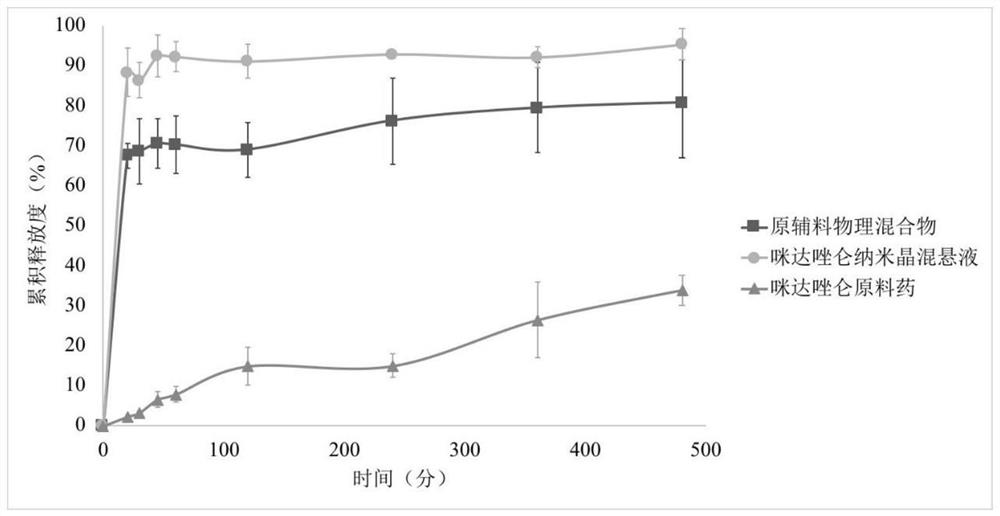
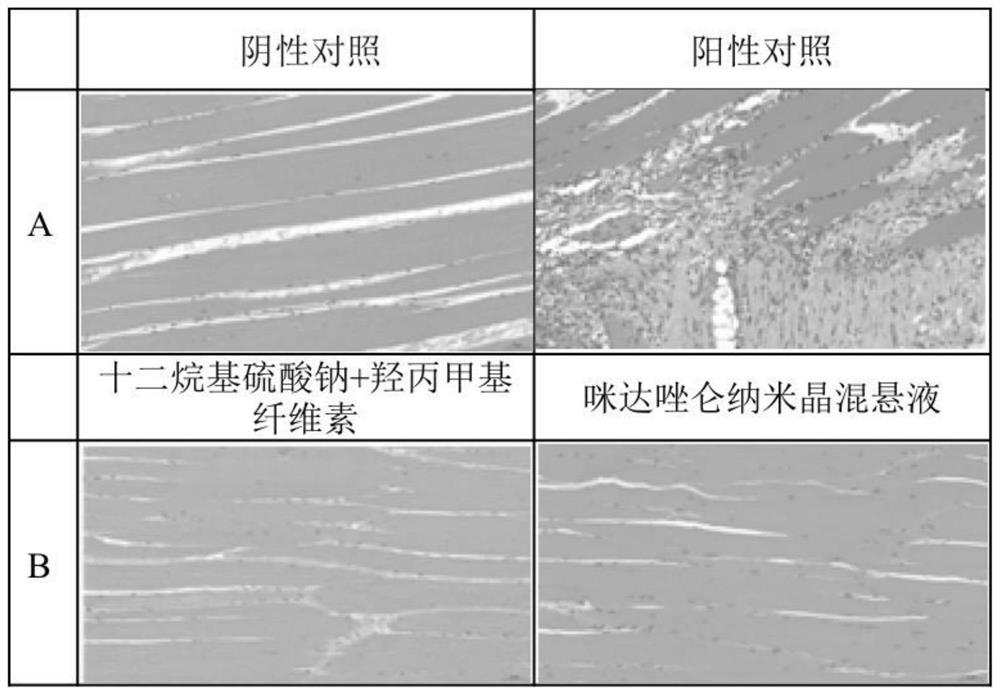
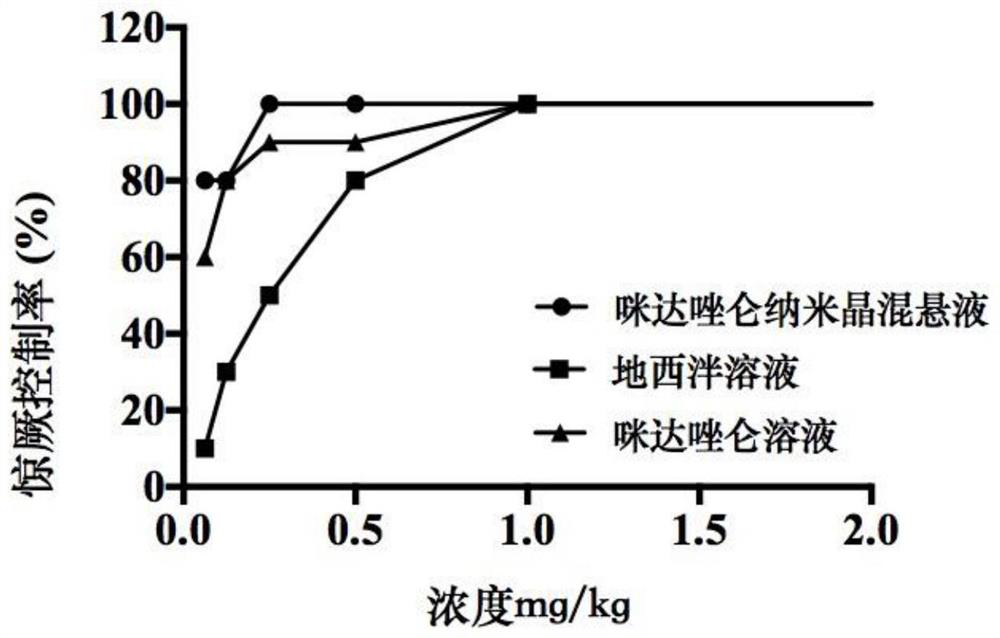
Application of midazolam nanocrystal in preparation of drugs for improving blood-brain barrier permeability
A technology of midazolam and blood-brain barrier, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Example 1 Preparation of midazolam nanocrystal suspension
[0228] Add 2.5g of HPMC E5 into 100ml of water, heat to 50°C, stir until completely dissolved, then add 1g of SDS, 5g of midazolam, and homogenize by ultrasonication for 5min to fully wet the midazolam and suspend evenly. Pour the primary mixture into a wet mill with an initial rotation speed of 1500rpm, and increase the rotation speed by 500rpm every 5min until the rotation speed reaches 3000rpm, and continue grinding for 1h. The ground nano-suspension was diluted 200 times, and the particle size of the prepared nano-crystals was detected to be 286.6 nm, the polydispersity index (PDI) was 0.124, and the Zeta potential was -23.4 mV.
Embodiment 2
[0263] Example 2 Preparation of midazolam nanocrystal suspension
[0264] Add 2.5g of HPMC E5 to 100ml of water, heat to 55°C, stir until completely dissolved, then add 0.5g of SDS and 5g of midazolam, and homogenize by ultrasonication for 5min to fully wet the midazolam and suspend evenly. Pour the initial mixture into a wet mill, the initial speed is 1500rpm, and the speed is increased by 500rpm every 5 minutes until it reaches 3000rpm, and the grinding is continued for 1h to obtain the product. The ground nanosuspension was diluted 200 times, and the particle size of the prepared midazolam nanocrystals was detected to be 267.3 nm, the polydispersity index (PDI) was 0.132, and the Zeta potential was -28.1 mV.
Embodiment 3
[0341] Example 3 Preparation of midazolam nanocrystal suspension
[0342] Add 2.5g of HPMC E5 into 100ml of water, heat to 60°C, stir to dissolve, then add 0.5g of SDS and 10g of midazolam, and homogenize by ultrasonication for 5min to fully wet the midazolam and suspend evenly. Pour the initial mixture into a wet mill, the initial speed is 1500rpm, and the speed is increased by 500rpm every 5 minutes until it reaches 3500rpm, and the grinding is continued for 2h to obtain the product. The ground nanosuspension was diluted 200 times, the detected particle size was 223.6nm, the polydispersity index (PDI) was 0.124, and the Zeta potential was -25.8mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com