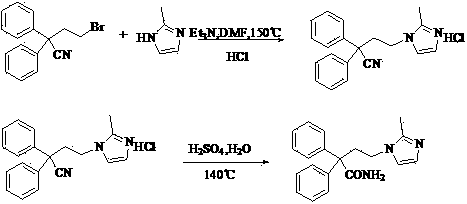
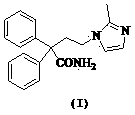
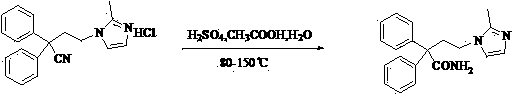Novel preparation method of imidafenacin intermediate and refined product thereof
A technology for midacin and intermediates, which is applied in the field of preparing relatively pure midacin-2,2-diphenylbutyramide, which can solve the problem of low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 4-(2-Methyl-1-imidazolyl)-2,2-diphenylbutanamide hydrobromide. Put 16.5 g (52 mmol) of crude 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyramide into 100 mL of isopropanol, and add 8.0 mL of hydrobromic acid and isopropanol while stirring Alcohol mixed solution (volume ratio 1:1), the solid gradually dissolves and becomes a nearly colorless transparent liquid. After keeping the reaction under stirring for half an hour, 100 mL of ethyl acetate was added to the reaction solution, stirred at room temperature for 1 hour, and a solid precipitated out. Suction filtration, and rinse the filter cake with an appropriate amount of ethyl acetate. The solid was collected and dried in a blast oven at 40°C to constant weight to obtain 19.5 g of off-white 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyramide hydrobromide, yield 98.9 %. M.p. 228.4-229.0°C. MS (m / z): 320[M+1] + . 1 H-NMR (DMSO- d 6 , 400 MHz) δ: 2.25 (3H, s), 2.73-2.74 (2H, m), 3.68-3.91 (2H, m), 6.81 (1H, s), 7.28-7.35...
Embodiment 2
[0026] 4-(2-Methyl-1-imidazolyl)-2,2-diphenylbutanamide. 19.5 g (0.051 mmol) of 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutanamide acetate obtained in Example 1 was dissolved in 900 mL of water. The aqueous solution was extracted with 900 mL of ether, and the inorganic layer was collected. Add 200 mL of ethanol to the obtained aqueous solution, and add 2 mol / L of KOH aqueous solution to the system while stirring, and a solid precipitates out. After stirring for 1 h, filter with suction. The filter cake was rinsed with 40% ethanol solution and rinsed with water several times. Collect the filter cake and dry it in a blast oven at 40°C to constant weight to obtain 14.8 g of off-white 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyramide with a yield of 91.0% ( Two-step total yield 90%). M.p. 192.3-193.0°C (CN101076521A 191-193°C). MS(m / z): 320[M+1] + . 1 H-NMR (DMSO- d 6 , 400 MHz) δ: 2.11 (3H, s), 2.69-2.73 (2H, m), 3.61-3.65 (2H, m), 6.75 (1H, d, J=1.0 MHz), 7.01 (1H, br s) ...
Embodiment 3
[0028] 4-(2-Methyl-1-imidazolyl)-2,2-diphenylbutanamide. Add 116 mL of ethyl acetate to 14.5 g (0.045 mmol) of the 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutyramide obtained in Example 2, slowly heat to reflux, and keep Reflux for 30 min, cool to room temperature and stir for crystallization for 5 h. Suction filtration, rinse the filter cake with a small amount of ethanol, collect the filter cake and dry it in a blast drying oven at 40°C to constant weight to obtain 13.4 g of off-white 4-(2-methyl-1-imidazolyl)-2,2-di The refined product of phenylbutyramide has a yield of 92.4% (the total yield of three steps is 83.1%). M.p.192.5-193.0℃
[0029] (CN101076521A 191-193°C). MS(m / z): 320[M+1] + . 1 H-NMR (DMSO- d 6 , 400 MHz) δ: 2.11 (3H, s), 2.69-2.73 (2H, m), 3.61-3.65 (2H, m), 6.75 (1H, d, J=1.0 MHz), 7.01 (1H, br s) , 7.04 (1H, d, J=1.0 MHz), 7.34-7.49 (11H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com