Patents
Literature
68 results about "Midazolam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Midazolam is used before surgery or a procedure.
Methods and compositions for improving drug safety
A pharmaceutical composition with improved safety includes a selected amount of a vomit-inducing agent, wherein the selected amount is less than an amount needed to induce vomit in a user; and a therapeutic agent. The therapeutic agent may be selected from a sleeping pill, an anxiolytic, a hypnotic, a contraceptive agent. The therapeutic agent may also be selected from diazepam, flunitrazepam, alprazolam, triazolam, fludiazepam, midazolam, estazolam, zopiclone, and a combination thereof. The vomit-inducing agent may be selected from emetine, cephaeline, and a combination thereof.
Owner:LOTUS PHARMA CO LTD
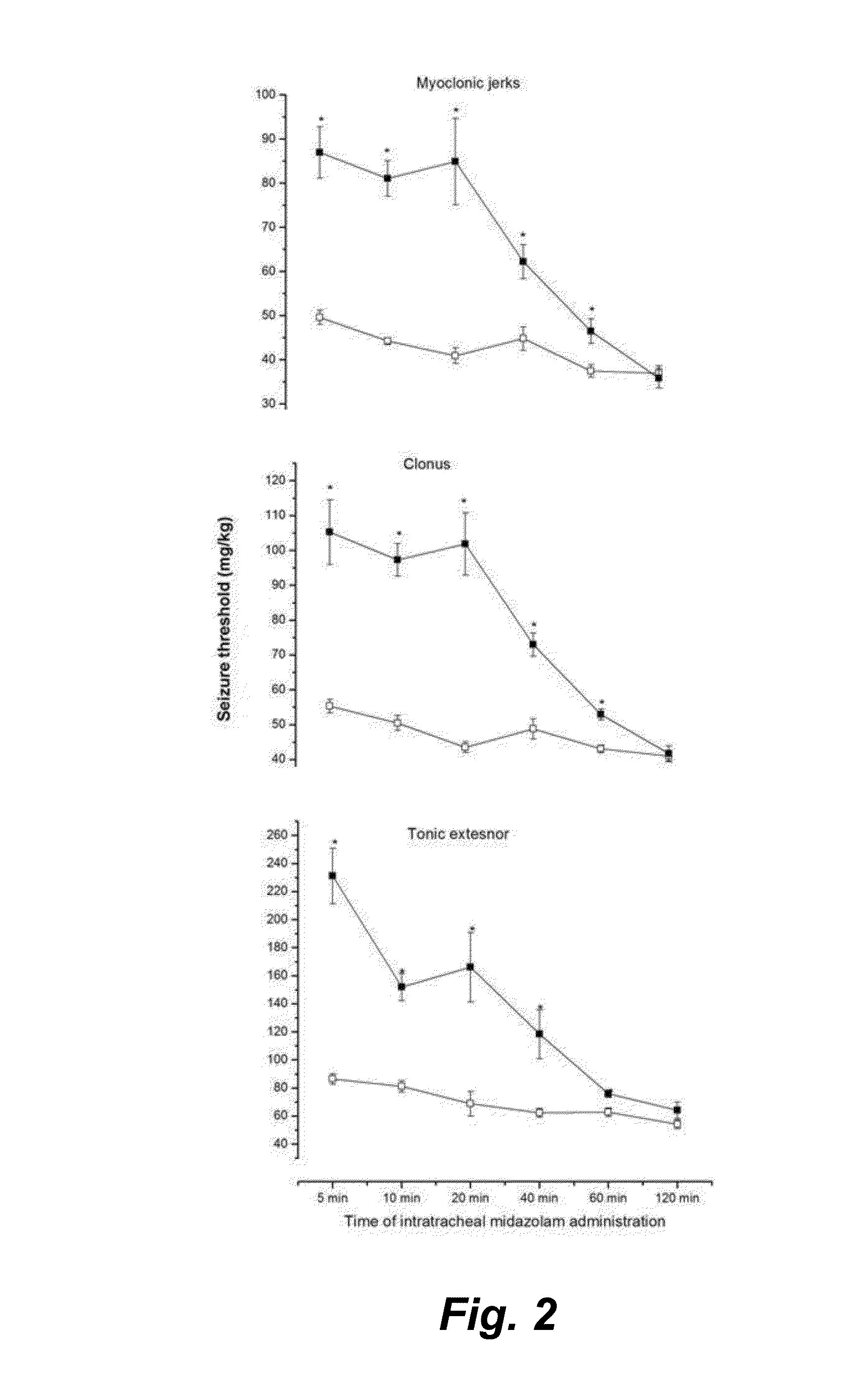
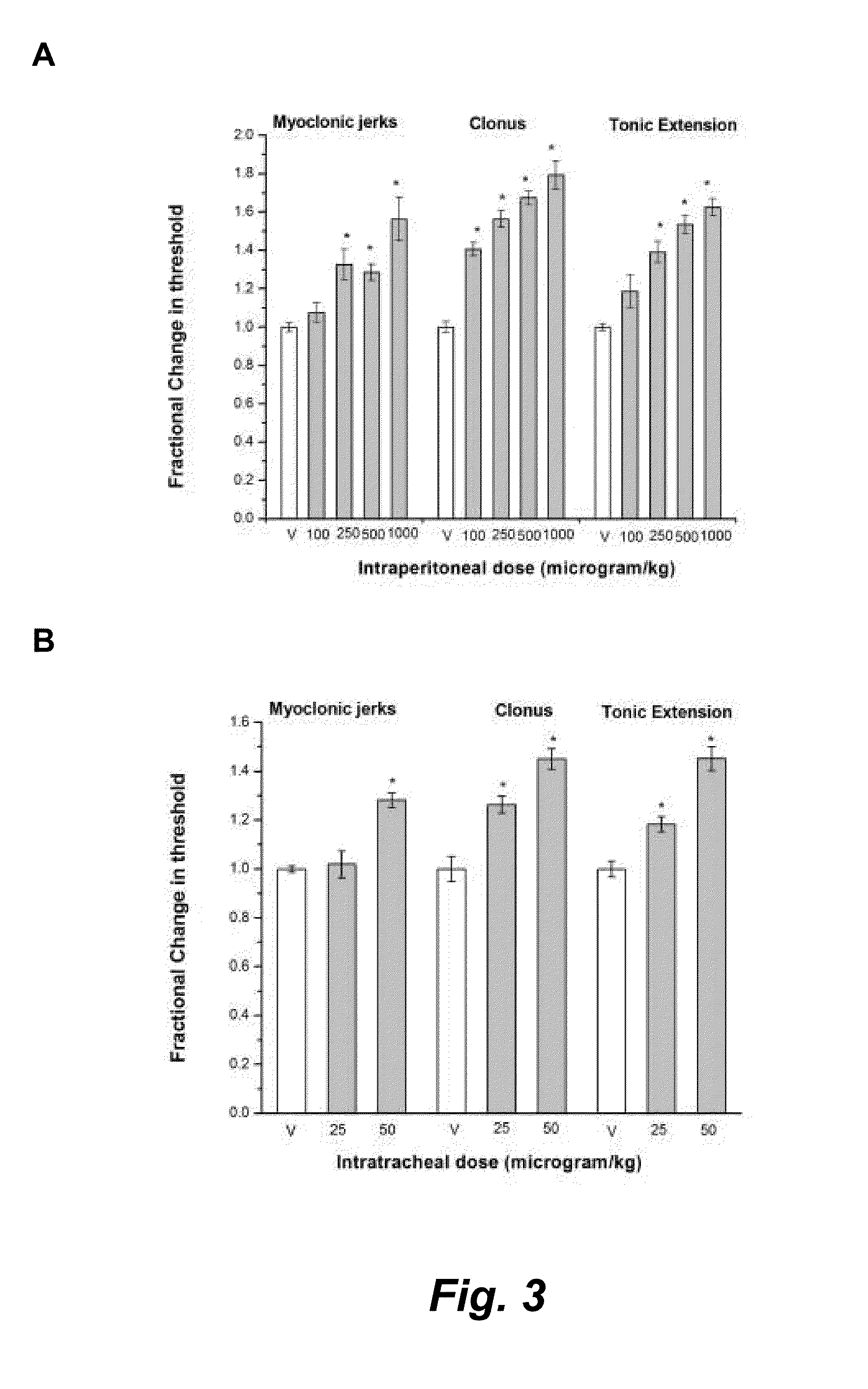
Intrapulmonary benzodiazepine for the treatment and prevention of seizures
This invention provides methods for the amelioration, termination and / or abortion of a seizure by the intrapulmonary administration of a benzodiazepine, for example, midazolam.
Owner:RGT UNIV OF CALIFORNIA
Pharmaceutical compositions comprising midazolam in a high concentration
ActiveUS20050153956A1Efficiently and comfortably administeredLittle irritationBiocideNervous disorderHigh concentrationSolvent
Compositions of midazolam, a benzodiazapine, in concentrations of 35-100 mg / ml are disclosed for the treatment of anxiety, epilepsy and epileptic seizures, invasive surgical procedures and diagnostic procedures and sedation. These compositions are particularly characterized by a solubilizer such an propylene glycol. Preferably, the compositions are aqueous solutions for intranasal administration.
Owner:MERKUS FRANCISCUS WILHELMUS HENRICUS MARIA
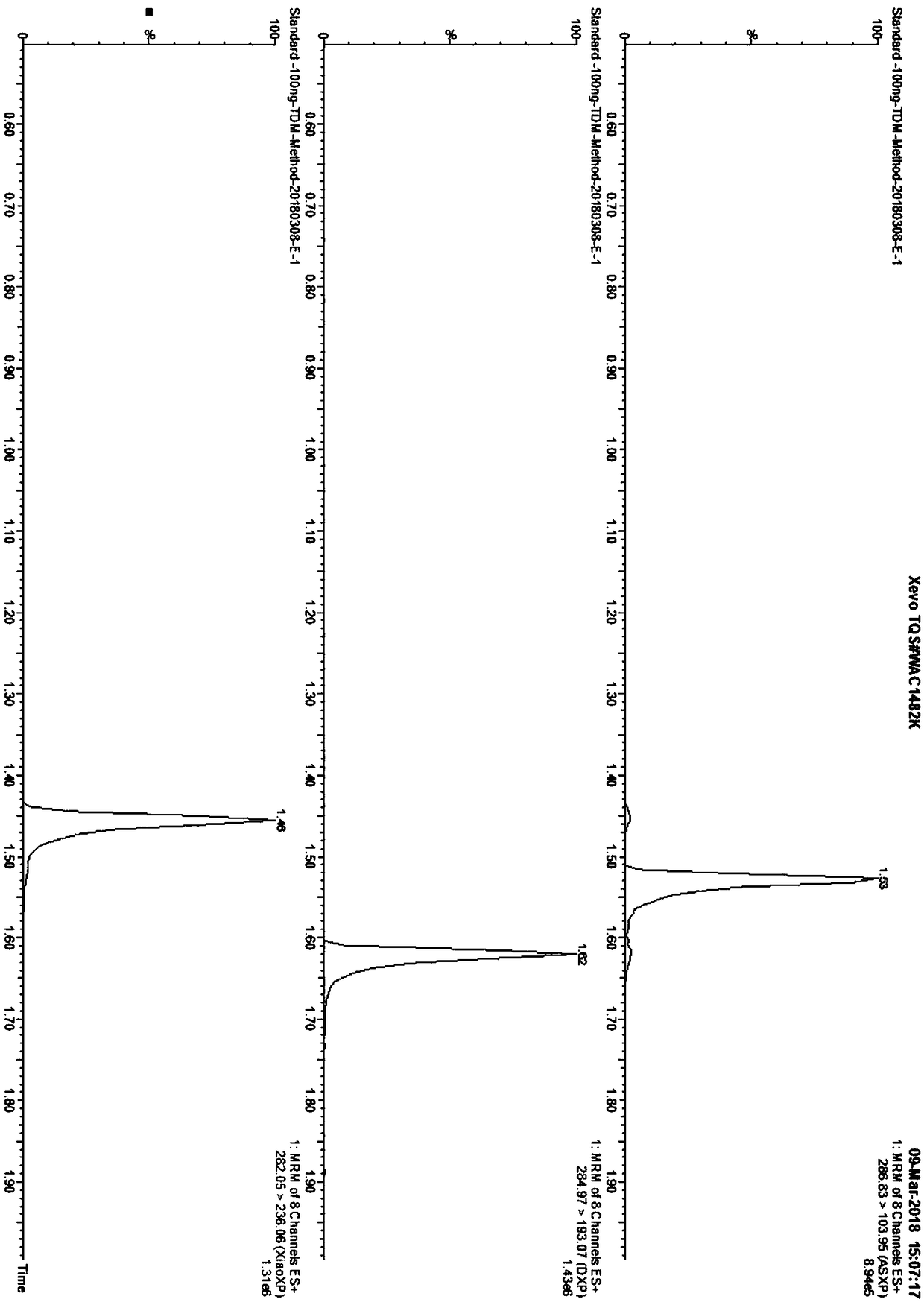
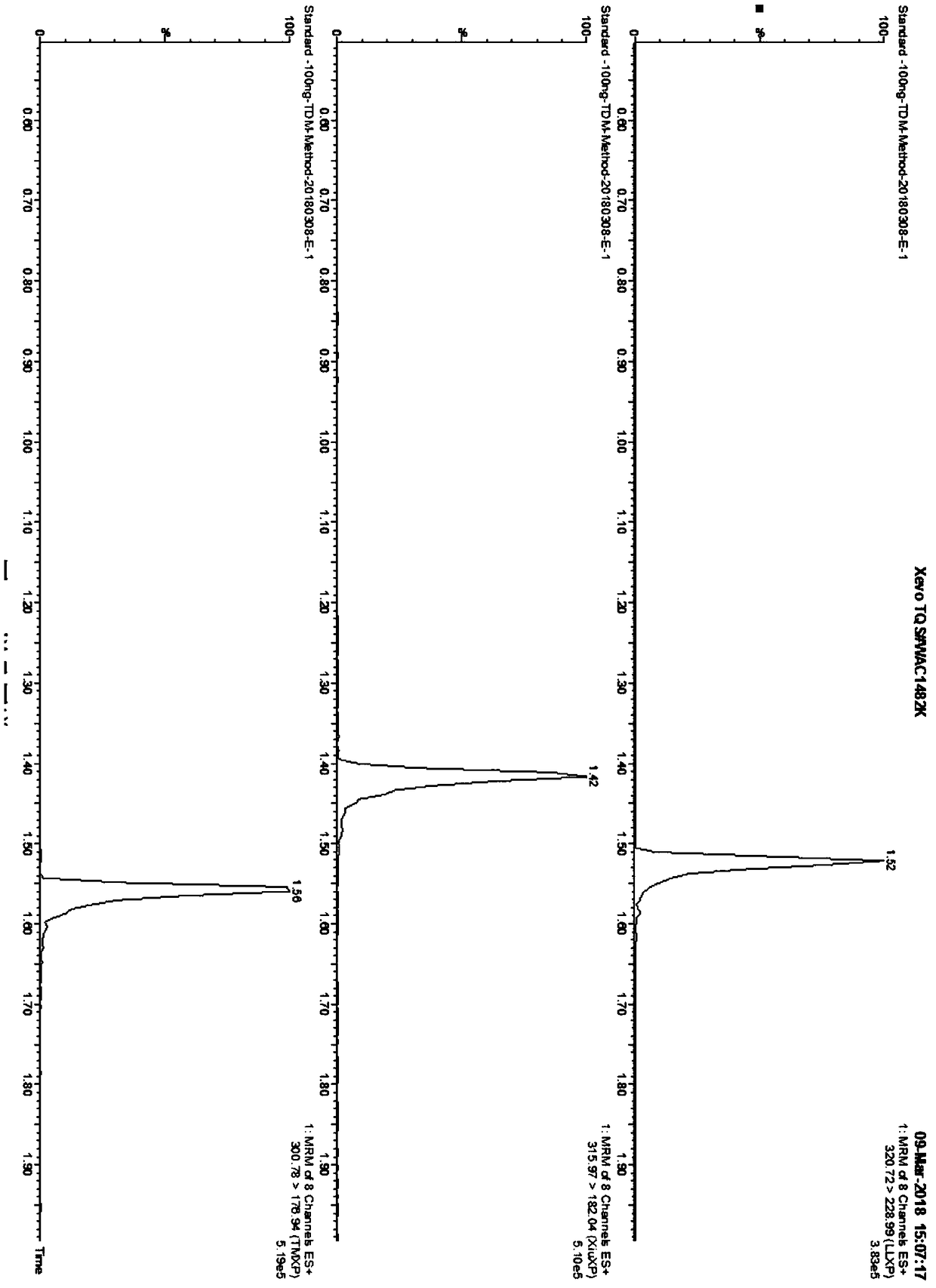
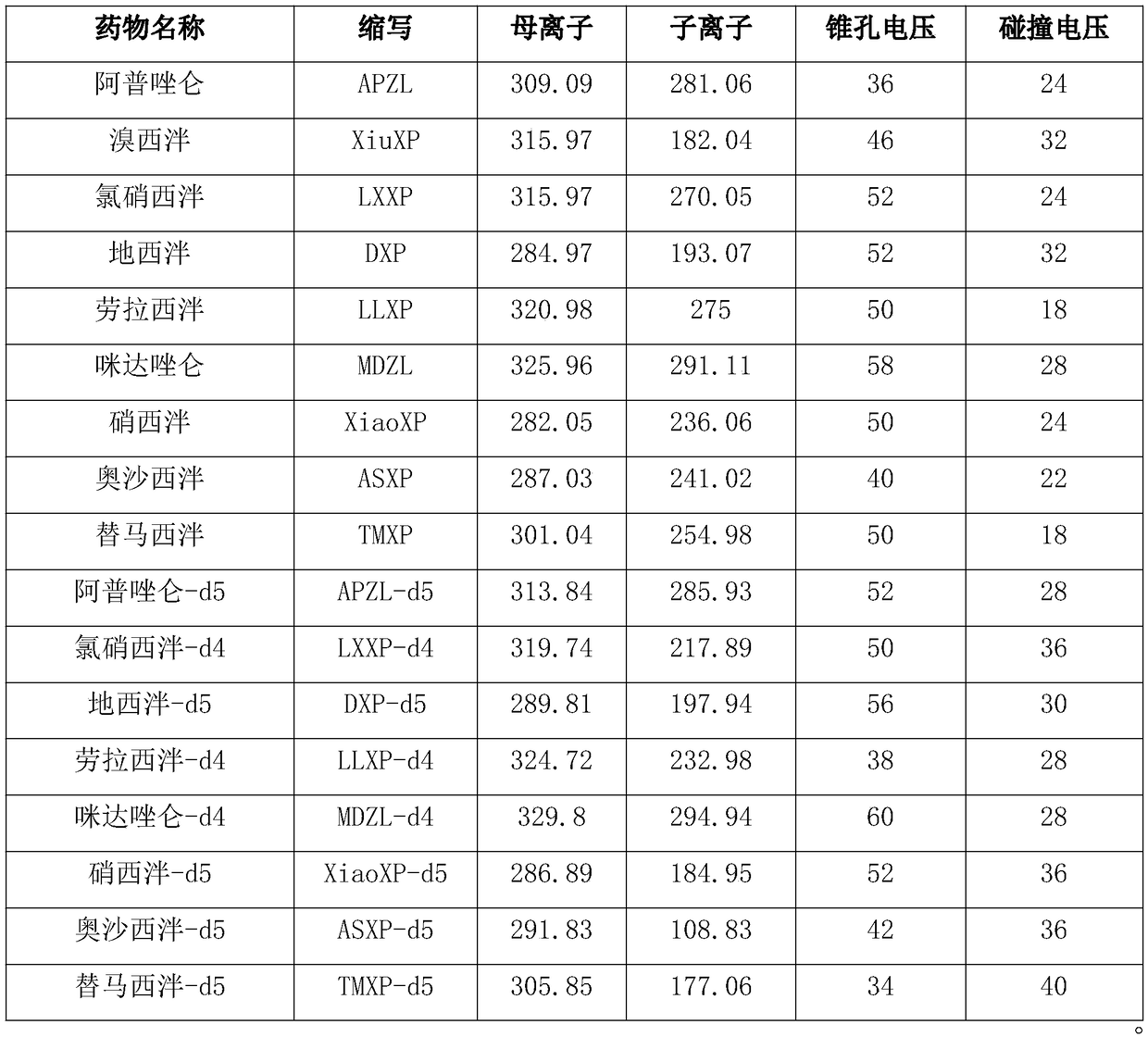
Kit for determining antianxiety/hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085265AReduce matrix effectThe test result is accurateComponent separationBromazepamTandem mass spectrometry
The invention provides a kit for determining antianxiety / hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry. The kit comprises the following constituents: drug standards comprising bromazepam, clonazepam, diazepam, lorazepam, midazolam, nitrazepam, oxazepam and Temazepam; drug internal standard compounds comprising alprazolam-d5, clonazepam-d4, diazepam-d5, lorazepam-d4, midazolam-d4, nitrazepam-d5, oxazepam-d5 and Temazepam-d5; drug extraction compositions comprising, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropanol solution and 10% of purified water; negative plasma; and diluent: 50% of carbinol water solution. The kit can be used for simultaneously determining antianxiety / hypnotic type drugs and active metabolites thereof, and has the advantages of short determination time and high flux.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Process for producing highly pure midazolam and salts thereof
InactiveUS20090069306A1High yieldHigh purityBiocideNervous disorderPharmaceutical SubstancesOrganic chemistry
Owner:CHEMAGIS
Pharmaceutical compositions comprising midazolam in a high concentration
InactiveUS20060009447A1Efficiently and comfortably administeredSmall volumeBiocideNervous disorderHigh concentrationSolvent
Owner:MERKUS FRANCISCUS WILHELMUS HENRICUS MARIA
Midazolam medicament composition as well as preparation method and application thereof
ActiveCN102309438APromote absorptionImprove bioavailabilityOrganic active ingredientsNervous disorderCurative effectBioavailability
The invention belongs to fields of pharmacy and pharmaceutics and relates to a midazolam medicament composition as well as a preparation method and application thereof. Particularly, the midazolam medicament composition comprises midazolam and / or pharmaceutically acceptable salts of midazolam, serving as main medicament components, and pharmaceutically acceptable auxiliary materials, wherein the content of the main medicament components is 0.2%-2%. The midazolam medicament composition provided by the invention is administered through an orifice, so that the bioavailability and curative effectof the midazolam medicament are improved, and favorable compliance can be achieved.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
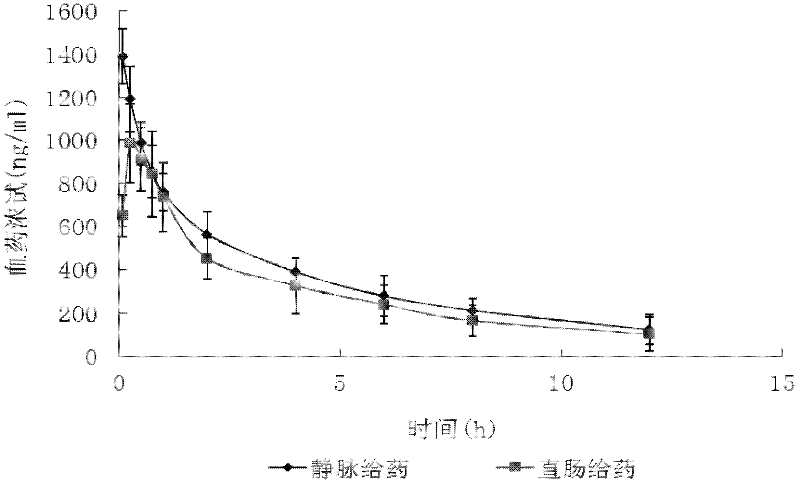
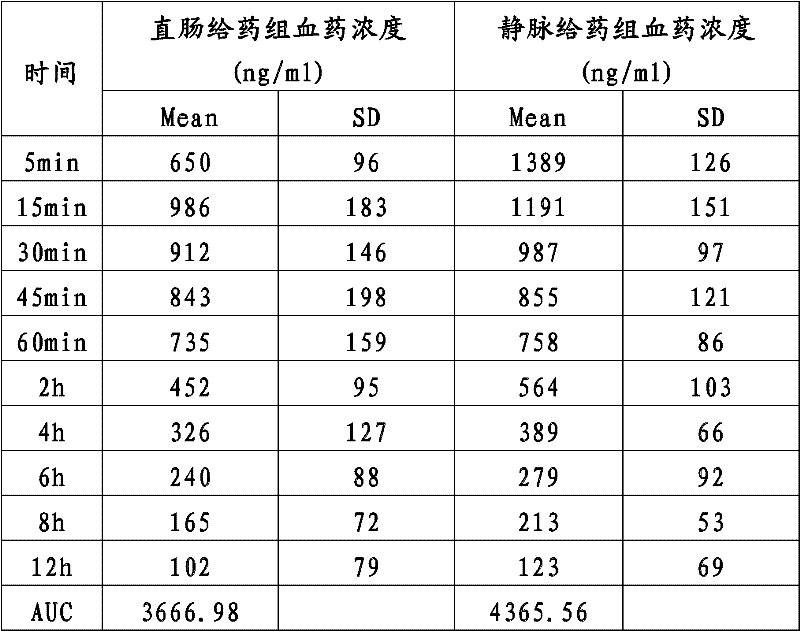
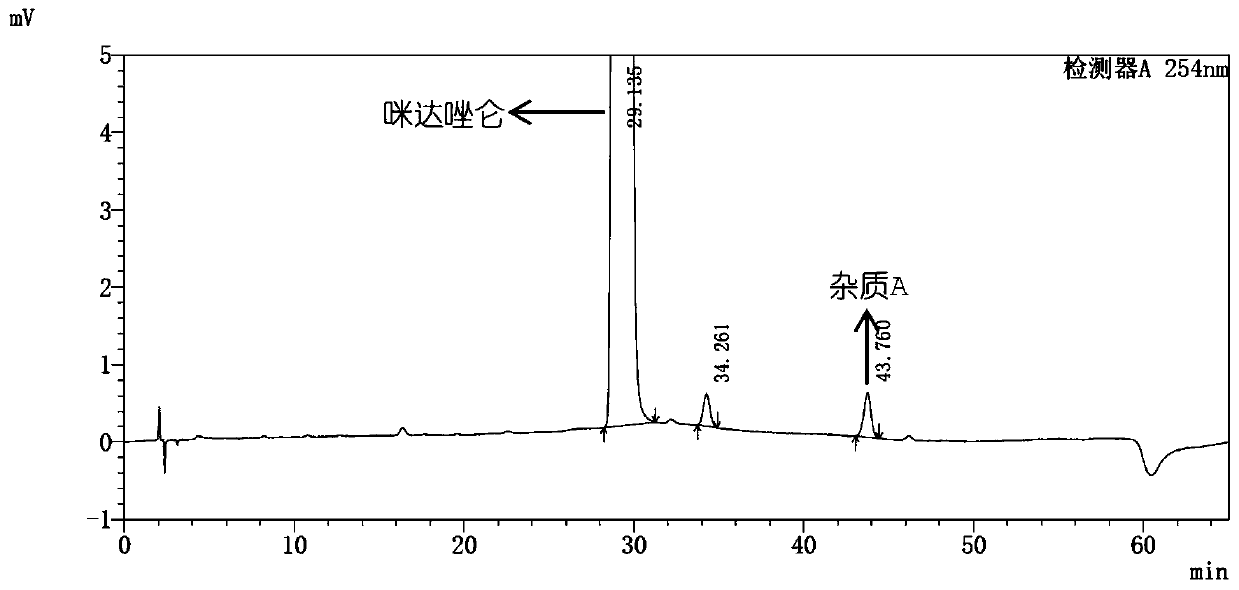
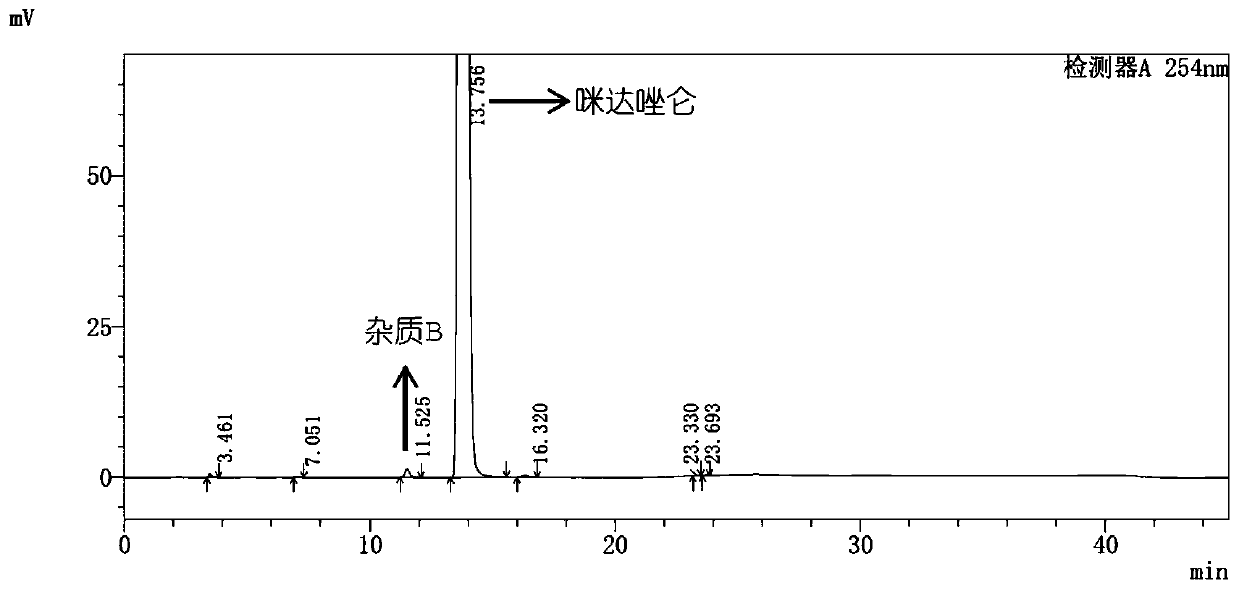
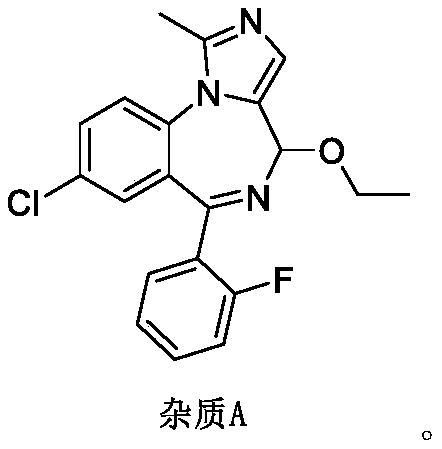
Impurity A and impurity B of midazolam or pharmaceutical composition thereof and application thereof
The invention belongs to the field of medical chemistry, and particularly relates to an impurity A and an impurity B of midazolam or a pharmaceutical composition of midazolam and a preparation methodthereof, and application of the impurity A and the impurity B as reference standard substances for quality control of midazolam or the pharmaceutical composition of midazolam.
Owner:NHWA PHARMA CORPORATION
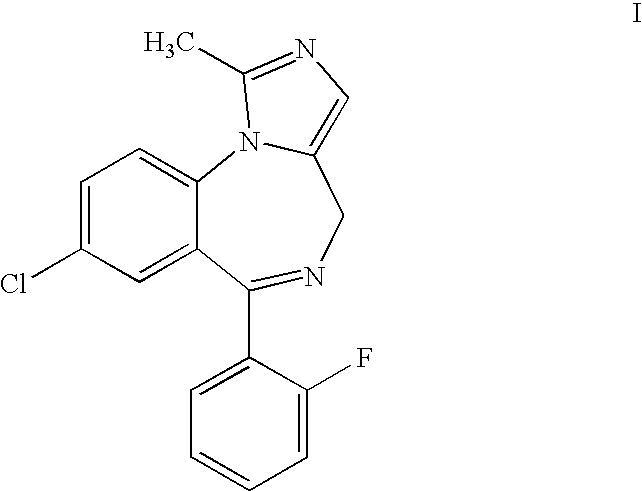
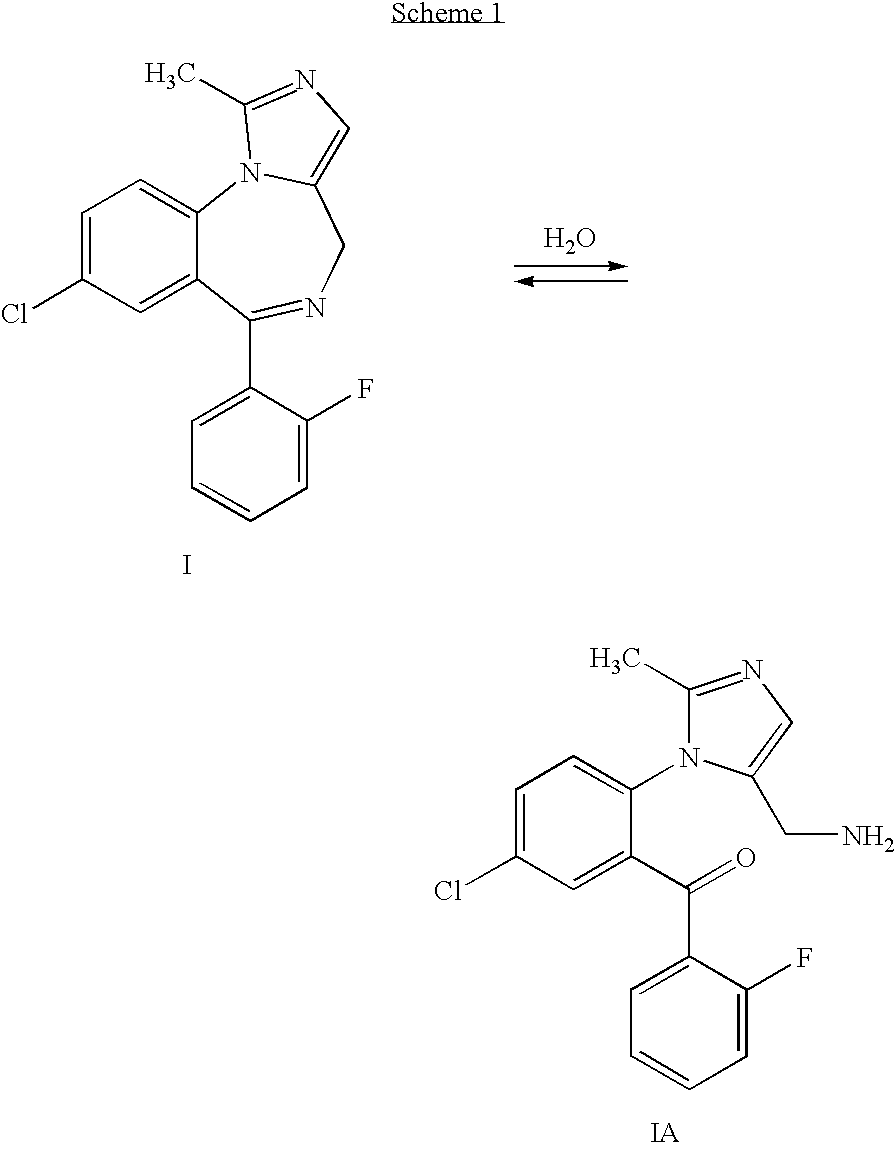
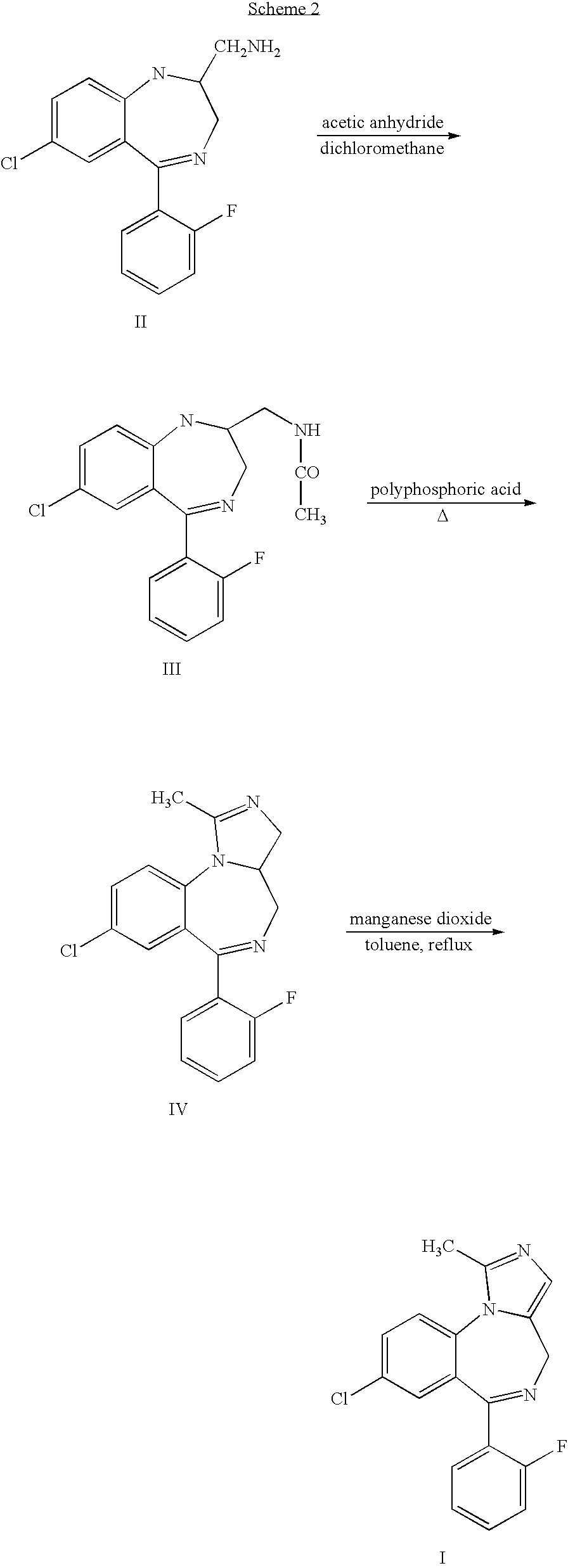
Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam
InactiveCN103319486AMaintain configurationReduce formationNervous disorderOrganic chemistryBenzodiazepineIsomerization
The invention relates to a method for preparation of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam through a selective decarboxylation reaction of formula (VII) with a DBU (1,8-diazabicyclo[5.4.0.]-undec-7-ene) novel catalyst in an NMP (N-methyl pyrrolidone) solvent. According to the invention, isomerization of 4H-imidazo[1, 5-a][1, 4]benzodiazepine is avoided, the midazolam synthesis yield is enhanced, and the post-treatment difficulty is reduced.
Owner:XUZHOU CARDIOVASCULAR INST +1
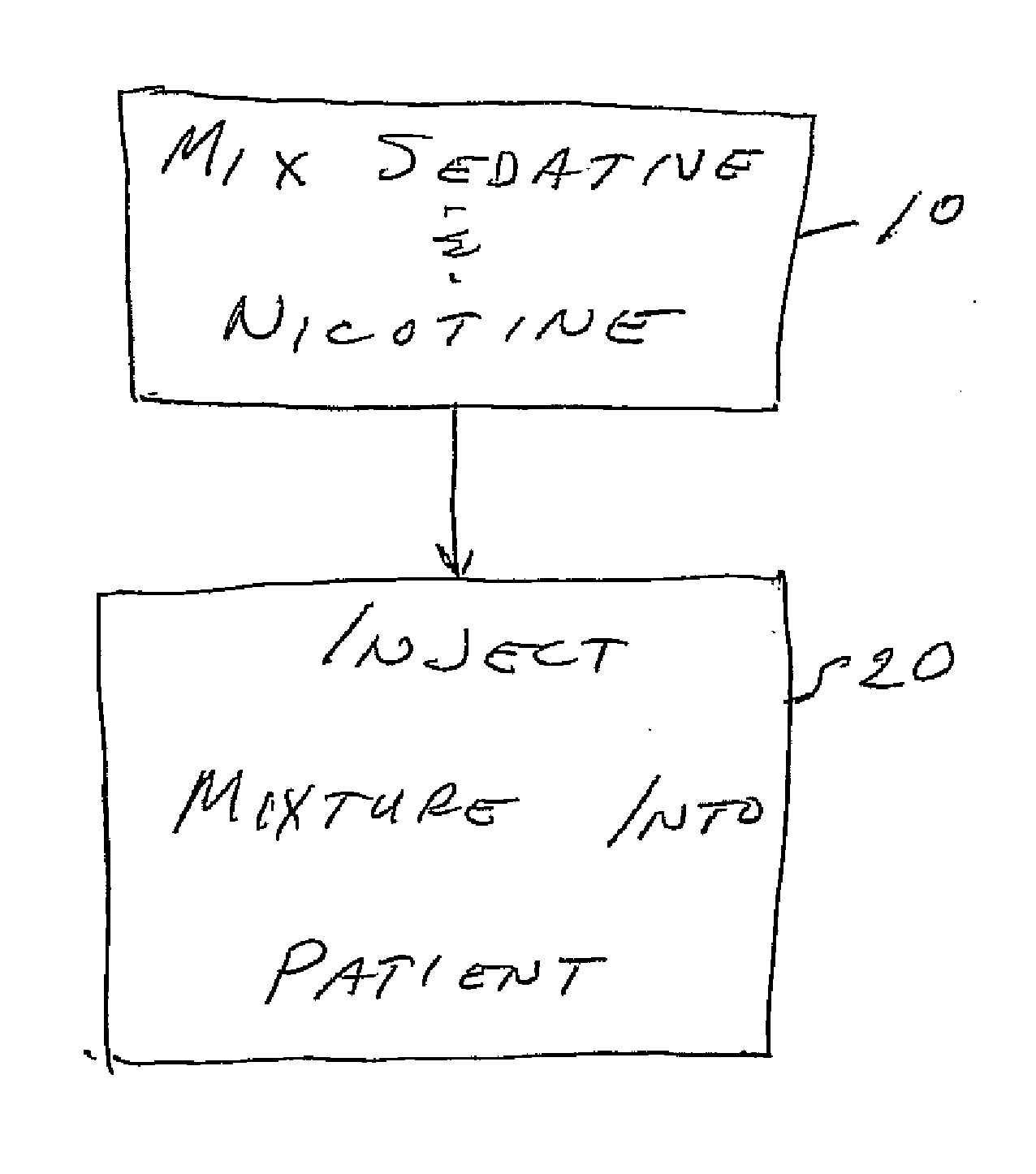
Sedative for use during eye surgery
InactiveUS20110046116A1Reduces twitchingReduces fidgetingBiocideNervous disorderMedicineTranquilizing Agents
A drug for sedating a patient, and especially a smoker, during eye surgery. The drug includes a sedative such as midazolam in a mix with nicotine. The mixture is injected into the patient prior to the eye surgery.
Owner:CUKROWSKI WALTER J
Midazolam combination as well as preparation method and application thereof
ActiveCN102462685AImprove physical stabilityGood chemical stabilityOrganic active ingredientsNervous disorderConvulsionMethyl-beta-cyclodextrin
The invention belongs to the field of medicines and pharmaceutics, and relates to a midazolam combination, a preparation method of the midazolam combination, and the application of the midazolam combination. Specifically, the midazolam combination comprises 2.5 to 8.0 percent (g / ml) of midazolam, 5 to 30 percent (g / ml) of cyclodextrin and 30 to 80 percent (ml / ml) of polyols through being calculated according to the total volume of the midazolam combination, wherein the cyclodextrin is carefully selected from one or more of beta cyclodextrin, hydroxypropyl-beta cyclodextrin, gamma cyclodextrin, dimethyl beta cyclodextrin, trimethyl beta cyclodextrin, hydroxypropyl beta cyclodextrin and HE-beta cyclodextrin; and the polyols are selected from one or more of propylene glycol, glycerol, polyoxyethylene castor oil and polyethylene glycol. The concentration of the midazolam in the combination can reach above 25mg / ml and the pH (potential of hydrogen) value of the midazolam is between 4.5 and 10.0. The invention additionally relates to the preparation method of the combination and the application of the combination during the preparation of medicines for curing epilepsy and achieving the effects of anti-anxiety, sedation, peaceful sleeping, muscular relaxation or anti-convulsion.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF
The present invention refers to a process for the preparation of 4H-imidazo[1,5-a][1,4]benzodiazepines, in particular Midazolam, through an efficient and selective decarboxylation reaction of the derivative compound of the 5-aminomethyl-1-phenyl-1H-imidazole-4-carboxylic acid of formula (II)avoiding the formation of the 6H-imidazo[1,5-a][1,4]benzodiazepines by-products and the ensuing process for the isomerisation of a 4H-imidazo[1,5-a][1,4]benzodiazepine product.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Novel probe medicament combination and preparation method and application thereof
InactiveCN101596321AReduced drug metabolism in vivoEstimated metabolismIn-vivo testing preparationsLiver ischemiaPharmaceutical drug
The invention relates to a novel cocktail probe medicament combination comprising caffeine, metoprolol, chlorzoxazone, tolbutamide and midazolam. The influence of hepatic ischemia preconditioning and hepatic ischemia-reperfu-sion injury on medicament metabolism activity of rat CYP1A2, CYP2C9, CYP2E1, CYP2D6, and CYP3A4 in vivo is evaluated through the change of pharmacokinetics parameters of the combination.
Owner:娄建石
Bronchoalveolar lavage fluid collection method for 16S rRNA gene detection and detection method
The invention discloses a bronchoalveolar lavage fluid collection method for 16S rRNA gene detection, which comprises the following steps: letting a subject inhale a bronchodilator through atomization, and leaving a venous indwelling needle; continuously supplying oxygen to a nasal catheter in the whole process of collecting bronchoalveolar lavage fluid while monitoring the transcutaneous oxygen saturation and judging the vital signs of the subject according to the transcutaneous oxygen saturation; performing surface anesthesia of superior nasal meatus and pharynx and larynx with 2% lidocaine, and then performing intravenous injection with 2-10mg of midazolam and 125-500mg of alfentanil; and letting a fiber bronchoscope enter the glottis area through the nasal meatus, and anesthetizing the glottis area with 4% lidocaine. The invention aims at providing the bronchoalveolar lavage fluid collection method for 16S rRNA gene detection and the detection method thereof, wherein the collection method is safe, simple and convenient, brings little pollution and has good repeatability.
Owner:张清玲 +3
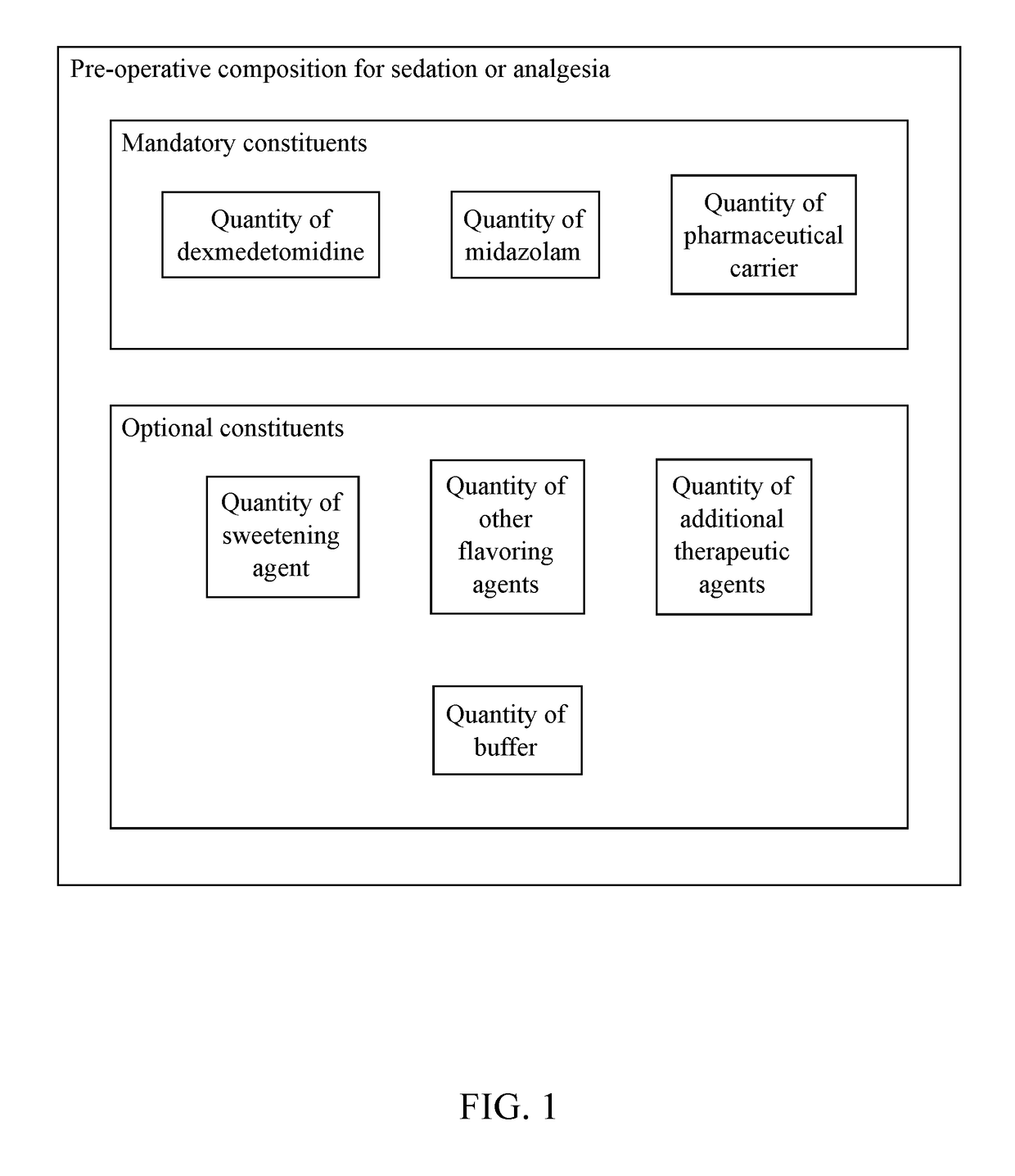
Pre-Operative Composition for Sedation or Analgesia
InactiveUS20170326154A1Organic active ingredientsPharmaceutical delivery mechanismDrug carrierPre operative
A pre-operative composition for sedation or analgesia is used to sedate a patient before a medical procedure. The pre-operative composition is formulated to prevent negative effects felt by the patient after the medical procedure. The pre-operative composition includes dexmedetomidine, midazolam, and some kind of pharmaceutical carrier. The pre-operative composition can be administered in a variety of methods to the patient. The preferred method of administering the pre-operative composition is either orally or by a syringe.
Owner:PARKER STEPHEN
Stable liquid inectable solution of midazolam and pentazocine
ActiveUS20190076411A1Induction of sedationReliable inductionOrganic active ingredientsInorganic non-active ingredientsPentazocineChemistry
The invention discloses compositions of clear injectable solution which comprises Midazolam, pentazocine, tonicity agent, chelating agent, and acids to adjust pH.
Owner:NEON LAB
Method for testing influence of midazolam on development of testicular interstitial cells cultured in vitro
ActiveCN114181885APromote differentiationPromote productionCell dissociation methodsMicrobiological testing/measurementTesticular Interstitial CellsMidazolam
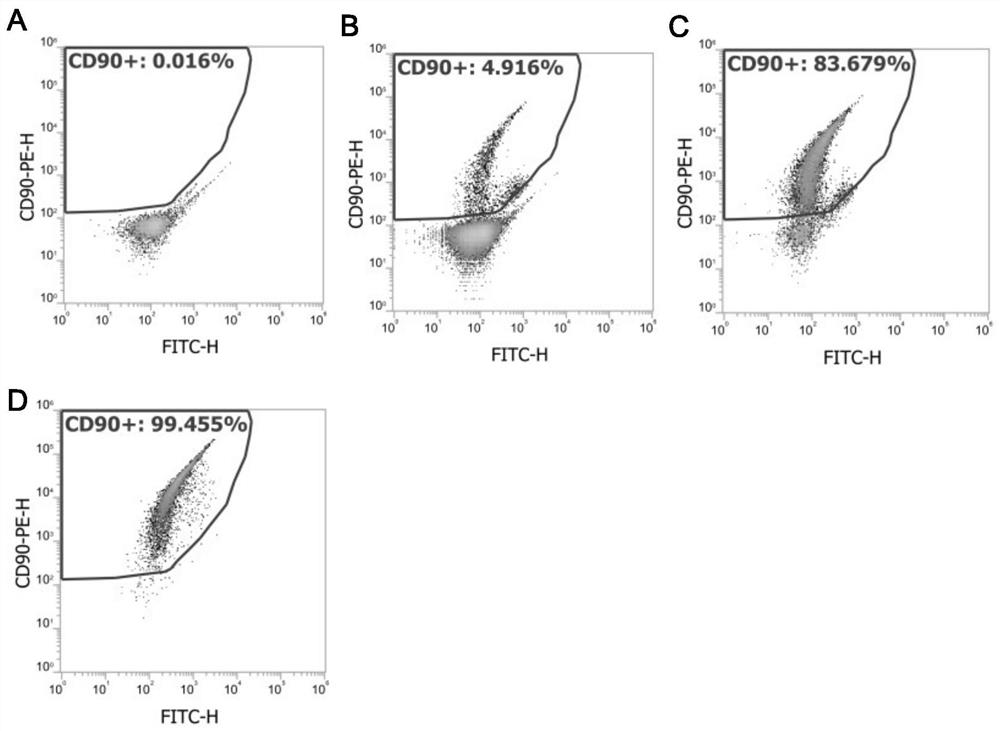
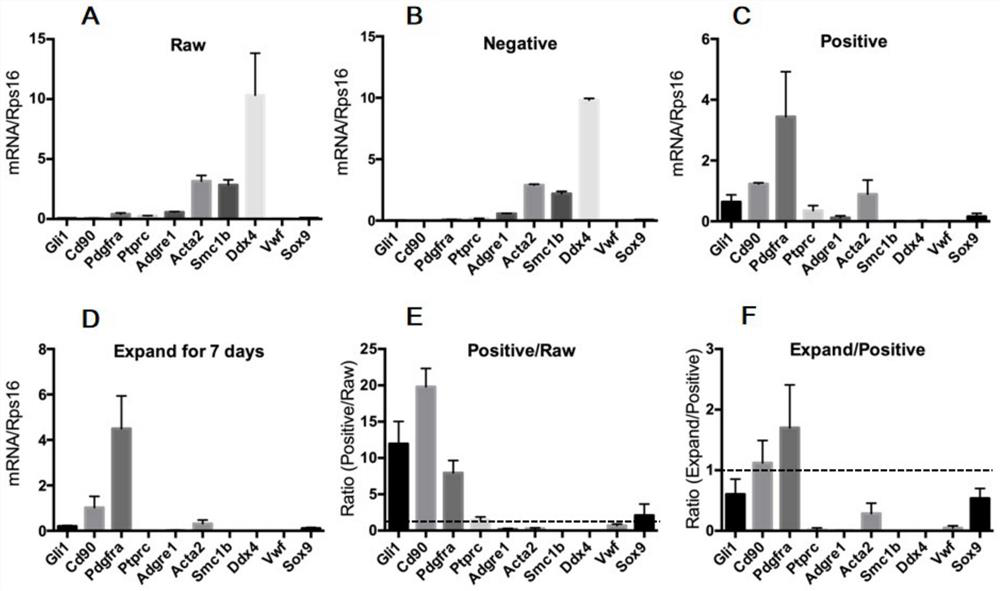
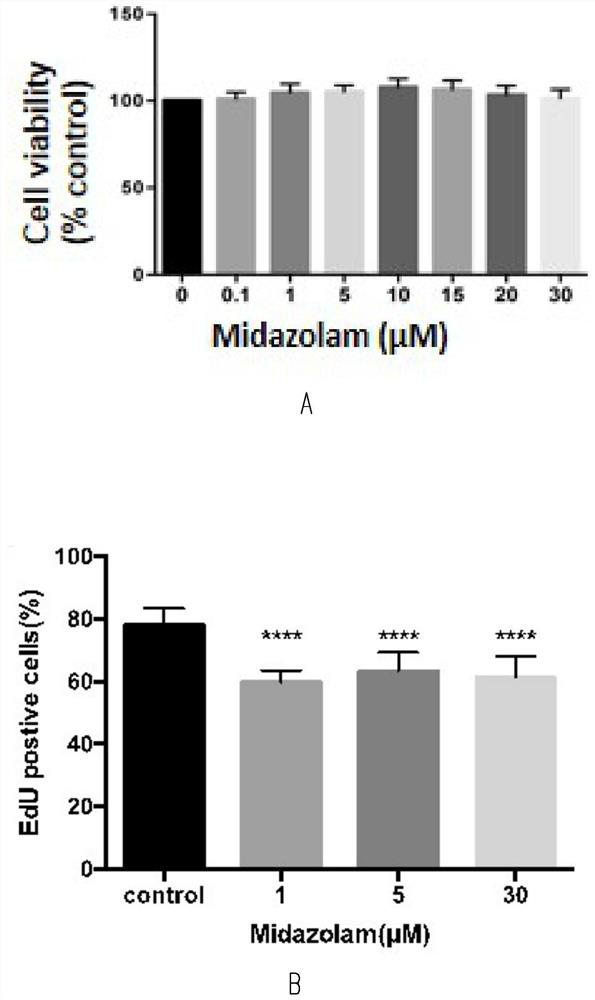
The invention discloses a method for testing influence of midazolam on development of testicular interstitial cells cultured in vitro. Comprising the following steps: culturing rats, separating CD90 positive cells, culturing testicular mesenchymal stem cells (SLC), detecting the influence of midazolam on proliferation and differentiation, amplifying the SLCs and detecting the cell activity, carrying out DAPI dyeing and counting, analyzing cells by a flow cytometer, analyzing testosterone (T) and progesterone (P4), carrying out mRNA and protein expression detection through QPCR (Quantitative Polymerase Chain Reaction) and WB (White Brown) experiments, and determining significant differences and drawing a chart through SNK (Sodium Nitrogen Kinase) detection. The steps are simple, and the influence of midazolam medicine on proliferation and differentiation of the testicular mesenchymal stem cells can be effectively verified.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Compound anesthetic for rat and preparation method thereof
InactiveCN104055780ASlight respiratory depressionMetabolism is less affected by renal functionAnaestheticsHeterocyclic compound active ingredientsPhysiologic toleranceDexmedetomidine
The invention discloses a compound anesthetic for a rat and a preparation method thereof and belongs to the field of preparation and application of animal compound anesthetics. The invention discloses the compound anesthetic for the rat for the first time. The compound anesthetic comprises dexmedetomidine, midazolam, oxycodone, a buffer solution and water for injection. The invention further discloses the preparation method of the compound anesthetic for the rat. The preparation method comprises the following steps: dissolving the above components in the water for injection; diluting; filtering; pilling in an ampoule bottle; and sterilizing to obtain. The compound anesthetic for the rat disclosed by the invention is quick in anesthesia induction, long in anesthesia maintenance time, good in antalgic and sedative effects and stable in revival, and the main influence on physiological and biochemical indexes of the rat is within the physiological range tolerance. The compound anesthetic is simple to operate and can be used for complex or long-term operations of the rat.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
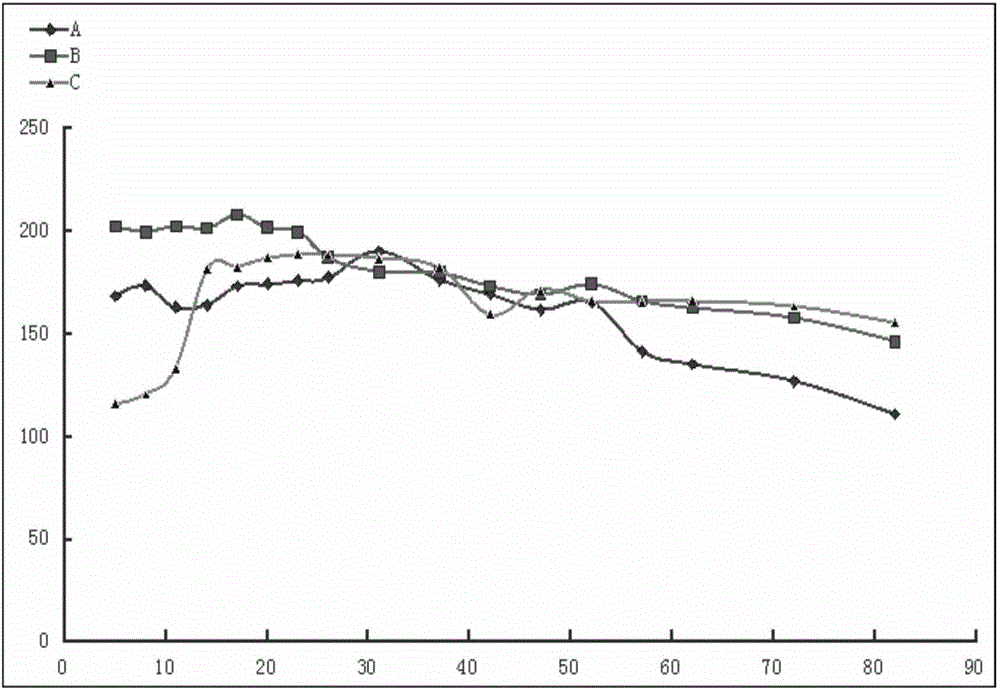
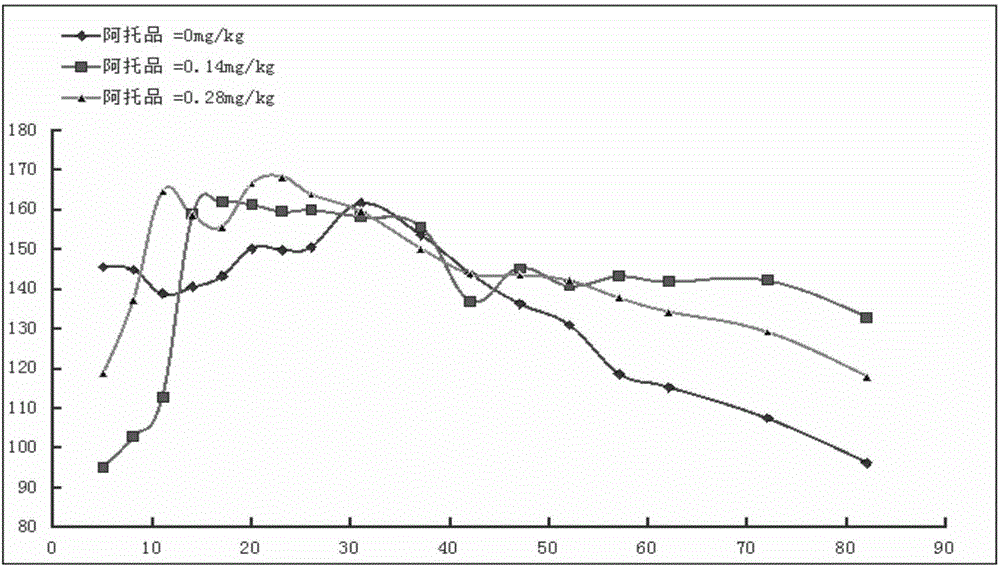
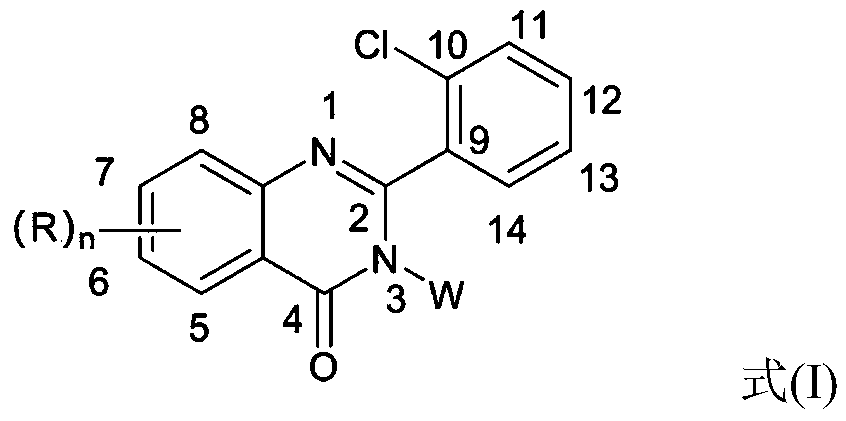
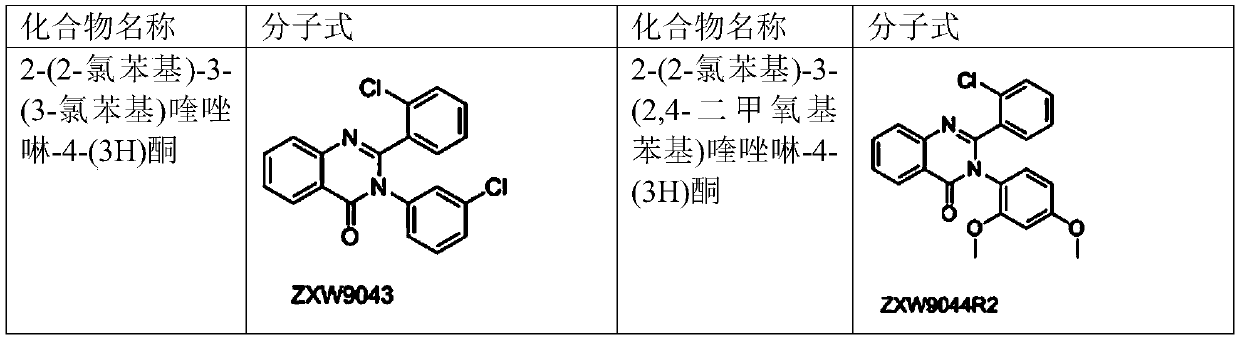
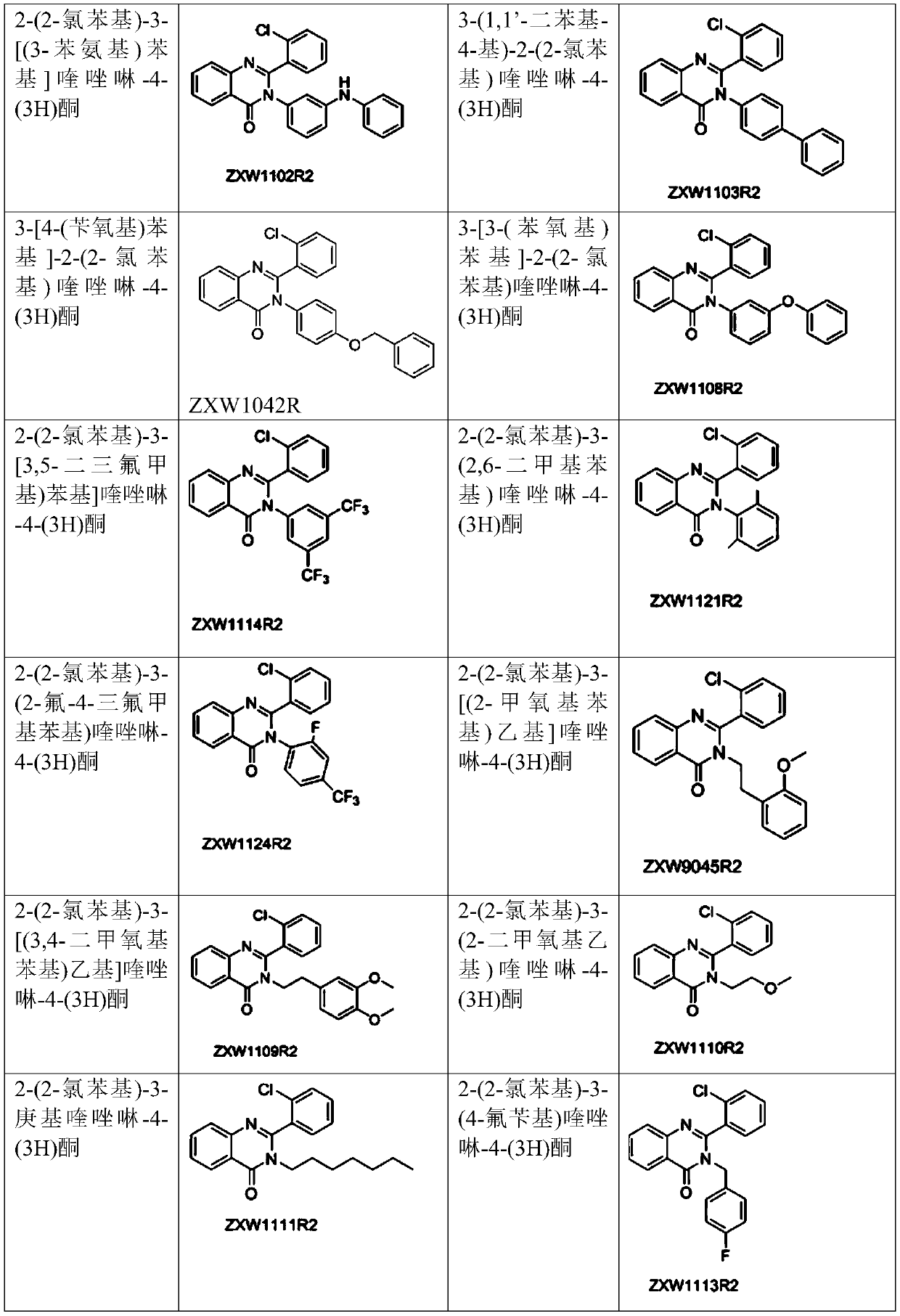
2-(2-chlorophenyl) quinazoline-4 (3H)-one derivative and preparation method and application thereof
ActiveCN110041272AStrong medicineFast metabolismAntibacterial agentsOrganic active ingredientsDiazepamHypnotic drug
The invention discloses a 2-(2-chlorophenyl) quinazoline-4 (3H)-one derivative shown in formula (I) or pharmaceutically acceptable salts thereof. Compared with the existing first-line sedative and hypnotic drugs diazepam and midazolam, the compound has stronger drug effect and faster metabolism speed, can reduce the next-day residual effect, and is expected to be developed into a novel high-efficiency low-toxicity sedative and hypnotic drug.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Compound anesthetic for racoon dogs as well as preparation method and application thereof
InactiveCN104161760ASlightly affectedGood anesthesiaOrganic active ingredientsNervous disorderLiver and kidneyMidazolam
The invention discloses a compound anesthetic for racoon dogs as well as a preparation method and application thereof, and belongs to the fields of preparation and application of compound anesthetics for racoon dogs. The compound anesthetic for the racoon dogs comprises the following components: dexmedetomidine, midazolam and water for injection. The invention further discloses a method for preparing the compound anesthetic for the racoon dogs, and the method comprises the following steps: (1) mixing the dexmedetomidine and the midazolam; (2) adding the water for injection and mixing evenly to obtain the compound anesthetic for the racoon dogs. Animal experiments prove that the compound anesthetic for the racoon dogs has an excellent anesthetic effect on the racoon dogs, and is quick and steady in induction and waking, and excellent and balanced in calming, pain easing and muscle relaxation effects; the compound anesthetic has a marginal effect on the circulatory system, the respiratory system, blood indexes and partial liver and kidney indexes, and can be applied to preparing the clinical anesthetics for the racoon dogs.
Owner:NORTHWEST A & F UNIV
Infusion bag of midazolam for intravenous use
ActiveUS20200268606A1Infusion devicesInorganic non-active ingredientsEngineeringPharmaceutical medicine
The invention relates to an infusion container comprising a sterile, ready-to-use, stable aqueous solution of Midazolam or a pharmaceutically acceptable salt thereof, suitable for direct intravenous infusion to a patient in need thereof. The invention also relates to a novel infusion container having plurality of ports that is suitable for delivering or receiving a sterile fluid, such as the said stable aqueous solution of Midazolam or a pharmaceutically acceptable salt thereof
Owner:SUN PHARMA INDS
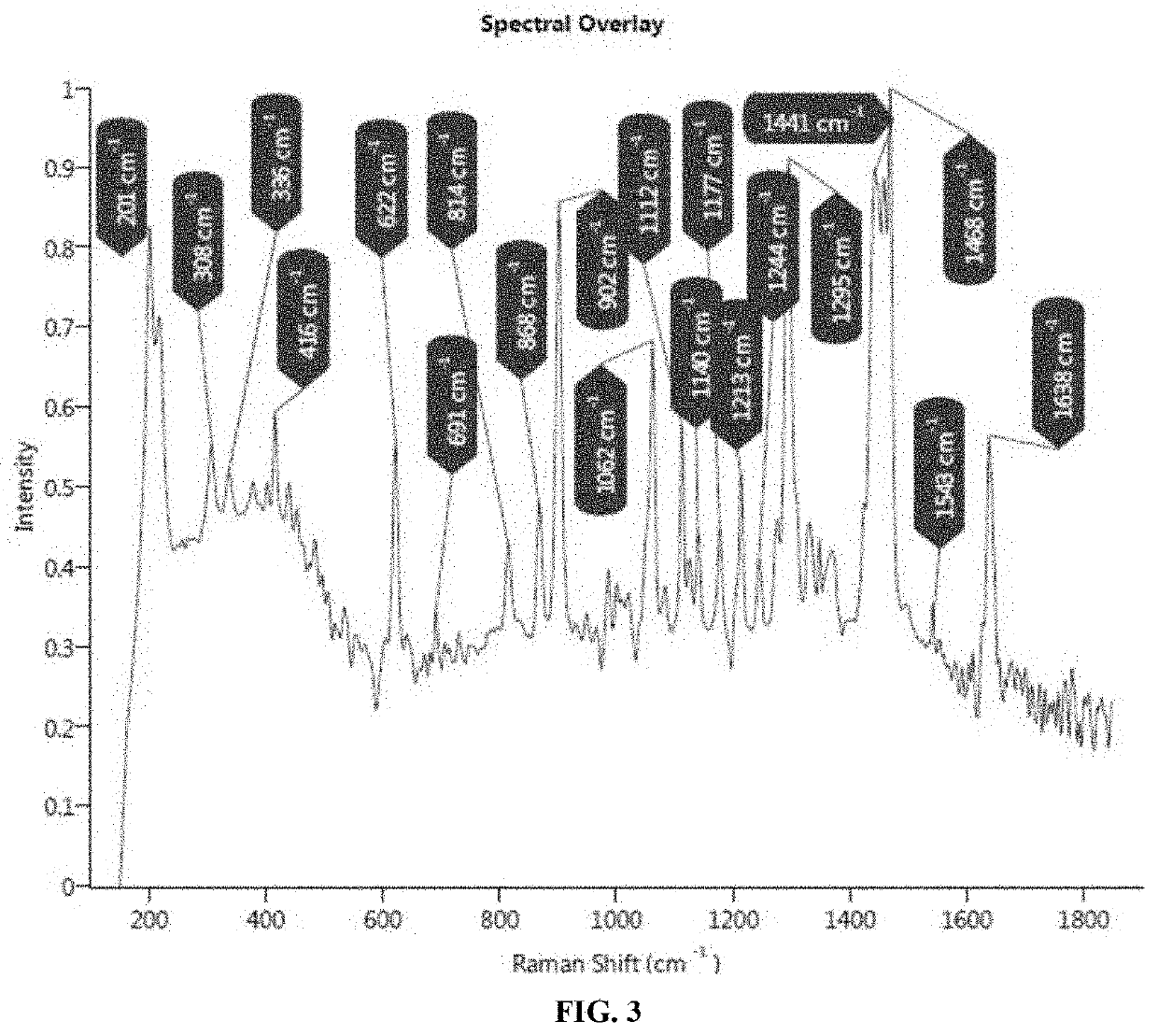
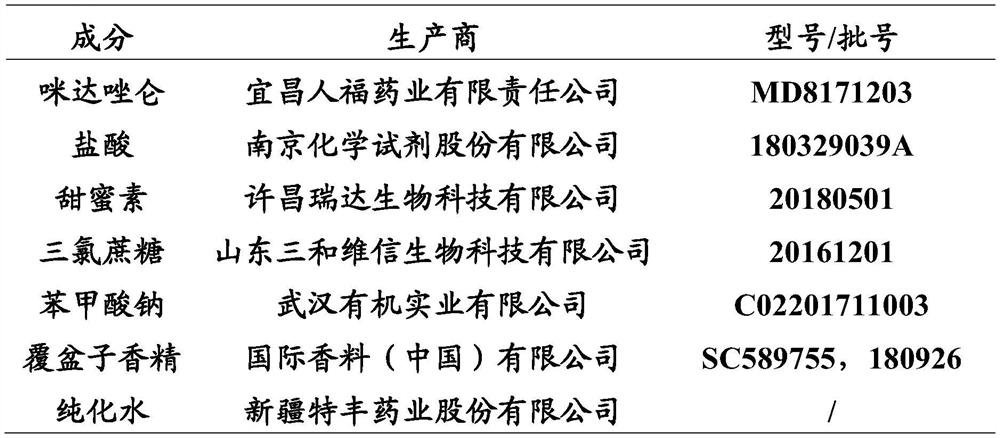
Midazolam liquid preparation, preparation method therefor and use of midazolam liquid preparation
ActiveCN113209013AReduce generationMeet children's clinical drug needsOrganic active ingredientsNervous disorderFormularySucrose
The invention provides a midazolam liquid preparation, a preparation method therefor and use of the midazolam liquid preparation. Particularly, the invention provides a liquid preparation containing midazolam or pharmaceutically acceptable salts thereof, sodium cyclamate, a halogenated saccharose sweetener and a raspberry flavoring essence, a preparation method for the liquid preparation and use of the liquid preparation in preparation or drugs for treating insomnia, epilepsy and anxiety or tranquilizers or anesthetics. Through a specific adjuvant combination, the liquid preparation prepared by the method has the advantages that the palatability of the midazolam can be effectively improved, and the requirements of children on clinical medication are met; and meanwhile, the liquid preparation is simple in formula, and the safety of the product is improved. Furthermore, due to selection of a pH regulator and / or a flavoring agent, the stability of the midazolam can be improved remarkably (for example, the generation of degraded substances is reduced, properties are not changed after storage, crystal precipitation is absent, and the like).
Owner:XINJIANG TEFENG PHARMA +1
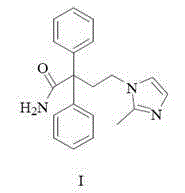
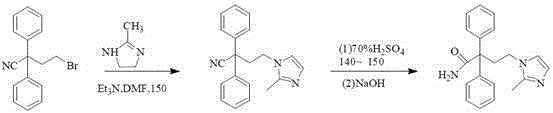
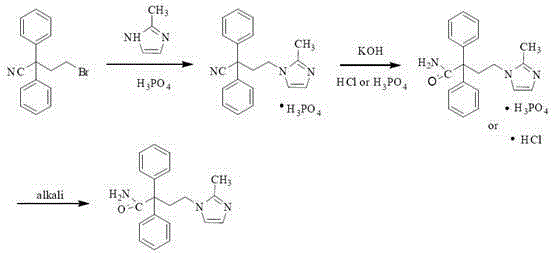
A kind of method for preparing midanaxin
The invention discloses a method for preparing imidafenacin. The method comprises the steps of hydrolyzing 4-bromo-2,2-diphenyl butyronitrile into acid amide under an alkaline condition, and then enabling the acid amide to react with 2-methylimidazole so as to obtain a target product. The preparation method provided by the invention is high in yield, economical, simple, convenient, friendly to human body and environment, and suitable for industrialized large-scale production.
Owner:JIANGZI QINGFENG PHARMACEUTICALS INC
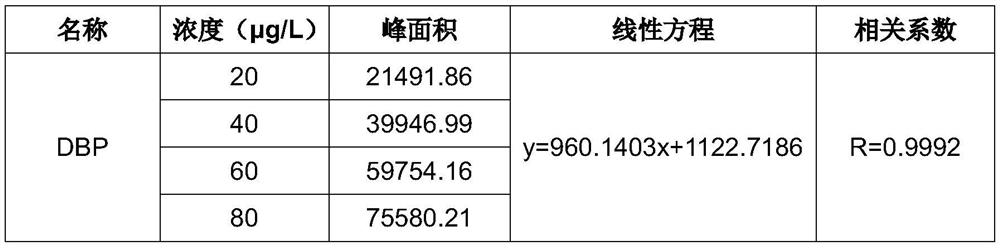
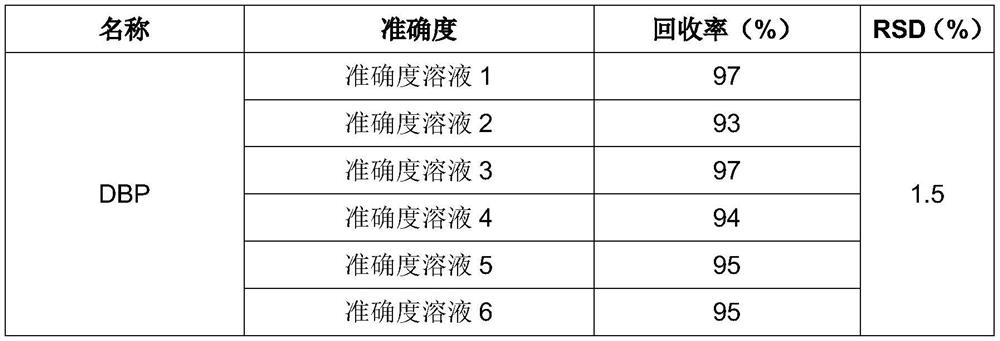
A kind of detection method of additive in midazolam medicine
The invention provides a method for analyzing the content of dibutyl phthalate in midazolam process liquid, which is characterized in that it comprises the following steps: S1: preparation of sample mother liquor; S2: preparation of sample solution; The obtained mother liquor of the sample was dissolved in the mixed solution, shaken evenly, left to stand, separated to obtain the organic phase, added anhydrous magnesium sulfate for dehydration, and the supernatant was taken for testing.
Owner:上海微谱检测科技集团股份有限公司
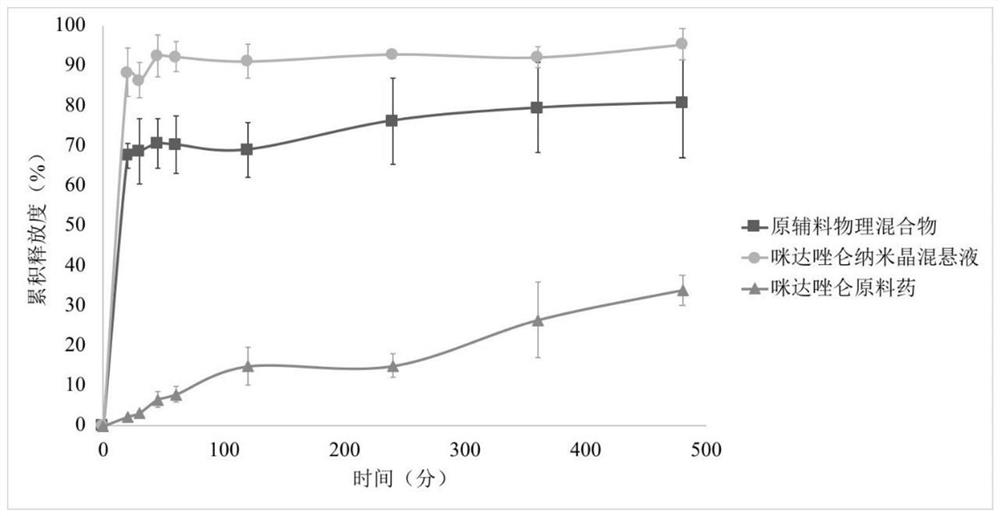
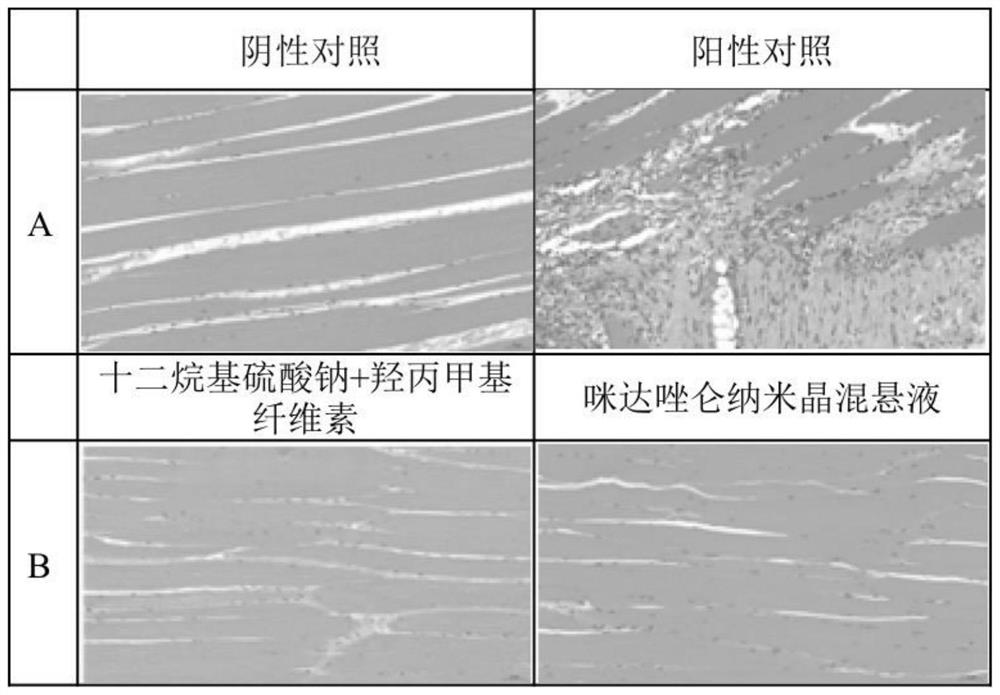
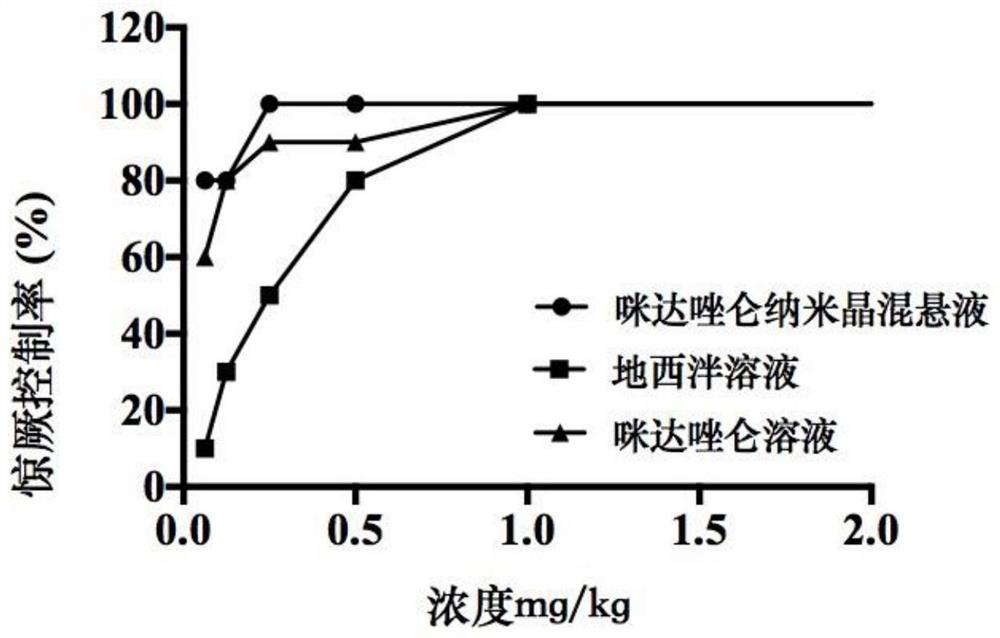
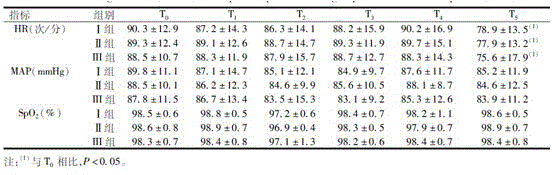
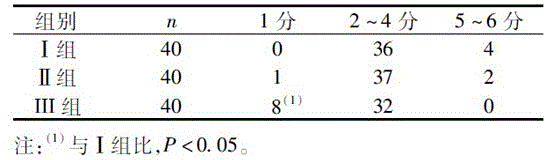
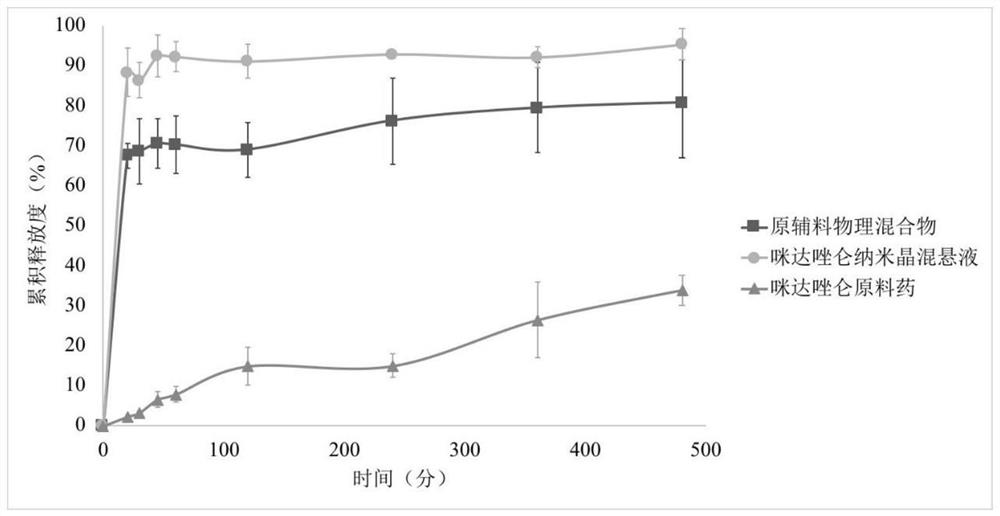
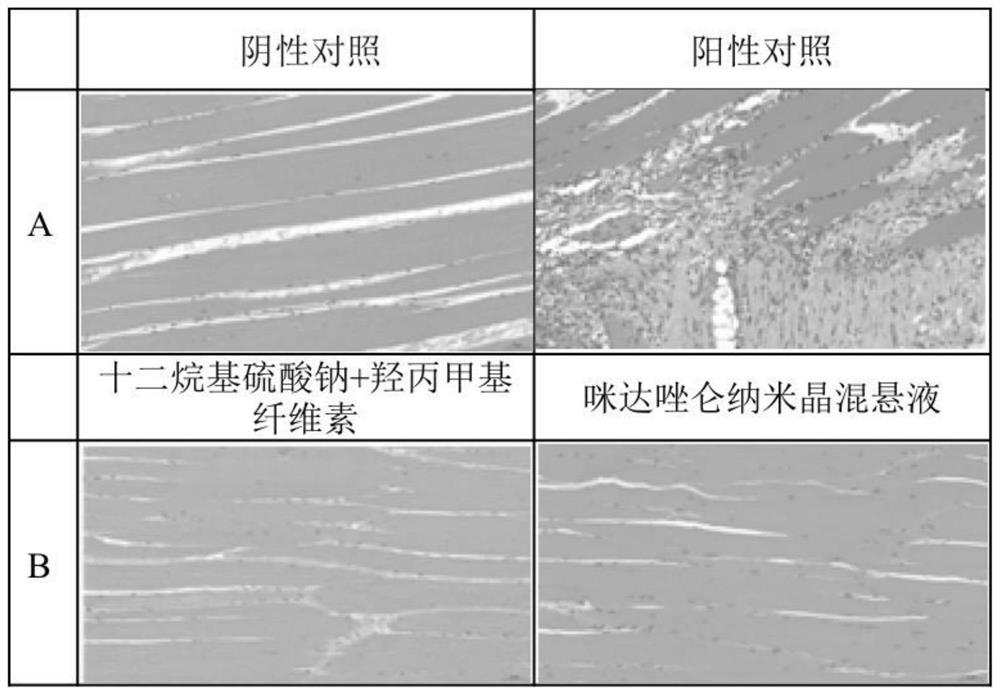
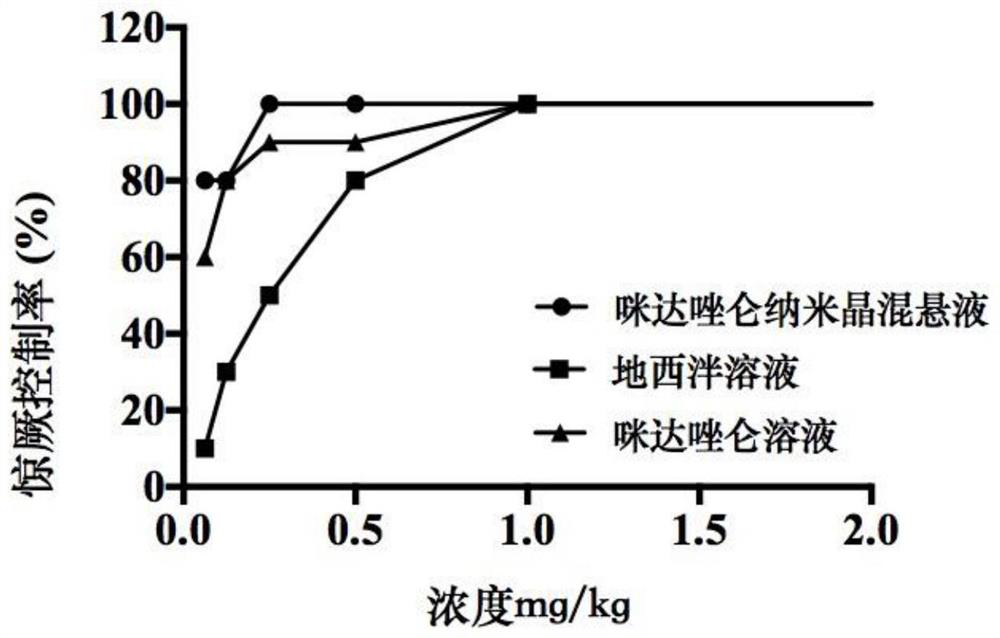
Midazolam nanocrystal suspension as well as preparation method and application thereof
ActiveCN112426406AImprove stabilityUniform particle size distributionOrganic active ingredientsNervous disorderBioavailabilityMidazolam
The invention provides an midazolam nanocrystal suspension as well as a preparation method and application thereof. The midazolam nanocrystal suspension is neutral, and is relatively small in irritation and good in safety; and in addition, the nanocrystal suspension improves the problem of insolubility of midazolam, improves the bioavailability, and has a good curative effect. The midazolam nanocrystal suspension is stable in preparation process and suitable for industrial production, and the nanocrystal suspension obtained through the process is good in stability, free of particle aggregationand layering phenomena and good in release and dissolution.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method for appendix excision under condition of epidural anesthesia
InactiveCN104523697AEnhanced inhibitory effectReduce anxietyOrganic active ingredientsAnaestheticsBenzodiazepineSedative Effects
The invention discloses a method for appendix excision under the condition of epidural anesthesia. Through midazolam administration and fentanyl-assistant application, the method realizes appendicectomy under the condition of epidural anesthesia. According to the method, midazolam is a water-soluble benzodiazepine narcotic and is combined with a benzodiazepine acceptor of a central nervous system so that normal nerve functions are inhibited, effects of anxiety resistance, memory loss and calmness are obtained, and calming effects of other narcotics are improved. The appendicectomy is carried out under the condition of midazolam- and fentanyl-assistant epidural anesthesia so that intraoperative tractive response is reduced and patient anxiety, fear and postoperative bad memory are eliminated. Midazolam and fentanyl are combined so that fentanyl can reduce a use amount of midazolam needed by loss of consciousness, improves consciousness inhibition effects of midazolam, makes consciousness disappear at a high Bis value, has calmness and forgetting effects obviously better than those of the traditional droperidol-fentanyl compound, and has ideal and stable anaesthesia effects. The method provides humanistic service for patients.
Owner:敖云霞
Pig intramuscular injection compound anesthetic and preparation method thereof
InactiveCN108186650AAnesthesia is effectiveModerate maintenance timeOrganic active ingredientsAnaestheticsSide effectThiazole
The invention discloses a pig intramuscular injection compound anesthetic and a preparation method thereof, and belongs to the field of veterinarian medicine. The anesthetic is prepared from a component A 2,4-dimethaniline thiazole, a component B midazolam, and a component C ketamine hydrochloride. In 100 mL of water, the mass ratio of the component A to the component B to the component C is 0.5:0.6:10; the preparation method comprises the steps that a 100 ml measuring cylinder is used for measuring 50 ml of distilled water, 1N hydrochloric acid is used for adjusting the PH value to 5.5, then,0.5 g of 2,4-dimethaniline thiazole, 10 g of ketamine hydrochloride and 0.6 g of midazolam are accurately weighed, poured in to be dissolved and mixed to be uniform, the distilled water is added to reach the constant volume being 100 ml, a degerming filter membrane is used for filtering, and subpackaging is performed to obtain the pig intramuscular injection compound anesthetic. The pig intramuscular injection compound anesthetic has the advantages of being real in anesthetic effect, proper in maintenance time and small in side effect, facilitating dosage and the like.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Application of midazolam nanocrystal in preparation of drugs for improving blood-brain barrier permeability
ActiveCN112426427AImprove stabilityUniform particle size distributionOrganic active ingredientsNervous disorderAntiepileptic drugPharmacologic therapy
The invention provides an application of a midazolam nanocrystal and a composition thereof in medicines for improving blood-brain barrier permeability. According to the midazolam nanocrystal, the solubility problem is solved, the bioavailability is improved, the problem that the treatment effect is poor when tablets and injection are used as anticonvulsant and antiepileptic drugs is solved, and the midazolam nanocrystal can effectively penetrate through a blood brain barrier and is used for treating epilepsy. In addition, the invention provides a technical platform, and the blood-brain barrierpermeability of the brain medicine is improved by the nanocrystalline preparation in the presence of a space protective agent and a charge stabilizer.
Owner:ACADEMY OF MILITARY MEDICAL SCI
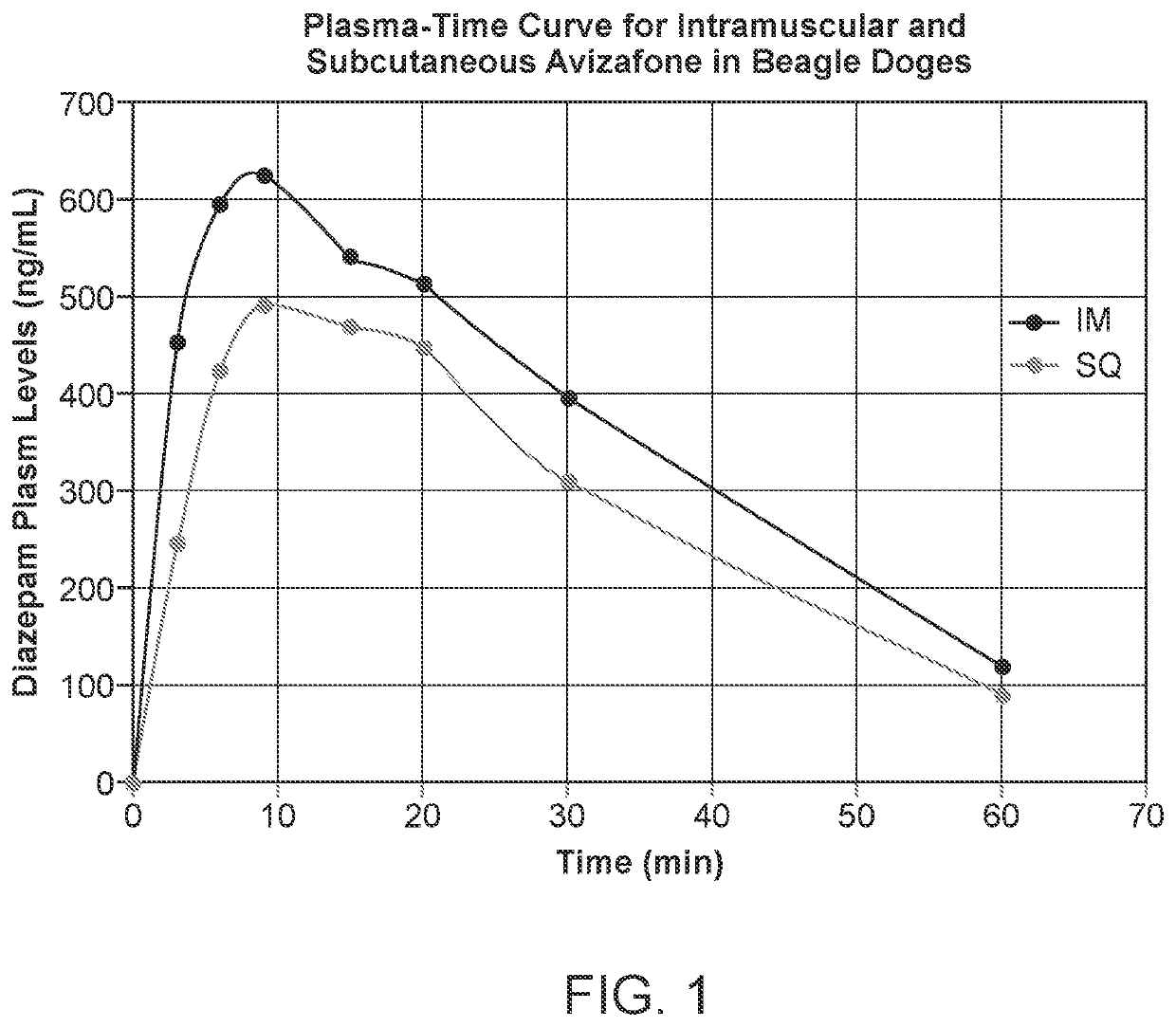
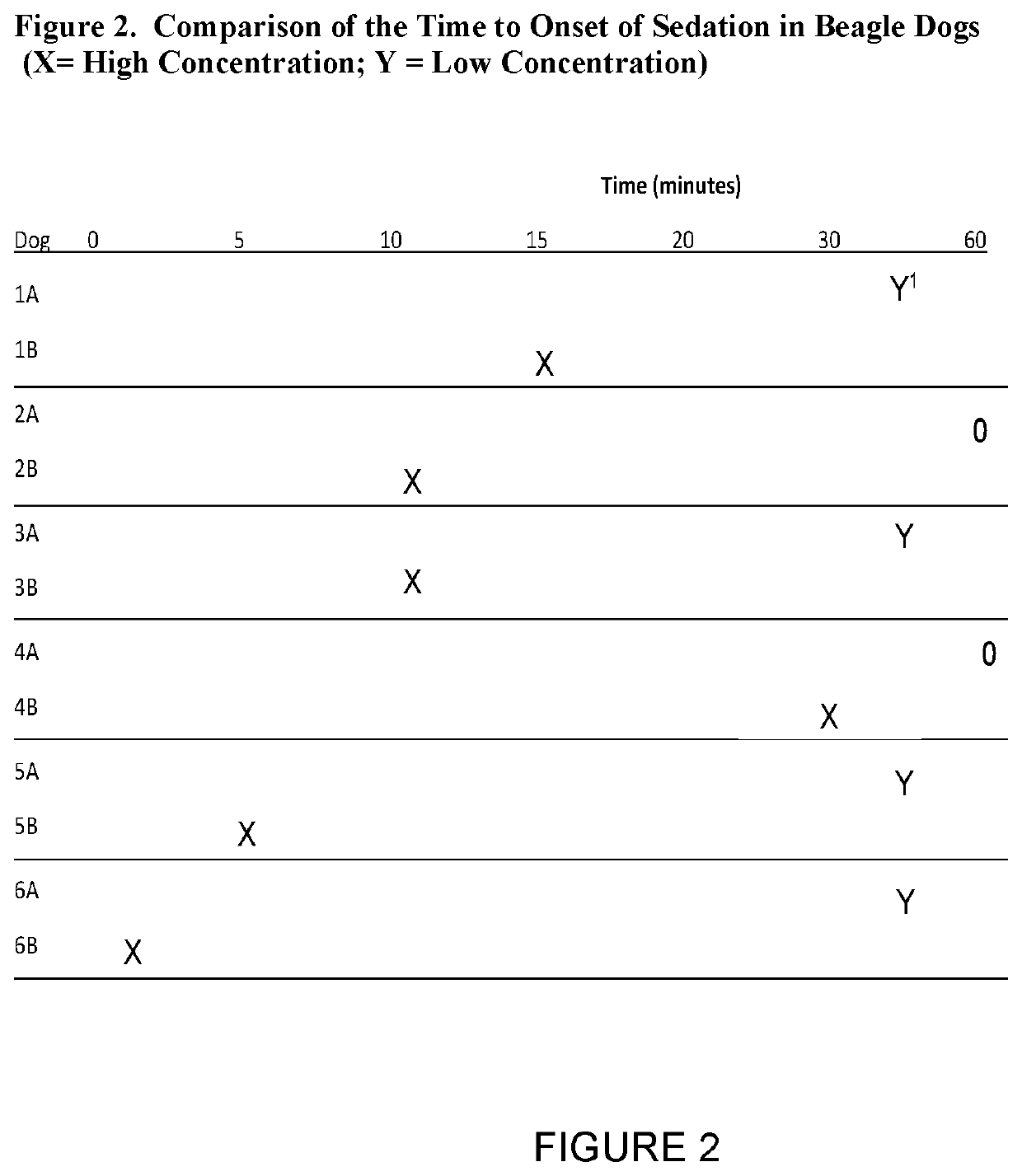
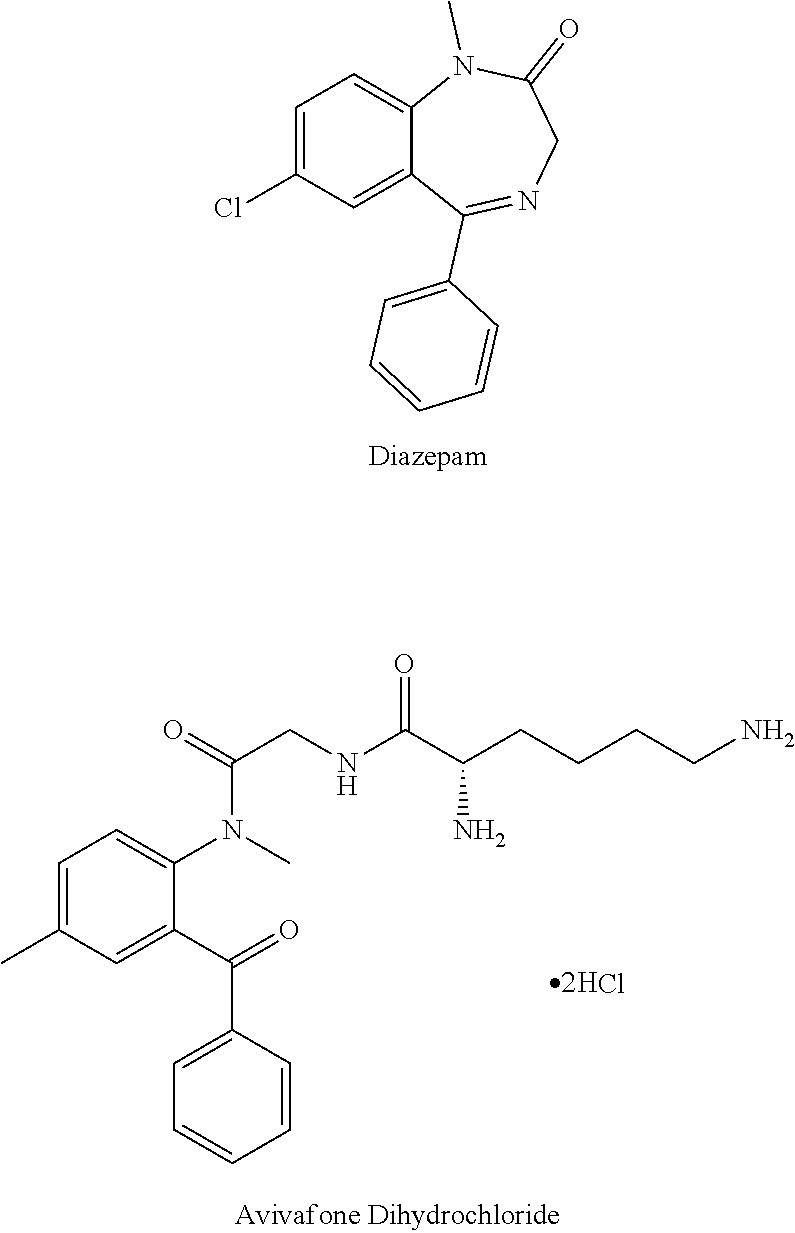
Parenteral delivery of avizafone
InactiveUS20220151963A1Swift sedationIncrease supplyOrganic active ingredientsNervous disorderBenzodiazepineBenzodiazine
Water-stable formulations of Avizafone and methods of use are described herein. The formulations may be administered to patients intravenously, intramuscularly, or subcutaneously. For serious COVID, or other viral infections, Avizafone is a rapidly sedating benzodiazepine that could be used interchangeably with midazolam augmenting the supply of drugs for swift and intubation.
Owner:SOLLIEVO PHARM INC
A kind of analysis method of compatibility between midazolam and production system
ActiveCN109632930BAccurate detectionWill not be disturbedPreparing sample for investigationMaterial analysis by electric/magnetic meansPhysical chemistryMidazolam
The invention provides a method for analyzing the compatibility between midazolam and a production system, comprising the following steps: S1: preparation of a sample mother liquor, dissolving midazolam and a mixed solution in a sodium chloride solution, and adjusting the pH to 3-4, add water to make up to the scale; S2: ICP-MS test, the mixed solution contains at least one of dilute hydrochloric acid, concentrated nitric acid, and hydrogen peroxide; the detection method provided by the present invention can accurately detect metal ions , and will not be interfered by C and F ions.
Owner:上海微谱检测科技集团股份有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam](https://images-eureka.patsnap.com/patent_img/40541304-9fa6-4cd5-9688-72d2ce57e726/BSA00000688184200032.png)
![Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam](https://images-eureka.patsnap.com/patent_img/40541304-9fa6-4cd5-9688-72d2ce57e726/FSA00000688184100011.png)
![Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam Method for synthesis of 4H-imidazo[1, 5-a][1, 4]benzodiazepine, especially midazolam](https://images-eureka.patsnap.com/patent_img/40541304-9fa6-4cd5-9688-72d2ce57e726/FSA00000688184100012.png)
![PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF](https://images-eureka.patsnap.com/patent_img/68fef1b9-9173-4e3c-876b-8c70c41a6514/US20110275799A1-20111110-D00000.png)
![PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF](https://images-eureka.patsnap.com/patent_img/68fef1b9-9173-4e3c-876b-8c70c41a6514/US20110275799A1-20111110-D00001.png)
![PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF PROCESS FOR THE SYNTHESIS OF 4H-IMIDAZO [1,5-a] [1,4] BENZODIAZEPINES, IN PARTICULAR MIDAZOLAM AND SALTS THEREOF](https://images-eureka.patsnap.com/patent_img/68fef1b9-9173-4e3c-876b-8c70c41a6514/US20110275799A1-20111110-C00001.png)