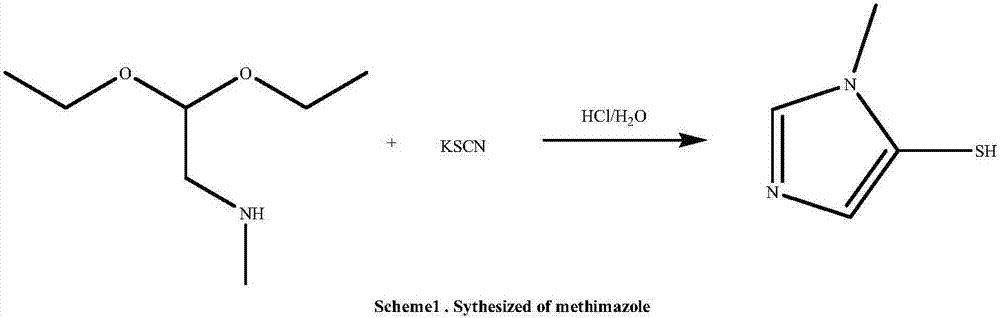
Methimazole synthesizing and purifying method
A technology of methimazole and a purification method is applied in the preparation field suitable for industrial production, can solve problems such as harsh operating conditions, unfavorable industrial production and the like, and achieves the effects of short production cycle, easy industrial production and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Mix methylaminoacetaldehyde ethylene acetal (294g, 2.0mol, 1.0eq), ammonium thiocyanate (198g, 2.6mol, 1.3eq) and deionized water (200mL) and stir to dissolve, then add benzyl triethyl ammonium chloride (22.8g, 2.6mol, 0.05eq), p-toluenesulfonic acid (38.1g, 0.2mol, 0.1eq), the temperature was raised to 50°C for condensation reaction for 8h, and the disappearance of raw materials was monitored by TLC (V PE / EA =15:1, KMnO 4 Color development), after the reaction is completed, add 200mL saturated saline, tetrahydrofuran (300mL*2), extract, separate liquids, combine the organic layers, dry over anhydrous sodium sulfate, concentrate to obtain 171g of the crude product of light red solid methimazole, the yield 75% with a purity of 94.7%.
[0022] Add 150g of the light red crude product methimazole into 300mL of methanol, heat to dissolve completely, add activated carbon to decolorize, reflux for 30min, filter while hot, cool to 0°C, filter, and vacuum dry at 45°C to obta...
Embodiment 2
[0024] Stir and dissolve methylaminoacetaldehyde ethylene acetal (221g, 1.5mol, 1.0eq), ammonium thiocyanate (206g, 2.7mol, 1.8eq), deionized water (200mL), and then add PEG-2000 (15g) The temperature was raised to 40°C, and then 98% concentrated sulfuric acid (40mL g, 0.74mol) was slowly added thereto, and the addition was completed in 1h, then the temperature was raised to 70°C for 5h, and TLC monitored the disappearance of the raw material (V PE / EA =15:1, KMnO 4 color development), after the reaction was completed, 200mL saturated saline and tetrahydrofuran (300mL*2) were added, extracted, separated, and the organic layers were combined, dried over anhydrous sodium sulfate, and concentrated to obtain 139.4g of a light red solid methimazole crude product. The yield is 81.4%, and the purity is 95.8%.
[0025] Add 120g of the light red crude product methimazole into 200mL of ethanol, heat to dissolve completely, add activated carbon to decolorize, reflux for 30min, filter ...
Embodiment 3
[0027] Stir and dissolve methylaminoacetaldehyde ethylene acetal (353g, 2.4mol, 1.0eq), ammonium thiocyanate (274g, 3.6mol, 1.5eq), deionized water (260mL), and then add tetrabutylammonium bromide (38.7g, 0.12mol, 0.05eq) was warmed up to 40°C, and then 37% concentrated hydrochloric acid (50mL, 0.6mol) was slowly added thereto for 1h, then the temperature was raised to 60°C for 6h, and TLC monitored the disappearance of the raw material (V PE / EA =15:1, KMnO 4 color development), after the reaction is completed, add 300mL saturated saline, 1,4-dioxane (450mL*2), extract, separate liquids, combine organic layers, dry over anhydrous sodium sulfate, concentrate to obtain light red solid formazan Thiamimazole crude product 255g, yield 93.2%, purity 96.8%.
[0028] Add 250g of the light red crude product methimazole into 500mL of acetone, heat to dissolve completely, add activated carbon to decolorize, reflux for 30min, filter while hot, cool to 0°C, filter, and vacuum dry at 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com