Preparation technology for imidafenacin
A midanacin and preparation process technology, applied in the field of medicinal chemistry, can solve the problems of raising the reaction temperature, losing the reactivity, reducing the nucleophilic activity, etc., and achieves the effects of lowering the reaction temperature, improving the reactivity and shortening the reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
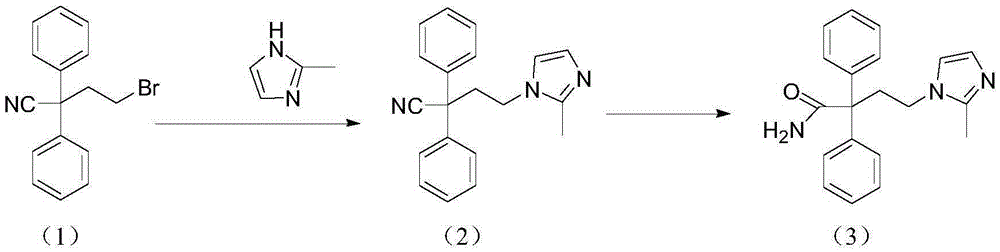
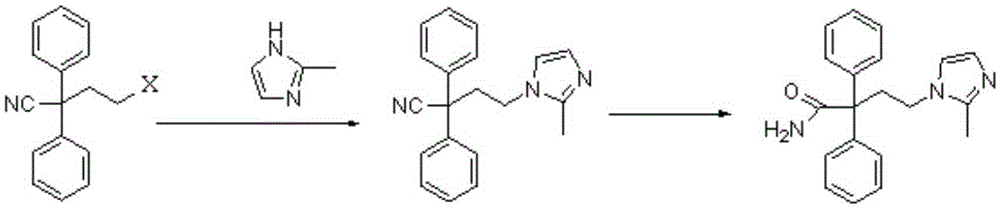
[0015] Mix 160g (4mol) sodium hydroxide with 1200ml propanol, add 300g (1mol) 2-bromoethyldiphenylacetonitrile and 96g (1.2mol) 2-methimazole, and 12g polyethylene glycol 200 at room temperature, Then the reaction was stirred at 20-30°C to obtain 4-(2-methyl-1-imidazolyl)-2-2-diphenylbutyronitrile; then, the temperature was raised to 70-75°C and the reaction was continued with stirring. After the reaction is completed, lower the temperature to room temperature, neutralize the pH value of the reaction system with hydrochloric acid to 8-9, crystallize at room temperature, filter, wash, and dry to obtain the crude product of midanacine. The combined yield of the two steps of substitution and hydrolysis reaches more than 78%. Crystallization, the purity can reach more than 99.5%, which meets the requirements of product quality standards.
Embodiment 2
[0017] Mix 448g (8mol) of potassium hydroxide with 1533ml of isopropanol, add 255.5g (1mol) of 2-chloroethyldiphenylacetonitrile and 128g (1.6mol) of 2-methimazole, and 25.6g of polyethylene glycol at room temperature Alcohol 800, then stirred and reacted at 20-30°C to obtain 4-(2-methyl-1-imidazolyl)-2-2-diphenylbutyronitrile; then, the temperature was raised to 75-85°C, and the stirred reaction was continued. After the reaction is completed, lower it to room temperature, neutralize the reaction system with hydrochloric acid, bring the pH value to 8-9, crystallize at room temperature, filter, wash, and dry to obtain the crude product of midanacine. The combined yield of the two steps of substitution and hydrolysis reaches more than 78%. Recrystallized, the purity can reach more than 99.5%, which meets the requirements of product quality standards.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com