Use of adenosine receptor signaling to modulate permeability of blood-rain barrier
A technology of adenosine receptor and blood-brain barrier, applied in the direction of medical preparations containing active ingredients, antibodies, antibody medical components, etc., can solve the problems of poor understanding of molecular mechanisms, limited endocytosis of BBB transport capacity, etc. To achieve the effect of enhanced treatment and improved treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0156] The following examples are provided to illustrate embodiments of the invention, but are in no way intended to limit the scope thereof.
[0157] Example 1 - Mice
[0158] cd73 has been described previously - / - Mice (Thompson et al., "Crucial Role for Ecto-5'-Nucleotidase (CD73) in Vascular Leakage During Hypoxia," J. Exp. Med. 200:1395-1405 (2004), which is hereby incorporated by reference in its entirety) And it has been backcrossed with C57BL / 6 for 14 generations. cd73 - / - Mice have no apparent susceptibility to infection and appear to be normal based on the size and cellular composition of their lymphoid organs and their T and B cell responses in in vivo and in vitro assays (Thompson et al., "Crucial Role for Ecto-5'-Nucleotidase( CD73) in Vascular Leakage During Hypoxia," J. Exp. Med. 200:1395-1405 (2004), which is hereby incorporated by reference in its entirety). Purchase C57BL / 6 and tcra with C57BL / 6 background from Jackson Laboratories - / - mice. Mice were b...
Embodiment 2-E
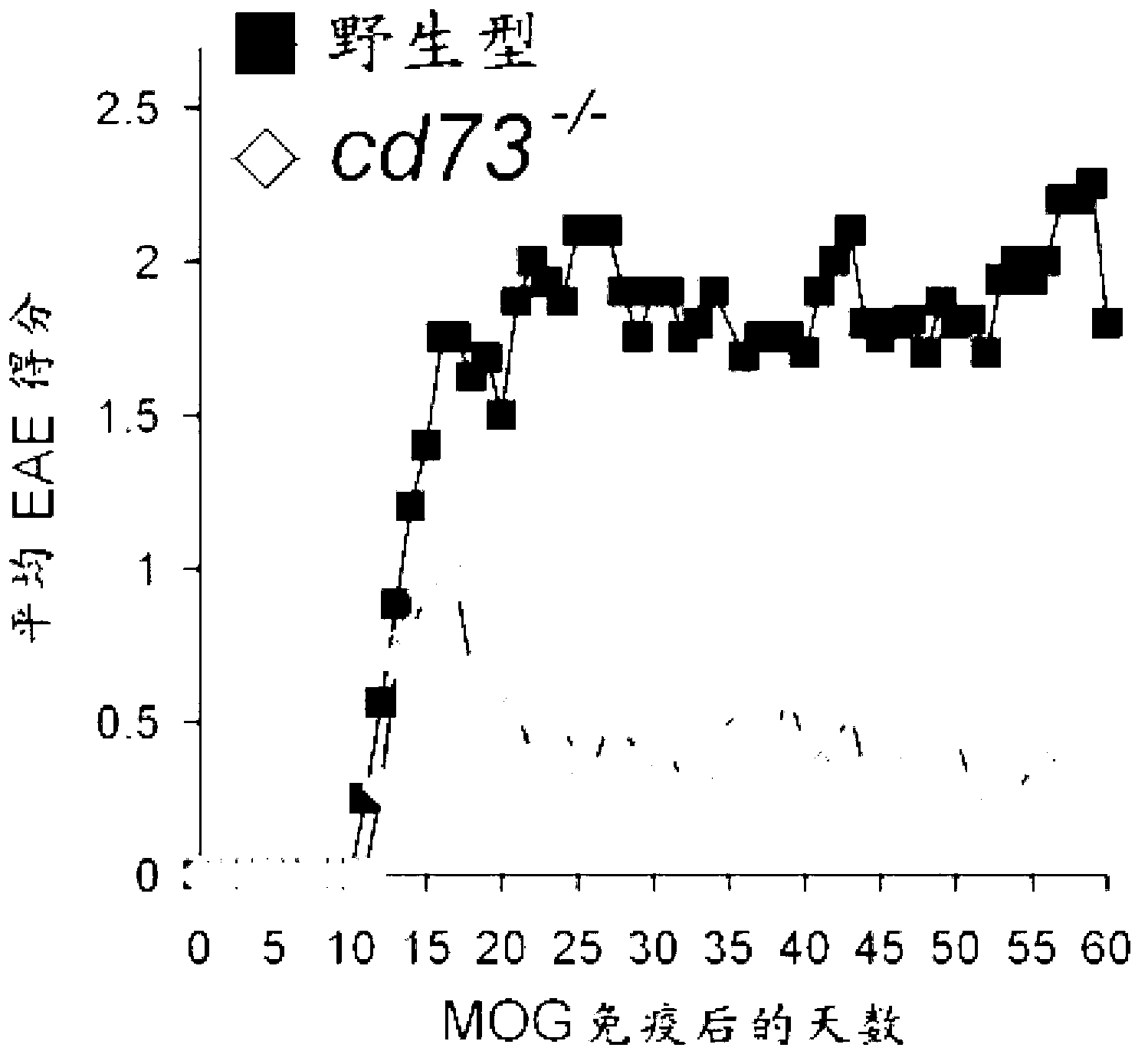
[0159] Example 2 - EAE induction and scoring
[0160] By subjecting mice to Swanborg, "Experimental Autoimmune Encephalomyelitis in Rodents as a Model for Human Demyelinating Disease," Clin. Immunol. Immunopathol. 77:4-13 (1995) and Bynoe et al., as hereby incorporated by reference in its entirety. Epicutaneous Immunization with Autoantigenic Peptides Induces T Suppressor Cells that Prevent Experimental Allergic Encephalomyelitis, "Immunity 19:317-328 (2003) described in the myelin oligodendrocyte glycoprotein ("MOG") EAE induction protocol to induce EAE. Briefly, MOG was injected subcutaneously into each flank 35-55 1:1 emulsion (50 μl) of peptide (3 mg / ml in PBS) (Invitrogen) and complete Freund's adjuvant (CFA, Sigma). Pertussis toxin (PTX, 20 ng) (Biological Laboratories Inc.) was reinjected intravenously (200 μl in PBS) at the time of immunization and 2 days later. Mice were scored daily for EAE based on severity of disease symptoms; 0=no disease, 0.5-1=tail weakness / li...
Embodiment 3
[0161] Example 3-T cell preparation and adoptive transfer
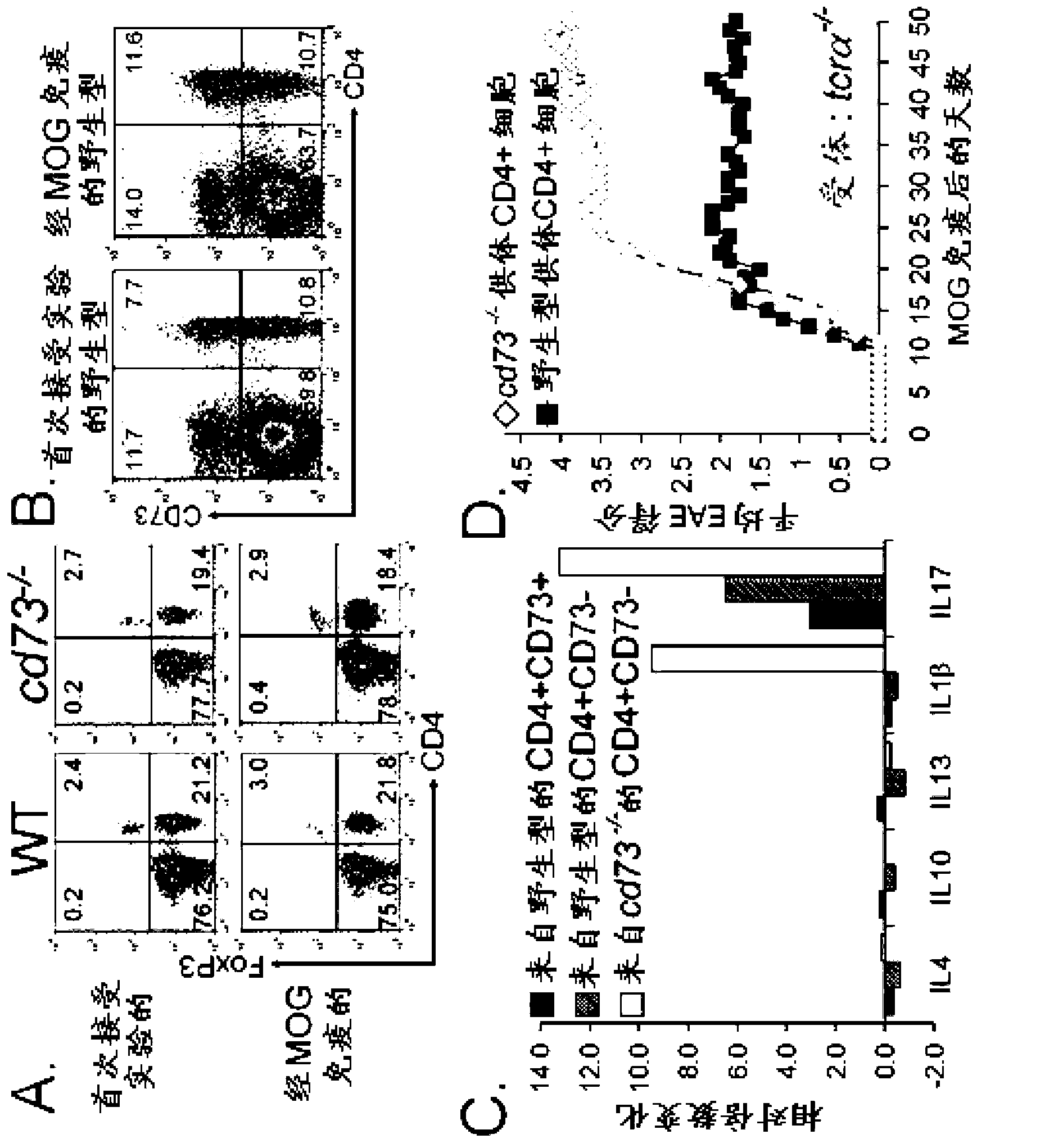
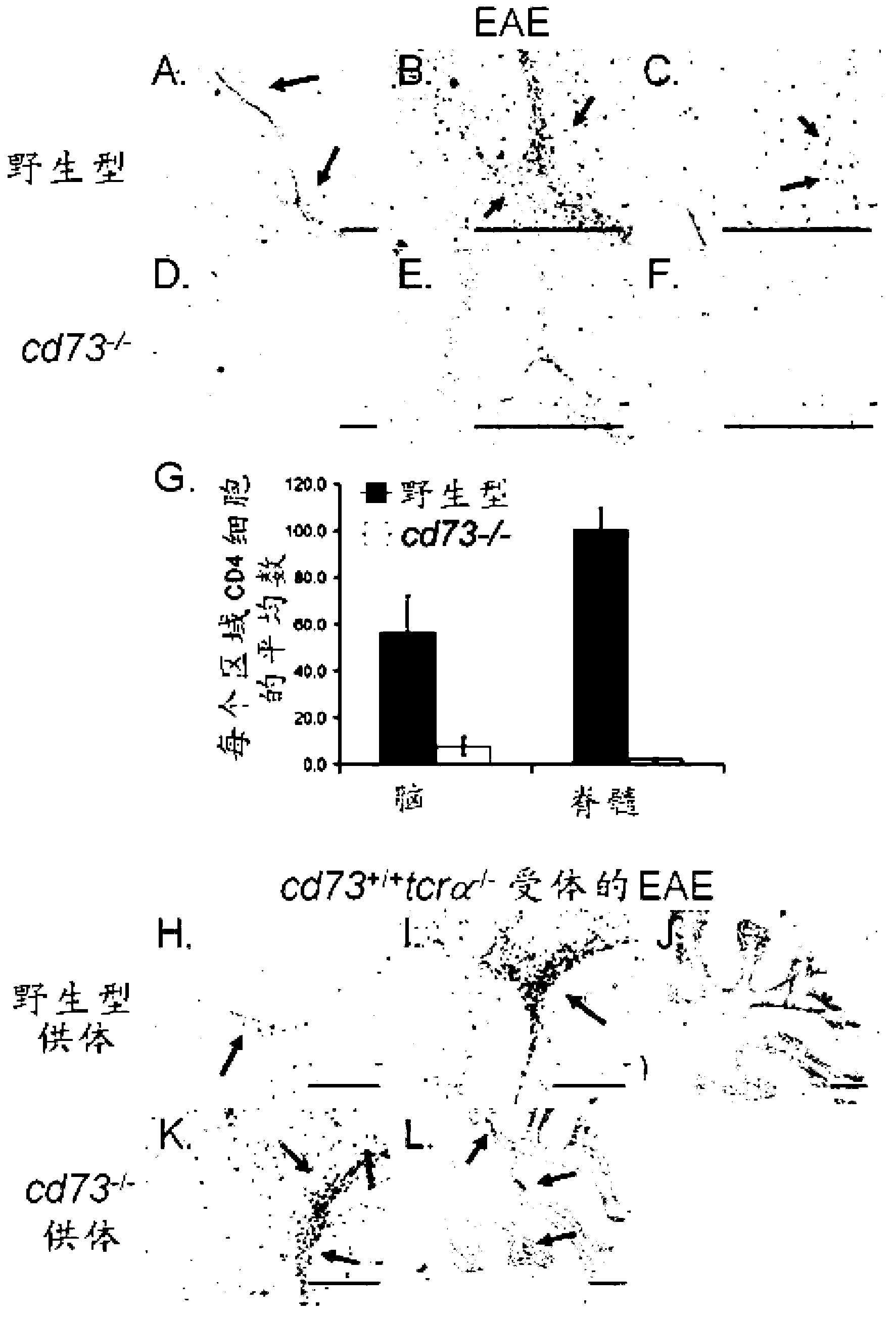
[0162] For MOG in CFA without PTX 35-55 Peptide primed mice. One week later, lymphocytes were harvested from the spleen and lymph nodes and mixed with ACK buffer (0.15M NH 4 Cl, 1mM KHCO 3 , 0.1mM EDTA, pH7.3) to lyse red blood cells. Combine cells with CD8(TIB-105), IA b,d,v,p,q,r (212.A1), FcR(2.4-G2), B220(TIB-164), NK1.1(HB191) antibodies were then incubated with BioMag goat anti-mouse IgG, IgM and goat anti-rat IgG (Qiagen). After negative magnetic enrichment, use CD4 directly +Cells may be further sorted into specific subpopulations. For sorting, cells were stained with antibodies to CD4 (RM4-5) and CD73 (TY / 23), and in some experiments CD25 (PC61), and then separated using a FACSAria (BD Biosciences). Typically >99% pure after sorting. For adoptive transfer, wash all CD4 + Or sort T cells and resuspend in sterile PBS. Recipient mice received ≤2.5×10 intravenously 6 cells in 200 μl sterile PBS.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com