Preparation method of drug microcarrier for acquired deafness based on microfluidic technology
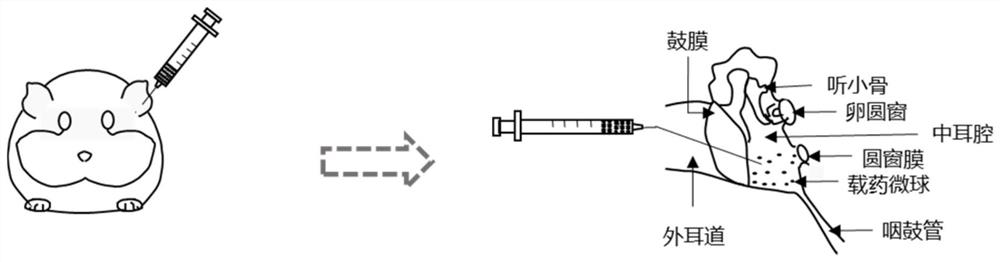
A microfluidic technology and microcarrier technology are applied in the field of preparation of drug microcarriers, which can solve the problems of increasing the risk of tympanic membrane perforation and middle ear infection, limiting the efficacy of hearing loss, and the loss of the eustachian tube of drugs, and reducing the permanent tympanic membrane. Risk of sexual perforation and infection, improved drug therapy, less systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] (1) Preparation steps of microfluidic loading:
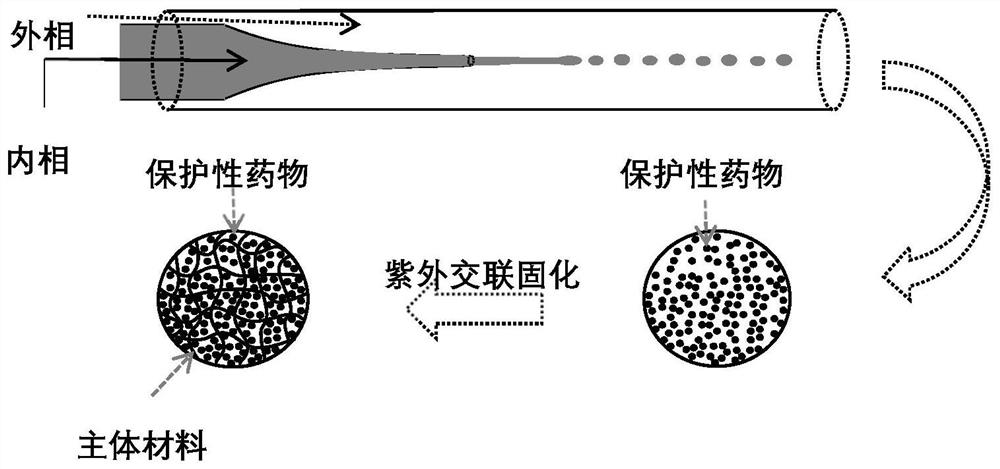
[0047] The glass capillary was drawn using a tube drawing machine, the tube opening was ground to a thickness below 50 μm by sandpaper, soaked in alcohol, and cleaned ultrasonically. Glass capillary microfluidic chip was assembled with drawn glass capillary, glass capillary with outer diameter of 1000 μm, 300 μm, 100 μm, glass slide, sampling needle and quick-drying adhesive.
[0048] (2) The preparation steps of drug microcarrier:
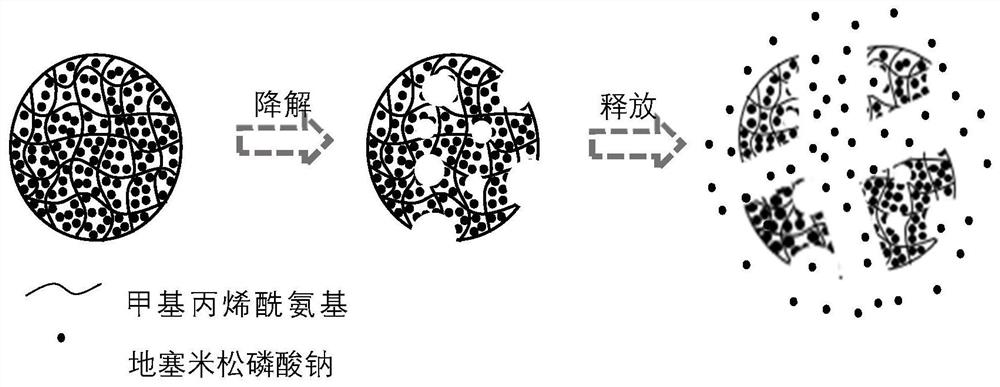
[0049] According to the hydrophilicity and hydrophobicity of hearing protection drugs and adjuvants, high biosafety and degradable materials with the same hydrophilicity and hydrophobicity are formulated into polymerizable polymer prepolymer solutions, and a certain proportion of hearing protection drugs and adjuvants are mixed. An appropriate proportion of ultraviolet photoinitiator HMPP can be added to the prepolymerization solution; choose a solution with good biological safety and incomp...
Embodiment 1
[0055] Preparation of methyl methacrylate gelatin (Dexsp-GelMA) microcarrier loaded with dexamethasone sodium phosphate-saponin and its antagonism against cisplatin-induced hair cell damage:
[0056] 1. Preparation of methyl methacrylate gelatin (Dexsp-GelMA) microcarrier loaded with dexamethasone sodium phosphate-saponin
[0057] (1) Fabrication of a single-emulsion microfluidic chip: a glass capillary was drawn using a tube puller, and the nozzle was ground to a size below 50 μm by sandpaper, soaked in alcohol, and cleaned ultrasonically. Glass capillary microfluidic chip was assembled with drawn glass capillary, glass capillary with outer diameter of 1000 μm, 300 μm, 100 μm, glass slide, sampling needle and quick-drying adhesive.
[0058] (2) Preparation of single emulsion template: see figure 1 , prepare each solution, 50mg dexamethasone sodium phosphate and 130ug saponin are dissolved in the mixed aqueous solution of 1ml 8% (w / v) GelMA and 6% (v / v) HMPP (phenylacetone) ...
Embodiment 2
[0073] Preparation of N-acetyl-L-cysteine-loaded poly(lactic-co-glycolic acid) (PLGA) microcarriers and its antagonism against cisplatin-induced hair cell damage by microfluidic technology:
[0074] 1. Preparation of polylactic-co-glycolic acid (PLGA) microcarriers of dexamethasone.
[0075] (1) Fabrication of a single-emulsion microfluidic chip: a single-emulsion microfluidic chip meeting the requirements was fabricated, and the steps were the same as in Example 1.
[0076] (2) Preparation of single emulsion template: prepare each phase solution, dissolve dexamethasone in polylactic acid-glycolic acid copolymer (PLGA) methanol and methylene chloride solution as the internal phase, and the external phase is 2wt% PVA solution, respectively Connect the Teflon tube through the syringe to the two-phase inlet of the microfluidic chip, and control the flow rate of the peristaltic pump to shear the two-phase fluid into monodisperse single-emulsion droplets with a particle size of les...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com