Endomorphin-derived peptide with blood-brain barrier permeability as well as synthesis and application of endomorphin-derived peptide
An endomorphin and blood-brain barrier technology, applied in the field of biochemistry, can solve the problems of not easily permeating the blood-brain barrier, short biological half-life, poor enzymatic hydrolysis stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
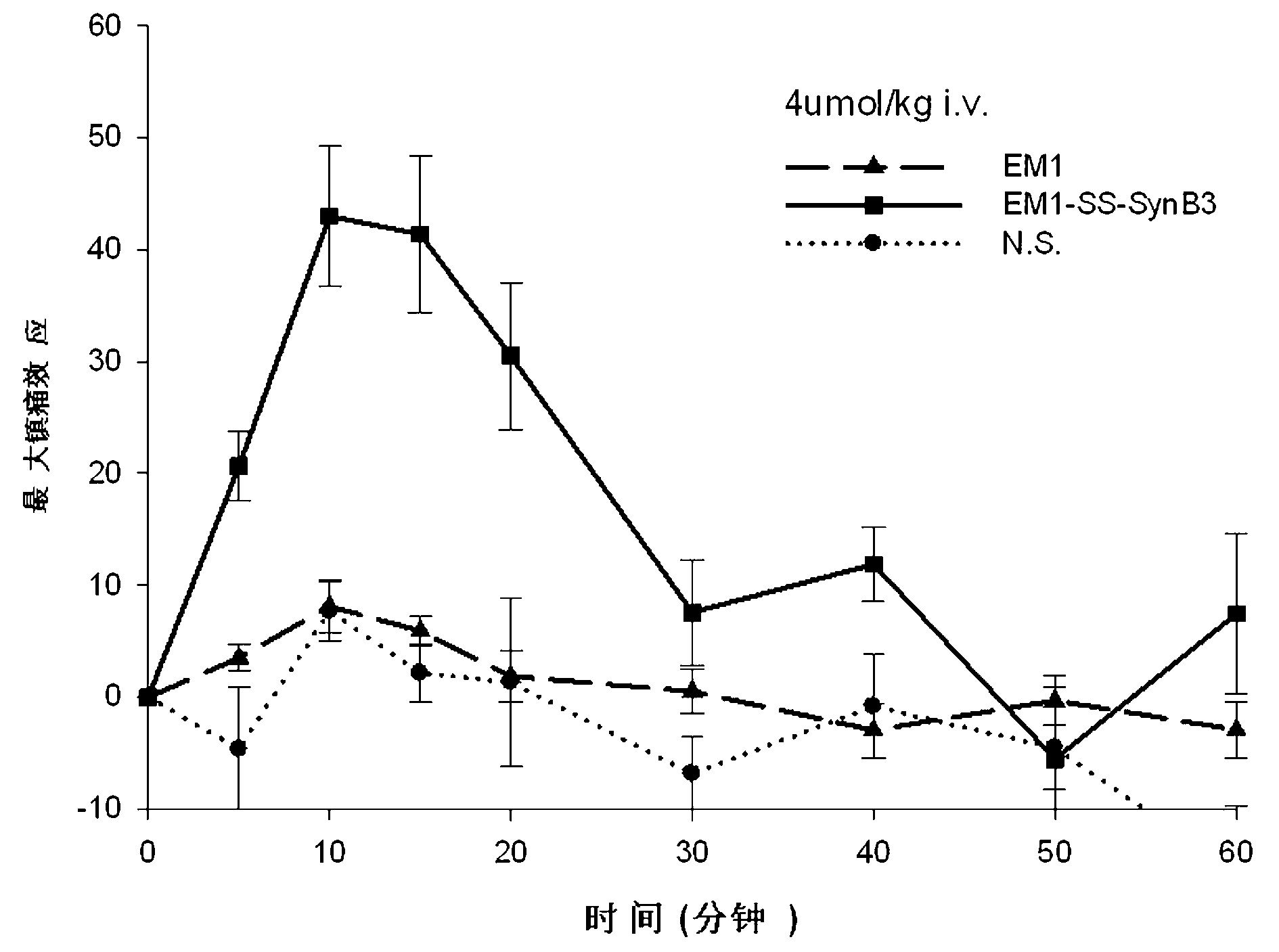
[0055] Example 1: Membrane-penetrating peptide-linked endomorphin-1 derivative peptide——EM1-SS-SynB3 synthesis
[0056] (1) Cysteine-containing endomorphin-1 (Tyr-Pro-Trp-Phe-Cys-NH 2 ) and the penetrating peptide SynB3 (Arg-Arg-Leu-Ser-Tyr-Ser-Arg-Arg-Arg-Phe-NH 2 ), the solid-phase synthesis of:
[0057] a. Resin pretreatment: Add Rink-Amide-MBHA resin with an amino molar mass of 0.5mmol / g into the reactor, add dichloromethane and stir for 30 min to fully swell the resin and then vacuum-drain the solvent;
[0058] b. Deprotection of F-moc: The deprotection reagent is piperidine / DMF=1:4 (V / V). Stir the deprotection reagent with the resin for 2 minutes and then drain it. Repeat 4 times to completely remove the Fmoc group. Finally, wash with DMF to remove the deprotection reagent.
[0059] c. Condensation: 0.6~0.8 mol Fmoc group-protected amino acid, N-hydroxybenzotriazole, O-benzotriazole-N,N,N',N'-tetramethylurea-hexa Dissolve fluorophosphate in DMF, add 1.2~1.6 mol of di...
Embodiment 2
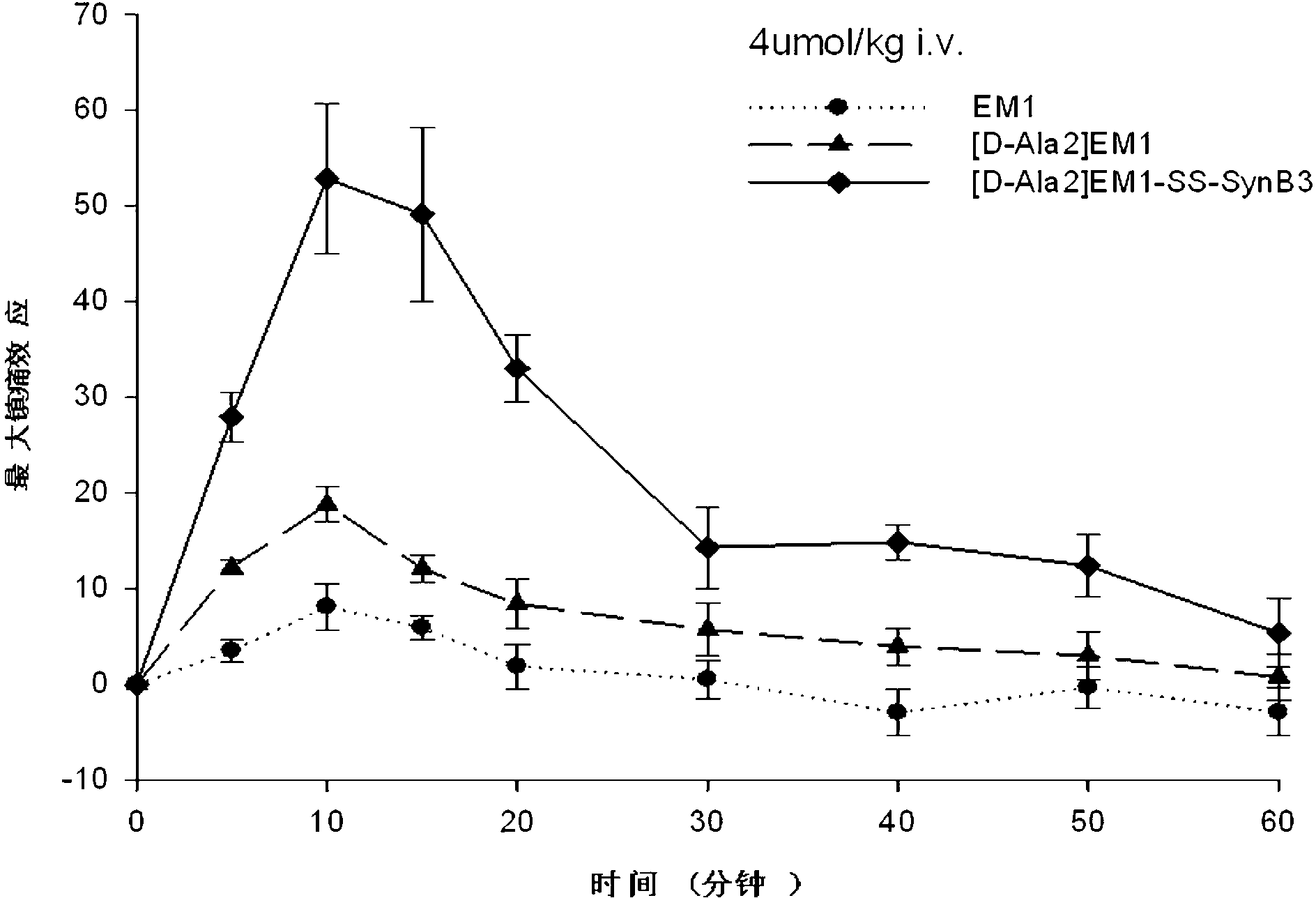
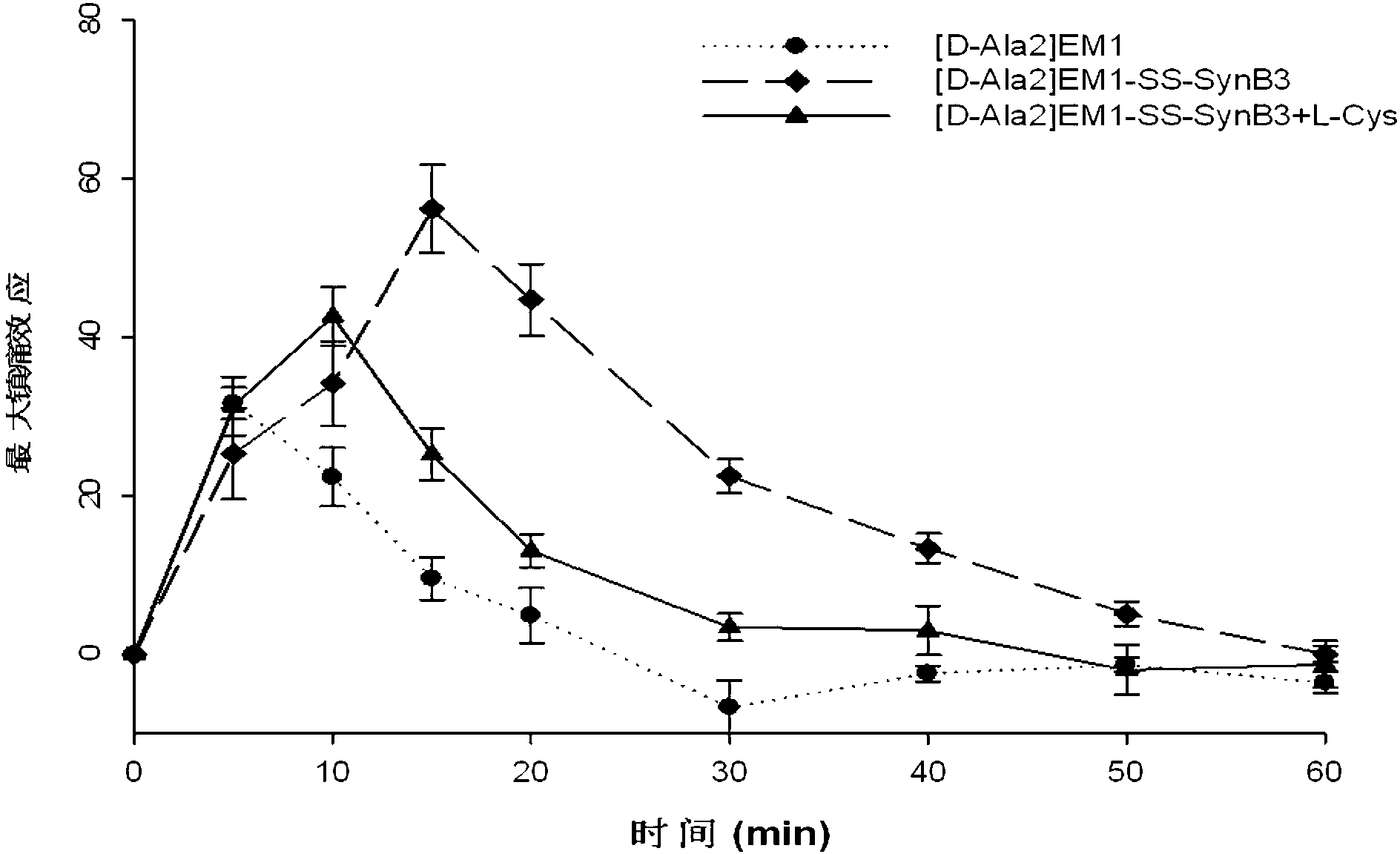
[0066] Example 2: Derivative peptides of endomorphin-1 analogues linked to penetrating peptides——[D-Ala 2 ] Synthesis of EM1-SS-SynB3
[0067] (1) Cysteine-containing endomorphin-1 analogs (Tyr-D-Ala-Trp-Phe-Cys-NH 2 ) and the penetrating peptide SynB3 (Arg-Arg-Leu-Ser-Tyr-Ser-Arg-Arg-Arg-Phe-NH 2 ) of solid-phase synthesis: with embodiment 1.
[0068] (2) Synthesis of PDP-SynB3 with activated sulfhydryl group: Dissolve the peptide SynB3 (1mmol) and the activator SPDP (2mmol) in 10ml of anhydrous methanol, stir and mix well, add 6mmol of N,N-diisopropylethyl Amine (DIEA), react at room temperature for 6~8h; use C 18 Reversed-phase HPLC column purification to obtain PDP-SynB3 with activated sulfhydryl group;
[0069] (3) Synthesis of endomorphin-derived peptides: the reaction bottle was pumped and replaced with argon. Sulfhydryl-activated PDP-SynB3 (1 mmol) was combined with cysteine-containing endomorphin-1 analog [D-Ala 2 ]EM1 (1.1mmol) was dissolved in 10ml methanol / wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com