Labeled implantable medical devices
A medical device, identification technology, applied in the direction of preventing wrong connection devices, coupling devices, parts of connecting devices, etc., can solve the risk of increasing patient morbidity and mortality, wrong ventricular stimulation or sensing, incomplete intermittent Connectivity, etc., to the effect of eliminating biocompatibility risks, reducing the risk of incorrect manufacturing, and reducing the risk of incorrect assembly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
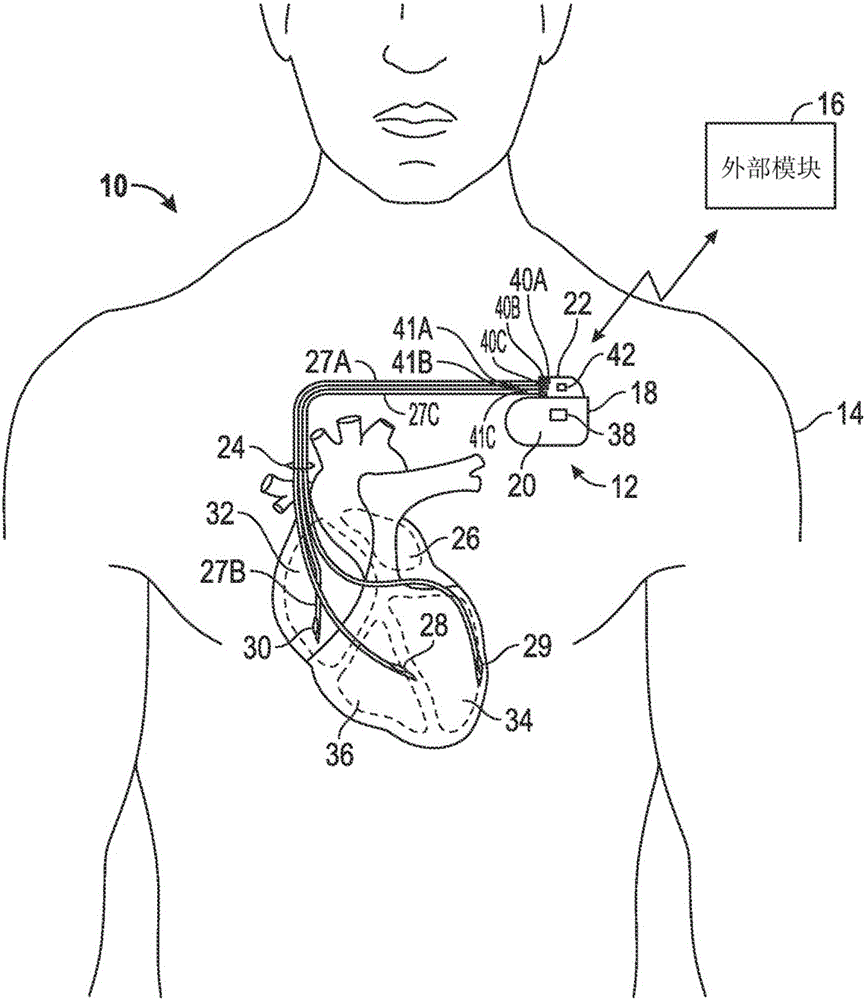
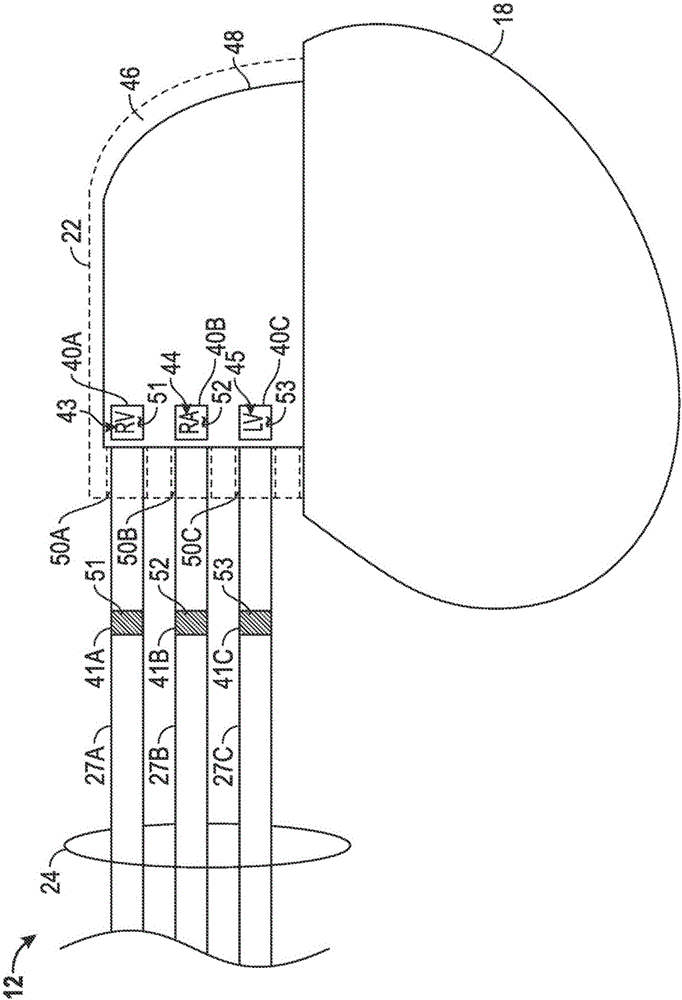
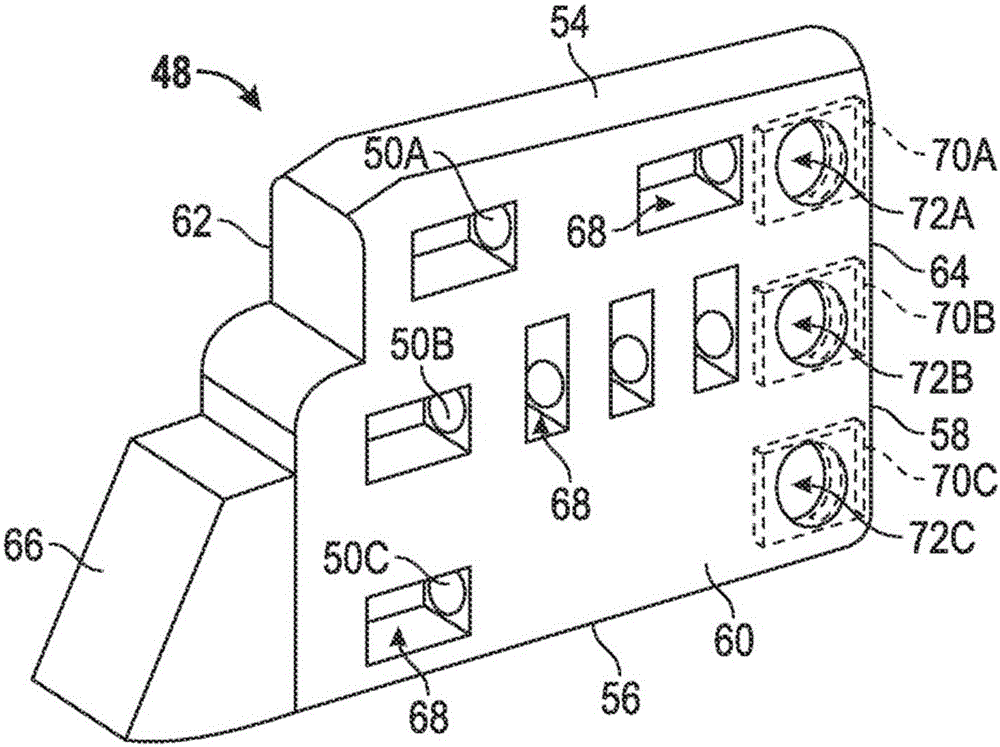
[0083] Example 1 may include a subject matter including an implantable medical device, this medical device including: a housing with an electrical circuit; a top cover coupled to the housing and including a core that defines a hole and includes a A first metal mark near a hole; and a guide assembly, the guide assembly includes at least one guide having a distal end and a proximal end, the at least one guide includes a second metal mark, the distal end includes at least one electrode, and The proximal end is received in the hole.
example 2
[0084] Example 2 may include the subject of Example 1, or may be selectively combined with the subject of Example 1 to selectively include a first metal identification and a second metal identification of at least one selected from titanium, tantalum, tungsten, and stainless steel .
example 3
[0085] Example 3 can include the subject matter of one or any combination of Examples 1 or 2, or can be selectively combined with the subject matter of one or any combination of Examples 1 or 2, to selectively include the first identification code containing the first The metal identification and the second metal identification including the second identification code, the first identification code and the second identification code are basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com