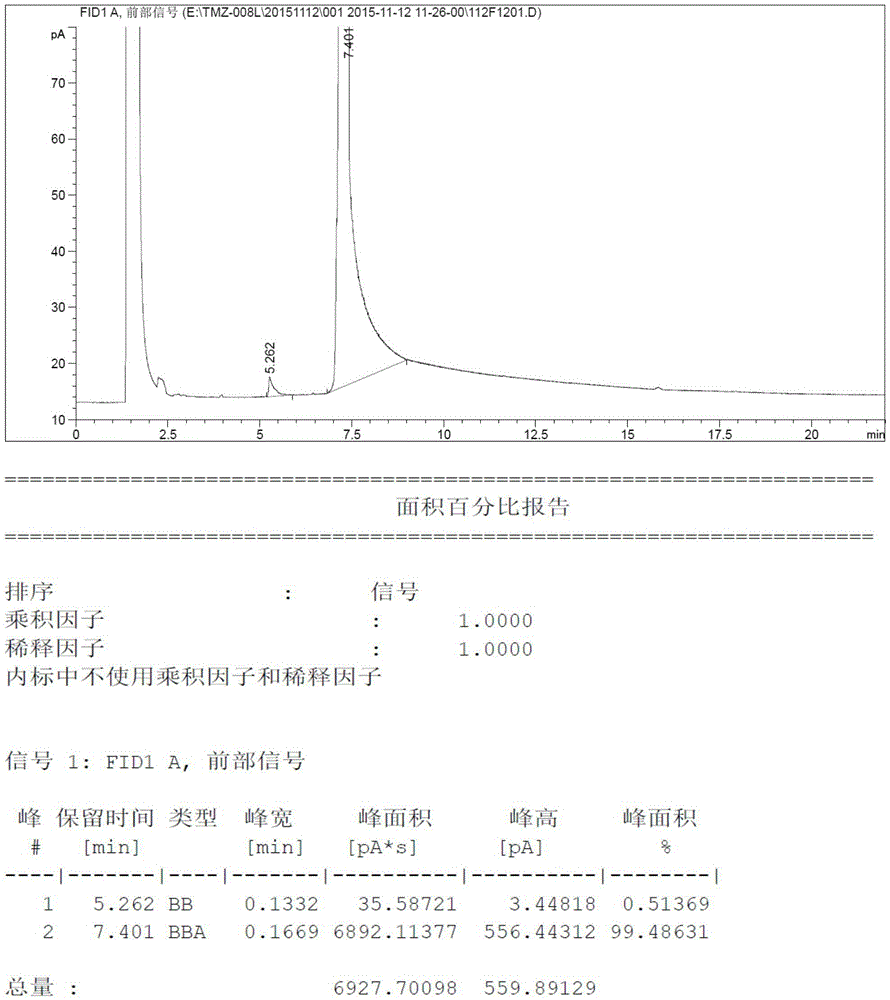
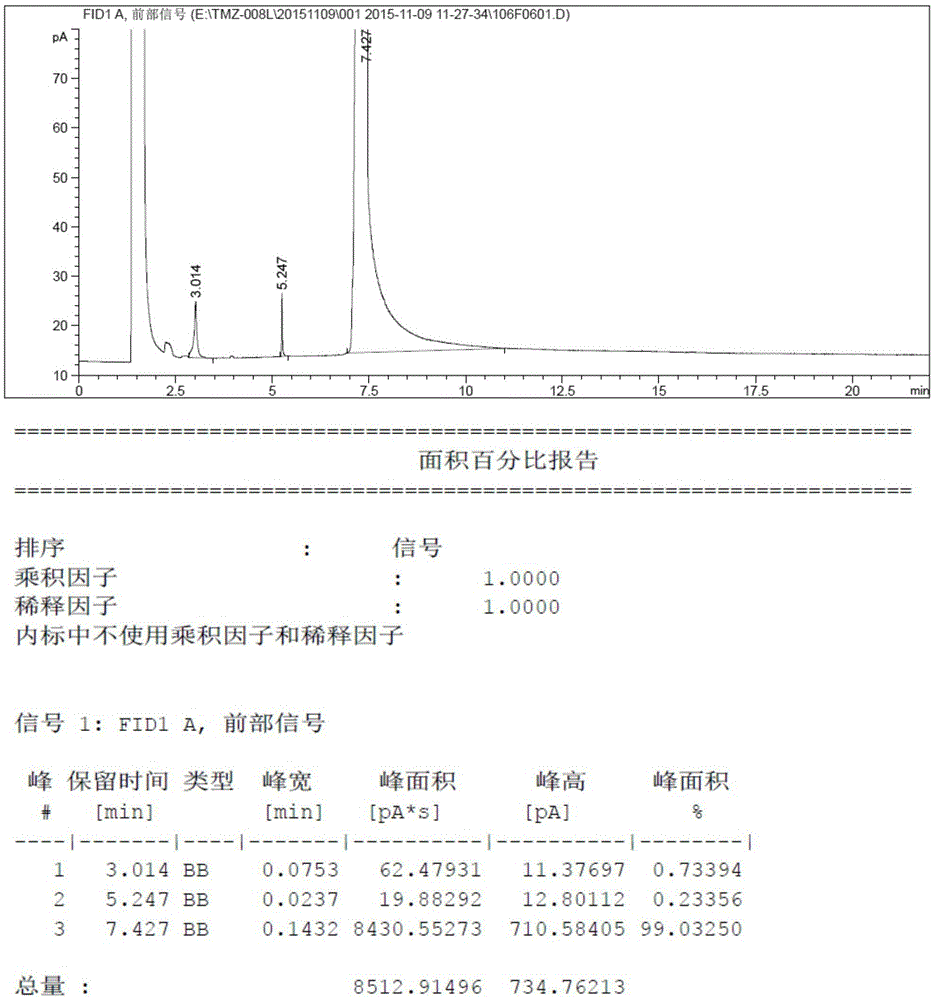
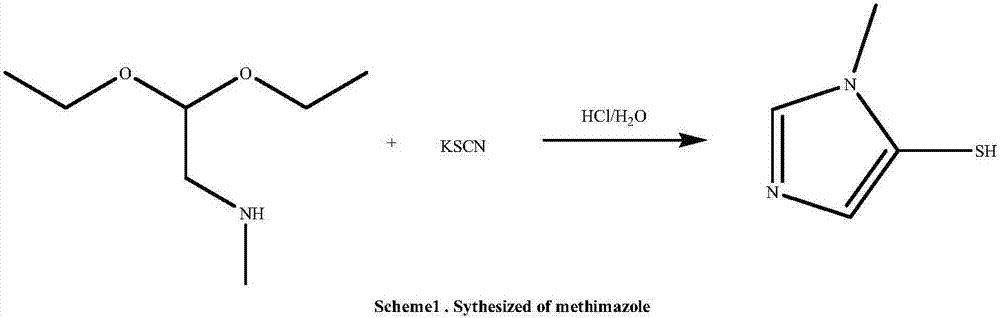
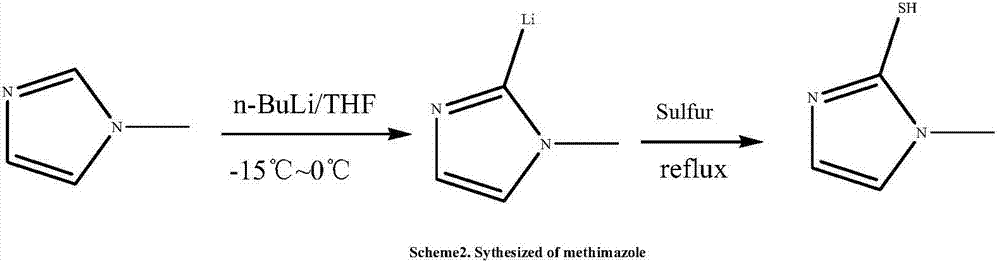
Patents
Literature
35 results about "Methimazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methimazole is used to treat overactive thyroid (hyperthyroidism).
Methods for the amelioration of episodes of acute or chronic ulcerative colitis
InactiveUS7928132B2Decreases endogenous amountAntibacterial agentsBiocideDrug compoundUlcerative colitis
Methods of ameliorating episodes of accute or chronic colitis are provided by using methimazole derivatives and tautomeric cyclic thiones in combination with another pharmaceutical compound.
Owner:OHIO UNIV
Methimazole derivatives and tautomeric cyclic thiones to inhibit cell adhesion
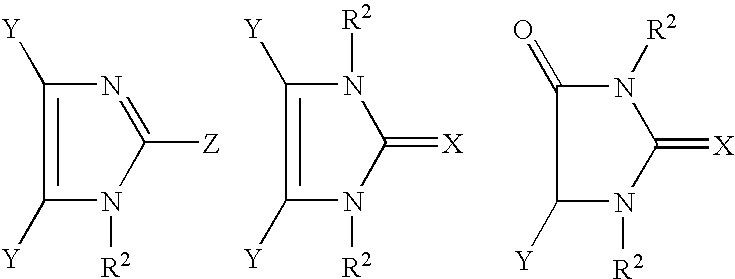
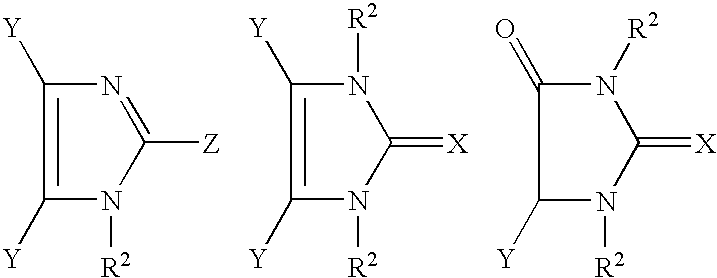
The present invention relates to novel compounds and methods of use for inhibition and prevention of cell adhesion and cell adhesion-mediated pathologies. This invention also relates to pharmaceutical formulations comprising these compounds and methods of using them for inhibition and prevention of cell adhesion and cell adhesion-mediated pathologies. The compounds and pharmaceutical compositions of this invention can be used as therapeutic or prophylactic agents. In particular, methimazole derivatives and tautomeric cyclic thiones have the ability to inhibit the adhesion and the migration of leukocytes. In addition to being active anti-inflammatories, the methimazole derivatives and tautomeric cyclic thiones and their physiologically tolerable salts, derivatives and prodrugs are generally suitable for the treatment (i.e., for the therapy and prophylaxis) of diseases that are based on the interaction between VCAM-1 and its ligands or can be influenced by an inhibition of this interaction. In particular, the methimazole derivatives and tautomeric cyclic thiones are suitable for the treatment of diseases that are caused at least partly by an undesired extent of leukocyte adhesion and / or leukocyte migration or are connected therewith, and for whose prevention, alleviation or cure the adhesion and / or migration of leukocytes should be decreased.
Owner:OHIO UNIV
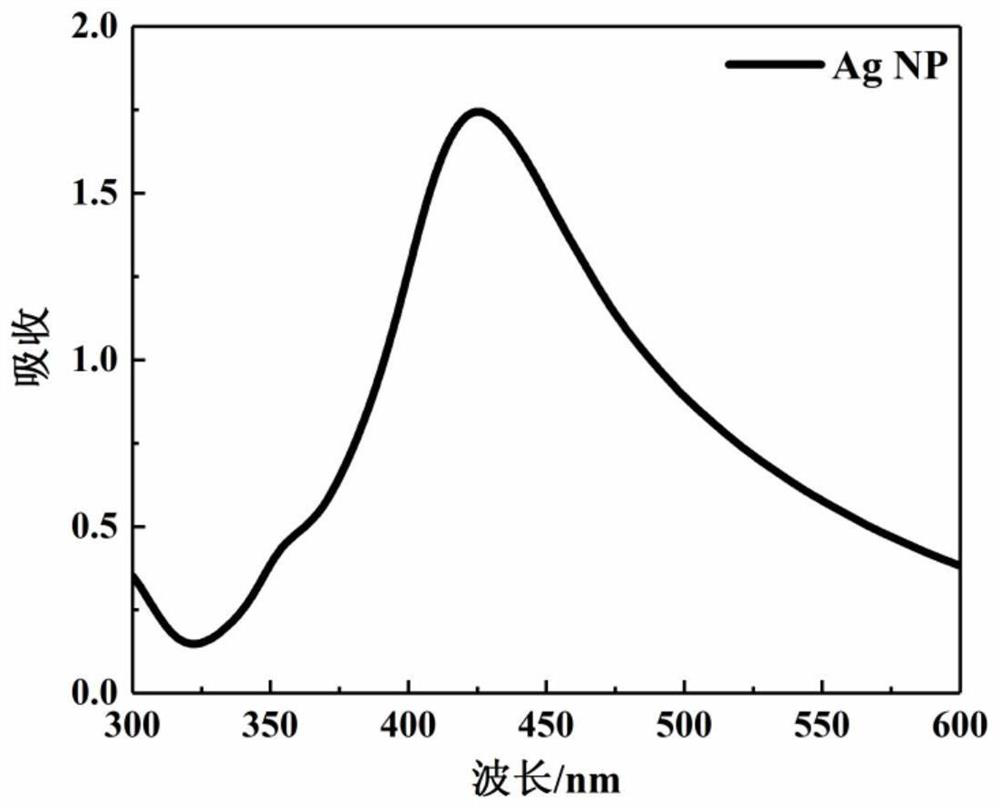
Method for detecting methimazole by surface-enhanced raman scattering
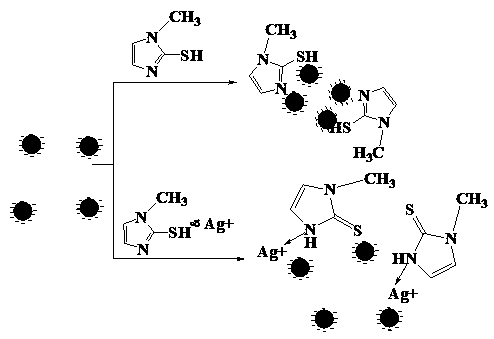
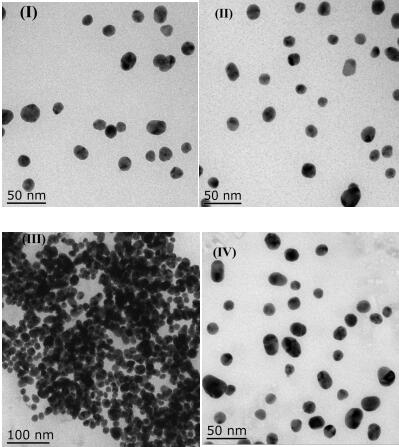
A method for analyzing or detecting methimazole (“MTZ”) comprising contacting a sample suspected of containing MTZ with the dendrimer-stabilized silver nanoparticles and performing surface-enhanced Raman scattering (SERS). Graphene-dendrimer-stabilized silver nanoparticles (G-D-Ag).
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
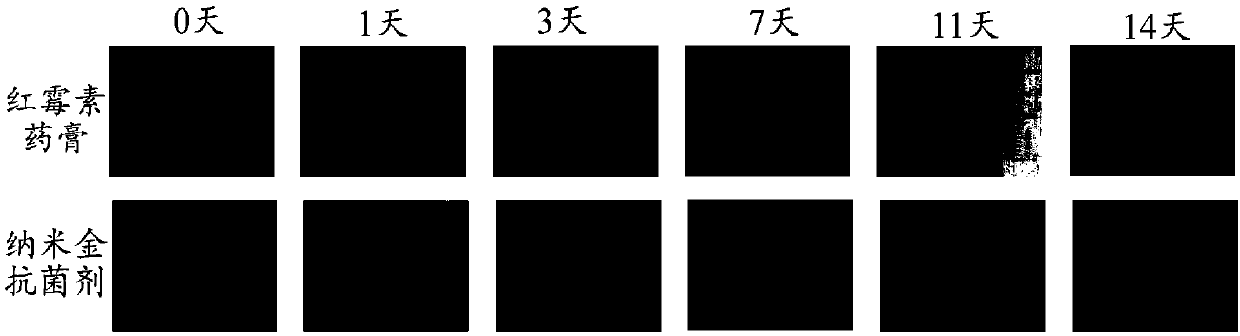
Preparation method of nanogold-containing antibacterial agent
ActiveCN107598185AHas antibacterial propertiesSimple processAntibacterial agentsMaterial nanotechnologyBiocompatibility TestingDistilled water
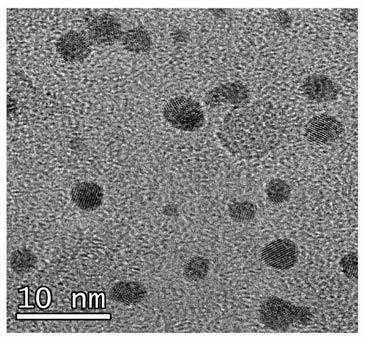
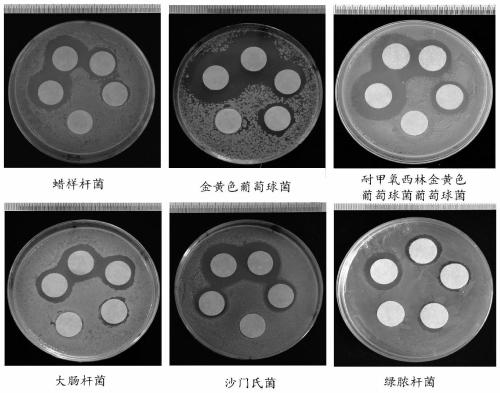
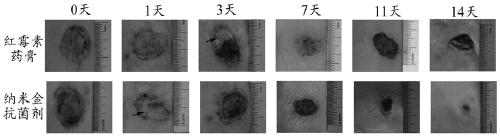
The invention belongs to the technical field of medicine and health and relates to a preparation method of a nanogold-containing antibacterial agent. The preparation method comprises the following steps that A, egg white is centrifuged, liquid supernatant is taken, and a reactant A is obtained; B, under the temperature condition of 20-30 DEG C, distilled water is added in the reactant A obtained in the step A to dilute till the mass fraction is 1-5%, a sodium hydroxide solution is used for adjusting the pH to be 9-11, uniform mixing is conducted, and a mixed solution is obtained; C, under thetemperature condition of 80-100 DEG C, a chloroauric acid solution is added in the mixed solution obtained in the step B to react for 20-30 min, and then a nanogold-containing solution is obtained; and D, under the temperature condition of 80-100 DEG C, a methimazole solution is added in the nanogold-containing solution obtained in the step C, stirring and mixing are conducted, and after reaction,the nanogold-containing antibacterial agent is obtained. The nanogold-containing antibacterial agent has the excellent antibacterial property and biocompatibility, the safety and reliability are achieved, and the preparation method of the nanogold-containing antibacterial agent is simple.
Owner:重庆和其美科技有限公司
Preparation method of methimazole molecularly imprinted polymer
InactiveCN102336869AHigh selectivityImprove stabilityOther chemical processesAlkali metal oxides/hydroxidesCross-linkFunctional monomer
The invention relates to a preparation method of a methimazole molecularly imprinted polymer. The method comprises the technical key points of: dissolving the template molecule methimazole, the functional monomer methacrylic acid and the cross-linking agent ethylene glycol dimethacrylate in a mole ratio of 1:4:5.81 into the pore forming agent absolute ethanol; adding the initiator azodiisobutyronitrile, conducting ultrasonic processing for 20min, introducing nitrogen for 15min, initiating polymerization under ultraviolet irradiation (lambda=365nm) for 12h; after the reaction, crushing and sieving the product, and taking polymer particles with a particle size of 80-125 micrometers, and washing the particles with a mixed solution of 50mL of methanol and 50mL 1.0mol L<-1> of hydrochloric acid under stirring, carrying out soxhlet extraction with a mixed solution of methanol and glacial acetic acid that are in a volume ratio of 9:1 to remove the template molecule, implementing vacuum drying at a temperature of 70DEG C for 10h, thus obtaining the methimazole molecularly imprinted polymer. The preparation method of the invention has low cost, simple synthesis process and easily controllable reaction conditions, and the obtained molecularly imprinted polymer can be used for concentration of the trace amount of methimazole existing in a sample and substrate purification, so that the method provided in the invention boasts great application value and market prospects.
Owner:TIANJIN UNIV OF SCI & TECH
Use of phenylmethimazoles, methimazole derivatives, and tautomeric cyclic thiones for the treatment of autoimmune/inflammatory diseases associated with toll-like receptor overexpression
ActiveUS20120238610A1Mitigating and preventing complicationBiocideSenses disorderDendritic cellFactor ii
Treatment of autoimmune and / or inflammatory diseases associated with overexpression of Toll-like receptor 3 (TLR3) as well as Toll-like receptor 4 (TLR4) and / or TLR3 / TLR4 signaling in nonimmune cells, monocytes, macrophages, and / or dendritic cells in association with related pathologies. The use of phenylmethimazoles, methimazole derivatives, and tautomeric cyclic thiones for the treatment of autoimmune and inflammatory diseases associated with TLR3 as well as TLR4 and / or TLR3 / TLR4 cellular signaling in association with related pathologies is disclosed. Methods of treating a subject having a disease or condition associated with abnormal TLR-3 as well as TLR-4 and / or TLR3 / TLR4 cellular signaling in association with related pathologies are also disclosed. The present disclosure also relates to the treatment of autoimmune-inflammatory pathologies and chemokine and cytokine-mediated diseases associated with TLR overexpression and signaling. The disclosure also relates to pharmaceutical formulations capable of inhibiting the IRF-3 / Type 1 IFN / STAT / ISRE / IRF-1 pathway associated with Toll-like receptor overexpression or signaling.
Owner:OHIO UNIV
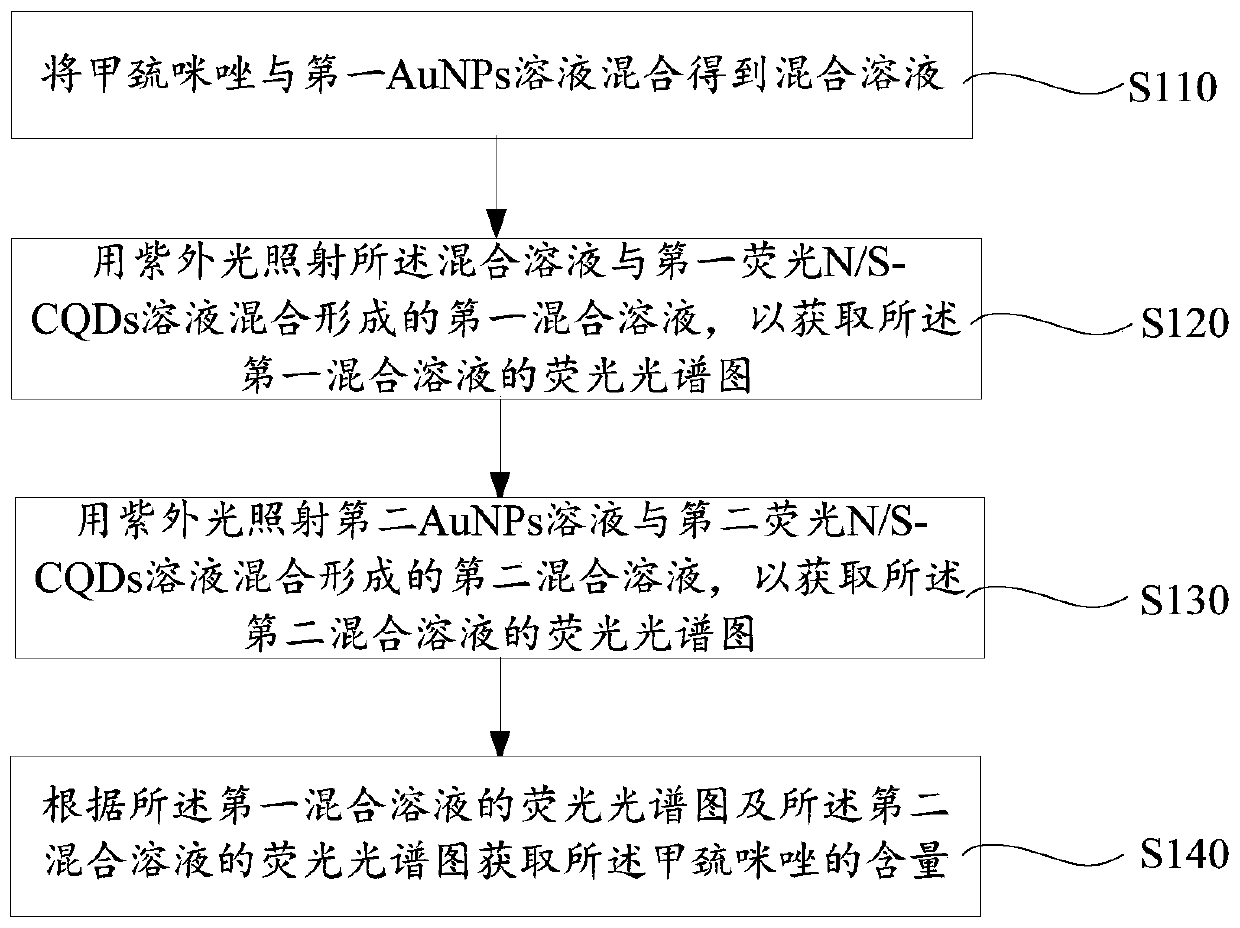
Methimazole detection method
ActiveCN107525791ALow costSimple and fast operationFluorescence/phosphorescenceFluorescence spectrometryUltraviolet lights
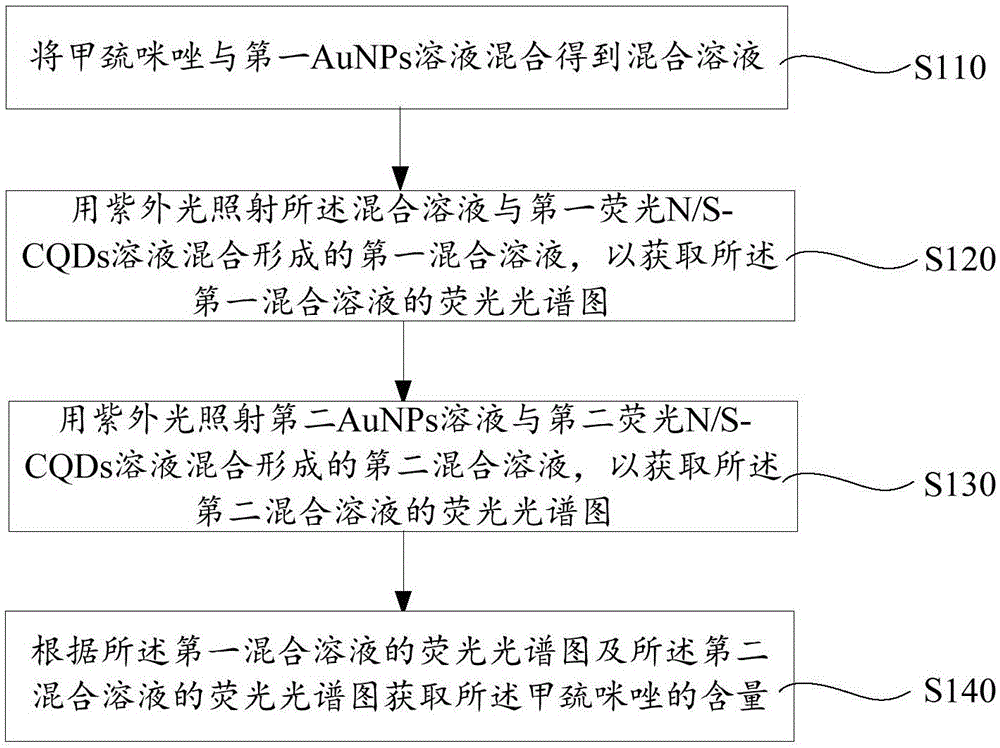
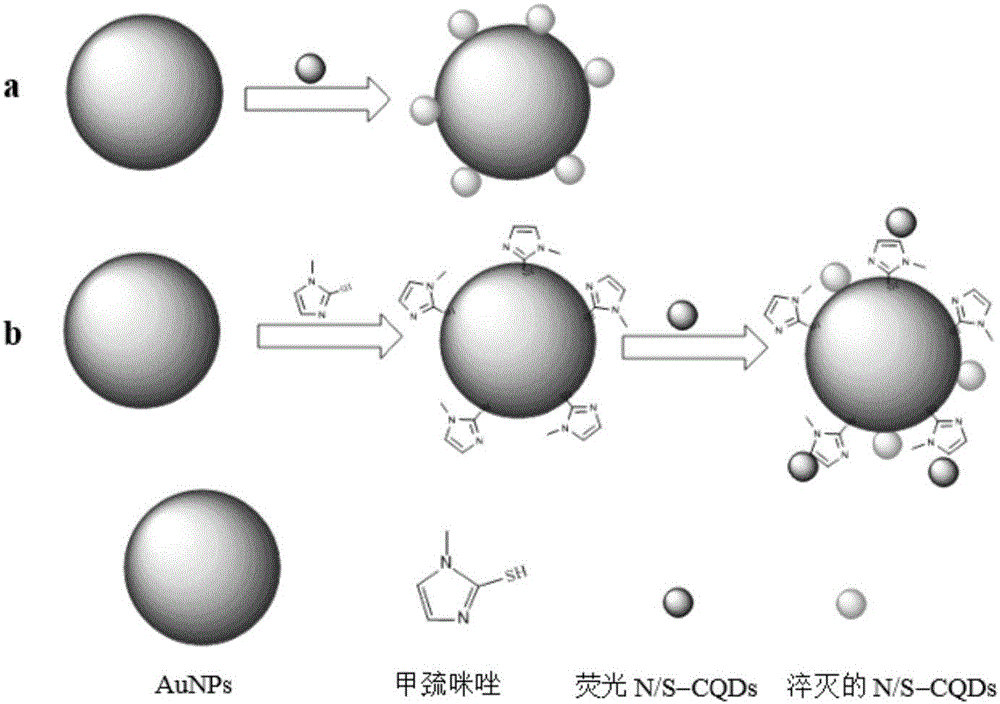
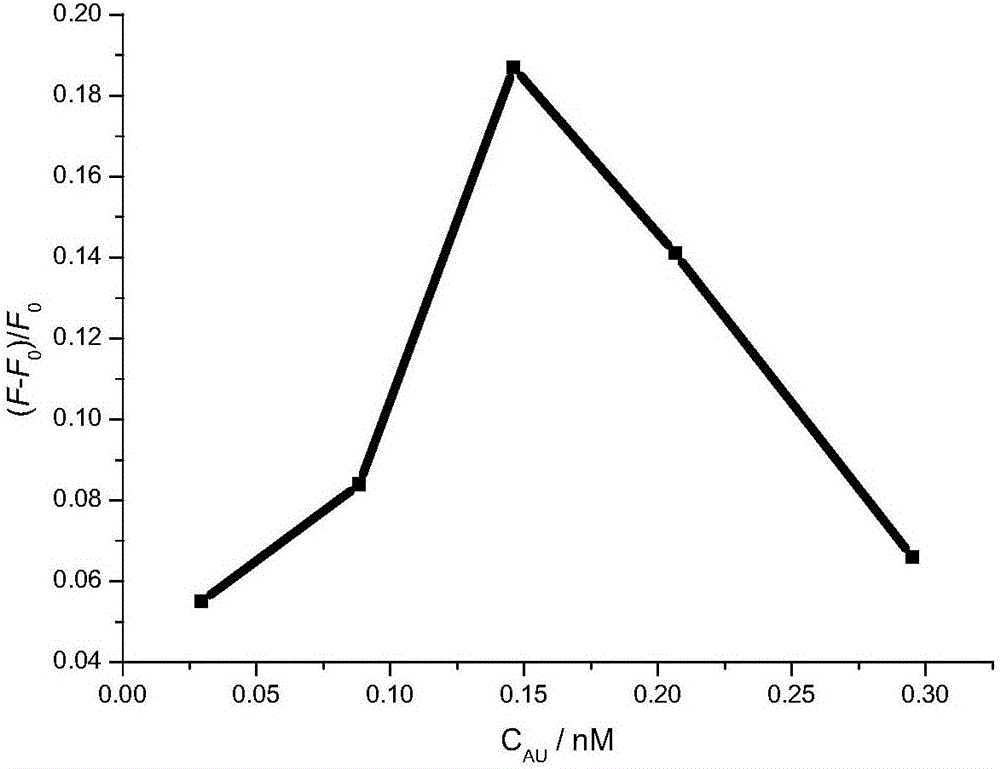
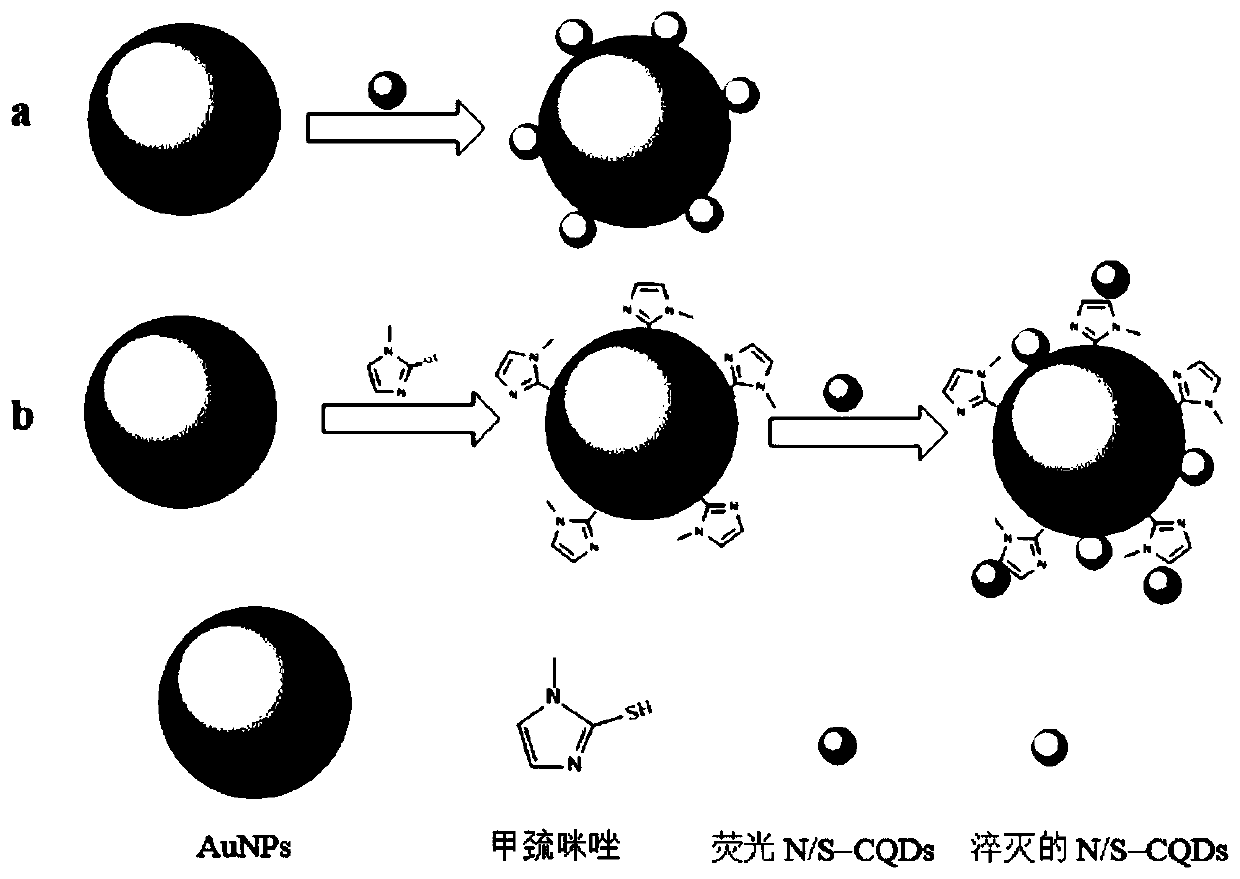
The invention provides a methimazole detection method, which comprises the following steps of mixing methimazole with a first AuNPs solution to obtain a mixed solution; irradiating a first mixed solution formed by mixing the mixed solution with a first fluorescent N / S-CQDs solution with ultraviolet light to obtain a fluorescence spectrogram of the first mixed solution; irradiating a second mixed solution formed by mixing a second AuNPs solution and a second fluorescent N / S-CQDs solution with ultraviolet light to obtain a fluorescence spectrogram of the second mixed solution; obtaining the content of the methimazole according to the fluorescence spectrogram of the first mixed solution and the fluorescence spectrogram of the second mixed solution. The methimazole detection method provided by the invention uses the fluorescent N / S-CQDs as energy donors and AuNPs as energy receptors to construct a complex fluorescent probe, and the methimazole is detected by using the complex fluorescent probe. Compared with high performance liquid chromatography, flow injection spectrophotometry and the like, the cost is low, and the operation is easy.
Owner:NANKAI UNIV
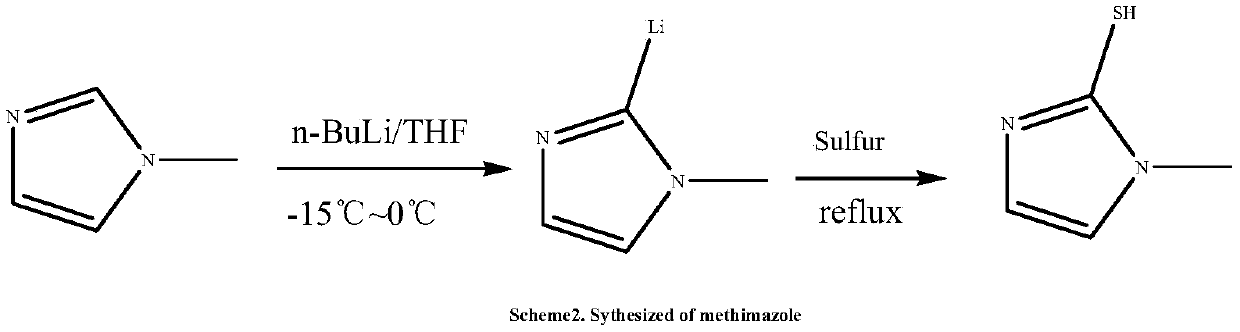
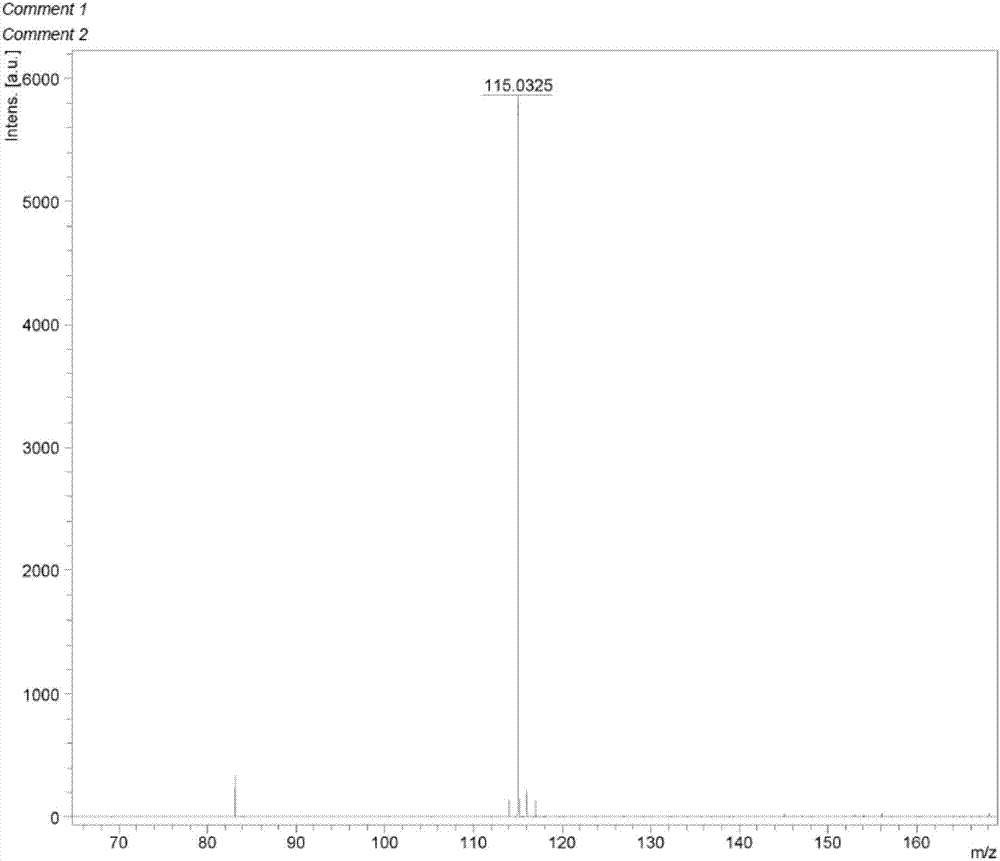
Preparation method of methimazole
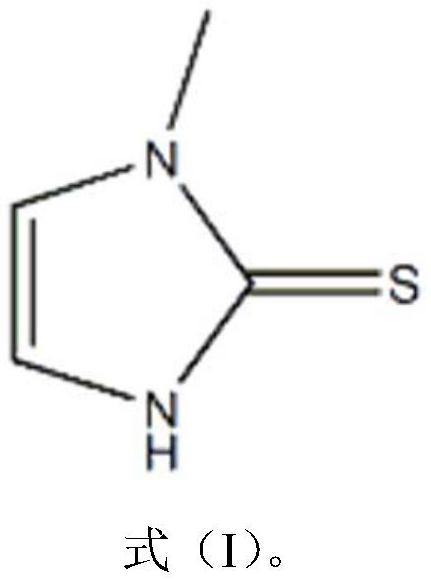
The invention relates to a preparation method of methimazole. The method specifically comprises the following step: enabling reaction of N-methylimidazole, N-butyllithium and powdered sulfur in an organic solvent to obtain methimazole. The preparation method disclosed by the invention is simple in process, mild in condition, simple to operate, easy for post treatment, applicable for industrialized production, and capable of greatly improving the reaction yield of methimazole.
Owner:WUHAN CONFORM PHARMA CO LTD
Methimazole synthesizing and purifying method
ActiveCN107162983AMild reaction conditionsOperational securityOrganic chemistryOrganic layerPolyethylene glycol
The invention discloses a methimazole synthesizing and purifying method, and belongs to the field of medicine synthesis. The method comprises the following steps: carrying out a condensation reaction on methylaminoacetaldehyde polyethylene glycol and ammonium thiocyanate which are used as raw materials in the presence of an acid catalyst and a phase transfer catalyst; and adding saturated salt water after the reaction is completed, uniformly mixing the obtained reaction product and the saturated salt water, adding an organic solvent to carry out extraction, drying and concentrating the obtained organic layer to obtain crude methimazole, heating and dissolving the crude product in a solvent, adding active carbon to decolorize the obtained solution, filtering the solution, and drying the filtered solution to obtain purified methimazole. The method is a preparation method simple to operate and suitable for industrial production.
Owner:CHANGZHOU TIANHUA PHARMA
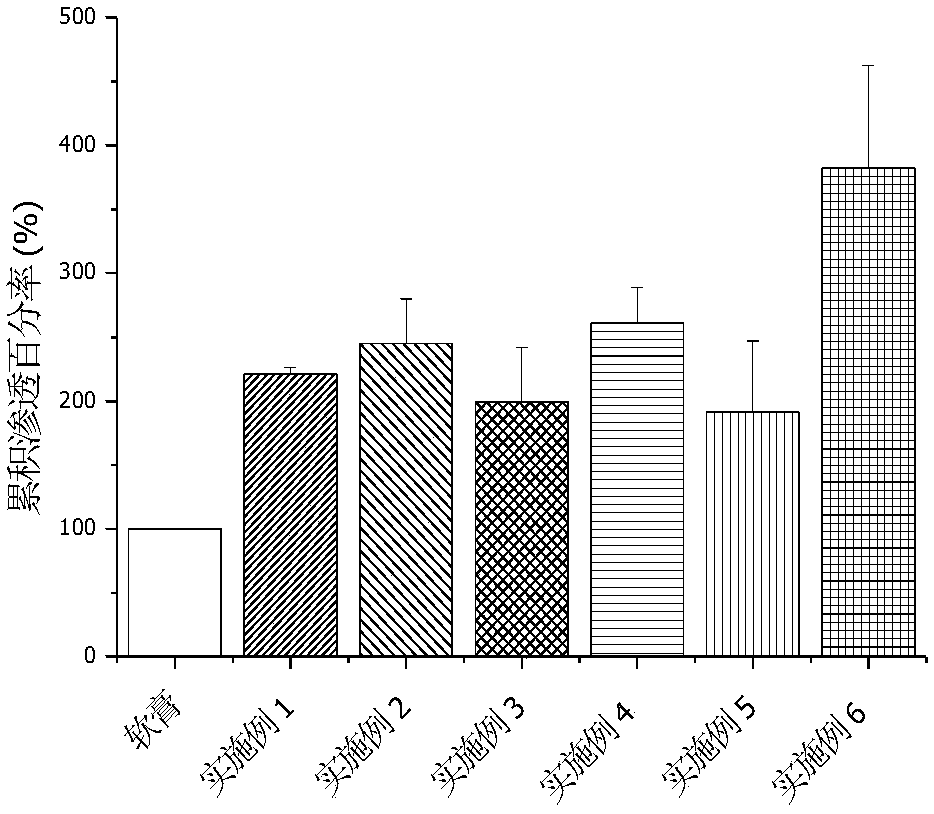
Methimazole microemulsion, methimazole microemulsion-based gel and preparation method and application of methimazole microemulsion
ActiveCN105030671AGood curative effectEnhanced ability to penetrate the skinOrganic active ingredientsAerosol deliverySolubilityTherapeutic effect
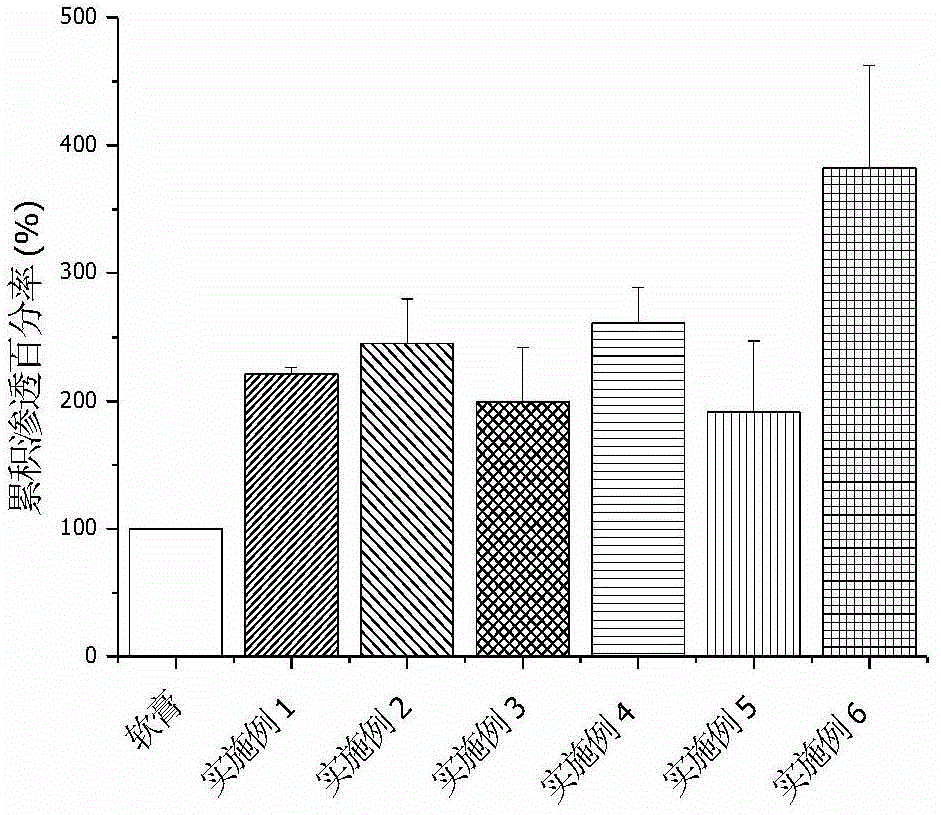
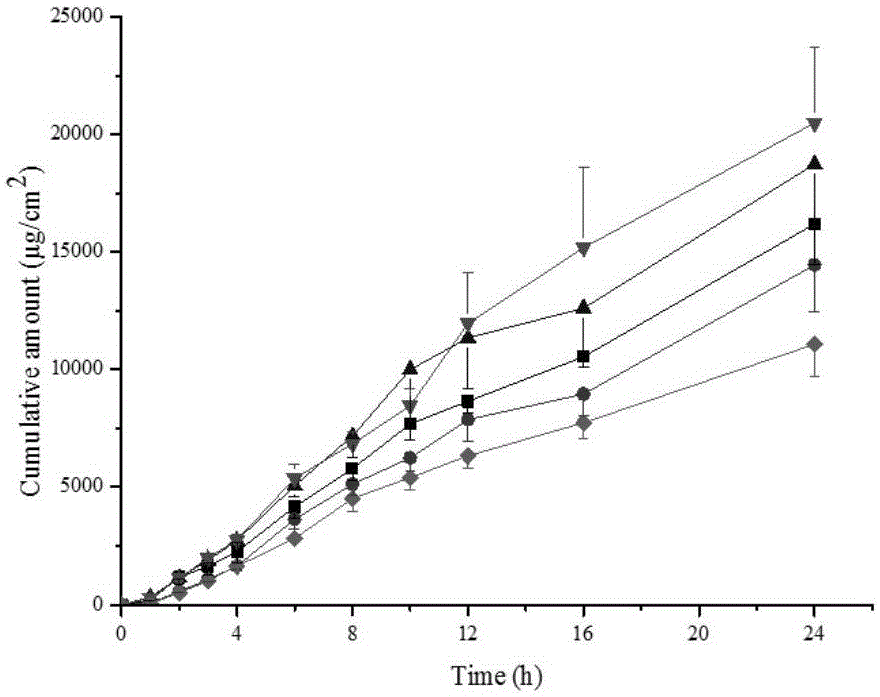
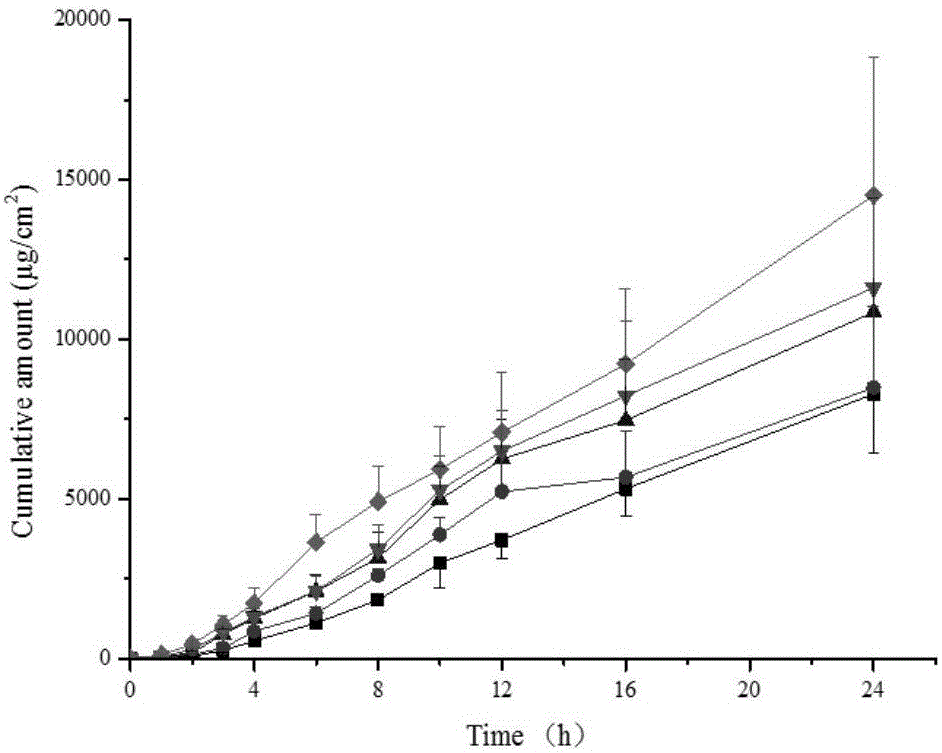
The invention relates to methimazole microemulsion, methimazole microemulsion-based gel and a preparation method and application of the methimazole microemulsion. The methimazole microemulsion comprises methimazole, oil, surfactant, cosurfactant, water and transdermal Enhancer according to a weight ratio of 1:(1.40-4.25):(5.13-15.45):(5.13-15.45):(1.40-8.32):(0.26:1.26). The methimazole microemulsion-based gel is prepared by adding gel matrix into the methimazole microemulsion, allowing swelling and performing uniform mixing. The methimazole microemulsion and the methimazole microemulsion-based gel are directly applicable to affected parts, are also applicable to the preparation of hyperthyroidism pharmaceuticals such as patches or plasters, and have the advantages such as good solubility, high penetration capacity, long acting time, evident improvement in therapeutic effect, fewer times of administration, and convenience of administration.
Owner:HARBIN MEDICAL UNIVERSITY
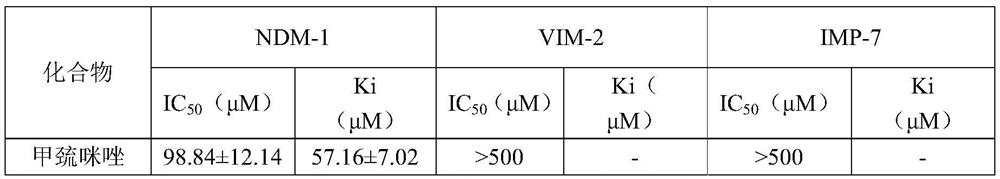
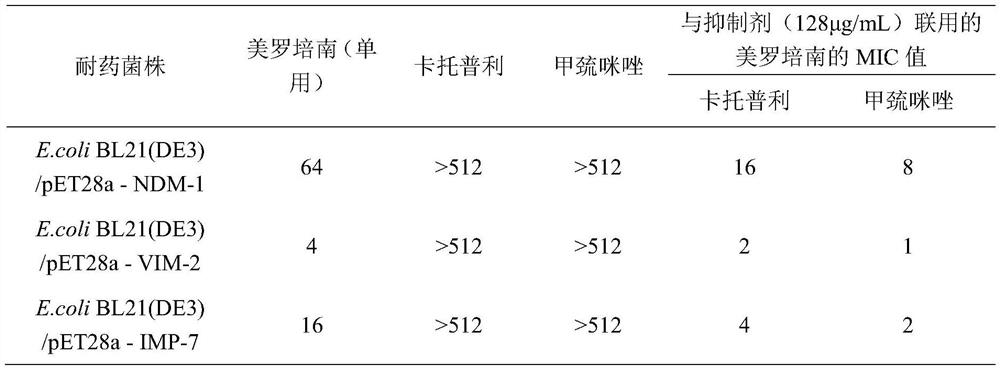
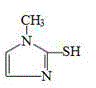
Application of methimazole serving in as and/or in preparation of metal beta-lactamase inhibitor
ActiveCN113209090AProtects against bacterial degradationIncreased sensitivityAntibacterial agentsOrganic active ingredientsHyperthyroidsPharmaceutical drug
The invention belongs to the field of medicines, and discloses application of methimazole in serving as and / or preparation of a metal beta-lactamase inhibitor. The invention discloses the application of the methimazole and / or the derivative thereof in serving as and / or preparation of the metal beta-lactamase inhibitor for the first time, the methimazole and / or the derivative thereof has a good inhibition effect on metal beta-lactamase, can protect antibiotics from being degraded by bacteria, improves the sensitivity of the bacteria to the antibiotics, and reversing drug resistance of the bacteria to the antibiotics; and meanwhile, the methimazole and / or the derivative thereof have a good synergistic effect when being combined with the antibiotics, and can be used as an adjuvant drug for inhibiting the bacteria. The methimazole has small toxic and side effects on human bodies under normal clinical use dosage, and has good safety in many years when being clinically used as a medicine for treating hyperthyroidism.
Owner:GENERAL HOSPITAL OF SOUTHERN THEATRE COMMAND OF PLA
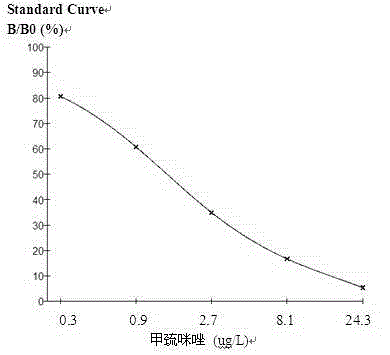
Enzyme linked immunosorbent assay kit for detecting methimazole residues in animal-derived food and application thereof
The invention relates to an enzyme linked immunosorbent assay kit for detecting methimazole residues in animal-derived food. The kit comprises the following components according to proportion: a coating plate, a standard substance, an enzyme-labeled substrate working solution, a substrate solution A, a substrate solution B, a terminating solution, a concentrated washing liquid and a concentrated extract. The kit sensitivity is 0.3 ppb, and the precision is as follows: intra assay variation coefficient (CV%) is 5%, and inter assay variation coefficient (CV%) is 10%; the accuracy is shown by recovery rate, and the recovery rate is in the range of 80-110%. IC 50 range is between 0.3 and 0.5 ppb, and the linear correlation coefficient |r| is not less than 0.9900, and the kit has the advantages of wide usage scope, convenient usage and accurate detection.
Owner:JIANGSU WISE SCI & TECH DEV
Pharmaceutical application of rapamycin in refractory graves disease
ActiveCN111658643BImprove plasma levelsNo adverse reactionOrganic active ingredientsImmunological disordersRapamycin treatmentGraves' disease
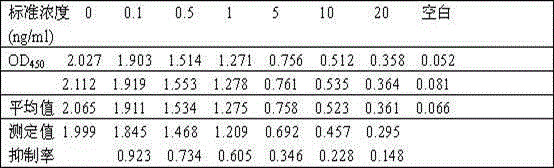
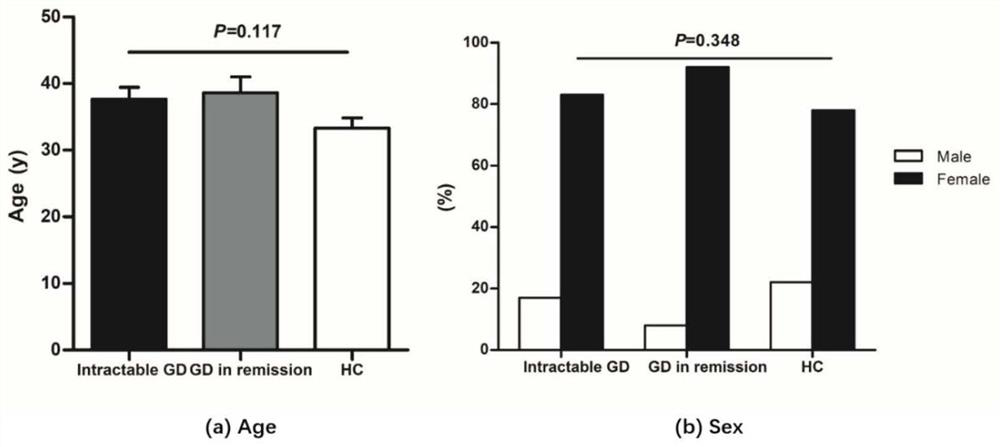
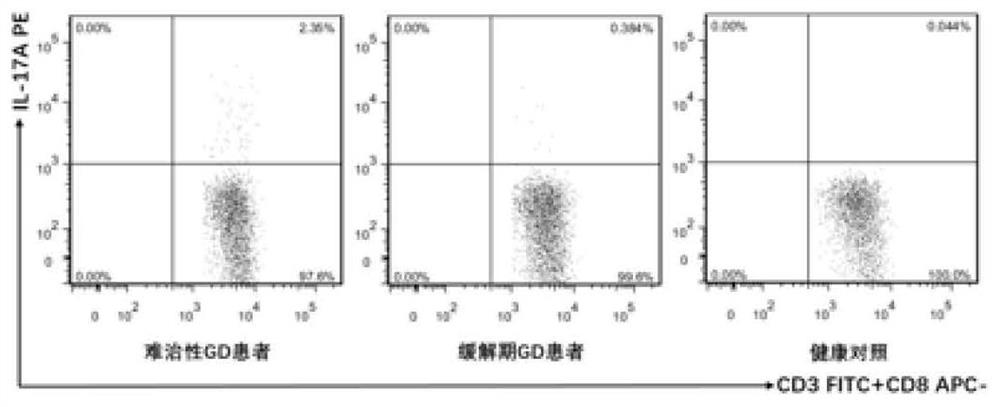
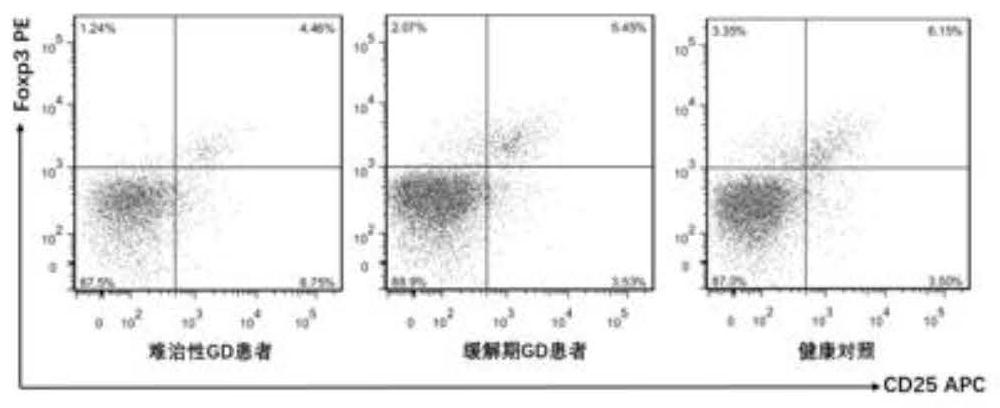
The present invention discloses for the first time the pharmaceutical application of rapamycin in the treatment of refractory GD. The combined treatment of rapamycin + methimazole can improve the TSH and TRAb levels of patients with refractory GD; and, the Treg / CD4+ in patients The ratio of T increased, the ratio of Th17 / CD4+T and the ratio of Th17 / Treg decreased; at the same time, the plasma levels of Th17 and Treg cell-related cytokines, including IL-17A, IL-6 and TGF-β1, were significantly improved. The dose of rapamycin for refractory GD is 0.5 mg / day, and the trough concentration of the drug is 2-4 ng / mL. There were no obvious adverse reactions in the combined treatment group of rapamycin + methimazole. Therefore, the experimental results of the present invention fully support the new application of rapamycin in the treatment of refractory GD, and the combined treatment of rapamycin + methimazole is a new direction for the treatment of refractory GD worth exploring.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Nanogold colorimetric method based on anti-agglomeration and determination of silver ions
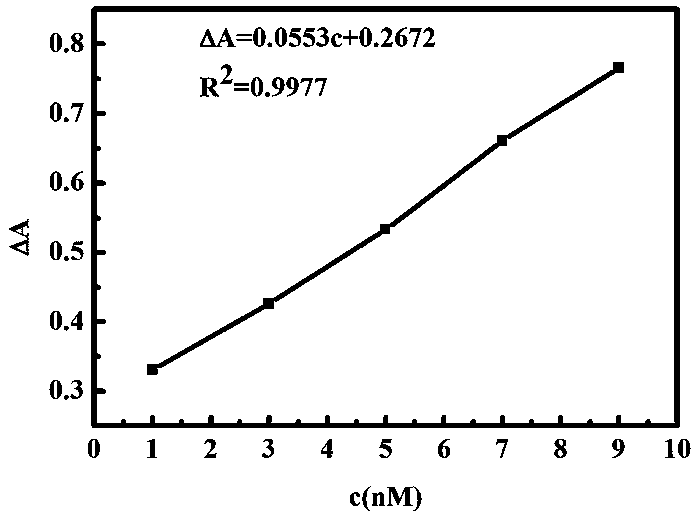
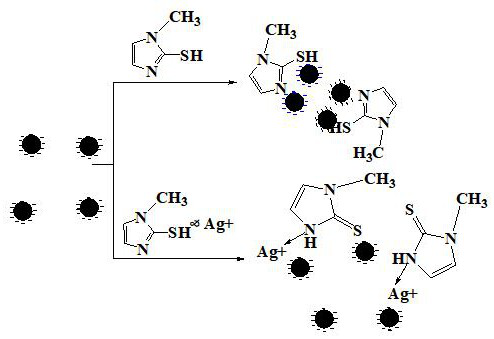
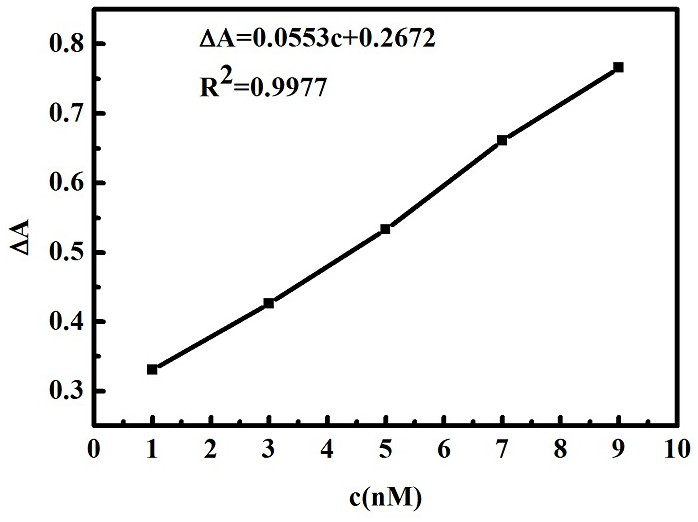
ActiveCN111157521AReduce agglomerationGood choiceMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationSilver ionBuffer solution
The invention provides a nanogold colorimetric method based on an anti-agglomeration principle and a method for determining the content of silver ions in a water sample by using the nanogold colorimetric method. The method comprises the following steps: taking 200 [mu] L of a nanogold solution prepared by reduction of sodium citrate, adding 200 [mu] L of a methimazole solution with a certain concentration, adding 400 [mu] L of a BR buffer solution, carrying out uniform shaking incubation for 5 min, carrying out ultraviolet-visible spectrum scanning, determining the absorbance of the solution at 520 nm and 660 nm, and taking the absorbance ratio A520 / A660 of the solution as A0; taking 200 [mu] L of methimazole with a certain concentration and 200 [mu] L of a silver ion solution with a certain concentration; adding 200 [mu] L BR buffer solution, shaking incubation for 10 minutes, adding 200 [mu] L of a nanogold solution; shaking and incubating for 5 minutes, carrying out ultraviolet-visible spectrum scanning, determining the absorbance of the solution at 520 nm and 660 nm, using the absorbance ratio A520 / A660 of the solution as A1, and the change of the absorbance ratio after the silver ions are added is in linear relation with the concentration of the silver ions so that the content of the silver ions in the water sample can be rapidly, sensitively and accurately measured. The method is good in selectivity, high in sensitivity and wide in detection range, only an ultraviolet spectrophotometer is needed for measurement without a large instrument, the operation requirement islow and the method has wide application prospects.
Owner:信阳学院
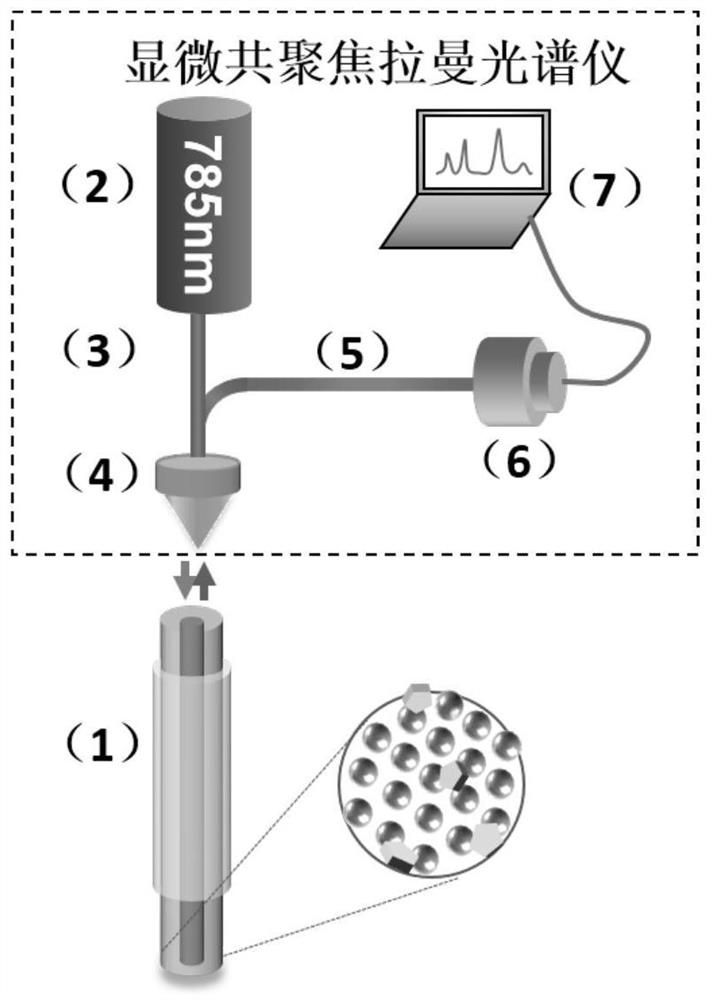
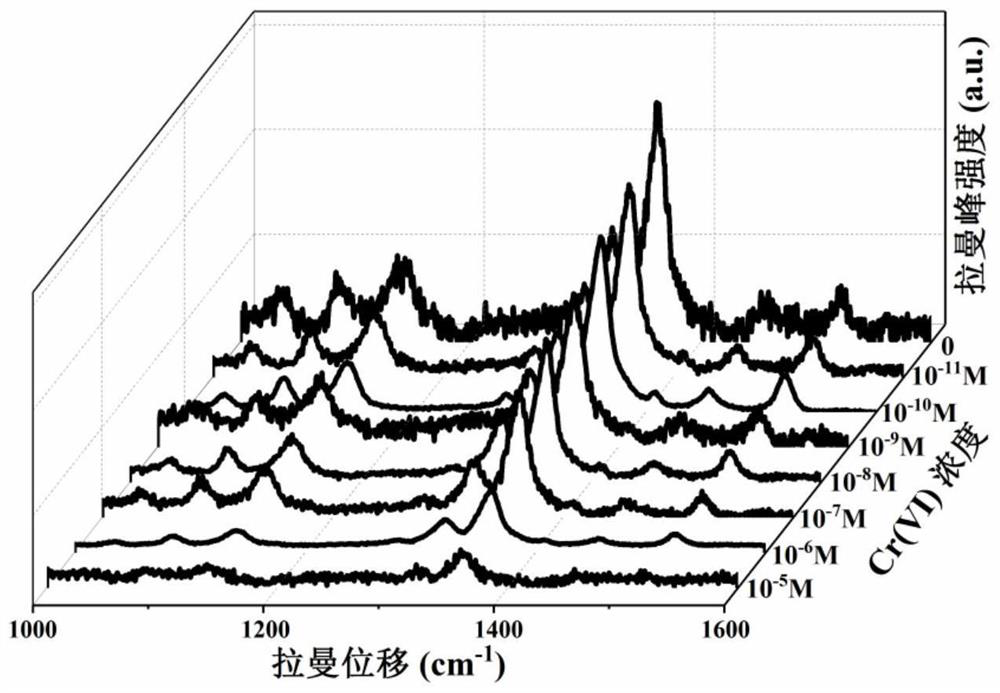
Silver-plated optical fiber Raman probe as well as preparation method and application thereof
PendingCN114199773AEnables ultra-sensitive detectionImprove antioxidant capacityRaman scatteringSignalling moleculesChemistry
The invention relates to the field of material, optics and environment detection, in particular to a silver-plated optical fiber Raman probe as well as a preparation method and application thereof, and methimazole is adopted as a Raman signal molecule to realize Cr (VI) detection. According to the silver-plated optical fiber Raman probe disclosed by the invention, the methimazole functionalization is adopted, so that the ultra-sensitive detection of Cr (VI) and the excellent oxidation resistance are realized. The preparation method disclosed by the invention is simple in process, high in sensitivity, good in repeatability, good in stability and good in anti-interference performance, and by adopting a stannous chloride assisted solvothermal method, morphology regulation and control of the silver nanoparticles on the end face of the optical fiber and strong interface adhesion of the optical fiber and the silver nanomaterial are realized. Specifically, the stannous chloride pretreatment process promotes compact and uniform growth of silver nanoparticles on the end face of the optical fiber on one hand, and enhances interface adhesion of the optical fiber and the silver nanoparticles on the other hand. By adopting methimazole functionalization, ultra-sensitive detection and excellent oxidation resistance of Cr (VI) are realized.
Owner:WUHAN UNIV OF TECH
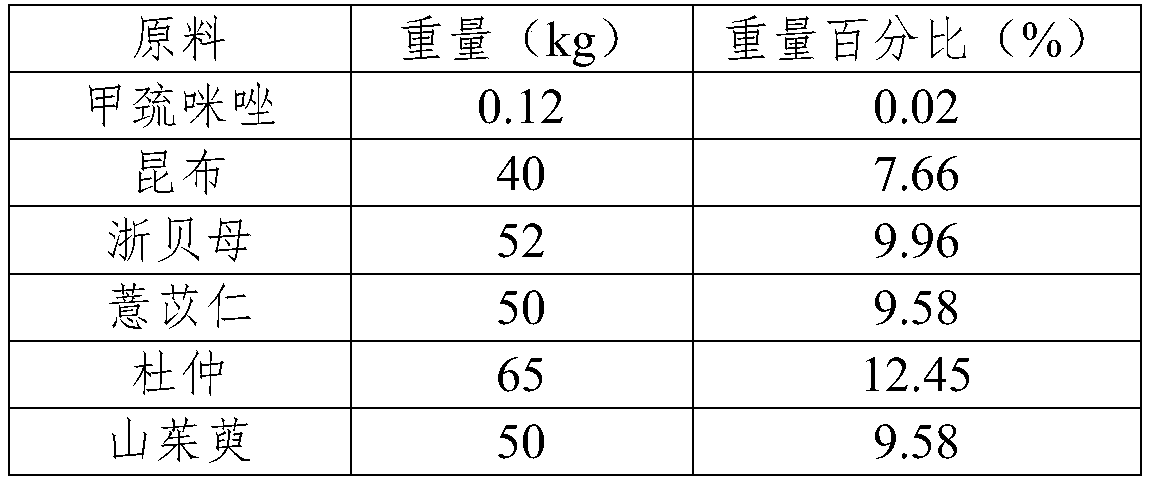
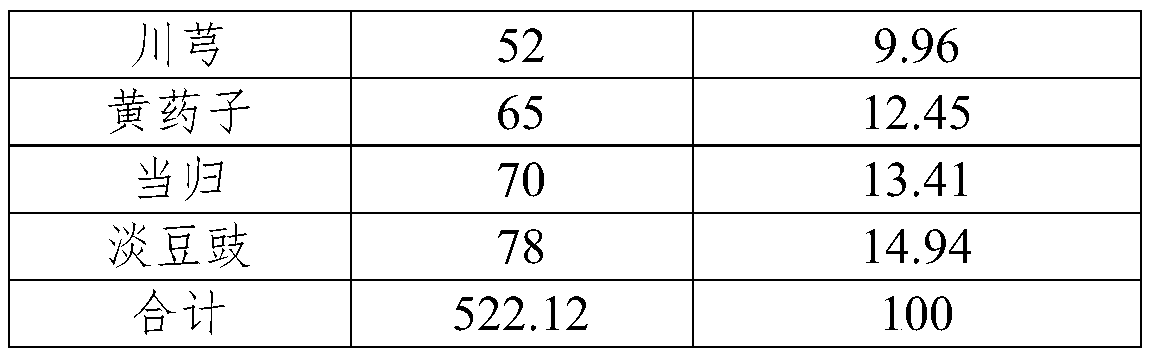
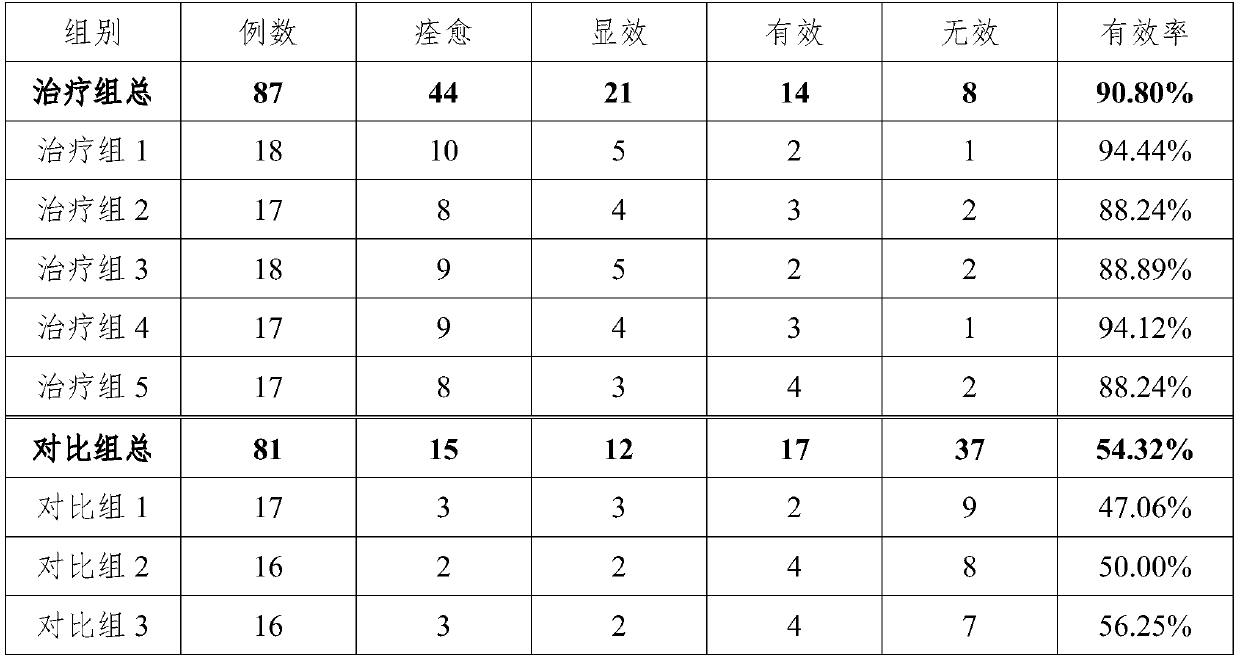
Pharmaceutical composition for treating hyperthyroidism, including its preparation and its preparation method and application
ActiveCN106822674BGood at eliminating phlegm and softening firmnessOrganic active ingredientsAlgae medical ingredientsAngelica Sinensis RootFritillaria thunbergii
Owner:蚌埠丰原涂山制药有限公司
Preparation process of 7-ATCA
The invention relates to a preparation process of 7-ATCA. The preparation process comprises the following steps: (1) mixing dimethyl carbonate with a boron trifluoride dimethyl carbonate complex, adding 7-ACA and methimazole tetrazolylazo, and stirring to react at 15-20 DEG C until the residue of 7-ACA is less than or equal to 1% by virtue of liquid detection; (2) cooling reaction liquid to 5-15 DEG C, and adding purified water with the temperature of 5-15 DEG C for hydrolysis; (3) after adding dichloromethane for once extraction, adding dichloromethane into a water layer for secondary extraction; (4) dropwise adding weak base into the water layer at 0-10 DEG C until the pH is 3.4-3.6, and stirring to grow crystals at 0-10 DEG C for 2h, wherein the temperature in the dropwise adding process does not exceed 10 DEG C; and (5) carrying out centrifugation, sequentially washing with purified water and acetone, and drying, so as to obtain 7-ATCA. By virtue of the preparation process, a 7-ATCA product with good crystal form and particle size can be obtained and is a white crystal, the purity can reach 99.5% or above, and the mass yield can reach 115% or above.
Owner:HENAN KANGDA PHARMA
A kind of preparation method containing nano-gold antibacterial agent
ActiveCN107598185BHas antibacterial propertiesSimple processAntibacterial agentsMaterial nanotechnologyAntibacterial agentPharmacology
The invention belongs to the technical field of medicine and health and relates to a preparation method of a nanogold-containing antibacterial agent. The preparation method comprises the following steps that A, egg white is centrifuged, liquid supernatant is taken, and a reactant A is obtained; B, under the temperature condition of 20-30 DEG C, distilled water is added in the reactant A obtained in the step A to dilute till the mass fraction is 1-5%, a sodium hydroxide solution is used for adjusting the pH to be 9-11, uniform mixing is conducted, and a mixed solution is obtained; C, under thetemperature condition of 80-100 DEG C, a chloroauric acid solution is added in the mixed solution obtained in the step B to react for 20-30 min, and then a nanogold-containing solution is obtained; and D, under the temperature condition of 80-100 DEG C, a methimazole solution is added in the nanogold-containing solution obtained in the step C, stirring and mixing are conducted, and after reaction,the nanogold-containing antibacterial agent is obtained. The nanogold-containing antibacterial agent has the excellent antibacterial property and biocompatibility, the safety and reliability are achieved, and the preparation method of the nanogold-containing antibacterial agent is simple.
Owner:重庆和其美科技有限公司
Application of hla-drb1*04:03 allele in evaluating the risk of drug eruption caused by methimazole
The invention discloses the application of HLA‑DRB1*04:03 allele in evaluating the risk of drug eruption caused by methimazole. The present invention claims the application of the material used for detecting the HLA‑DRB1*04:03 allele in the preparation of the kit. The present invention also protects a kit comprising materials for detecting HLA‑DRB1*04:03 alleles. Functions of the kit: (a) evaluate the risk of the subject’s drug eruption caused by methimazole; (b) evaluate the risk of the subject’s drug eruption caused by methimazole; carry HLA‑DRB1*04:03, etc. Subjects with alleles have a higher risk of drug eruption after taking methimazole than subjects without HLA‑DRB1*04:03 alleles; (c) evaluate whether subjects are suitable for taking methimazole; ( d) Evaluate whether the subject is suitable for taking methimazole; subjects carrying the HLA‑DRB1*04:03 allele are not suitable for taking methimazole.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
A kind of synthesis and purification method of methimazole
ActiveCN107162983BMild reaction conditionsOperational securityOrganic chemistryPtru catalystPolyethylene glycol
The invention discloses a methimazole synthesizing and purifying method, and belongs to the field of medicine synthesis. The method comprises the following steps: carrying out a condensation reaction on methylaminoacetaldehyde polyethylene glycol and ammonium thiocyanate which are used as raw materials in the presence of an acid catalyst and a phase transfer catalyst; and adding saturated salt water after the reaction is completed, uniformly mixing the obtained reaction product and the saturated salt water, adding an organic solvent to carry out extraction, drying and concentrating the obtained organic layer to obtain crude methimazole, heating and dissolving the crude product in a solvent, adding active carbon to decolorize the obtained solution, filtering the solution, and drying the filtered solution to obtain purified methimazole. The method is a preparation method simple to operate and suitable for industrial production.
Owner:CHANGZHOU TIANHUA PHARMA
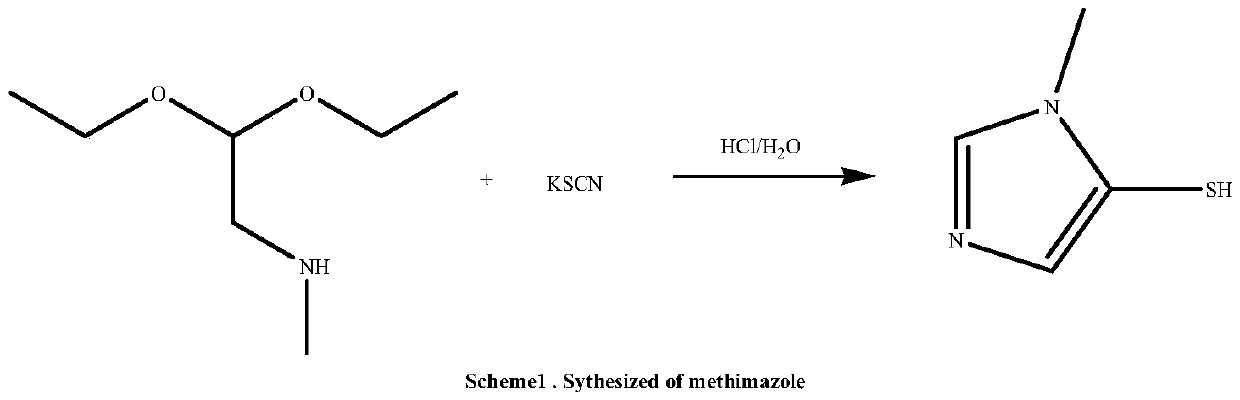
Methimazole preparation method
The invention discloses a methimazole preparation method, which comprises: 1) carrying out an ammonolysis reaction on dimethylchloroacetal and a methylamine methanol solution at a temperature of 125-135 DEG C, adding the alkoxide of an alkali metal after completing the reaction, carrying out a neutralization reaction, filtering, distilling to remove the methanol, and carrying out pressure reducing distillation to obtain an intermediate methylamino dimethoxyethane; and 2) adding sodium thiocyanate and the methylamino dimethoxyethane obtained in the step 1) into purified water, adding hydrochloric acid at a room temperature in a dropwise manner, carrying out a reaction for 12-15 h at a temperature of 50-60 DEG C, carrying out pressure reducing distillation to remove the water after completing the reaction, adding ethyl acetate and a drying agent, carrying out heating reflux for 30 min, carrying out hot filtering, distilling the filtrate until the remaining volume of the ethyl acetate is 1 / 3, and carrying out cooling crystallization to obtain the methimazole. According to the present invention, the preparation method has advantages of low cost, short route, convenient operation and environmental protection, and the high-quality methimazole can be obtained through the method.
Owner:BEIJING WANPENGLANGGE PHARMA TECH
Nano-gold colorimetric method based on anti-agglomeration and determination of silver ions
ActiveCN111157521BReduce agglomerationGood choiceMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationSilver ionPhotochemistry
The invention provides a nano-gold colorimetric method based on the principle of anti-agglomeration and a method for measuring the content of silver ions in water samples. The method comprises the following steps: take 200 μL of the nano-gold solution prepared by sodium citrate reduction, add 200 μL of a certain concentration of methimazole solution, then add 400 μL of BR buffer solution, shake well and incubate for 5 minutes, perform ultraviolet-visible spectrum scanning, and measure the concentration of the solution at 520 The absorbance at nm and 660 nm, the ratio of the absorbance of the solution A 520 / A 660 as A 0 ; Then take 200 μL of methimazole at a certain concentration and 200 μL of silver ion solution at a certain concentration, add 200 μL of BR buffer solution, shake well and incubate for 10 minutes, then add 200 μL nano-gold solution, shake well and incubate for 5 minutes, and perform UV-visible spectrum scanning. Measure the absorbance of the solution at 520 nm and 660 nm, and the absorbance ratio of the solution A 520 / A 660 as A 1 , after adding silver ions, the change of absorbance ratio has a linear relationship with the concentration of silver ions, so the content of silver ions in water samples can be quickly, sensitively and accurately determined. The invention has good selectivity, high sensitivity and wide detection range, only needs ultraviolet spectrophotometer measurement, does not need large-scale instruments, has low operation requirements, and has wide application prospects.
Owner:信阳学院
Medicine for treating acute pharyngitis
InactiveCN106619645AGood reliefEasy to takeSulfur/selenium/tellurium active ingredientsPill deliveryAcute PharyngitisSide effect
The invention discloses a medicine for treating acute pharyngitis. The medicine for treating acute pharyngitis comprises the following main raw materials in parts by weight: 5-12 parts of tea polyphenol, 8-15 parts of methimazole, 13-18 parts of vitamin E, 5-9 parts of phenprobamate, 11-16 parts of allicin, 2-6 parts of calcium gluconate, 4-10 parts of acyclovir and 5-8 parts of bromhexine hydrochloride. The medicine is used for treating acute pharyngitis, is convenient to take and small in side effect, has effects of clearing heat and relieving sore-throat and can be mainly adopted to treat acute pharyngitis, and practical tests show that the medicine has a remarkable alleviation function on acute pharyngitis.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
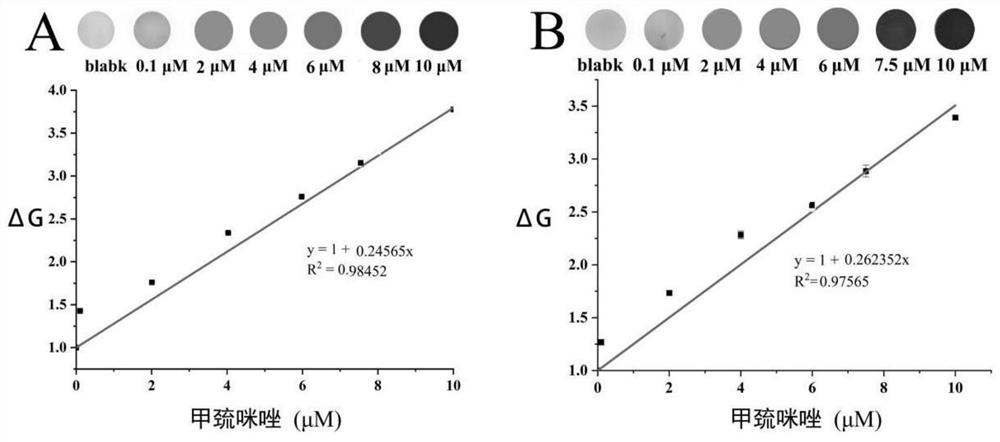
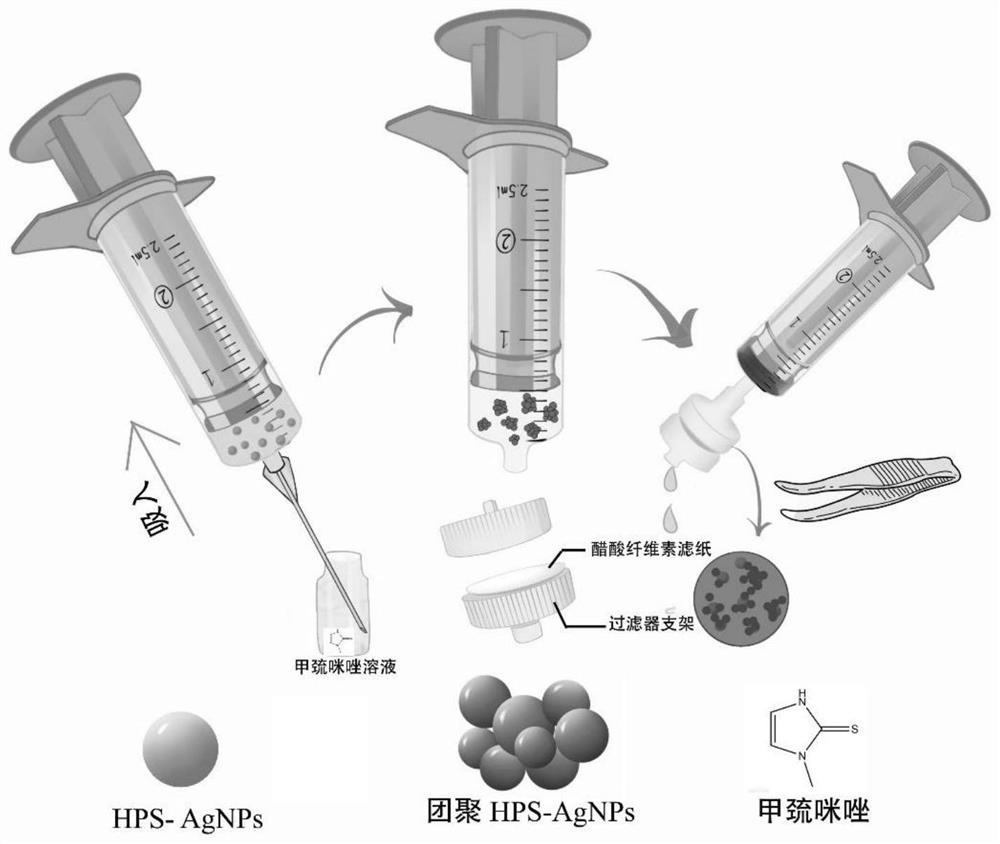
Radix hedysari polysaccharide functionalized silver nanoparticle colorimetric sensor as well as preparation method and application thereof in detection of methimazole
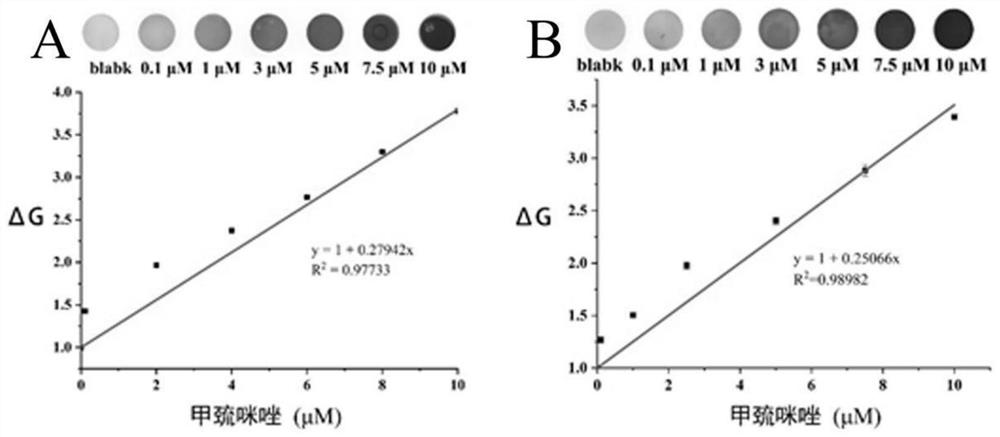
PendingCN114813596AGood dispersionLarge particle sizeMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsColorimetric sensorPolysaccharide
The invention provides a preparation method of a hedysarum polybotrys polysaccharide functionalized silver nanoparticle colorimetric sensor, which comprises the following steps: heating a silver nitrate solution to 60-90 DEG C, adjusting the pH value to 8-14, quickly adding a hedysarum polybotrys polysaccharide solution at one time under stirring for reaction, stopping heating and cooling until the solution is changed from transparent to orange yellow, and filtering to obtain a silver nitrate solution; the hedysarum polybotrys polysaccharide functionalized silver nanoparticle colorimetric sensor is obtained. An injector paper-based methimazole colorimetric detection device prepared by applying the hedysarum polybotrys polysaccharide functionalized silver nanoparticle colorimetric sensor can be used for quantitatively detecting methimazole. The detection device is low in cost, simple and convenient to operate, high in sensitivity and strong in specificity, is successfully applied to naked eye colorimetric detection of methimazole in human and animal serum, human and animal urine and other samples, and can realize quantitative analysis of colorimetric detection results only through naked eye observation or application of a smart phone and computer software in a real scene; or estimating the concentration of methimazole by using a colorimetric card.
Owner:LANZHOU UNIVERSITY
A kind of herbicidal composition containing imazethapyr and clethodim
ActiveCN104381262BImprove the effect of prevention and controlLower doseBiocideAnimal repellantsChemical compositionActive component
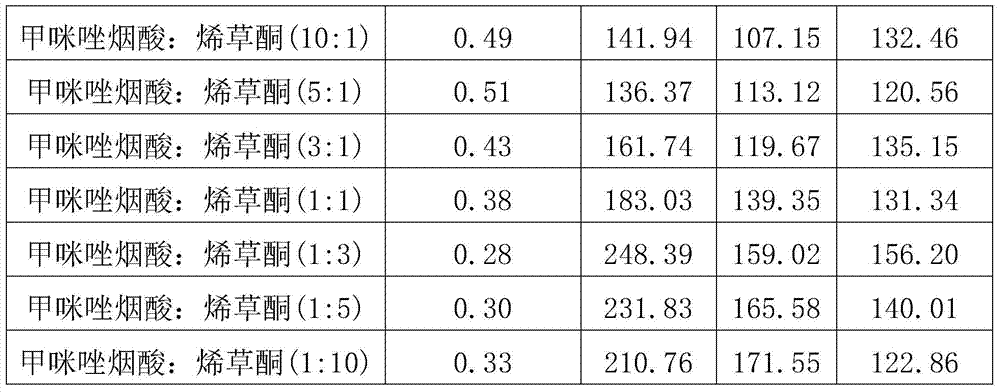
The invention discloses a weeding composition containing imazameth and clethodim. The weeding composition comprises active components, that is, imazameth and clethodim, and the balance of pesticide additives, wherein the mass part ratio of imazameth to clethodim is (1:10) to (10:1); the mass sum of imazameth and clethodim accounts for 1-80% of the weeding composition percentage by mass. The weeding composition disclosed by the invention has a remarkable synergism function, is applicable to prevention and treatment on annual weeds in peanut fields, and is relatively applicable to a post-emergence spraying method.
Owner:QIDONG CHUANGLU NEW MATERIAL CO LTD
A kind of preparation technology of 7-atca
The invention relates to a preparation process of 7-ATCA. The preparation process comprises the following steps: (1) mixing dimethyl carbonate with a boron trifluoride dimethyl carbonate complex, adding 7-ACA and methimazole tetrazolylazo, and stirring to react at 15-20 DEG C until the residue of 7-ACA is less than or equal to 1% by virtue of liquid detection; (2) cooling reaction liquid to 5-15 DEG C, and adding purified water with the temperature of 5-15 DEG C for hydrolysis; (3) after adding dichloromethane for once extraction, adding dichloromethane into a water layer for secondary extraction; (4) dropwise adding weak base into the water layer at 0-10 DEG C until the pH is 3.4-3.6, and stirring to grow crystals at 0-10 DEG C for 2h, wherein the temperature in the dropwise adding process does not exceed 10 DEG C; and (5) carrying out centrifugation, sequentially washing with purified water and acetone, and drying, so as to obtain 7-ATCA. By virtue of the preparation process, a 7-ATCA product with good crystal form and particle size can be obtained and is a white crystal, the purity can reach 99.5% or above, and the mass yield can reach 115% or above.
Owner:HENAN KANGDA PHARMA
Pharmaceutical composition for treating hyperthyroidism, preparation containing same, preparation method and application thereof
ActiveCN106822674AReduce adverse reactionsImprove toleranceOrganic active ingredientsAlgae medical ingredientsChinese traditionalAngelica sinensis
Owner:蚌埠丰原涂山制药有限公司
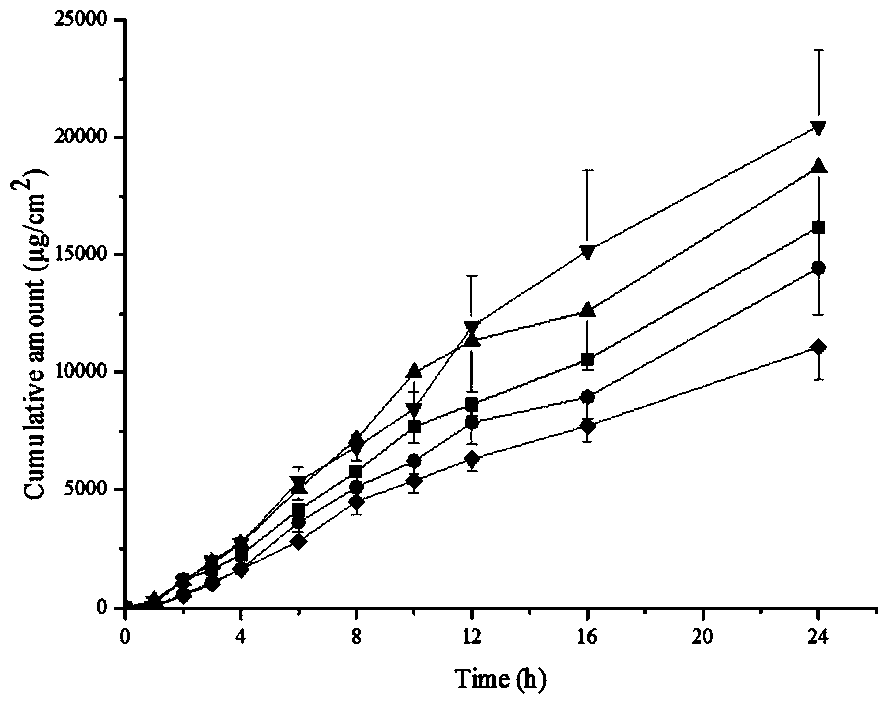
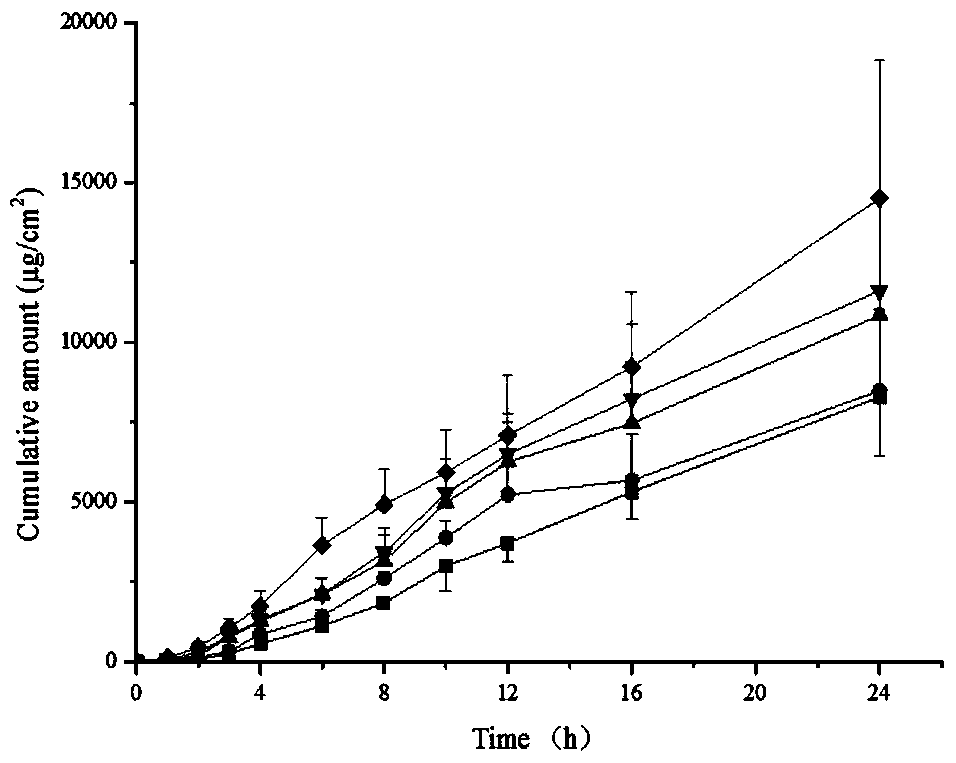
Methimazole microemulsion, methimazole microemulsion gel and preparation method and application thereof
ActiveCN105030671BGood curative effectImprove solubilityOrganic active ingredientsAerosol deliverySolubilityTherapeutic effect
The invention relates to methimazole microemulsion, methimazole microemulsion-based gel and a preparation method and application of the methimazole microemulsion. The methimazole microemulsion comprises methimazole, oil, surfactant, cosurfactant, water and transdermal Enhancer according to a weight ratio of 1:(1.40-4.25):(5.13-15.45):(5.13-15.45):(1.40-8.32):(0.26:1.26). The methimazole microemulsion-based gel is prepared by adding gel matrix into the methimazole microemulsion, allowing swelling and performing uniform mixing. The methimazole microemulsion and the methimazole microemulsion-based gel are directly applicable to affected parts, are also applicable to the preparation of hyperthyroidism pharmaceuticals such as patches or plasters, and have the advantages such as good solubility, high penetration capacity, long acting time, evident improvement in therapeutic effect, fewer times of administration, and convenience of administration.
Owner:HARBIN MEDICAL UNIVERSITY
Medicine for treating acute pharyngitis
InactiveCN106619592AGood reliefEasy to takeRespiratory disorderHeterocyclic compound active ingredientsSide effectAcute Pharyngitis
The invention discloses a medicine for treating acute pharyngitis. The medicine for treating the acute pharyngitis is prepared from the following main raw materials in parts by weight: 5 to 12 parts of cefalexin, 8 to 15 parts of methimazole, 13 to 18 parts of vitamin E, 5 to 9 parts of phenprobamate, 11 to 16 parts of analginum, 2 to 6 parts of calcium gluconate, 4 to 10 parts of acyclovir and 5 to 8 parts of bromhexine hydrochloride. The medicine disclosed by the invention is used for treating the acute pharyngitis; the medicine is convenient to take, small in side effect, has the effects of clearing heat and relieving sore throat, and has the major function of treating the acute pharyngitis; practical inspection shows that the medicine has an obvious relieving effect on the acute pharyngitis.
Owner:ZHENGZHOU ZHENGXIAN PHARMA CO LTD
Methimazole detection method
ActiveCN107525791BLow costSimple and fast operationFluorescence/phosphorescenceFluoProbesPhoto irradiation
The invention provides a methimazole detection method, which comprises the following steps of mixing methimazole with a first AuNPs solution to obtain a mixed solution; irradiating a first mixed solution formed by mixing the mixed solution with a first fluorescent N / S-CQDs solution with ultraviolet light to obtain a fluorescence spectrogram of the first mixed solution; irradiating a second mixed solution formed by mixing a second AuNPs solution and a second fluorescent N / S-CQDs solution with ultraviolet light to obtain a fluorescence spectrogram of the second mixed solution; obtaining the content of the methimazole according to the fluorescence spectrogram of the first mixed solution and the fluorescence spectrogram of the second mixed solution. The methimazole detection method provided by the invention uses the fluorescent N / S-CQDs as energy donors and AuNPs as energy receptors to construct a complex fluorescent probe, and the methimazole is detected by using the complex fluorescent probe. Compared with high performance liquid chromatography, flow injection spectrophotometry and the like, the cost is low, and the operation is easy.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com