Method for separating imidafenacin and related substances thereof by using high performance liquid chromatography
A technology of new midanna and related substances, applied in the field of analytical chemistry, can solve problems such as incomplete removal of intermediates, affecting the purity and quality of drugs, and achieve quality controllable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Instruments and Conditions
[0041] High performance liquid chromatography: Shimadzu: LC-20AT, CBM-20A, SIL-20AC, SPD-M20A, CTO-10ASvp;
[0042] Column: C 18 (ES, 250×4.6mm, 5μm)
[0043] Mobile phase A: 0.02mol / L dipotassium hydrogen phosphate (pH 7.0)
[0044] B: Acetonitrile
[0045] Elute with a concentration gradient:
[0046] T(min) 0 25 40 50 60 75 76 85 B% 30 30 60 60 70 70 30 30
[0047] Flow rate: 1.0mL / min
[0048] Detection wavelength: 210nm
[0049] Column temperature: 30°C
[0050] Injection volume: 10μL
[0051] Experimental steps:
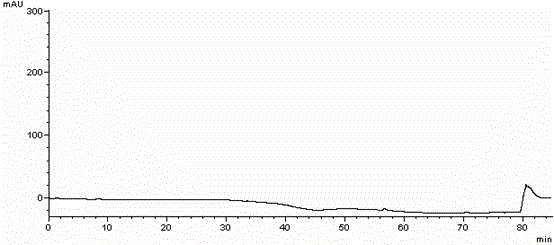
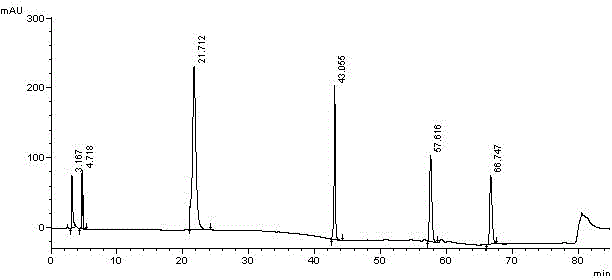
[0052] Take an appropriate amount of midanacin and its related substances, dissolve the samples in acetonitrile respectively, and prepare a sample solution containing about 0.5 mg / mL of midanacin and its related substances; another appropriate amount of acetonitrile is used as a blank solvent. Perform HPLC analysis according to the above conditions, and record the chromatograms. se...
Embodiment 2
[0054] Instruments and Conditions
[0055] High performance liquid chromatography: Shimadzu: LC-20AT, CBM-20A, SIL-20AC, SPD-M20A, CTO-10ASvp;
[0056] Column: C 18 (ES, 250×4.6mm, 5μm)
[0057] Mobile phase A: 0.02mol / L dipotassium hydrogen phosphate (pH 7.0)
[0058] B: Acetonitrile
[0059] Elute with a concentration gradient:
[0060] T(min) 0 5 55 65 75 B% 30 30 75 30 30
[0061] Flow rate: 1.0mL / min
[0062]Detection wavelength: 210nm
[0063] Column temperature: 30°C
[0064] Injection volume: 10μL
[0065] Experimental steps:
[0066] Take an appropriate amount of midanacin and its related substances, dissolve the samples in acetonitrile respectively, and prepare a sample solution containing about 0.5 mg / mL of midanacin and its related substances; another appropriate amount of acetonitrile is used as a blank solvent. Perform HPLC analysis according to the above conditions, and record the chromatograms. see attached results Figur...
Embodiment 3
[0068] Instruments and Conditions
[0069] Chromatographic column: Phenyl (Ultimate, 250×4.6mm, 5μm)
[0070] Mobile phase A: 0.02mol / L dipotassium hydrogen phosphate (pH 7.0)
[0071] B: Acetonitrile
[0072] Elute with a concentration gradient:
[0073] T (min) 0 5 55 56 65 B(%) 30 30 75 30 30
[0074] Detection wavelength: 210nm
[0075] Column temperature: 30°C
[0076] Injection volume: 10μL
[0077] Experimental steps:
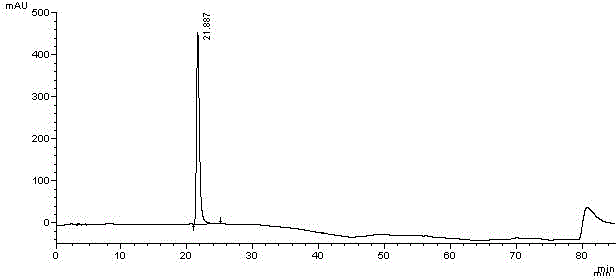
[0078] Take an appropriate amount of midanacin and its related substances, dissolve the samples in acetonitrile respectively, and prepare a sample solution containing about 0.5 mg / mL of midanacin and its related substances; another appropriate amount of acetonitrile is used as a blank solvent. Perform HPLC analysis according to the above conditions, and record the chromatograms. see attached results Figure 6~7 , Image 6 The chromatographic peak with a retention time of 20.805min is midanacine, and the remaining chro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Flow | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com