Preparation method of carbon-coated lithium titanate
A carbon-coated lithium titanate and titanium source technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of reduced material gram capacity, low electronic conductivity, low energy density, etc., to improve high-rate performance and High temperature performance, simple and practical preparation process, wide range of raw material sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Weigh 1mol of TiO 2 and 0.8 mol of LiCl, dispersed in 200 mL of distilled water; add 12 g of capric acid, heat to 60 ° C and disperse with vigorous stirring for 5 hours to obtain a uniform milky white thick suspension; transfer the suspension to a sealable reaction and heated to 220°C in a container, and allowed to react at a constant temperature of 220°C for 12 hours; after the reaction was completed, the reaction product was cooled to room temperature, washed repeatedly with a mixture of alcohol and water, centrifuged, and dried to collect the precursor; And, the collected precursor was transferred to an atmosphere furnace filled with nitrogen, baked at 800° C. for 10 hours, and collected after cooling to obtain carbon-coated lithium titanate.
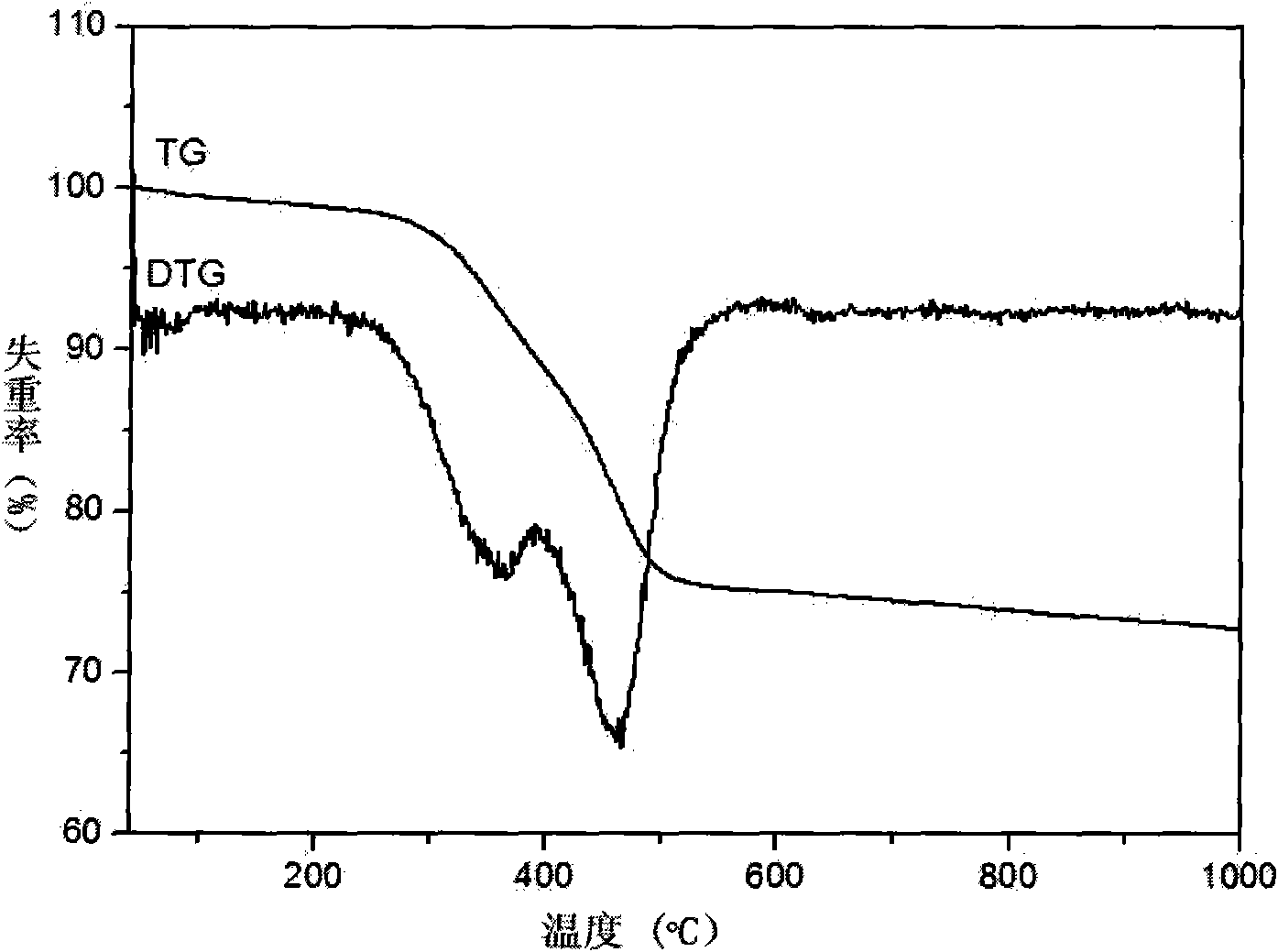
[0032] figure 1 It is the thermogravimetric curve (TG) figure of precursor in the embodiment 1 of the present invention, from figure 1 It can be seen that the coating amount of carbon in Example 1 is about 25%....
Embodiment 2
[0033] Embodiment 2: Weigh 1mol of TiO 2 and 0.9 mol of LiNO 3 , dispersed in 200mL of deionized water; add 1.2g capric acid, heat to 100°C and disperse vigorously for 1 hour to obtain a uniform milky white thick suspension; transfer the suspension into a closeable reactor and Heating to 80°C, allowing it to react at a constant temperature of 80°C for 48 hours; after the reaction is completed, the reaction product is cooled to room temperature, washed repeatedly with a mixed solution of alcohol and water, centrifuged, and dried to collect the precursor; and, the The collected precursors were transferred to an atmosphere furnace filled with argon, baked at 600°C for 24 hours, and collected after cooling to obtain carbon-coated lithium titanate.
Embodiment 3
[0034] Embodiment 3: Weigh 1mol of TiO 2and 1.0mol of LiF, dispersed in 200mL of water; add 6g of nonanoic acid, heat to 80°C and disperse vigorously for 2 hours to obtain a uniform milky white thick suspension; transfer the suspension to a closed reactor and heating to 350°C, allowing it to react at a constant temperature of 350°C for 3 hours; after the reaction is completed, the reaction product is cooled to room temperature, washed repeatedly with a mixed solution of alcohol and water, centrifuged, and dried to collect the precursor; and , the collected precursor was transferred to an atmosphere furnace filled with argon and nitrogen gas mixture, baked at 950°C for 5 hours, and collected after cooling to obtain carbon-coated lithium titanate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Graininess | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com