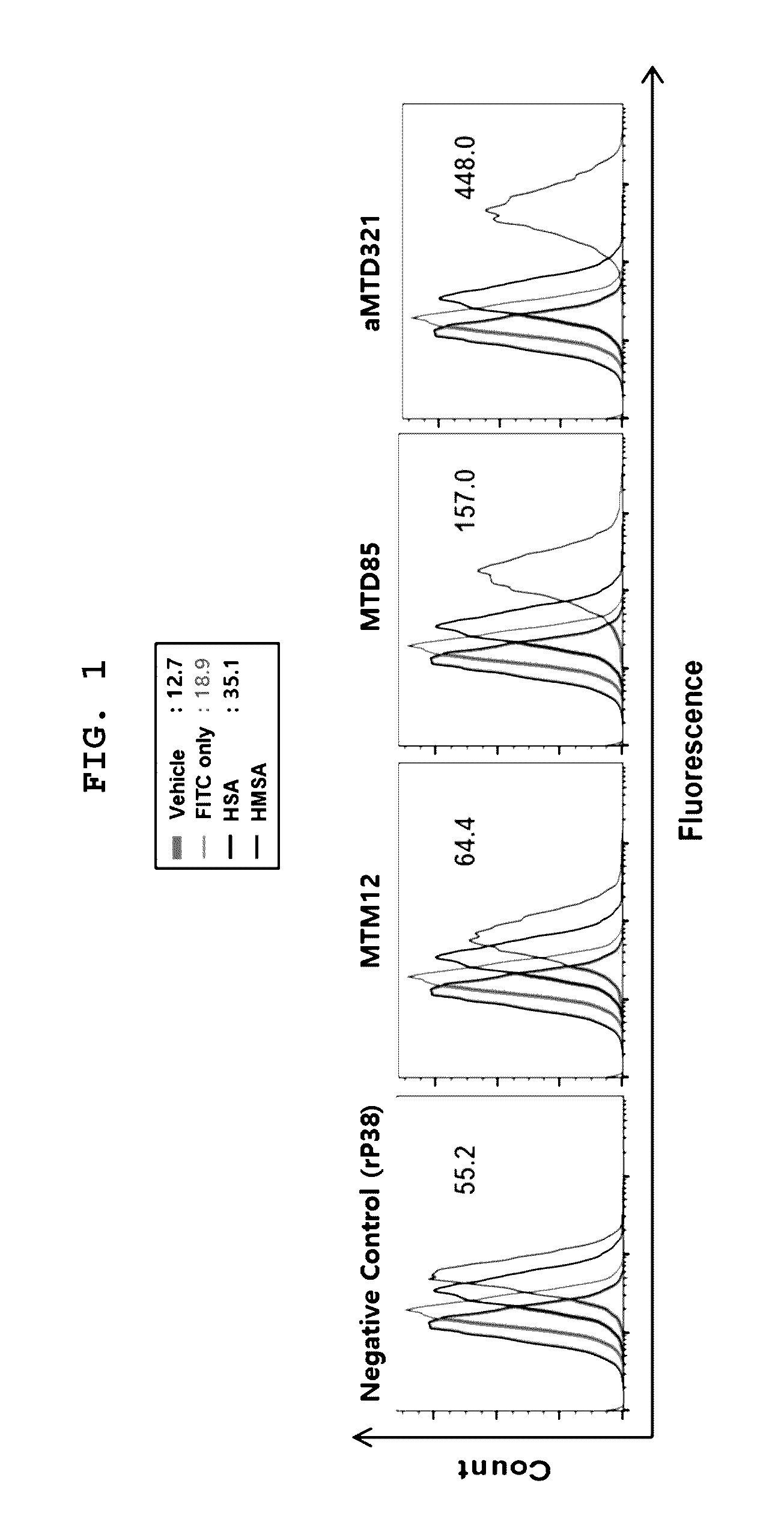
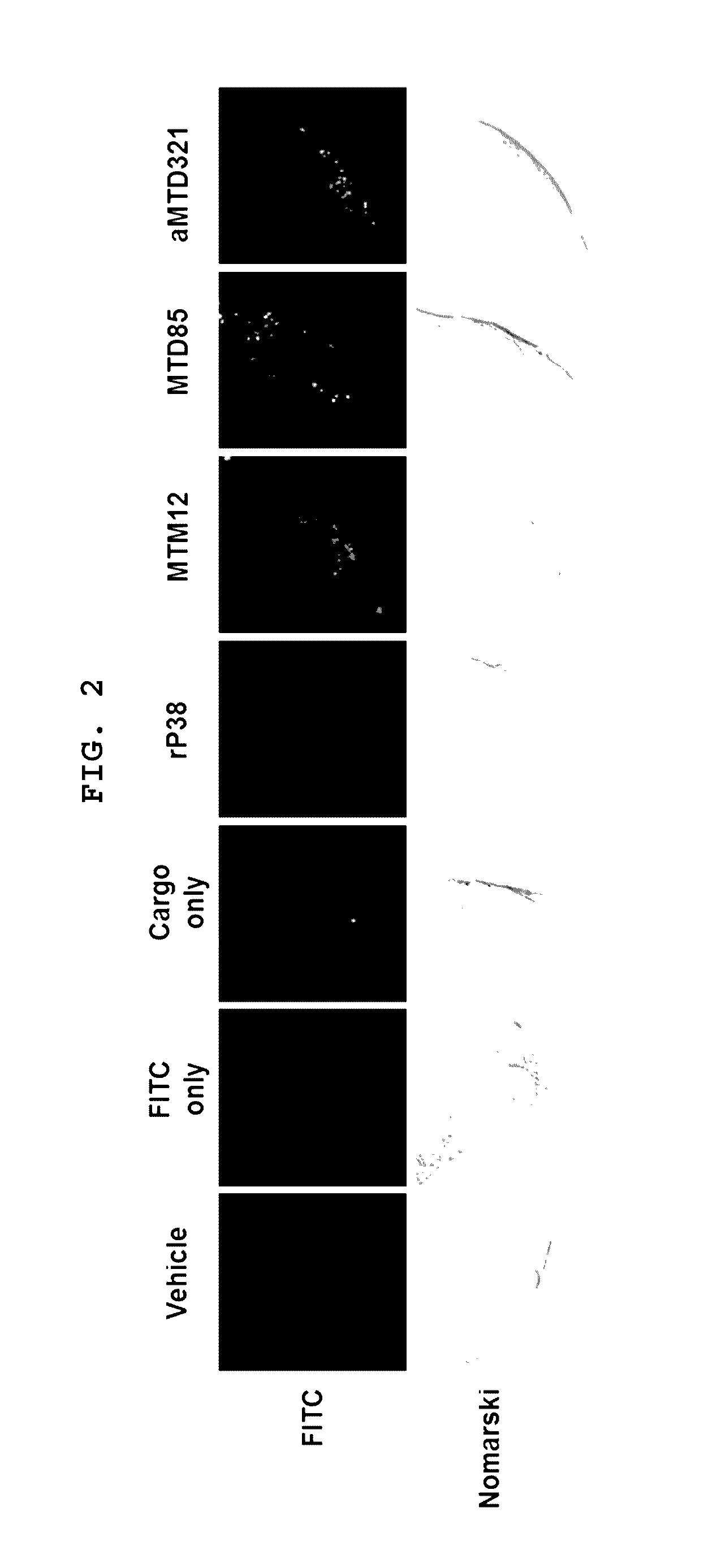
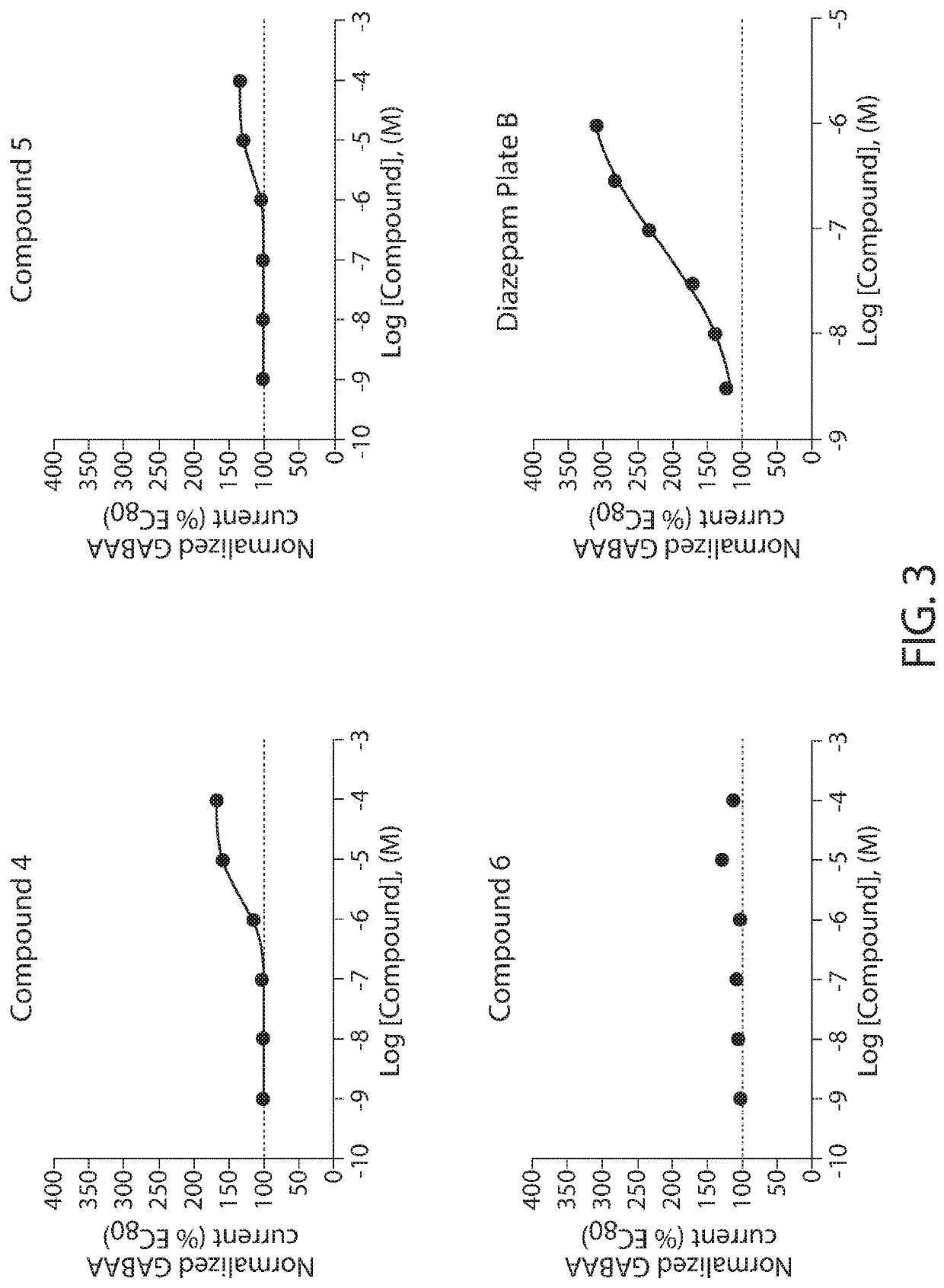
Patents
Literature
45 results about "Bbb permeability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
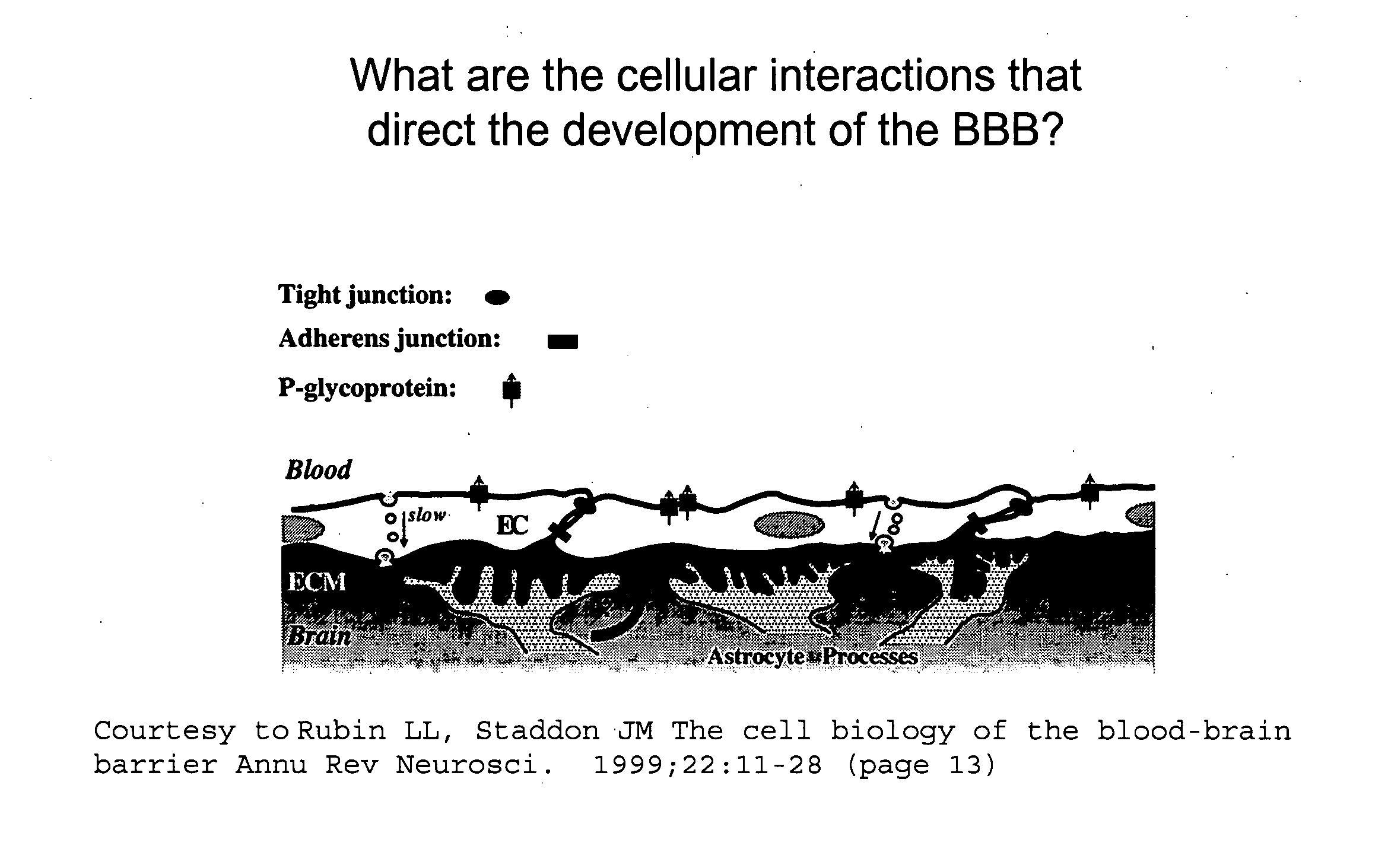
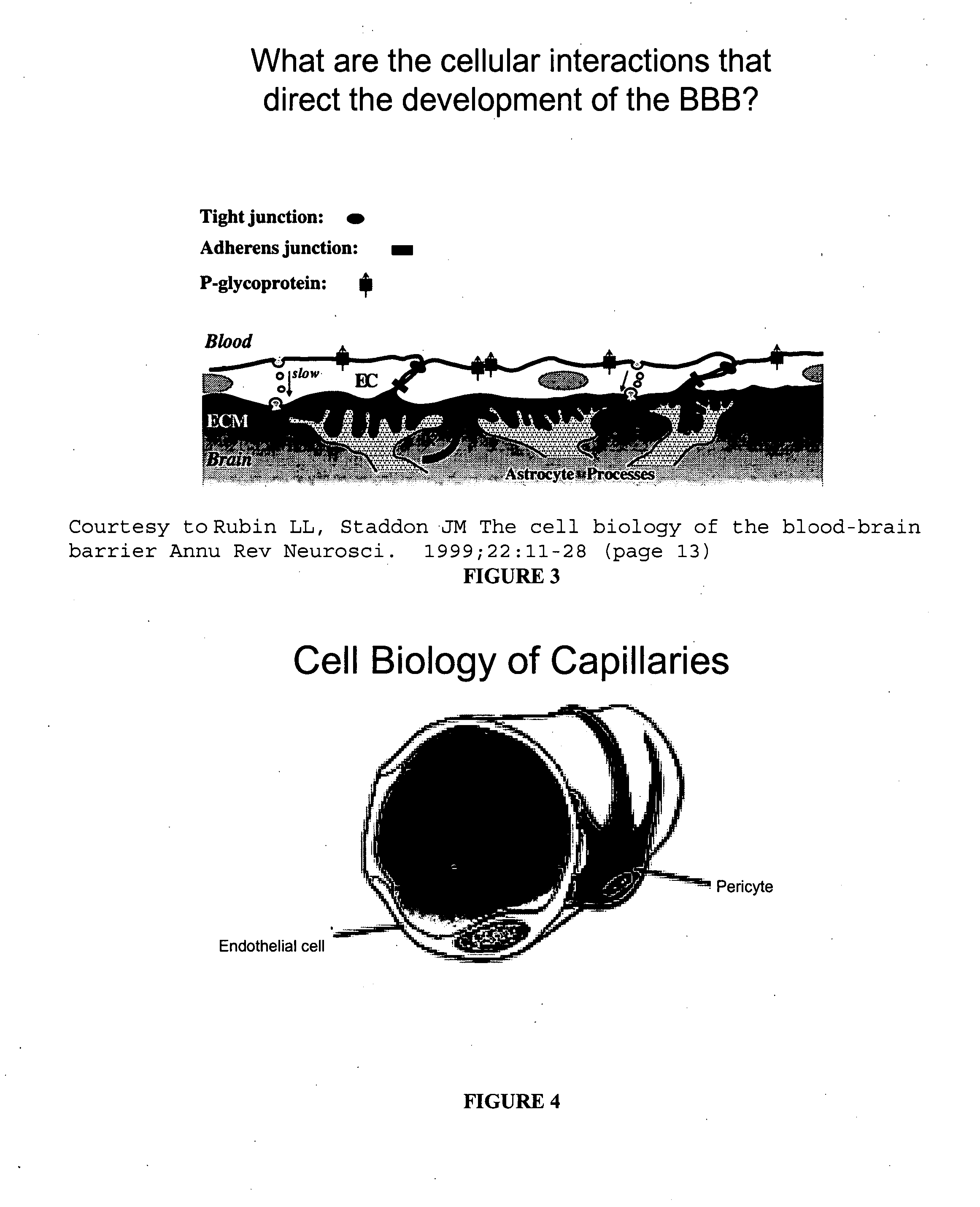
Permeability of blood-brain barrier
InactiveUS20080025959A1Increased and decreased expressionCompounds screening/testingOrganic active ingredientsCell Surface ProteinsBbb permeability
This relates to a discovery of a cell surface protein, namely NgR2, that is implicated in regulation of blood-brain barrier (BBB). In addition, the invention relates methods for identifying agents that modulate BBB permeability. Furthermore, the invention relates to methods of delivering therapeutic agents to the central nervous system by increasing BBB permeability.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
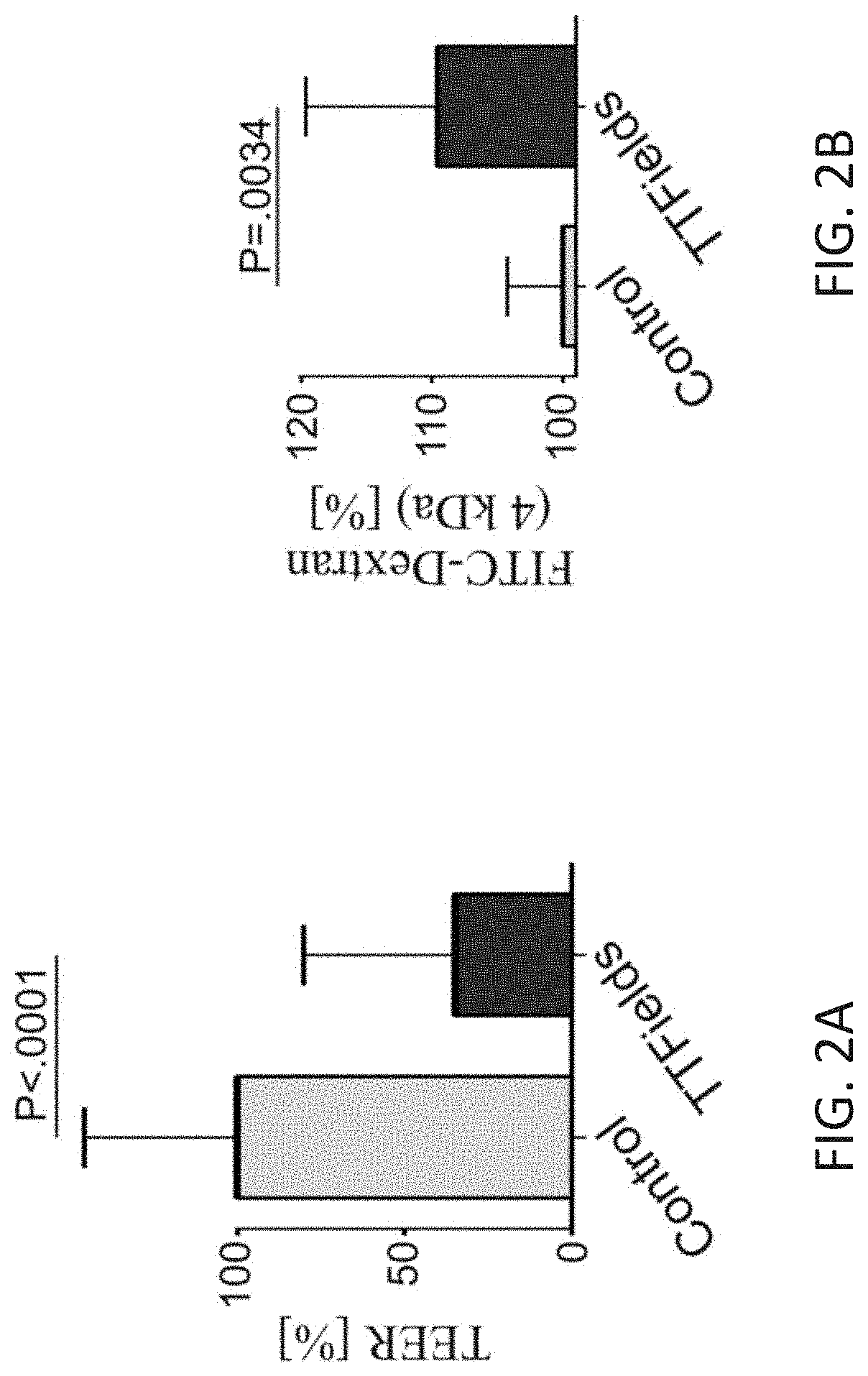
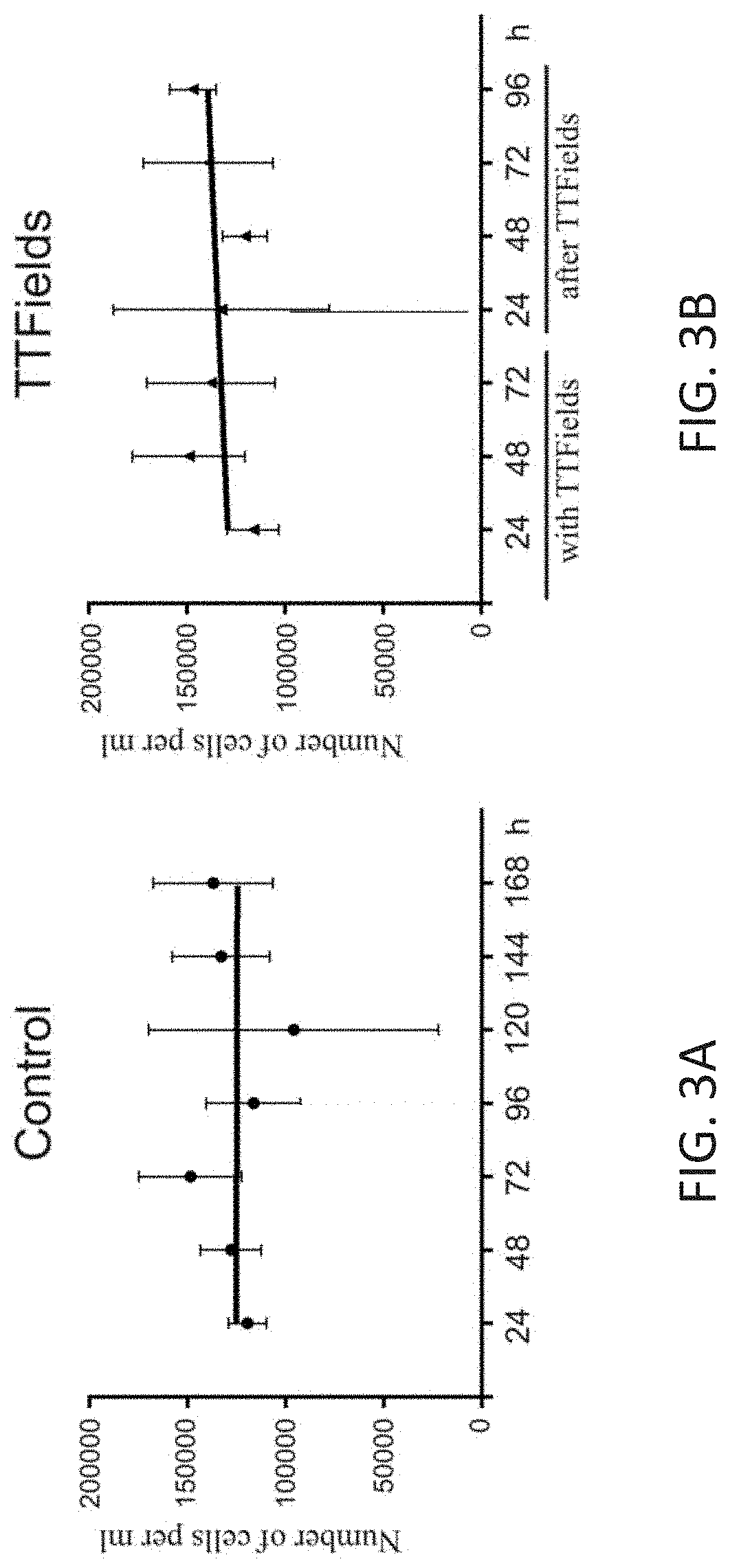
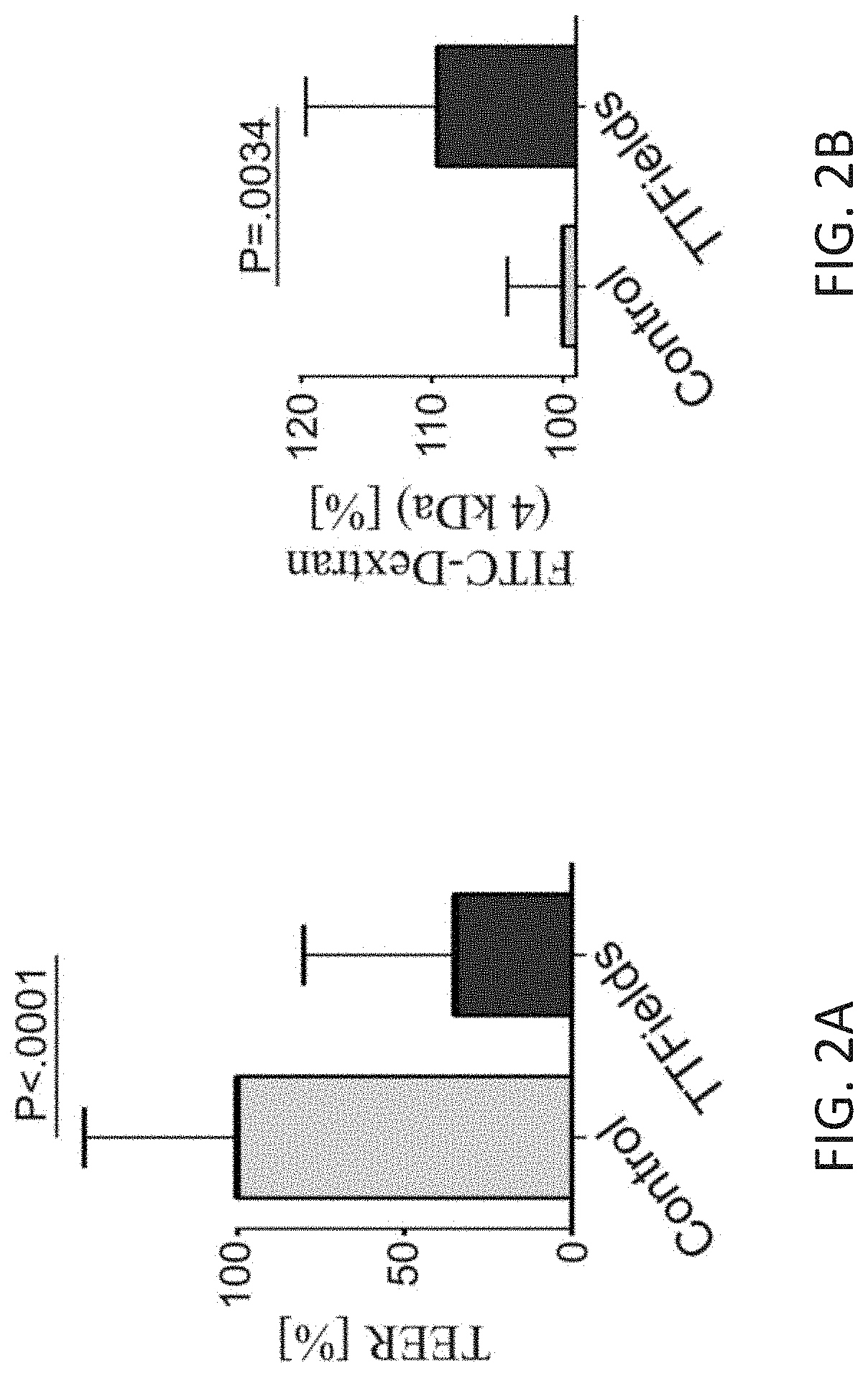
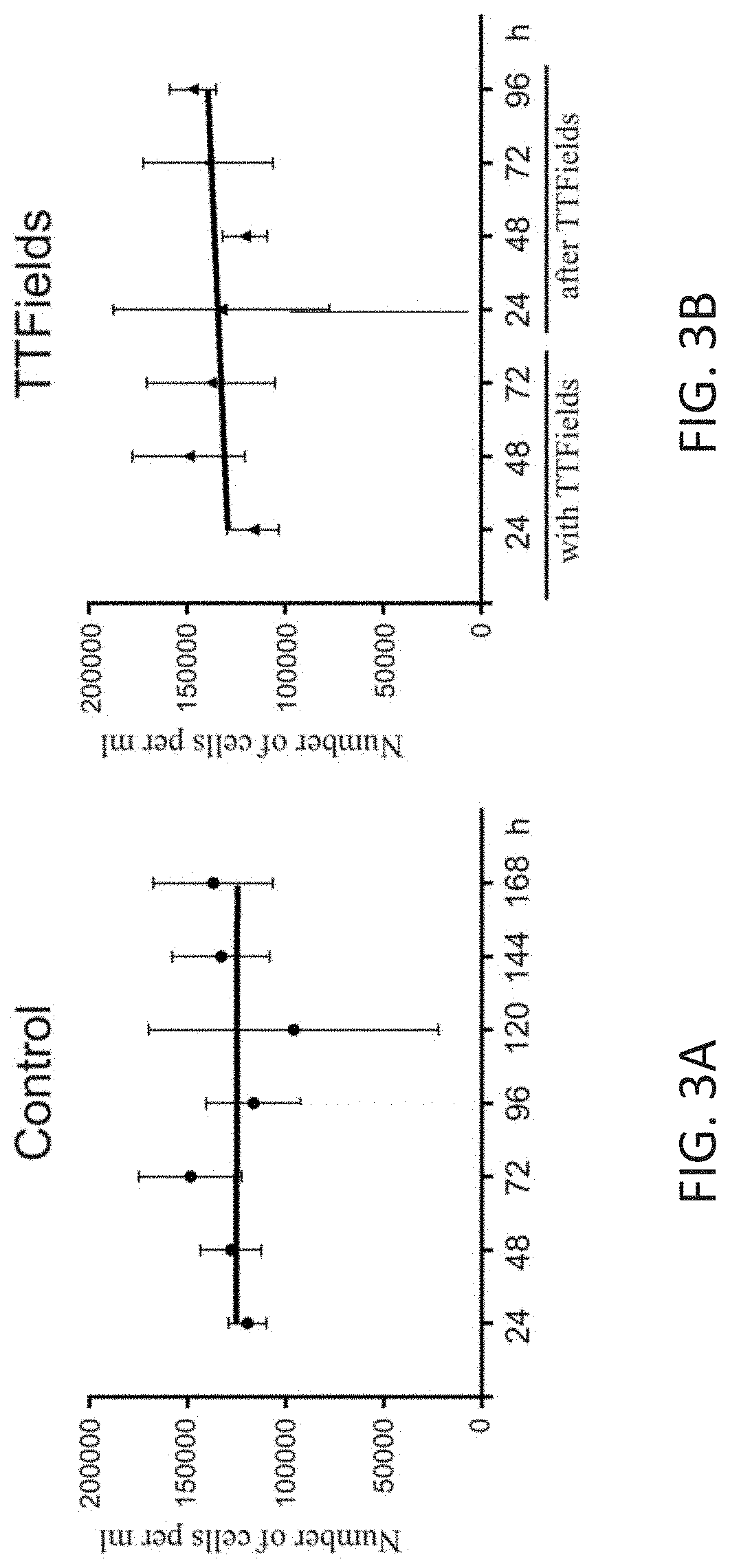
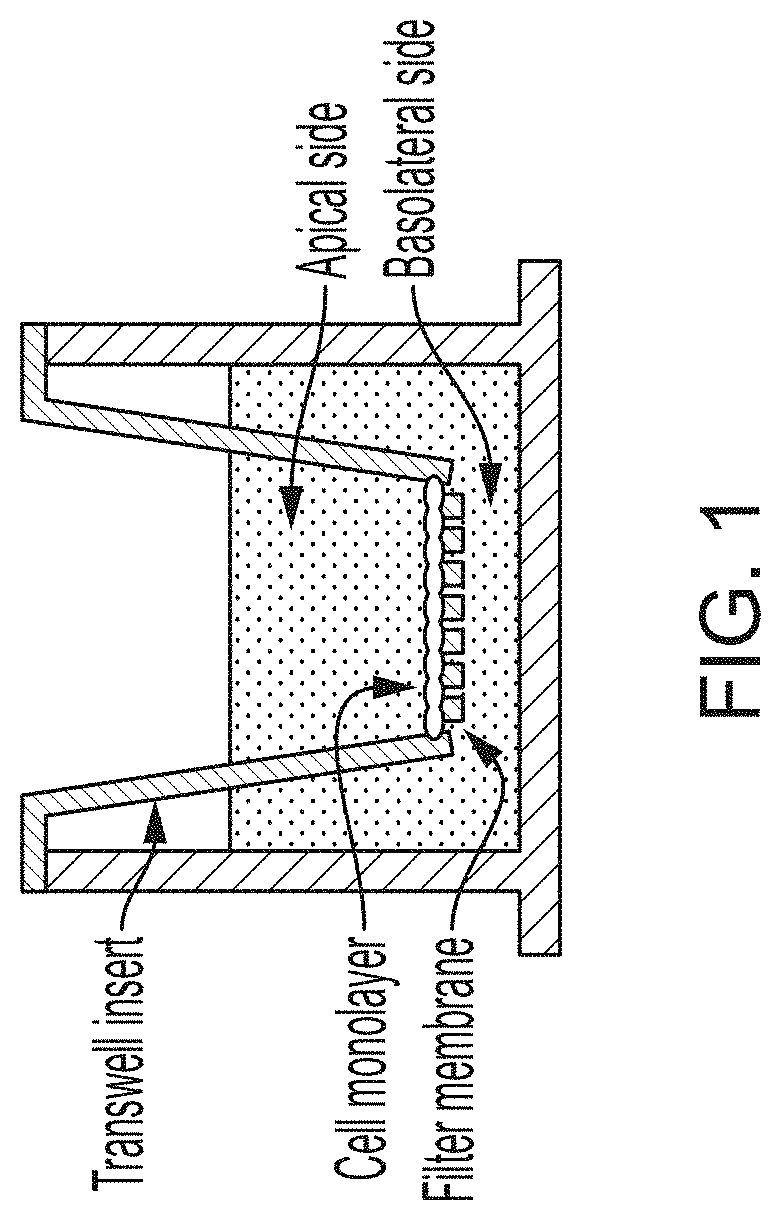
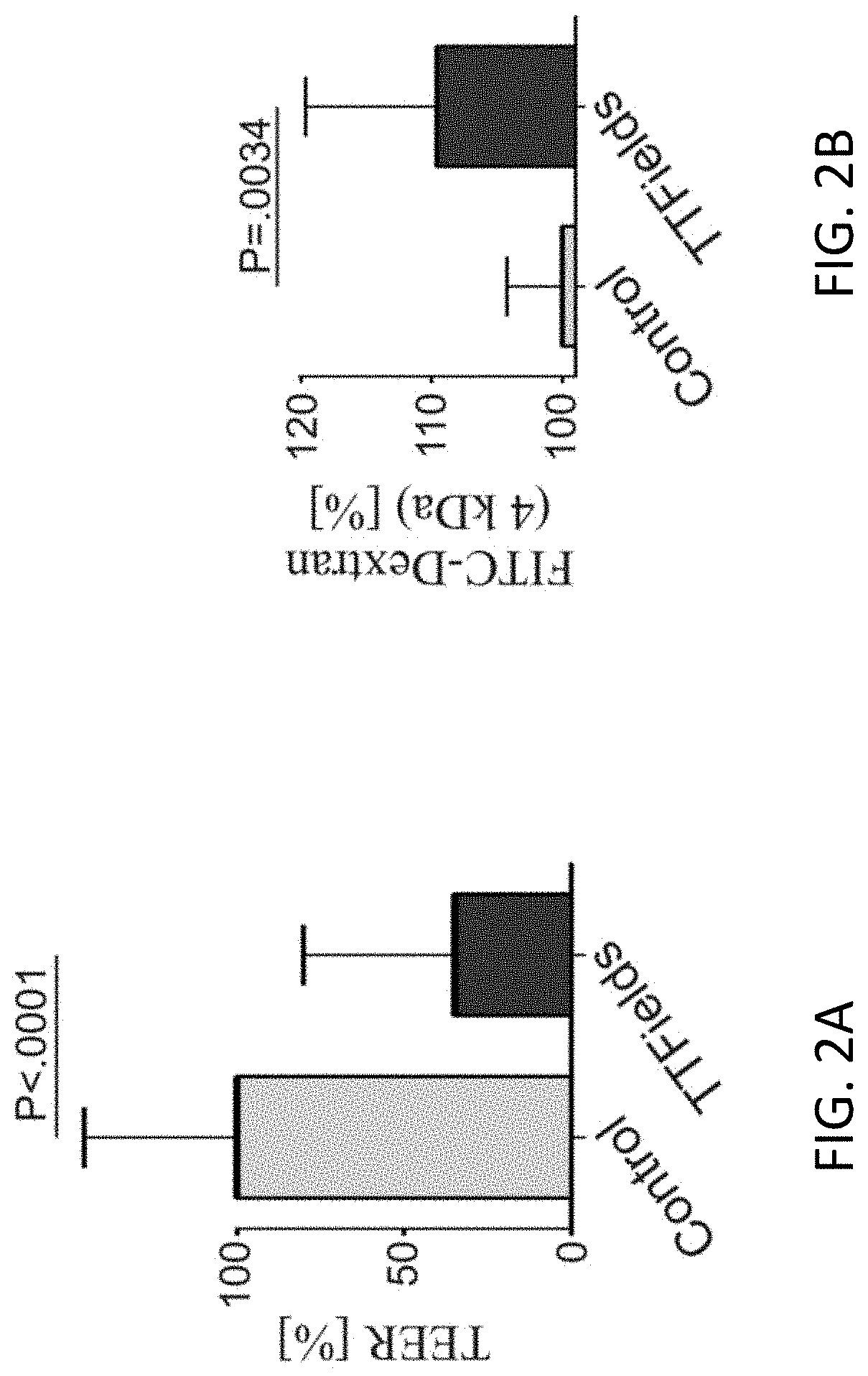
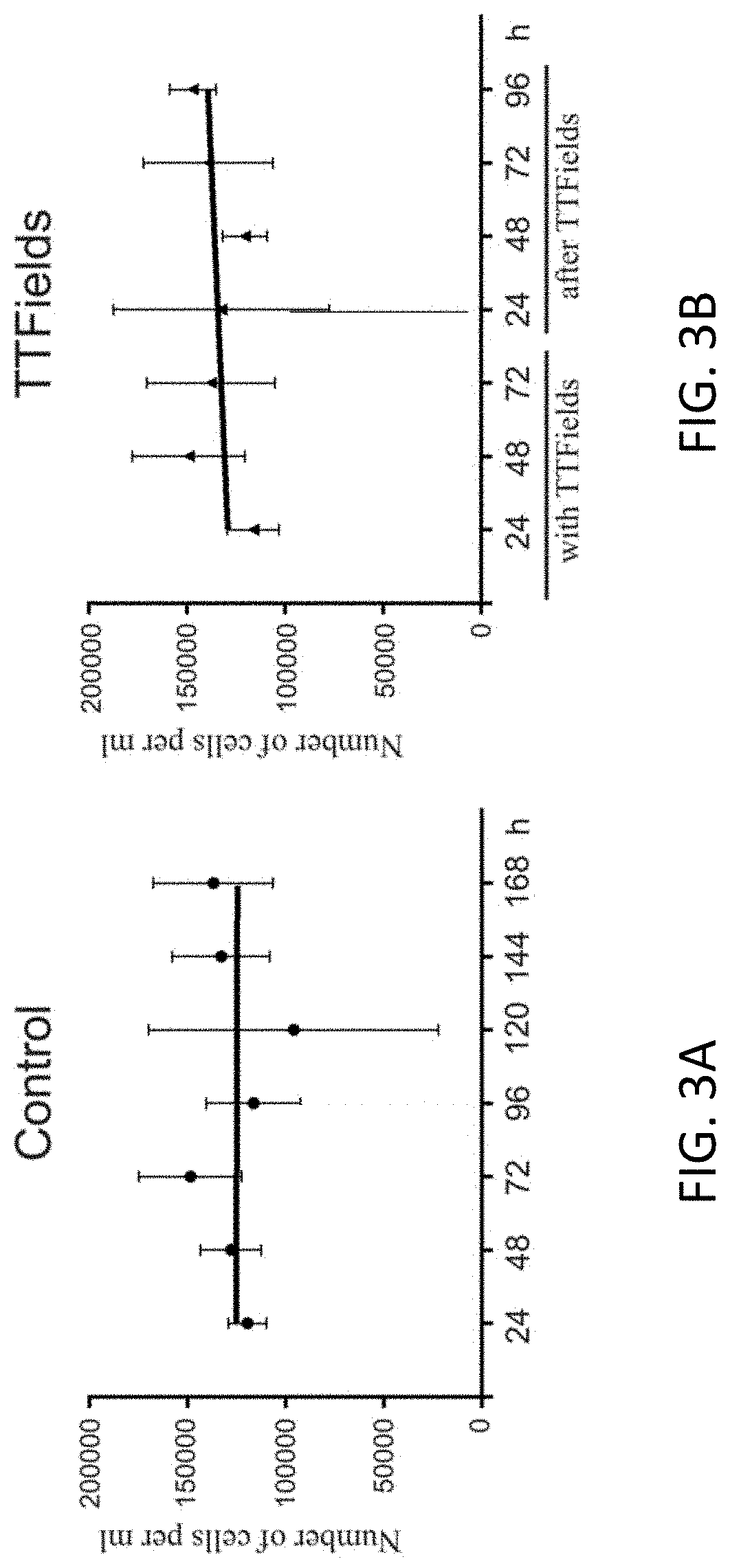
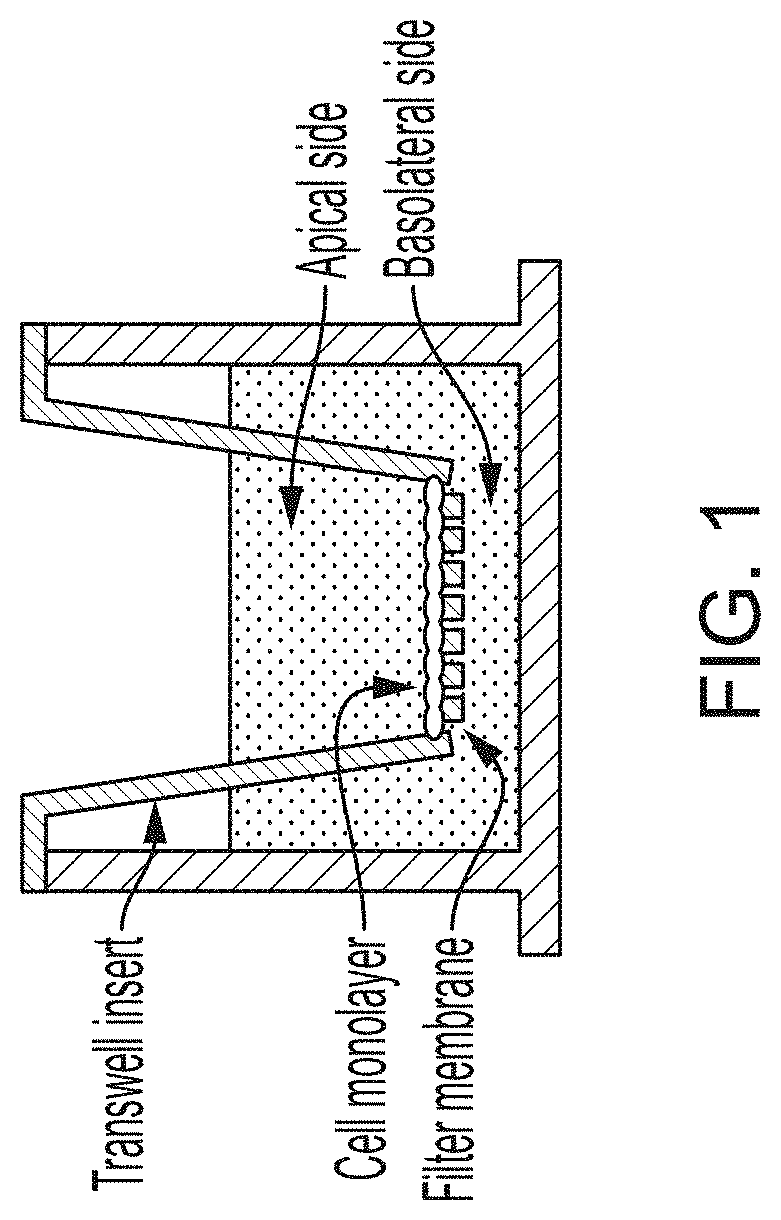
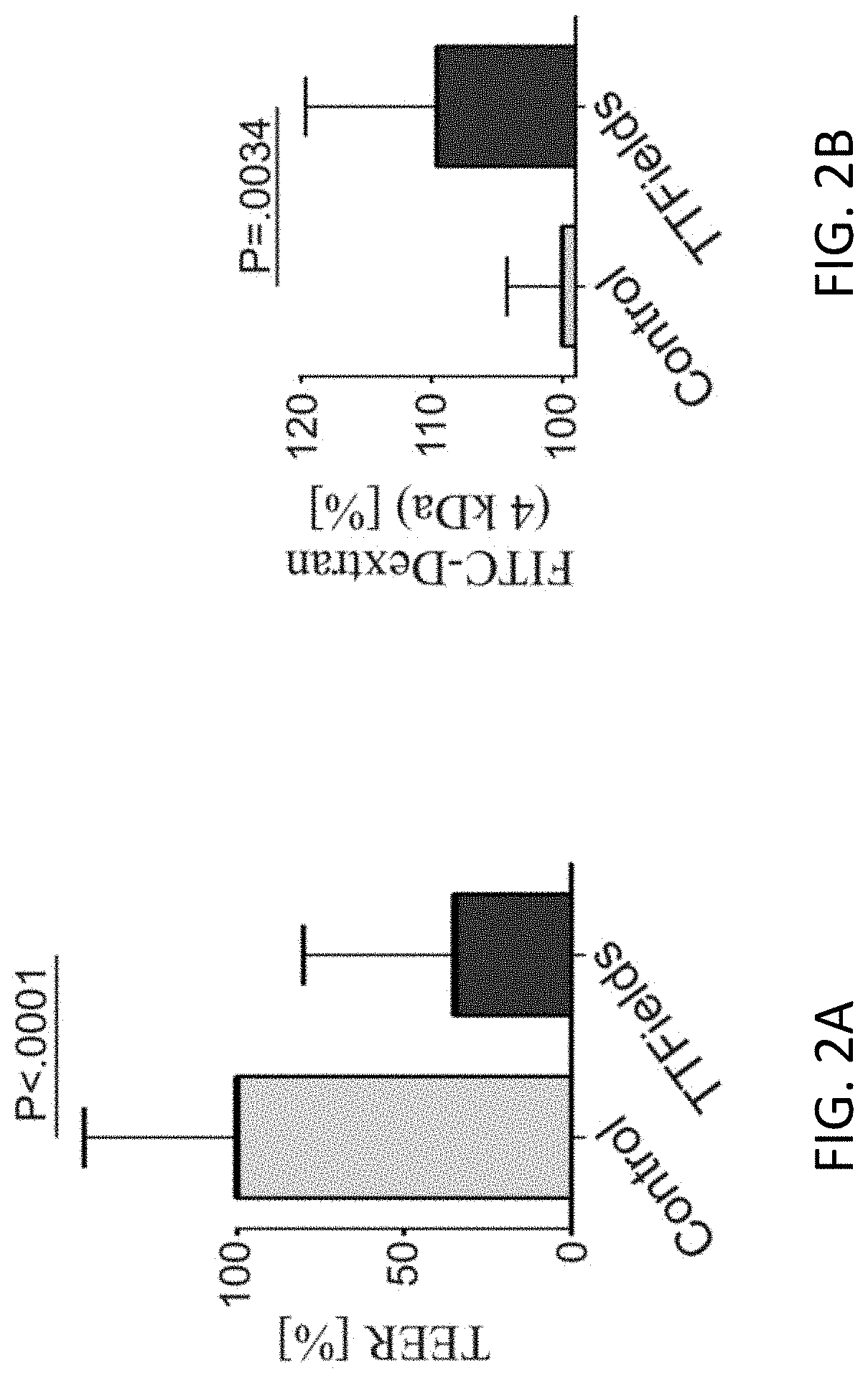
Using Alternating Electric Fields to Increase Permeability of the Blood Brain Barrier
ActiveUS20200061360A1Improve blood-brain barrier permeabilityPrevent tumorElectrotherapyMedical devicesBbb permeabilityBiomedical engineering
Certain substances (e.g., large molecules) that ordinarily cannot traverse the blood brain barrier can be introduced into the brain by applying an alternating electric field to the brain for a period of time, wherein the frequency of the alternating electric field is selected so that application of the alternating electric field increases permeability of the blood brain barrier. In some embodiments, the frequency of the alternating electric field is less than 190 kHz (e.g., 100 kHz). Once the permeability of the blood brain barrier has been increased, the substance is able to cross the blood brain barrier.
Owner:NOVOCURE GMBH
Using Alternating Electric Fields to Increase Permeability of the Blood Brain Barrier
ActiveUS20200061361A1Improve blood-brain barrier permeabilityPrevent tumorElectrotherapyMedical devicesElectric fieldBbb permeability
Certain substances (e.g., large molecules) that ordinarily cannot traverse the blood brain barrier can be introduced into the brain by applying an alternating electric field to the brain for a period of time, wherein the frequency of the alternating electric field is selected so that application of the alternating electric field increases permeability of the blood brain barrier. In some embodiments, the frequency of the alternating electric field is less than 190 kHz (e.g., 100 kHz). Once the permeability of the blood brain barrier has been increased, the substance is able to cross the blood brain barrier.
Owner:NOVOCURE GMBH
Using alternating electric fields to increase permeability of the blood brain barrier
Certain substances (e.g., large molecules) that ordinarily cannot traverse the blood brain barrier can be introduced into the brain by applying an alternating electric field to the brain for a period of time, wherein the frequency of the alternating electric field is selected so that application of the alternating electric field increases permeability of the blood brain barrier. In some embodiments, the frequency of the alternating electric field is less than 190 kHz (e.g., 100 kHz). Once the permeability of the blood brain barrier has been increased, the substance is able to cross the blood brain barrier.
Owner:NOVOCURE GMBH
Using Alternating Electric Fields to Increase Permeability of the Blood Brain Barrier
ActiveUS20200269037A1ElectrotherapyPharmaceutical delivery mechanismBbb permeabilityBiomedical engineering
Certain substances (e.g., large molecules) that ordinarily cannot traverse the blood brain barrier can be introduced into the brain by applying an alternating electric field to the brain for a period of time, wherein the frequency of the alternating electric field is selected so that application of the alternating electric field increases permeability of the blood brain barrier. In some embodiments, the frequency of the alternating electric field is less than 190 kHz (e.g., 100 kHz). Once the permeability of the blood brain barrier has been increased, the substance is able to cross the blood brain barrier.
Owner:NOVOCURE GMBH
Use of adenosine receptor signaling to modulate permeability of blood-brain barrier
InactiveUS20130224110A1Permeability of BBB is increasedFacilitates CNS entryBiocideHeavy metal active ingredientsMedicineBbb permeability
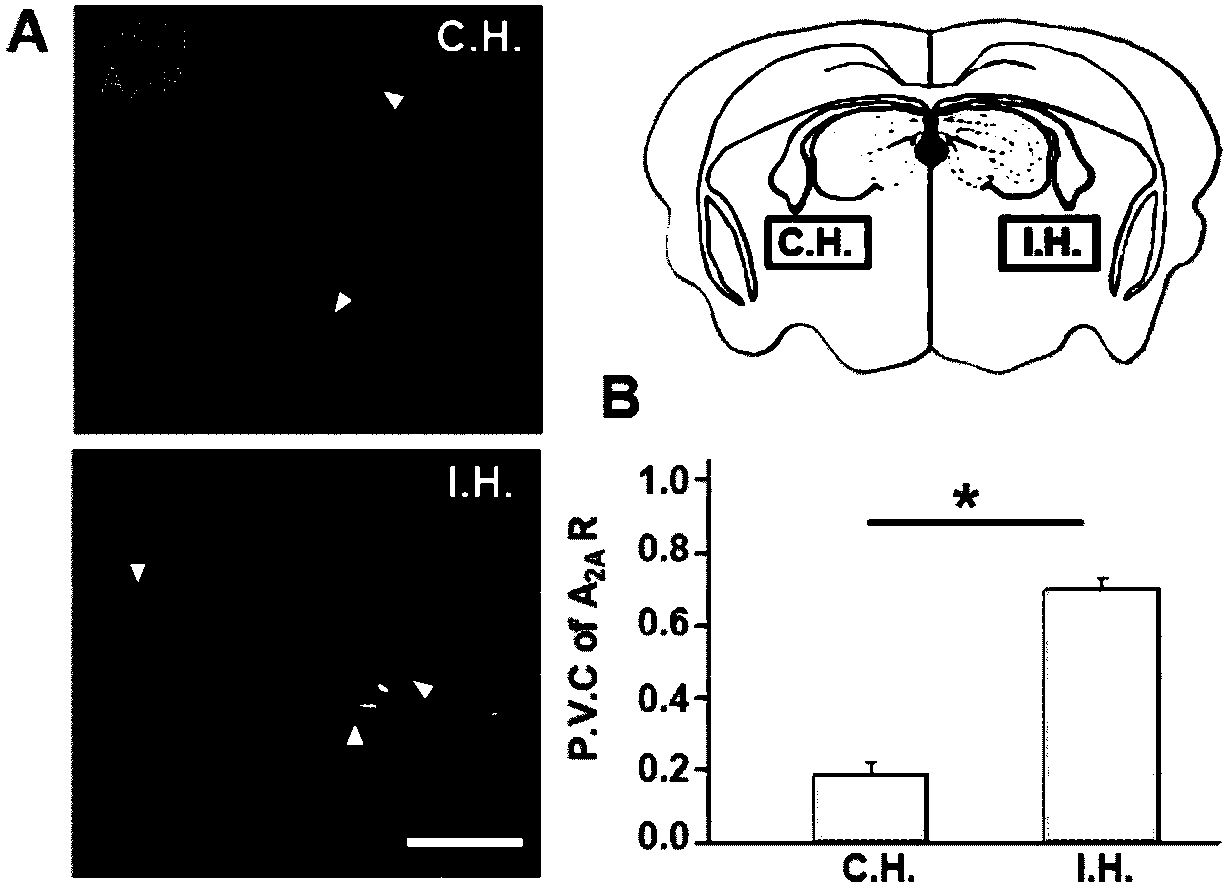
The present invention relates to a method of increasing blood brain barrier (“BBB”) permeability in a subject. This method involves administering to the subject an agent or agents which activate both of the A1 and A2A adenosine receptors. Also disclosed is a method to decrease BBB permeability in a subject. This method includes administering to the subject an agent which inhibits or blocks the A2A adenosine receptor signaling. Compositions relating to the same are also disclosed.
Owner:CORNELL UNIVERSITY
Use of adenosine receptor signaling to modulate permeability of blood-rain barrier
The present invention relates to a method of increasing blood brain barrier ("BBB") permeability in a subject. This method involves administering to the subject an agent or agents which activate both of the A1 and A2A adenosine receptors. Also disclosed is a method to decrease BBB permeability in a subject. This method includes administering to the subject an agent which inhibits or blocks the A2A adenosine receptor signaling. Compositions relating to the same are also disclosed.
Owner:CORNELL UNIVERSITY
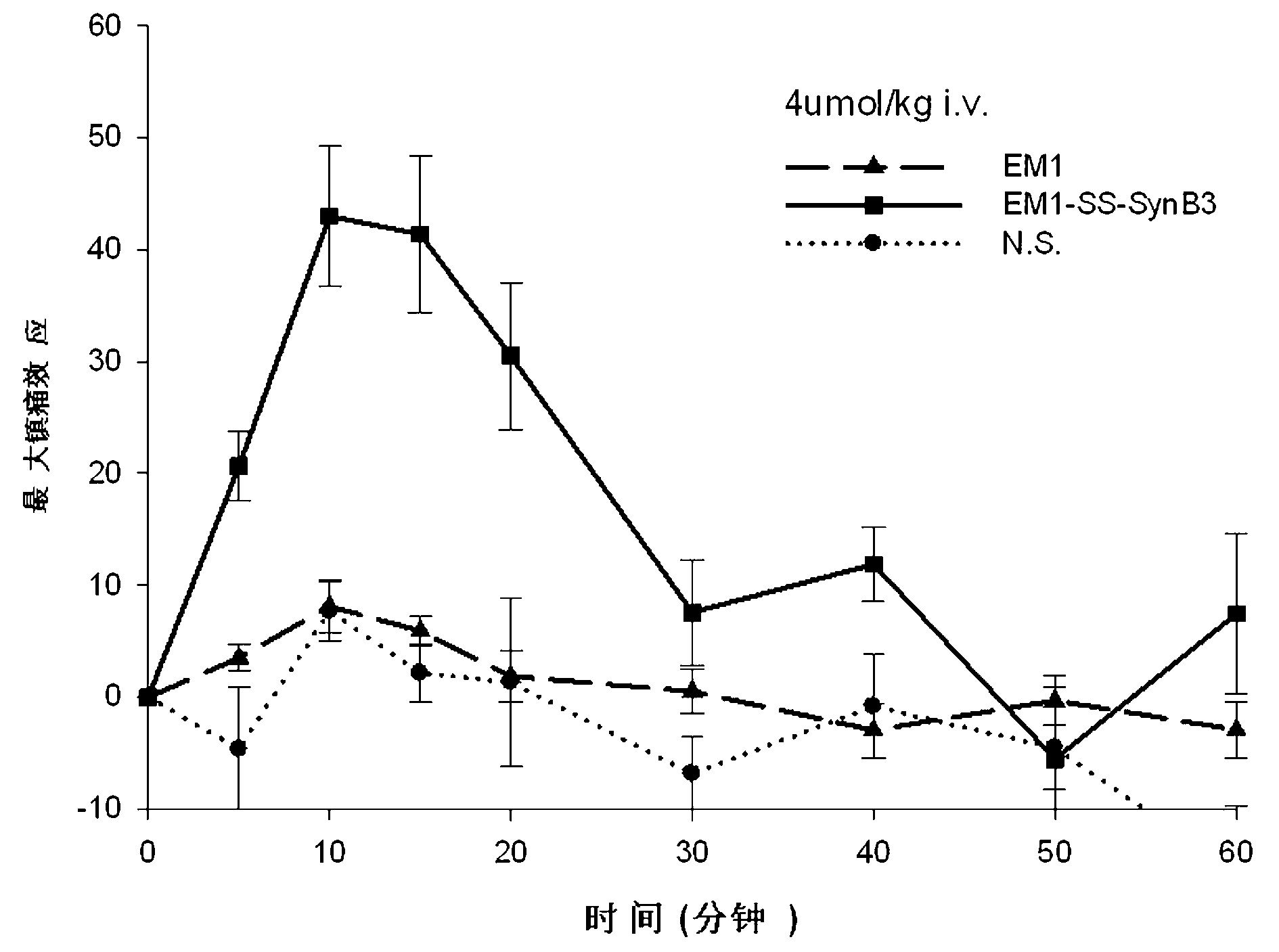
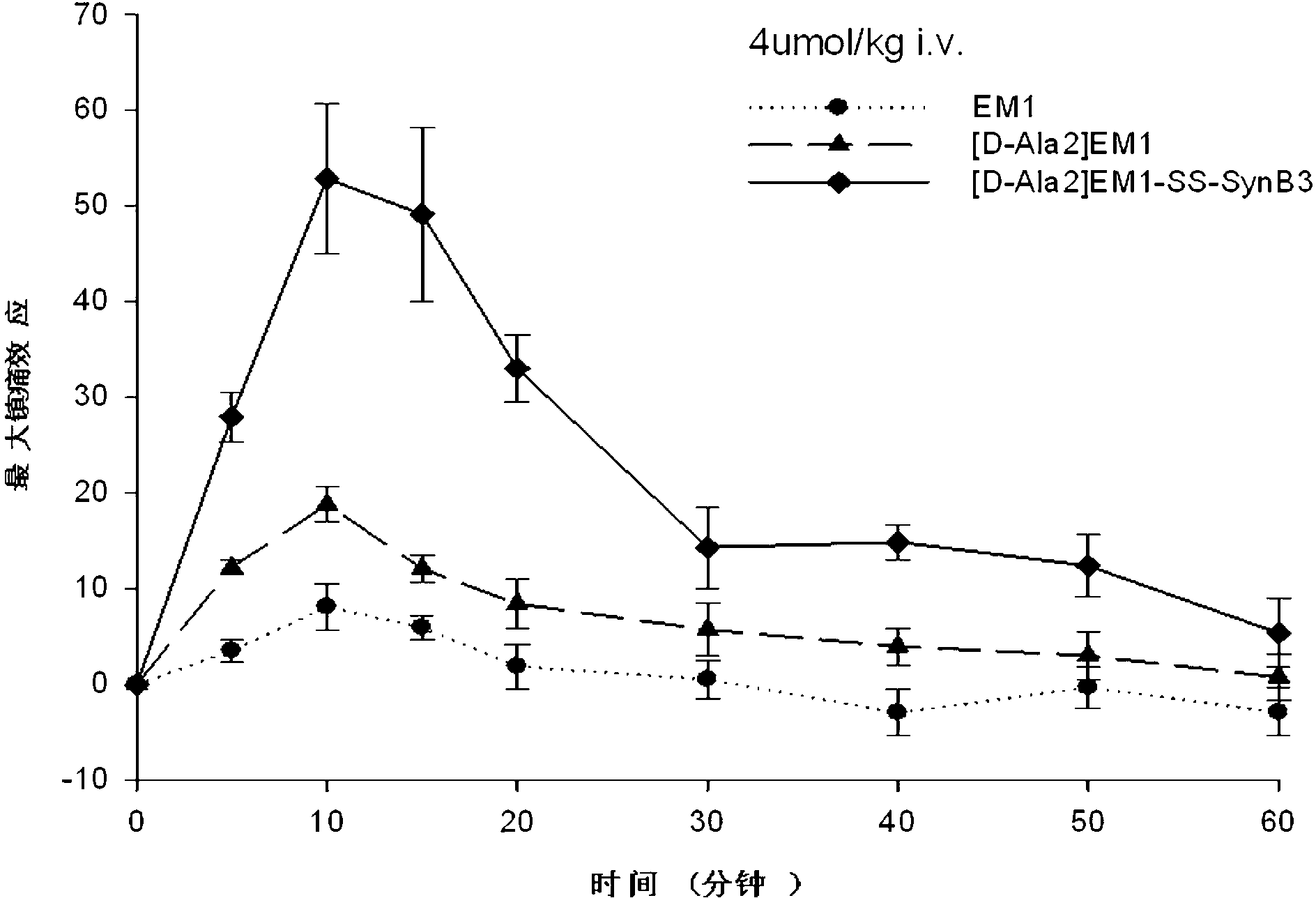
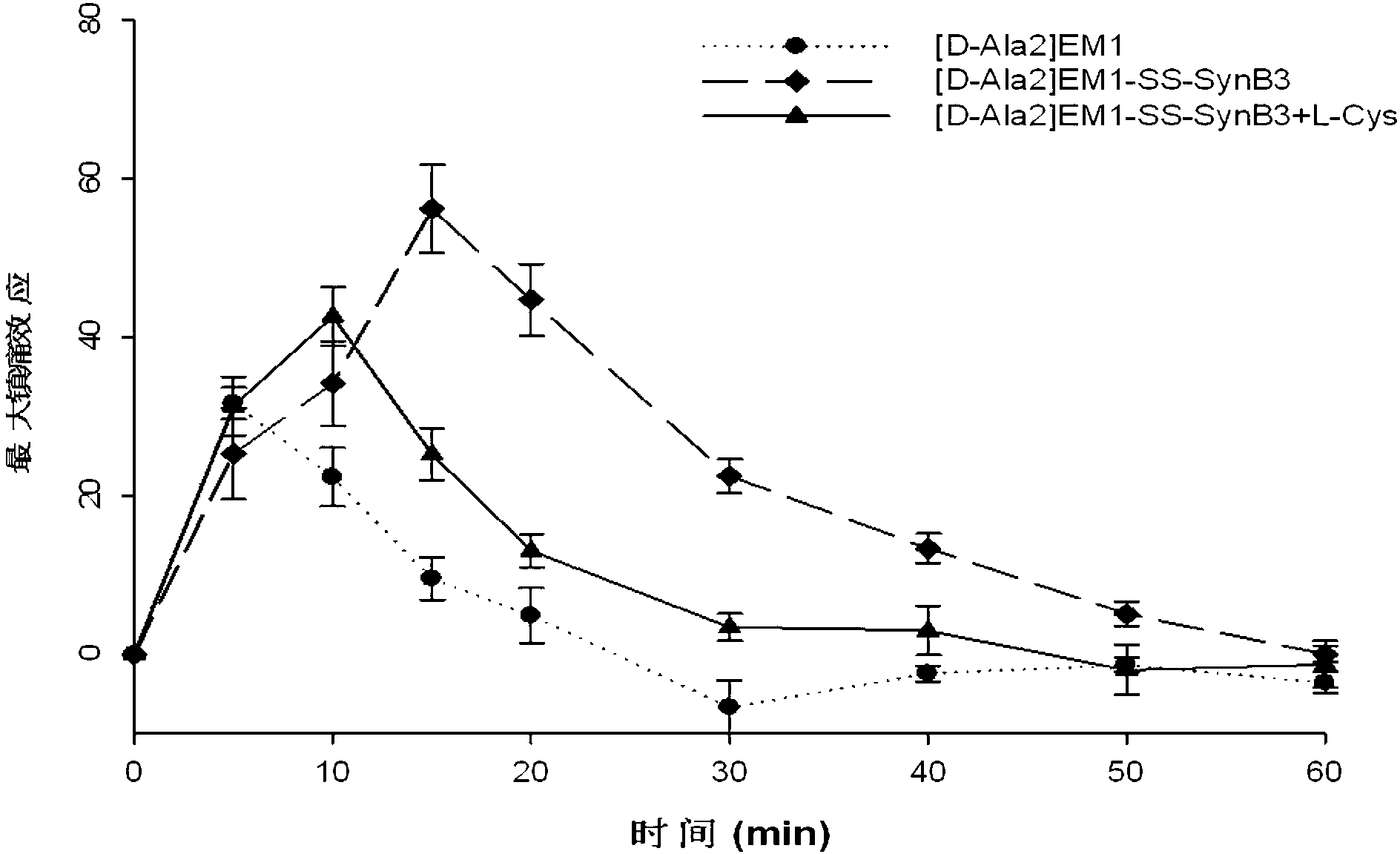
Endomorphin-derived peptide with blood-brain barrier permeability as well as synthesis and application of endomorphin-derived peptide
ActiveCN103012596AIncreased Brain UptakeWithout compromising integrityNervous disorderTetrapeptide ingredientsMeningesAdsorption effect
The invention discloses endomorphin-derived peptides with blood-brain barrier permeability. The endomorphin-derived peptides are formed by connecting cell-penetrating peptide SynB3 with the carbon terminal of EM1 (Endomorphin1) and analogue (D-A1a2) EM1 through a disulfide bond; good BBB (Blood-Brain Barrier) permeability of the SynB3 is used to mediate the EM1 and the analogue (D-A1a2) EM1 to permeate the blood-brain barrier to reach the center by means of adsorption effect; and the characteristics that the disulfide bond is stable in blood and is easily reduced to break in meninx are utilized, the disulfide bond is broken under the action of a meningeal reductase to release free endomorphin-derived peptide so that the pain killing effect of the endomorphin-derived peptide can be exerted. A pharmacodynamic experiment proves that the endomorphin-derived peptides can be peripherally administrated besides the obviously improved pain killing activity to provide a wide prospect for developing clinical application of a neuropeptide medicine.
Owner:LANZHOU UNIVERSITY
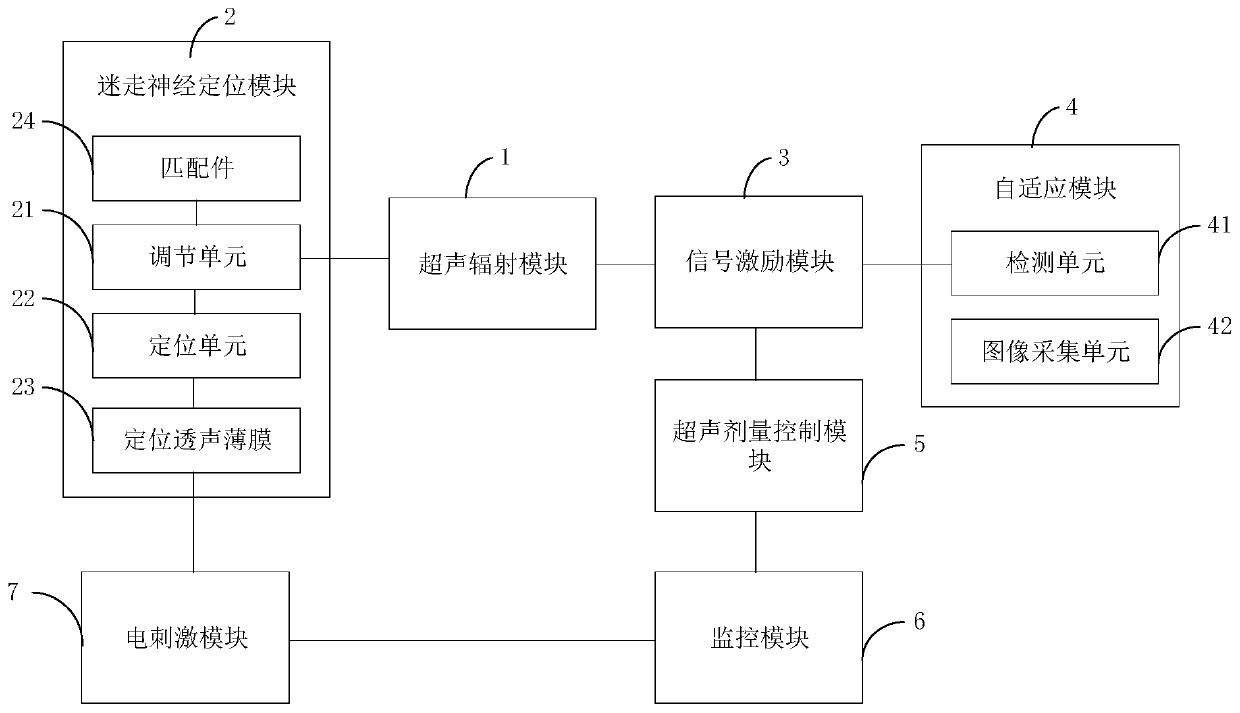
Blood brain barrier permeability regulation and control device, and method and storage medium
ActiveCN110811690ARegulate permeabilityImprove obstaclesUltrasound therapyElectrotherapyUltrasonic radiationControl ultrasound
The invention is suitable for the technical field of control, and provides a blood-brain barrier permeability regulation and control device, and a method and a storage medium. The device comprises anultrasonic radiation module, and a vagus nerve positioning module and a signal excitation module which are connected with the ultrasonic radiation module; the vagus nerve positioning module is used for acquiring position information of the vagus nerve and determining a radiation position according to the position information of the vagus nerve; the signal excitation module is used for sending an excitation signal to the ultrasonic radiation module when the ultrasonic radiation module is located at the radiation position, and the excitation signal is used for controlling the ultrasonic radiation module to radiate ultrasonic waves to the vagus nerves so as to regulate and control the blood-brain barrier permeability. By regulating and controlling the permeability of the blood-brain barrier,the obstruction of the blood-brain barrier to macromolecular substances can be improved, so that the macromolecular substances can be smoothly transferred into the brain.
Owner:SHENZHEN INST OF ADVANCED TECH
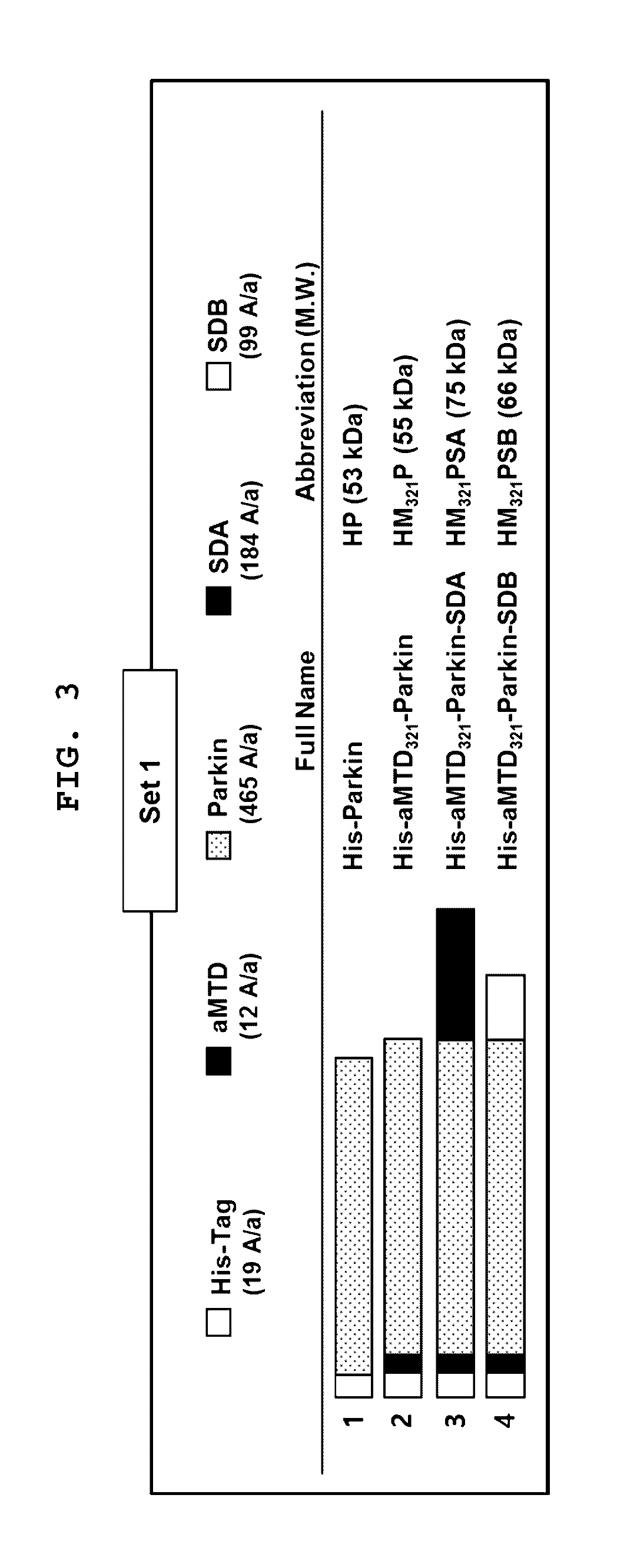
Development of Improved Cell-Permeable (iCP) Parkin Recombinant Protein as a Protein-Based Anti-Neurodegenerative Agent for the Treatment of Parkinson's Disease-Associated Phenotypes by Utilizing BBB-Penetrating Protein Delivery System MITT, Enabled by Advanced Macromolecule Transduction Domain (aMTD)
InactiveUS20170029798A1Improve solubilityHigh yieldPolypeptide with localisation/targeting motifNervous disorderNeuron cell deathSolubility
Macromolecule intracellular transduction technology based on improved cell-permeable Parkin recombinant protein (iCP-Parkin) has been developed as a protein-based anti-neurodegenerative agent for efficient BBB-penetration to effectively deliver the recombinant protein into the brain. Parkin protein, a dopaminergic neuronal cell death inhibitor, has been fused with a newly developed advanced macromolecule transduction domain (aMTD) and solubilization domain (SD) to increase the solubility / yield and cell- / tissue-permeability of the recombinant protein. In addition, our newly developed aMTD / SD-fused recombinant iCP-Parkin protein has shown BBB-permeability. Both in vitro and in vivo, our iCP-Parkin recombinant protein improved motor skills, a typical phenotype of Parkinson's disease, by increasing dopamine level in the brain by suppressing apoptosis of dopaminergic neuron cells. In conclusion, iCP-Parkin could be applicable in clinical studies as a protein-based anti-neurodegenerative agent to treat Parkinson's disease by protecting dopaminergic neuron cells and regulating the secretion of dopamine.
Owner:JO DAEWOONG +1
Application of nanogold in preparation of reagent for measuring blood-brain barrier permeability
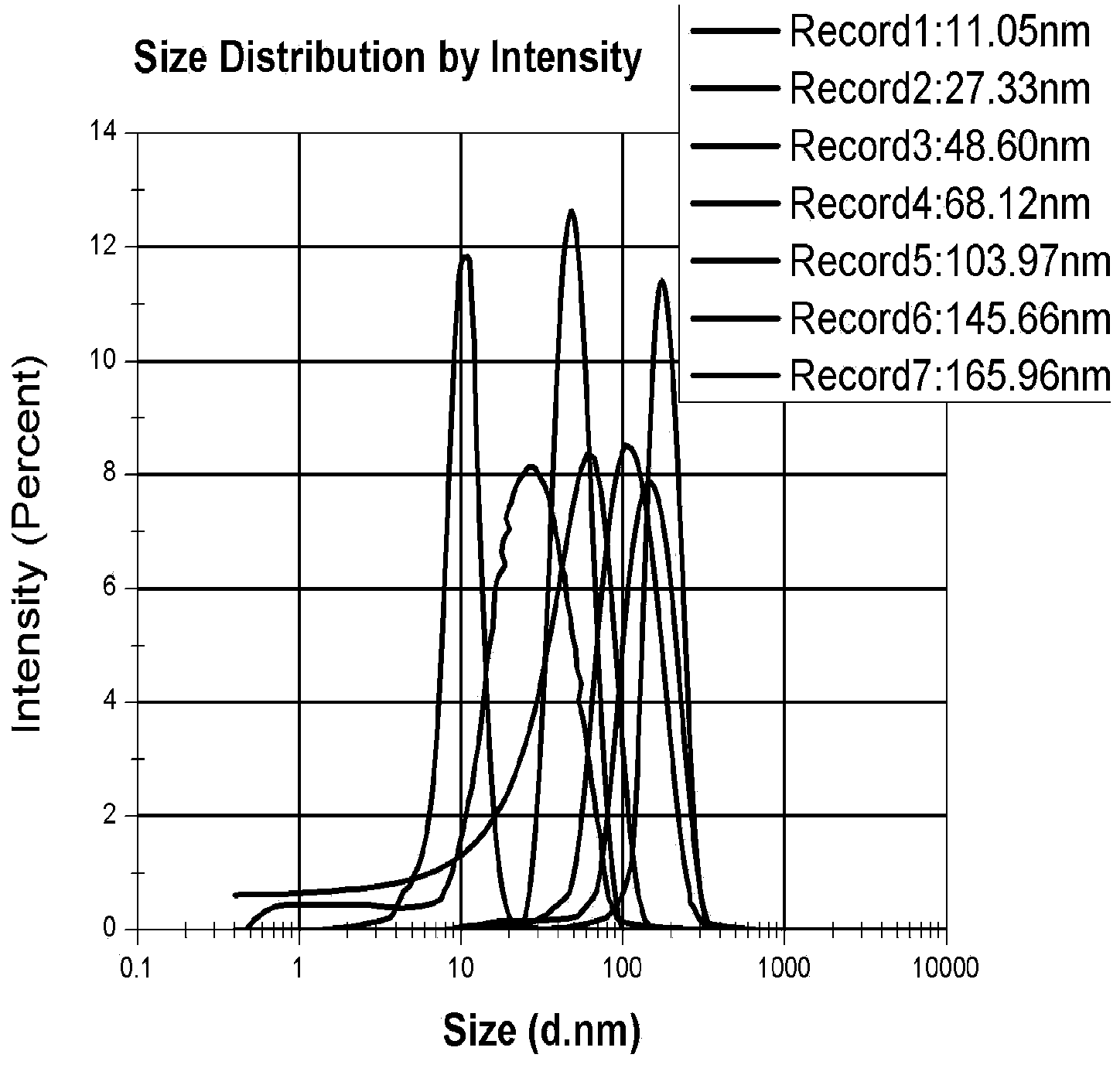
ActiveCN103800920AAccurately reflectSimple ingredientsColor/spectral properties measurementsIn-vivo testing preparationsBlood brain barrier permeabilityMaterials science
The invention provides the application of nanogold in preparation of a reagent for measuring blood-brain barrier permeability. The nanogold is formed by the following nanogold particles with the particle sizes by weight percent: 1 part of nanogold particles with the particle size range of 9-15nm and the average particle size of 11+ / -0.5nm, 1 part of nanogold particles with the particle size range of 18-31nm and the average particle size of 27.5+ / -0.5nm, 1 part of nanogold particles with the particle size range of 41-63nm and the average particle size of 48.5+ / -0.5nm, 1 part of nanogold particles with the particle size range of 50-76nm and the average particle size of 68+ / -0.5nm, 1 part of nanogold particles with the particle size range of 93-114nm and the average particle size of 104+ / -0.5nm, 1 part of nanogold particles with the particle size range of 134-156nm and the average particle size of 145.5+ / -0.5nm and 1 part of nanogold particles with the particle size range of 151-176nm and the average particle size of 166+ / -0.5nm. According to the application, the quantitative measurement for the blood-brain barrier permeability of animals with cerebral trauma can be completed by the nanogold particles with different particle sizes; the number of nanogold permeating into the blood-brain barrier can be measured, and the largest pore of the opening of the blood-brain barrier can be estimated according to the particle sizes of the nanogold permeating into the blood-brain barrier; therefore, a quantitative measurement standard is provided for various blood-brain barrier damage models in animal experiments.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
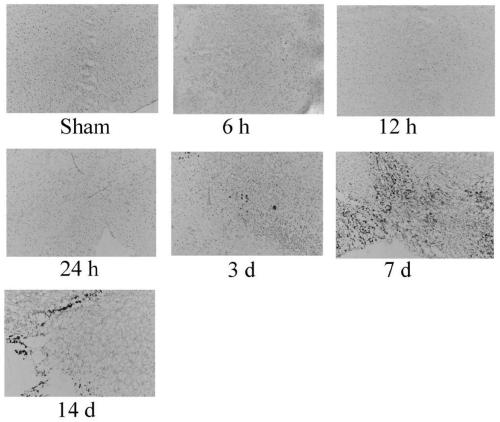
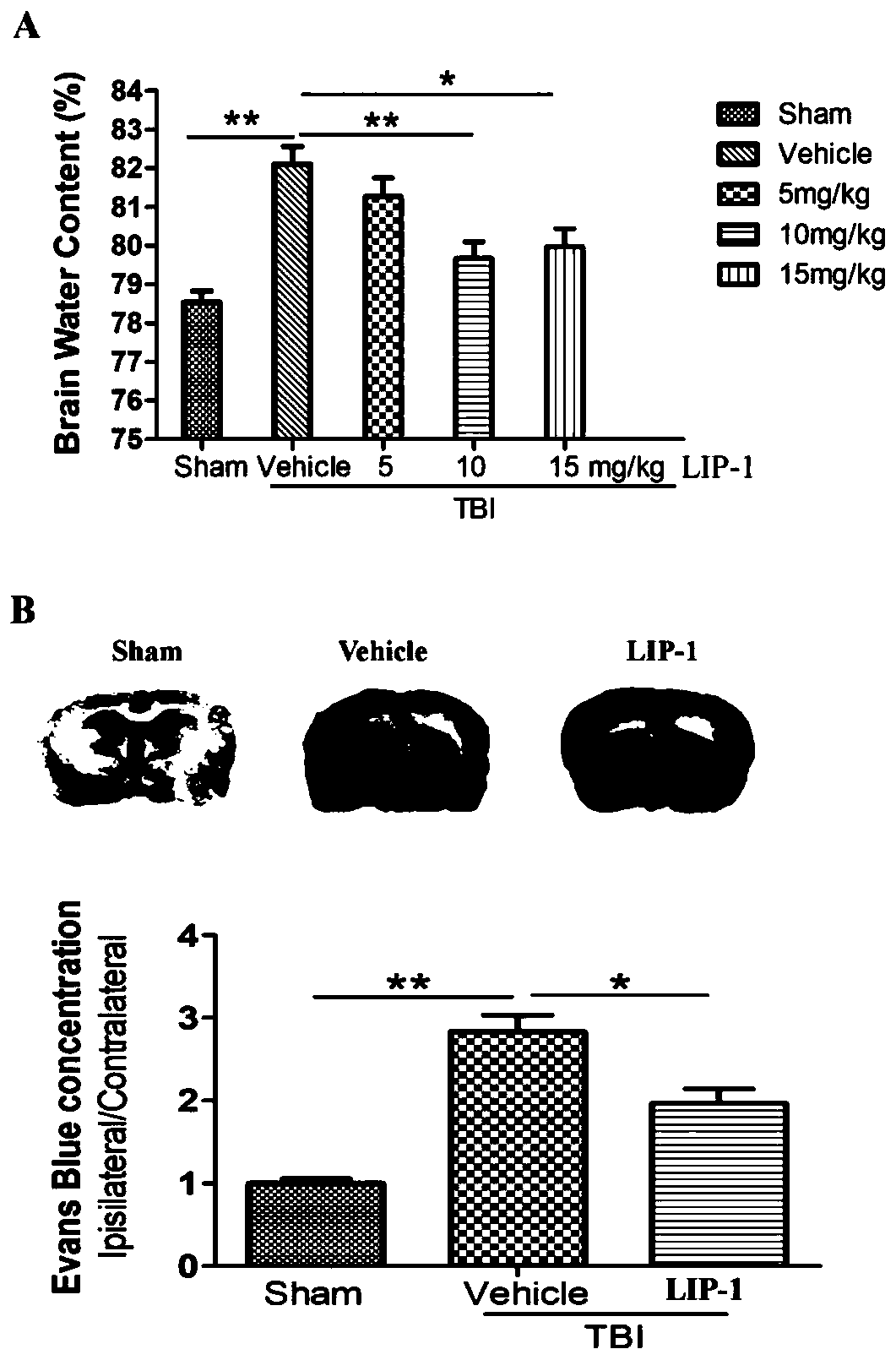
Application of ferroptosis inhibitor Liproxstatin-1 to preparation of medicine for treating traumatic brain injury
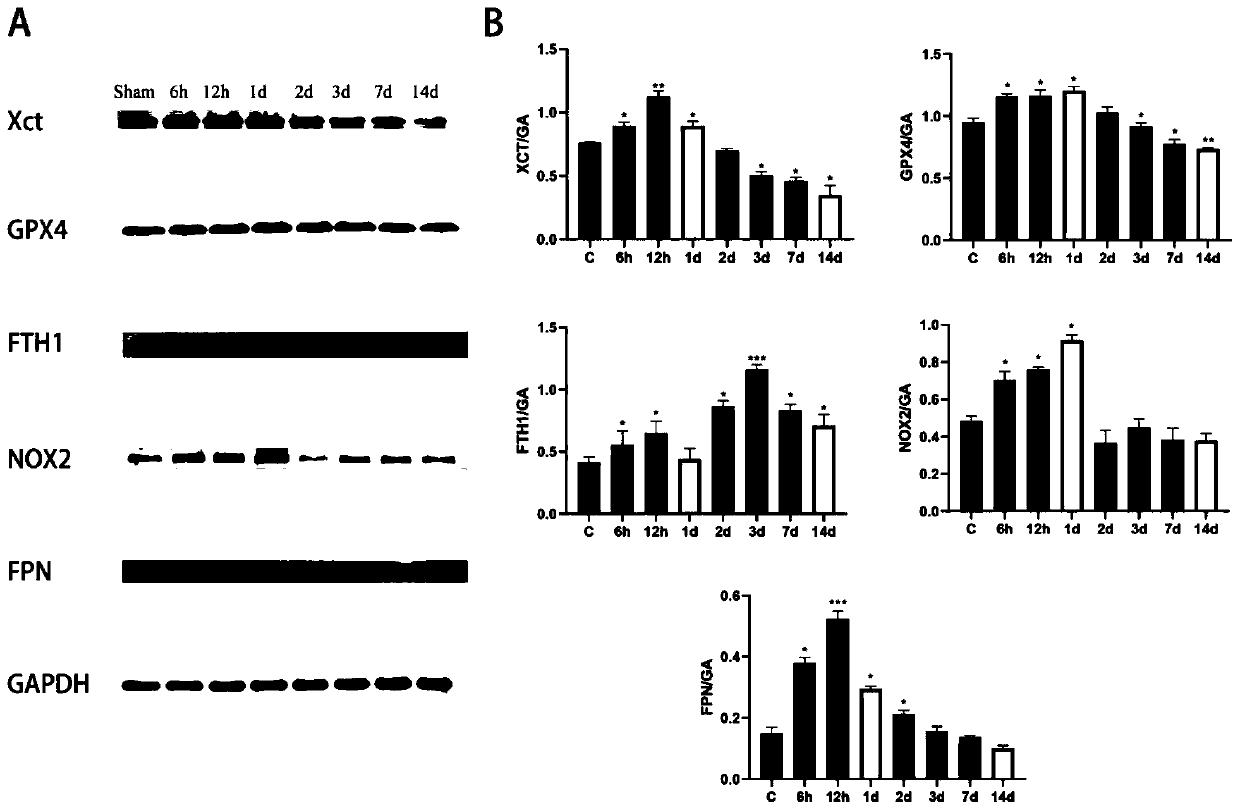
InactiveCN111135177AReduce cerebral edemaReduce permeabilityOrganic active ingredientsNervous disorderDepressantBiomedicine
The invention discloses application of ferroptosis inhibitor Liproxstatin-1 to preparation of medicine for treating traumatic brain injury, and belongs to the technical field of biomedicines. The system of the ferroptosis inhibitor Liproxstatin-1 illustrates a time-course changing process (from 6h to 14d) of ferroptosis-related protein expression and occurrence of iron deposits after traumatic brain injury; meanwhile, the Liproxstatin-1 is dosed through an abdominal cavity after a person suffers from traumatic brain injury for 1 hour, which proves that the Liproxstatin-1 can alleviate cerebraledema and blood-brain barrier permeability caused by the traumatic brain injury; the findings proves that the Liproxstatin-1 has an improvement effect on exercise and learning memory impairment caused by the traumatic brain injury; the confirmation shows that the Liproxstatin-1 can obviously improve anxiety and cognitive functions caused by TB1; and in addition, revelation shows that a protectioneffect of the Liproxstatin-1 is related with up-regulation of FTH1 and Nrf2 and down-regulation of expression of NOX2 caused by reversion of TB1. An evidence is provided for using of the Liproxstatin-1 as a novel medicine for treating the traumatic brain injury.
Owner:SUZHOU UNIV
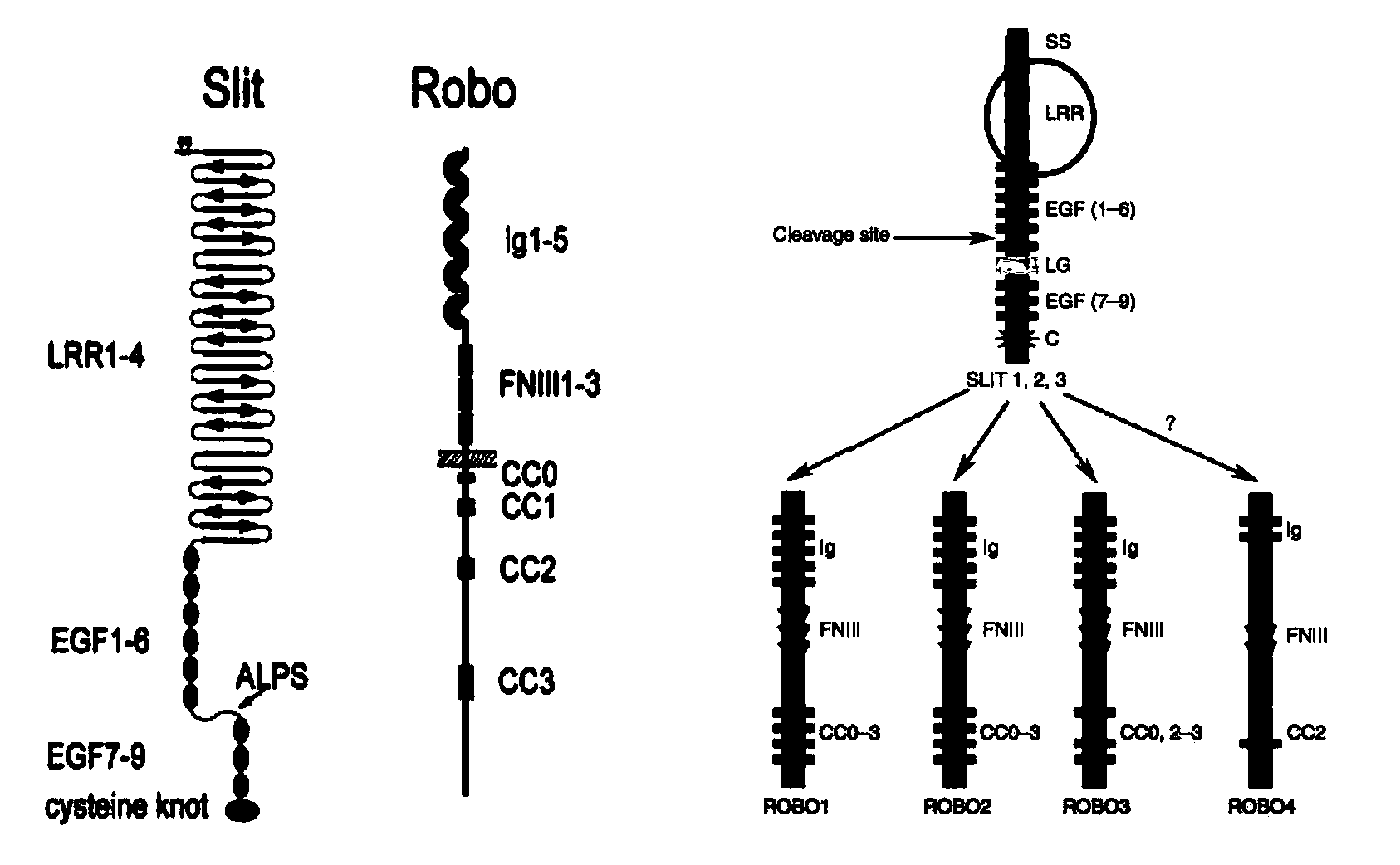
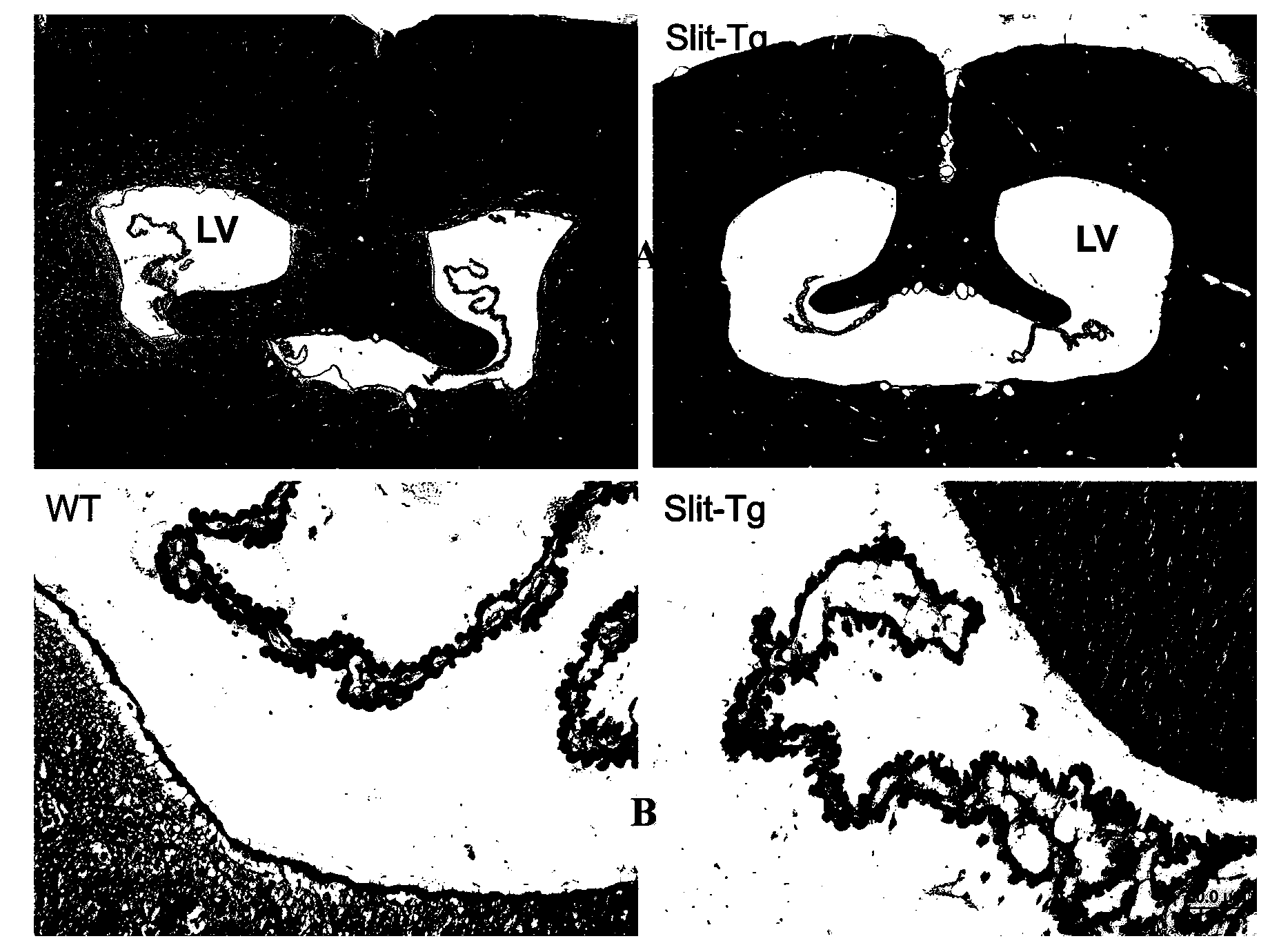
Construction method and application of transgenic animal with blood-brain barrier permeability enhanced
InactiveCN103409461AIncrease blood-brain barrier permeabilityReduce forksVector-based foreign material introductionTesting medicinal preparationsEpitheliumStaining
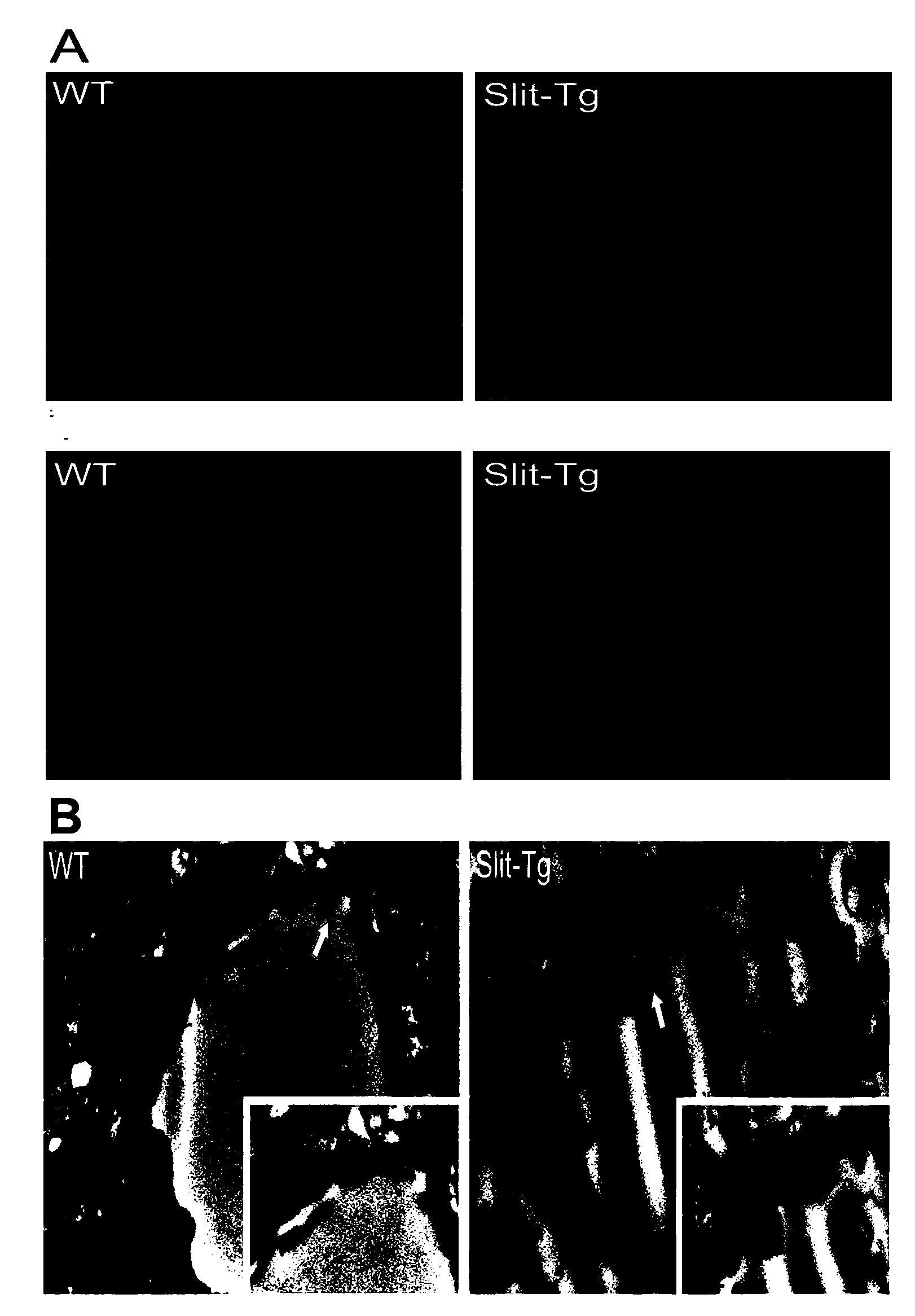
The invention discloses a construction method and an application of a transgenic mouse with blood-brain barrier permeability enhanced. The construction method of the transgenic mouse with blood-brain barrier permeability enhanced comprises the step of modifying genes of a mouse, so that expression quantity of slit protein is increased, and a transgenic animal model with blood-brain barrier permeability enhanced is obtained; Study shows that overexpression of slit protein can cause that a tricorn of the mouse is enlarged, a choroid plexus structure is seriously destroyed, and tight junction of epithelium cells is lost; observation on a vascular structure of the brain of a Slit-Tg mouse by virtue of immunofluorescent staining shows that bifurcation of blood vessels of the brain of the Slit-Tg mouse is less, more microvessels exist, the shapes of vessels are incomplete and the distribution of the vessels is irregular; a transmission electron microscope map also shows that endothelial cells of the Slit-Tg mouse are damaged and highly irregular and basement membranes of vessels are incomplete. Evansblueassay detects that the permeability of the mouse is enhanced, i.e., the detection result shows that the blood-brain barrier permeability of the Slit-Tg mouse is enhanced, the Slit-Tg mouse can be applied to an animal experiment of central nervous system drugs and the Slit-Tg mouse can be used for screening, research and development as well as therapeutic effect evaluation on nervous system drugs.
Owner:GUANGDONG PHARMA UNIV
Preparation method and application of cyclosporine A-loaded efficient brain-targeted drug delivery system
ActiveCN112675149AReduce solubilityAvoid damageCyclic peptide ingredientsMacromolecular non-active ingredientsInflammatory factorsCyclosporins
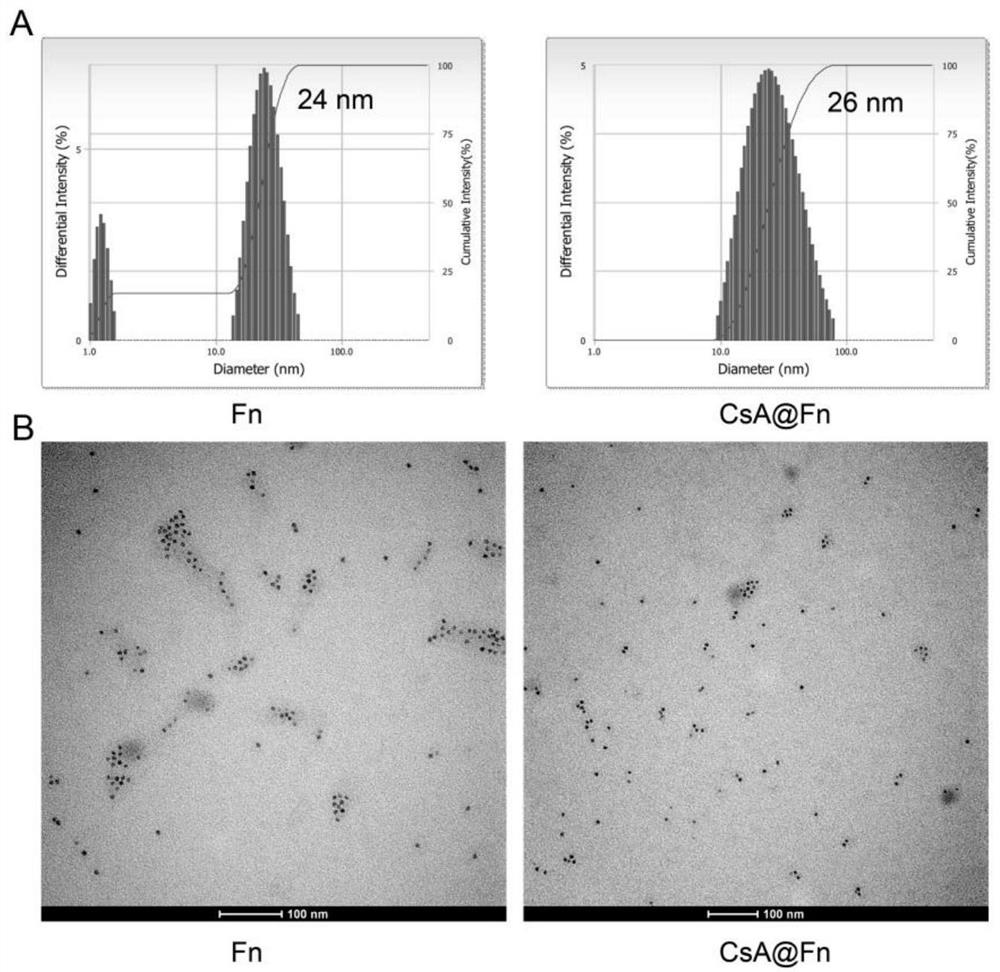
The invention relates to the field of biological pharmacy, and discloses a preparation method and application of a cyclosporine A-loaded efficient brain-targeted drug delivery system. The drug delivery system is composed of CsA and Fn; by utilizing the characteristic that ferritin is denatured in acetone and can be renatured in water, the CsA is wrapped in a cage structure (CsA(at)Fn) of the Fn through a solvent evaporation method; the CsA(at)Fn can be mediated by transferrin receptors on the surfaces of brain capillary endothelial cells to penetrate through a blood brain barrier and be accumulated in an ischemic area; on one hand, the CsA(at)Fn van protect the integrity of the blood brain barrier and reduce accumulation of inflammatory factors in a cerebral ischemic area; and on the other hand, the CsA(at)Fn taken by neuronal cells can inhibit the opening of mitochondrial permeability conversion pores of the neuronal cells, reduce the release of mitochondrial ROS and cytochrome C, inhibit the apoptosis of the neuronal cells and play a role in neuroprotection.
Owner:AIR FORCE MEDICAL UNIV
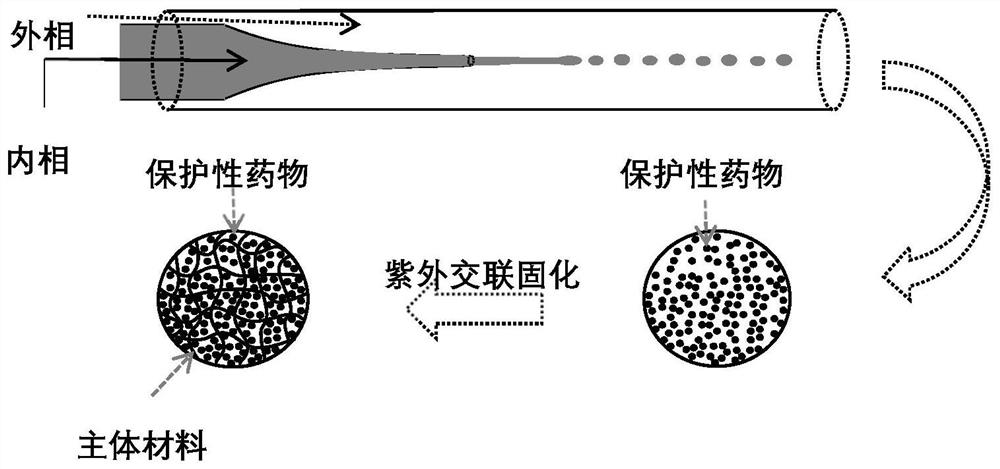
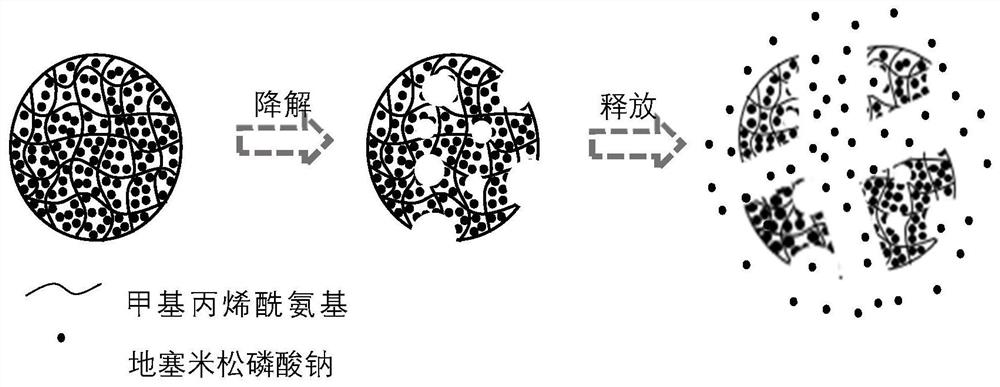
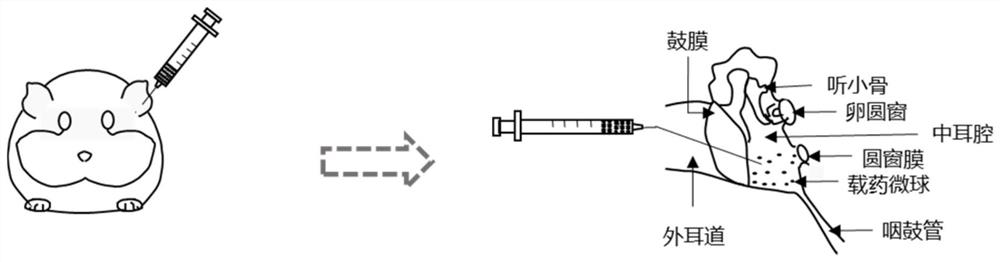
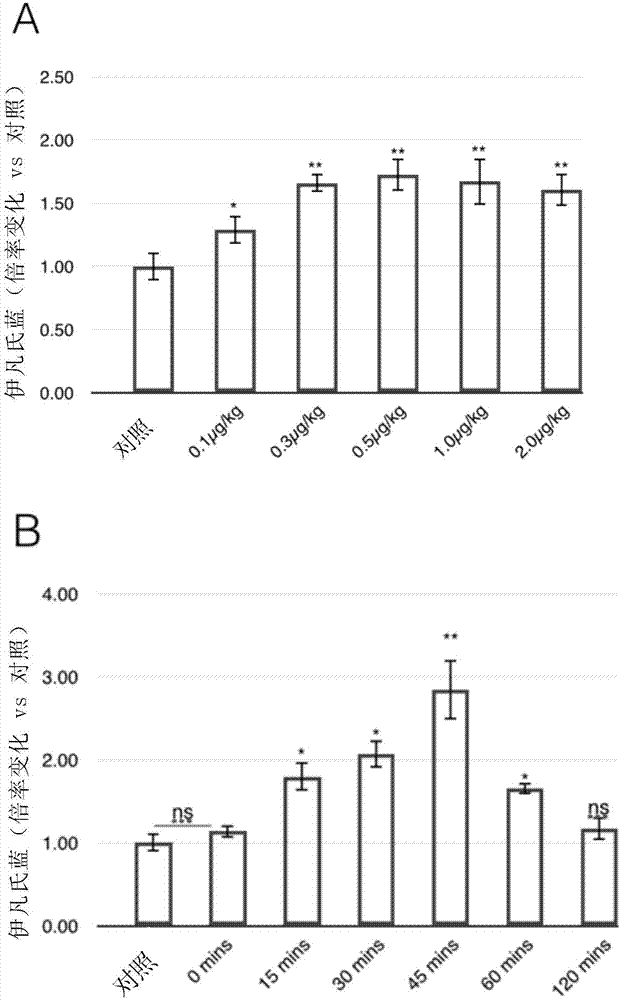
Preparation method of drug microcarrier for acquired deafness based on microfluidic technology
InactiveCN113577030AWide range of optionsThe size is easy to controlOrganic active ingredientsSenses disorderProtective drugsMiddle ear
The invention relates to a preparation method of a drug microcarrier for acquired deafness based on a microfluidic technology, which comprises the following steps of: controlling the generation of internal and external two-phase fluid by utilizing the micro-fluidic technology; the internal and external two-phase fluid generation process comprises the following steps: S1, preparing micron-scale liquid drops capable of being used for tympanic injection by utilizing shearing force and interfacial tension between two-phase fluids which are mutually insoluble, and in-situ entrapping hearing protective drugs and an auxiliary agent for increasing the permeability of a round window membrane; and S2, solidifying the liquid drops through polymerization reaction to form the drug microcarrier. Compared with the prior art, the prepared medicine microcarrier can be used for treating acquired deafness through tympanic injection, the middle ear cavity is filled with the medicine microcarrier through tympanic injection, the medicine is slowly released through natural degradation of the microcarrier and enters the inner ear through the round window membrane, and the effect of treating acquired deafness is achieved; the drug microcarrier has the advantages of small side effect and injury, lasting effect, obvious effect, simple operation and the like.
Owner:FUDAN UNIV
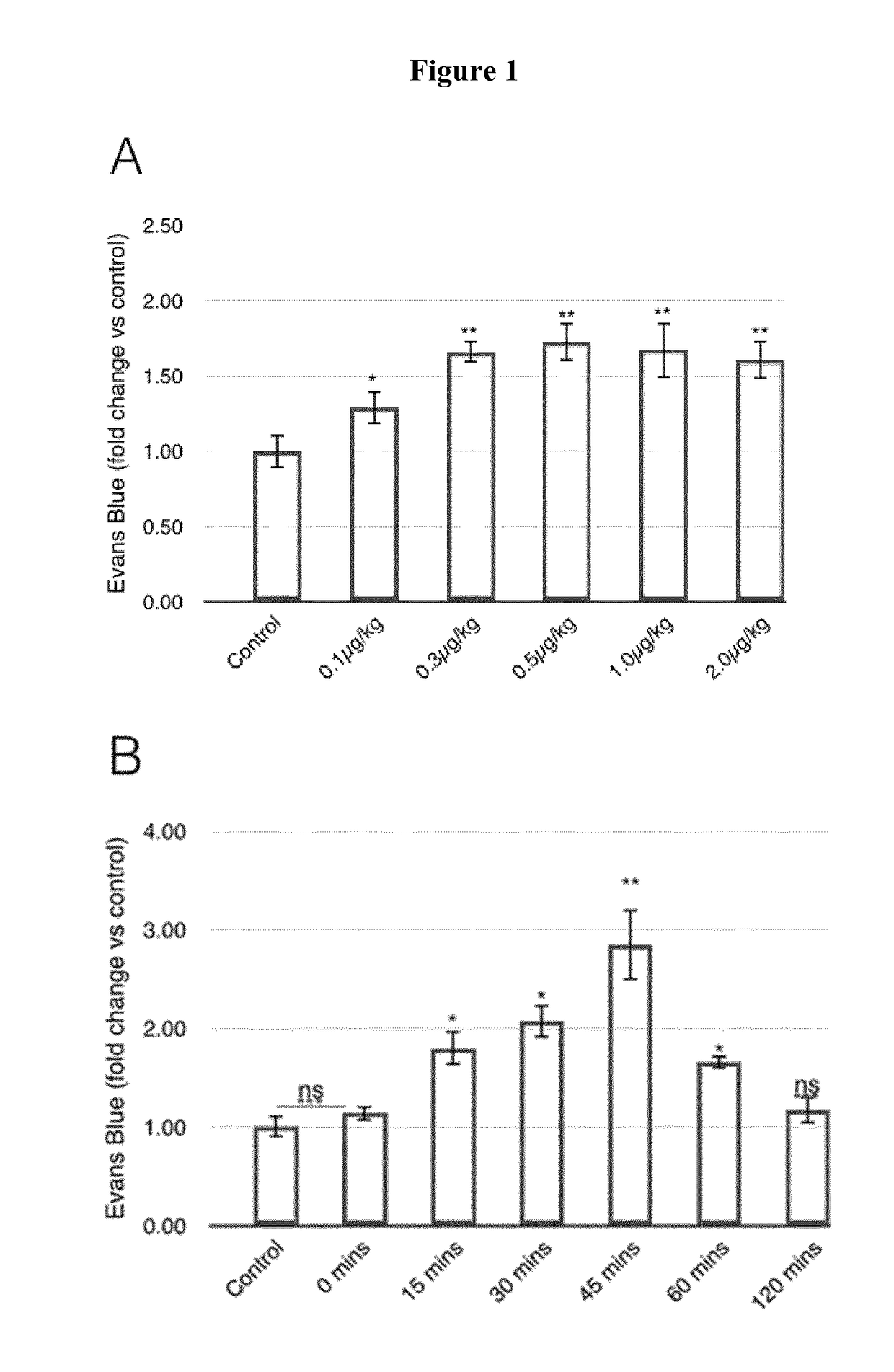
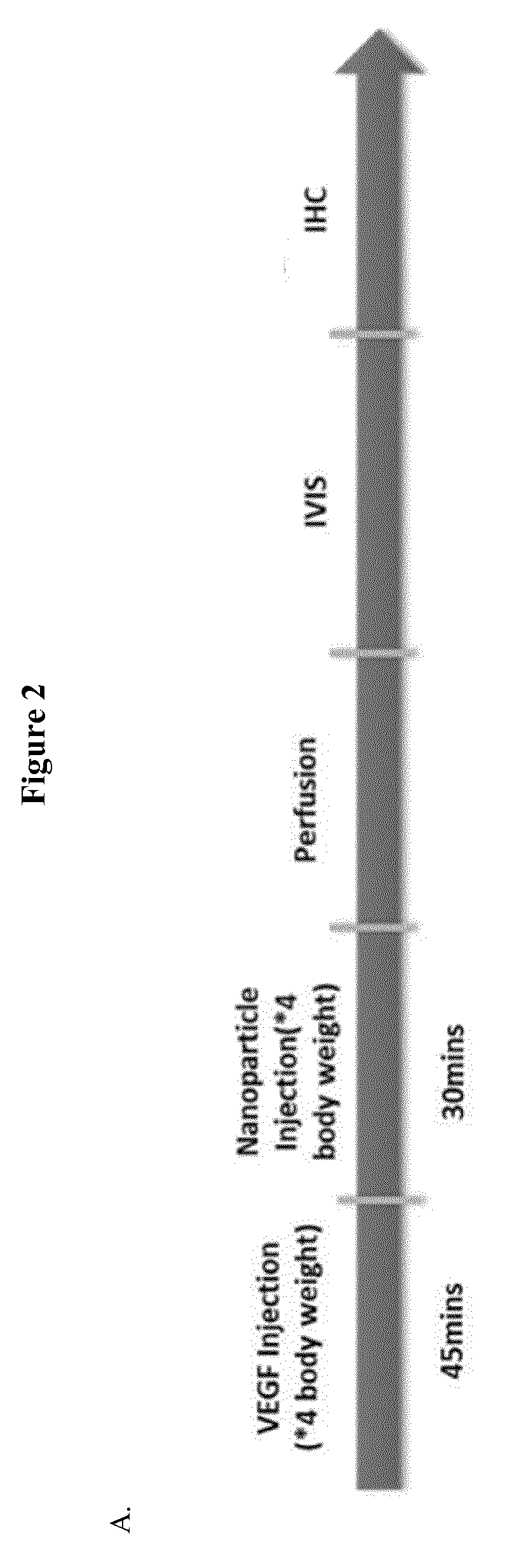
Methods for enhancing permeability to blood-brain barrier and uses thereof
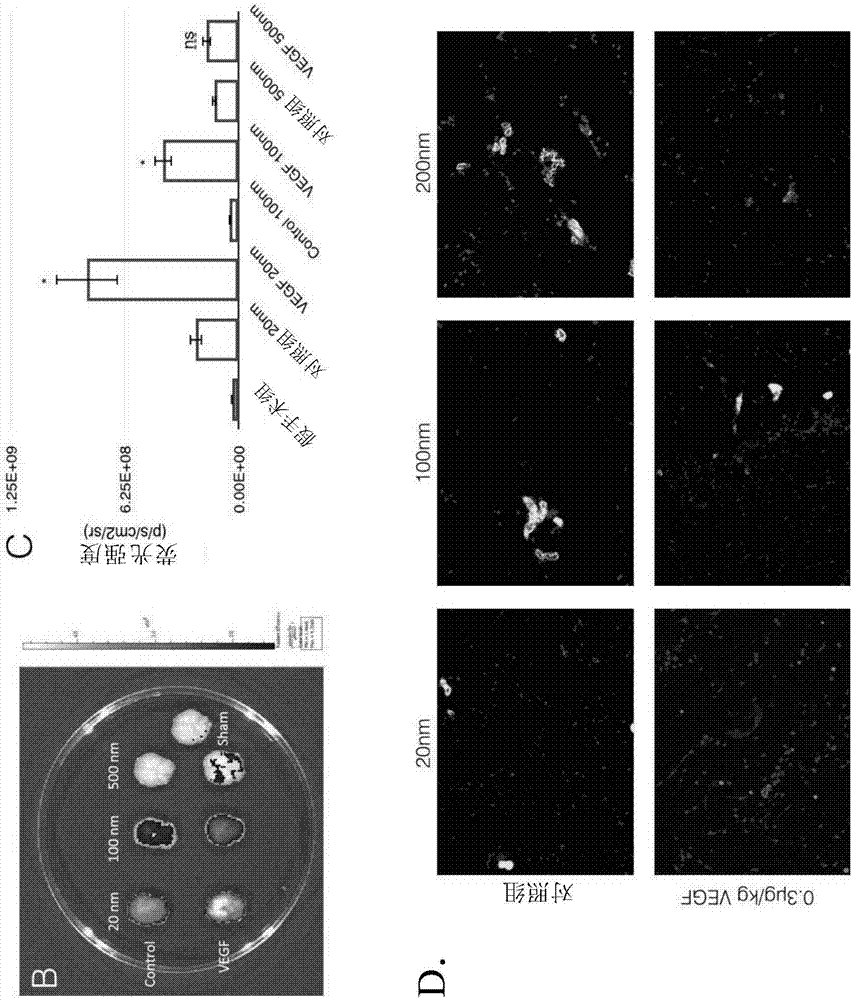
Disclosed herein is a method of facilitating the delivery of an agent across blood-brain barrier (BBB) of a subject. The method includes administering to the subject in sequence or concomitantly, an effective amount of a growth factor selected from the group consisting of, vascular endothelial growth factor (VEGF), insulin-like growth factor I (IGF-1), IGF-II, a portion thereof and a combination thereof; and an agent that is any of a therapeutic agent or an imaging agent. The administered amount of the growth factor is capable of transiently increasing BBB permeability of the subject and thereby allowing the agent to be delivered across BBB. Also disclosed herein is a method of treating a subject suffering from a brain tumor, a brain stroke, a neuropsychiatric disorder and / or a neurodegenerative disease.
Owner:ACAD SINIC
Method for regulating and controlling blood brain barrier permeability and application of method for regulating and controlling blood brain barrier permeability
ActiveCN113424797AImprove permeabilityPermeability can be adjustedLigasesFermentationMice brainMedicine
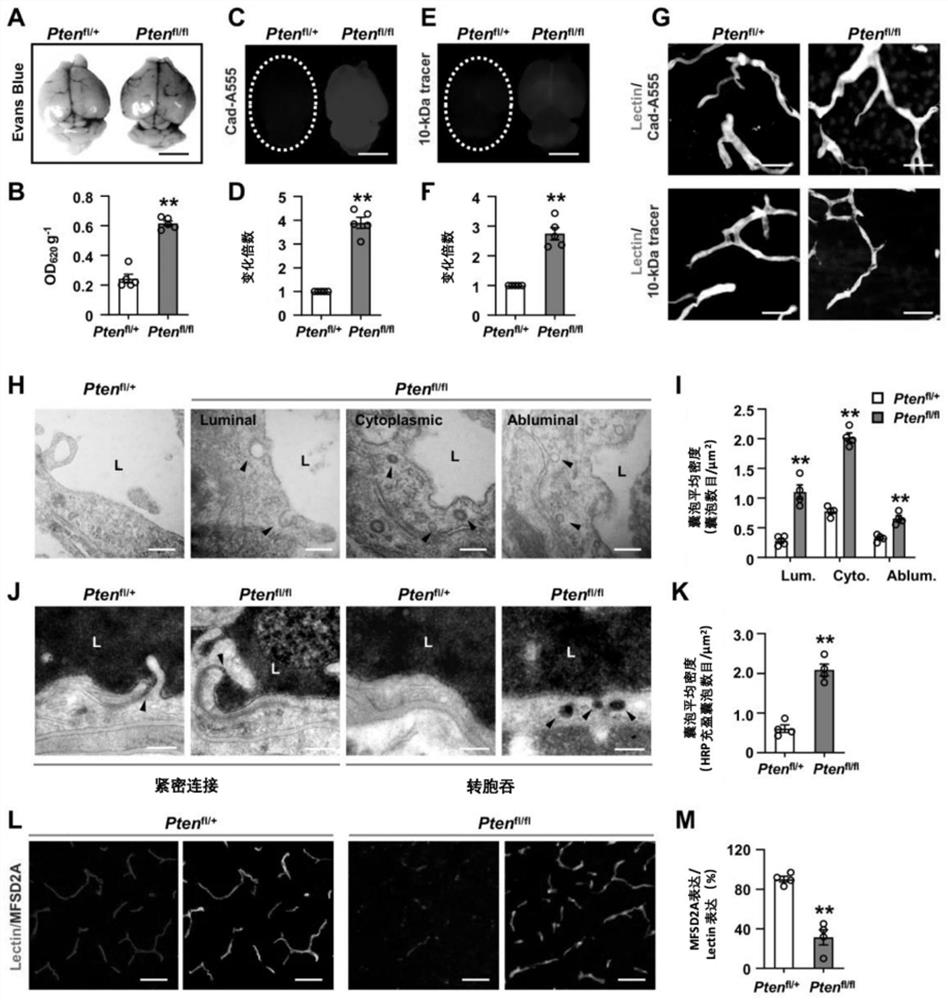
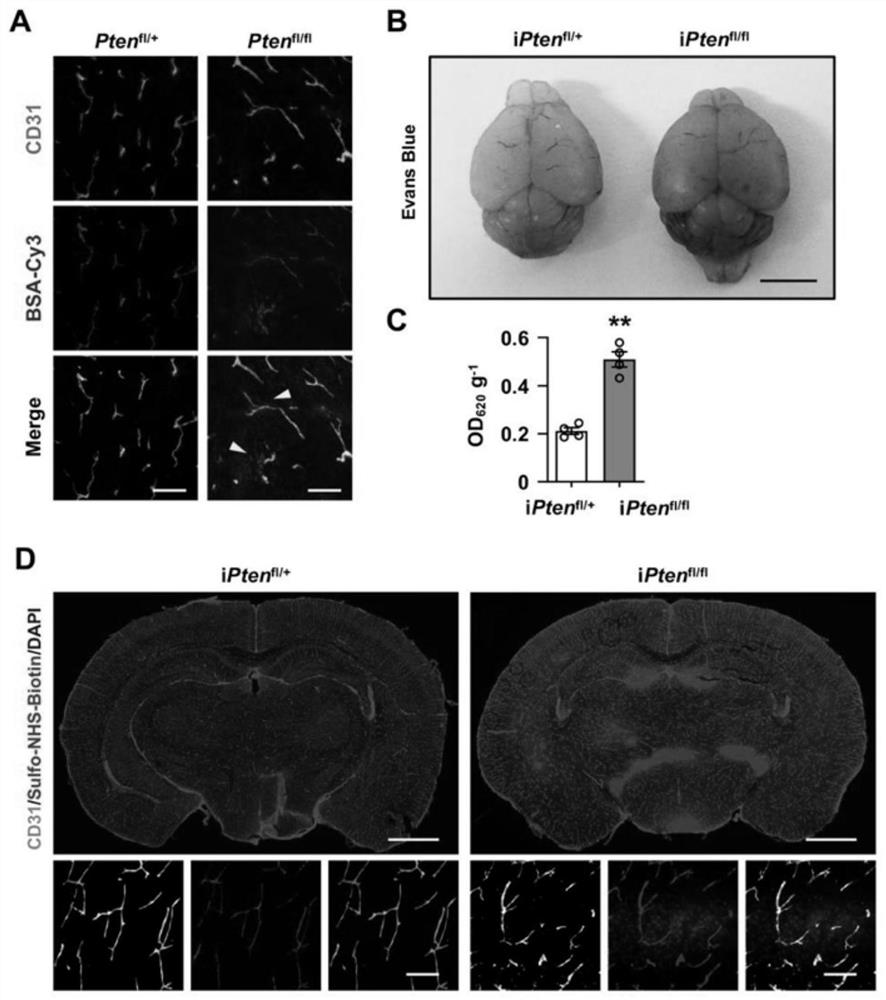
The invention discloses a method for regulating and controlling blood brain barrier permeability and application of the method for the regulating and controlling blood brain barrier permeability. Through specific knockout of a fifth exon, a sixth exon and a seventh exon of a PTEN gene in a mouse cerebrovascular endothelial cell, increase of the blood brain barrier permeability and blood brain barrier leakage of a mouse can be observed; recombinant adeno-associated viruses expressing NEDD4-2 protein are constructed and injected into the mouse, it is found that the number of vesicles in cerebral vascular endothelial cells of the mouse is increased, the increase of the vesicles also leads to increase of the blood brain barrier permeability and the blood brain barrier leakage of the mouse. it is proved that PTEN deletion can cause blood brain barrier leakage caused by overexpression of NEDD4-2-Mfsd2a, therefore, the blood brain barrier permeability can be regulated and controlled by regulating and controlling the content or activity of the PTEN protein or the NEDD4-2 protein, and the PTEN protein or the NEDD4-2 protein can be applied to clinical preparation or development of related medicines for regulating and controlling the blood brain barrier permeability or cerebrovascular related medicines.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Agitation micelle for treatment of cerebral arterial thrombosis
InactiveCN108066284AImprove delivery efficiencyGood neuroprotective efficacyOrganic active ingredientsNervous disorderSide effectCerebral arterial thrombosis
The invention belongs to the technical field of biology and relates to agitation micelle for treatment of cerebral arterial thrombosis as well as a preparation method and an application thereof. A drug delivery system comprises a functional group across BBB (blood brain barrier), a neuroprotection drug and a nano drug delivery system, wherein the functional group is an adenosine A2A receptor agitation group capable of reversibly regulating BBB permeability, and a micromolecular agonist is linked with a PEG end of the outer surface of a micelle drug carrier system in a chemical bond connectionmanner. The nano drug delivery system can enters the stroke area safely and initiatively across BBB, and the long-term neuroprotection function is provided through sustained and controlled release ofthe neuroprotection drug. The drug delivery system has great clinical significance in improving the treatment efficacy of patients suffering from cerebral arterial thrombosis and reducing the toxic and side effects of drugs used currently.
Owner:FUDAN UNIV
Application of butylphthalide or derivatives thereof in preparation of medicaments for treating or preventing radiation brain damage
InactiveCN103356521AOrganic active ingredientsNervous disorderCirculating endothelial cellVascular endothelium
The invention discloses an application of butylphthalide or derivatives thereof in preparation of medicaments for treating or preventing radiation brain damage. The mechanism of action for treating the radiation brain damage by using the butylphthalide is that oxygen free radical balance in brain tissues subjected to radiation damage can be improved and the damage effect of oxygen free radicals can be relieved by the butylphthalide; the permeability of blood-brain barrier of a rat with radioactive brain damage can be reduced by reducing the number of circulating endothelial cells of the rat after whole brain irradiation, and the radioactive brain damage is protected, i.e., acting target cells of the butylphthalide are mainly vascular endothelial cells; and the AQP4 (Aquaporin 4) effect can be adjusted so as to protect the blood-brain barrier.
Owner:SHIJIAZHUANG PHARMA GRP NBP PHARMA CO LTD +1
Methods for enhancing permeability to blood-brain barrier, and uses thereof
InactiveUS20170283493A1Reduce deliveryNervous disorderPeptide/protein ingredientsInsulin-like growth factorMedicine
Disclosed herein is a method of facilitating the delivery of an agent across blood-brain barrier (BBB) of a subject. The method includes administering to the subject in sequence or concomitantly, an effective amount of a growth factor selected from the group consisting of, vascular endothelial growth factor (VEGF), insulin-like growth factor I (IGF-1), IGF-II, a portion thereof and a combination thereof; and an agent that is any of a therapeutic agent or an imaging agent. The administered amount of the growth factor is capable of transiently increasing BBB permeability of the subject and thereby allowing the agent to be delivered across BBB. Also disclosed herein is a method of treating a subject suffering from a brain tumor, a brain stroke, a neuropsychiatric disorder and / or a neurodegenerative disease.
Owner:ACAD SINIC
Drug combination for analgesia, anesthesia or drug rehabilitation
InactiveCN104352499AImprove permeabilityInhibition-dependentOrganic active ingredientsNervous disorderMedication injectionTolerability
The invention provides a drug combination for analgesia, anesthesia or drug rehabilitation. The drug combination is composed of a tetrodotoxin injection and an opioid medical injection which are packaged separately. The tetrodotoxin injection is composed of tetrodotoxin containing a buffer agent and a high penetrating agent for opening of the blood-brain barrier (BBB) or a BBB carrier. The opioid medical injection is a commercially available injection containing an opioid receptor stimulant. In the drug combination for analgesia, anesthesia or drug rehabilitation, the BBB-opening high penetrating agent or the BBB carrier can raise BBB permeability and bioavailability of tetrodotoxin and the opioid medicine, and tetrodotoxin has a synergistic effect of de-addiction and synergism. Through cooperation of the three drugs, the drug therapeutic effect can be enhanced, duration of drug effect is prolonged, dosage of the opioid medicine is reduced, and tolerance and dependency of the opioid medicine are decreased or eliminated.
Owner:赵继红 +3
Compositions and methods for reducing tactile dysfunction, anxiety, and social impairment
ActiveUS20210206771A1Reducing tactile dysfunctionReduce tactile dysfunctionNervous disorderOrganic chemistryRett syndromePhelan-McDermid syndrome
The present invention features novel peripherally-restricted non-benzodiazipene analogs with reduced blood brain barrier permeability and methods of use thereof for reducing tactile dysfunction, social impairment, and anxiety in a subject diagnosed with Autism Spectrum Disorder, Rett syndrome, Phelan McDermid syndrome, or Fragile X syndrome, or for treating touch over-reactivity, pain, or mechanical allodynia.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
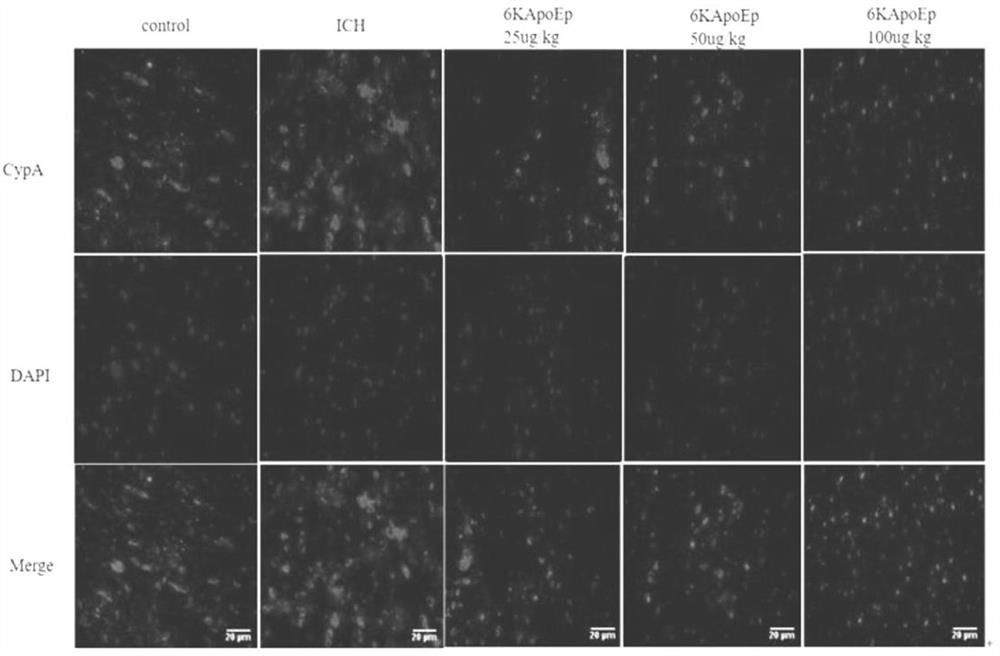
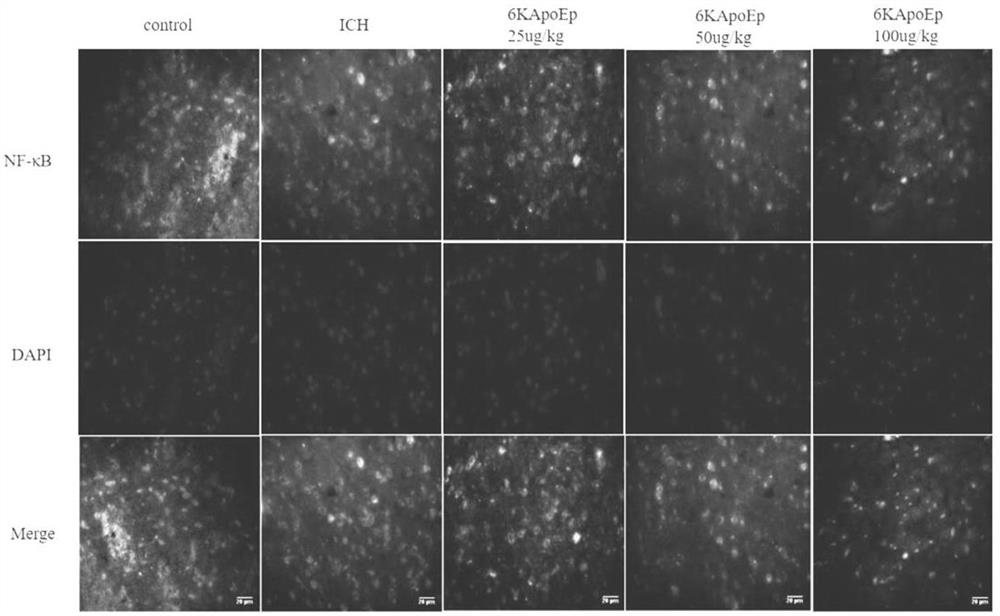
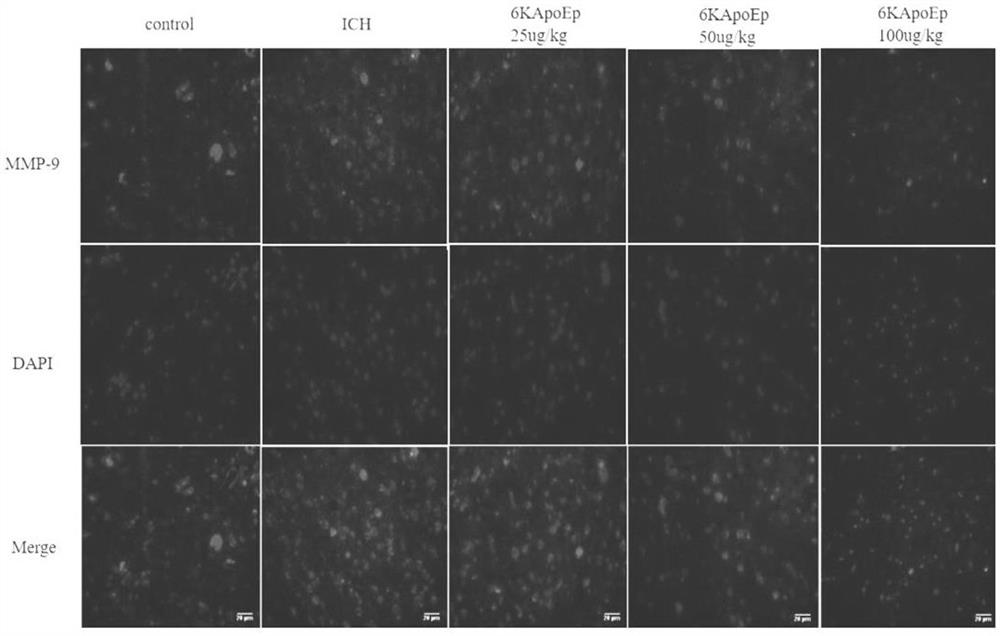
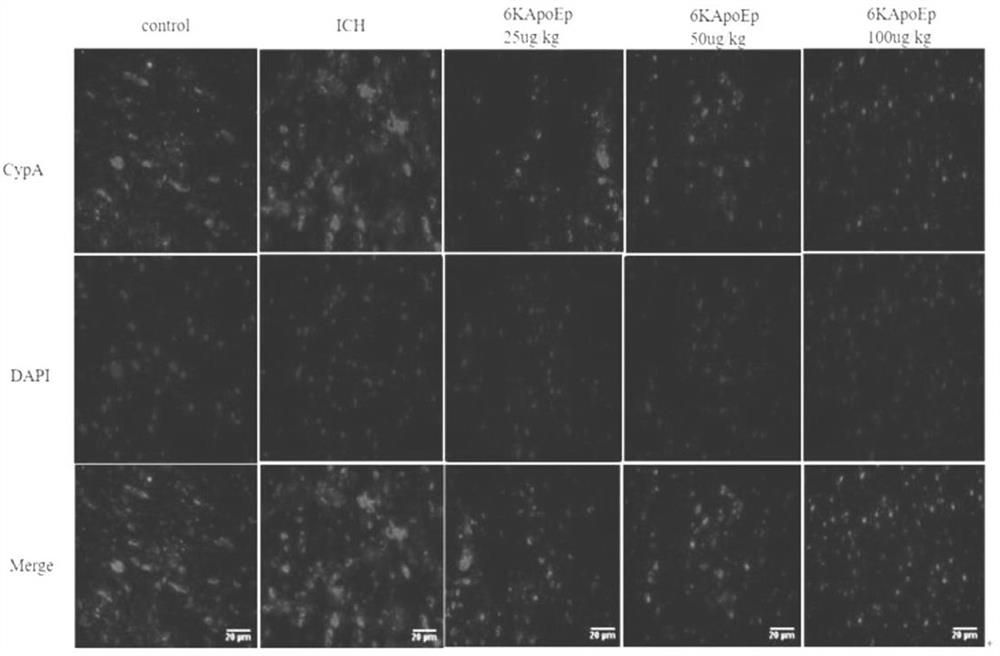
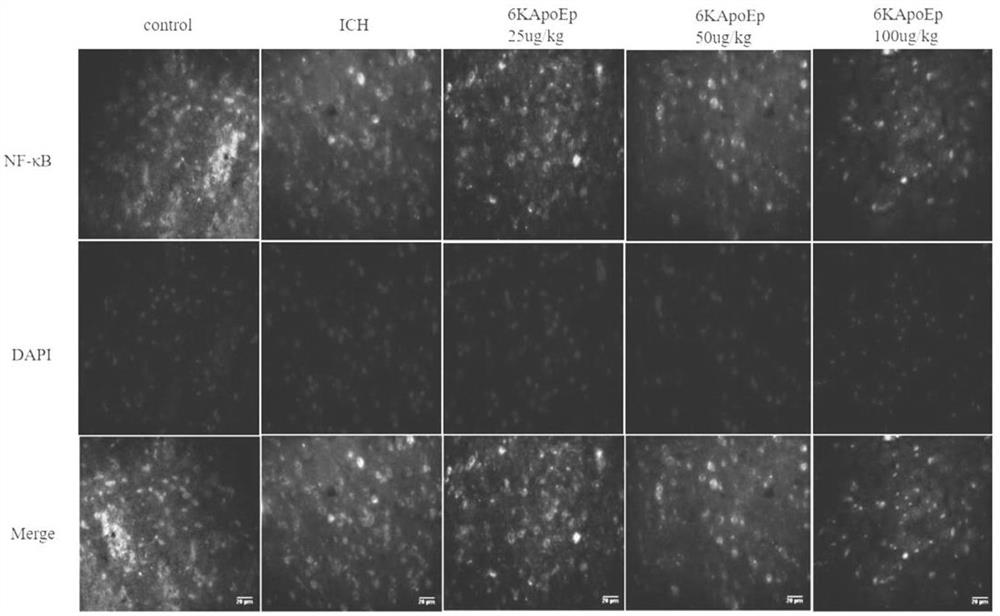
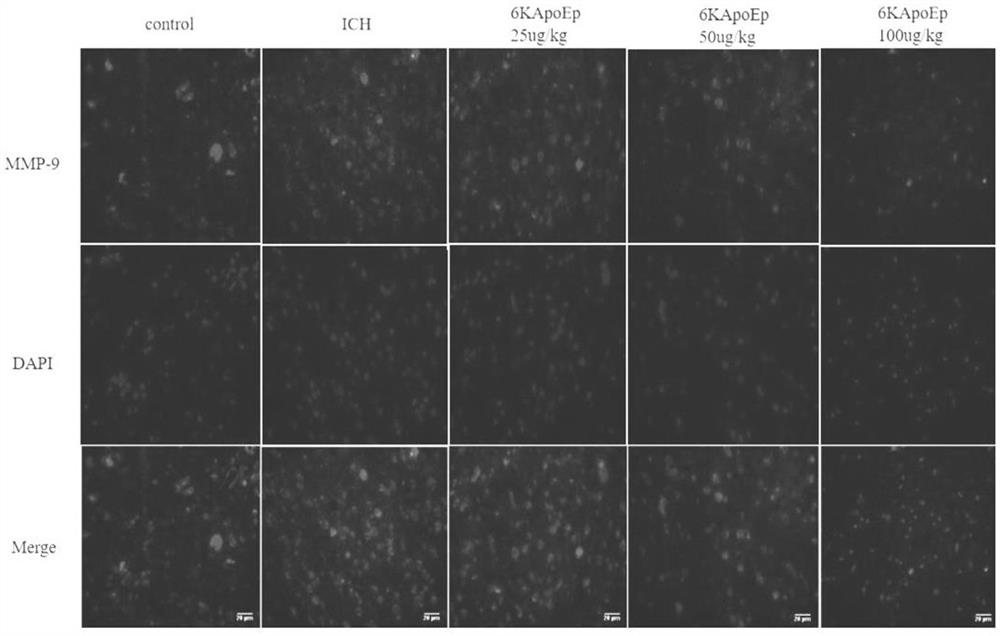
Application of apolipoprotein E receptor mimetic peptide 6KApoEp in preparation of medicine for treating secondary brain injury after cerebral hemorrhage
ActiveCN113230382AReduce expressionReduce generationNervous disorderCell receptors/surface-antigens/surface-determinantsInjury brainAPOLIPOPROTEIN RECEPTOR
The invention discloses an application of apolipoprotein E receptor mimetic peptide 6KApoEp in preparation of a medicine for treating secondary brain injury after cerebral hemorrhage, and discloses a protein sequence of the apolipoprotein E receptor mimetic peptide 6KApoEp, a construction method of the protein sequence of the apolipoprotein E receptor mimetic peptide 6KApoEp and preparation of an application mixed solution; after the 6KApoEp is applied, the expression of CypA can be reduced; by inhibiting activation of a / MMP-9 pathway, cerebral edema and blood-brain barrier damage after cerebral hemorrhage and generation of proinflammatory factors are relieved; the brain water content is reduced, the BBB permeability is improved, the increase of blood-brain barrier related proteins such as claudin-5, occludin and ZO-1 is promoted, the inflammatory response after cerebral hemorrhage is regulated and controlled by inhibiting CypA / NF-KB / MMP-9, the water content of brain tissues is reduced, and the blood-brain barrier permeability is improved, so that the secondary brain injury after cerebral hemorrhage is relieved, and the neuroprotective effect on cerebral hemorrhage is achieved; the inflammatory response after cerebral hemorrhage is reduced, and the blood-brain barrier permeability is improved.
Owner:王丽琨
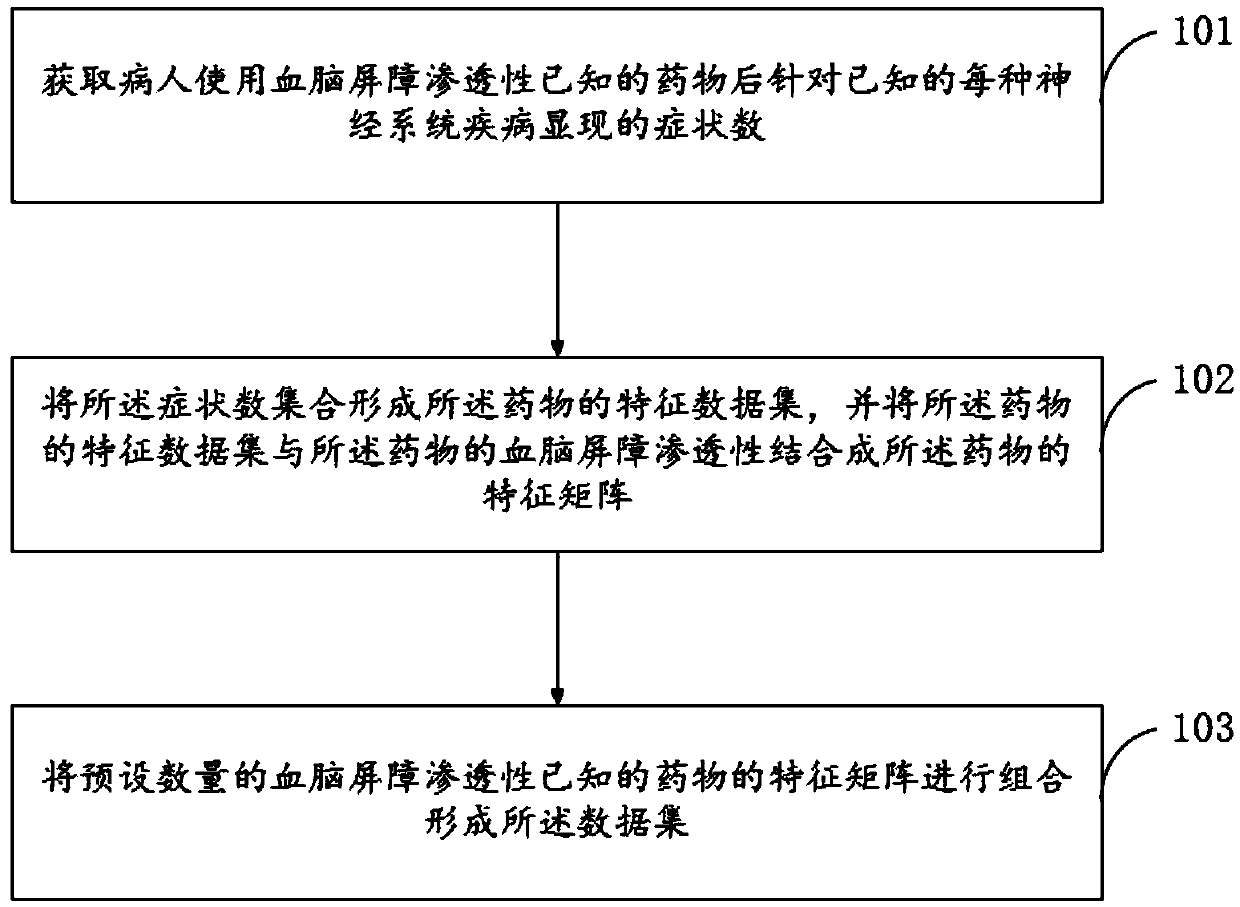
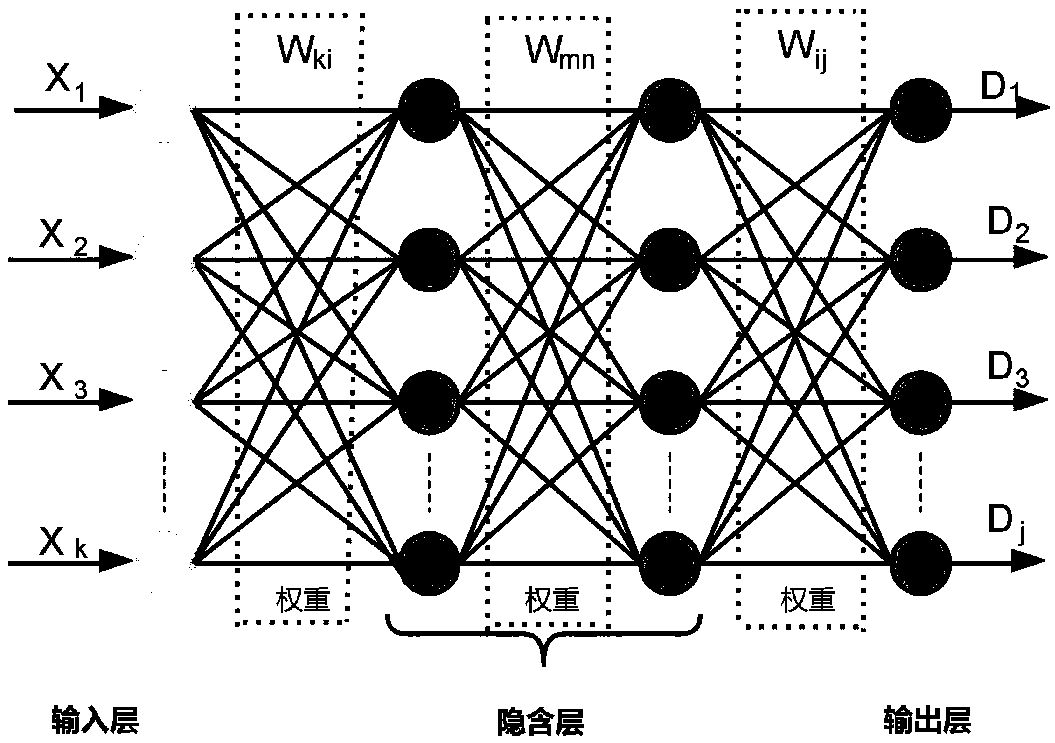
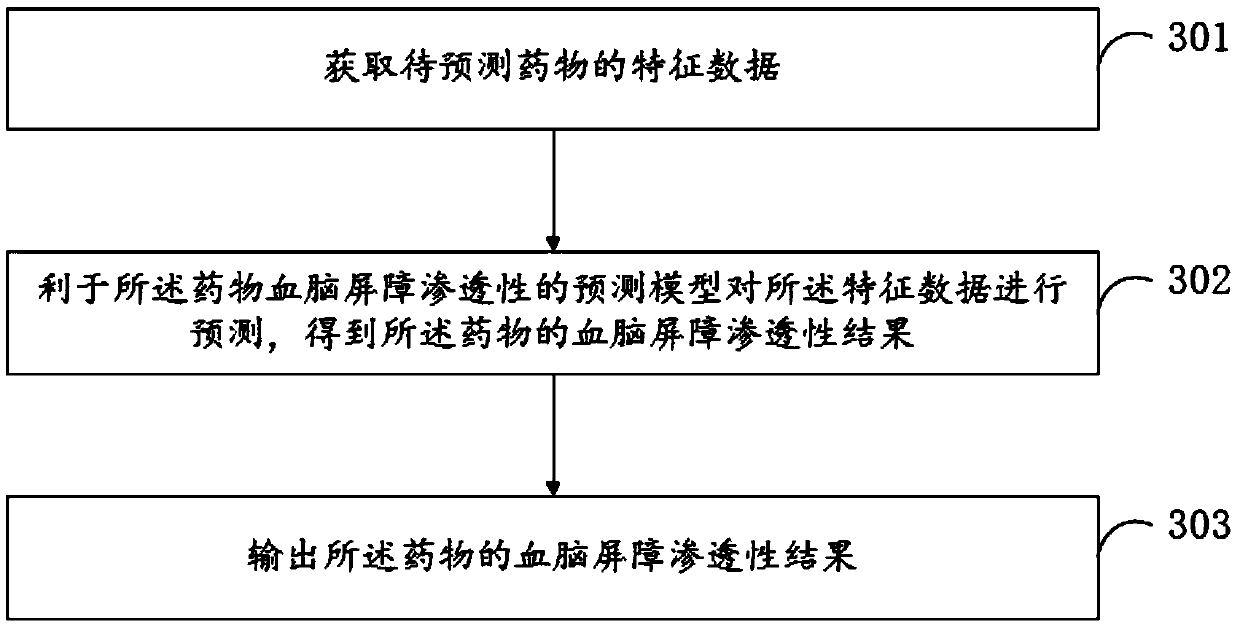
Establishing method for data set in drug BBB (Blood Brain Barrier) permeability prediction, and data model
ActiveCN109545389AWide range of predictionsSuitable for a wide rangeDrug referencesNeural learning methodsData setBlood brain barrier penetration
The invention relates to the field of computers, and especially relates to an establishing method for a data set in drug BBB (Blood Brain Barrier) permeability prediction, and a data model. The methodcomprises the steps: obtaining a number of symptoms manifested by a patient for each known neurological disease after using a drug having the known BBB permeability; using the number of symptoms to form a characteristic data set of the drug and combining the BBB permeability of the drug in the characteristic data set of the drug into a characteristic matrix of the drug; and combining a predetermined number of characteristic matrices of drugs with known BBB permeability to form a data set. According to the invention, the data set is formed by correlating the BBB permeability of the drug with the h known BBB permeability and the therapeutic effect of the drug on the nervous system diseases, so that the data set is applicable to a wider range, and the trained BBB permeability prediction model applicable to the data set also can accurately predict the BBB permeability of the drug in a wider range.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA ZHONGSHAN INST
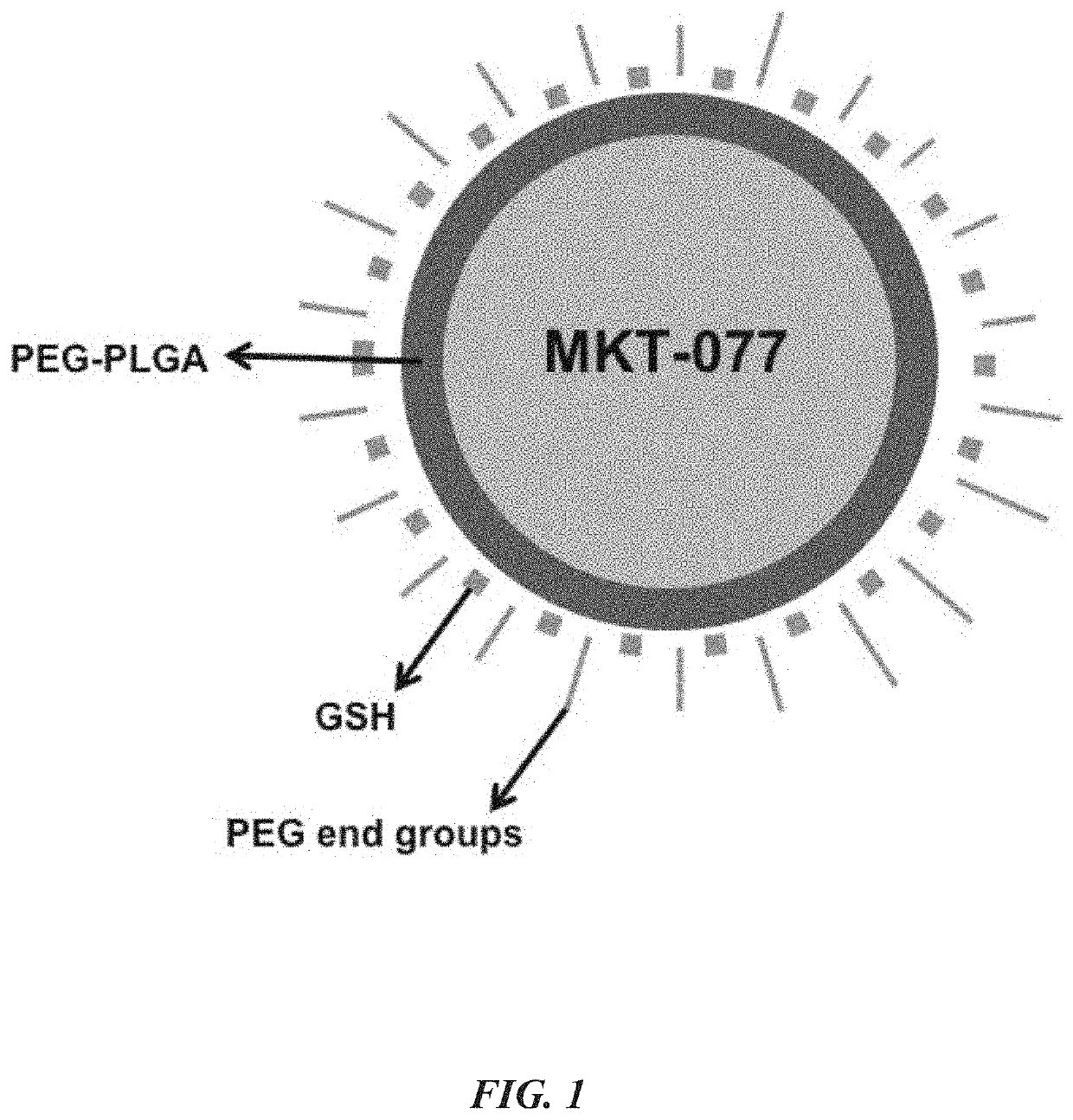
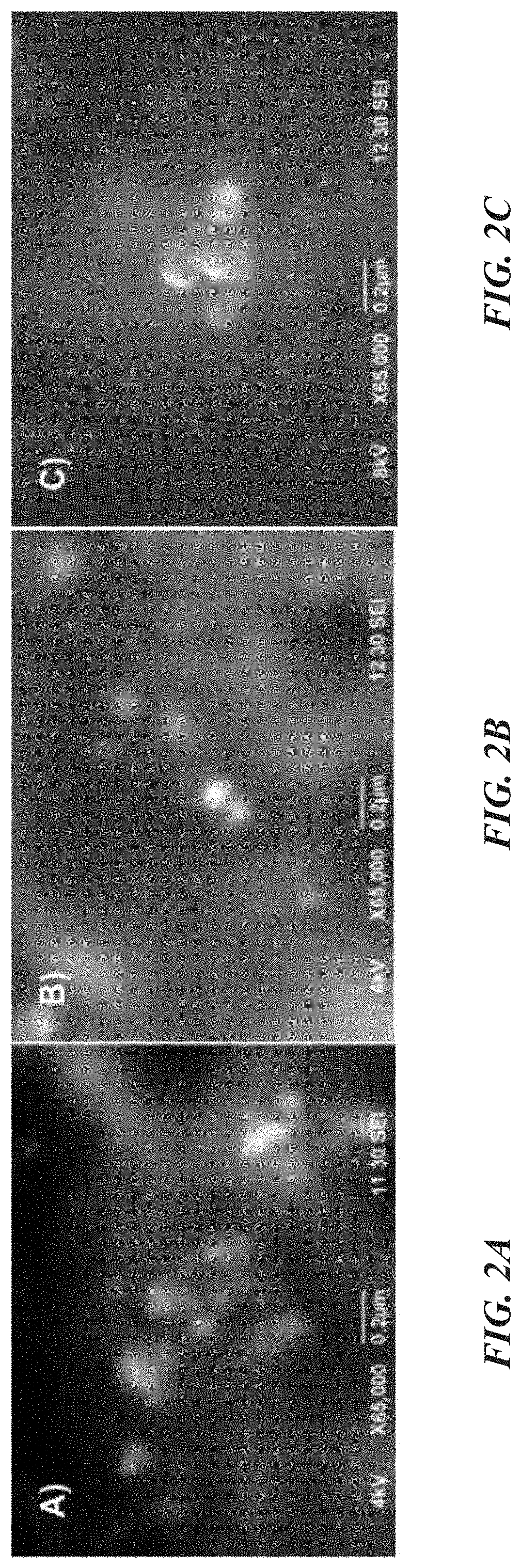
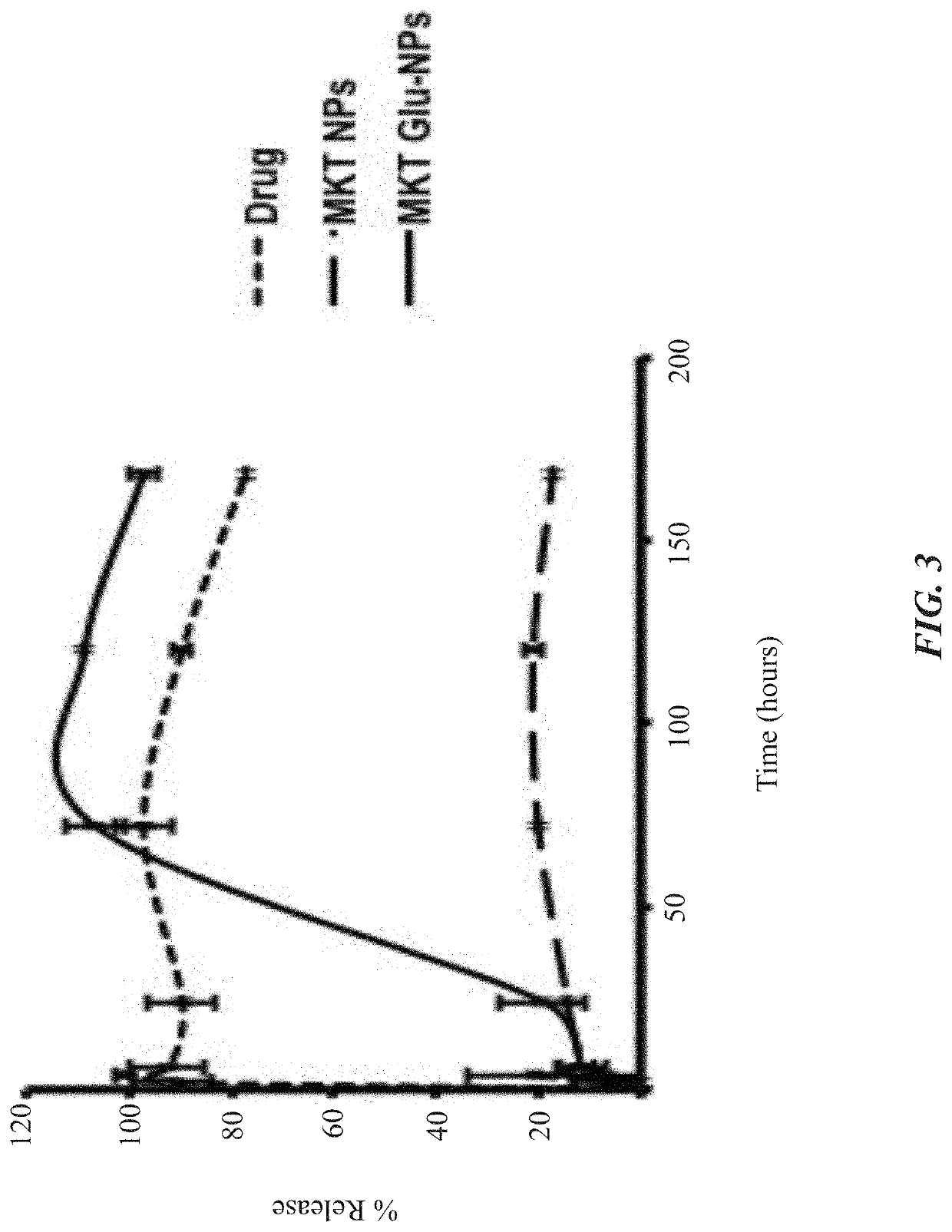
Glutathione-coated nanoparticles for delivery of MKT-077 across the blood-brain barrier
Nanoparticle based MKT formulation. MKT is encapsulated by poly(ethylene glycol)ylated (PEGylated) poly-(lactide-co-glycolide) (PLGA) to form nanoparticles (NPs). To induce trans-BBB permeability, glutathione (GSH) is coated on the resulting NPs. Newly generated MKT-NPs showed BBB permeability and tau reduction in experimental models. Specifically, brain-targeting MKT NPs were developed with a glutathione coating, characterized, and shown to permeate BBB permeation insert models as a therapeutic for Alzheimer's disease and related tauopathies.
Owner:UNIV OF SOUTH FLORIDA
Application of apolipoprotein e receptor mimetic peptide 6kapoep in the preparation of drugs for the treatment of secondary brain injury after cerebral hemorrhage
ActiveCN113230382BReduce expressionReduce generationNervous disorderCell receptors/surface-antigens/surface-determinantsInjury brainAPOLIPOPROTEIN RECEPTOR
The invention discloses the application of apolipoprotein E receptor mimic peptide 6KApoEp in the preparation of drugs for treating secondary brain injury after cerebral hemorrhage, and discloses the protein sequence of apolipoprotein E receptor mimic peptide 6KapoEp, apolipoprotein E receptor The construction method of the protein sequence of the analog peptide 6KapoEp, and the preparation of the application mixture, after the application of 6KApoEp, the expression of CypA can be reduced, and the cerebral edema and blood-brain barrier damage and inflammation after cerebral hemorrhage can be alleviated by inhibiting the activation of the MMP-9 pathway Factor production; reduce brain water content, improve BBB permeability, promote the increase of blood-brain barrier-related proteins such as claudin-5, occludin, ZO-1, regulate cerebral hemorrhage by inhibiting CypA / NF-KB / MMP-9 Post-inflammatory response, reduce brain tissue water content, improve blood-brain barrier permeability, thereby reducing secondary brain damage after cerebral hemorrhage, play a neuroprotective role in cerebral hemorrhage, reduce post-cerebral hemorrhage inflammatory response, improve blood-brain barrier permeability permeability effect.
Owner:王丽琨
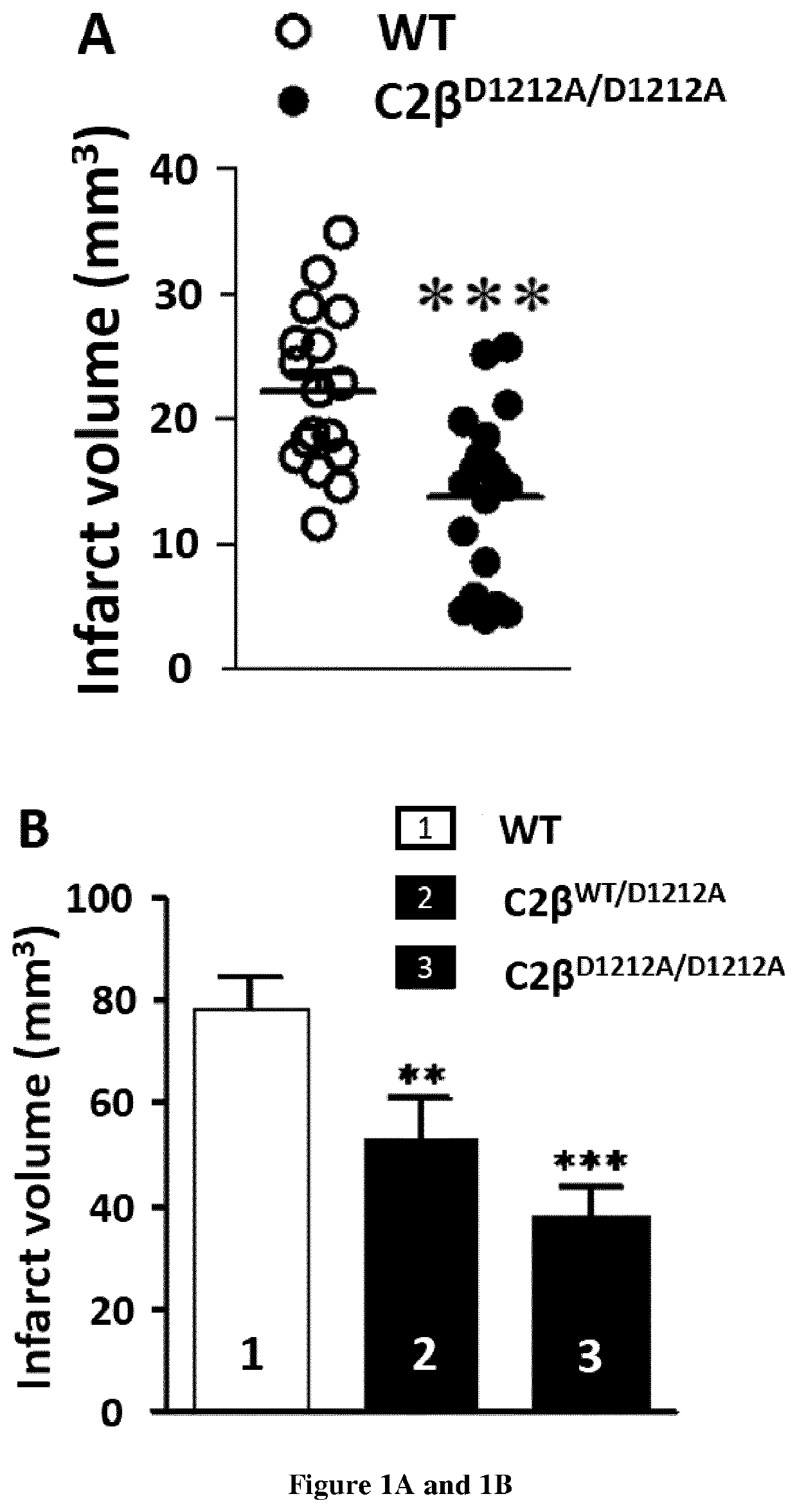
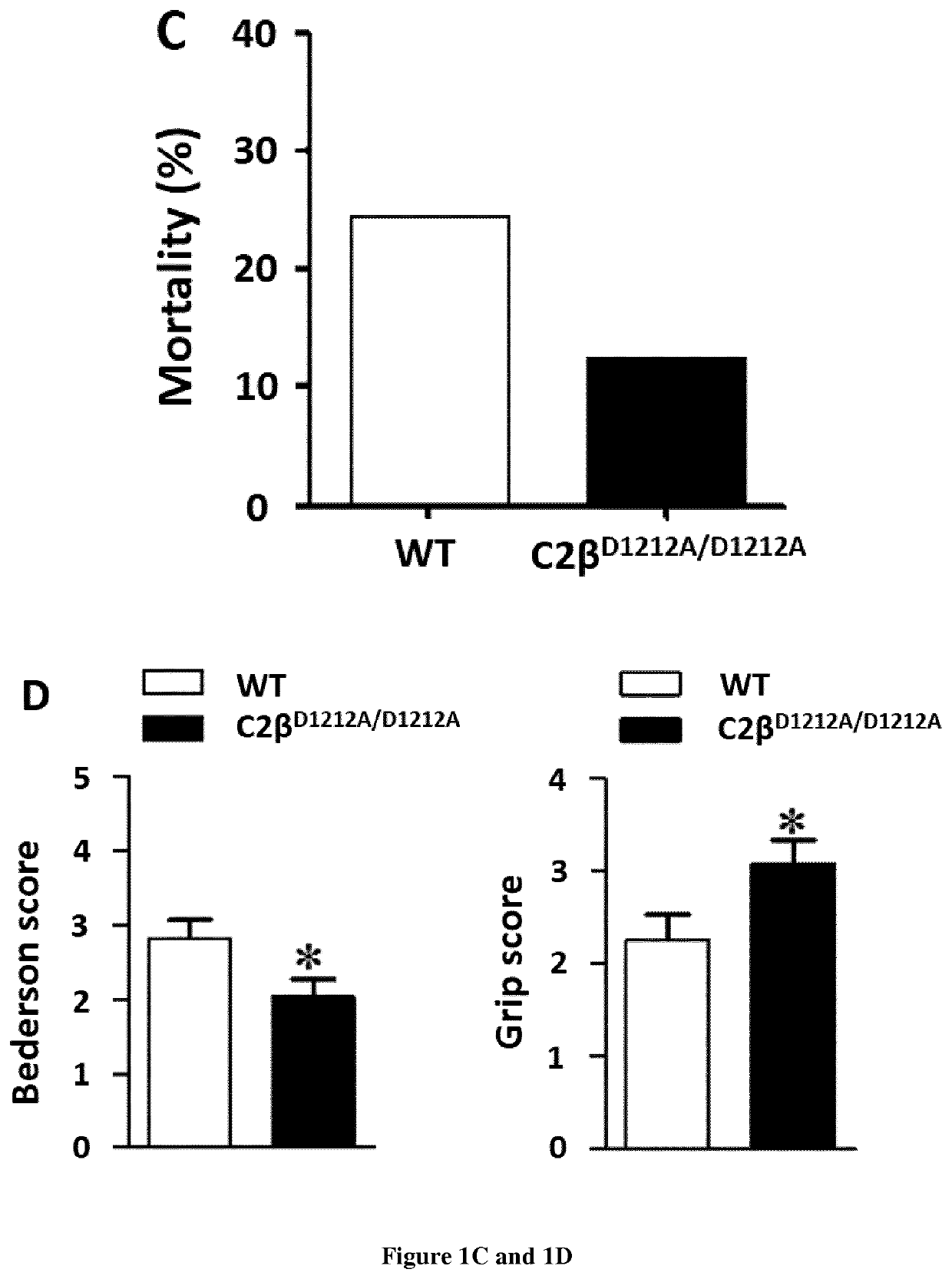
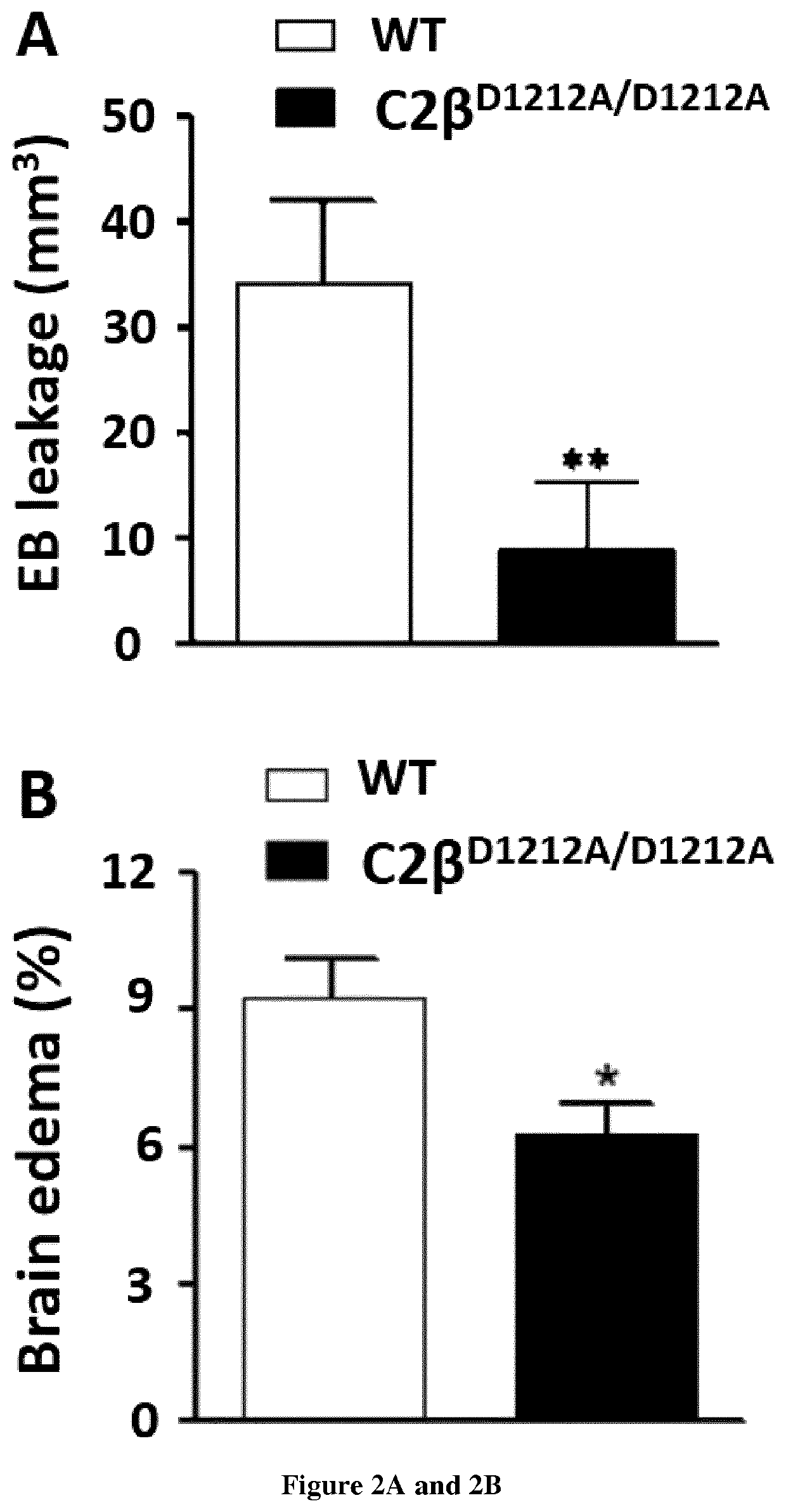
Use of pi3kc2b inhibitors for the preservation of vascular endothelial cell barrier integrity
InactiveUS20210238605A1Reduce infarct sizePreventing and reducing edemaOrganic active ingredientsBiological material analysisDiseaseVascular endothelium
Ischemic conditions are a leading cause of death for both men and women. Ischemia, a condition characterized by reduced blood flow and oxygen to an organ. Re-establishment of blood flow, or reperfusion, and re-oxygenation of the affected area following an pot ischemic episode is critical to limit irreversible damage. However, reperfusion also associates potentially damaging consequences. For NI instance, increased vascular permeability is an important contributor to edema and tissue damage following ischemic events. Here the inventors shows that genetic inhibition of PI3K-C2β reduces cerebral infarction in two ischemia / reperfusion (UR) models and improves neurological outcome. The genetic inhibition stabilizes the blood-brain barrier (BBB) after ischemic stroke and reduces inflammation. Accordingly, the present invention relates to a method for the preservation of vascular endothelial cell barrier integrity in a patient in need thereof comprising administering to the subject a therapeutically effective amount of a PI3KC2β inhibitor.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +5
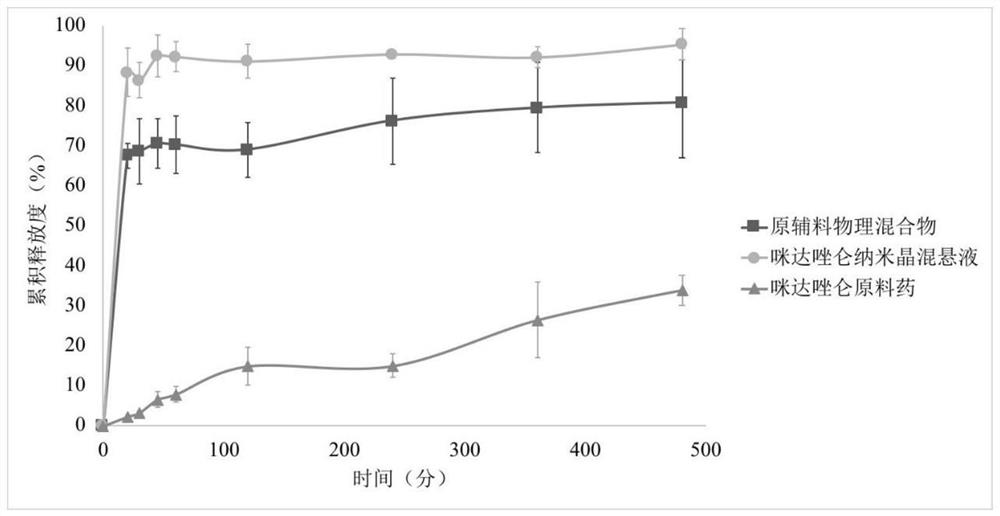
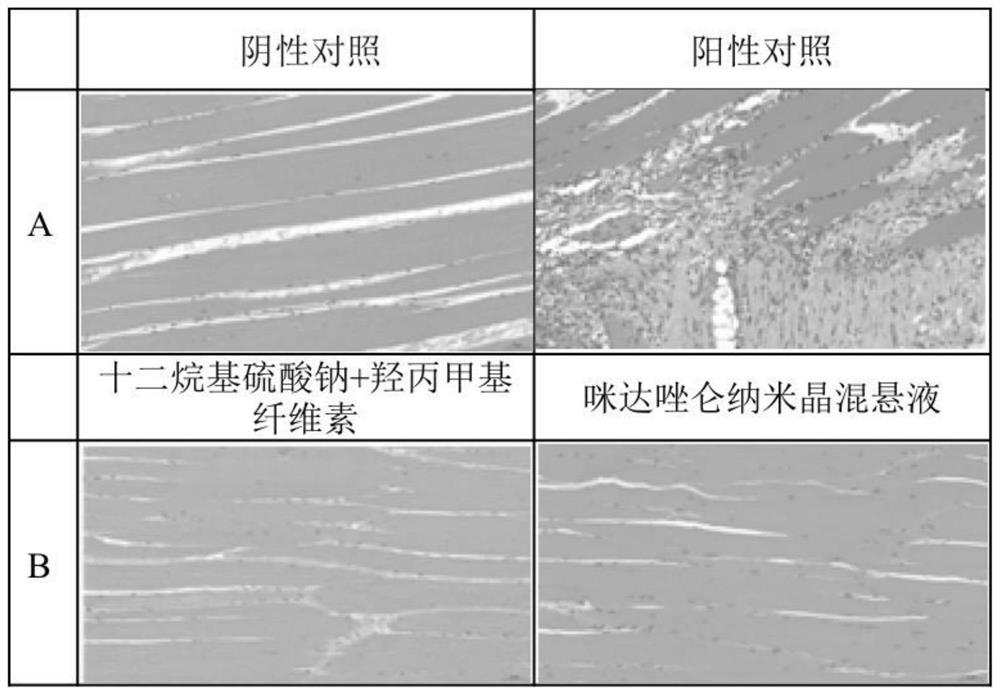
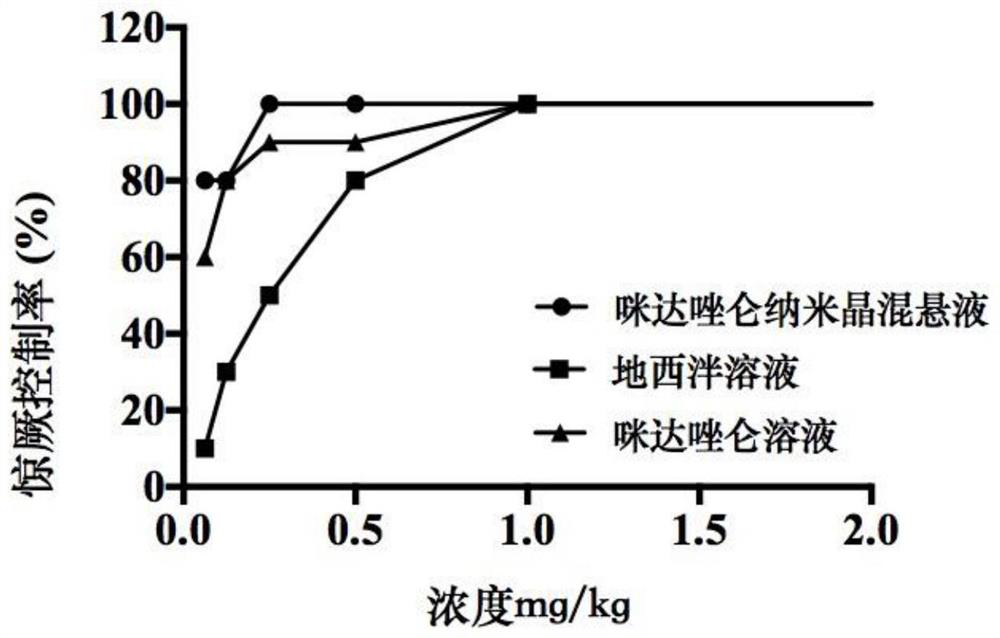
Application of midazolam nanocrystal in preparation of drugs for improving blood-brain barrier permeability
ActiveCN112426427AImprove stabilityUniform particle size distributionOrganic active ingredientsNervous disorderAntiepileptic drugPharmacologic therapy
The invention provides an application of a midazolam nanocrystal and a composition thereof in medicines for improving blood-brain barrier permeability. According to the midazolam nanocrystal, the solubility problem is solved, the bioavailability is improved, the problem that the treatment effect is poor when tablets and injection are used as anticonvulsant and antiepileptic drugs is solved, and the midazolam nanocrystal can effectively penetrate through a blood brain barrier and is used for treating epilepsy. In addition, the invention provides a technical platform, and the blood-brain barrierpermeability of the brain medicine is improved by the nanocrystalline preparation in the presence of a space protective agent and a charge stabilizer.
Owner:ACADEMY OF MILITARY MEDICAL SCI
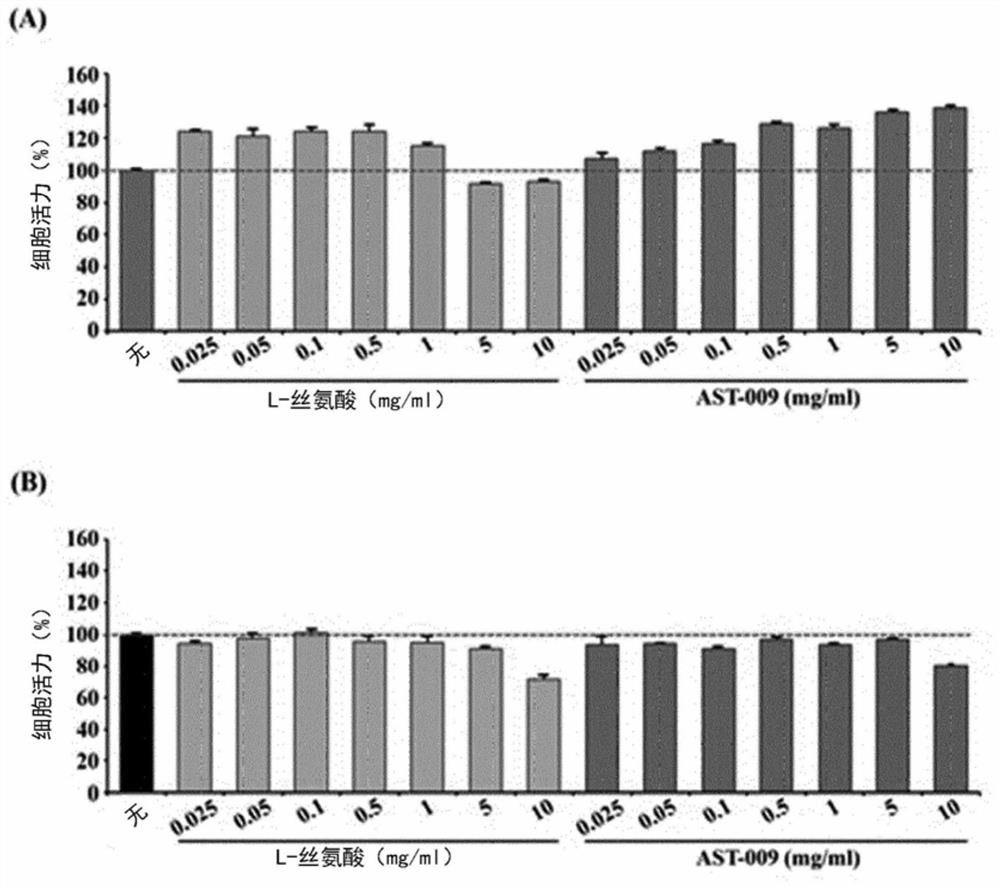
Serine derivative compound for preventing or treating central nervous system diseases
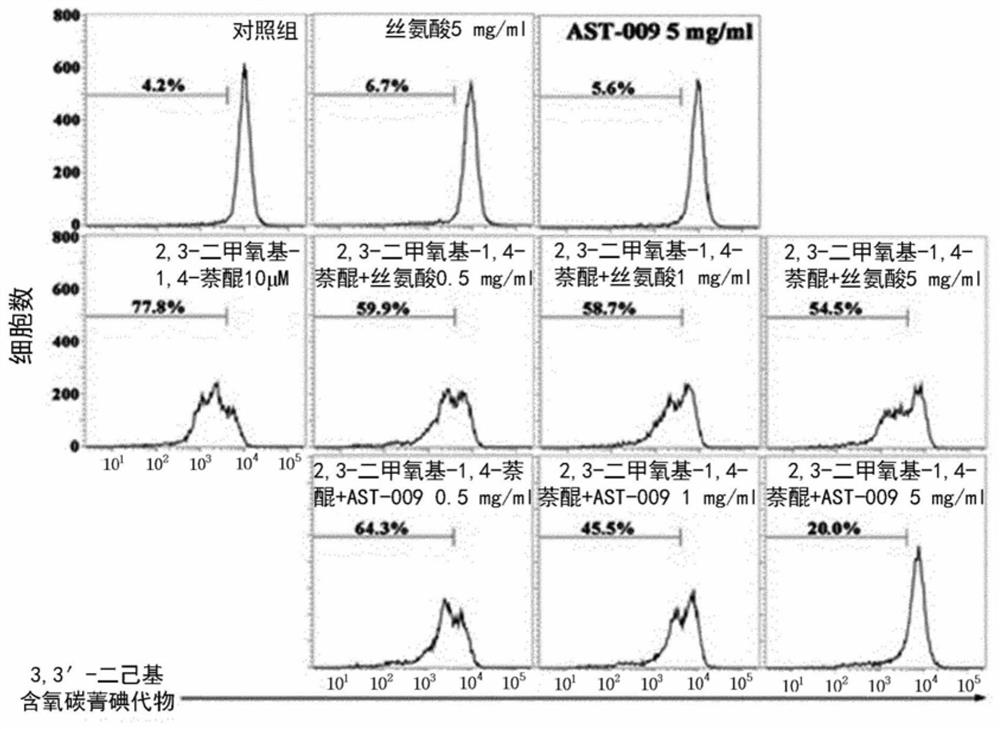
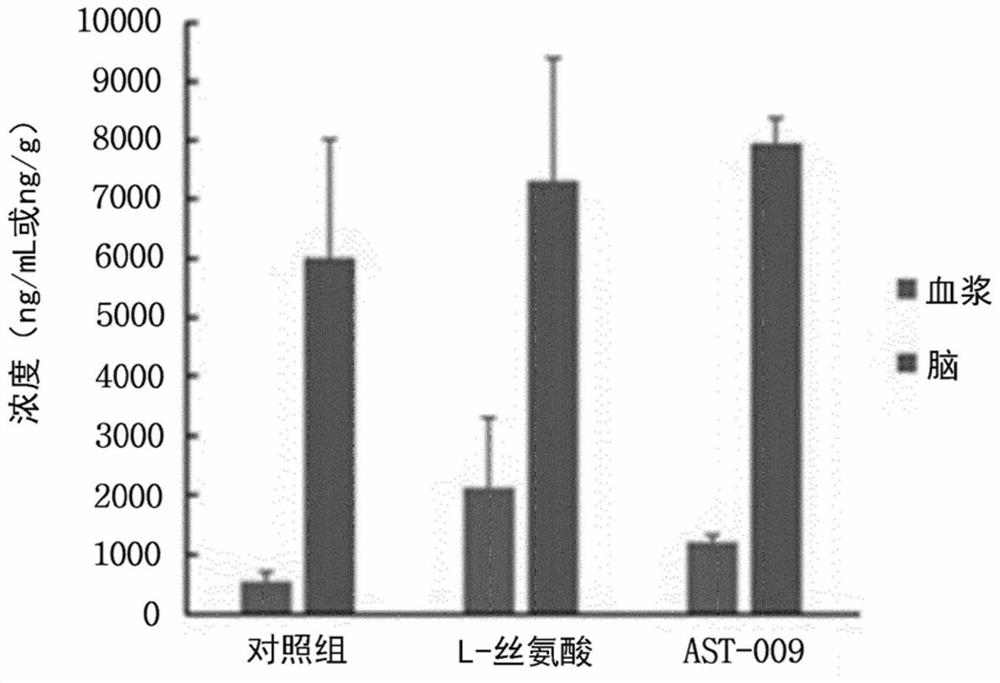
PendingCN112585154AEasy to solveGood treatment effectNervous disorderMicrobiological testing/measurementPharmaceutical medicineBbb permeability
The present invention relates to a novel serine derivative compound having improved blood-brain barrier (BBB) permeability, and a use of same, and more specifically, to a novel serine derivative compound having further improved BBB permeability compared to L-serine, and a pharmaceutical composition comprising the compound as an effective compound for preventing or treating / alleviating central nervous system diseases, such as cognitive disorders, intellectual disabilities, microcephaly, epilepsy, neurodevelopmental disorders, dementia, autism spectrum disorders, Down syndrome, Rett syndrome, fragile X syndrome, Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis. A compound of chemical formula (I) or a pharmaceutically acceptable salt thereof asan effective component for the pharmaceutical composition of the present invention for preventing or treating central nervous system diseases exhibits significantly improved BBB permeability comparedto L-serine, and activates the propagation of neurons, and through the neuroprotective effect that inhibits oxidative stress-induced mitochondrial membrane potential disruption and / or endoplasmic reticulum stress-induced apoptotic neuronal cell death, exhibits desirable effects in the prevention, treatment, and alleviation of central nervous system diseases, such as cognitive disorders, intellectual disabilities, microcephaly, epilepsy, neurodevelopmental disorders, dementia, autism spectrum disorders, Down syndrome, Rett syndrome, fragile X syndrome, Alzheimer's disease, Parkinson's disease,Huntington's disease, and amyotrophic lateral sclerosis. Thus, the compound is an extremely useful invention in the drug industry, food industry, and livestock industry.
Owner:アストロゲン カンパニーリミティド
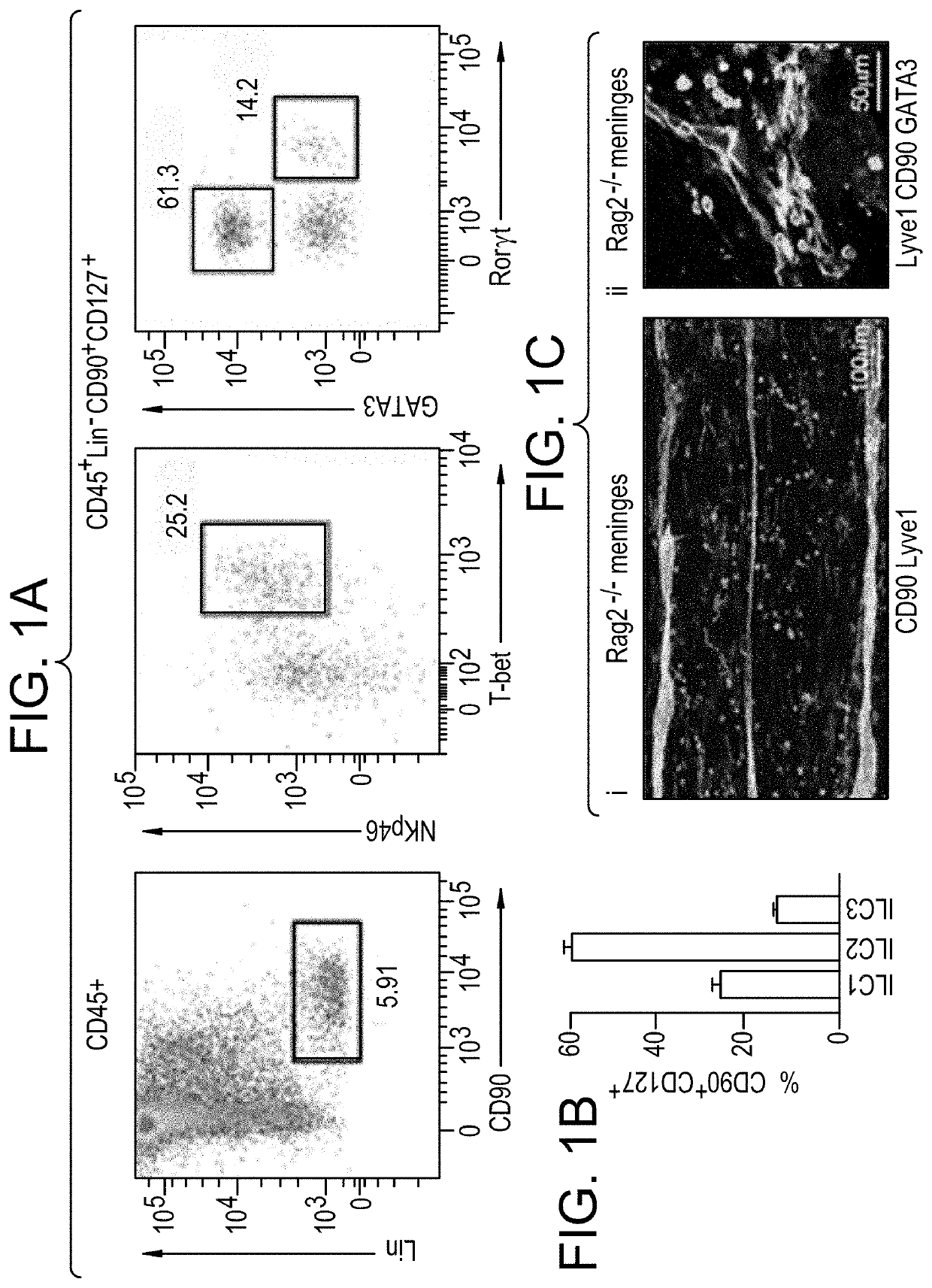
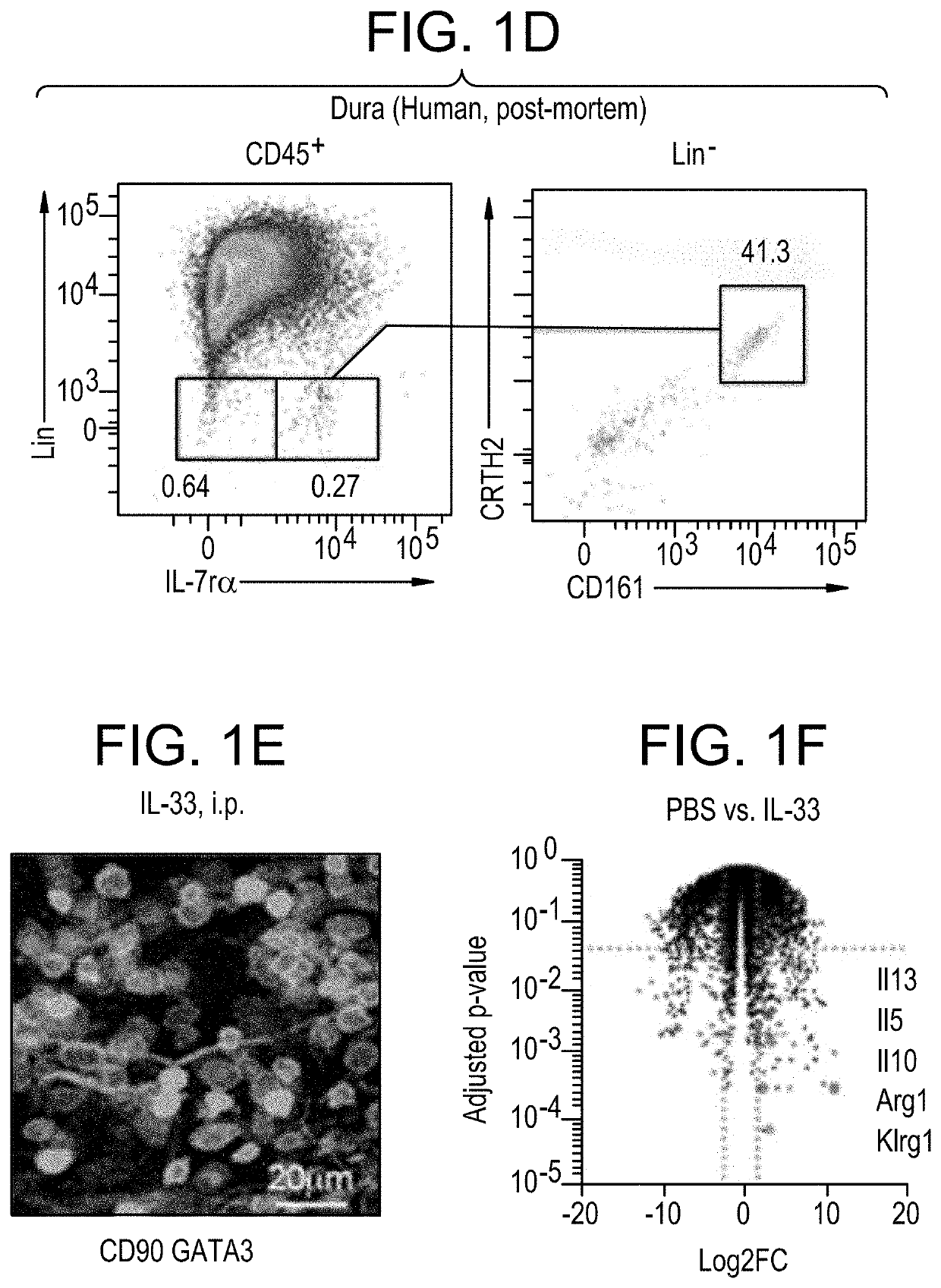
Suppression of Microglial Activation with Innate Lymphoid Cells
InactiveUS20200016205A1High activityIncrease the number ofNervous disorderPeptide/protein ingredientsLymphocytic cellMedicine
Compositions and methods of using ILC2 to reduce microglial activation or to reduce blood-brain barrier (BBB) permeability are described. Also described are methods and compositions that use an agent that increases the number of activated ILC2 to reduce microglial activation or BBB permeability.
Owner:JANSSEN PHARMA NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com