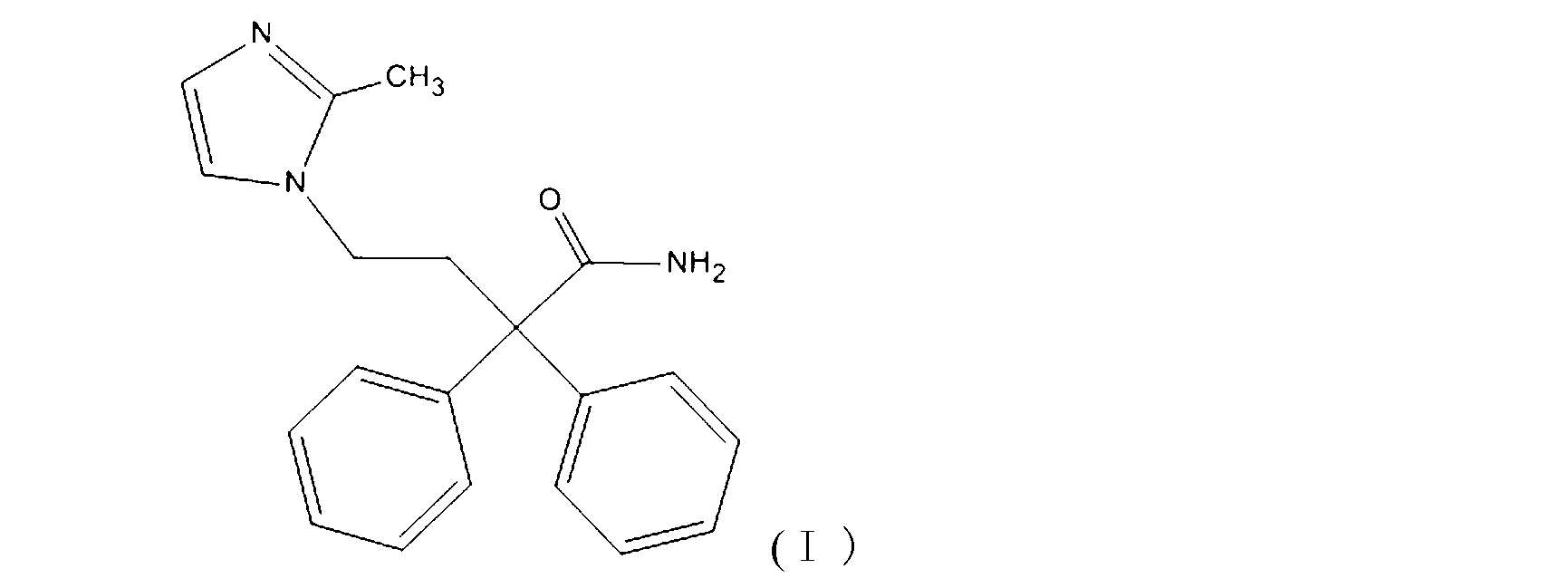
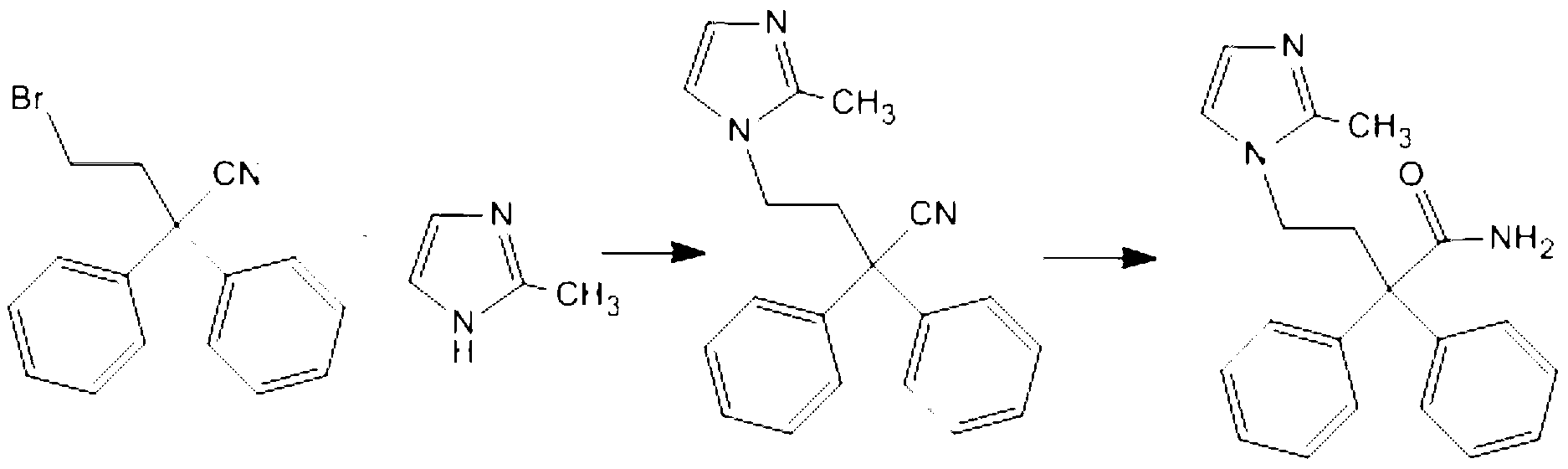
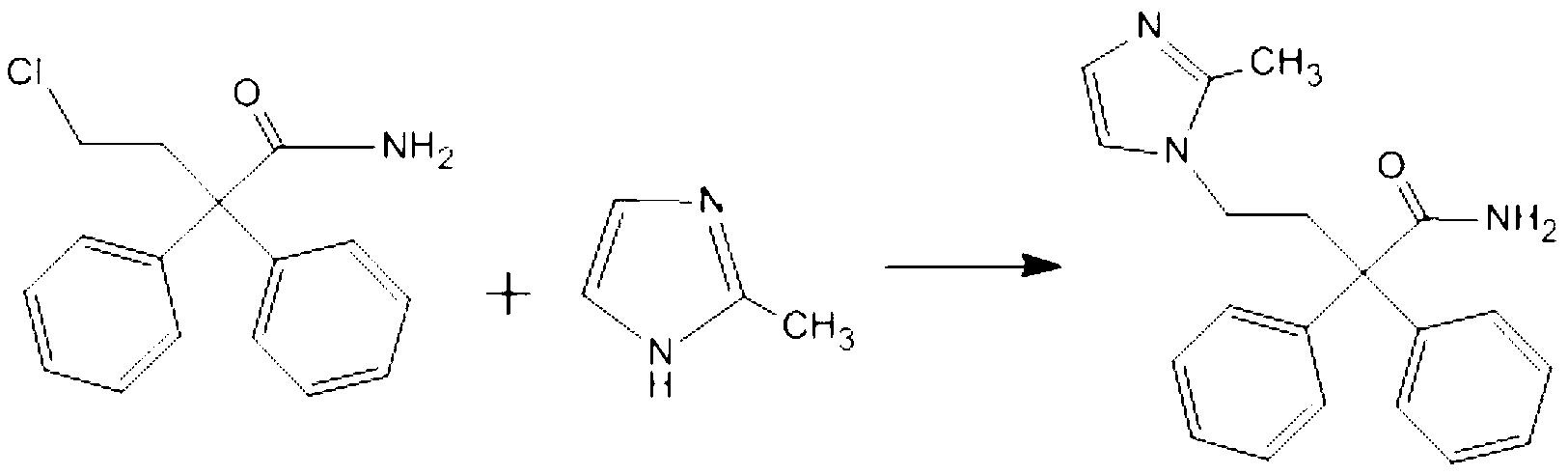
Preparation method of imidafenacin
A technology of midanacin and methylimidazole, applied in the field of medicine, can solve problems such as being unsuitable for industrialized production, having high requirements on production equipment, and failing to solve problems, and achieves the effects of short steps, improved yield, and favorable production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 In a 100ml three-necked flask, add 30ml of DMF, 2.73g (10mmol) of 4-chloro-2,2-diphenylbutanamide, and 1.23g (15mmol) of 2-methylimidazole, heat and stir, and when the reaction temperature rises to At 40°C, 3ml of 5mol / L NaOH solution was slowly added dropwise, and the reaction temperature was controlled not to exceed 45°C. After the dropwise addition was completed, the temperature was raised to 50°C. The reaction was monitored by TLC, and the reaction was completed after stirring for 1 hour. In the separatory funnel, add 30ml CH to the separatory funnel 2 Cl 2 and 30 ml of water, separate the organic phase and the aqueous phase, wash the camera twice with water, evaporate the organic phase to dryness under reduced pressure, and recrystallize the solid obtained by evaporation to dryness under reduced pressure with ethyl acetate to obtain 2.49 g of a white powdery solid. rate of 78%.
[0032] MS(ESI + , m / e): 320.17[M+H] +
[0033] 1 H-NMR (400MHz, CDCl ...
Embodiment 2
[0034] Example 2 In a 100ml three-necked bottle, add CH 2 Cl 2 30ml, 2.73g (10mmol) of 4-chloro-2,2-diphenylbutanamide, 1.23g (15mmol) of 2-methylimidazole, heat and stir, when the reaction temperature rises to 40℃, slowly add 5mol / L dropwise NaOH solution 3ml, control the reaction temperature not to exceed 45 ℃, after the dropwise addition is completed, the temperature is raised to 50 ℃, monitor the reaction with TLC, stir for 1.5 hours after the reaction is completed, pour the reaction solution into a separatory funnel, and then put it in the separatory funnel Add 30ml of water to it, separate the organic phase and the aqueous phase, and use CH for the aqueous phase. 2 Cl 2 Extract twice, combine the organic phases, evaporate the organic phase to dryness under reduced pressure, and recrystallize the solid obtained by evaporation under reduced pressure with ethyl acetate to obtain 2.39 g of a white powdery solid with a yield of 75%.
Embodiment 3
[0035] Example 3 In a 100ml three-necked bottle, add CHCl 3 30ml, 2.73g (10mmol) of 4-chloro-2,2-diphenylbutanamide, 1.23g (15mmol) of 2-methylimidazole, heat and stir, when the reaction temperature rises to 40℃, slowly add 5mol / L dropwise NaOH solution 3ml, control the reaction temperature not to exceed 45 ℃, after the dropwise addition is completed, the temperature is raised to 50 ℃, monitor the reaction with TLC, stir for 1.5 hours after the reaction is completed, pour the reaction solution into a separatory funnel, and then put it in the separatory funnel Add 30ml of water to it, separate the organic phase and the aqueous phase, and use CH for the aqueous phase. 2 Cl 2 Extracted twice more, combined cameras, evaporated the organic phase to dryness under reduced pressure, and recrystallized the solid obtained by evaporation under reduced pressure with ethyl acetate to obtain 2.32 g of a white powdery solid with a yield of 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com