Orally rapidly disintegrating tablet comprising imidafenacin
一种迅速崩解、口腔内的技术,应用在含有效成分的医用配制品、有机活性成分、非有效成分的医用配制品等方向,达到给药容易、崩解性优异、光稳定性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
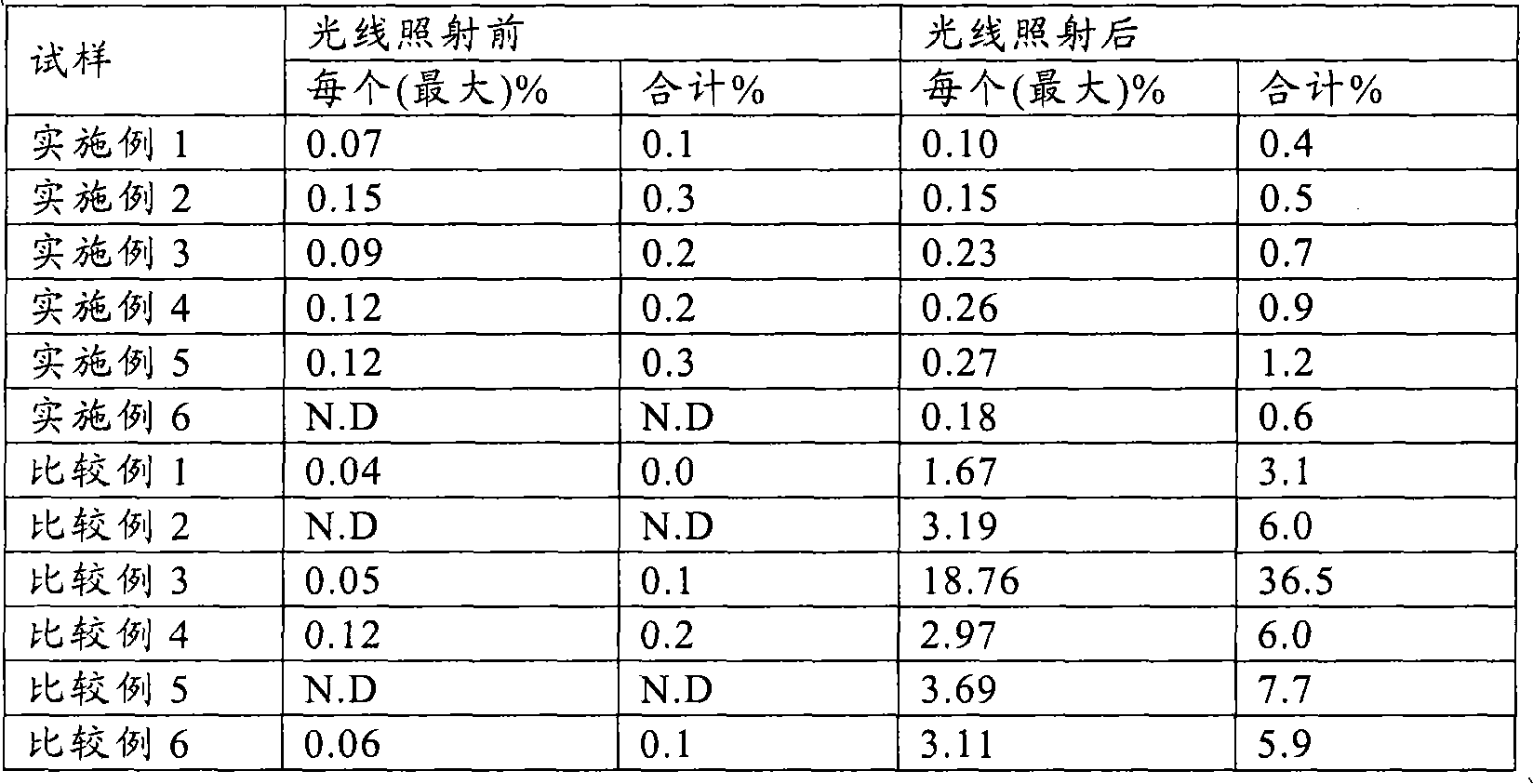
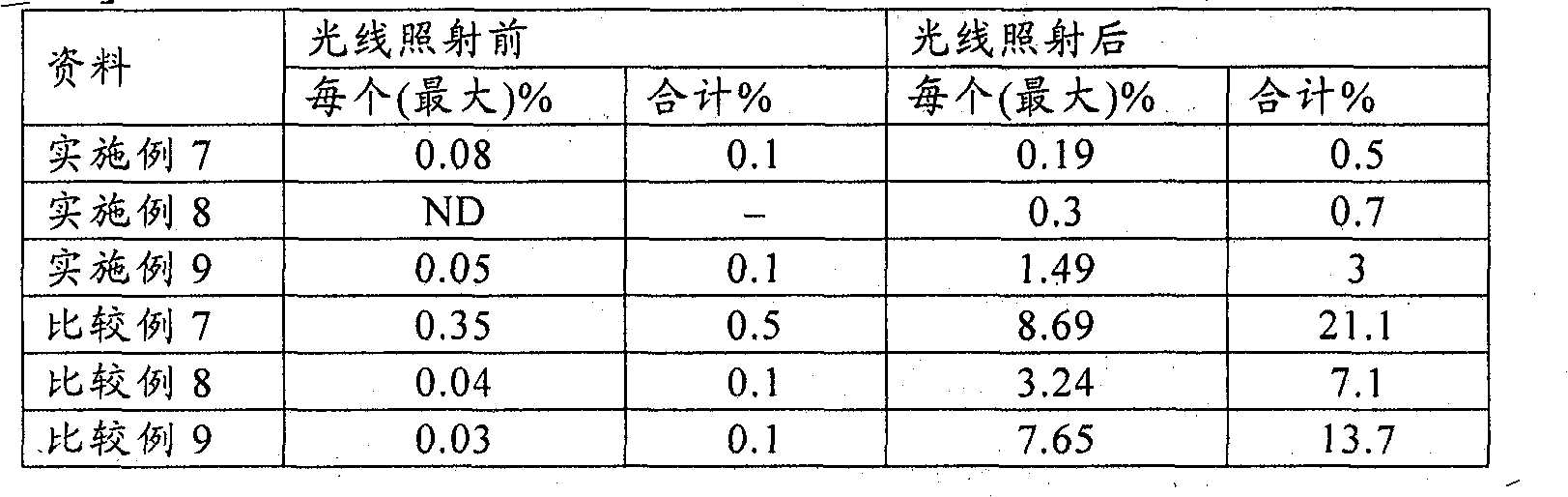
Examples
preparation example Construction
[0039] In the preparation of the orally rapidly disintegrating tablet of the present invention, the coated granules containing midanacin or midanacin granules are mixed with a composition containing an excipient and a disintegrating agent. The amount of the composition containing the excipient and the disintegrating agent is, for example, 1 to 50 parts by mass, preferably 3 to 25 parts by mass, relative to 1 part by mass of the coated granulated product containing midanacine or midanacin granules. parts by mass.
[0040] The coated granules or midancin granules containing excipients and disintegrants, for example, can be prepared in a V-shaped mixer, a diamond mixer (diamond mixer), a drum Mixing in a mixer or the like.
[0041] Then, the resulting mixture is compression-molded to prepare the orally rapidly disintegrating tablet of the present invention.
[0042]Compression molding can be performed suitably using a tablet press such as a rotary tablet press, for example. Ho...
Embodiment 1
[0068] 4.0 g of midanacin and 2 g of Kollidon 90F (BASF) were dissolved in a mixed solution of 118.2 g of pure water and 275.8 g of ethanol. 394 g of Starch 1500G (Japanese カラコン) was loaded into a rotary fluidized bed granulator (manufactured by Dalton, NQ-160), and the solution was coated by top spray method (spray liquid volume 15g / min, spray pressure 0.1MPa, air supply temperature 70° C.) to obtain granules containing midanacin.
[0069] Separately, 60 g of a stomach-soluble polymer, EUDRAGIT EPO (Rohm GmbH & Co. KG), and 30 g of magnesium stearate (vegetable) (Taihei Chemical Industry) were dissolved in a mixed solution of 273 g of pure water and 637 g of ethanol.
[0070] 300 g of granulated matter containing midanacin was charged into a rotary fluidized bed granulator (manufactured by Dalton, NQ-160), and the solution was applied by the top spray method (spray liquid volume 20 g / min, spray pressure 0.15 MPa, Air supply temperature 80°C) to obtain coated particles.
[0...
Embodiment 2
[0073] 25.0 g of midanacin and 12.5 g of Kollidon 90F (BASF) were dissolved in a mixed solution of 738.75 g of pure water and 1723.75 g of ethanol. Starch 1500G (Japanese カラコン) 4962.5g is packed into a fluidized bed granulator (manufactured by FLOINT INDUSTRY, FL-5), and the solution is coated by the top spray method (spray liquid amount 100g / min, spray pressure 0.3MPa, air supply temperature 70° C.) to obtain midanacin-containing granules (average particle diameter: 128 μm). Separately, 750 g of EUDRAGITEPO (Rohm GmbH & Co. KG) and 375 g of magnesium stearate (vegetable) (Taihei Chemical Industry) were dissolved in a mixed solution of 3412.5 g of pure water and 7962.5 g of ethanol. 3750 g of the granulated matter containing midanacin was charged into a fluidized bed granulator (manufactured by Floint Industries, FL-5), and the solution was coated by the top spray method (spray liquid volume 100 g / min, spray pressure 0.3 MPa, Supply air temperature: 80° C.) to obtain coated p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com