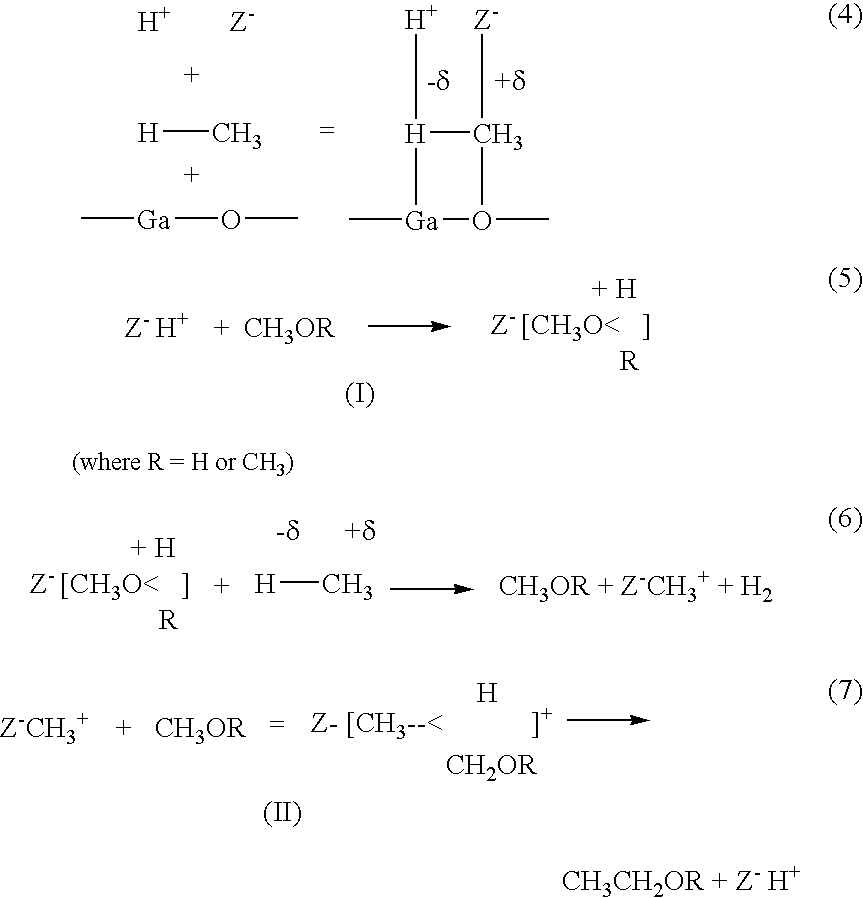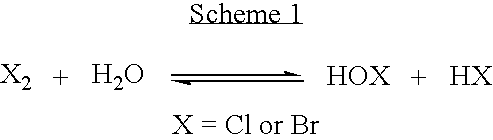Patents
Literature
397 results about "Non oxidative" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The non oxidative phase is basically a series of reversible reactions where two products react to form two products only which in turn react to form two products and so on . No reducing equivalent is formed, no carbon atom goes out of the reaction .
Method for producing carbon coated nano stage lithium iron phosphate by precipitation
InactiveCN101393982AAvoid synthetic stepsEasy to controlElectrode manufacturing processesIron saltsPhosphate
The invention discloses a precipitation method for preparing nanometer level iron phosphate lithium coated with carbon. The method comprises the following steps: firstly, weighing iron salt, deionized water and a compound of metallic elements; after the stirring and the mixing are performed, adding a phosphorous compound and citric acid diluted with water to the mixture; after the stirring is performed again, adding a precipitation agent to the mixture and controlling to the neutrality; stirring to react in a container, and after the static placement, respectively adding the deionized water, a carbon source and lithium salt to mix uniformly after the precipitate is filtered and washed; stirring again to react, and drying the water at 30 to 160 DEG C and warming up at the heating rate under the protection of non-oxidized gas after a product is crashed; baking at a constant temperature of 450 to 850 DEG C, cooling down to a room temperature at a cooling rate or with a stove, and finally obtaining the nanometer level ferric phosphate lithium coated with the carbon after crashing is performed. The precipitation method has the advantage that the raw material cost and the processing cost are low because bivalent iron is taken as the raw material. The iron phosphate lithium prepared by using the process has the characteristics of good physical processing performance and good electrochemistry performance, and is suitable for industrialized production.
Owner:南京海泰纳米材料有限公司
Process for the simultaneous conversion of methane and organic oxygenate to C2 to C10 hydrocarbons
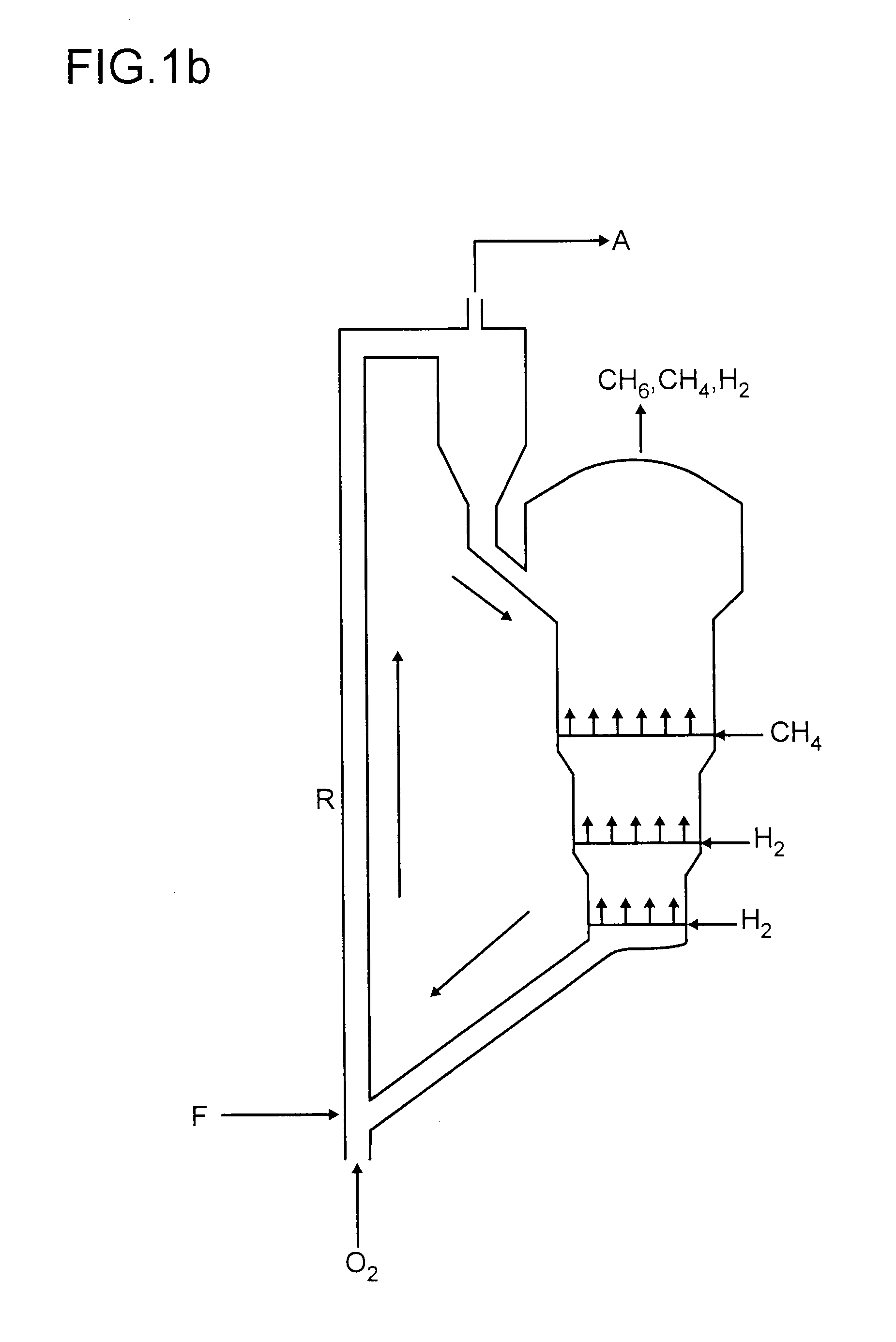
A process for the non-oxidative conversion of methane simultaneously with the conversion of an organic oxygenate, represented by a general formula: CnH2n+1OCmH2m+1, wherein C, H and O are carbon, hydrogen and oxygen elements, respectively; n is an integer having a value between 1 and 4; and m is an integer having a value between zero and 4, to C2+ hydrocarbons, particularly to gasoline range C6–C10 hydrocarbons and hydrogen, using a bifunctional pentasil zeolite catalyst, having strong acid and dehydrogenation functions, at a temperature below 700° C. is disclosed. In this process the moles of methane converted per mole of oxygenate converted is above 1.0, depending upon the process conditions.
Owner:COUNCIL OF SCI & IND RES
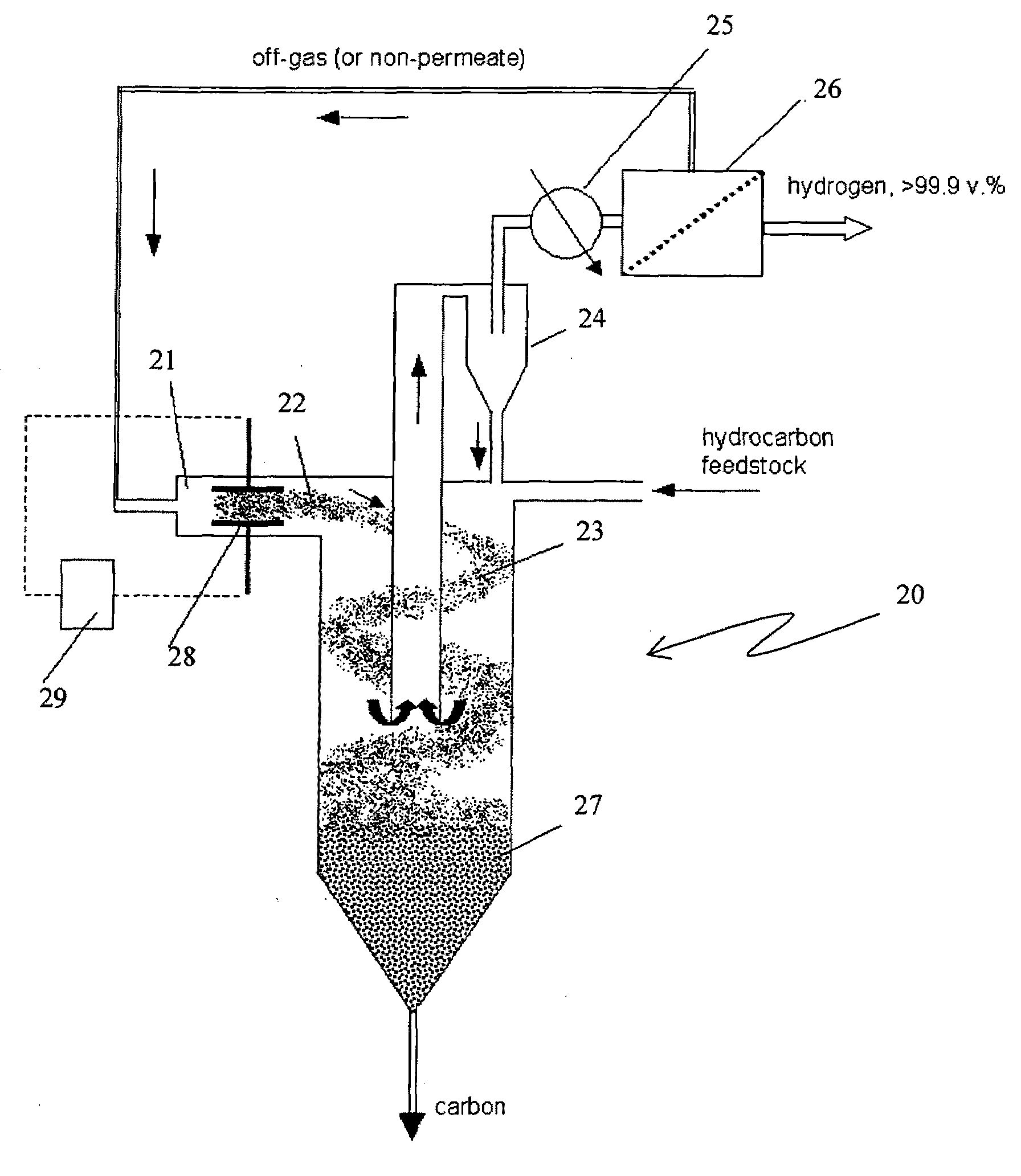
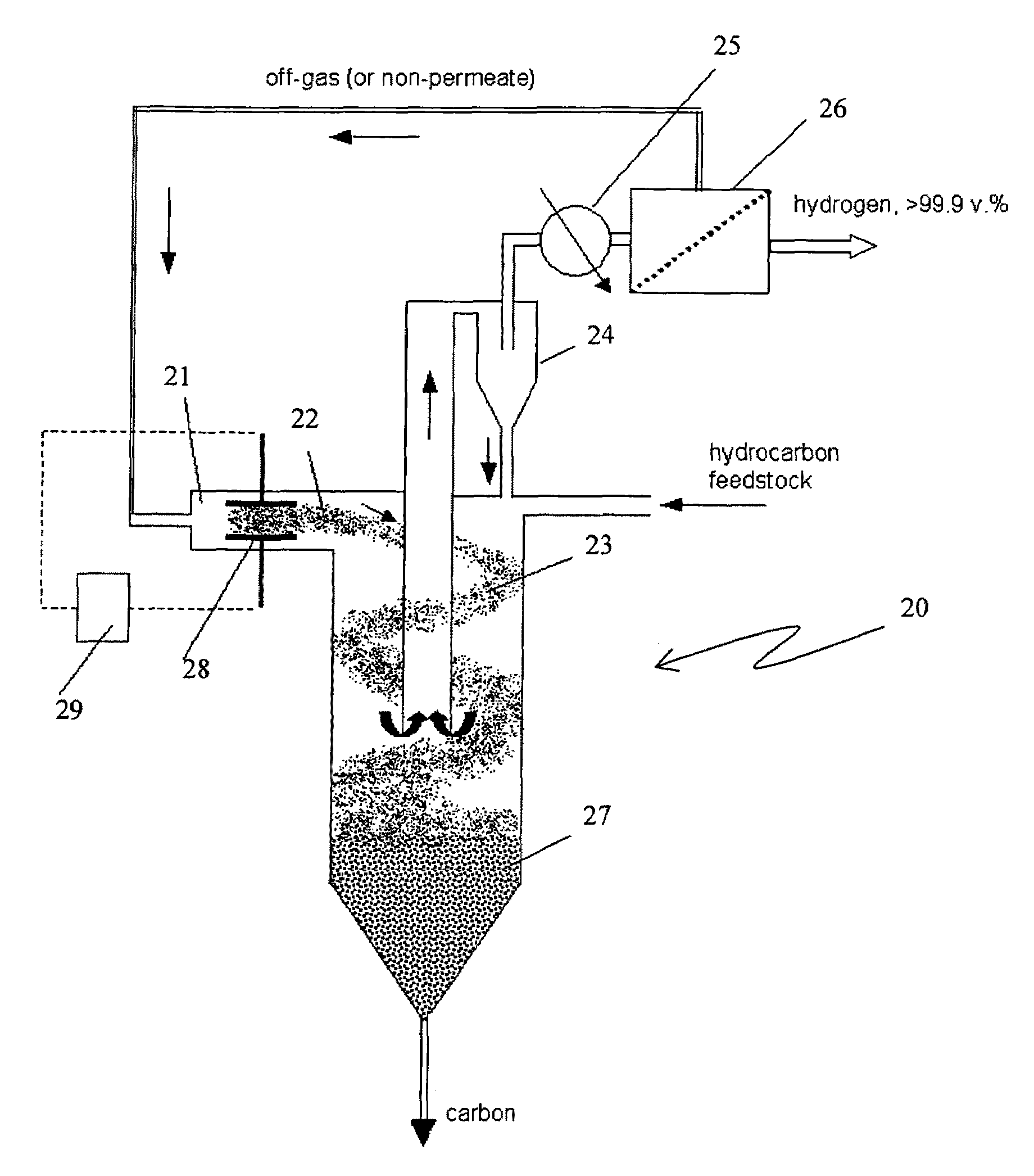
Process and apparatus for hydrogen and carbon production via carbon aerosol-catalyzed dissociation of hydrocarbons
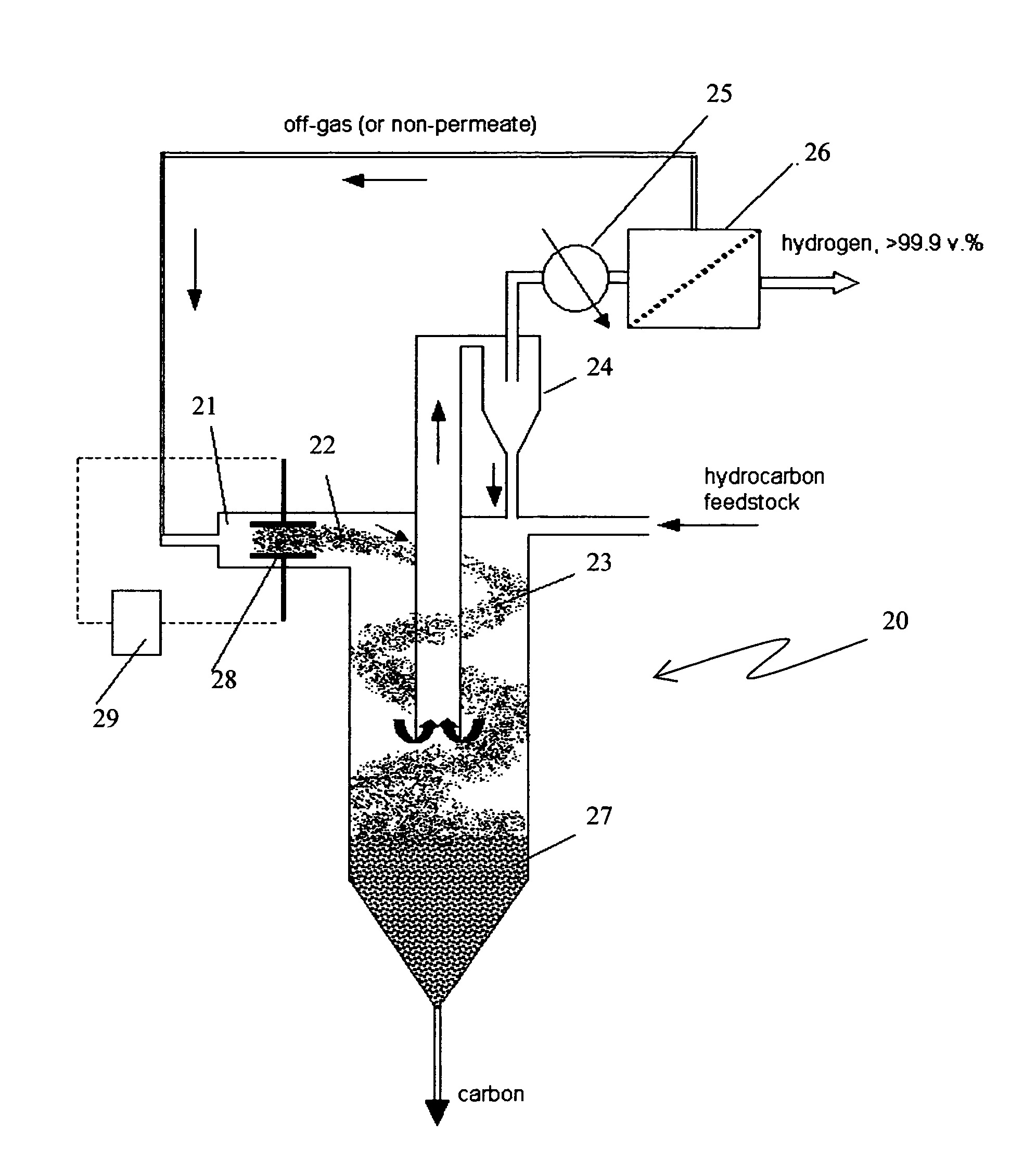
The present invention relates to a novel process for sustainable, continuous production of hydrogen and carbon by catalytic dissociation or decomposition of hydrocarbons at elevated temperatures using in-situ generated carbon particles. Carbon particles are produced by decomposition of carbonaceous materials in response to an energy input. The energy input can be provided by at least one of a non-oxidative and oxidative means. The non-oxidative means of the energy input includes a high temperature source, or different types of plasma, such as, thermal, non-thermal, microwave, corona discharge, glow discharge, dielectric barrier discharge, or radiation sources, such as, electron beam, gamma, ultraviolet (UV). The oxidative means of the energy input includes oxygen, air, ozone, nitrous oxide (NO2) and other oxidizing agents. The method, apparatus and process of the present invention is applicable to any gaseous or liquid hydrocarbon fuel and it produces no or significantly less CO2 emissions compared to conventional processes.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
High temperature polyurethane/urea elastomers
InactiveUS6964626B1High and low temperature resistance propertiesHigh and low temperature resistance propertyV-beltsRopes and cables for vehicles/pulleyElastomerPolyester
The present invention relates to molded polyurethane / urea elastomers, and specifically to improved polyurethane / urea elastomers having high temperature stability to about 140–150° C. and low temperature flexibility at about −35–(−40)° C., for use in dynamic applications. These elastomers are particularly useful for application in belts, specifically in automotive timing or synchronous belts, V-belts, multi V-ribbed or micro-ribbed belts, flat belting and the like. The polyurethane / urea elastomers of the present invention are prepared by reacting polyisocyanate prepolymers with symmetric primary diamine chain extenders, mixtures of symmetric primary diamine chain extenders and secondary diamine chain extenders, or mixtures of symmetric primary diamine chain extenders and non-oxidative polyols, which are all chosen to eliminate the need for catalysts via standard molding processes, and to improve phase separation. The polyisocyanate prepolymers are reaction products of polyols which are nonoxidative at high temperatures, such as polycarbonate polyols, polyester polyols, or mixtures thereof, with organic polyisocyanates which are either compact, symmetric and aromatic, such as para-phenylene diisocyanate, 1,5-naphthalene diisocyanate, and 2,6-toluene diisocyanate, or are aliphatic and possess trans or trans,trans geometric structure, such as trans-1,4-cyclohexane diisocyanate and trans,trans-4,4′-dicyclohexylmethyl diisocyanate.
Owner:THE GATES CORP
Composite, preparation method and application thereof in lithium ion secondary battery
ActiveCN106816594AImprove performanceRaw materials are easy to getMaterial nanotechnologySilicaCarbon coatingSilicon oxide
The invention discloses a composite. The composite comprises nanometer silicon, a lithium-containing compound and a carbon coating, or comprises nanometer silicon, a silicon oxide, a lithium-containing compound and a carbon coating. The method comprises the following steps: (1) mixing a carbon-coated silicon oxide and a lithium-source solid phase; and (2) thermally processing a prefabricated lithium precursor obtained in step (1) in a vacuum or non-oxidative atmosphere to obtain the composite. The method is simple, small in requirement on equipment, and low in cost; the obtained composite is stable in structure; the structure and property cannot be degraded after long-term storage; a battery prepared from a negative electrode material containing the composite is high in lithium taking-off capacity, high in first coulomb efficiency, and high in circulating performance; the charging capacity is equal to or above 1920mAh / g; the discharging capacity is equal to or above 1768mAh / g; and the first effect is equal to or more than 90.2%.
Owner:BTR NEW MATERIAL GRP CO LTD
Metal supported MCM-22 molecular sieve hollow sphere bifunctional catalyst preparation method and application thereof
ActiveCN101618336AHigh reactivityHigh selectivityMolecular sieve catalystsCatalyst activation/preparationMolecular sieveActive component
The invention relates to a metal supported MCM-22 molecular sieve hollow sphere bifunctional catalyst preparation method and an application thereof, belonging to the molecular sieve catalysis technology. The method is characterized in that carbon black sphere particles are used as template, molecular sieve hollow spheres with multi-stage pore path structure is prepared by rotation hydrothermal crystallization and by using metal active components the prepared molecular sieve hollow spheres are loaded and modified. The prepared Mo / HMCM-22 molecular sieve hollow sphere bifunctional catalyst can be used in methane non-oxidative aromatization reaction system. The effect and the benefit of the invention is that the preparation method of the molecular sieve hollow spheres has simple operation and low cost, and the prepared hollow sphere catalyst has excellent catalytic performance in the methane non-oxidative aromatization reaction, high methane conversion rate and aromatics yield and very long catalyst life.
Owner:DALIAN UNIV OF TECH
Method for improving catalytic property of methane aromatization catalyst
InactiveCN101618337AImprove catalytic performanceImprove bindingMolecular sieve catalystsHydrocarbonsLevel structureActive component
The invention relates to a method for improving the catalytic property of methane aromatization catalyst, belonging to the technical field of molecular sieve catalysis. The invention is characterized in that on the condition of no-second template, the crystal growth of zeolite molecular sieve is controlled by reasonablely controlling zeolite molecular sieve synthesis conditions such as synthesis formulation, charging sequence, ageing time, crystallization temperature, crystallization time and the like, small molecular sieve crystal is used for assembly and intergrowth to form MCM-22 and ZSM-5 molecular sieve assembly with multi-stage pore path structure, Mo or Re used can be adopted as active component to perform loading and modification to the prepared molecular sieve assembly, and the invention provides Mo-based MCM-22 and ZSM-5 molecular sieve assembly catalysts and an application thereof in methane non-oxidative aromatization reaction. The effects and the benefits of the invention is that the operation is simple, the cost is low and in methane non-oxidative aromatization reaction, the multi level structure assembly catalyst prepared by the method of the invention has better catalytic performance compared with the traditional catalyst.
Owner:DALIAN UNIV OF TECH
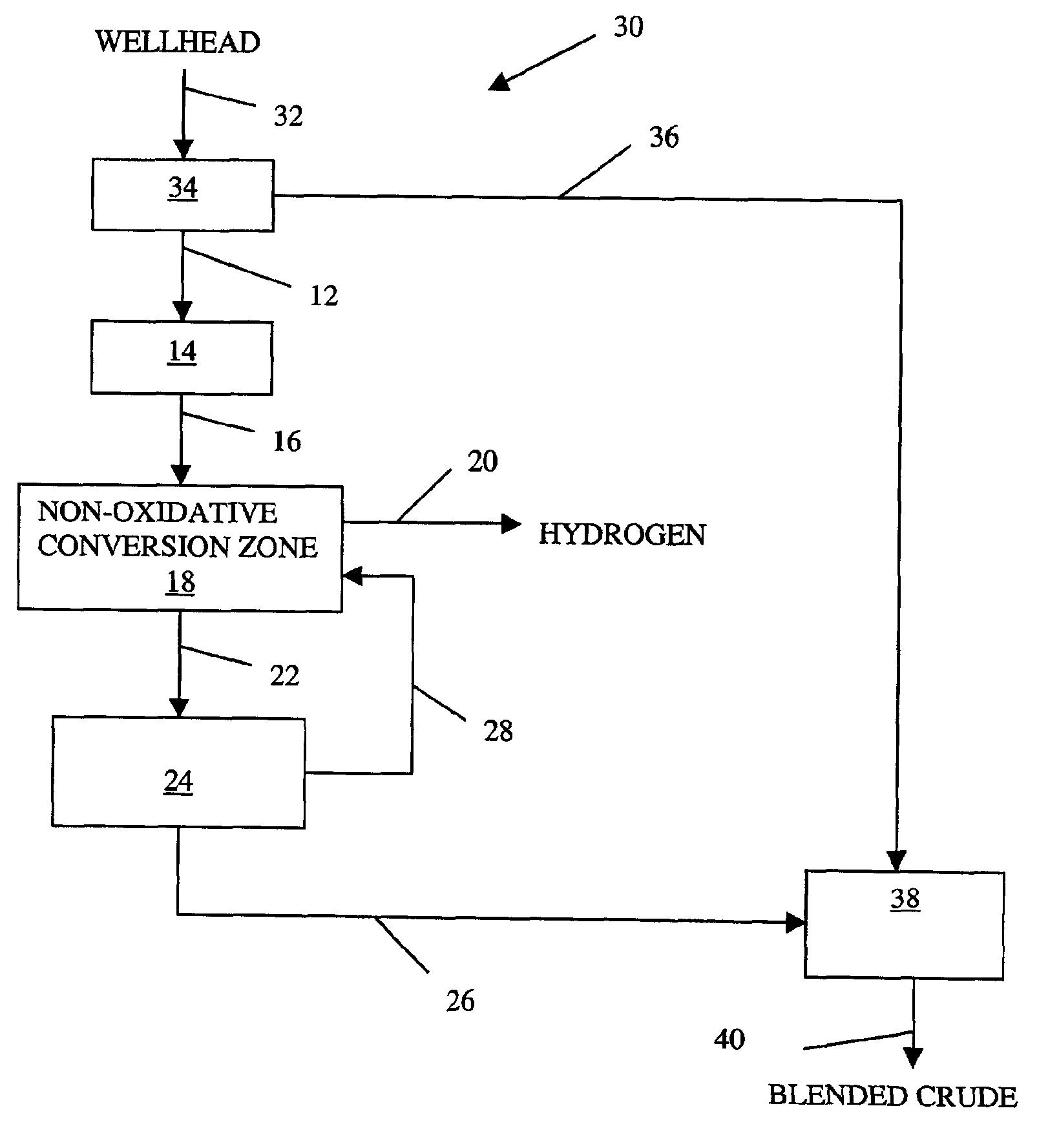
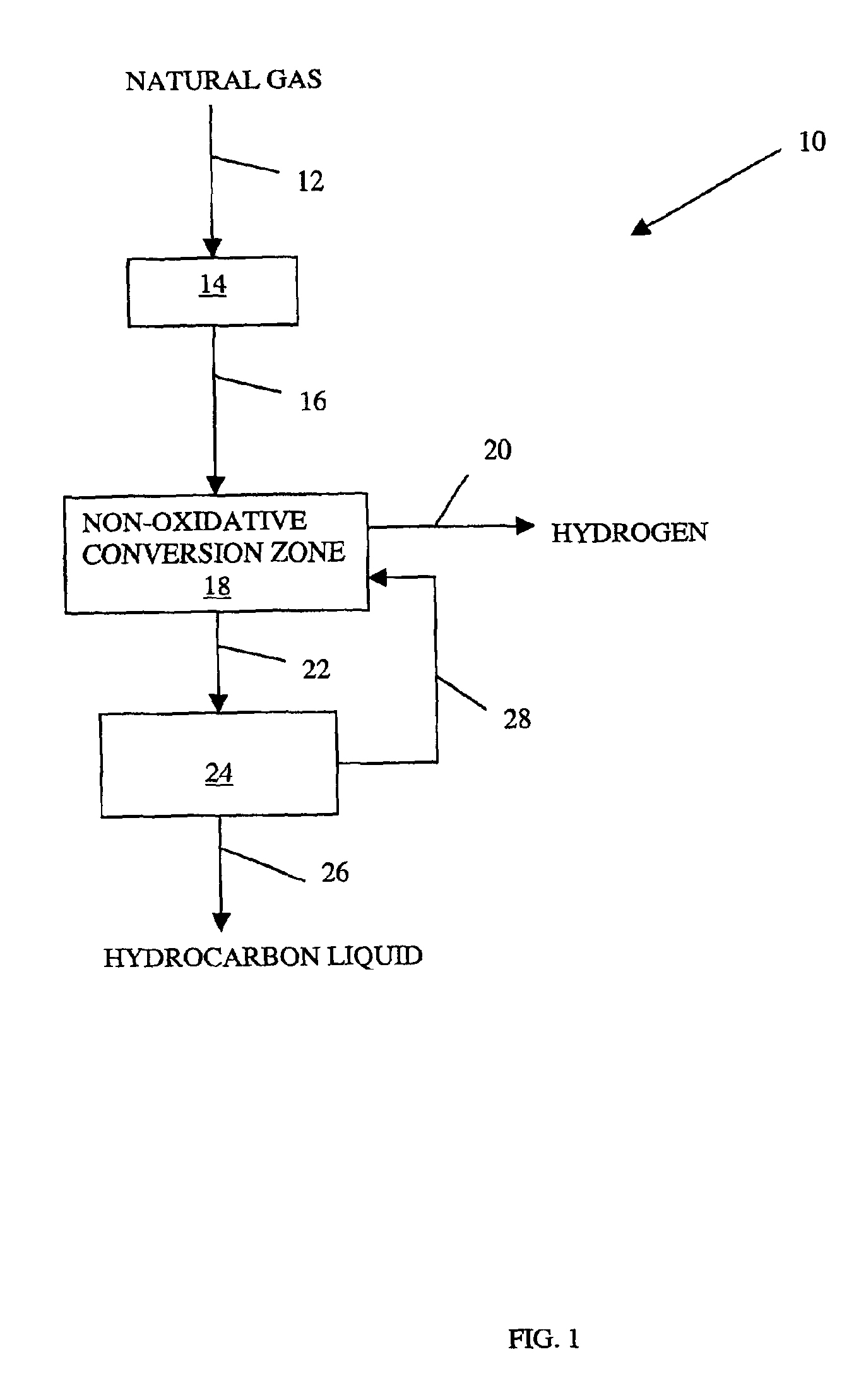
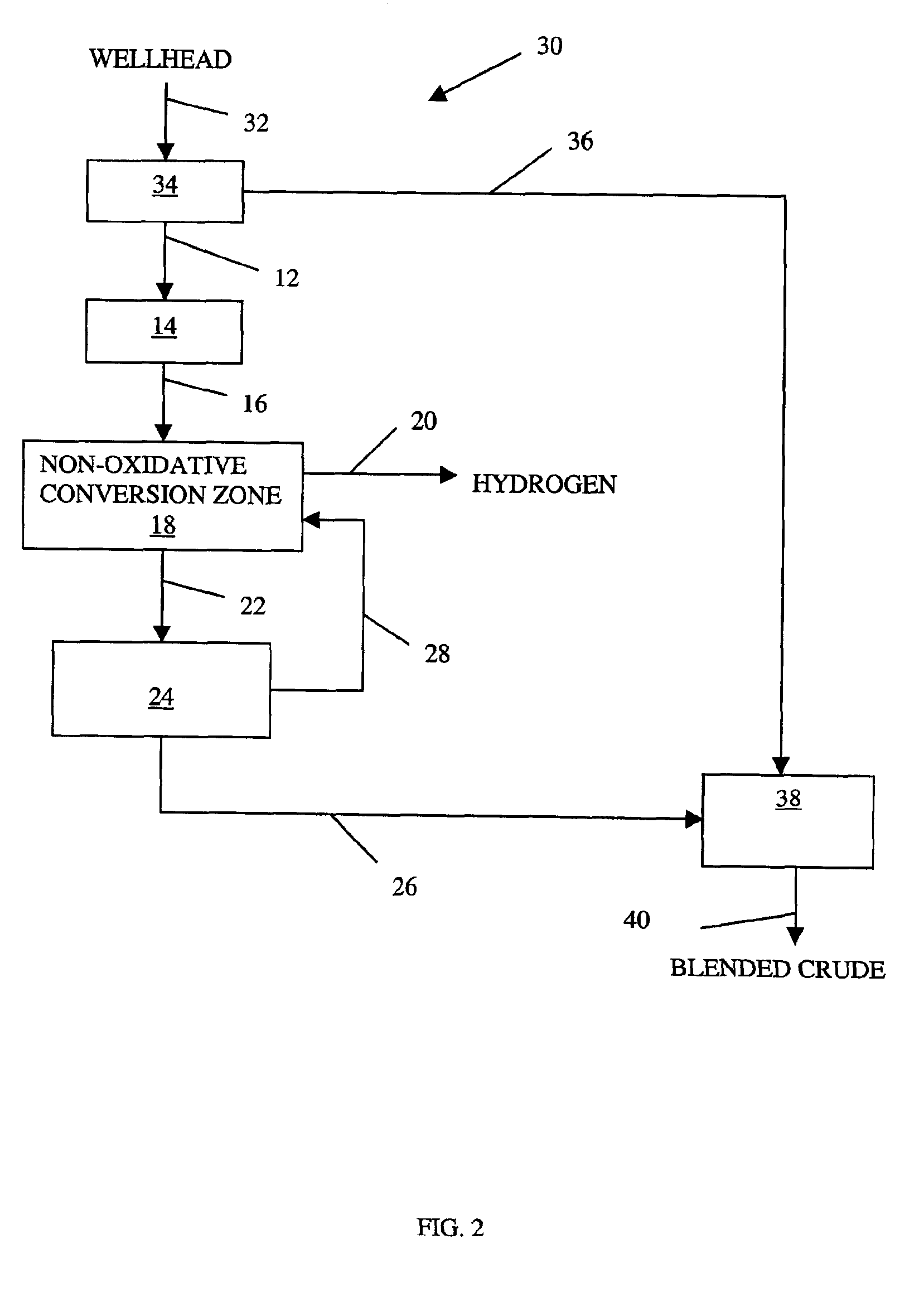
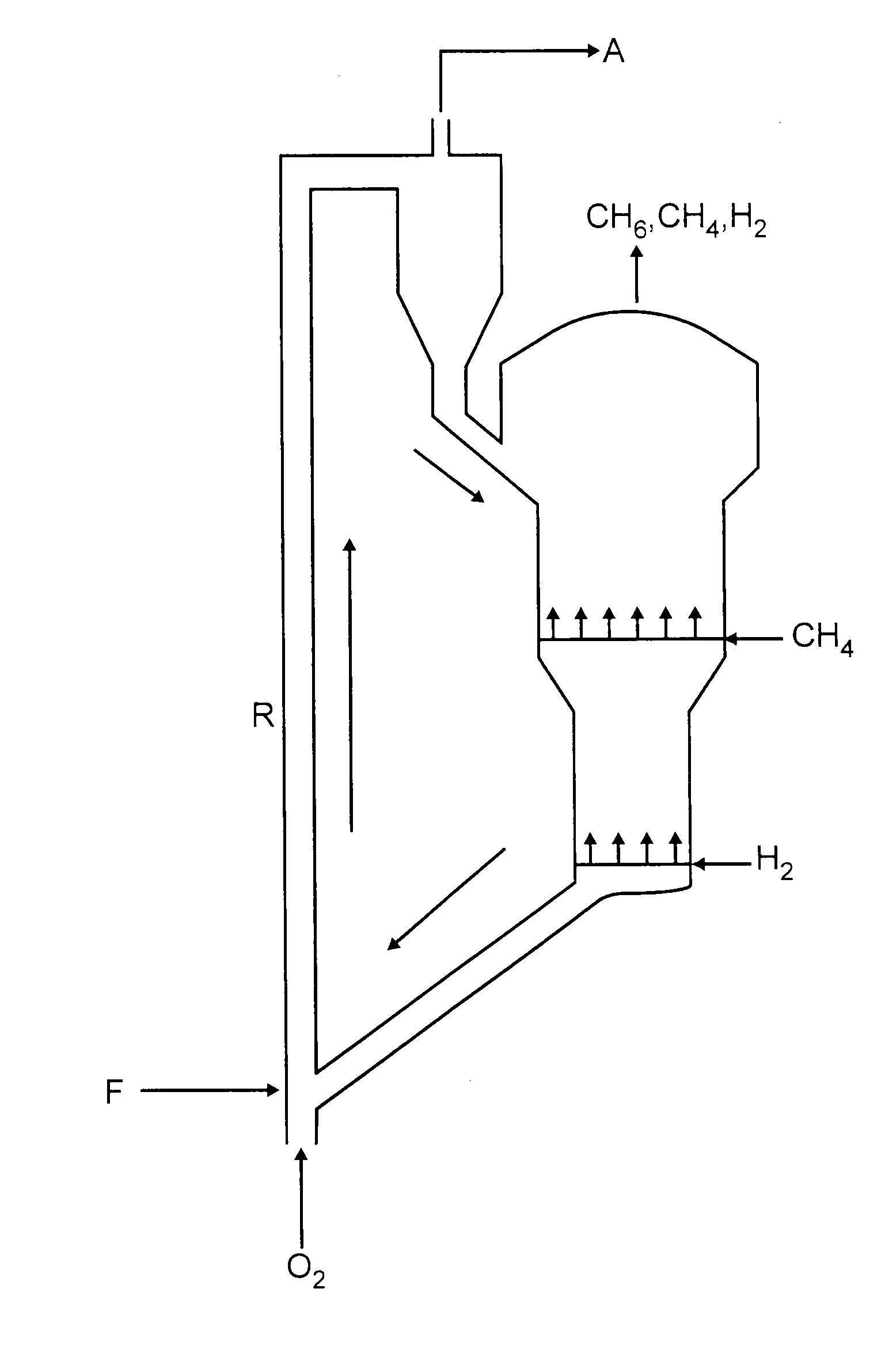
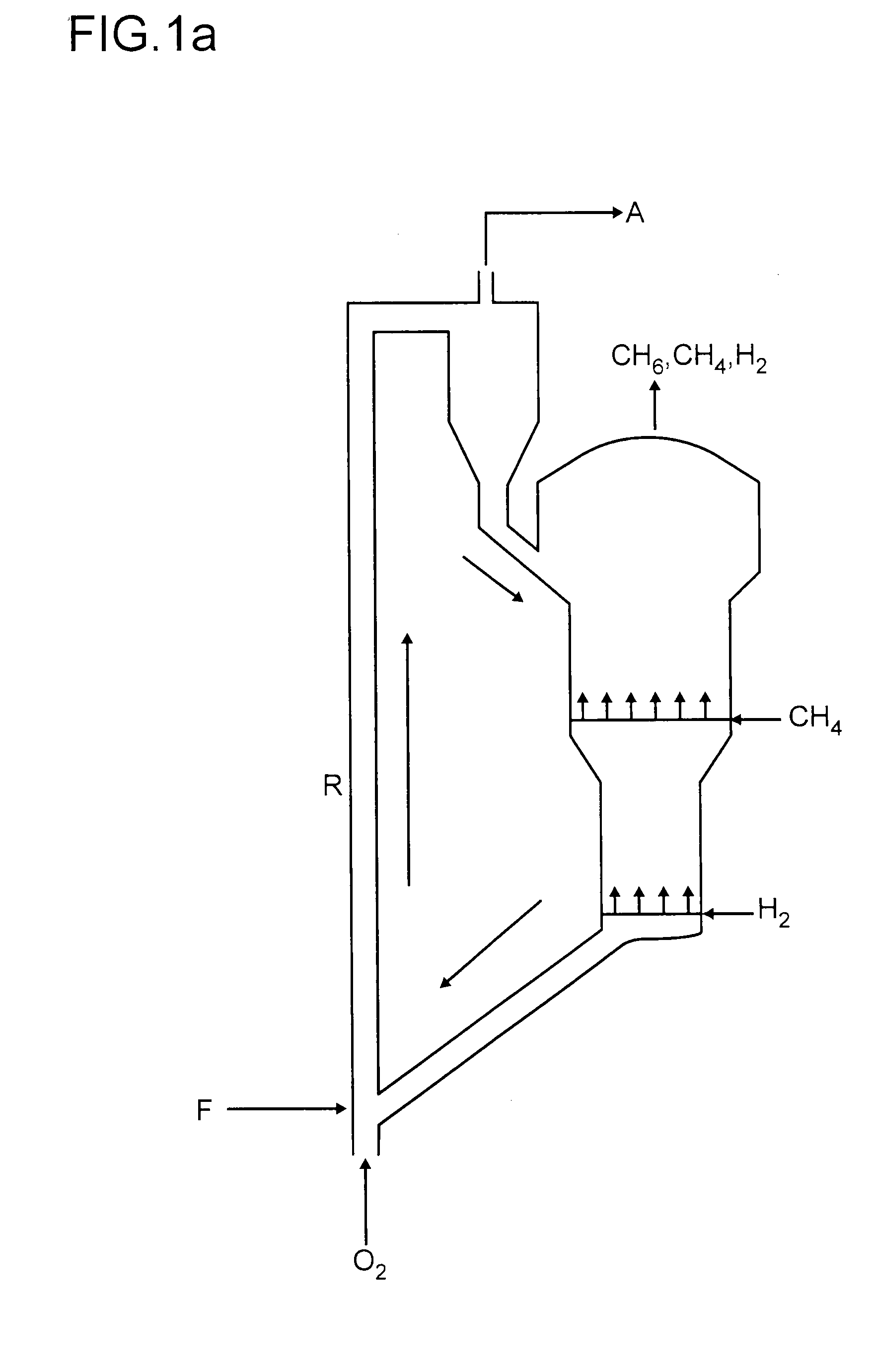
Non-oxidative conversion of gas to liquids
InactiveUS7019184B2Improve economySave powerHydrocarbon by isomerisationHydrocarbons from unsaturated hydrocarbon additionHydrogenGas to liquids
The present invention provides a process for natural gas in the form, e.g., of stranded gas or associated gas to transportable liquids. More particularly, the present invention provides a process in which the gas is non-oxidatively converted to aromatic liquid, preferably in proximity to the welihead, which may be onshore or offshore. In one aspect, the present invention provides integration of separation of wellhead fluids into associated gas and crude with blending of the aromatic liquid derived from the gas with the crude. Alternatively, or in combination, in another aspect, the present invention provides integration of conversion of byproduct hydrogen to power with non-oxidative conversion of gas to aromatic liquid.
Owner:CONOCO INC
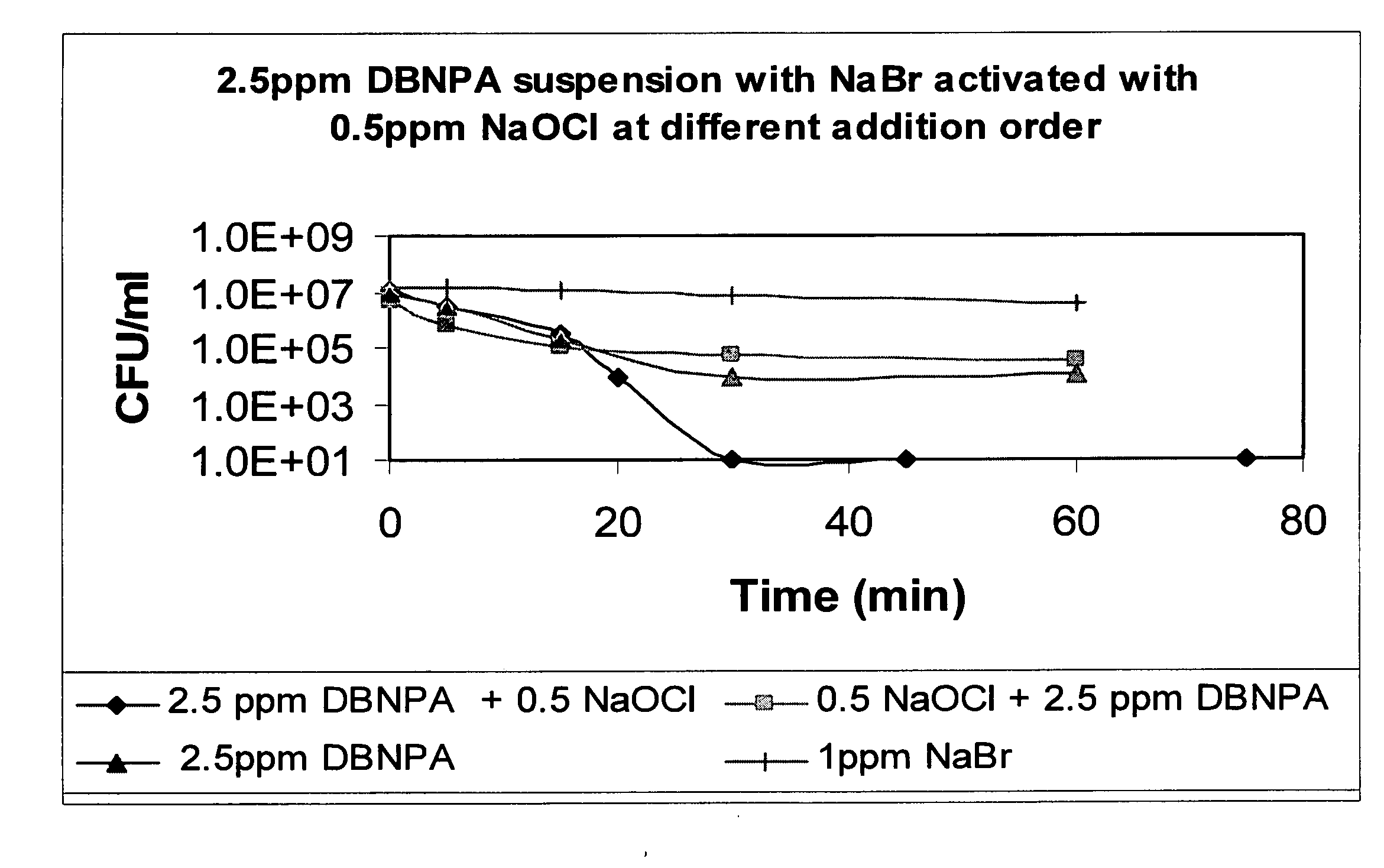
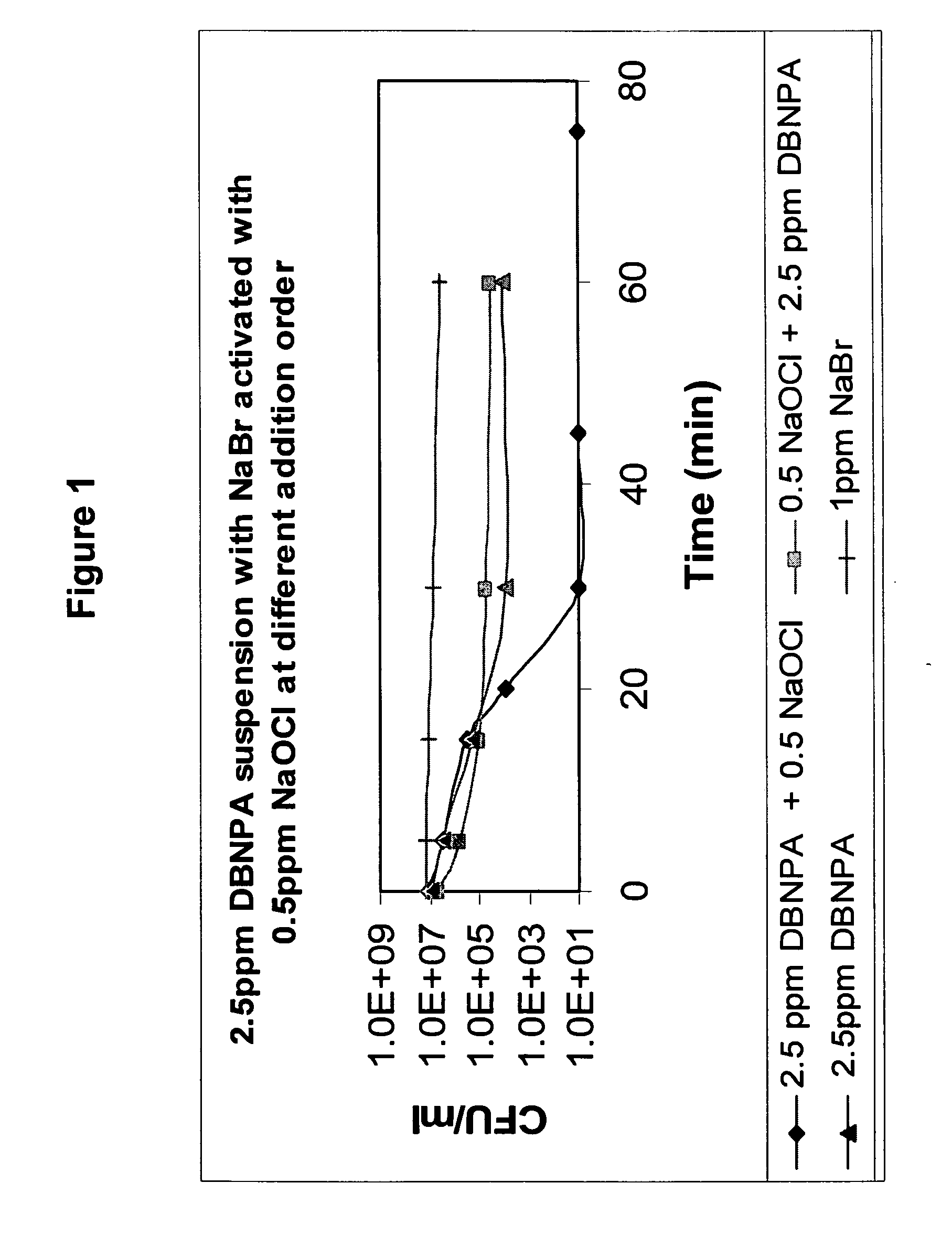
Formulations Containing a Non-Oxidative Biocide and a Source of Active Halogen and Use Thereof In Water Treatment
InactiveUS20090117202A1Effective water treatmentStable efficacyBiocidePeroxide active ingredientsHalogenNon oxidative
Novel formulations containing a non-oxidative biocide, such as DBNPA, and a source of an in situ produced active biocide, such as a concentrated aqueous solution of an inorganic halide salt, are disclosed. These novel formulations are particularly effective in the treatment of water, and are characterized by high stability, desirable rheological properties and an excellent biocidal activity.
Owner:BROMINE COMPOUNDS
Blackening method for lithium tantalite crystal substrate
ActiveCN105463581AEasy to control resistivityUniform colorPolycrystalline material growthAfter-treatment detailsNon oxidativeReducing atmosphere
The invention discloses a blackening method for a lithium tantalite crystal substrate. According to the method, a fluoride material and the to-be-treated lithium tantalite crystal substrate are contacted sufficiently, and the lithium tantalite crystal substrate is subjected to reductive heat treatment at the temperature of 450-600 DEG C in non-oxidative reducing atmosphere for 5-24 h. The volume resistivity of the lithium tantalite crystal substrate after blackening is 10<9>-10<12> omega cm. The method has the advantages that the method is simple, reliable and easy to operate, and the obtained lithium tantalite crystal substrate has good resistivity repeatability and low cost and is applicable to batch production.
Owner:上海召业申凯电子材料有限公司
Process-saving manufacturing method of sintered Nd-Fe-B series magnet
ActiveCN102956337ASave rare earth resourcesSimplify the manufacturing processInorganic material magnetismInductances/transformers/magnets manufactureHydrogen pressureRare earth
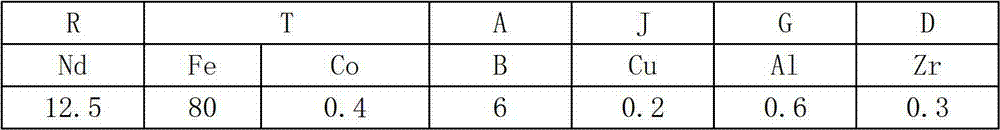
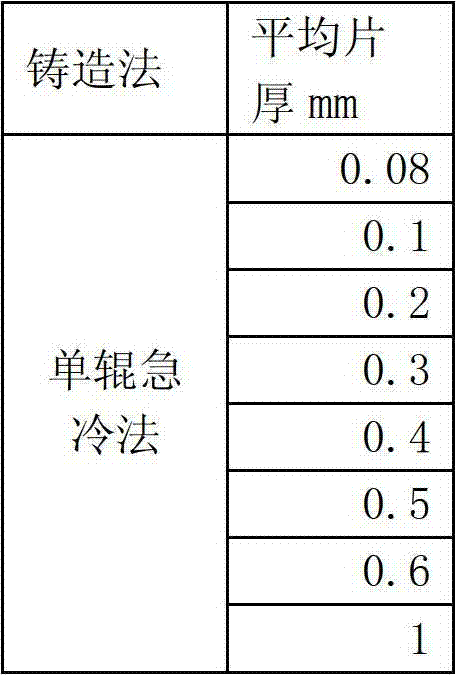
The invention discloses a process-saving manufacturing method of a sintered Nd-Fe-B series magnet. In a process of manufacturing the Nd-Fe-B series sintered magnet with oxygen content being below 2,500 ppm in the sintered magnet, a lamellar alloy raw material obtained by processes before a hydrogen demolishment pulverization process and with average thickness of 0.1-0.5 mm is used in a hydrogen demolishment pulverization process; hydrogen demolishment pulverization of not more than 24 hours is kept under the hydrogen pressure being above 0.01 MPa and below 1 MPa; then air stream pulverization is not performed; forming is directly performed by using a magnetic field forming method; and sintering is performed at the temperature of 900-1,140 DEG C under vacuum or in inert gas. The method realizes that the air stream pulverization process can be omitted to fulfill the aims of effectively utilizing precious rare earth resources, simplifying processes and performing low-cost production, and in addition, an oxidation effect which cannot be avoided in the air stream pulverization method in any way can also be prevented, so that the process is substantially a non-oxidative process, and large-scale manufacturing of ultrahigh-performance magnet is made possible.
Owner:FUJIAN CHANGJIANG GOLDEN DRAGON RARE EARTH CO LTD
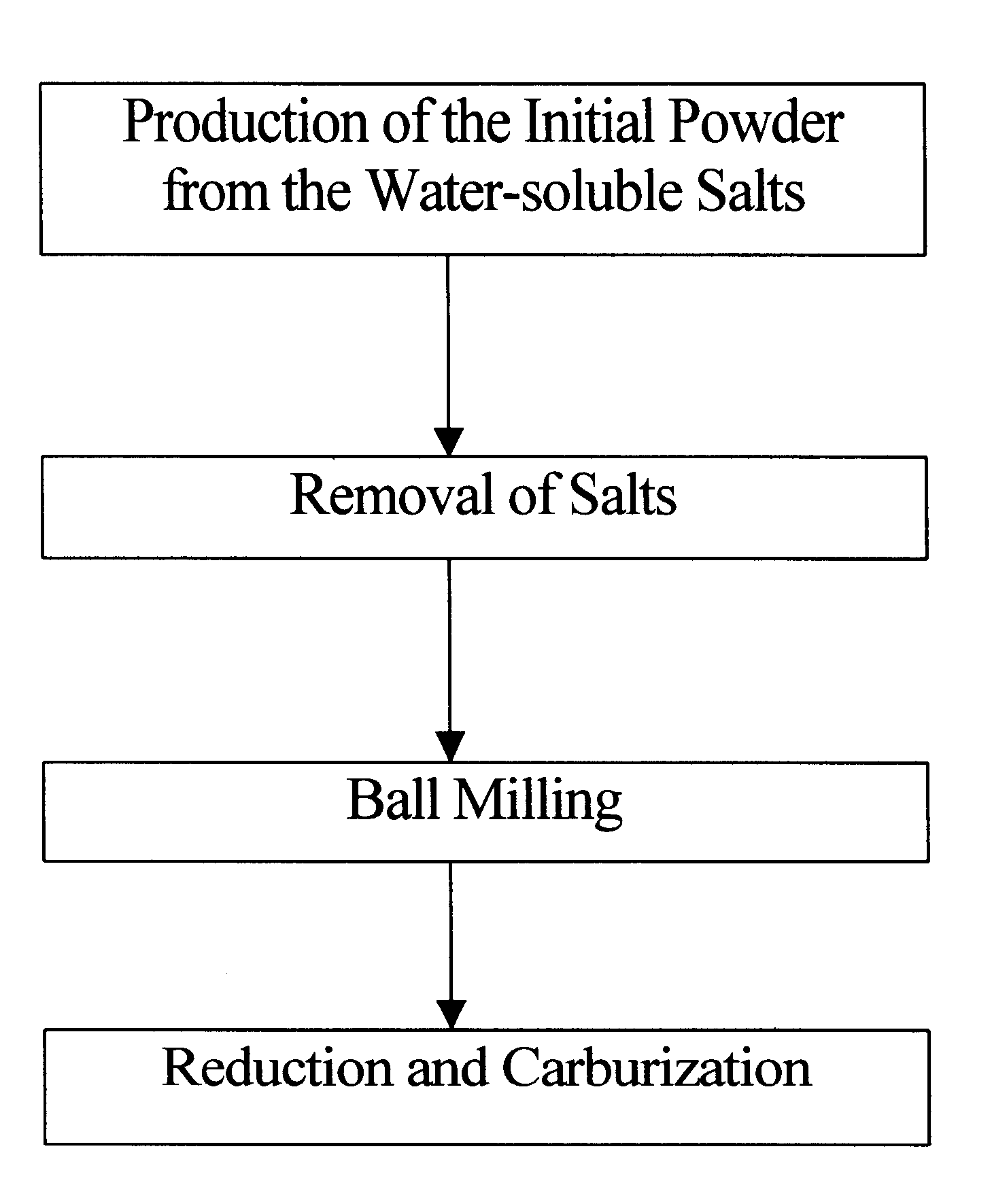
Method of producing nanophase WC/TiC/Co composite powder
InactiveUS6293989B1Increase surface areaImprove responseTransportation and packagingMetal-working apparatusNon oxidativeWater soluble
The present invention relates to a method of producing nanophase WC / TiC / Co composite powder by means of a mechano-chemical process comprising a combination of mechanical and chemical methods. For this purpose, the present invention provides a method of producing nanophase WC / TiC / Co composite powder, said method comprising as follows: a process of producing an initial powder by means of spray-drying from water-soluble salts containing W, Ti, and Co; a process of heating to remove the salts and moisture contained in the initial powder after spray-drying; a process of mechanically ball-milling to grind oxide powder after removing the salts and moisture therefrom, and to homogeneously mix the powder with an addition of carbon; and a process of heating the powder after milling, for reduction and carburization, in an atmosphere of reductive gas or non-oxidative gas.
Owner:KOREA INST OF MASCH & MATERIALS +1
Apparatus for hydrogen and carbon production via carbon aerosol-catalyzed dissociation of hydrocarbons
A novel process and apparatus is disclosed for sustainable, continuous production of hydrogen and carbon by catalytic dissociation or decomposition of hydrocarbons at elevated temperatures using in-situ generated carbon particles. Carbon particles are produced by decomposition of carbonaceous materials in response to an energy input. The energy input can be provided by at least one of a non-oxidative and oxidative means. The non-oxidative means of the energy input includes a high temperature source, or different types of plasma, such as, thermal, non-thermal, microwave, corona discharge, glow discharge, dielectric barrier discharge, or radiation sources, such as, electron beam, gamma, ultraviolet (UV). The oxidative means of the energy input includes oxygen, air, ozone, nitrous oxide (NO2) and other oxidizing agents. The method, apparatus and process of the present invention is applicable to any gaseous or liquid hydrocarbon fuel and it produces no or significantly less CO2 emissions compared to conventional processes.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Method for preparing carbon-coating ferric phosphate lithium
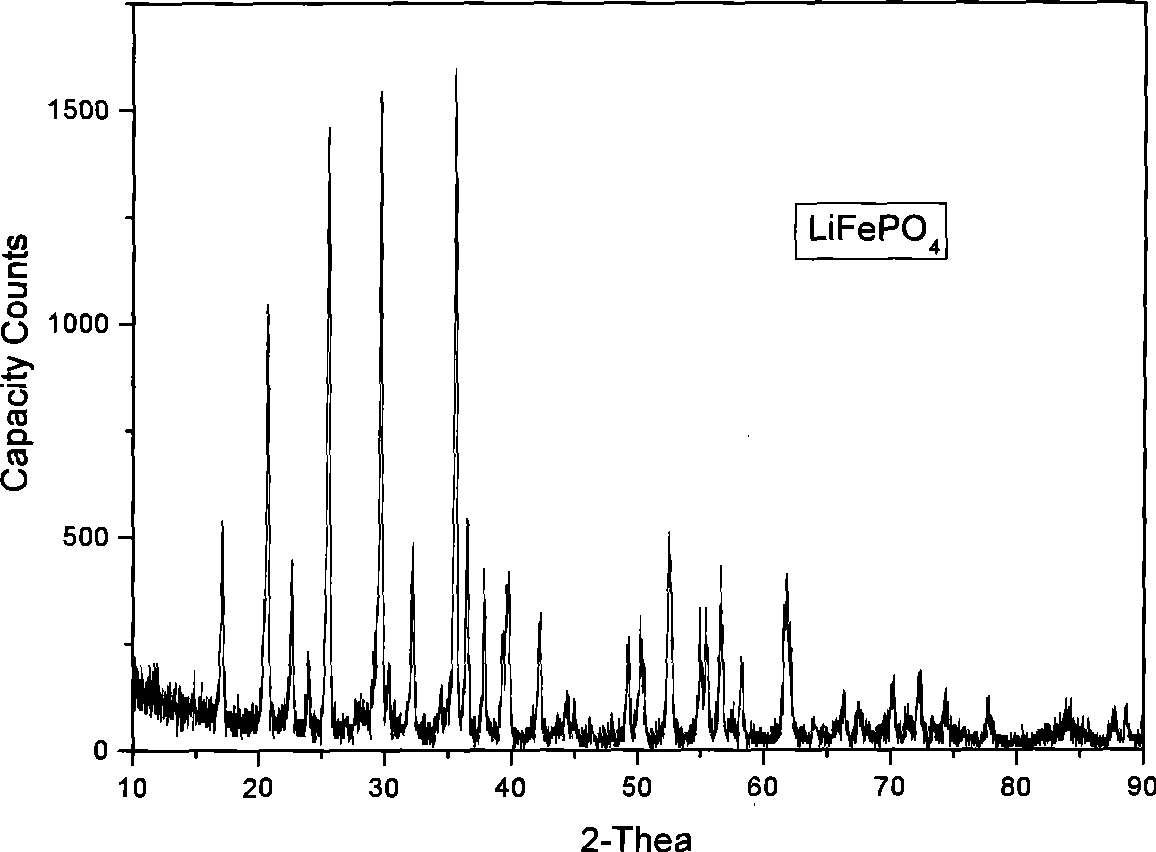
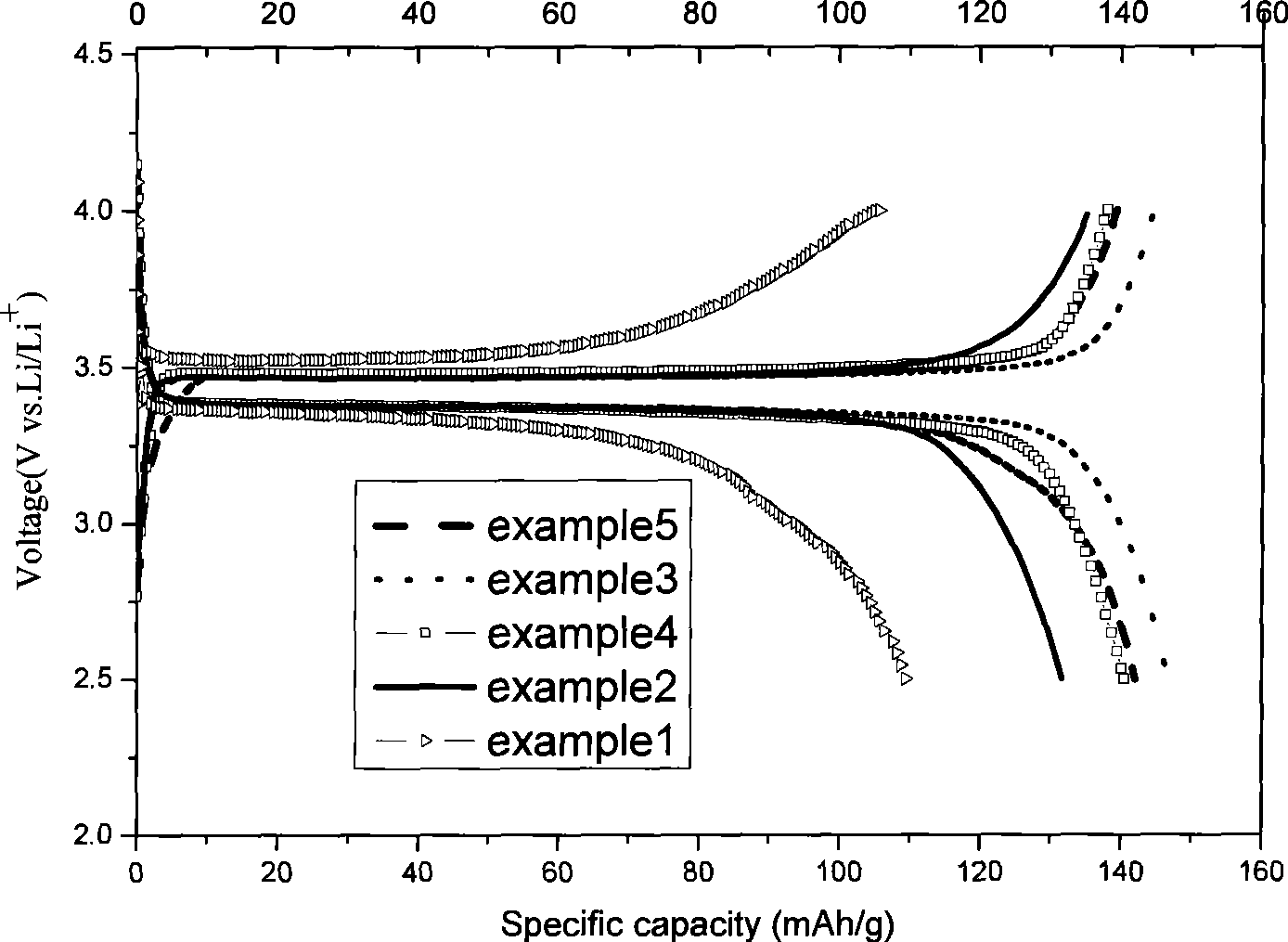
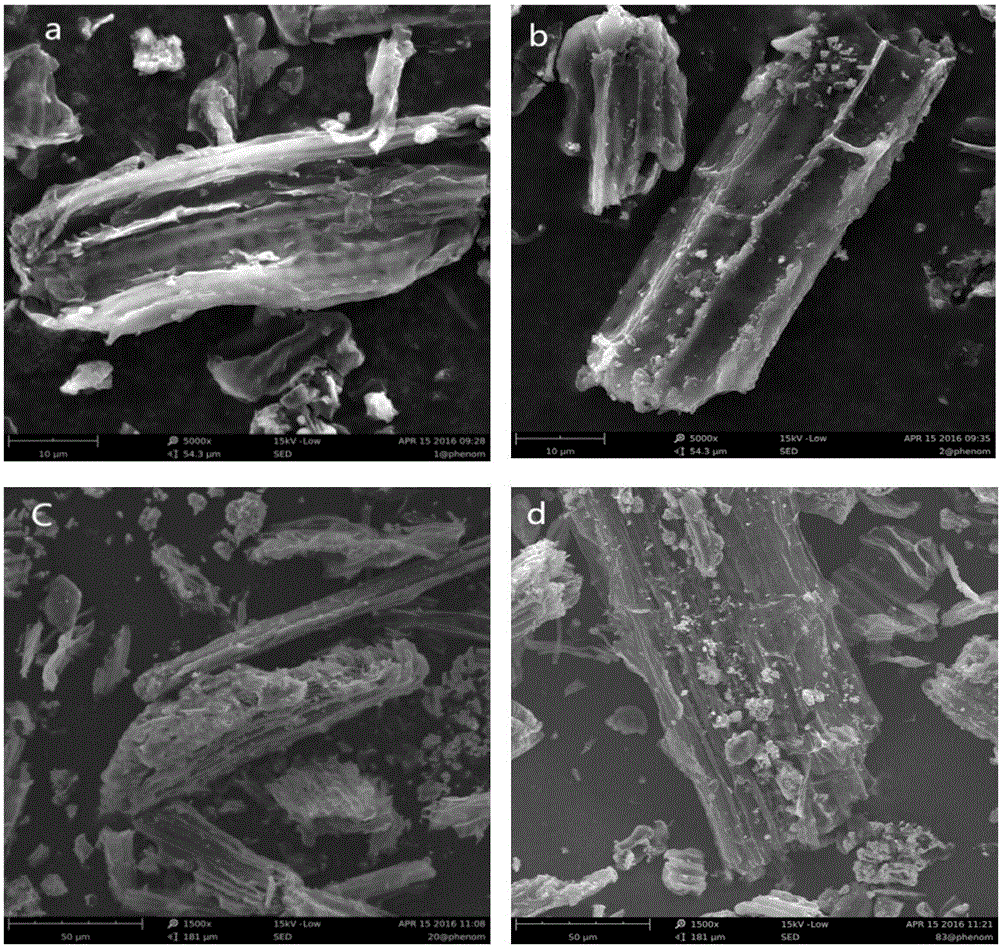
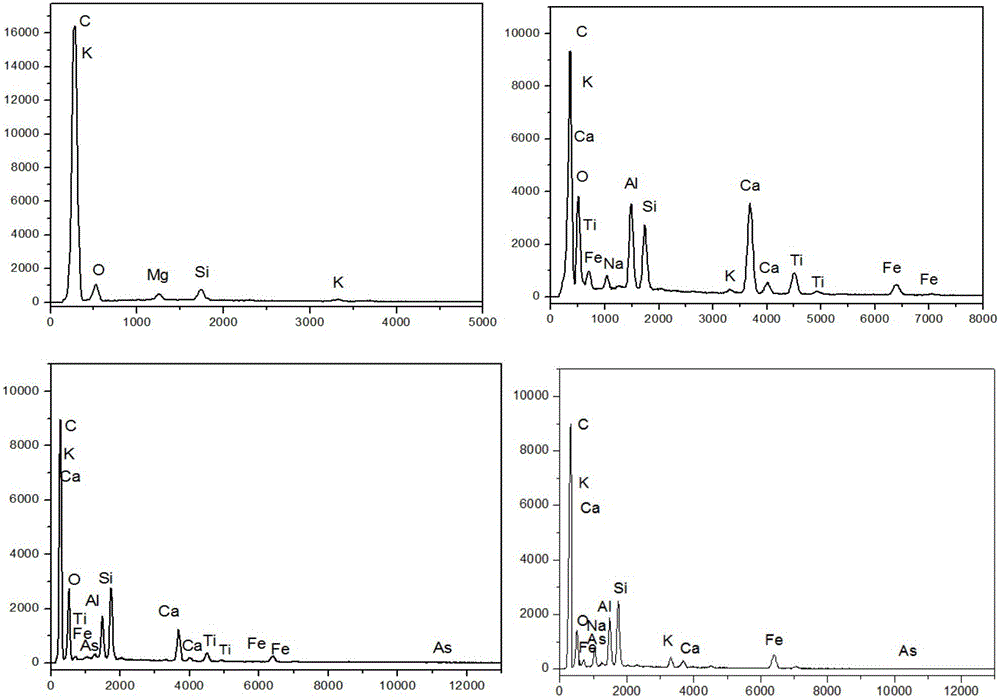
ActiveCN101442117AImprove surface structureImprove electrochemical performanceElectrode manufacturing processesPhosphateNon oxidative
The invention discloses a method for preparing carbon-coated lithium iron phosphate (LiFePO4 / C). The method comprises the following steps: a, LiOH.H2O, reduced Fe powder and H3PO4 are weighed according to the molar ratio of 1:1:1 and are stirred in an aqueous solution so as to react for 2 to 10 hours under nitrogen protection; a carbon source is added to a reaction system, and an obtained suspension as a reaction product is subjected to spray drying through a high-speed centrifugal spray drying machine, so as to obtain a LiFePO4 / C precursor; and b, the LiFePO4 / C precursor is transferred to a tubular furnace in inert or non-oxidative atmosphere and treated for 6 to 24 hours at a temperature of between 200 and 750 DEG C, so as to obtain the LiFePO4 / C. As the method adopts the Fe powder as a raw material, the specific discharge capacity of a LiFePO4 / C anode material prepared by a coprecipitation method under the multiplying power between 0.1 and 2C is obviously improved.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER +1
Modified biochar material for removing arsenic, and preparation and application thereof
InactiveCN106362685AImprove adsorption capacityWide variety of sourcesOther chemical processesWater contaminantsRed mudWastewater
The invention relates to a modified biochar material for removing arsenic, and preparation and application thereof. The preparation method comprises the following steps: adding biomass raw material powder into an ultrasonically-dispersed suspension of red mud and water, stirring the suspension to mix uniformly, and performing solid-liquid separation; roasting a obtained mixture of biomass and the red mud in a non-oxidizing atmosphere at the roasting temperature of 550 to 650 DEG C to obtain a carbonized product, namely, the modified biochar material. The modified biochar material is wide in raw material source, is convenient to prepare, has relatively high adsorption performance for arsenic-containing wastewater, is easy for large-scale production, and has a good application prospect.
Owner:CENT SOUTH UNIV
Process and apparatus for hydrogen and carbon production via carbon aerosol-catalyzed dissociation of hydrocarbons
The present invention relates to a novel process for sustainable, continuous production of hydrogen and carbon by catalytic dissociation or decomposition of hydrocarbons at elevated temperatures using in-situ generated carbon particles. Carbon particles are produced by decomposition of carbonaceous materials in response to an energy input. The energy input can be provided by at least one of a non-oxidative and oxidative means. The non-oxidative means of the energy input includes a high temperature source, or different types of plasma, such as, thermal, non-thermal, microwave, corona discharge, glow discharge, dielectric barrier discharge, or radiation sources, such as, electron beam, gamma, ultraviolet (UV). The oxidative means of the energy input includes oxygen, air, ozone, nitrous oxide (NO2) and other oxidizing agents. The method, apparatus and process of the present invention is applicable to any gaseous or liquid hydrocarbon fuel and it produces no or significantly less CO2 emissions compared to conventional processes.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
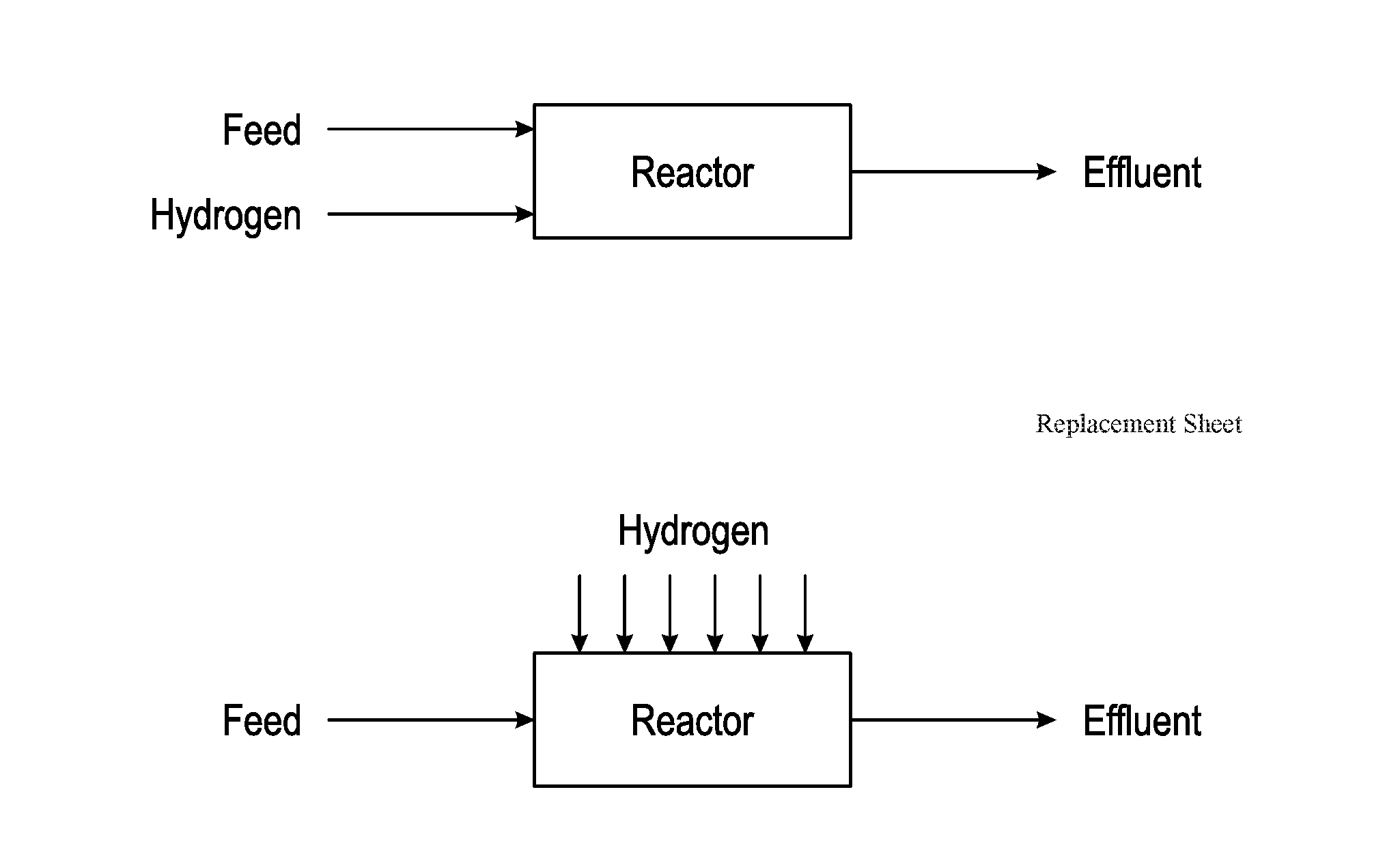
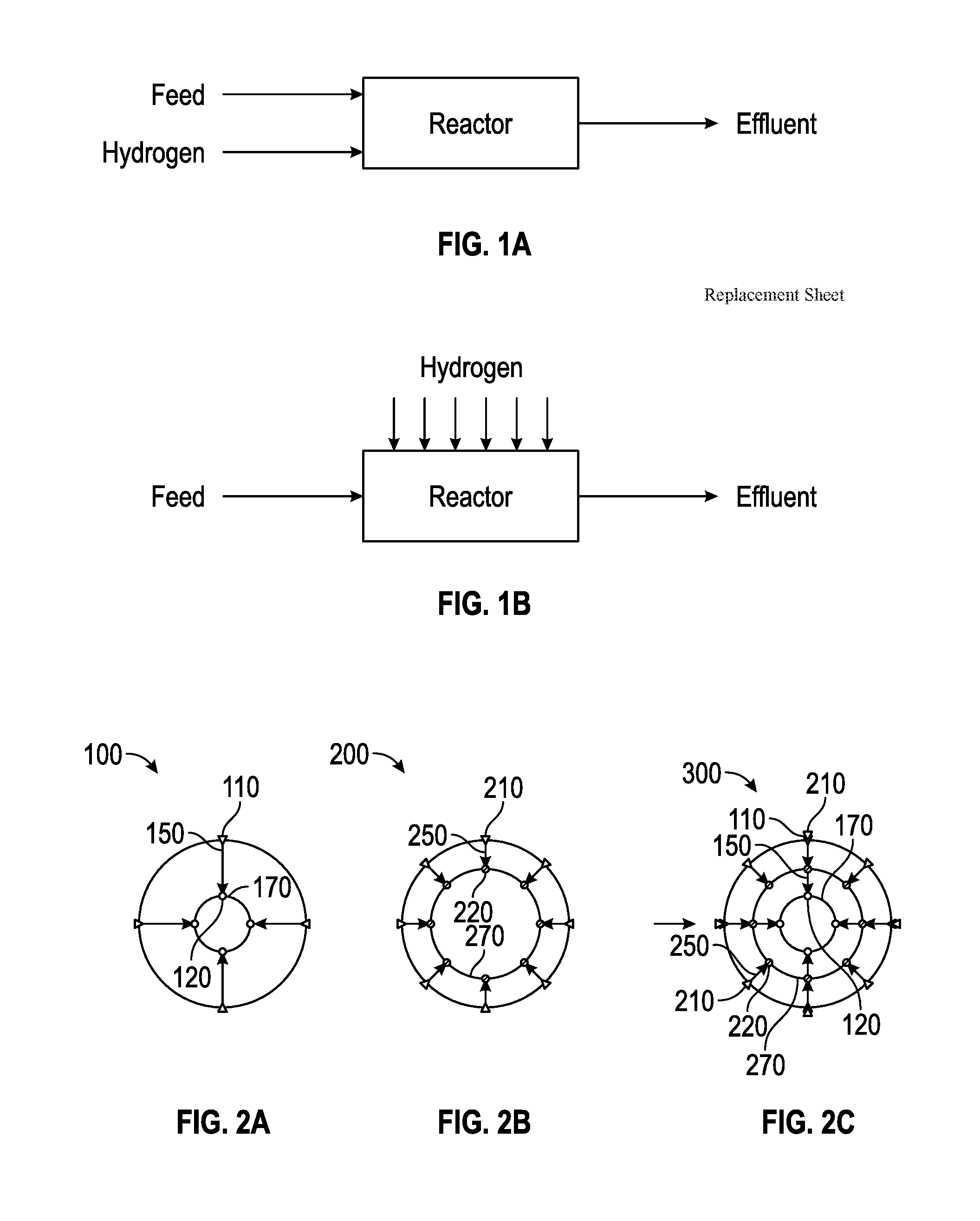
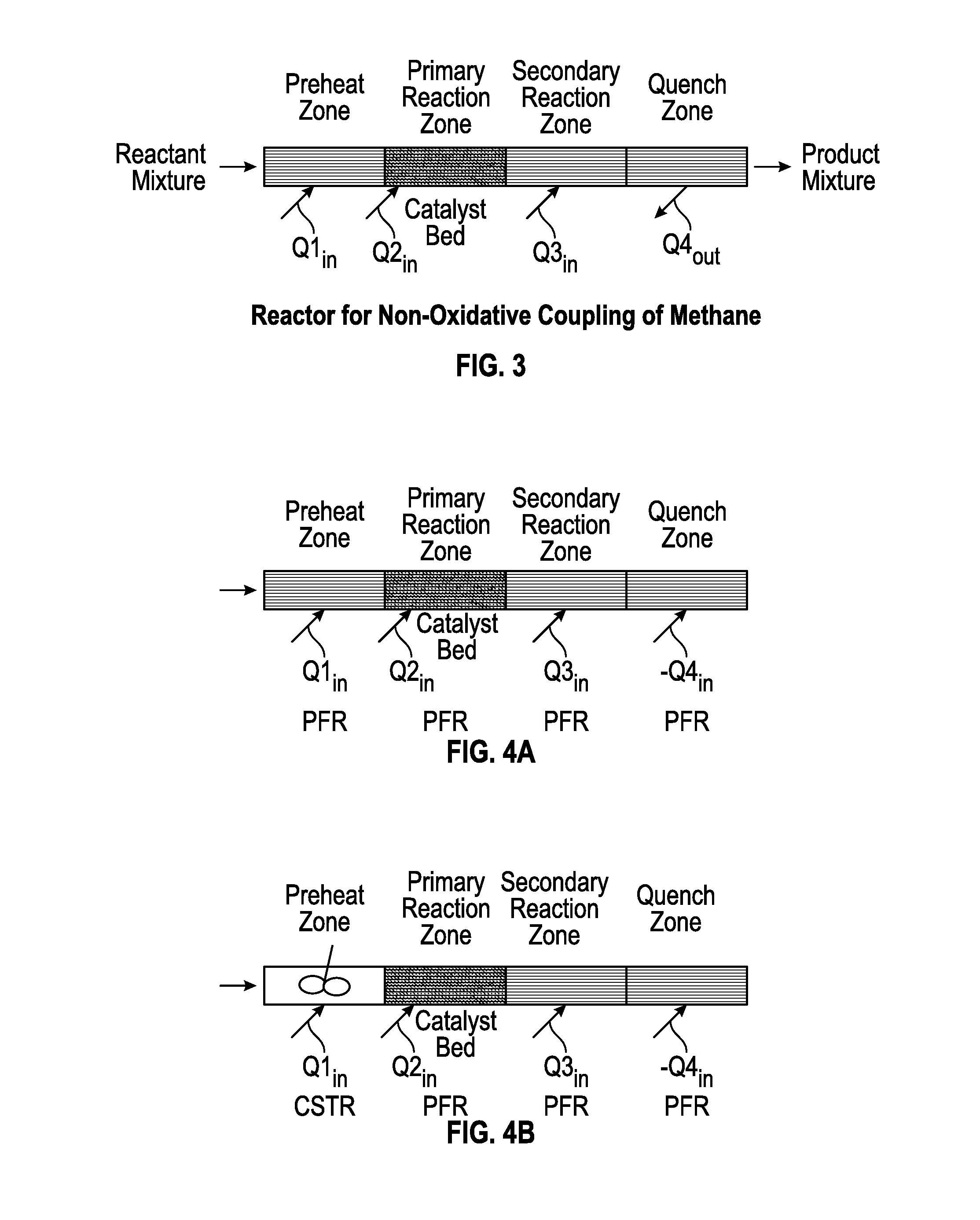
Method for Producing Hydrocarbons by Non-Oxidative Coupling of Methane
A method for producing C2+ hydrocarbons and H2 comprising (a) introducing to a reactor a reactant mixture comprising methane, (b) heating the reactant mixture to a preheating temperature to yield a heated mixture, (c) generating free radicals in the heated mixture to form a primary effluent mixture comprising free radicals, C2+ hydrocarbons, H2, and unreacted methane, (d) reacting the primary effluent mixture in a secondary reaction zone to form a secondary effluent mixture comprising C2+ hydrocarbons, H2, free radicals, and unreacted methane, at a secondary reaction zone temperature that is greater than the preheating temperature, wherein a free radicals amount in the primary effluent mixture is greater than a free radicals amount in the secondary effluent mixture, (e) cooling the secondary effluent mixture to a quench temperature lower than the secondary reaction zone temperature to yield a product mixture comprising C2+ hydrocarbons and H2, and (f) recovering the product mixture.
Owner:SABIC GLOBAL TECH BV
Preparation method of carbon-coated lithium titanate
The invention discloses a preparation method of carbon-coated lithium titanate, comprising the following steps of: 1) weighing and dispersing lithium source and titanium source in distilled water or deionized water; 2) adding long-chain aliphatic carboxylic acid to the water, heating the water to 30 to 100 DEG C, followed by uniform stirring and dispersion in order to obtain suspension; 3) transferring the suspension into a closed reaction container for being heated so that the suspension is subjected to reaction at constant temperature; 4) cooling reaction products to room temperature upon the end of the reaction, repeatedly washing with mixed liquid of alcohol and water, centrifuging and drying the reaction products, and collecting precursor; and (5) putting the collected precursor in an atmosphere furnace filled with non-oxidative gas to be roasted, and then cooling the roasted precursor to result in the carbon-coated lithium titanate. Compared with the prior art, the preparation method according to the invention achieves the formation of uniform carbon-coated layers under the protection of non-oxidative gas by means of surface coordination of long-chain aliphatic carboxylic acid and lithium titanate, thus the conductivity of lithium titanate can be improved and the high-rate and high temperature performances of lithium ion secondary battery can also be enhanced.
Owner:DONGGUAN AMPEREX TECH +1
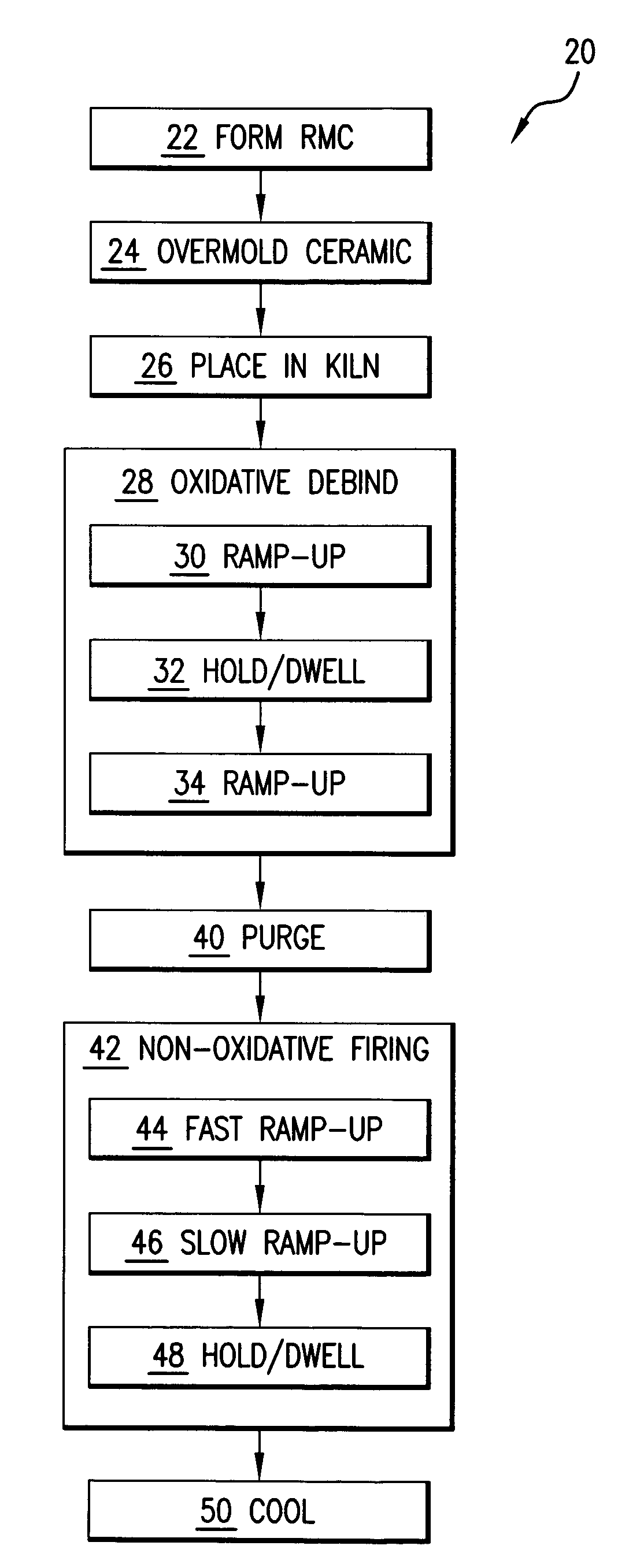
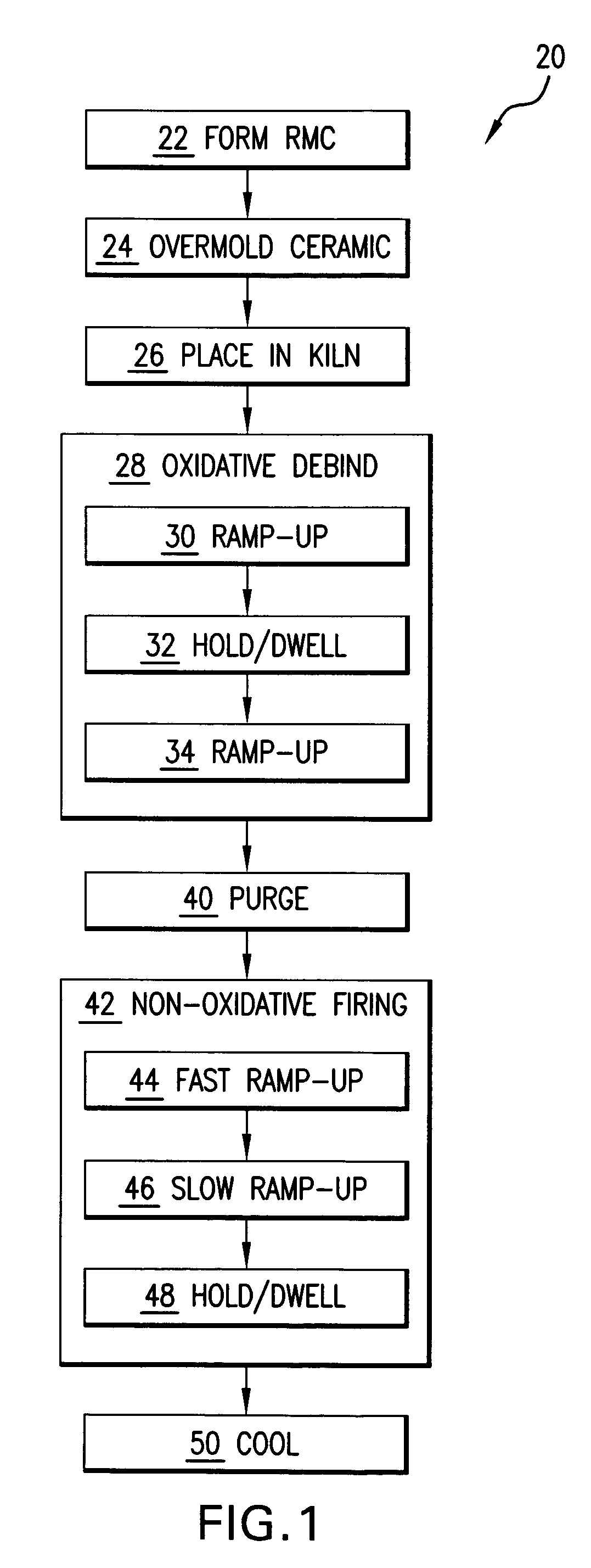
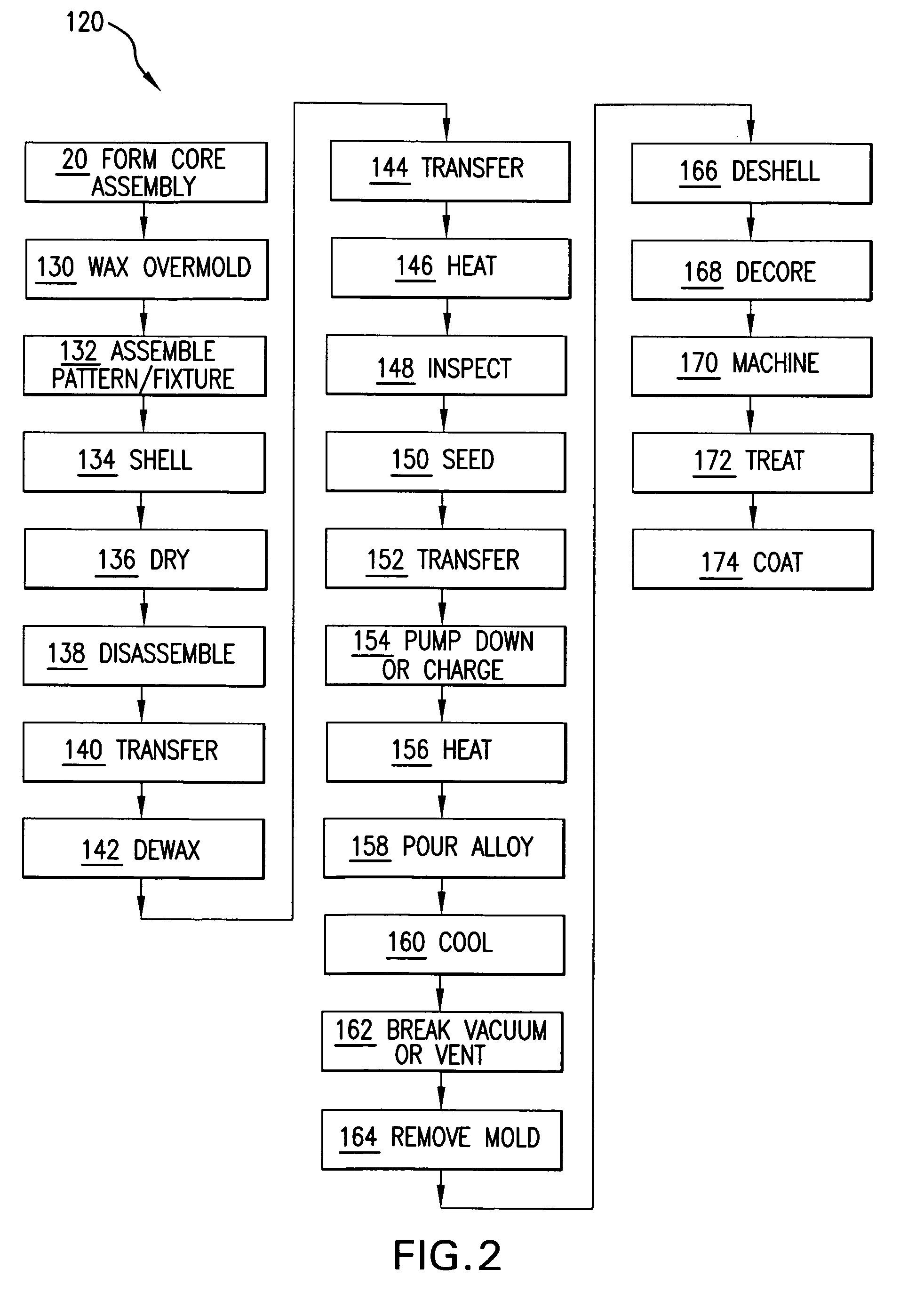
Method for firing a ceramic and refractory metal casting core
InactiveUS7861766B2Insufficient temperatureAvoid a lot of timeFoundry mouldsFoundry coresInvestment castingNon oxidative
In an investment casting process, a composite core is formed as a combination of ceramic casting core element and a non-ceramic casting core element. The core is heated in an oxidative atmosphere and then heated in a non-oxidative atmosphere.
Owner:RAYTHEON TECH CORP
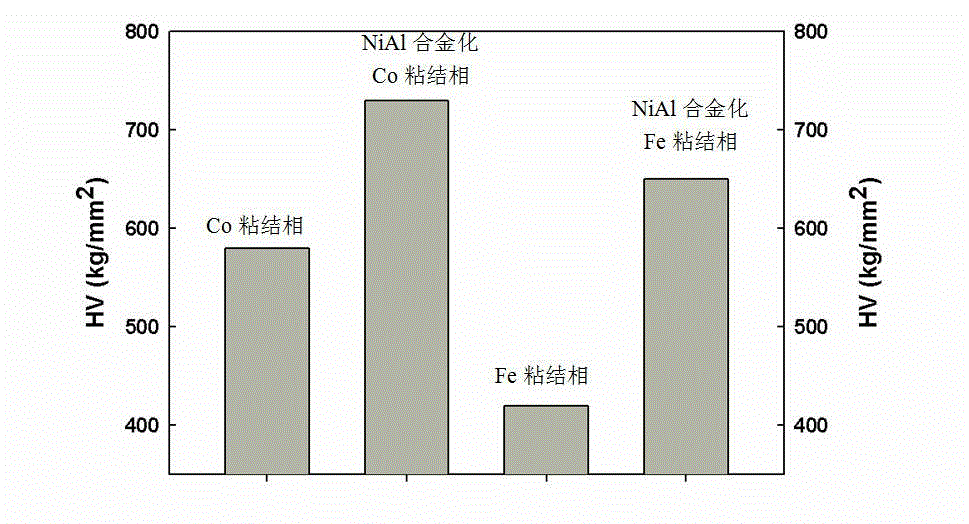
High-temperature-resistant and wear-resistant hard alloy and preparation method thereof
ActiveCN102978499AGuaranteed temperatureEnsure that the temperature of the powder does not exceed the temperatureHydrogen atmosphereWear resistant
The invention discloses a high-temperature-resistant and wear-resistant hard alloy with a NiAl alloying bonding phase. WC and / TiC is used as a hard phase, NiAl alloying Co and / Fe is used as a bonding phase, and the volume ratio of the hard phase to the bonding phase is equal to 10-40%. The preparation method comprises the following steps of: uniformly mixing 0.03-21.04wt% of nickel powder and aluminum powder with carbide powder based on the component proportion of Ni-50at.%Al; placing the mixture in a graphite container to spread out, heating the mixture to 660-1300 DEG C at a non-oxidative atmosphere, preserving heat, and then naturally cooling to obtain a mixture of a carbide and NiAl; grinding, crushing and screening to obtain mixed powder; carrying out deoxidation pretreatment at a hydrogen atmosphere of 400+ / -50 DEG C; carrying out wet grinding on 45.77-96.34wt% of mixed powder and the balance of Co and / Fe powder; carrying out spray-drying on the mixed material subjected to wet grinding and pressing; and sintering a compaction in a liquid phase at low pressure at the temperature of 1350-1550 DEG C to obtain the high-temperature-resistant and wear-resistant hard alloy.
Owner:ZHUZHOU HARD ALLOY GRP CO LTD
Process for preparing aromatics from methane
InactiveUS20120022310A1Extended service lifeShort transport distanceCatalystsHydrocarbon preparation catalystsPtru catalystNon oxidative
The present invention relates to a process for carrying out endothermic, heterogeneously catalyzed reactions in which the reaction of the starting materials is carried out in the presence of a mixture of inert heat transfer particles and catalyst particles, where the catalyst particles are regenerated in a nonoxidative atmosphere at regular intervals and the heat of reaction required is introduced by separating off the inert heat transfer particles, heating the heat transfer particles in a heating zone and recirculating the heated heat transfer particles to the reaction zone. The process of the invention is particularly suitable for the nonoxidative dehydroaromatization of C1-C4-aliphatics in the presence of zeolite-comprising catalysts.
Owner:BASF AG
Method for preparing fiber of preventing acarid and antibacterial cellulose
ActiveCN101050560AGood anti-mite effectReduce degradationBiocideFibre treatmentPolymer scienceCellulose fiber
The present invention discloses a preparation method of acarid-resisting anti-bacterial cellulose fiber. Said cellulose fiber is made up by adopting cellulose spinning dope containing acarid-resisting anti-bacterial agent through the processes of spinning, non-oxidative desulphurization and drying at 120 deg.C. The acarid-resisting anti-bacterial agent content in the spinning dope is 1-10%.
Owner:潍坊欣龙生物材料有限公司
Inoxidability permanent natural black hair-dyeing and preparing method thereof
InactiveCN101229110AEasy to acceptEasy to useCosmetic preparationsHair cosmeticsBlack paintNon oxidative
The invention relates to a non-oxidative permanent type natural melanoid hair water and a preparation method thereof. The melanoid hair water is prepared with natural materials and is formed by No. 1 hair dye and No. 2 hair dye. The No. 1 hair dye takes clove as an osmotic agent, takes cysteine as a reducing agent, takes nutgall, myrobalan and gallnut as the paints and takes angelica as a nutrition component; the No. 2 hair dye takes copperas as a visualization reagent and leads the copperas to be aggregated with the dye component of the No. 1 hair dye into a black paint giant molecule. The invention, on the basis of the application of a traditional Chinese medicine osmotic agent, also applies a natural reducing agent of the cysteine to cut off a hair keratin disulfide and increase the hair fasciola clearances, to lead the traditional Chinese medicine paint molecule to be easier to enter the hair fasciola clearances and lead the melanoid hair to be blacker and firmer; while the hair dye is easy to wash when contacting the scurf and can be used only by heating for one time. The invention is easyto be accepted by more consumers.
Owner:SHANGHAI JIAO TONG UNIV
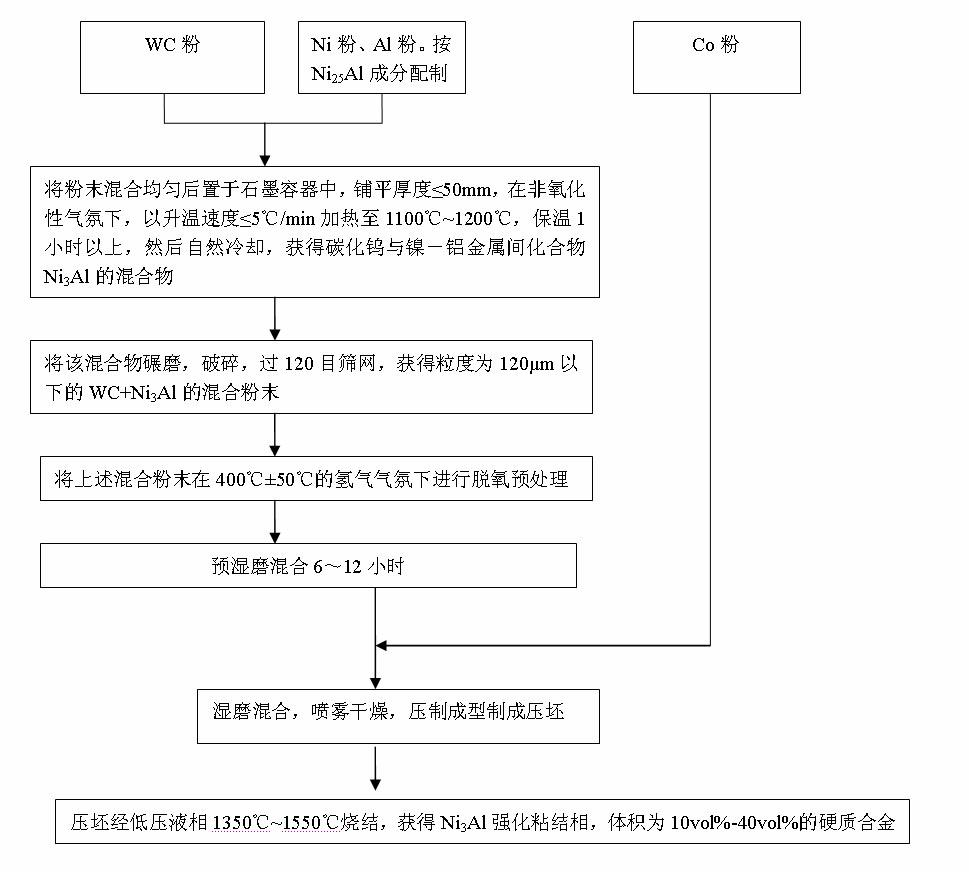
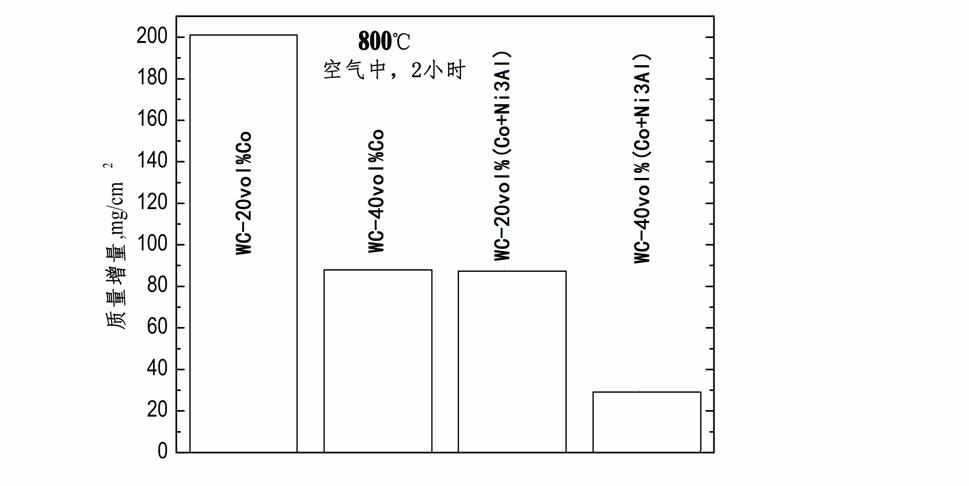
WC-Co hard alloy with binding phase enhanced by Ni3Al and preparation method thereof
The invention discloses a toughness-enhanced hard alloy with a Co binding phase enhanced by Ni3Al. The hard phase of the hard alloy is WC, and the binding phase is Co and Ni3Al of which the volume percent is 10-40%. The preparation method of the hard alloy comprises the following sequential steps of: uniformly mixing 2.07-16.05wt% of Ni powder and Al powder with WC powder according to a composition ratio of Ni25Al; placing the uniformly mixed powder in a graphite container at the thickness not more than 50 mm, heating to 1100-1200 DEG C in a non-oxidative atmosphere at a speed not more than 5DEG C / min, maintaining the temperature for more than 1 hour, and naturally cooling to obtain a mixture of WC and Ni3Al; grinding, pulverizing and screening to obtain mixed powder with a particle sizebelow 120 mu m; carrying out deoxidation pretreatment in 400+ / -50 DEG C hydrogen; carrying out pre-wet-grinding and mixing on 83.26-97.62wt% of mixed powder obtained after deoxidation pretreatment for 6-12 hours, then adding the balance of Co powder, and carrying out wet grinding for 18-36 hours; carrying out spray drying and pressure shaping on the wet-ground mixed material; and carrying out low-pressure liquid-phase sintering on the pressed blank at 1350-1550 DEG C to obtain the WC-Co hard alloy with the binding phase enhanced by Ni3Al. The hard alloy has the advantages that: a gamma' phaseis dispersed and distributed in the binding phase, the binding phase is uniformly distributed, and the alloy has high compactness, high strength, good wear resistance and excellent high-temperature oxidation resistance and corrosion resistance.
Owner:ZHUZHOU HARD ALLOY GRP CO LTD
Copper-containing nanoparticles and manufacturing method therefor
InactiveUS20110193034A1Efficient preparationGood dispersionGroup 1/11 organic compounds without C-metal linkagesPigmenting treatmentDecompositionCompound (substance)
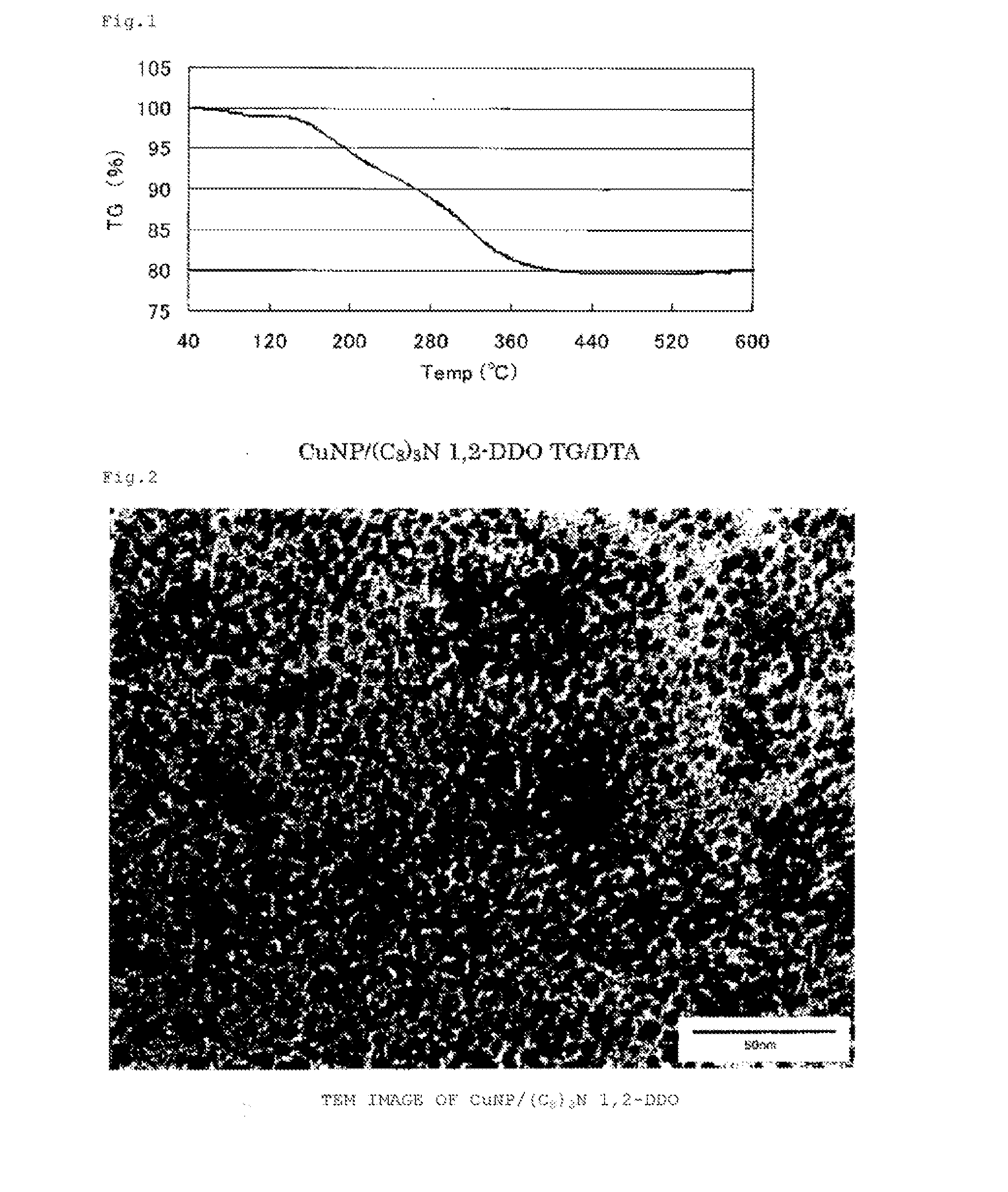
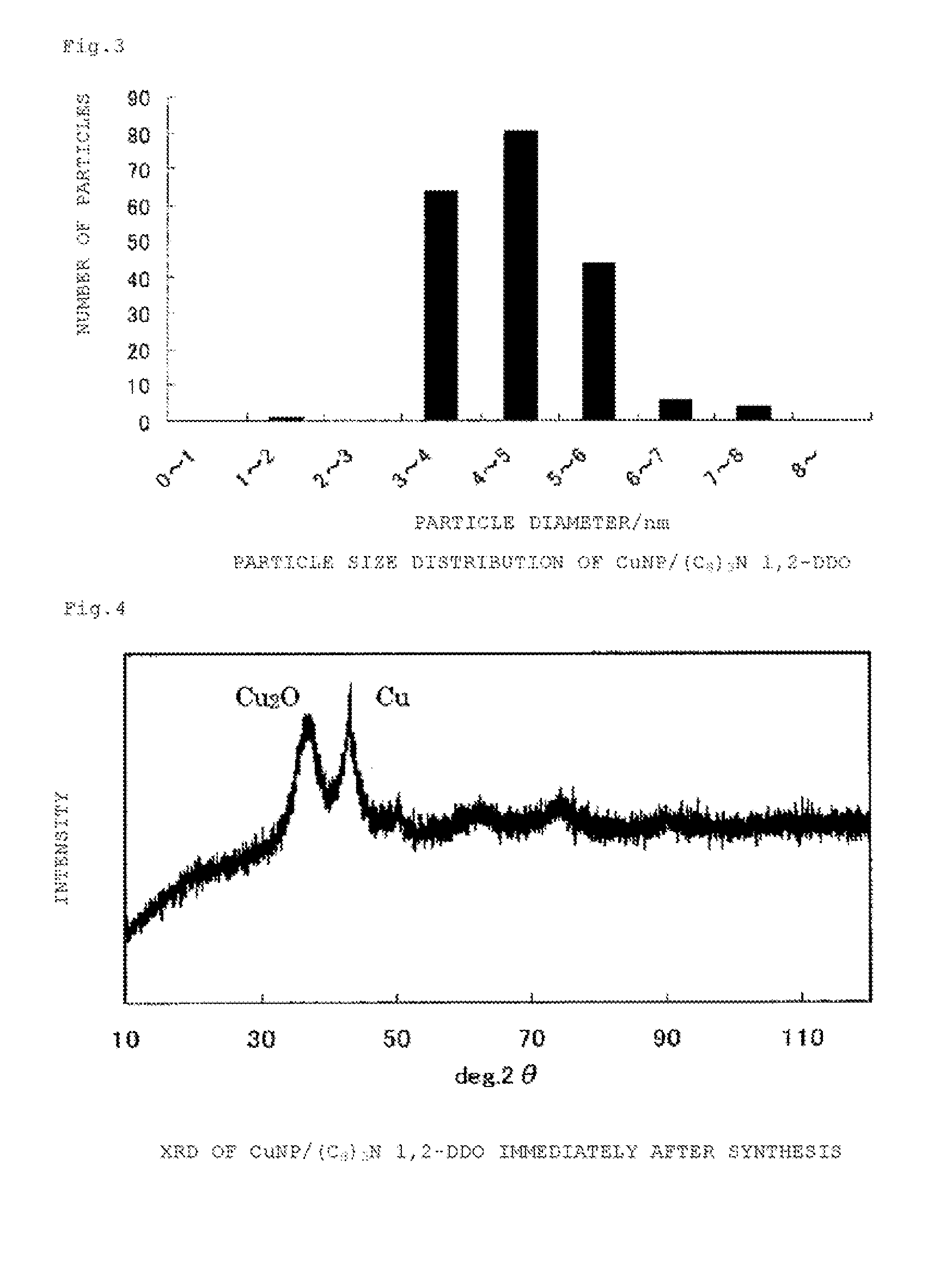
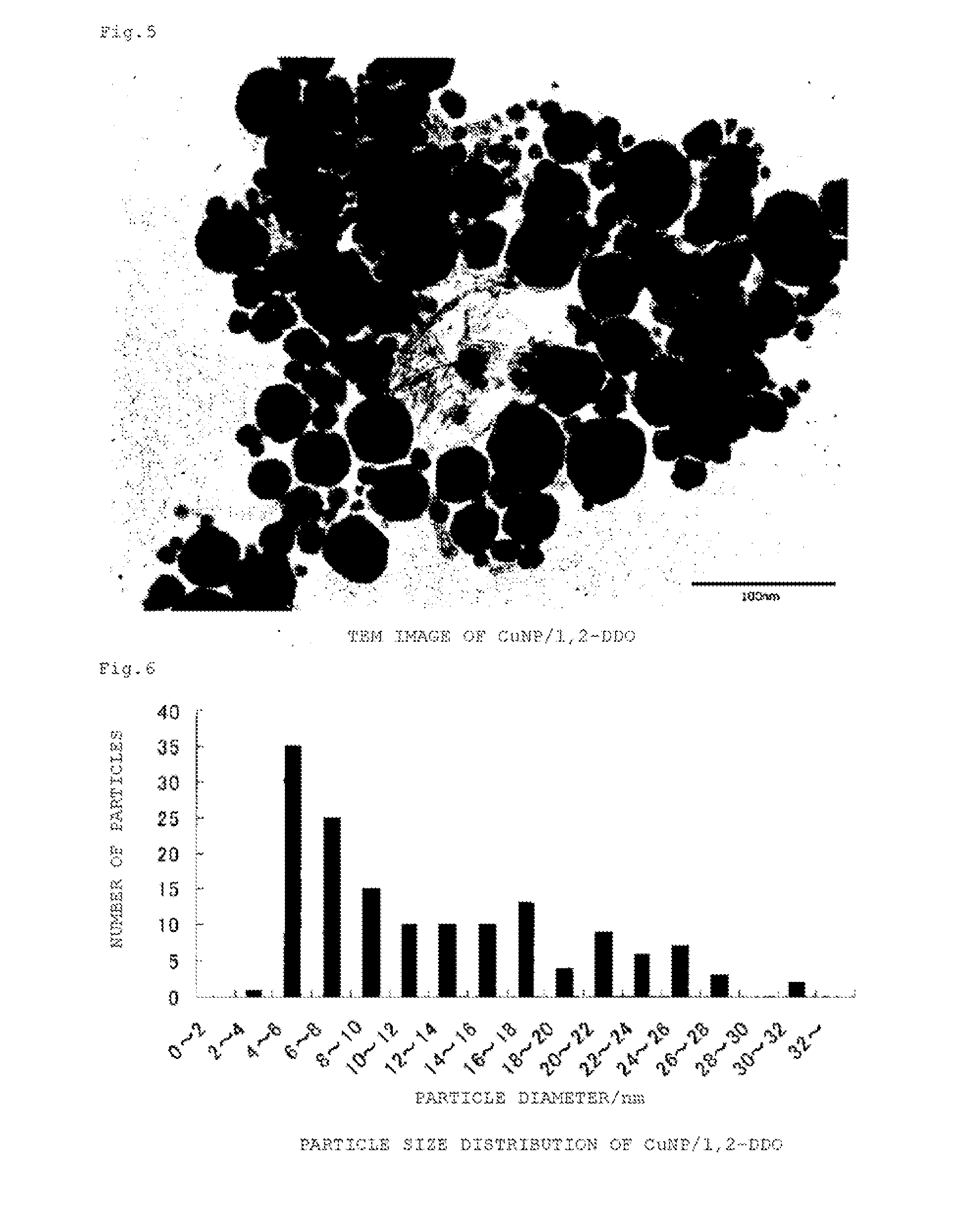
Copper-containing nanoparticles with excellent oxidation resistance is provided. The present invention relates to a method for manufacturing copper-containing nanoparticles including obtaining copper-containing nanoparticles that contain an organic component by heat treating an organic copper compound at a temperature equal to or higher than a decomposition initiation temperature of the compound and lower than a complete decomposition temperature of the compound in a non-oxidative atmosphere in the presence of an organic material containing a 1,2-alkanediol having 5 or more carbon atoms and / or a derivative thereof.
Owner:DAIKEN CHEM +1
Nanometer oxide ceramic purification and adsorption material with decomposition and bactericidal performance
InactiveCN103537255AImprove protectionKeep healthyBiocideOther chemical processesOxide ceramicHigh concentration
Owner:王泽辉
Modified non-metal impure nanometer TIO* photocatalyst and its preparing method
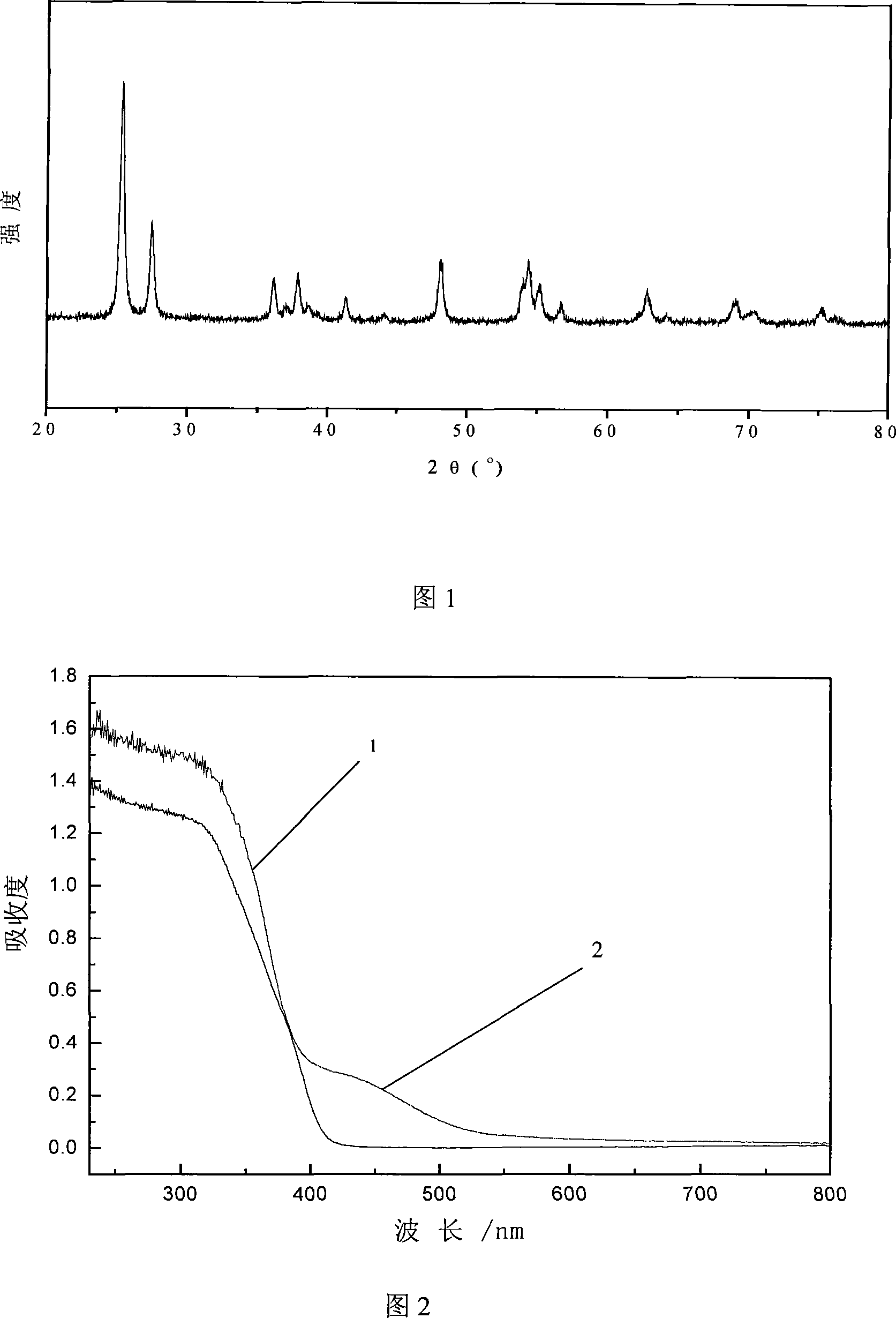
InactiveCN101157027AImprove photocatalytic performanceNo deactivation was foundMetal/metal-oxides/metal-hydroxide catalystsNon oxidativeOxygen
The invention relates to nonmetallic doped nanometer TiO2 photocatalyst through being modified and the preparation method thereof. The chemical formula of the photocatalyst of the invention is TiO2-XYX / WO3, TiO2-XYX / ZnO, or TiO2-XYX / Cu2O, the crystal grain size is 10 to 200 nm, crystal grains comprises anatase, or simultaneously comprises two crystal forms of anatase and rutile, the range of ultraviolet-visible absorption spectrum is of 230 to 600 nm, and Y is N, C, or S. The invention has the preparation method that the precursors TiN, TiC, or TiS2 of a non-metallic compound containing Ti are dipped in the solution of a compound of W, Zn, or Cu containing certain concentration, and are dried with the normal pressure or the reduced pressure, the obtained powder is calcined under the aerobic or non-oxidative condition, the calcination temperature is at 200 to 900 DEG C, and the reaction time needs 0.1 to 10 hours.
Owner:ZHEJIANG UNIV +1
Method for concentrating precious metals contained in leaching residue discharged from copper hydrometallurgical process
A method for concentrating precious metals contained in the leaching residue discharged from a copper hydrometallurgical process by removing pyrite from the residue. A method for concentrating precious metals in the leaching residue containing pyrite, elementary sulfur, precious metals and gangue, discharged from a copper hydrometallurgical process which comprises steps of leaching in an acidic, aqueous solution, reducing the copper-containing leaching liquor and electrolysis for copper recovery to treat copper sulfide ores, comprising (1) pyrolysis step in which the leaching residue is thermally treated at 550° C. or higher in a non-oxidative atmosphere, to produce the calcined ore containing pyrrhotite, precious metals and gangue, and (2) a re-leaching step in which the calcined ore is re-leached in an acidic, aqueous solution, to be separated into the re-leaching residue and iron-leached liquor, the former containing elementary sulfur, precious metals and gangue.
Owner:SUMITOMO METAL MINING CO LTD
Carbon-silicon composite material and preparation method and application thereof
ActiveCN107204438ASimple preparation processReduce energy consumptionElectrode thermal treatmentSecondary cellsNon oxidativeSolvent
The invention provides a carbon-silicon composite material and a preparation method and application thereof. The preparation method includes following steps: dispersing a silicon nanomaterial, a carbon nanomaterial and a binder into a solvent to form size, and preparing to obtain carbon-silicon composite macro body; thermally treating the obtained carbon-silicon composite macro body in a non-oxidative atmosphere to obtain the carbon-silicon composite material. The carbon-silicon composite material has a covalent carbon grid structure, and the silicon nanomaterial is embedded in the covalent carbon grid structure, so that the carbon-silicon composite material integrates advantages of the covalent carbon grid structure composed of the silicon nanomaterial, carbon converted by part of the binder and the carbon nanomaterial, mechanical performance and electron transmission stability of the composite material are improved, and the composite material presents extremely excellent charging-discharging specific capacity and circulating stability when serving as a lithium ion battery electrode and has good industrial application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Selective liquid-liquid extraction of oxidative desulfurization reaction products
InactiveUS20130075305A1Minimizes co-extractionReduce complexityRefining with oxygen compoundsTreatment with plural serial refining stagesCo extractionSulfone
The present invention provides selective extraction of sulfoxides, or sulfoxides in combination with sulfones, from hydrocarbon mixtures containing these compounds. A significant advantage of the invention is that oxidation products resulting from oxidative desulfurization of hydrocarbon feedstocks are selectively extracted with minimum co-extraction of non-oxidized products such as valuable hydrocarbon fuel components.
Owner:SAUDI ARABIAN OIL CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com