Method for detecting imidafenacin in human plasma through HPLC-MS/MS combination
A midanacin and human detection technology, applied in the field of biological analysis, can solve the problems of unsuitable biological sample detection, unreachable blood concentration of midanacin, long analysis time, etc., and achieves low quantitative lower limit and accuracy. good, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
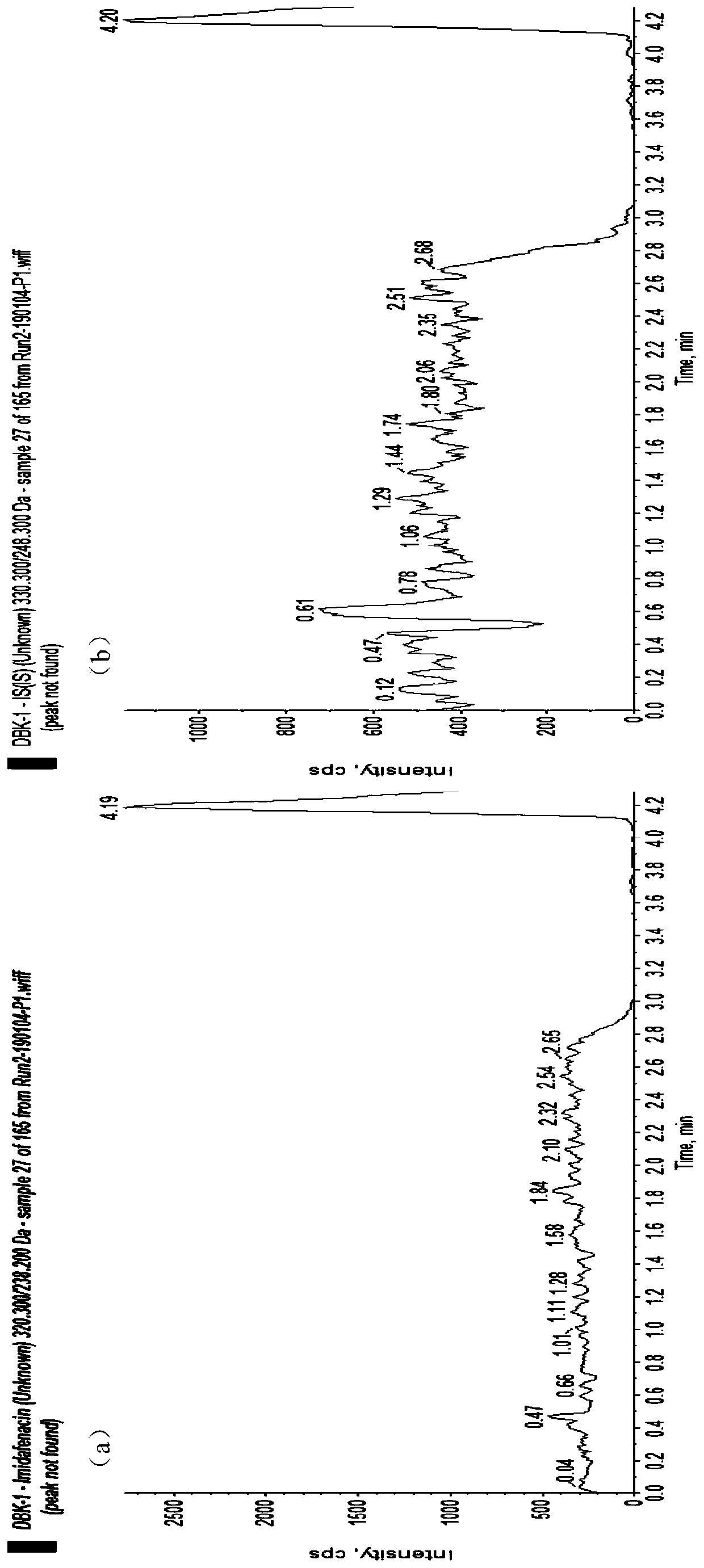
[0044] The detection method of the present invention is further illustrated by the following examples, but these examples do not constitute any limitation to the present invention.
[0045] Materials and Methods
[0046] 1. Instruments and reagents
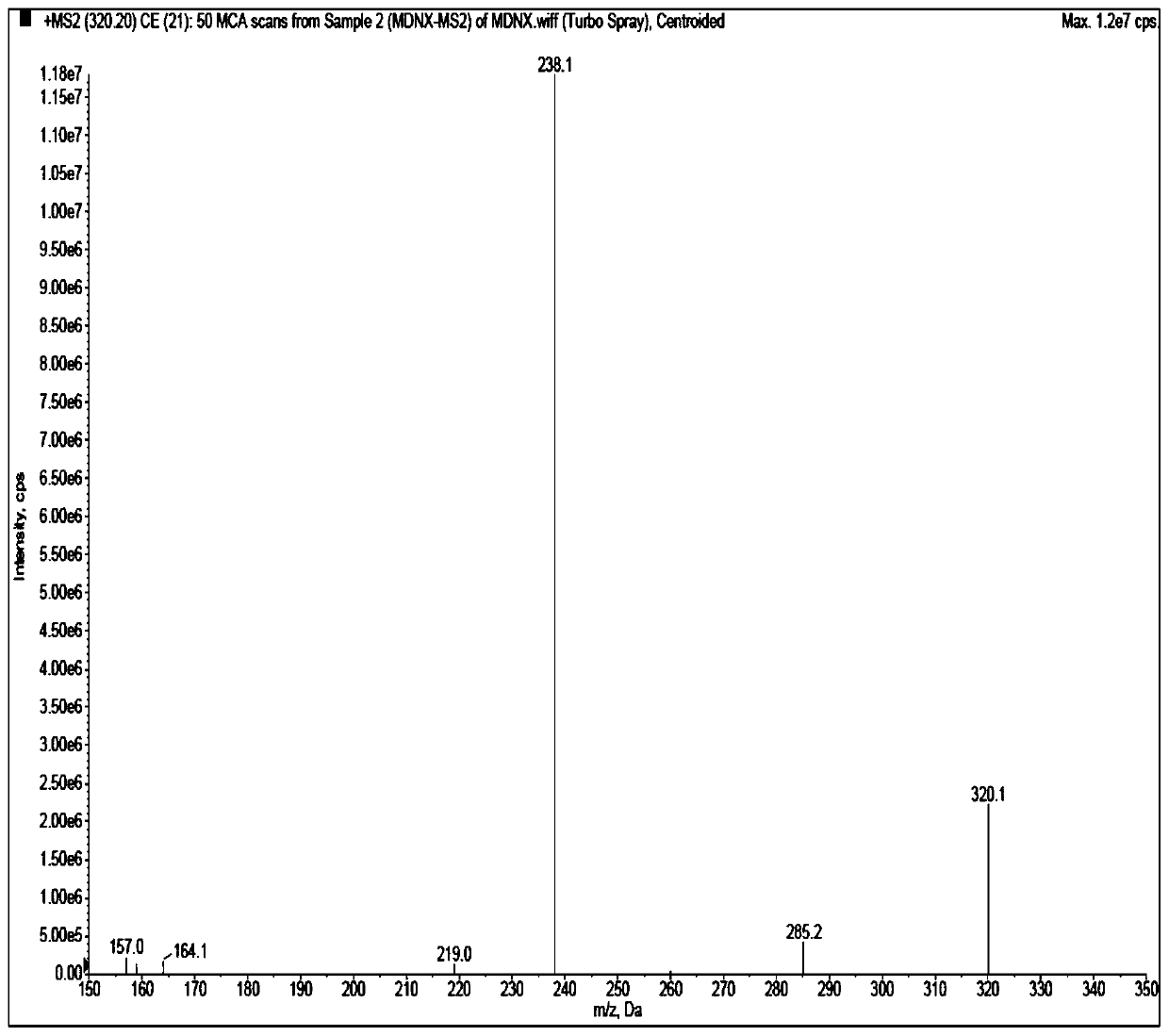
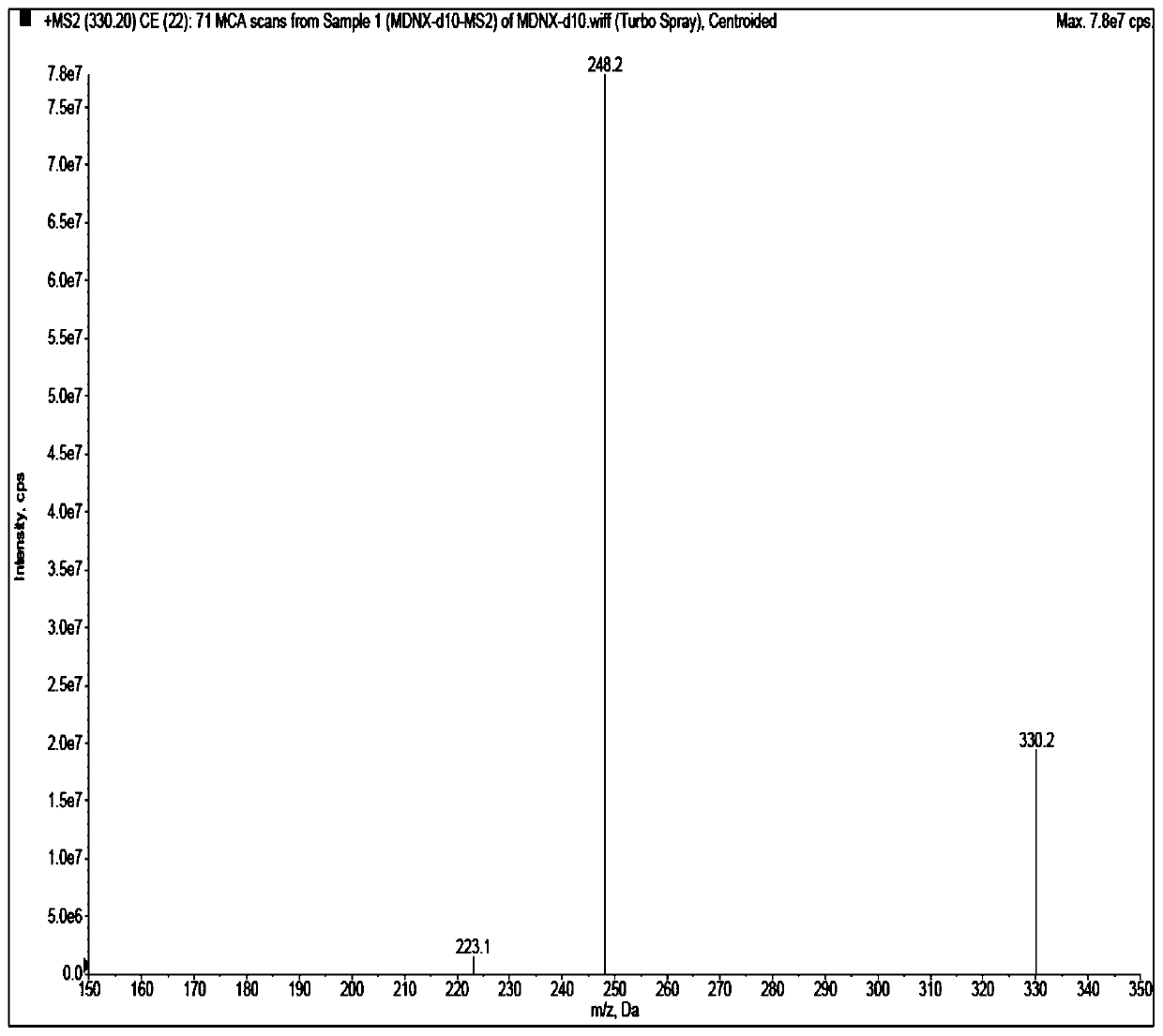
[0047] High-phase liquid chromatography (Shimadzu LC-30AD series); mass spectrometry (API 5500, Applied Biosystems / Sciex); pure water meter (MilliDirectQ, Millipore); microbalance (XP6, METTLER TOLEDO); centrifuge (HeraeusMuitifuge X1R, ThermoFisher) ; Oscillator (LPD2500, LE PARD); Nitrogen blowing instrument (Organomation Microvap 11803; NG150-1A, Lept Scientific Instrument Co., Ltd.).
[0048] Acetonitrile (Merck, HPLC grade), water (ultrapure water, laboratory-made), acetic acid (Aladdin, HPLC grade), ammonium acetate (Aladdin, HPLC grade), isopropanol (Anhui Shilian Special Solvent Co., Ltd., HPLC grade), methanol (Merck, HPLC grade). Blank plasma was obtained from healthy subjects. Midanacin (TLC, Lot No.: 1418-068A1), M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com