Novel chiral chromatographic column fixed phase and preparation method thereof
A chiral stationary phase, chiral technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problem of serious tailing of eluted components, easy loss of coated stationary phase, and poor stability of stationary phase. and other problems, to achieve the effect of simple and easy method, low cost and good binding force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
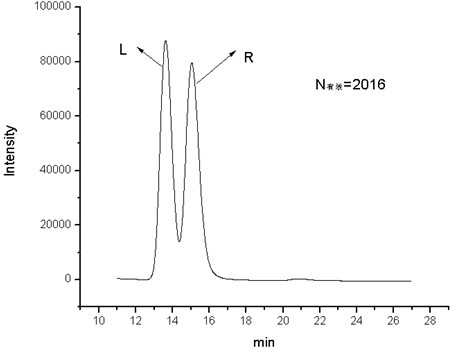
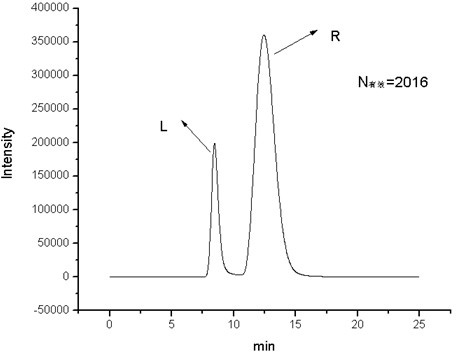
Image
Examples
Embodiment 1
[0028] 1. Pretreatment of the carrier: Take 1.0g of silica gel balls, put them into a round bottom flask, then add an appropriate amount of concentrated hydrochloric acid, heat to reflux for 2 hours, cool down, filter, wash the solid with water to pH=7, rinse the solid with acetone and ether in turn, and drain it spare.
[0029] 2. L-ValCONH (CH 2 ) 17 CH 3 synthesis:
[0030] 1) Synthesis of Z-L-ValCOOH
[0031] After reacting 0.5 mol of L-ValCOOH with 0.5 mol of benzyloxychloride and 0.5 mol of NaOH in an ice-water bath for 12 hours, Z-L-ValCOOH was obtained after extraction, acidification, dehydration, recrystallization and drying.
[0032] 2) Z-L-ValCONH (CH 2 ) 17 CH 3 Synthesis
[0033] Weigh 0.2 mol of Z-L-ValCOOH in a three-necked flask, add 500 mL of ethyl acetate, heat and stir to dissolve. Add 0.2 mol of DCC, stir for 1 hour, add 0.2 mol of triethylamine, weigh 0.2 mol of octadecylamine and dissolve it in 100 ml of chloroform, add it to the mixture, and sti...
Embodiment 2
[0040]1. Pretreatment of the carrier: Take 1.0g of silica gel balls, put them into a round bottom flask, then add an appropriate amount of concentrated hydrochloric acid, heat to reflux for 2 hours, cool down, filter, wash the solid with water to pH=7, rinse the solid with acetone and ether in turn, and drain it spare.
[0041] 2. D-ValCONH (CH 2 ) 17 CH 3 synthesis
[0042] 1) Synthesis of Z-D-ValCOOH
[0043] React 0.5 mol of D-ValCOOH with 0.5 mol of benzyloxychloride and 0.5 mol of NaOH in an ice-water bath for 12 hours, then extract, acidify, remove water, recrystallize, and dry to obtain Z-D-ValCOOH.
[0044] 2) Z-D-ValCONH (CH 2 ) 17 CH 3 Synthesis
[0045] Weigh 0.2 mol of Z-D-ValCOOH in a three-necked flask, heat and stir 500 mL of ethyl acetate to dissolve. Add 0.2 mol of DCC, stir for 1 hour, add 0.2 mol of triethylamine, weigh 0.2 mol of octadecylamine and dissolve it in 100 ml of chloroform, add it to the mixture, and stir in an ice bath for 2 hours. Sti...
Embodiment 3
[0052] 1. Pretreatment of the carrier: Take 1.0g of silica gel balls, put them into a round-bottomed flask, then add an appropriate amount of concentrated hydrochloric acid, heat to reflux for 2 hours, cool down, filter, wash the solid with water to pH=7, wash the solid with acetone and ether in turn, and then pump Dry.
[0053] 2. L-PheCONH (CH 2 ) 17 CH 3 synthesis
[0054] 1) Synthesis of Z-L-PheCOOH
[0055] After reacting 0.5 mol of L-PheCOOH with 0.5 mol of benzyloxychloride and 0.5 mol of NaOH in an ice-water bath for 12 hours, Z-L-PheCOOH was obtained after extraction, acidification, dehydration, recrystallization, and drying.
[0056] 2) Z-L-PheCONH (CH 2 ) 17 CH 3 Synthesis
[0057] Weigh 0.2 mol of Z-D-PheCOOH in a three-necked flask, heat and stir 500 mL of ethyl acetate to dissolve. Add 0.2 mol of DCC, stir for 1 hour, then add 0.2 mol of triethylamine, weigh 0.2 mol of octadecylamine and dissolve in 100 ml of chloroform, add to the mixture, and stir in i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com