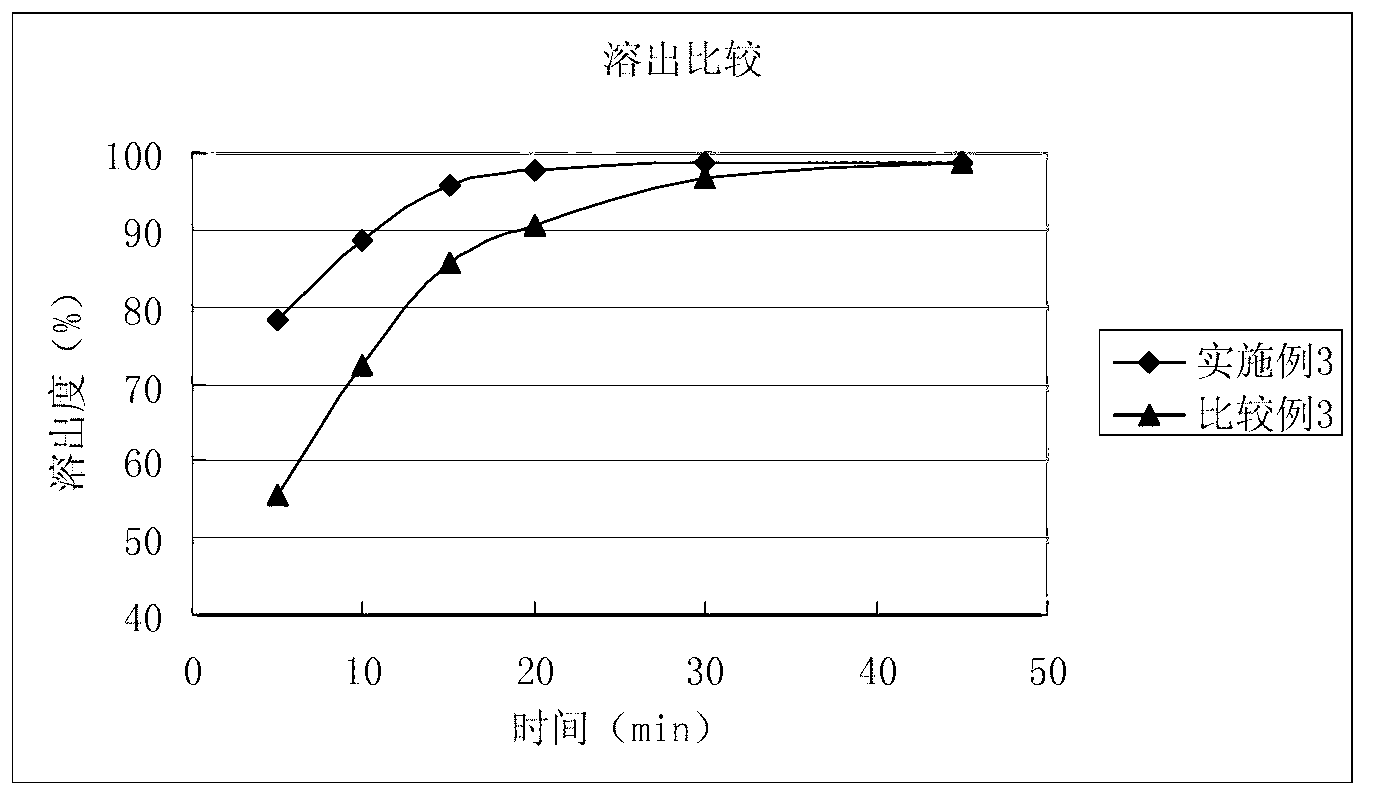
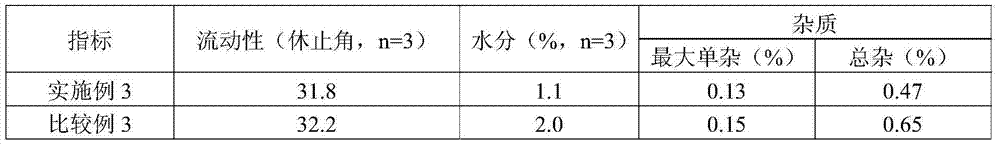
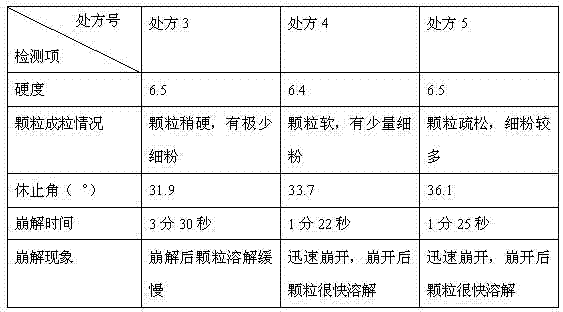
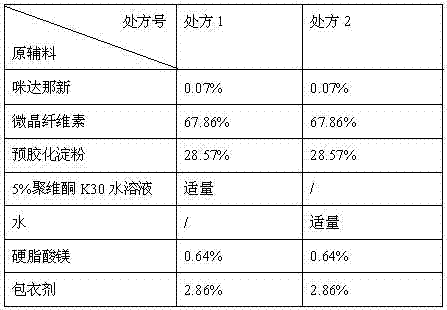
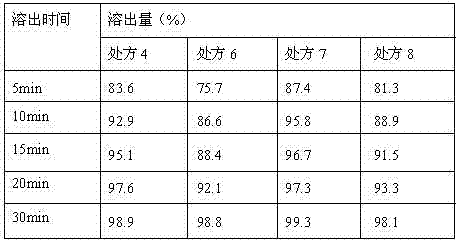
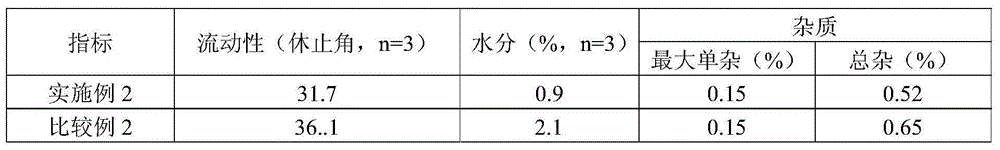
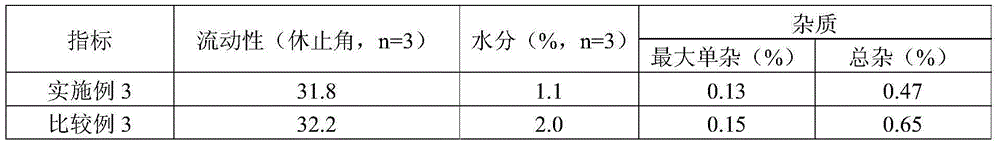
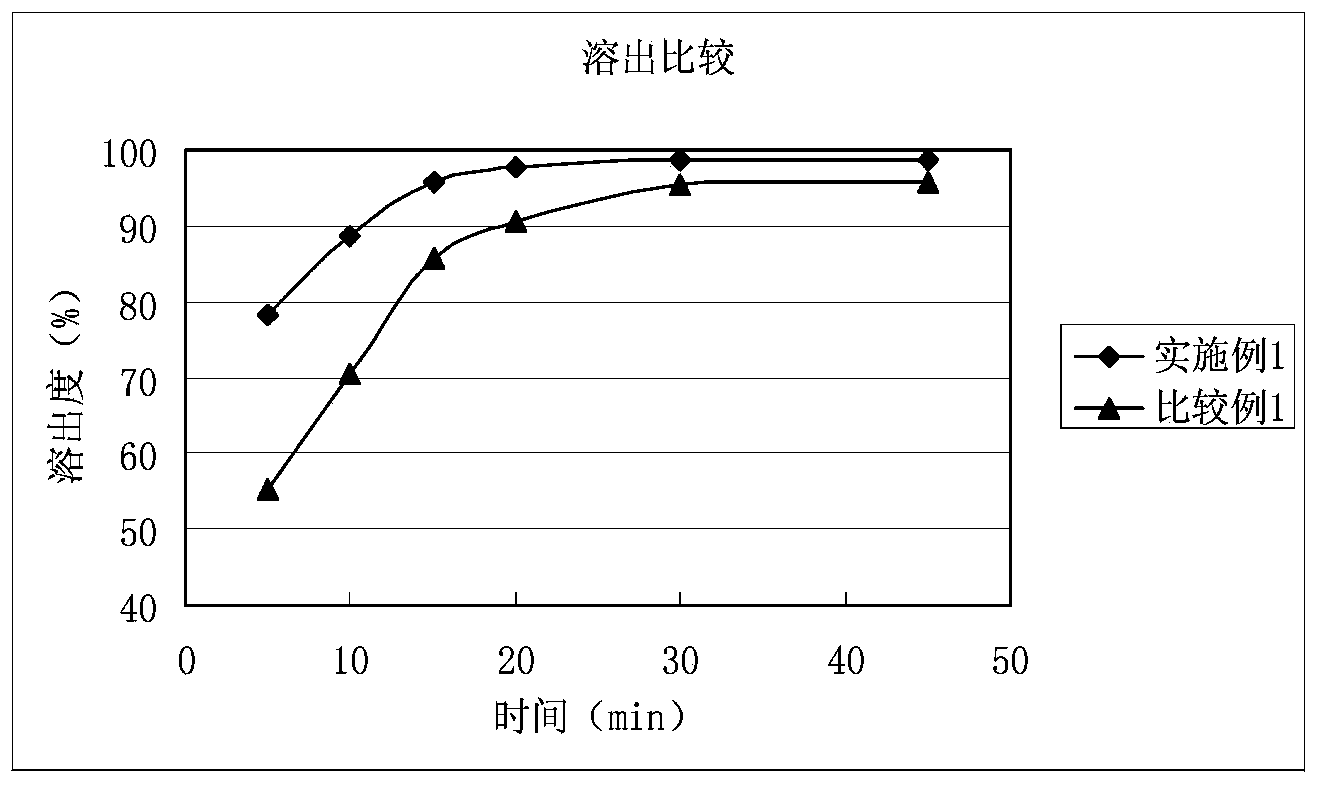
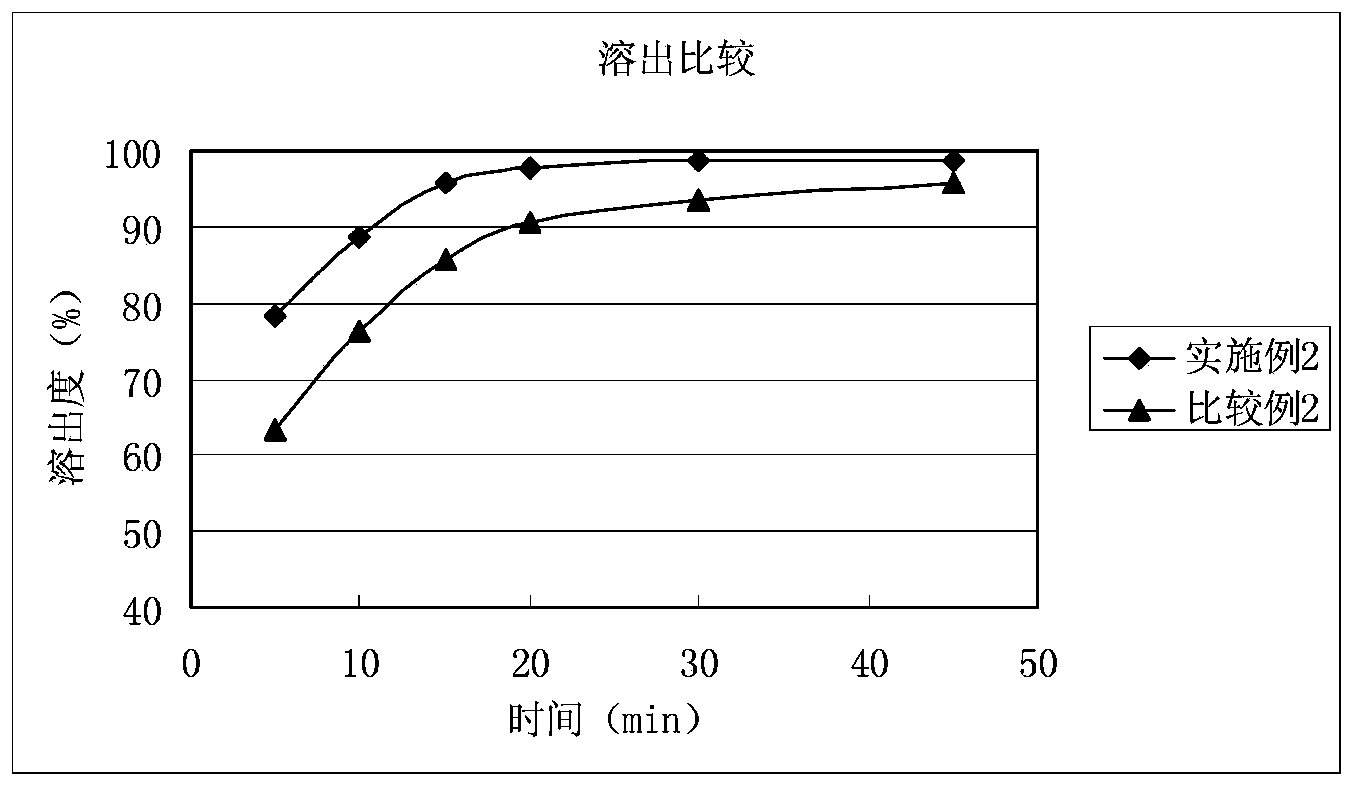
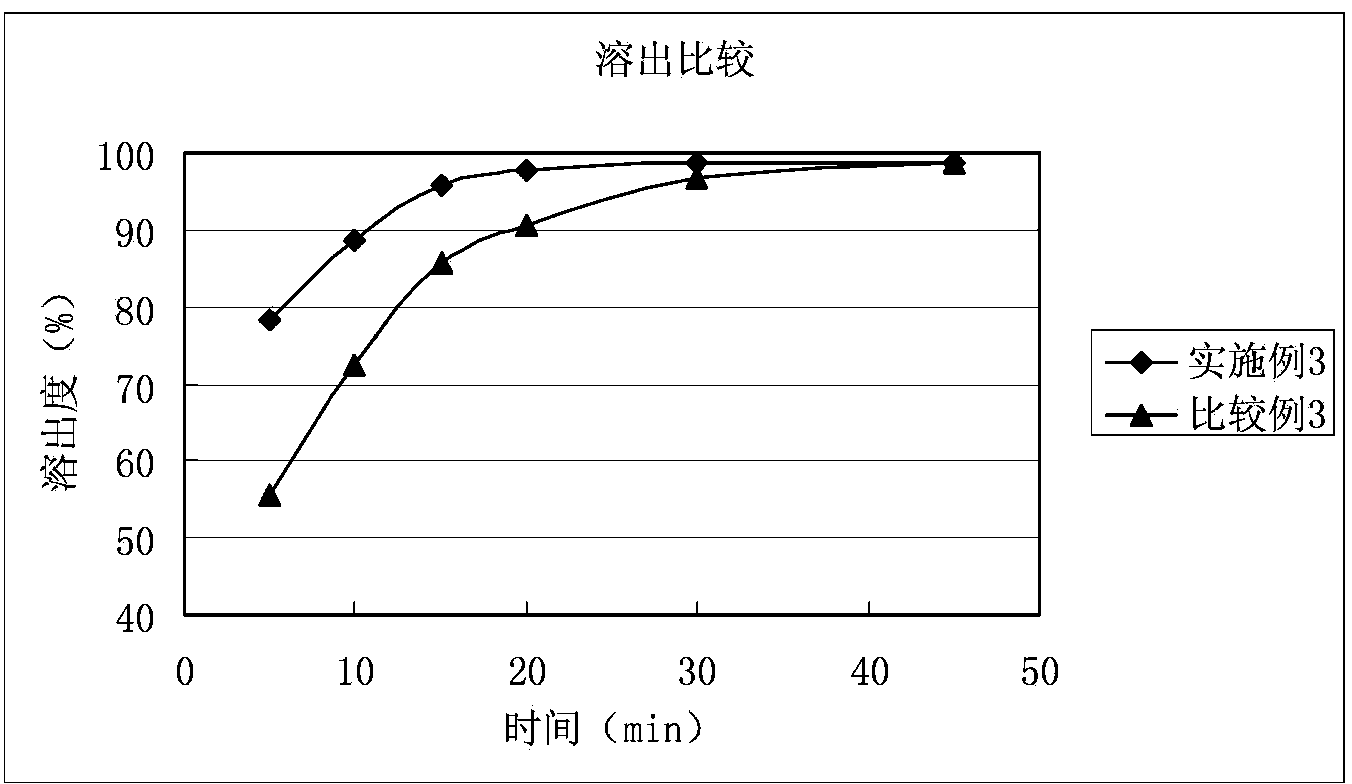
Solid composition for improving content uniformity and dissolution rate of imidafenacin
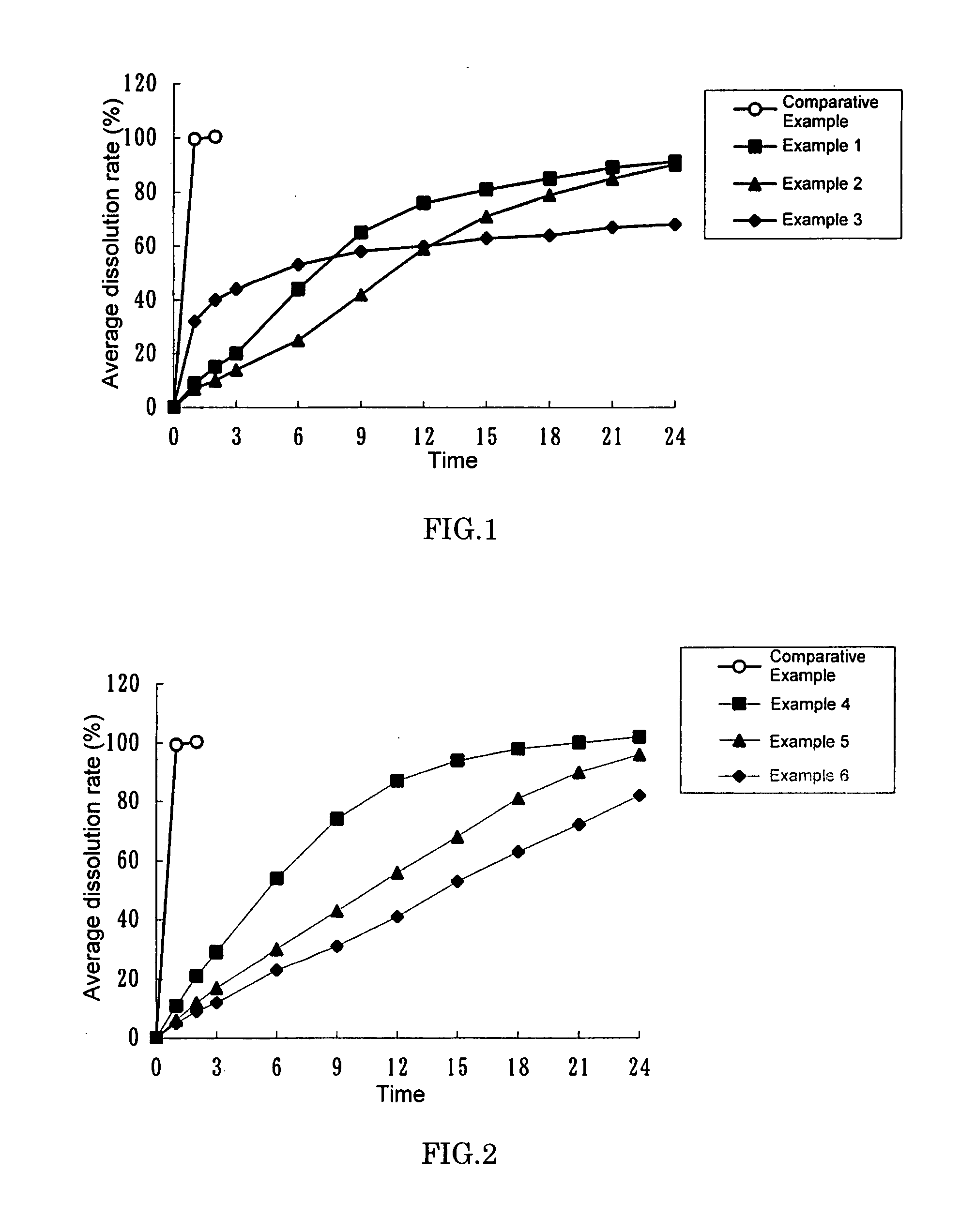
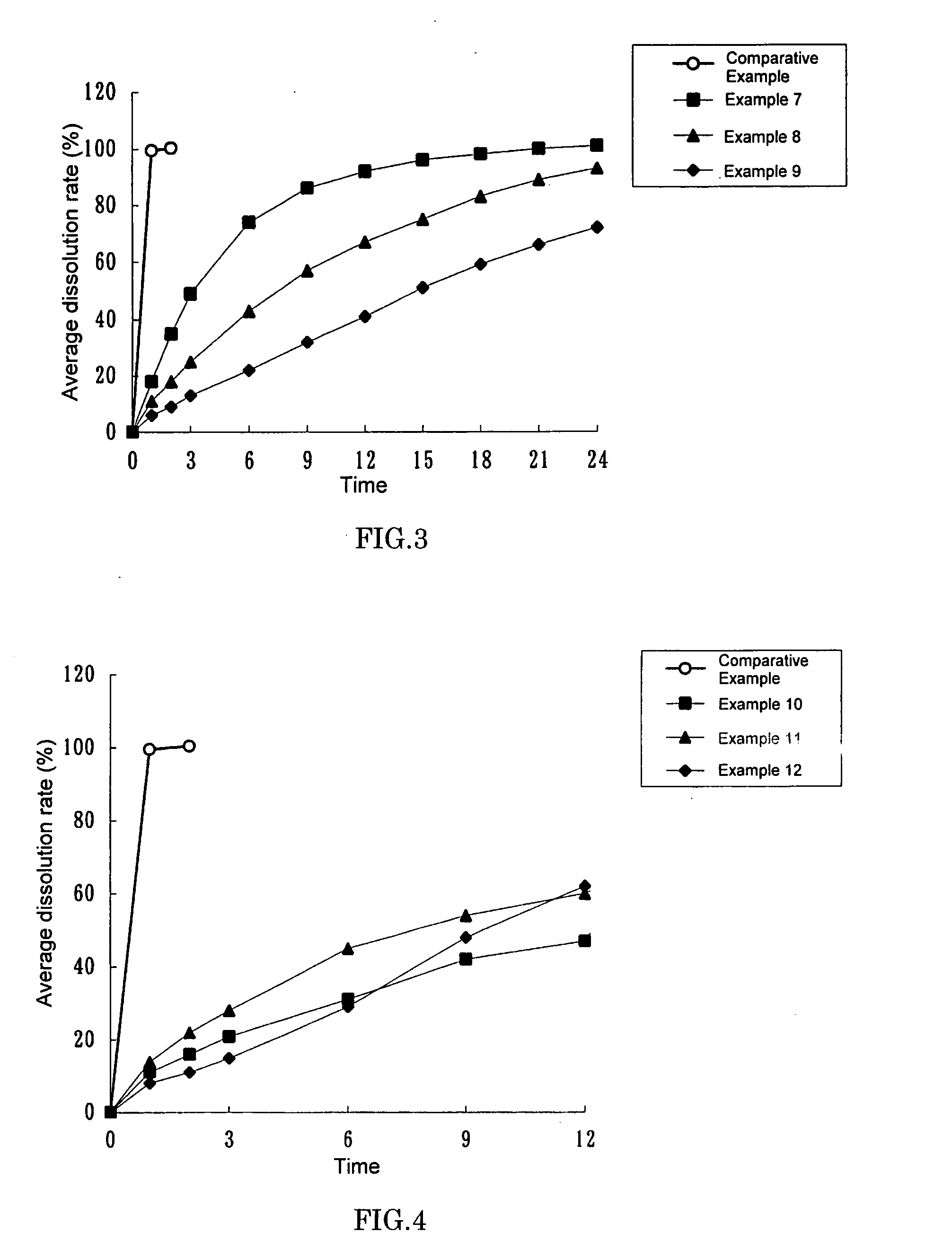
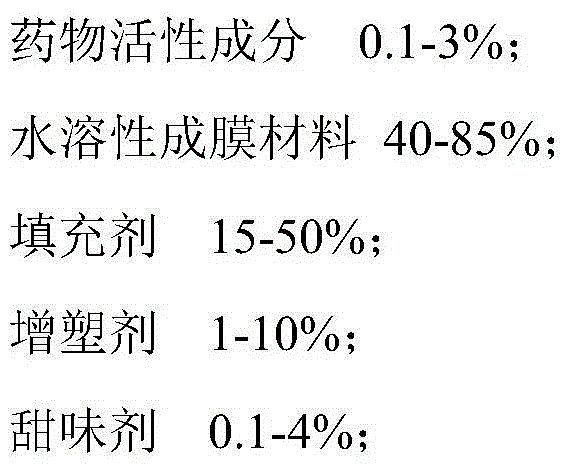
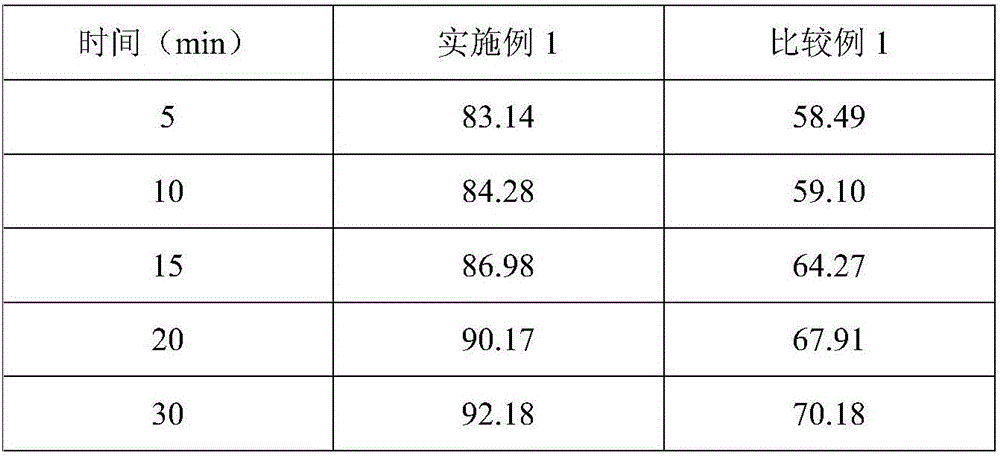
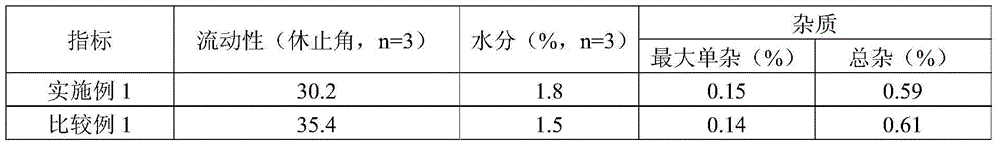
The invention relates to a solid composition containing imidafenacin. The solid composition can improve the dissolubility and content uniformity of main medicines effectively.
Owner:BEIJING D VENTUREPHARM TECH DEV
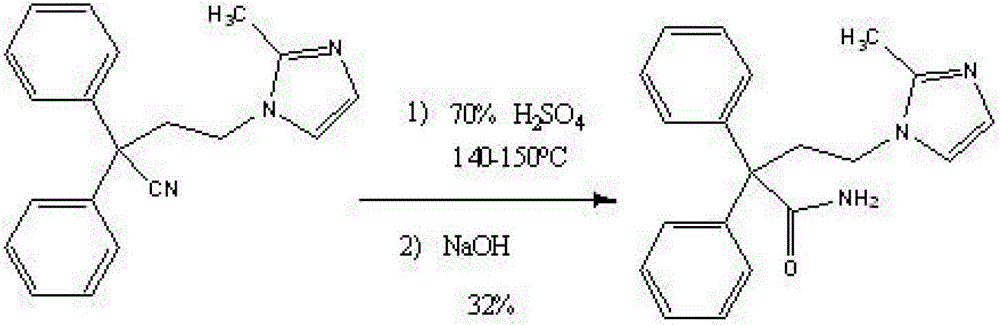
Improved method for preparing imidafenacin
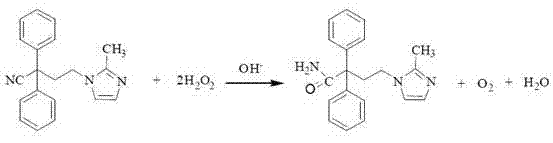
The invention discloses an improved method for preparing imidafenacin. The method is as follow: 4-(2-methyl-imidazol-1-yl)-2,2-diphenyl butyronitrile or phosphate of 4-(2-methyl-imidazol-1-yl)-2,2-diphenyl butyronitrile is reacted with peroxide by improved Radziszewski at the temperature of 40-60 DEG C in the presence of methanol and / or ethanol, dimethyl sulfoxide and alkali. The preparation method provided by the invention has high yield, large number of acid-base is avoided, and the method is a simple, economic and environment-friendly preparation method.
Owner:BEIJING COLLAB PHARMA
Multiple Unit Oral Sustained Release Preparation and Production Method Thereof
InactiveUS20080107727A1Sustained releaseControl the rate of diffusion of imidafenacin in waterPowder deliveryOrganic active ingredientsMolecular networkBlood level
A multiple-unit oral sustained release preparation is provided which allows controlled release of imidafenacin [4-(2-methyl-1-imidazolyl)-2,2-diphenylbutylamide]. The preparation serves to ensure a prolonged effect of imidafenacin and prevent rapid elevation in the blood levels of imidafenacin.Specifically, granules or powders comprising imidafenacin dispersed in a water-insoluble polymer or a higher alcohol are used in the preparation. These preparations achieve sustained release of imidafenacin since the molecular network structure that the water-insoluble polymer or the higher alcohol forms during the preparation of the granules or powders serves to control the rate of diffusion of imidafenacin in water. Granules comprising a core granule having two layers of an inner imidafenacin coating and an outer water-insoluble polymer coating are used in the preparation. The water-insoluble polymer coating serves to control the rate of diffusion of imidafenacin in water and ensure sustained release of imidafenacin. The multiple-unit oral sustained release preparation is provided in the forms of capsules and tablets containing the granules or powders that allow controlled release of imidafenacin. The preparation achieves controlled release of imidafenacin over a prolonged period of time.
Owner:KYORIN PHARMA CO LTD +1
Imidafenacin oral fast dissolving film and preparation method and application thereof
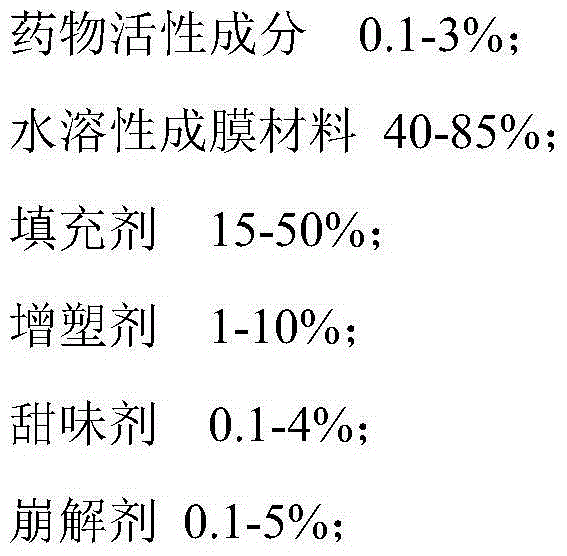
The invention belongs to the technical field of medicine, and particularly relates to an imidafenacin oral fast dissolving film and a preparation method and application thereof. The film agent comprises, by weight, 0.1%-3% of imidafenacin, 40%-85% of film-forming materials, 15%-50% of filling agents, 1%-10% of plasticizers, 0.1%-4% of sweetening agents and 0-5% of disintegrating agents. When the imidafenacin oral fast dissolving film is placed on the tongue, the film can be dissolved rapidly in saliva, medicine can be released, medicine taking through water is not needed, the danger of blocking throats does not exist, the imidafenacin oral fast dissolving film is suitable for being taken by elderly patients, the adaptability of the patients is improved, and carrying is convenient.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
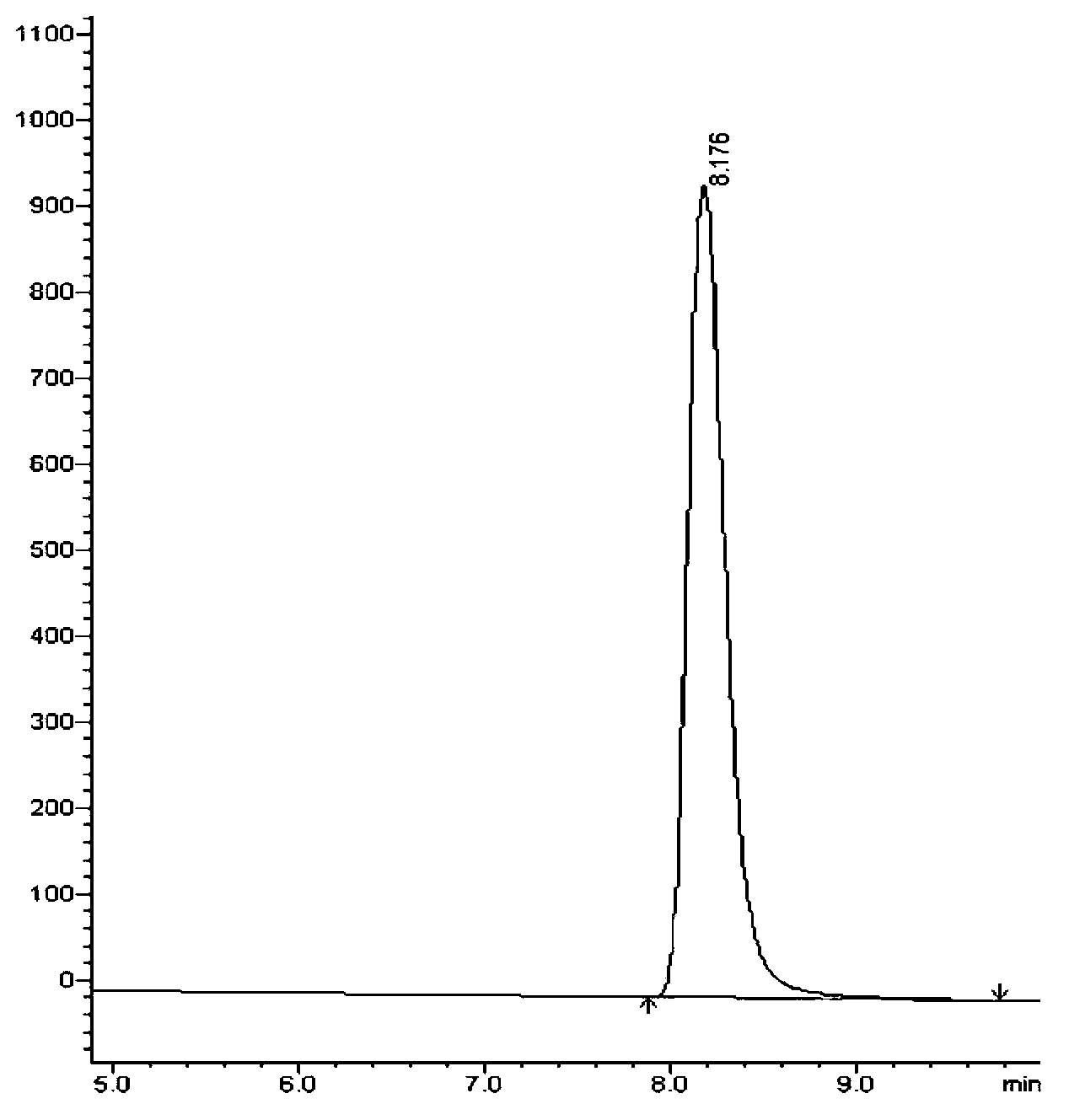
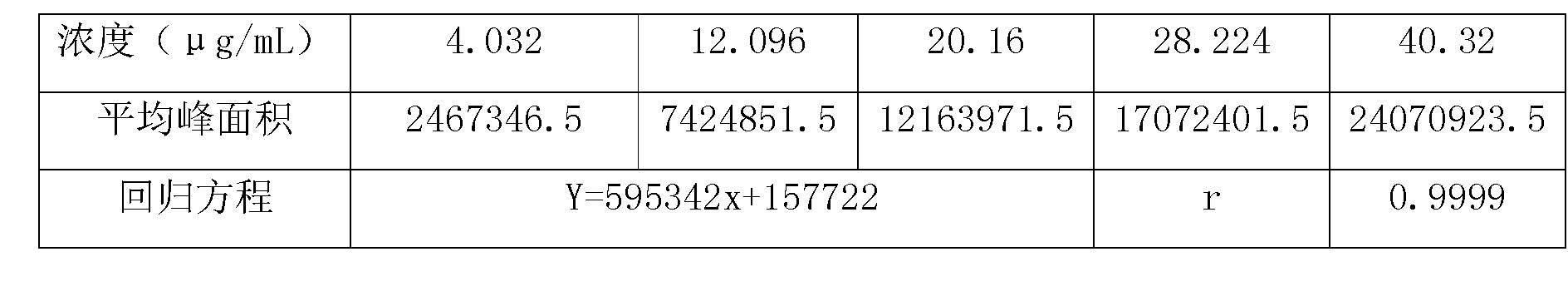
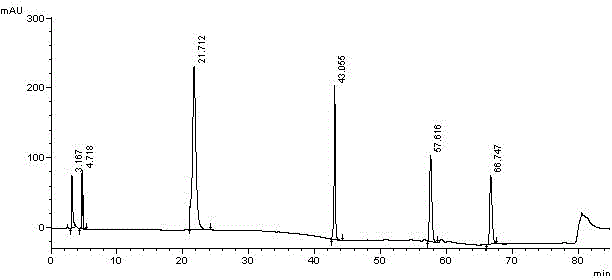
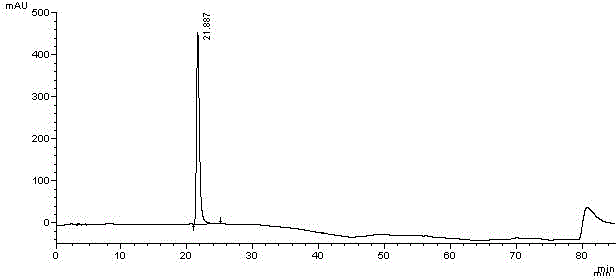
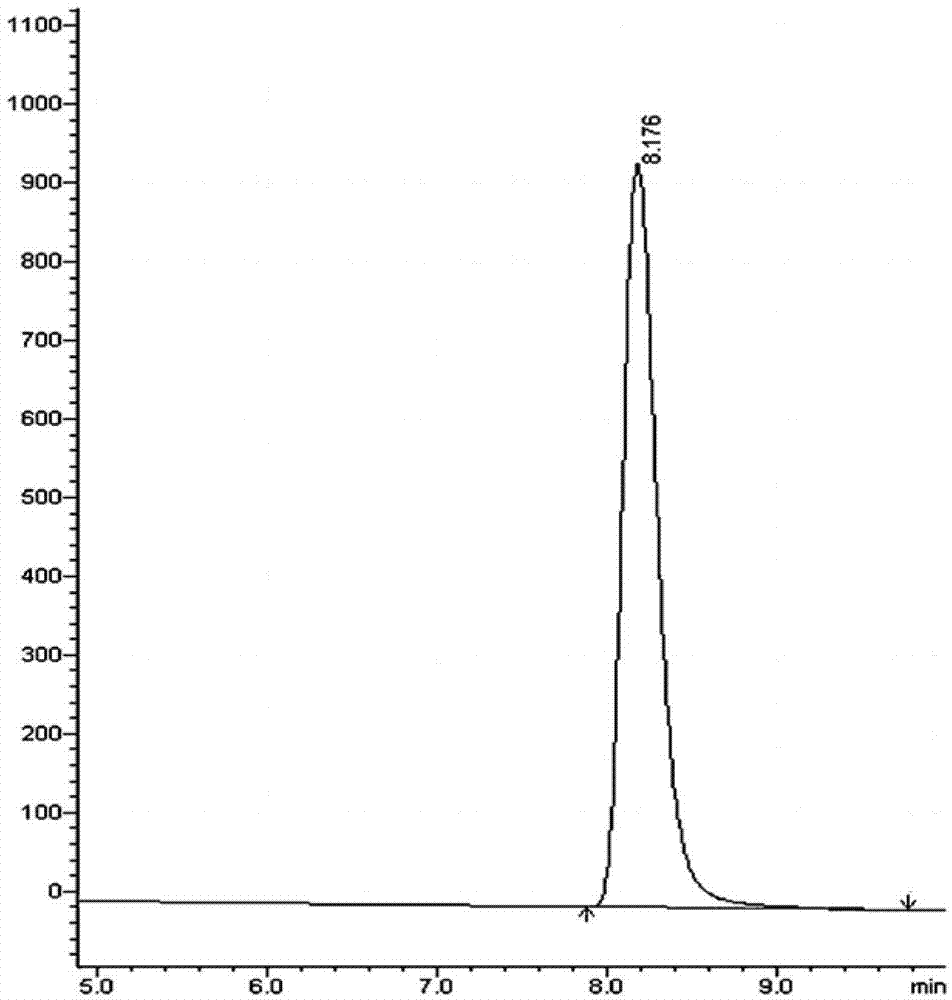
Methods for determining the content of imidafenacin and detecting related substances
ActiveCN103063795AHigh precisionThe content determination result is accurateComponent separationImidafenacinAdditive ingredient
The present invention discloses methods for determining the content of imidafenacin and detecting related substances. According to the methods, high-performance liquid chromatography is mainly used for determining and detecting the content of active pharmaceutical ingredient imidafenacin, and imidafenacin related substances. The methods are time-saving and labor-saving, high in precision, accurate in content determination results, and good in repeatability and recovery, and the method is validated, and can be used for routine analysis and quality control of imidafenacin materials and preparation samples.
Owner:NANJING CORE TECH CO LTD
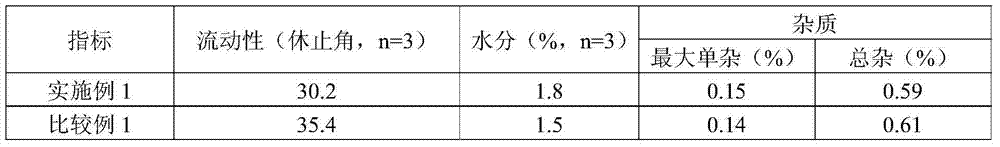
Imidafenacin tablet and preparation method thereof
ActiveCN103054822ASignificant progressImprove the disintegration effectOrganic active ingredientsUrinary disorderWater basedPolyvinyl alcohol
The invention discloses an imidafenacin tablet which is prepared from the components including imidafenacin, water-soluble polymer, a filling agent, a disintegrating agent and lubricant; the water-soluble polymer is selected from one or more of poloxamer, polyethyleneglycol, hydroxypropyl methylcellulose, polyvinyl alcohol and copovidone and accounts for 0.1 percent to 3 percent (w / w) of the total weight of the whole tablet; and the disintegrating agent is pregelatinized starch or a mixture of common starch and pregelatinized starch and accounts for 15 percent to 50 percent (w / w) of the total weight of the whole tablet. The tablet provided by the invention can be dissolved in a water-based medium at an unexpected high level in relative time and has a very good dissolution characteristic and remarkable physical stability.
Owner:NANJING CORE TECH CO LTD
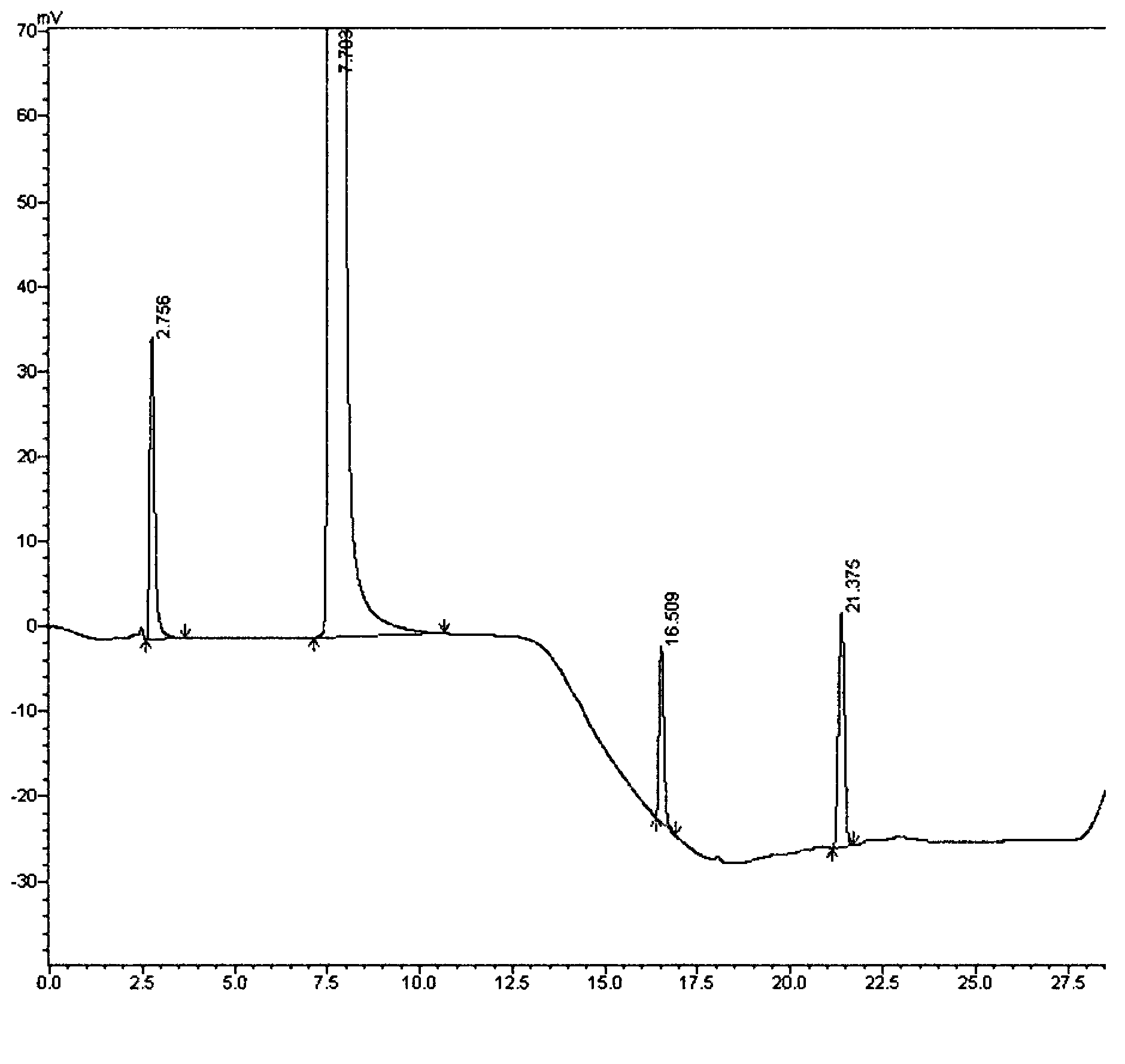
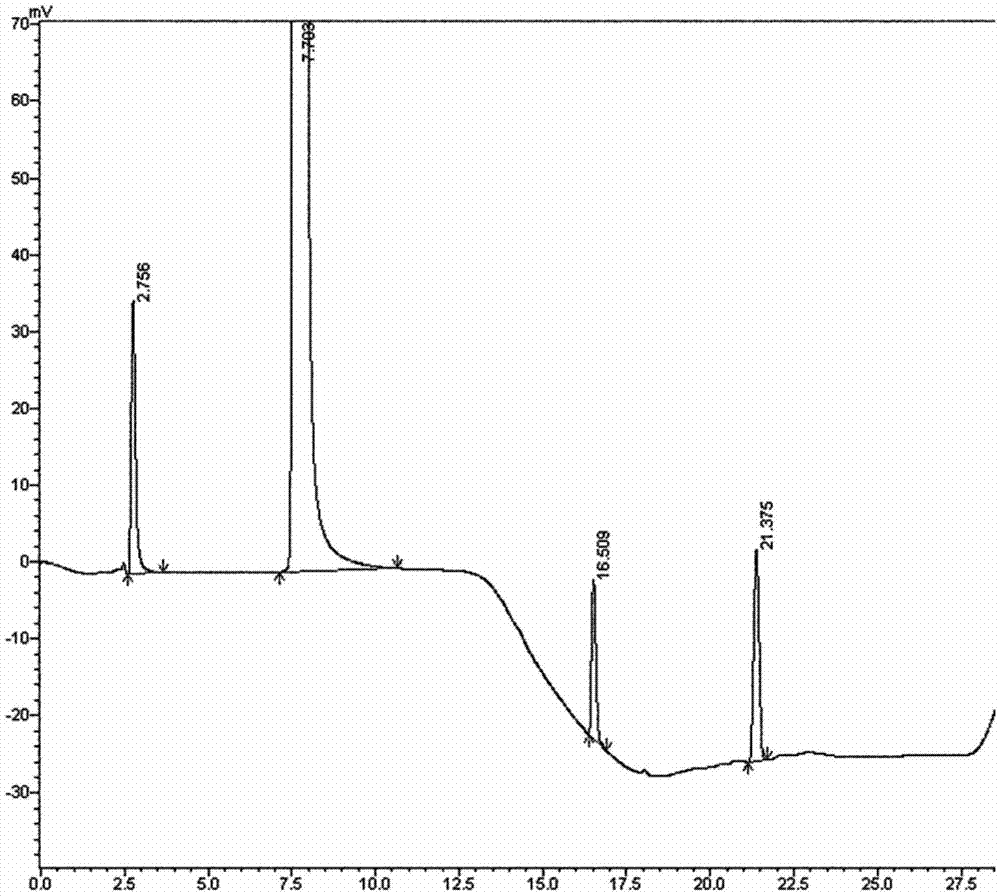
Method for separating imidafenacin and related substances thereof by using high performance liquid chromatography
ActiveCN104614468AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining imidafenacin and related substances thereof by using liquid chromatography. The method can be used for quantitatively determining the contents of imidafenacin and related substances thereof by using phenyl silane bonded silica gel as a chromatographic column of fillers and a certain proportion of buffer salt solutions-organic phases as mobile phases, thus effectively controlling the quality of imidafenacin. The method has strong specificity and high precision and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Imidafenacin film-coated tablet and preparation method thereof
InactiveCN103479594AImprove uniformityGuarantee of good quality uniformityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineImidafenacin
The invention provides an imidafenacin film-coated tablet. The imidafenacin film-coated tablet comprises raw and auxiliary materials in parts by weight as follows: 1-5 parts of imidafenacin, 700-850 parts of pregelatinized starch, 600-700 parts of microcrystalline cellulose, 3-6 parts of lubricants and appropriate coating agents. The invention further provides a preparation method of the film-coated tablet. According to research findings, the imidafenacin can dissolve completely even in 5min in a specific prescription ratio of the tablet; meanwhile, a specific fluidized bed granulation process is adopted, so that the uniformity of the content of the imidafenacin in the preparation can be remarkably improved; and the content of main drugs in the imidafenacin tablet is quite low, so that the quality uniformity of drugs is well guaranteed with the preparation method.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
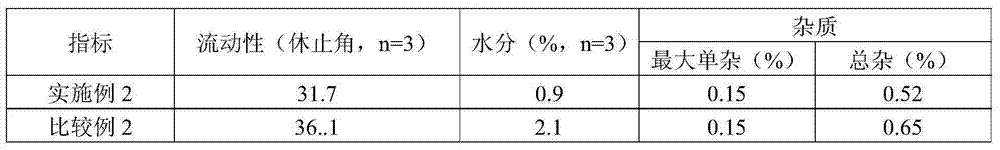
Tablet containing imidafenacin and preparation method thereof
ActiveCN107753455AIncrease contentGood content uniformityOrganic active ingredientsUrinary disorderAdhesiveDissolution
The invention discloses a tablet containing imidafenacin and a preparation method thereof. The tablet or a tablet core contains an active ingredient imidafenacin, a filling agent, an adhesive and a lubricating agent. The preparation method comprises the following steps: dissolving the active ingredient into an ethanol solvent or a mixed solvent of ethanol and water; adding the adhesive and water to prepare an adhesive solution; and granulating and tabletting with the filling agent and the lubricating agent. According to the method disclosed by the invention, the finished product can be rapidlydissolved out, and the raw materials and the adhesive are dissolved in the ethanol solution in steps and added into other mixed powder in a solution state, so that the content uniformity can be greatly improved; and a reduced pressure drying process is adopted to isolate from air, and the oxidative degradation impurities can be well reduced. According to the technical scheme, the tablet disclosedby the invention has the advantages of being rapid in dissolution, less in oxidative impurities, excellent in content uniformity and the like, and the preparation process is simple and suitable for large-scale production.
Owner:NANJING HEALTHNICE MEDICAL TECH
Percutaneously absorbed preparation
ActiveUS20130211352A1Good skin permeabilityStable absorptionBiocideOrganic active ingredientsIsostearic acidSkin permeability
The purpose of the invention is to produce an imidafenacin-containing percutaneously absorbed preparation, wherein the drug not only is not allowed to crystallize but also has adequate skin penetration. The imidafenacin-containing percutaneously absorbed preparation comprises isostearic acid and a fatty acid ester, which function as crystallization-preventing agents.
Owner:HISAMITSU PHARM CO INC
Pharmaceutical composition containing imidafenacin
ActiveCN104274422AImprove stabilityHigh dissolution rateOrganic active ingredientsPharmaceutical delivery mechanismImidafenacinMicrocrystalline cellulose
The invention relates to a pharmaceutical composition containing imidafenacin and a preparation method of the pharmaceutical composition. Imidafenacin is mixed with pregelatinized starch and microcrystalline cellulose according to a certain ratio. The composition obtained by the invention is simple in prescription, mature in preparation process and good in dissolution effect.
Owner:KUNMING JIDA PHARMA
Orally rapidly disintegrating tablet comprising imidafenacin
InactiveUS20110002988A1Disintegrates quicklyEasy to manageOrganic active ingredientsBiocideCompression moldingImidafenacin
Provided herein is an imidafenacin-containing orally rapidly disintegrating tablet which is excellent in the photostability.The orally rapidly disintegrating tablets comprises (1) a granulated product containing imidafenacin or imidafenacin particles, which is or are coated with povidone or a gastric juice-soluble polymer; and (2) a composition containing an excipient and a disintegrating agent, wherein the resulting composition is subjected to compression molding.
Owner:KYORIN PHARMA CO LTD
Preparation method of imidafenacin
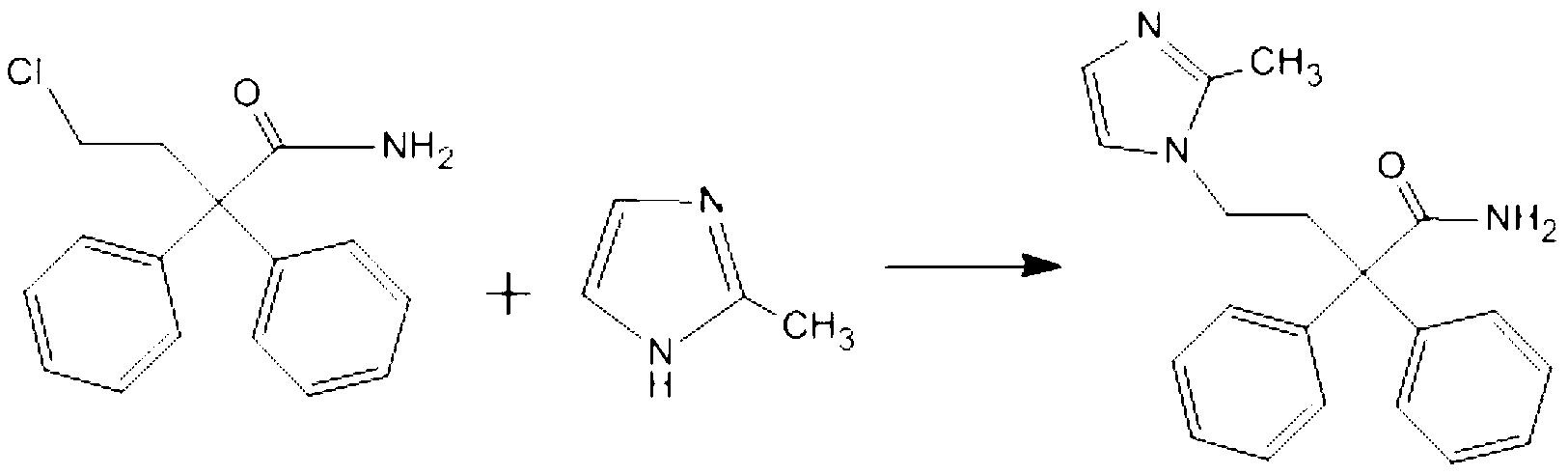
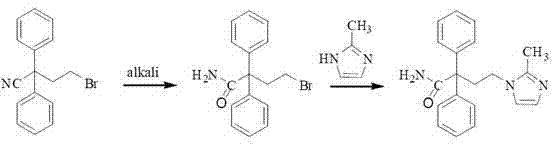
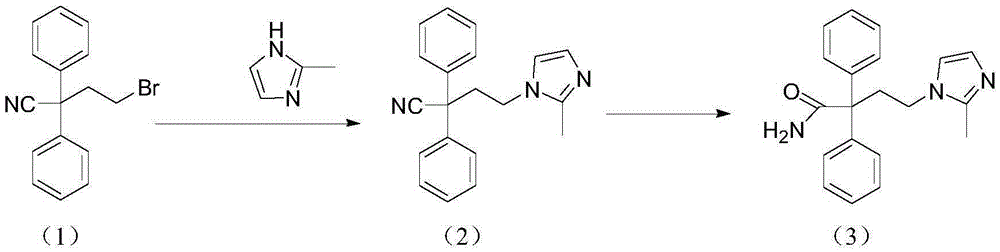
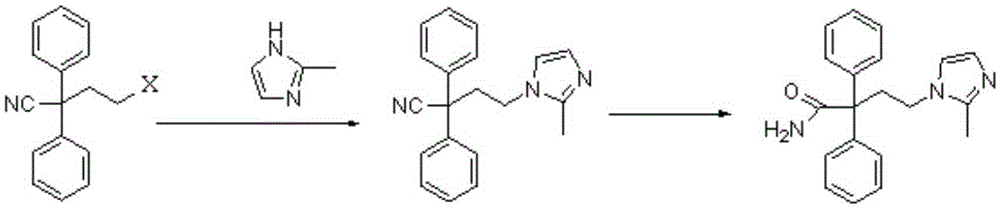
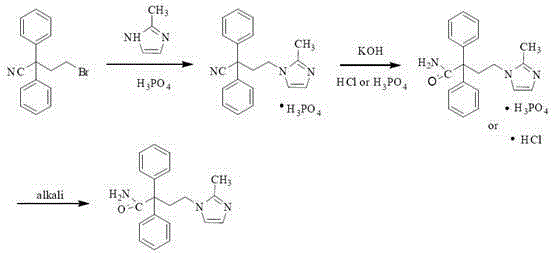
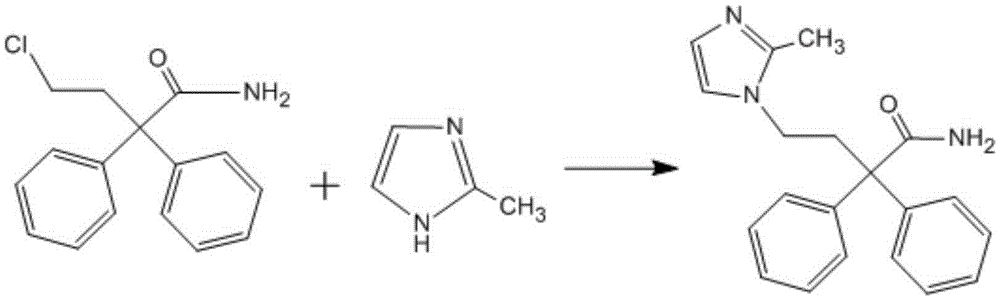
The present invention discloses a preparation method of imidafenacin. In the method, 4-chloro-2, 2-diphenylbutaneamide and 2-methylimidazole are taken as raw materials for reaction in the presence of alkali, and then ethyl acetate is used for recrystallization. The method has advantages of high yield, mild reaction condition, and simple purification and is suitable for industrialized production.
Owner:陕西步长高新制药有限公司
Preparation method of imidafenacin
The invention discloses a method for preparing 4-(2-methyl-1H-imidazole-1-yl)-2,2-diphenyl butyrylamide (1). The target product of the method is a new drug imidafenacin for treating overactive bladder. The invention provides a new synthetic route and a new preparation method; and the new method is simple and convenient to operate, mild in reaction conditions, easy to control, high in yield, good in product purity, free of pollution on the environment and suitable for industrial mass production.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
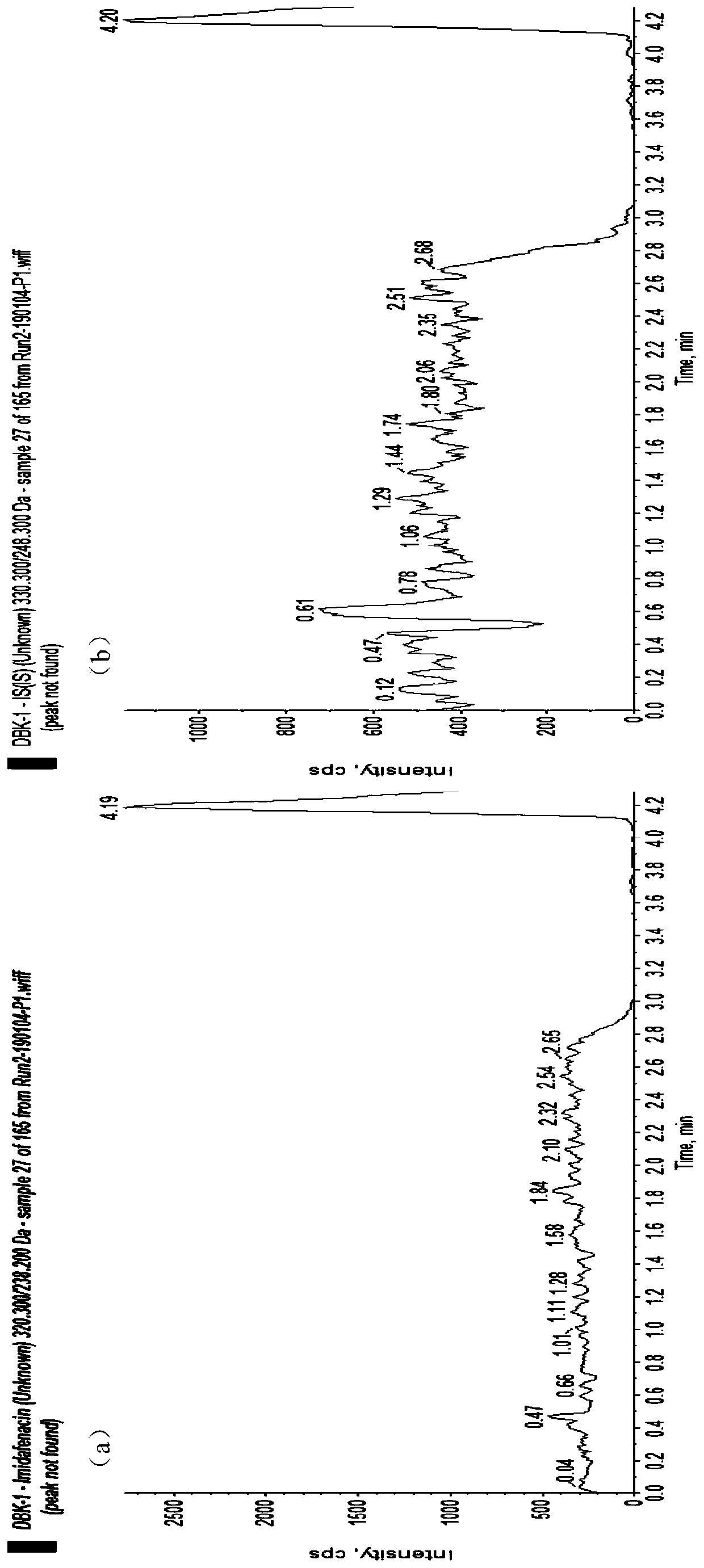
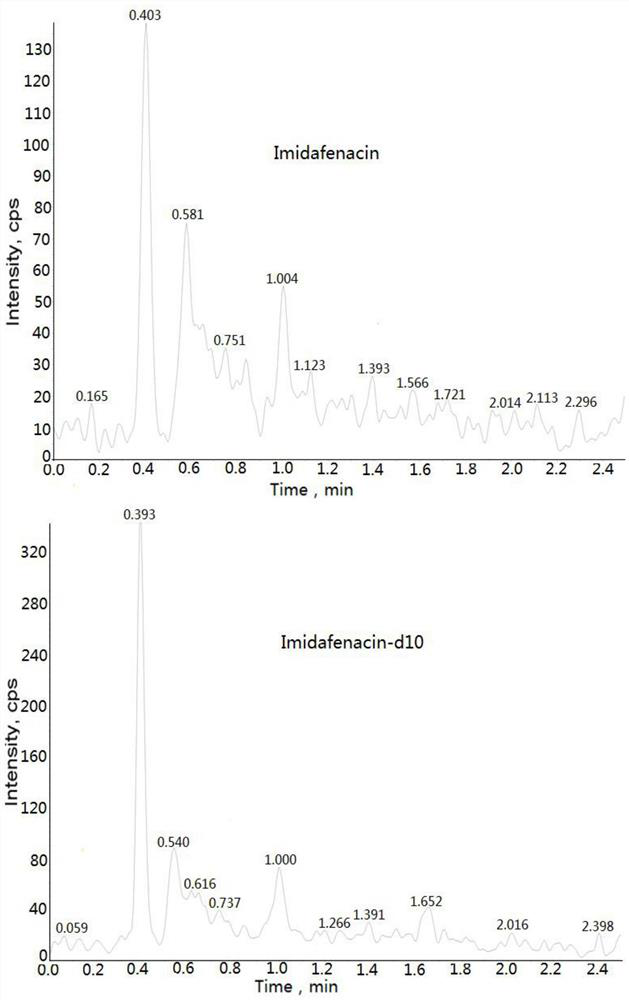
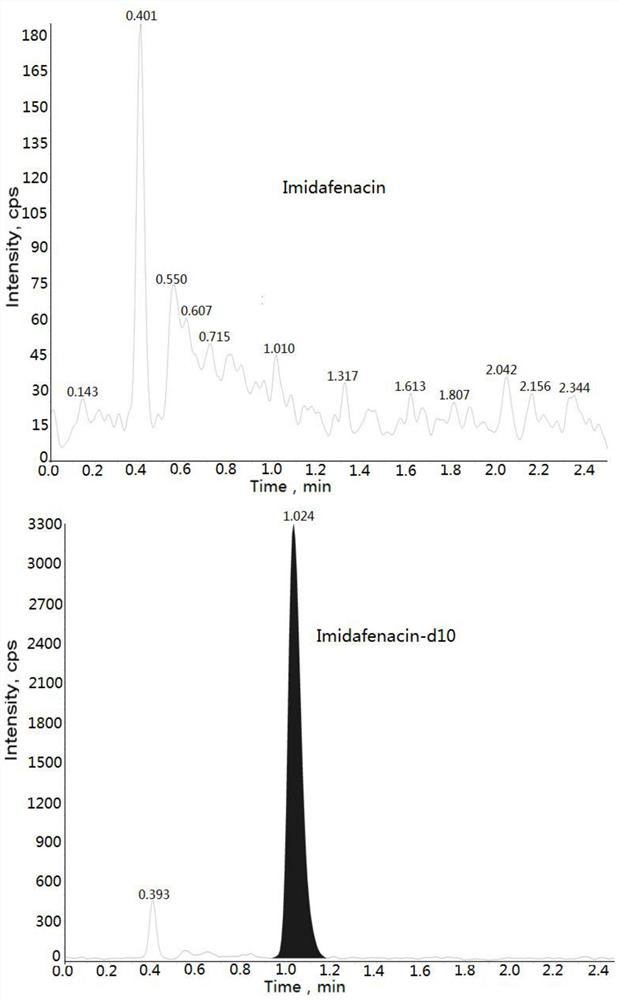
Method for detecting imidafenacin in human plasma through HPLC-MS/MS combination
ActiveCN110554104AHigh column efficiencyHigh sensitivityComponent separationRetention timeGradient elution
The invention relates to a method for detecting imidafenacin in human plasma through HPLC-MS / MS combination, and belongs to the field of biological analysis. The detection method comprises the following steps: (1) pretreatment of a human plasma sample; (2) liquid chromatography-mass spectrometry combined detection: a mobile phase A and a mobile phase B are adopted as a mixed mobile phase for gradient elution, the mobile phase A is acetonitrile, and the mobile phase B is an ammonium acetate aqueous solution; and (3) measurement of the concentration of imidafenacin in human plasma. According tothe method, with imidafenacin-d10 as an internal standard, gradient elution is carried out by adopting Waters, ACQUITY UPLC BEH C8 chromatographic columns, the deuterated internal standard and a to-be-detected object have the same retention time, chemical property and matrix effect, and the reproducibility and accuracy for detecting the concentration of imidafenacin in blood plasma are relativelygood. The method provided by the invention can be used for evaluating the bioequivalence of each dosage form of imidafenacin.
Owner:NANJING HEALTHNICE MEDICAL TECH +1
Imidafenacin tablet preparation method
InactiveCN106361716ASolve the uniformity problemImprove practicalityOrganic active ingredientsUrinary disorderDrug contentFiller Excipient
The invention discloses an imidafenacin tablet preparation method. The preparation method comprises the following steps: taking a formulated amount of filler, disintegrant and adhesive, and uniformly mixing to obtain an auxiliary material for later use; dispersing and dissolving a formulated amount of imidafenacin and cosolvent in anhydrous ethanol to obtain a main drug for later use; and uniformly spraying the main drug into the auxiliary material, adding lubricant, uniformly mixing, determining the content of an intermediate compound, and tabletting. According to the imidafenacin tablet preparation method, the main drug is sprayed into the premixed medicinal auxiliary material through a spray gun, thereby well solving the uniformity problem of the tablet under the condition of low drug content. Besides, the low moisture filler is used as the filler of the imidafenacin tablet, so that direct tabletting can be performed without a drying process, and the direct tabletting process is simpler and easier to implement in comparison with wet granulation, fluidized bed, boiling granulation, spray drying and other processes, thereby being suitable for industrial production, being beneficial to product quality control, energy saving and consumption reduction, lowering the production cost, and having favorable practicality.
Owner:SHENYANG PHARMA UNIVERSITY
Imidafenacin film-coated tablet and preparation method thereof
InactiveCN103479594BImprove uniformityGuarantee of good quality uniformityOrganic active ingredientsUrinary disorderImidafenacinDrug product
The invention provides an imidafenacin film-coated tablet. The imidafenacin film-coated tablet comprises raw and auxiliary materials in parts by weight as follows: 1-5 parts of imidafenacin, 700-850 parts of pregelatinized starch, 600-700 parts of microcrystalline cellulose, 3-6 parts of lubricants and appropriate coating agents. The invention further provides a preparation method of the film-coated tablet. According to research findings, the imidafenacin can dissolve completely even in 5min in a specific prescription ratio of the tablet; meanwhile, a specific fluidized bed granulation process is adopted, so that the uniformity of the content of the imidafenacin in the preparation can be remarkably improved; and the content of main drugs in the imidafenacin tablet is quite low, so that the quality uniformity of drugs is well guaranteed with the preparation method.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD
Method for preparing imidafenacin
ActiveCN103772286ASimple methodSuitable for industrialized mass productionOrganic chemistryImidafenacinHydrolysis
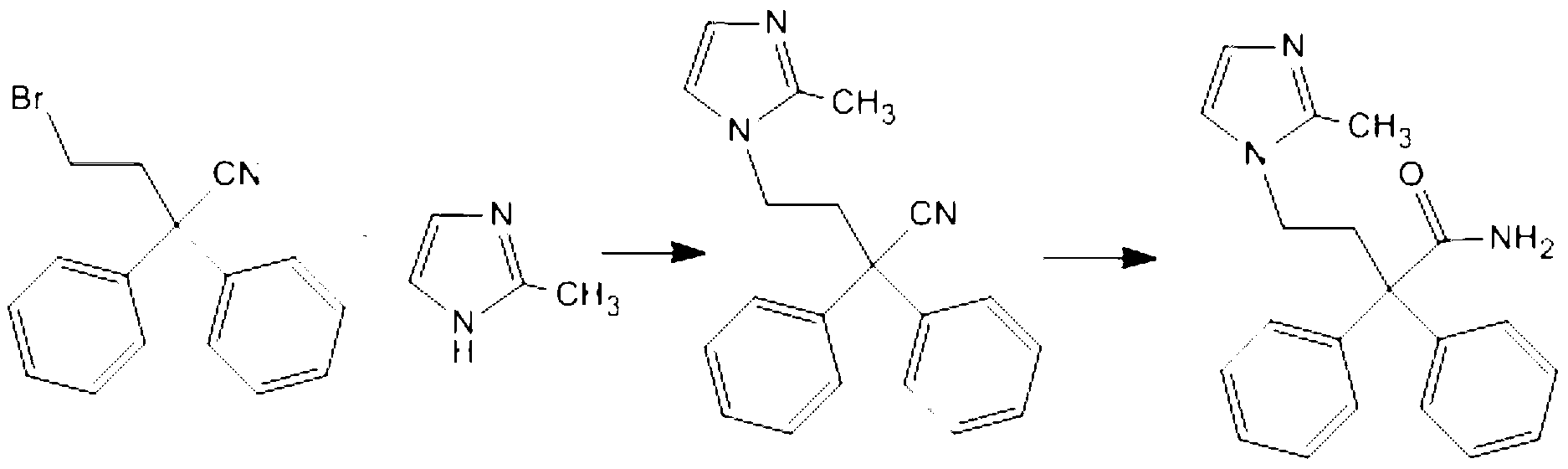
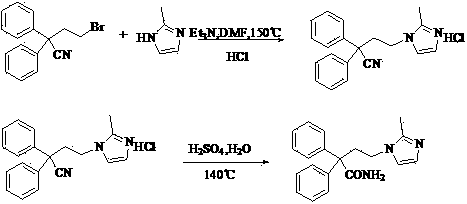
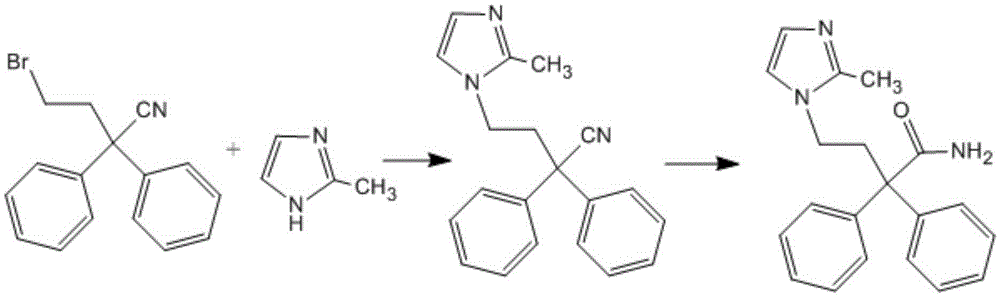
The invention discloses a method for preparing imidafenacin. The method comprises the steps of hydrolyzing 4-bromo-2,2-diphenyl butyronitrile into acid amide under an alkaline condition, and then enabling the acid amide to react with 2-methylimidazole so as to obtain a target product. The preparation method provided by the invention is high in yield, economical, simple, convenient, friendly to human body and environment, and suitable for industrialized large-scale production.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Application of imidafenacin in preparation of drugs for pre-anesthetic medication
The invention discloses application of imidafenacin in preparation of drugs for pre-anesthetic medication. Preclinical pharmacological tests prove that imidafenacin not only can be used for pre-anesthetic medication, but also has effects superior to those of atropine, thus opening up a new path for clinical pre-anesthetic medication.
Owner:张蕊
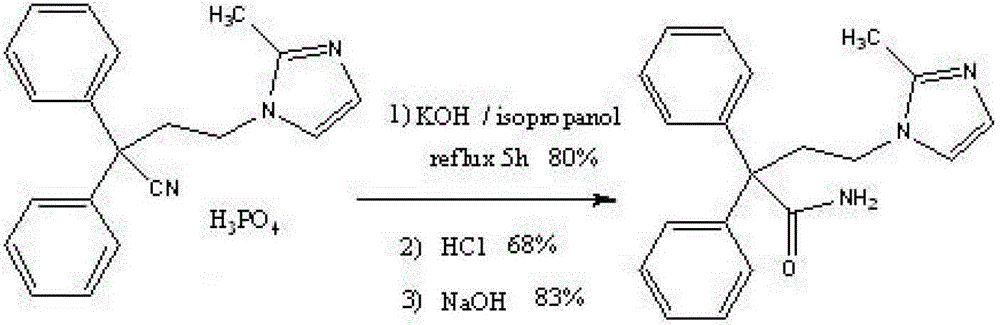
Novel preparation method of imidafenacin intermediate and refined product thereof
The invention belongs to the field of medicine chemistry, and specifically relates to a preparation method of relatively pure imidafenacin ( 4-(2-methyl-1- imidazolyl)-2,2-dibenzyl butyramide) and intermediate prepared by the method. 4-(2-methyl-1- imidazolyl)-2,2-dibenzyl butyronityile hydrolyze in the presence of alkaline to generate product, the product reacts with an inorganic acid or an organic acid to form salt, then the salt is decomposed to get rid of impurities in the product, and finally the product is recrystallized in an organic solvent to remove the residual solvent. The preparation method has the characteristics of simpleness, high yield, and good reproducibility, and is suitable for industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
Preparation technology for imidafenacin
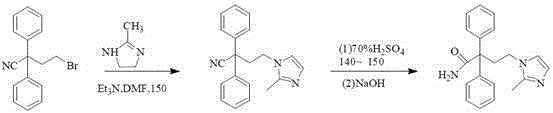
The invention discloses a preparation technology for imidafenacin and belongs to the pharmaceutical chemistry field. The method is as follows: 2-halogenated ethyl diphenylacetonitrile and 2-methyl imidazole are employed as initial raw materials, alcohol compounds are employed as a solvent, polyethylene glycol is employed as a phase-transfer catalyst, replacement and hydrolysis reactions are combined in an alkali metal hydroxide condition, and imidafenacin is prepared through one step. The technology is advantageous in that reaction steps are reduced, dosage of 2-methyl imidazole and the reaction temperature are lowered substantially, the reaction time is shortened, the synthesis yield is raised obviously, and the preparation technology is suitable for industrial production.
Owner:KAIFENG PHARMA GRP +2
A kind of method for preparing midanaxin
The invention discloses a method for preparing imidafenacin. The method comprises the steps of hydrolyzing 4-bromo-2,2-diphenyl butyronitrile into acid amide under an alkaline condition, and then enabling the acid amide to react with 2-methylimidazole so as to obtain a target product. The preparation method provided by the invention is high in yield, economical, simple, convenient, friendly to human body and environment, and suitable for industrialized large-scale production.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
Method for production of orally rapidly disintegrating tablet comprising imidafenacin as active ingredient
InactiveUS20100323090A1Disintegrates quicklyImprove photostabilityOrganic active ingredientsPretreated surfacesCompression moldingImidafenacin
The present invention herein provides an imidafenacin-containing orally rapidly disintegrating tablet which is excellent in the photostability.The present invention comprises the steps of: (A) granulating imidafenacin together with starch to thus give a granulated product having an imidafenacin concentration ranging from 0.001 to 3% by mass and a starch concentration ranging from 40 to 99.999% by mass; (B) covering the granulated product prepared in the step (A) with a non-cellulosic coating agent; and (C) blending the granulated product obtained in the preceding step (B) with an excipient and a disintegrating agent and then forming the resulting mixture into a tablet according to the compression molding technique.
Owner:KYORIN PHARMA CO LTD
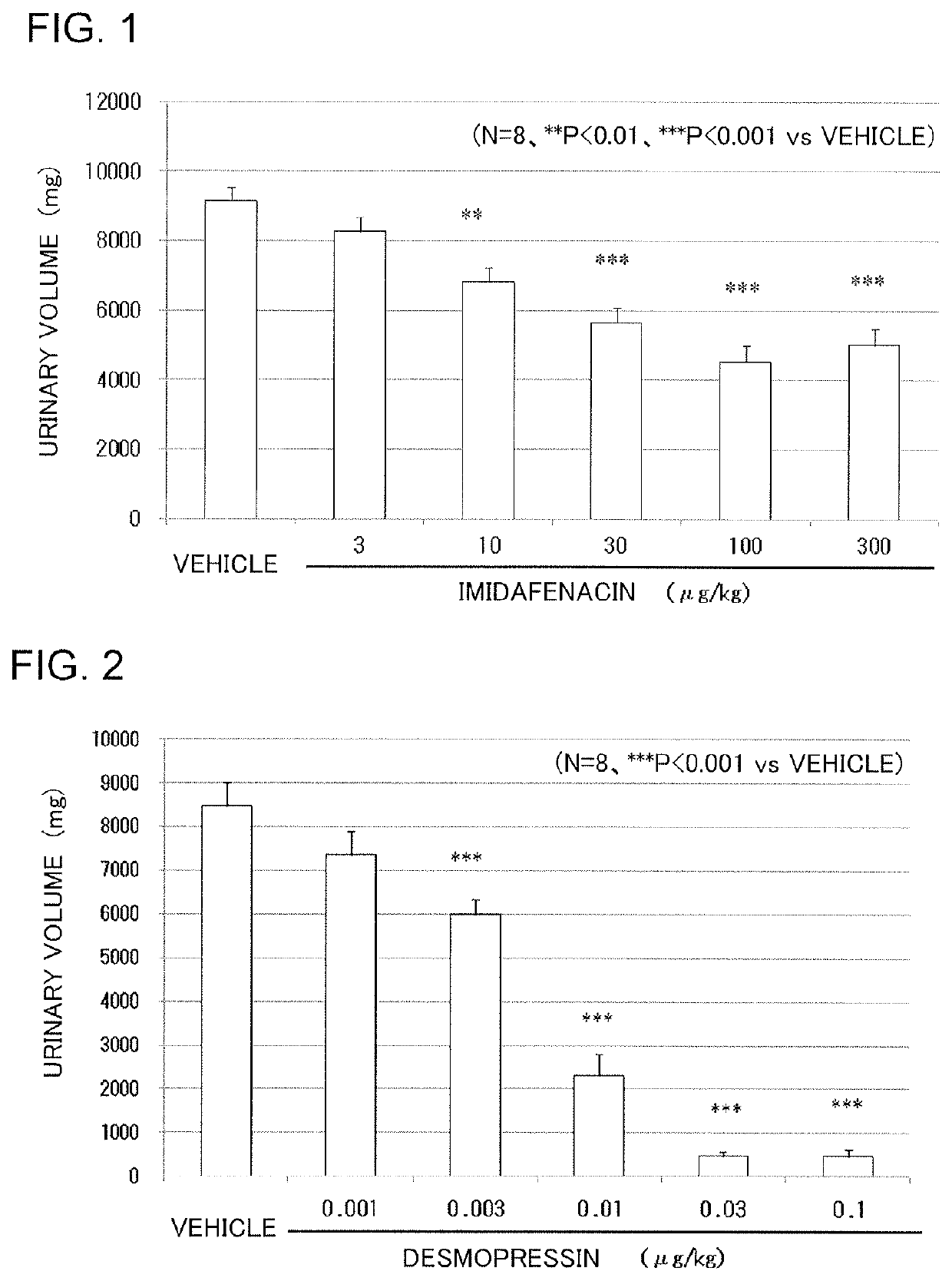
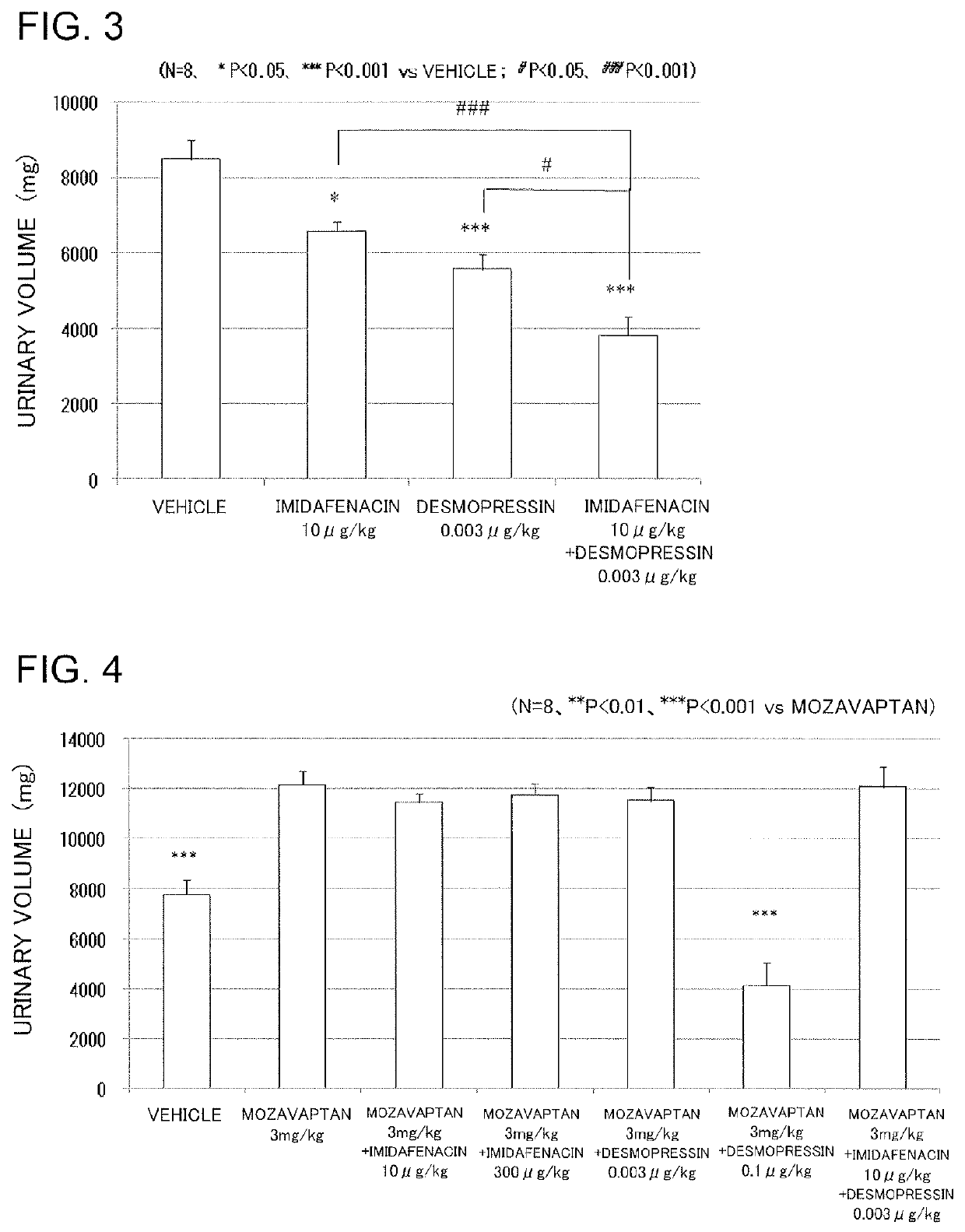
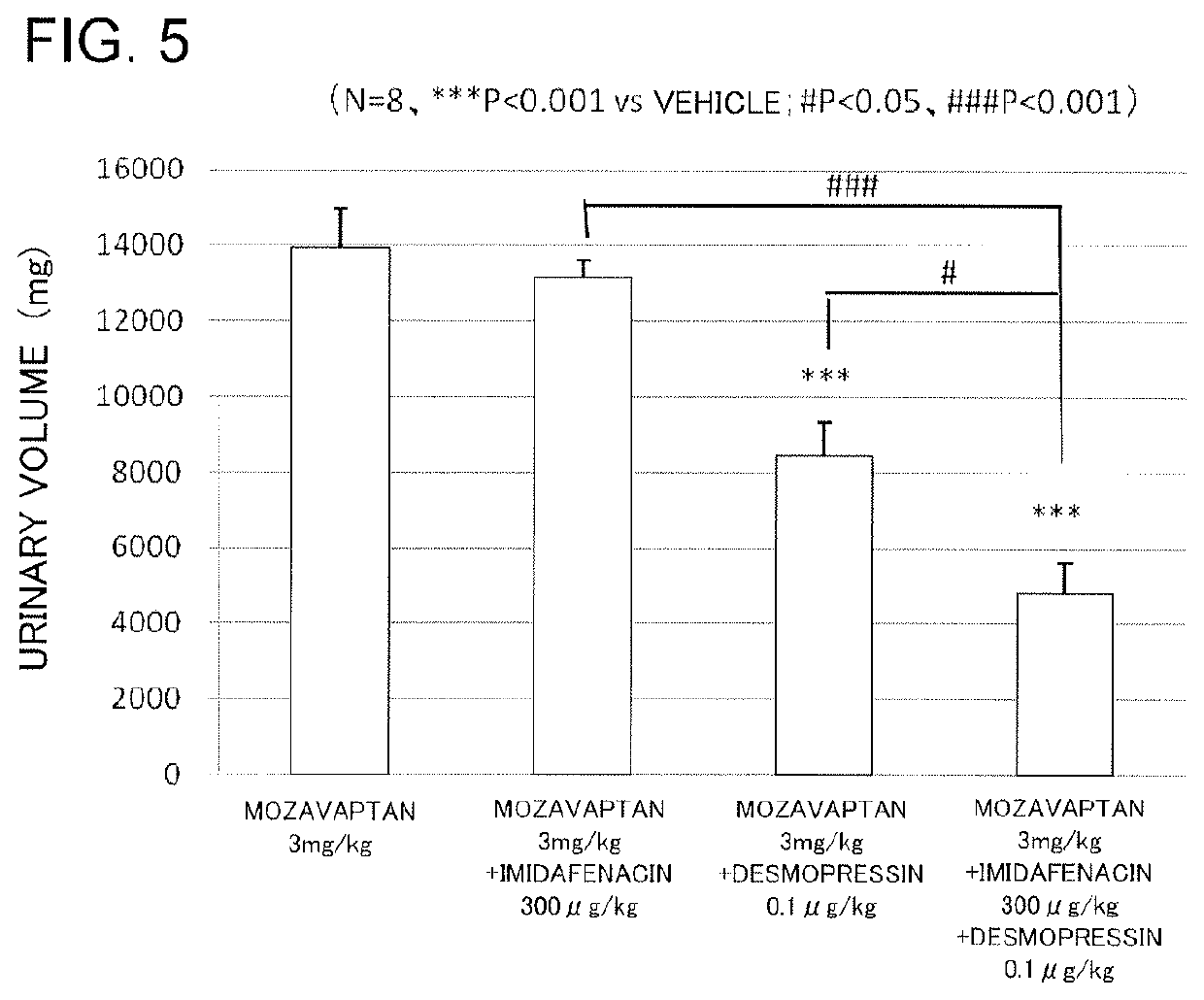
Vasopressin-like action enhancer
ActiveUS10517851B2Improve actionPrevent and treat pollakiuriaOrganic active ingredientsPeptide/protein ingredientsImidafenacinAgonist
A problem to be solved by the present invention is to provide a method for ameliorating pollakiuria and nocturia, in particular, nocturia caused by nocturnal polyuria by finding a composition that enhances the antidiuretic action of vasopressin or a vasopressin V2 receptor agonist. As a result of the studies, it has been found that imidafenacin enhances the antidiuretic action of vasopressin or the vasopressin V2 receptor agonist, whereby the present invention has been completed. According to the present invention, a composition containing imidafenacin can enhance the antidiuretic action of vasopressin or the vasopressin V2 receptor agonist, making it possible to ameliorate pollakiuria and nocturia, in particular, nocturia caused by nocturnal polyuria.
Owner:KYORIN PHARMA CO LTD
Imidafenacin tablet and preparation method thereof
ActiveCN103054822BSignificant progressImprove the disintegration effectOrganic active ingredientsPill deliveryWater basedPolyvinyl alcohol
The invention discloses an imidafenacin tablet which is prepared from the components including imidafenacin, water-soluble polymer, a filling agent, a disintegrating agent and lubricant; the water-soluble polymer is selected from one or more of poloxamer, polyethyleneglycol, hydroxypropyl methylcellulose, polyvinyl alcohol and copovidone and accounts for 0.1 percent to 3 percent (w / w) of the total weight of the whole tablet; and the disintegrating agent is pregelatinized starch or a mixture of common starch and pregelatinized starch and accounts for 15 percent to 50 percent (w / w) of the total weight of the whole tablet. The tablet provided by the invention can be dissolved in a water-based medium at an unexpected high level in relative time and has a very good dissolution characteristic and remarkable physical stability.
Owner:NANJING CORE TECH CO LTD
Methods for determining the content of imidafenacin and detecting related substances
ActiveCN103063795BHigh precisionThe content determination result is accurateComponent separationAdditive ingredientImidafenacin
The present invention discloses methods for determining the content of imidafenacin and detecting related substances. According to the methods, high-performance liquid chromatography is mainly used for determining and detecting the content of active pharmaceutical ingredient imidafenacin, and imidafenacin related substances. The methods are time-saving and labor-saving, high in precision, accurate in content determination results, and good in repeatability and recovery, and the method is validated, and can be used for routine analysis and quality control of imidafenacin materials and preparation samples.
Owner:NANJING CORE TECH CO LTD
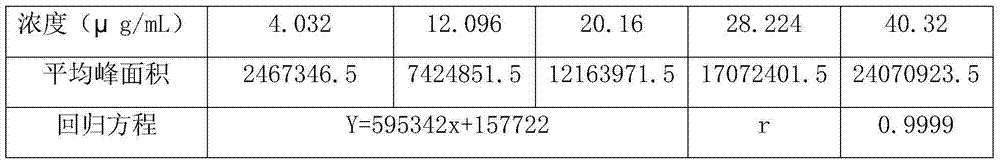
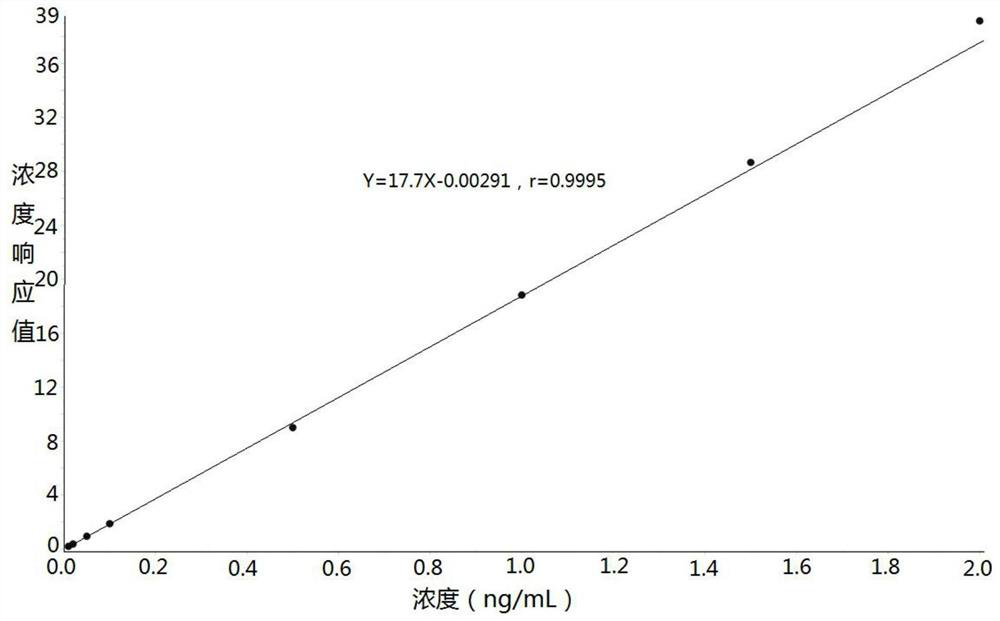
Method for determining concentration of imidafenacin in blood plasma by liquid chromatography-mass spectrometry
InactiveCN112730701AThe pretreatment method is simpleSuitable for routine determinationComponent separationBlood concentrationOrganic solvent
The invention discloses a method for determining the concentration of imidafenacin in blood plasma by liquid chromatography-mass spectrometry. The method adopts a liquid chromatography-mass spectrometry system for determination and comprises the following steps: taking a sample to be detected, adding a certain amount of mixed organic solvent for extraction and pretreatment, separating by using a chromatographic column, and detecting by using a mass spectrum detector. The method is rapid, accurate, high in sensitivity and simple and convenient to operate, and provides a basis for measuring the blood concentration of imidafenacin; the linear range of the plasma standard curve of the method is 0.01-2ng / mL, the intra-batch precision RSD and the inter-batch precision RSD are both smaller than + / -15%, and the method is suitable for measuring the concentration of imidafenacin in blood plasma.
Owner:徐州立顺康达医药科技有限公司
A kind of method for preparing midanaxin
The present invention discloses a preparation method of imidafenacin. In the method, 4-chloro-2, 2-diphenylbutaneamide and 2-methylimidazole are taken as raw materials for reaction in the presence of alkali, and then ethyl acetate is used for recrystallization. The method has advantages of high yield, mild reaction condition, and simple purification and is suitable for industrialized production.
Owner:陕西步长高新制药有限公司
A kind of method for separating midanacin and related substances by high performance liquid chromatography
ActiveCN104614468BEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilanes
The invention belongs to the field of analytical chemistry and discloses a method for separating and determining imidafenacin and related substances thereof by using liquid chromatography. The method can be used for quantitatively determining the contents of imidafenacin and related substances thereof by using phenyl silane bonded silica gel as a chromatographic column of fillers and a certain proportion of buffer salt solutions-organic phases as mobile phases, thus effectively controlling the quality of imidafenacin. The method has strong specificity and high precision and is simple and convenient to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com