Method for preparing carbon-coating ferric phosphate lithium
A technology of carbon-coated lithium iron phosphate and carbon source, which is applied in electrode manufacturing and other directions to achieve the effects of improving electrochemical performance, increasing discharge specific capacity and low material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) Add 0.1mol / L phosphoric acid solution to 0.1mol reduced iron powder under nitrogen atmosphere, mix under stirring, control the temperature at 70°C, react for 5 hours, then add 0.1mol / L LiOH solution dropwise to react 4 hours, the control temperature is 90°C, and the above reaction product is spray-dried by a high-speed centrifugal spray dryer to obtain the LiFePO4 / C precursor;
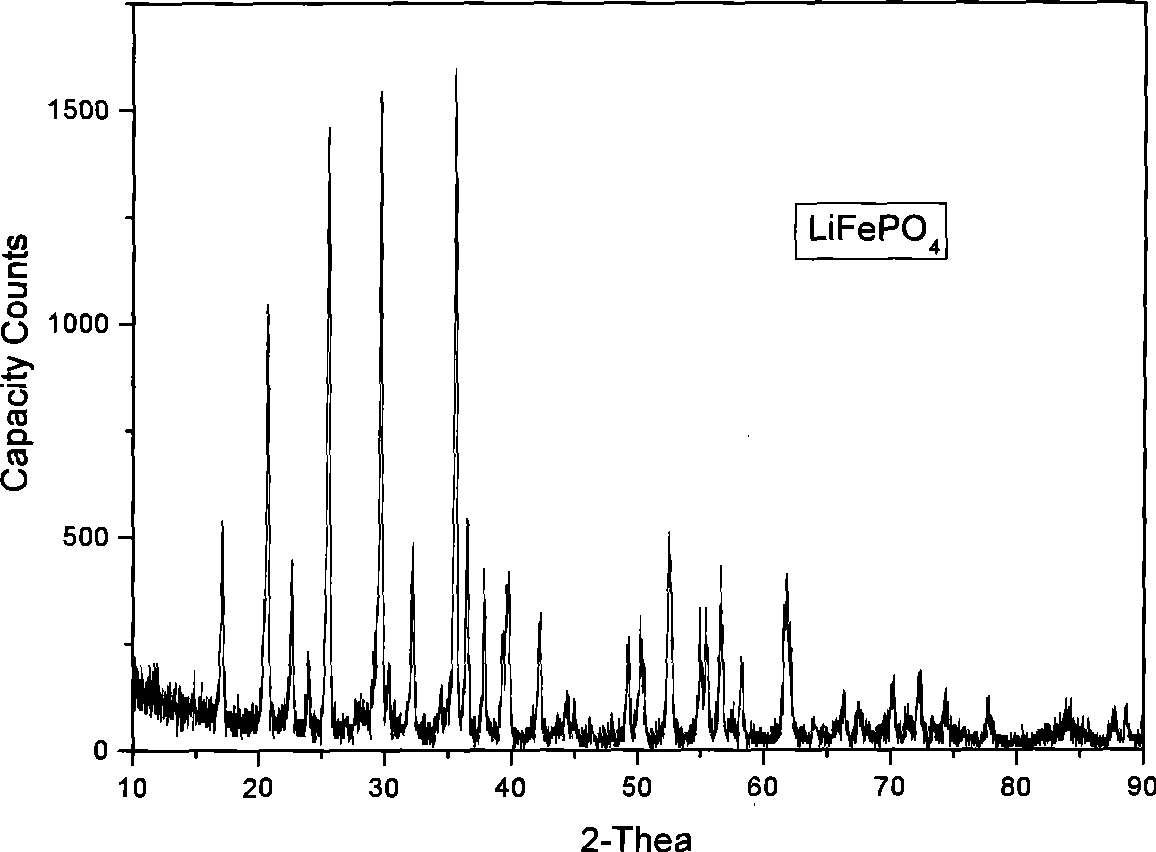
[0020] (2) In an inert or non-oxidizing atmosphere, the LiFePO4 / C precursor was transferred to a tube furnace and treated at 200 °C for 3 hours, then at 700 °C for 5 hours to obtain lithium iron phosphate. figure 1 The prepared lithium iron phosphate material (LiFePO 4 ) of the XRD spectrum, showing that the synthesized lithium iron phosphate has a good crystal structure.
Embodiment 2
[0022] (1) Add 0.1mol / L phosphoric acid solution to 0.1mol / L phosphoric acid solution under nitrogen atmosphere, add 0.1mol reduced iron powder and 0.004mol glucose, mix under stirring, control the temperature at 70°C, react for 5 hours, then add 0.1mol / L phosphoric acid dropwise LiOH solution was reacted for 4 hours, and the temperature was controlled to be 90° C., and the above reaction product was spray-dried by a high-speed centrifugal spray dryer to obtain the LiFePO4 / C precursor;
[0023] (2) In an inert or non-oxidizing atmosphere, the LiFePO4 / C precursor was transferred to a tube furnace and treated at 200 °C for 3 hours, and then at 700 °C for 5 hours to obtain coated carbonic lithium iron phosphate.
Embodiment 3
[0025] (1) Add 0.1mol / L phosphoric acid solution to 0.1mol / L phosphoric acid solution under nitrogen atmosphere, add 0.1mol reduced iron powder and 0.004mol ascorbic acid, mix under stirring, control the temperature at 70°C, react for 5 hours, then add 0.1mol / L phosphoric acid dropwise LiOH solution was reacted for 4 hours, and the temperature was controlled to be 90° C., and the above reaction product was spray-dried by a high-speed centrifugal spray dryer to obtain the LiFePO4 / C precursor;
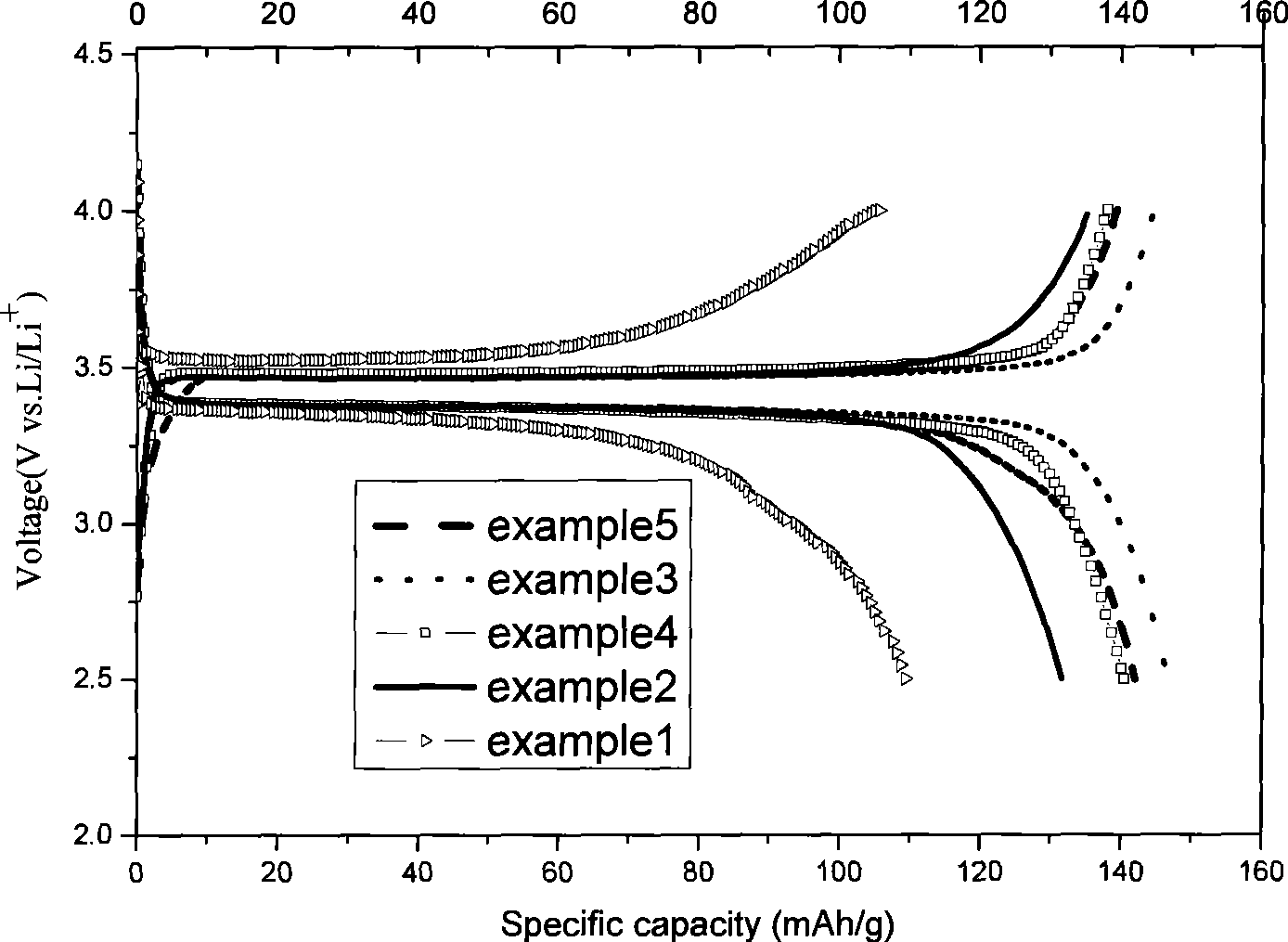
[0026] (2) In an inert or non-oxidizing atmosphere, the LiFePO4 / C precursor was transferred to a tube furnace and treated at 200 °C for 3 hours, and then at 700 °C for 5 hours to obtain coated lithium iron phosphate. figure 2 It is a TEM photo of the material, indicating that the surface of lithium iron phosphate is covered with a loose carbon layer. from image 3It can be seen from the first charge and discharge curve of the material that the first discharge capacity of the material a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com