Agitation micelle for treatment of cerebral arterial thrombosis
A technology of ischemic stroke and micelles, applied in the biological field, can solve problems such as difficult standardized production and clinical transformation, complex nano-drug structure, etc., to achieve long-term neuroprotective efficacy, optimal neuroprotective efficacy, and improved delivery efficiency effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
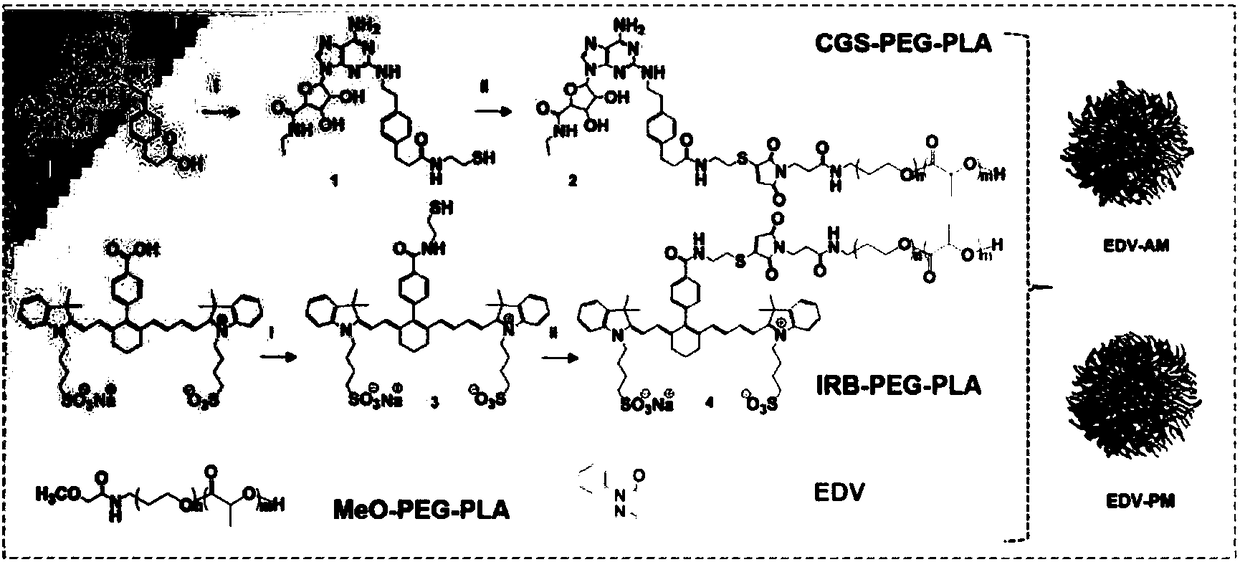
[0050] Example 1: Preparation of CGS21680-HS
[0051] CGS-21680 (5.4mg, 10μmol), HATU (7.6mg, 20μmol) and triethylamine (2.0mg, 20μmol) were dissolved in 1.0ml of anhydrous DMF, stirred at room temperature for 30min, 2-mercaptoethylamine (1.2mg , 15 μmol) was dissolved in 150 μL of anhydrous DMF, added dropwise to the reaction solution, and stirred at room temperature for 24 h. Purified by silica gel column to obtain the pure product CGS21680-HS.
Embodiment 2
[0052] Embodiment 2: Preparation of CGS-PEG 2K -PLA 2K
[0053] Mal-PEG 2K -PLA 2K (10mg, 2.5μmol) and CGS21680-HS (1.4mg, 2.5μmol) were dissolved in PBS (pH 7.4), stirred at room temperature for 24h to react, and the reaction product was dialysis bag (M W =4000kd) dialyzed for 48h to obtain pure CGS-PEG 2K -PLA 2K , using this method to obtain IRB (a near-infrared dye IR783B)-PEG 2K -PLA 2K for subsequent NIR experiments.
Embodiment 3
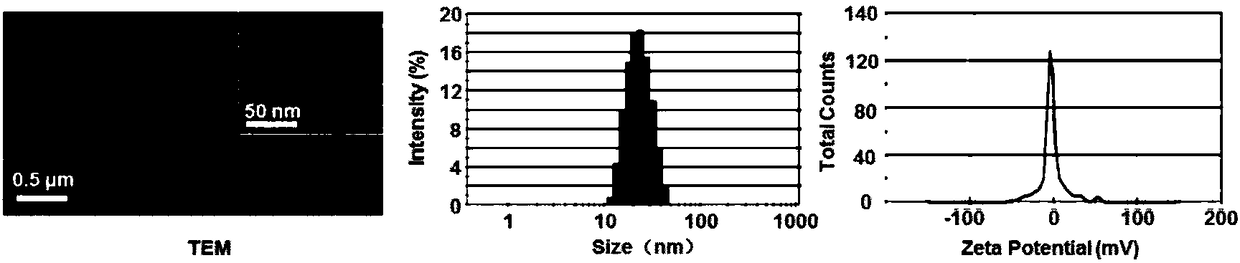
[0054] Real case 3: Preparation of agonist micelles
[0055] Agonist micelles were prepared by thin film hydration method, MeO-PEG-PLA (33.4mg, 8.4μmol), IRB-PEG-PLA (0.7mg, 0.2μmol), CGS-PEG-PLA (0.1mg, 0.03μmol) and EDV (1.2mg, 6.7μmol) was dissolved in 1ml of acetonitrile. Sonicate in ice bath for 15s, redissolve in 3.0ml of PBS solution after rotary evaporation at 40℃. Control micelles were prepared in the same way but the side chains of the agonist target head were removed. Use a particle size analyzer to measure the particle size and zata potential of the micelles, and use a transmission electron microscope to observe the ion state, such as figure 1 , regular micelles with a diameter of 19 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com