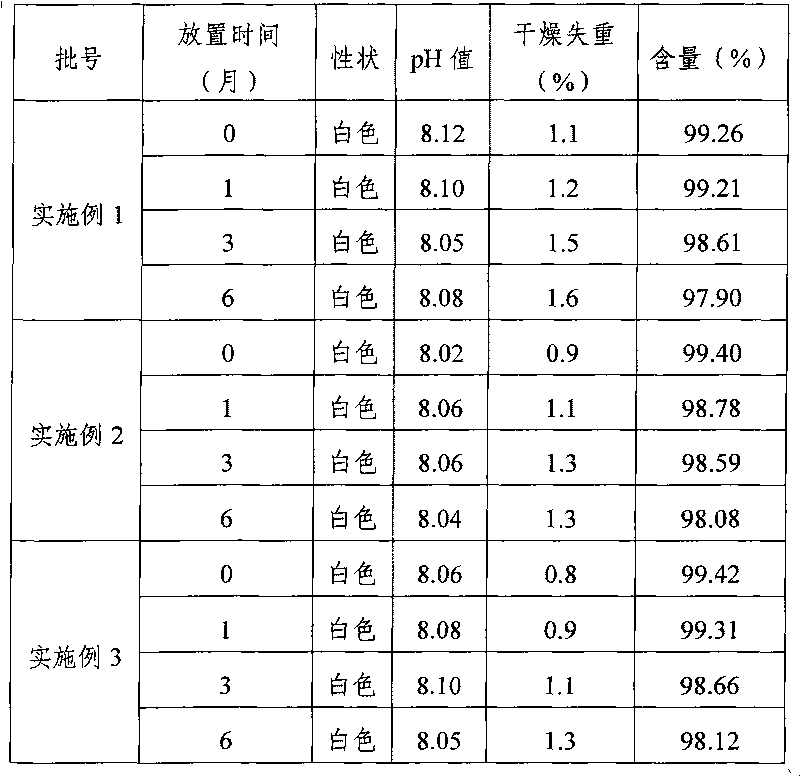
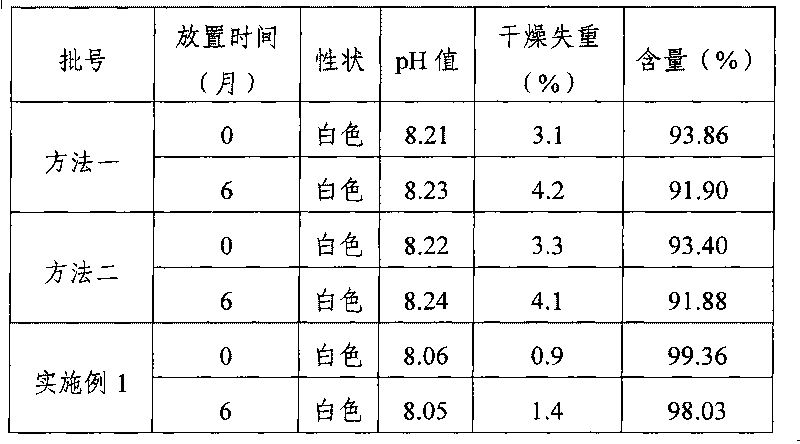
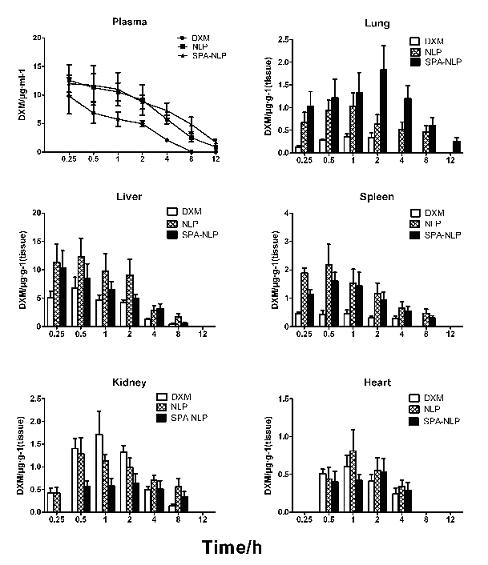
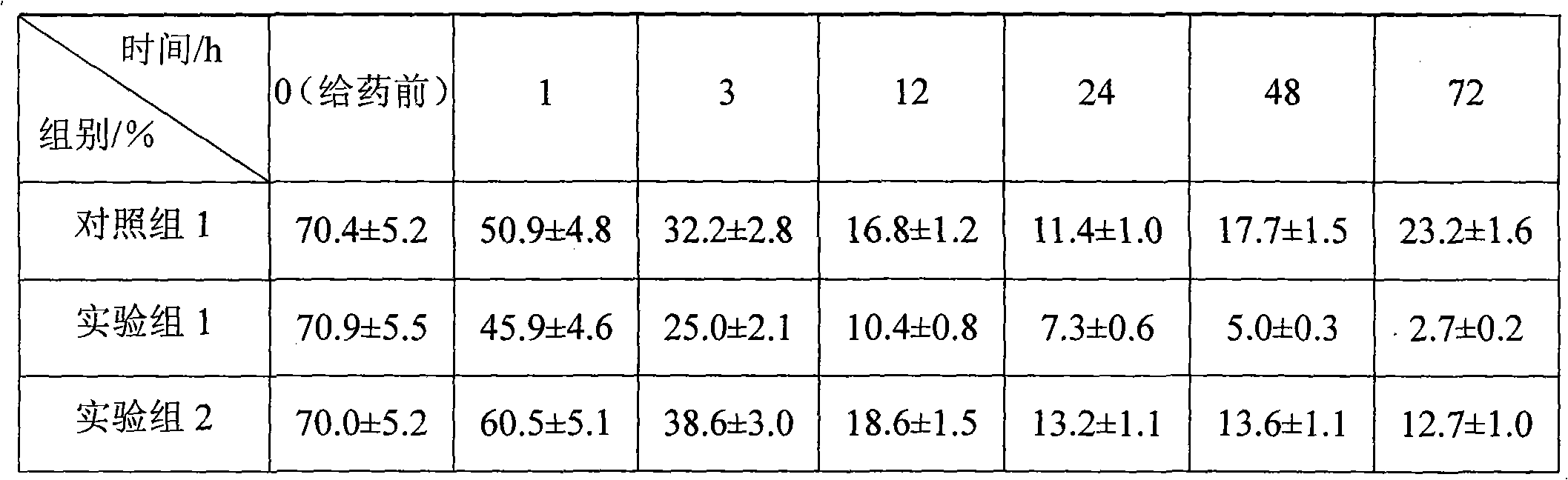
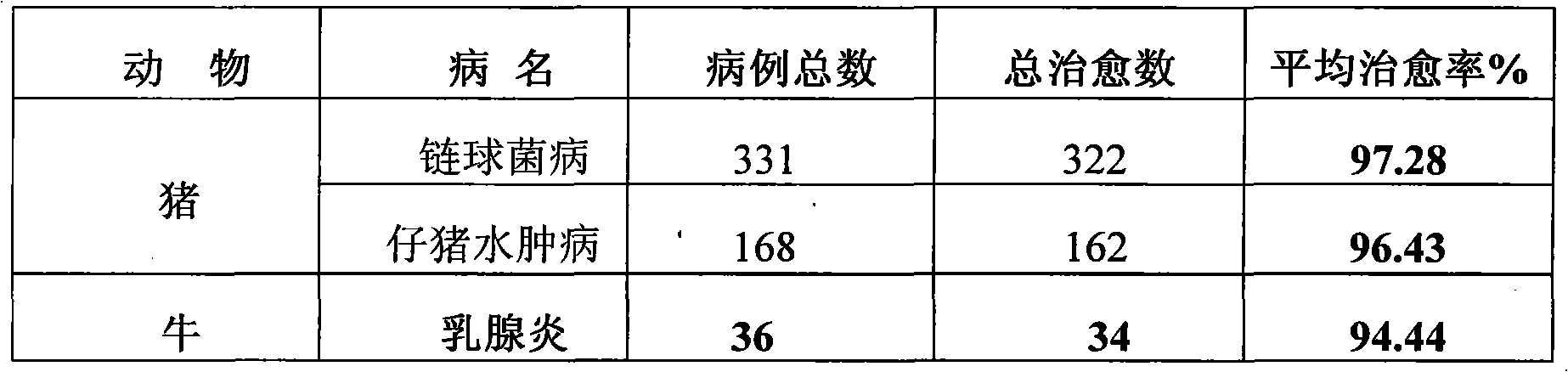
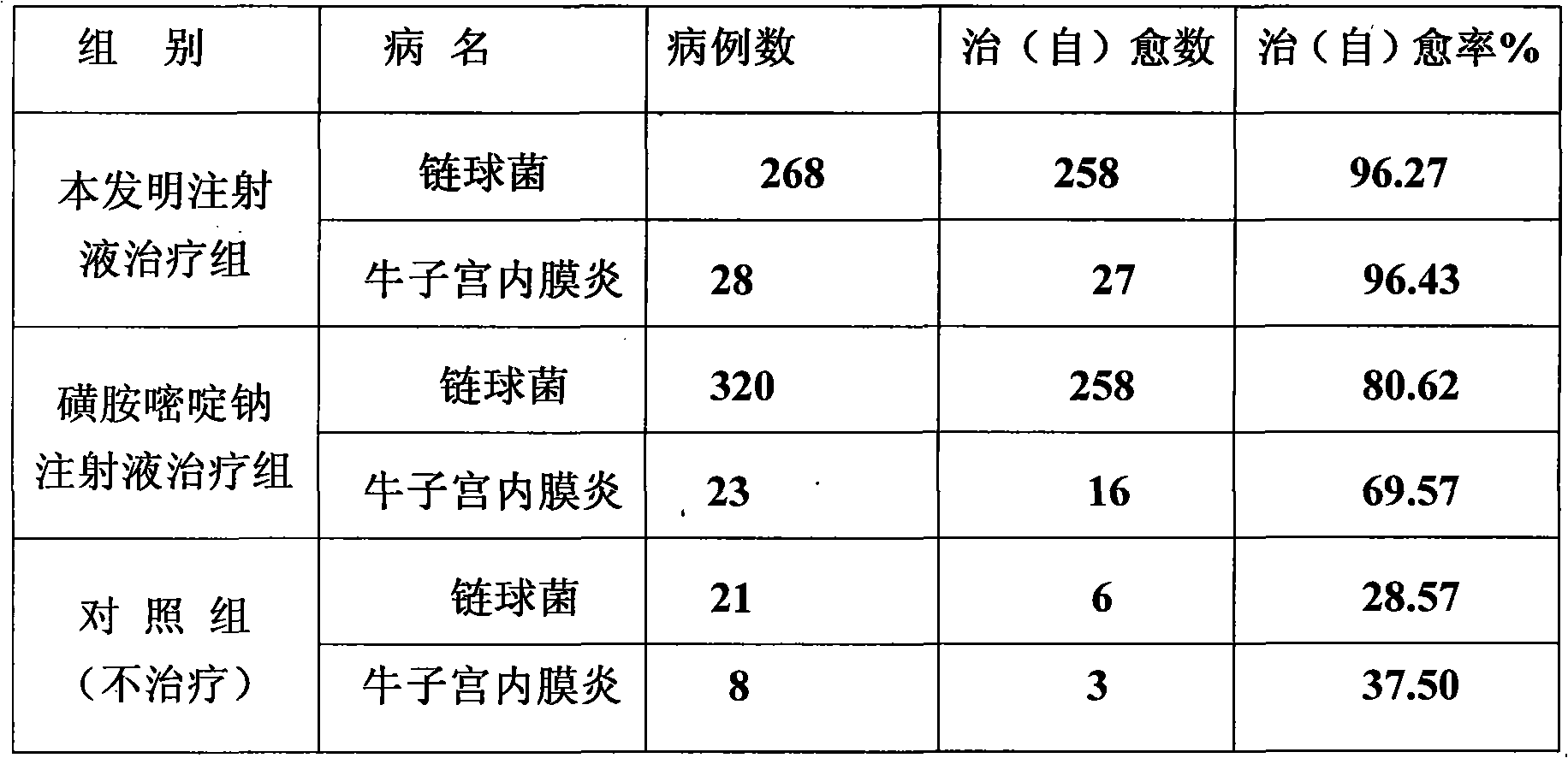
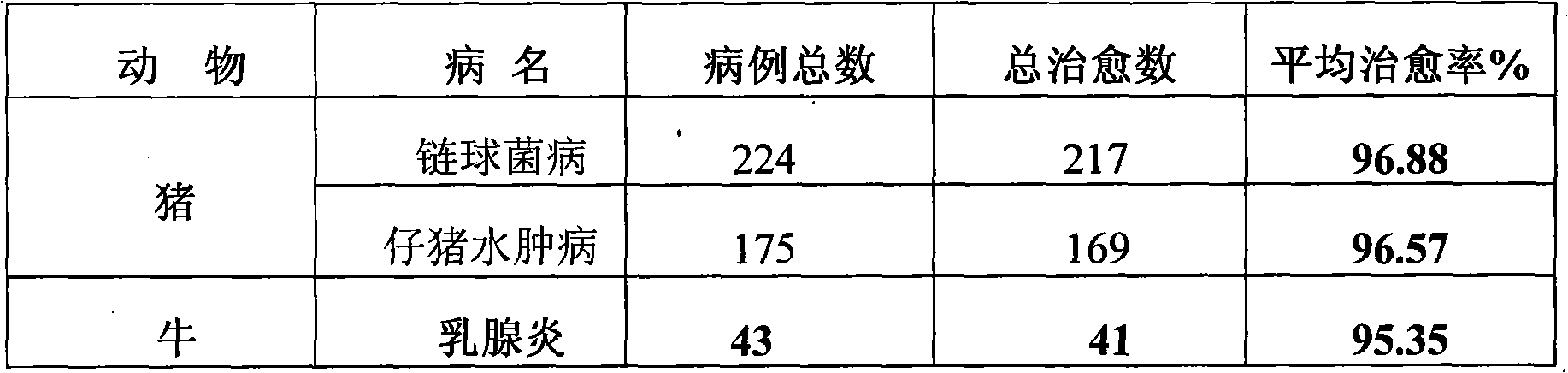
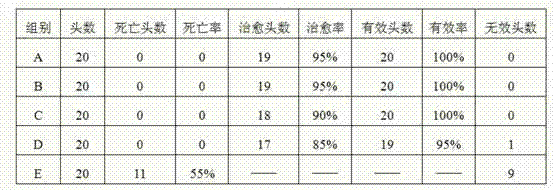
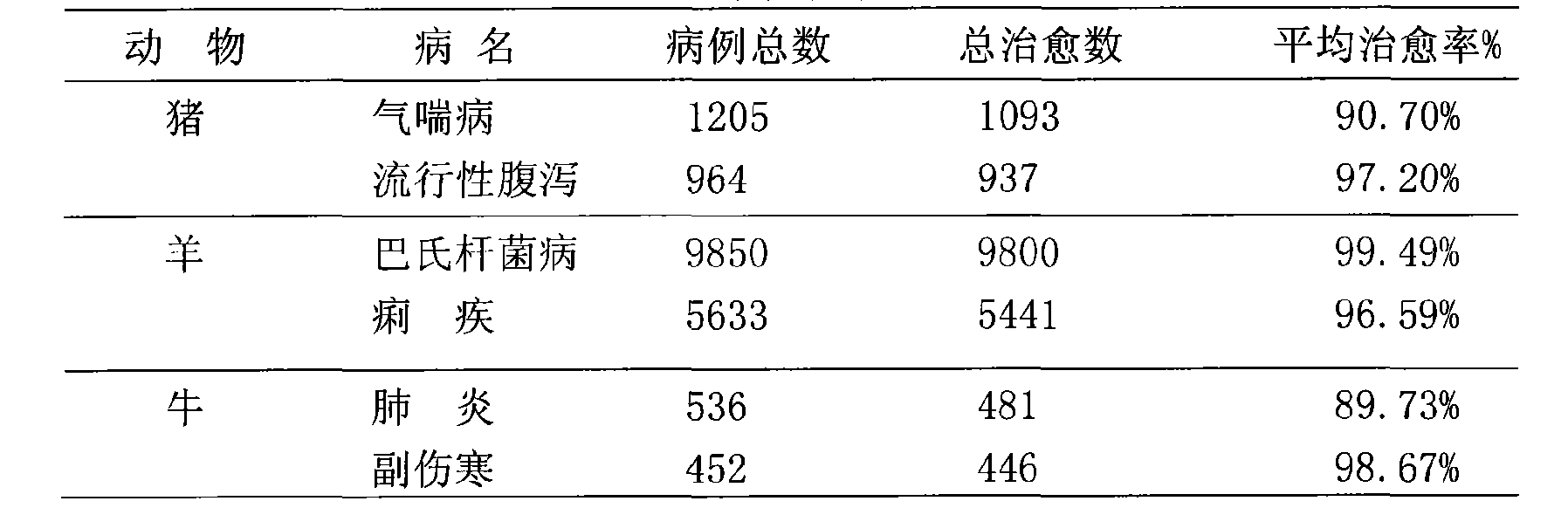
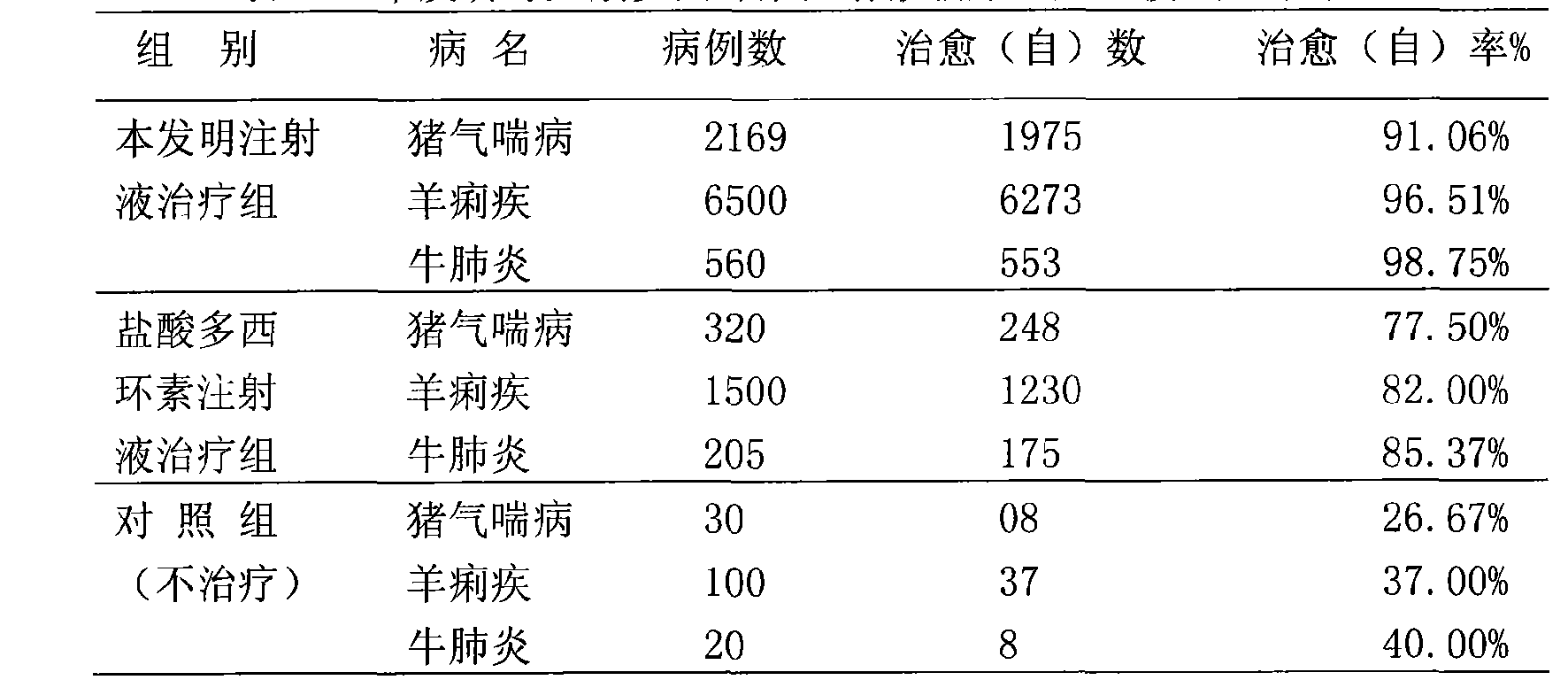
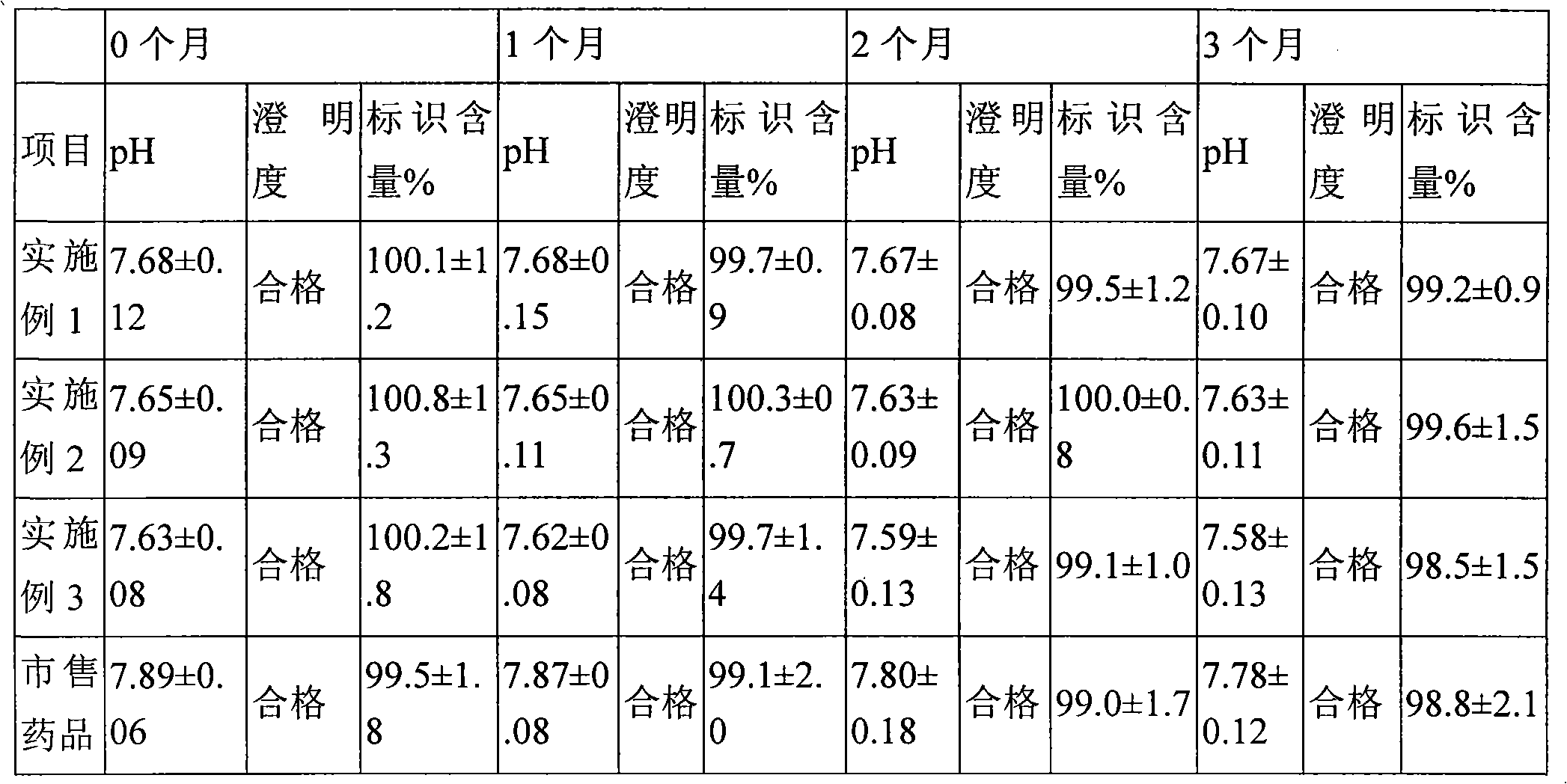
Patents
Literature
125 results about "Dexamethasone Sodium Phosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
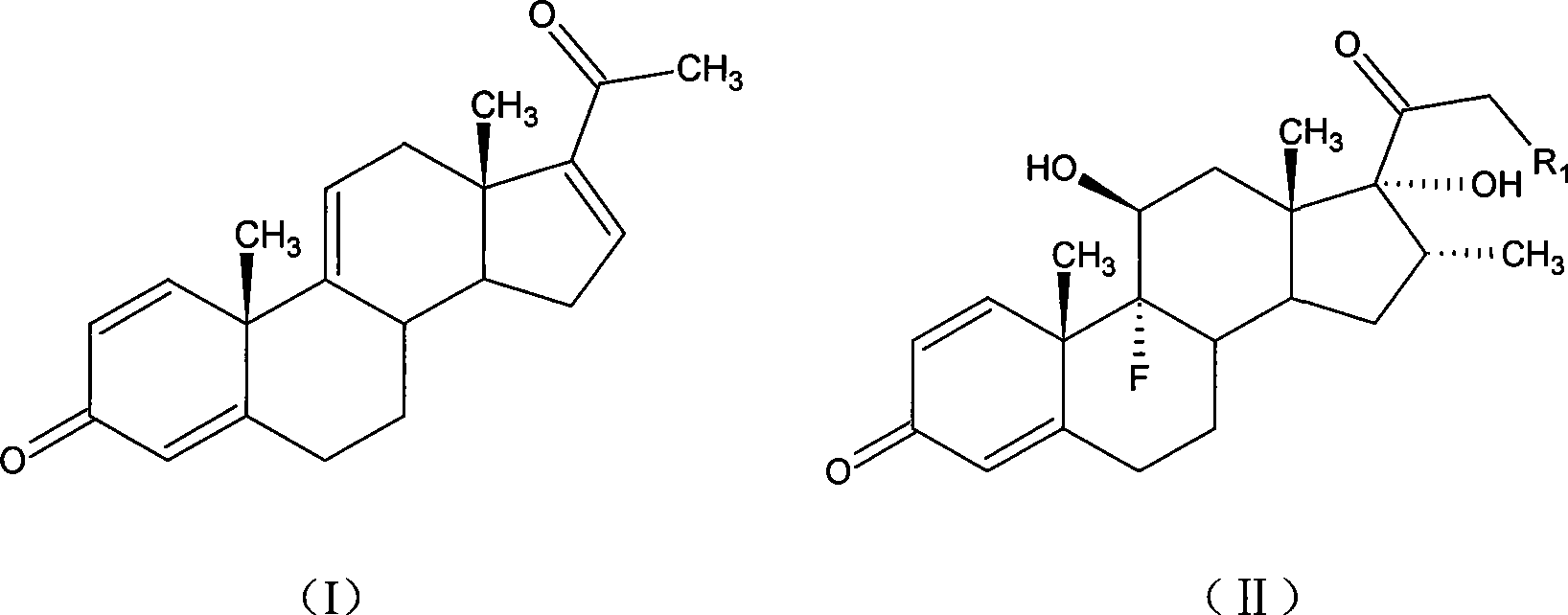
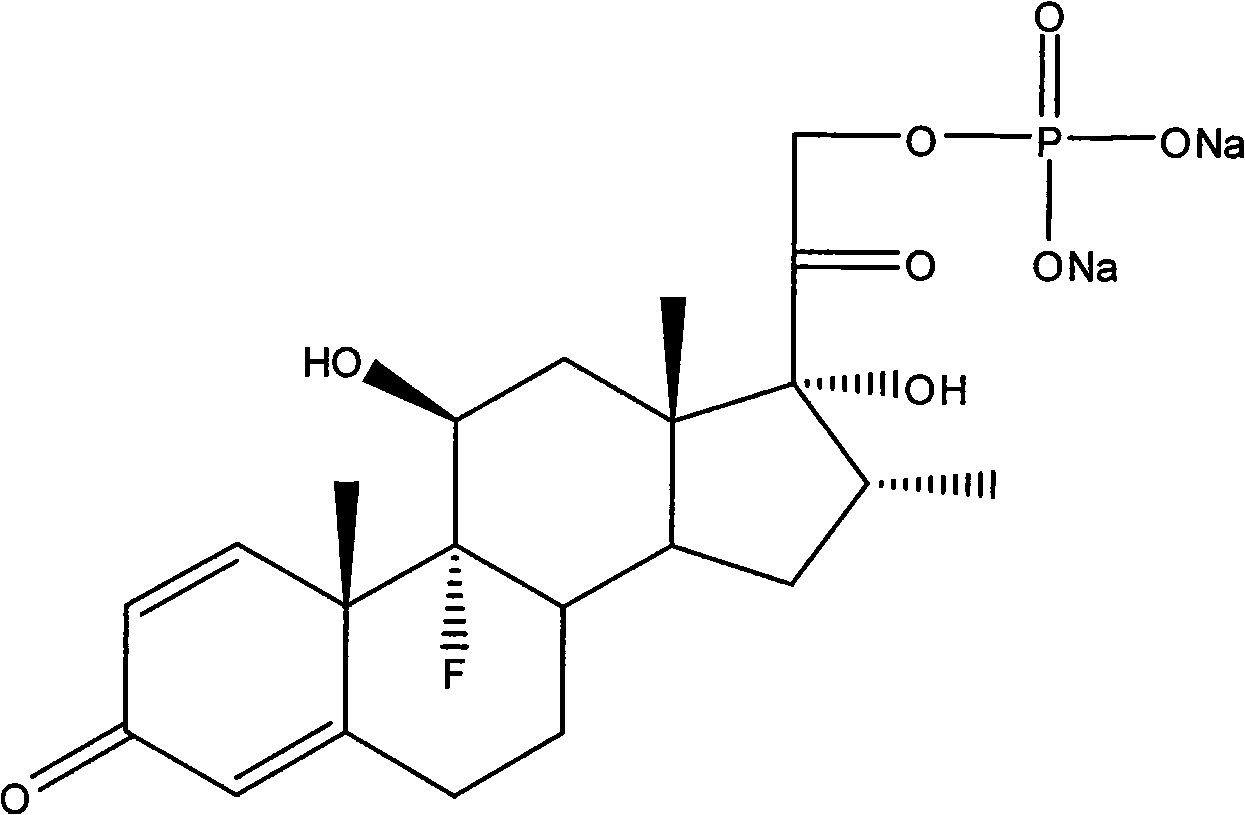
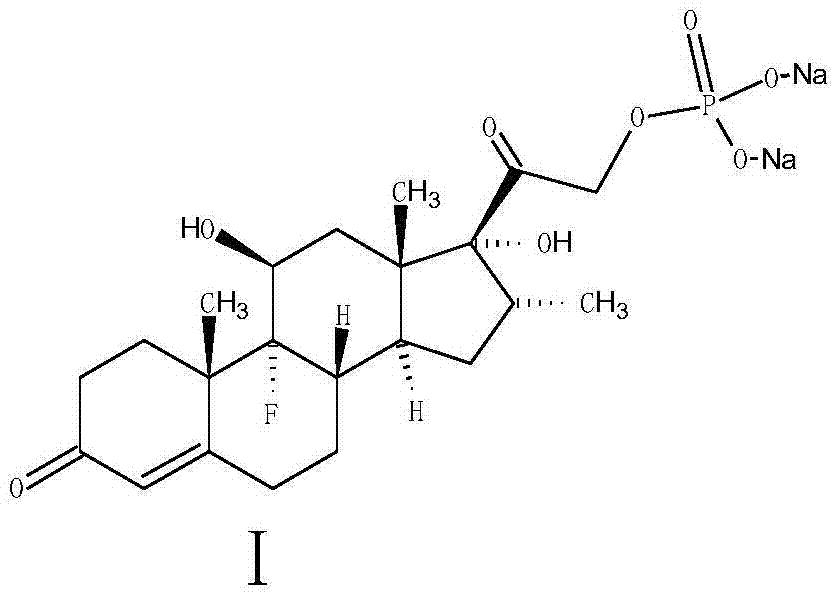
A sodium phosphate salt form of Dexamethasone, a synthetic adrenal corticosteroid with potent anti-inflammatory properties. In addition to binding to specific nuclear steroid receptors, dexamethasone also interferes with NF-kB activation and apoptotic pathways. This agent lacks the salt-retaining properties of other related adrenal hormones. (NCI04)
Method for preparing dexamethasone and series products thereof
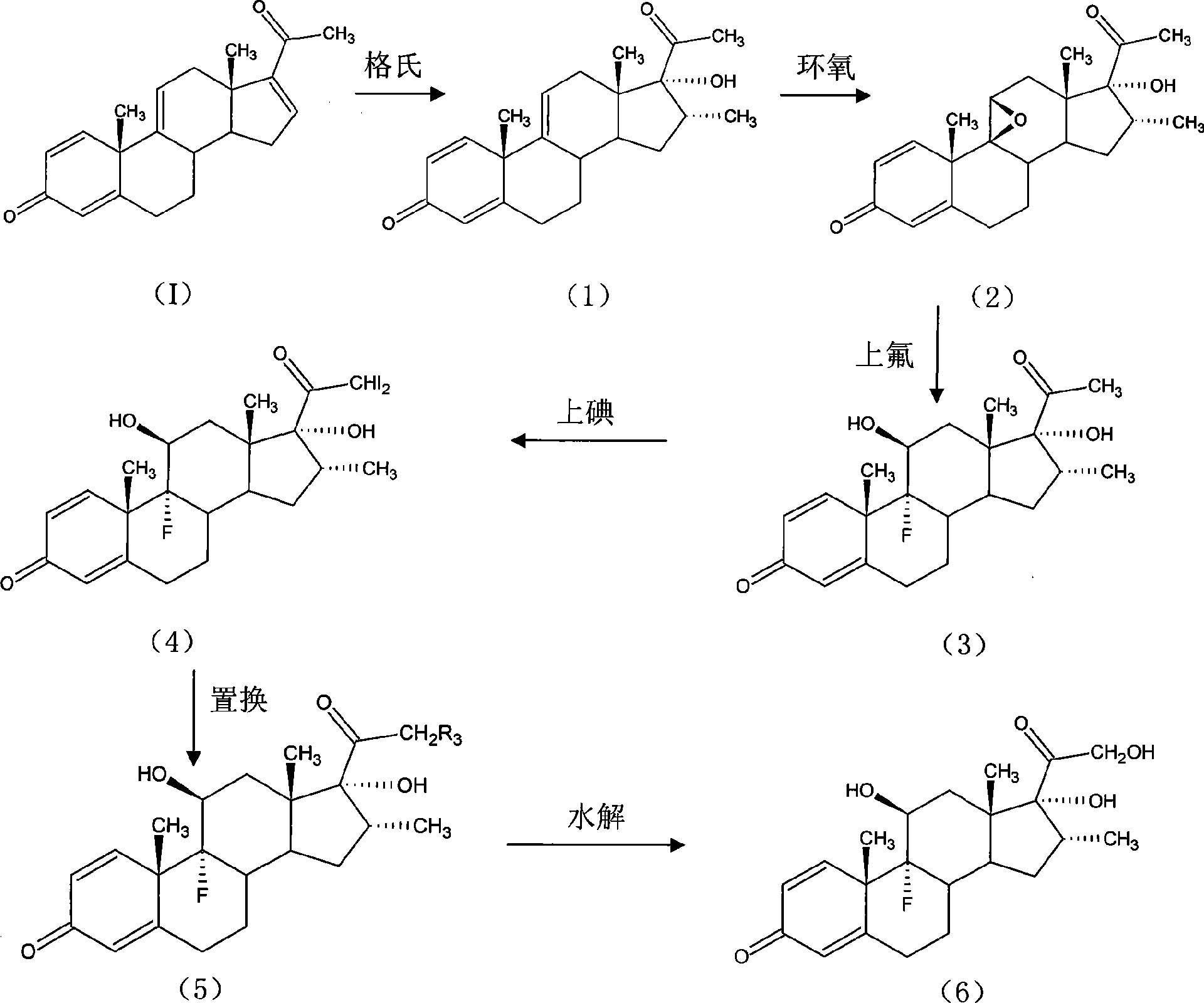
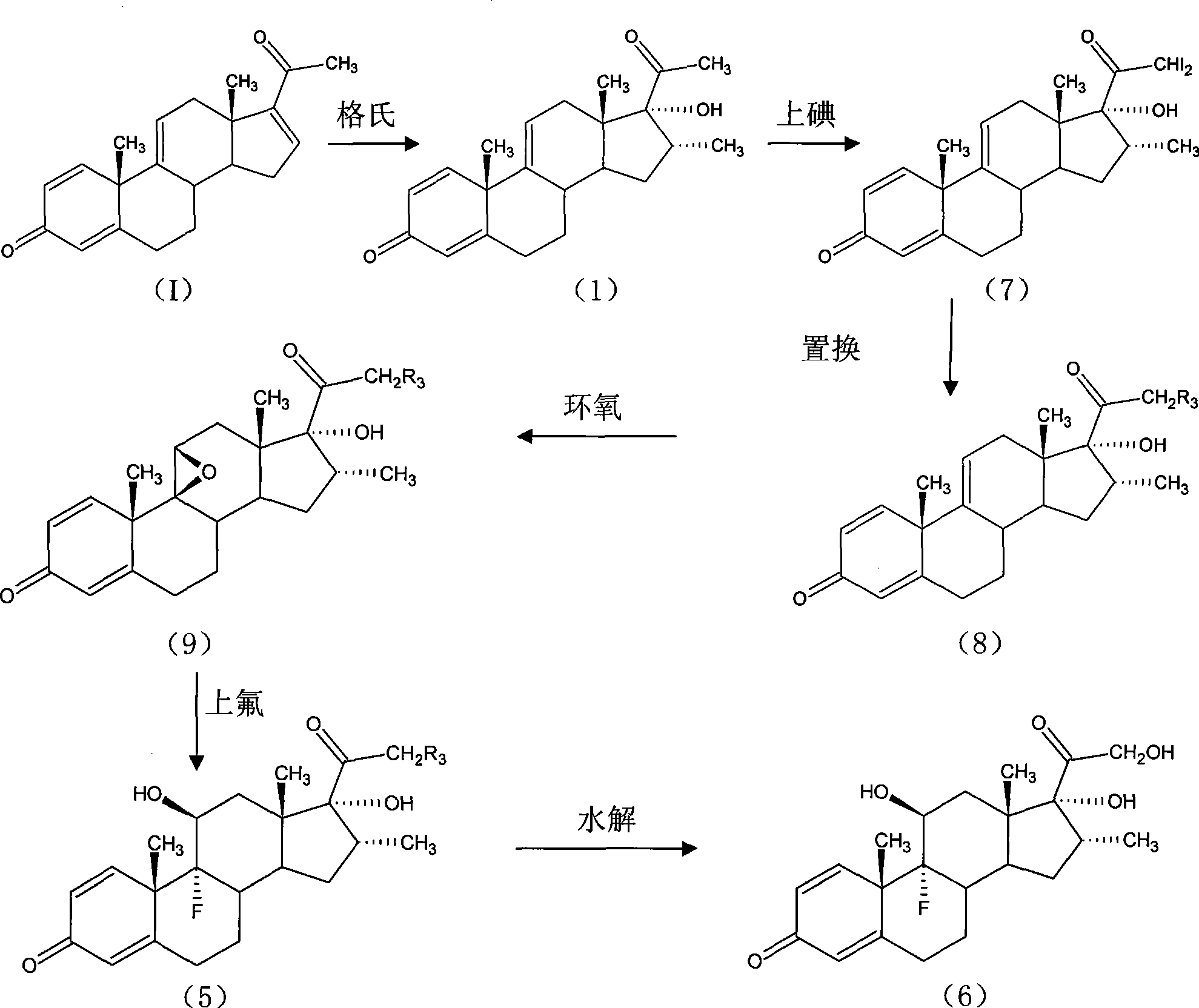
The invention provides a completely new process route for synthesizing dexamethasone and series products thereof. The invention adopts 1, 4, 9, 16-tetraene-pregna-3, 20-diketone as the original material which is modified by 9, 11bits, 16, 17 bits and 21 bits so as to obtain the dexamethasone and the series products thereof such as dexamethasone acetate and dexamethasone sodium phosphate and the like. The process has the advantage that the invention adopts the existing intermediates of manufacturers as the original material; the route is simple; the materials are available; the use of expensive accessories is avoided; the yield and the cost are dramatically better than that of the prior methods used for synthesizing the dexamethasone and derivatives thereof; moreover, the adoption of the existing intermediates realizes the combined-line prodction of the betamethasone series products and the dexamethasone series products, thus greatly reducing the manufacturing cost and the industrial manufacturing condition.
Owner:TIANJIN TIANYAO PHARM CO LTD
Medicine for preventing and treating acute altitude stress
InactiveCN104721202APrevention and treatment of acute hypertensive reactionsAntinoxious agentsHeterocyclic compound active ingredientsDiazepamMedicine
The invention discloses a medicine for preventing and treating acute altitude stress. The medicine comprises the following raw materials in percentage by mass: 0.23-1.15 percent of dexamethasone sodium phosphate, 1.14-5.68 percent of diazepam, 45.45-48.41 percent of aminophylline and a pharmaceutically acceptable excipient. Since capsules for treating acute altitude stress comprise 0.23-1.15 percent of dexamethasone sodium phosphate, 1.14-5.68 percent of diazepam and 45.45-48.41 percent of aminophylline, acute altitude stress can be treated effectively, the effective rate of treatment can be above 87%, and the maximum effective rate of treatment is up to 92.5%.
Owner:中国人民解放军西藏军区总医院
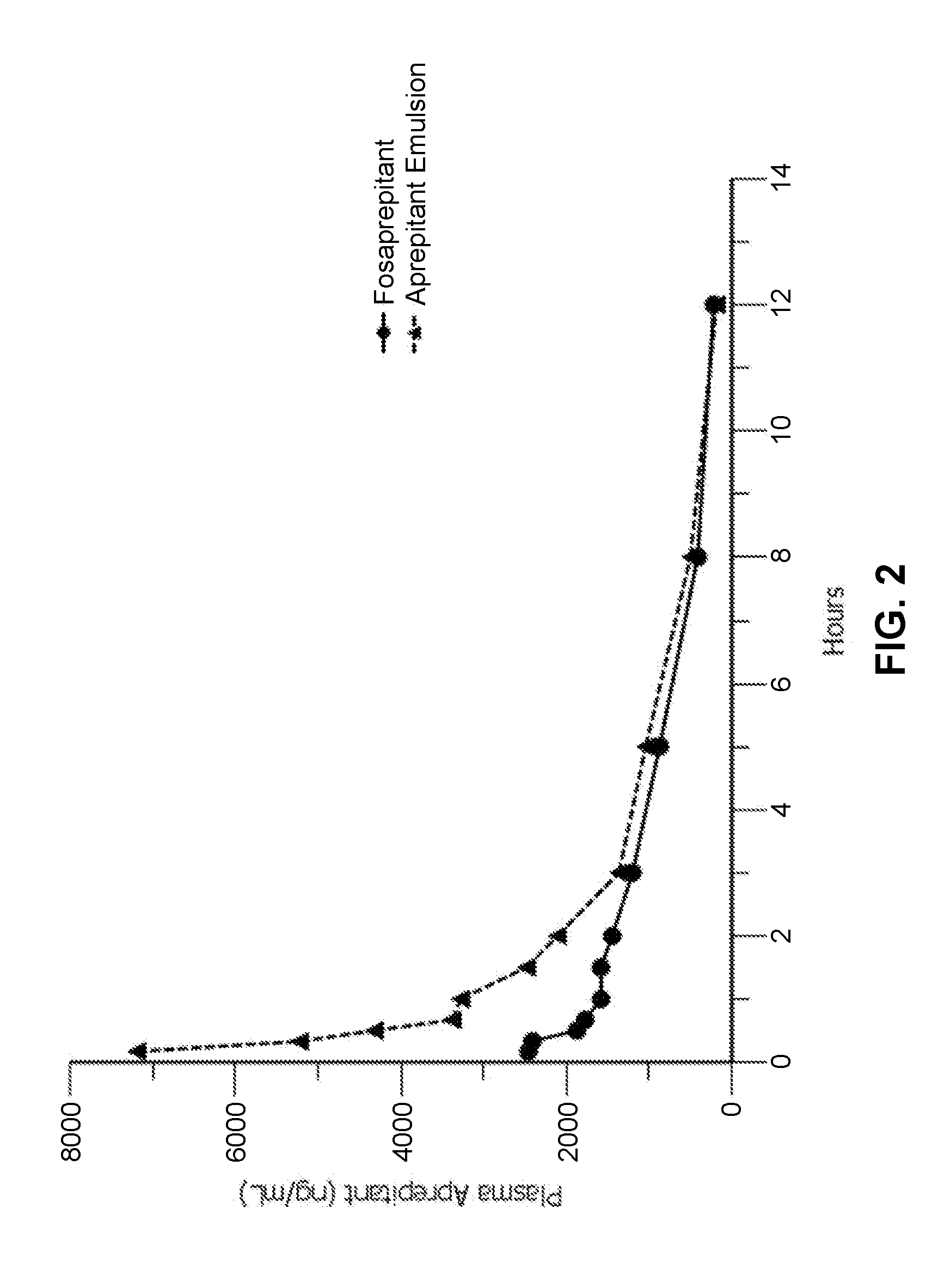
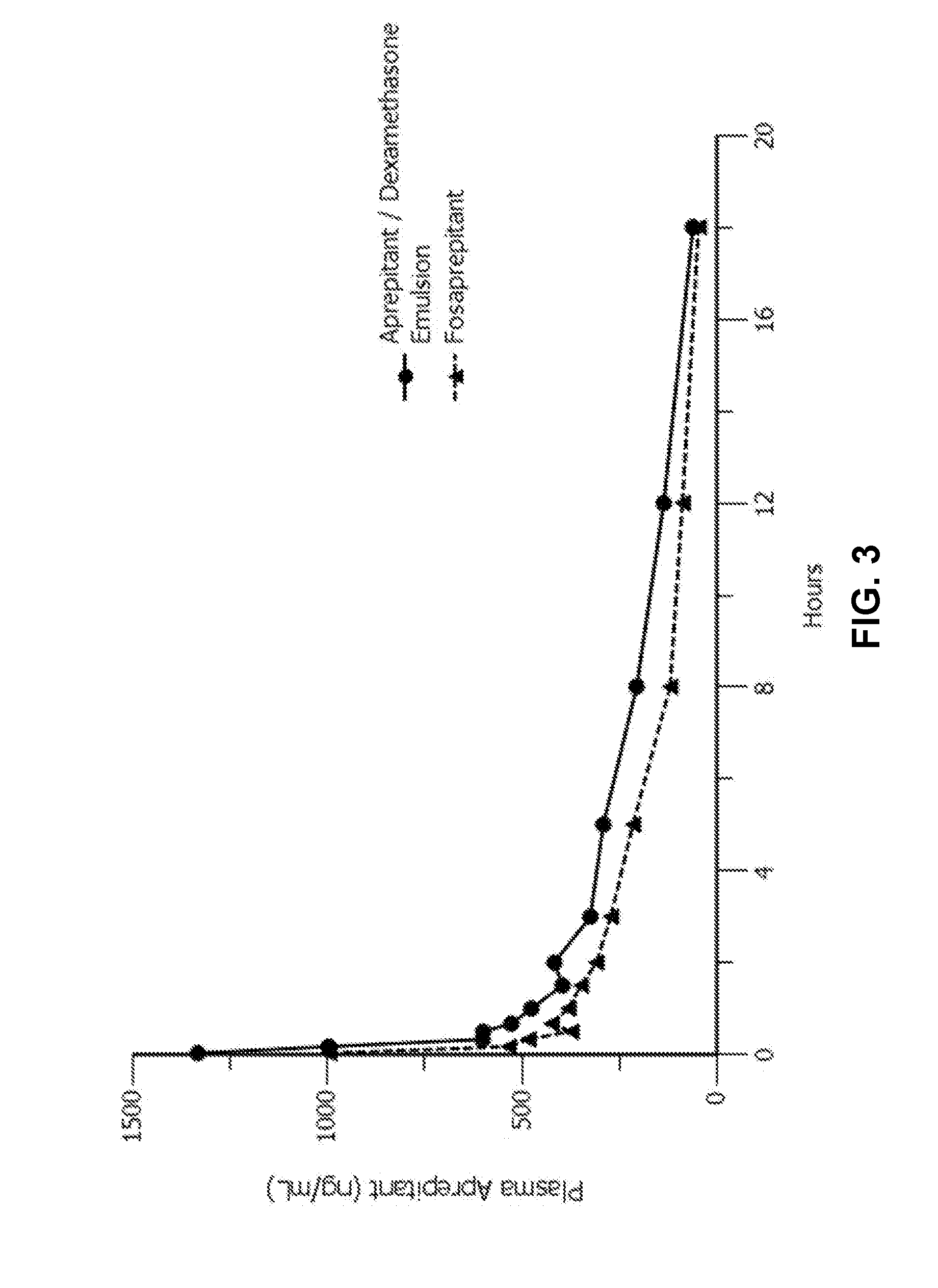
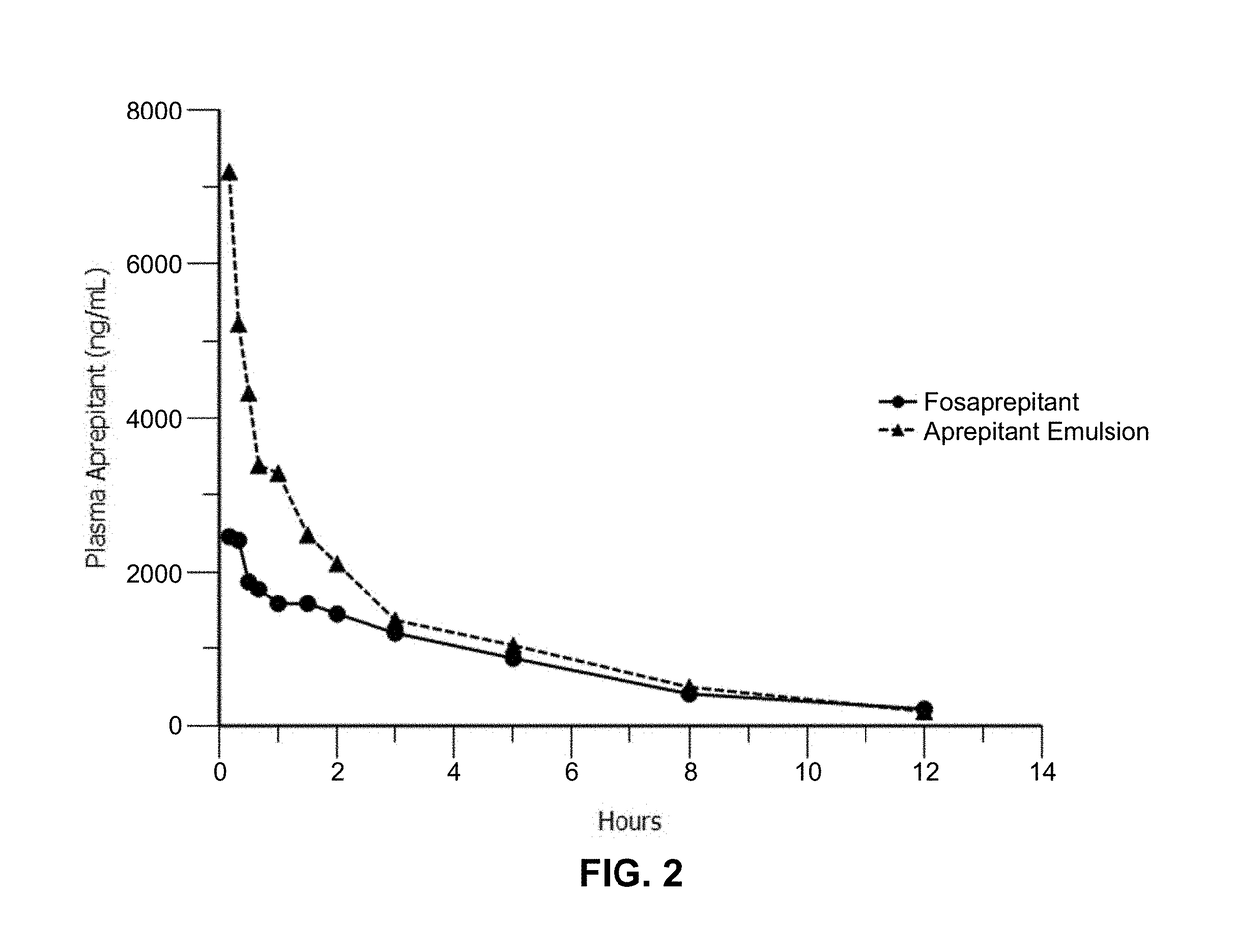
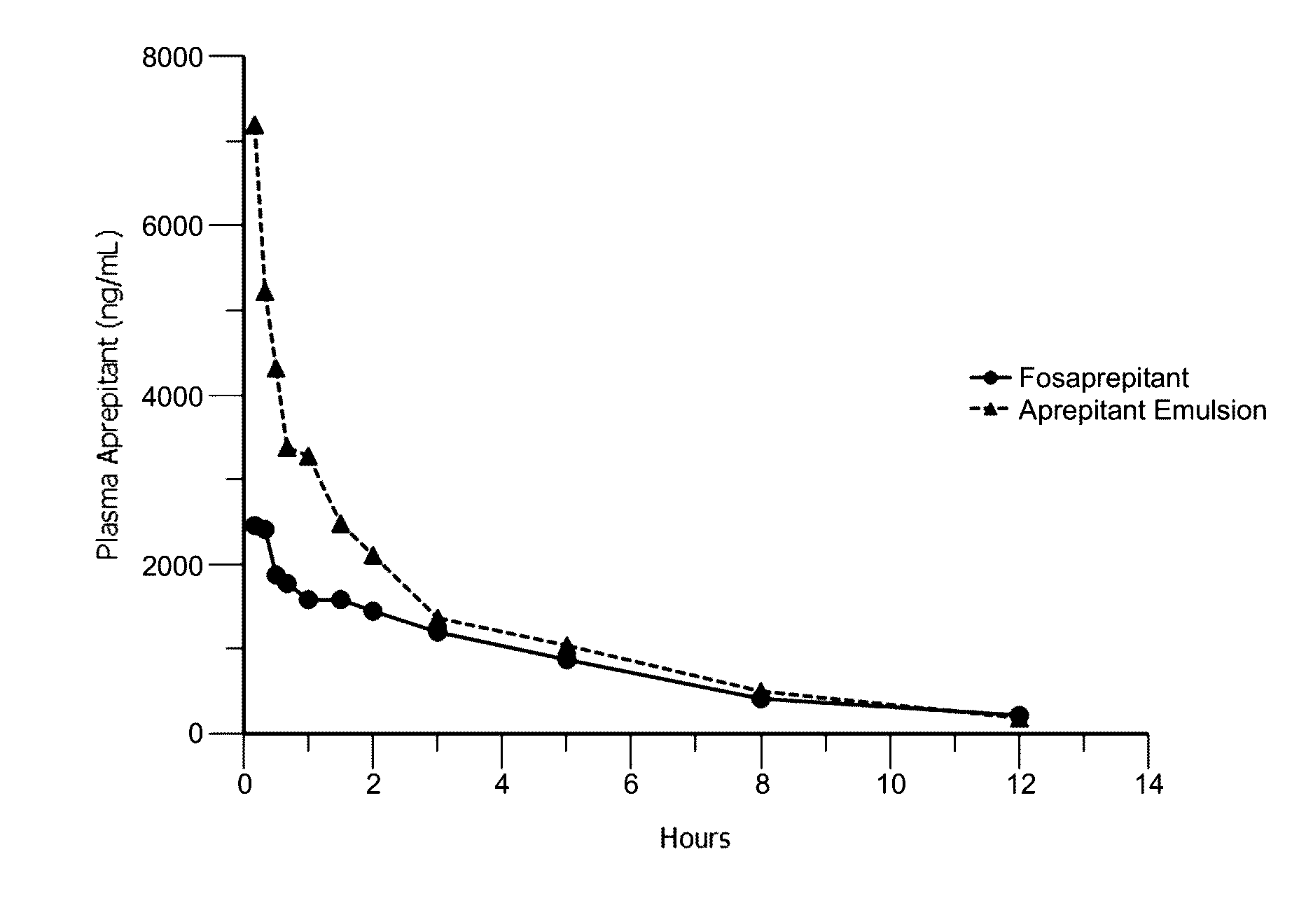
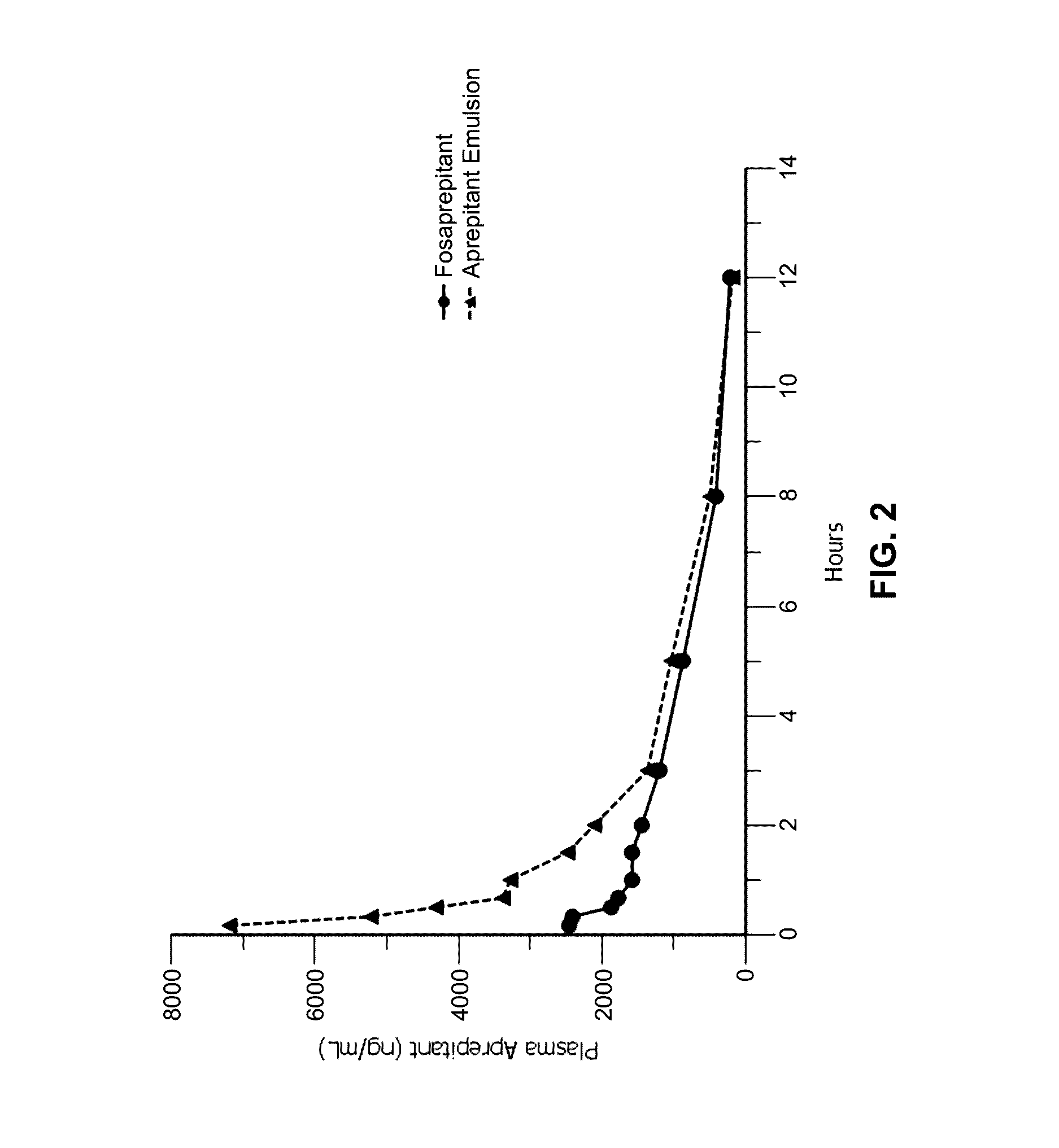
Emulsion formulations of aprepitant
ActiveUS20160082013A1Organic active ingredientsDigestive systemOral treatmentPharmaceutical formulation
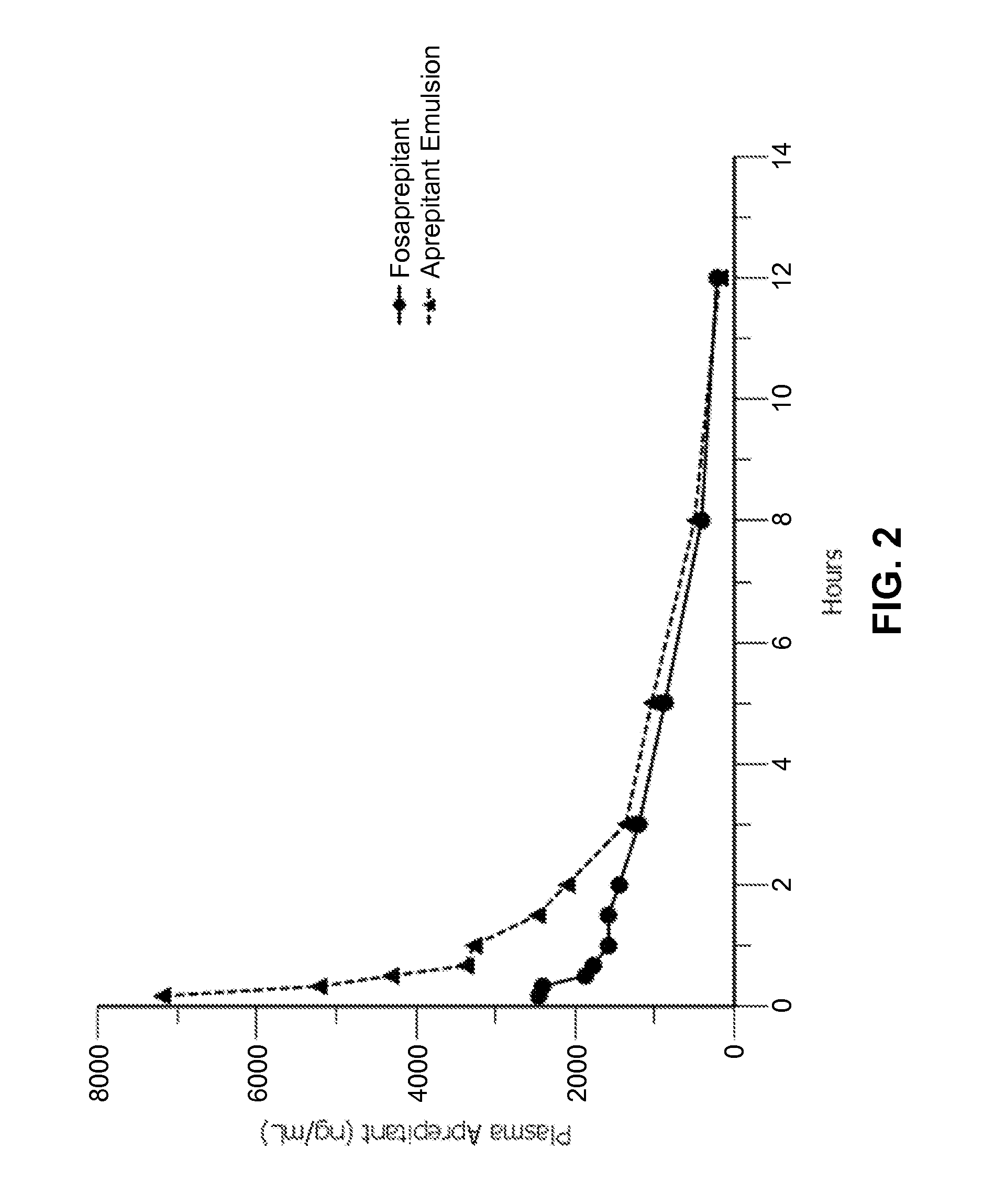
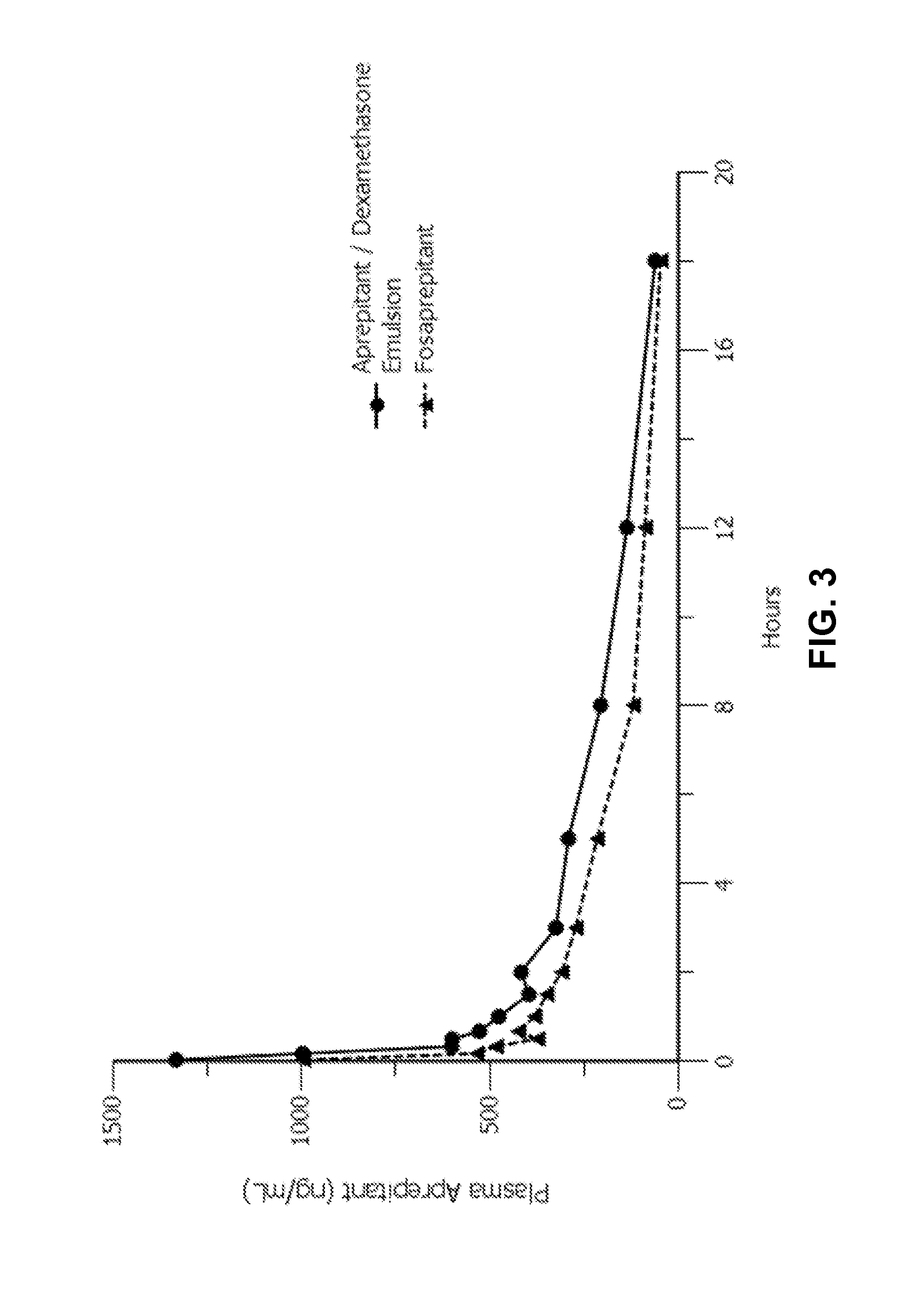
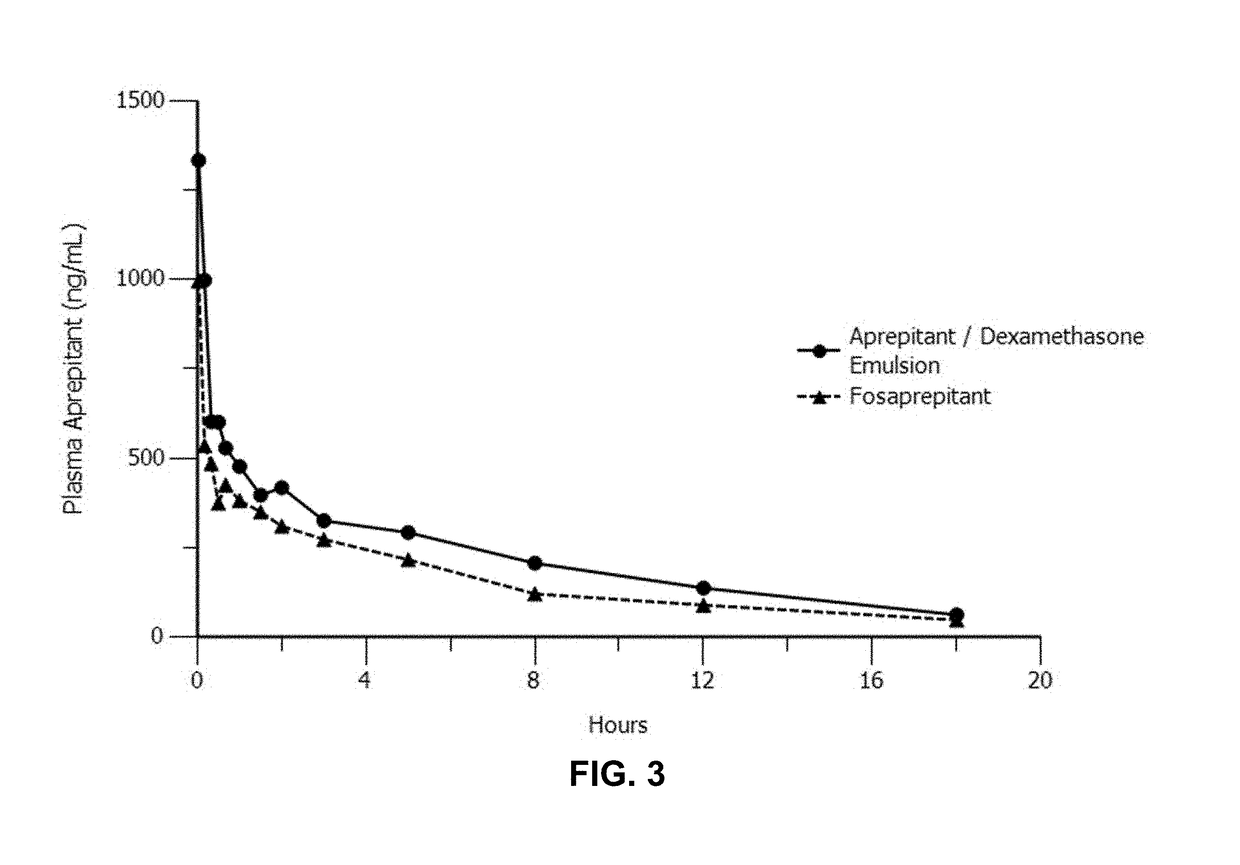
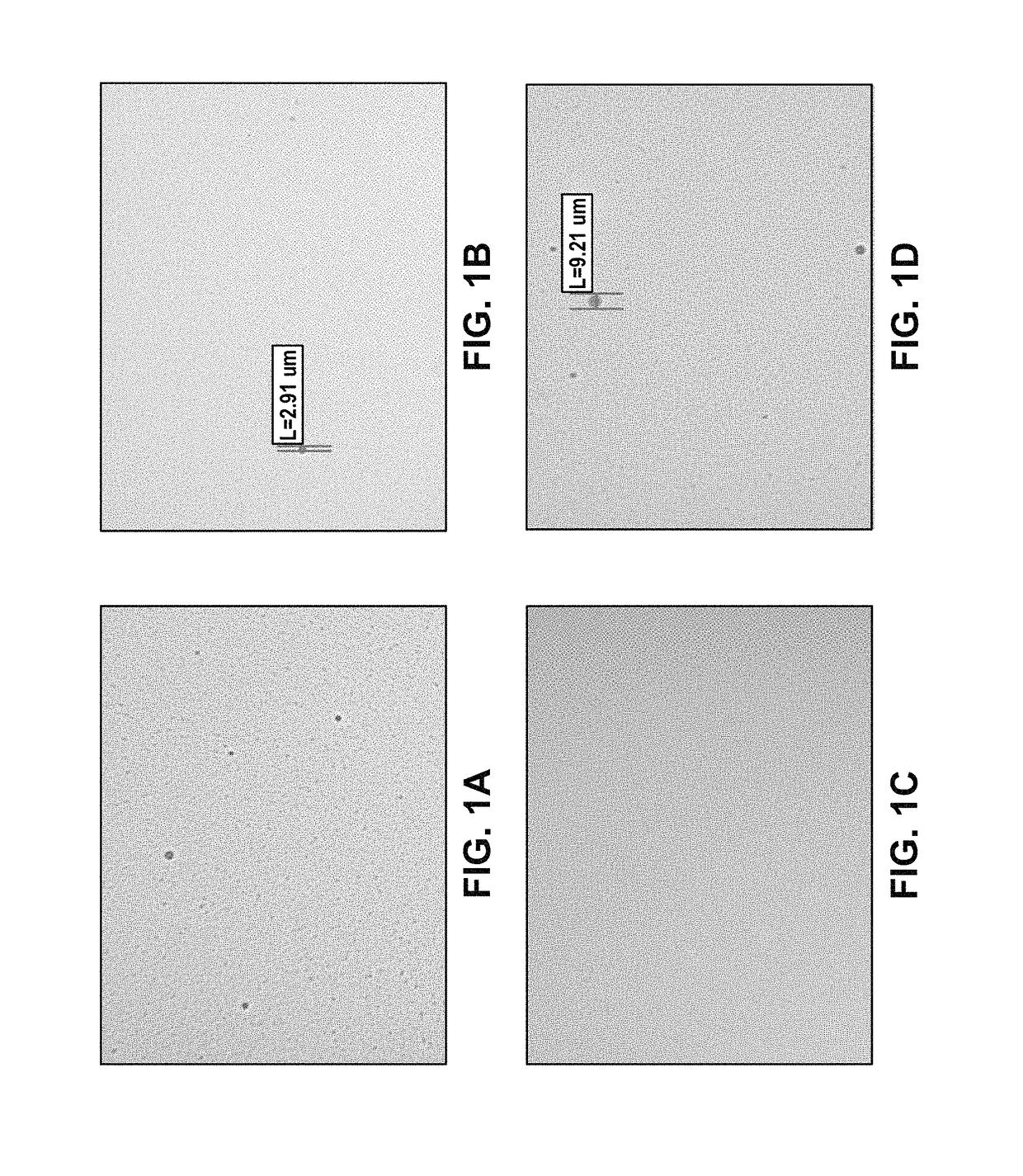
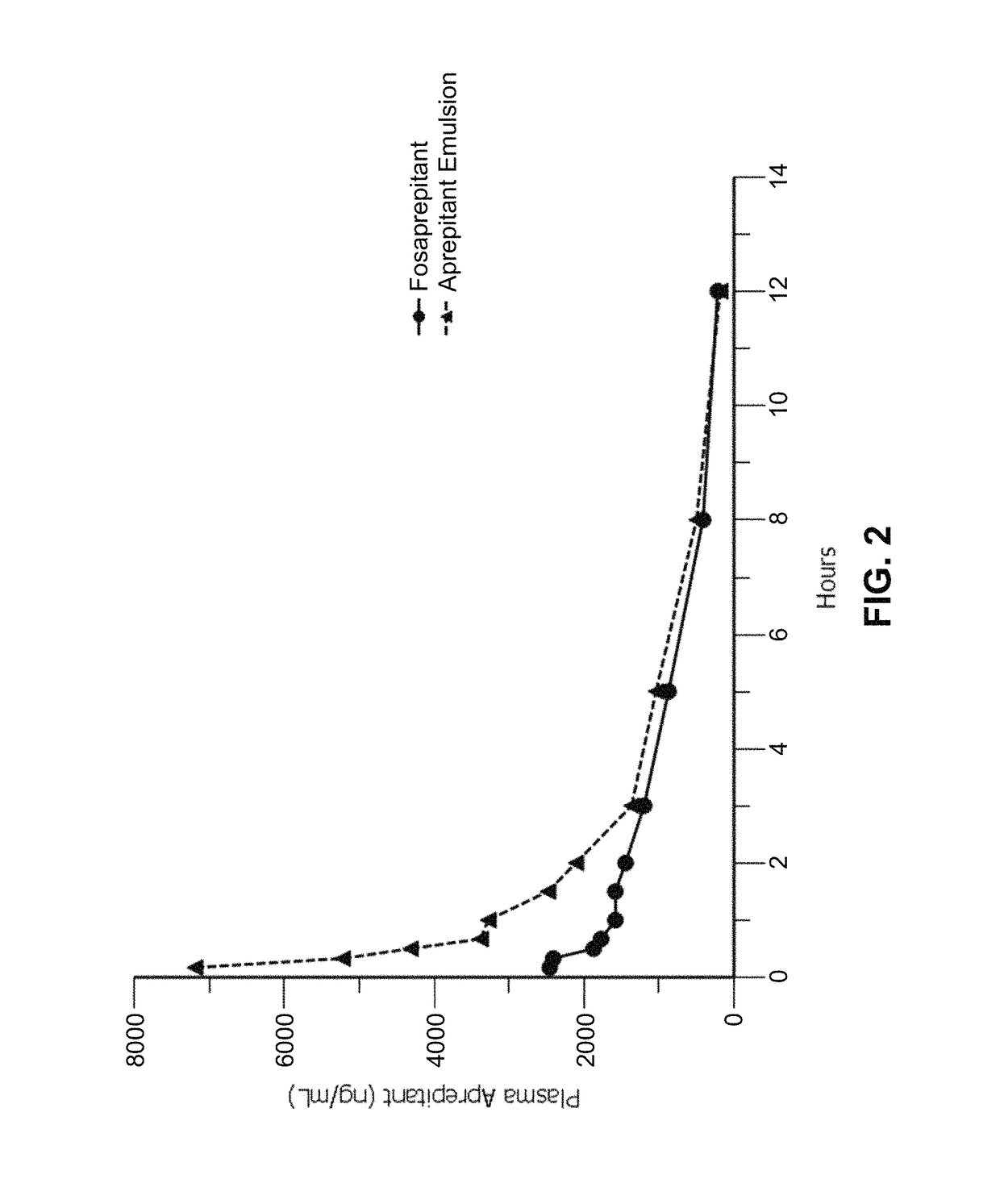
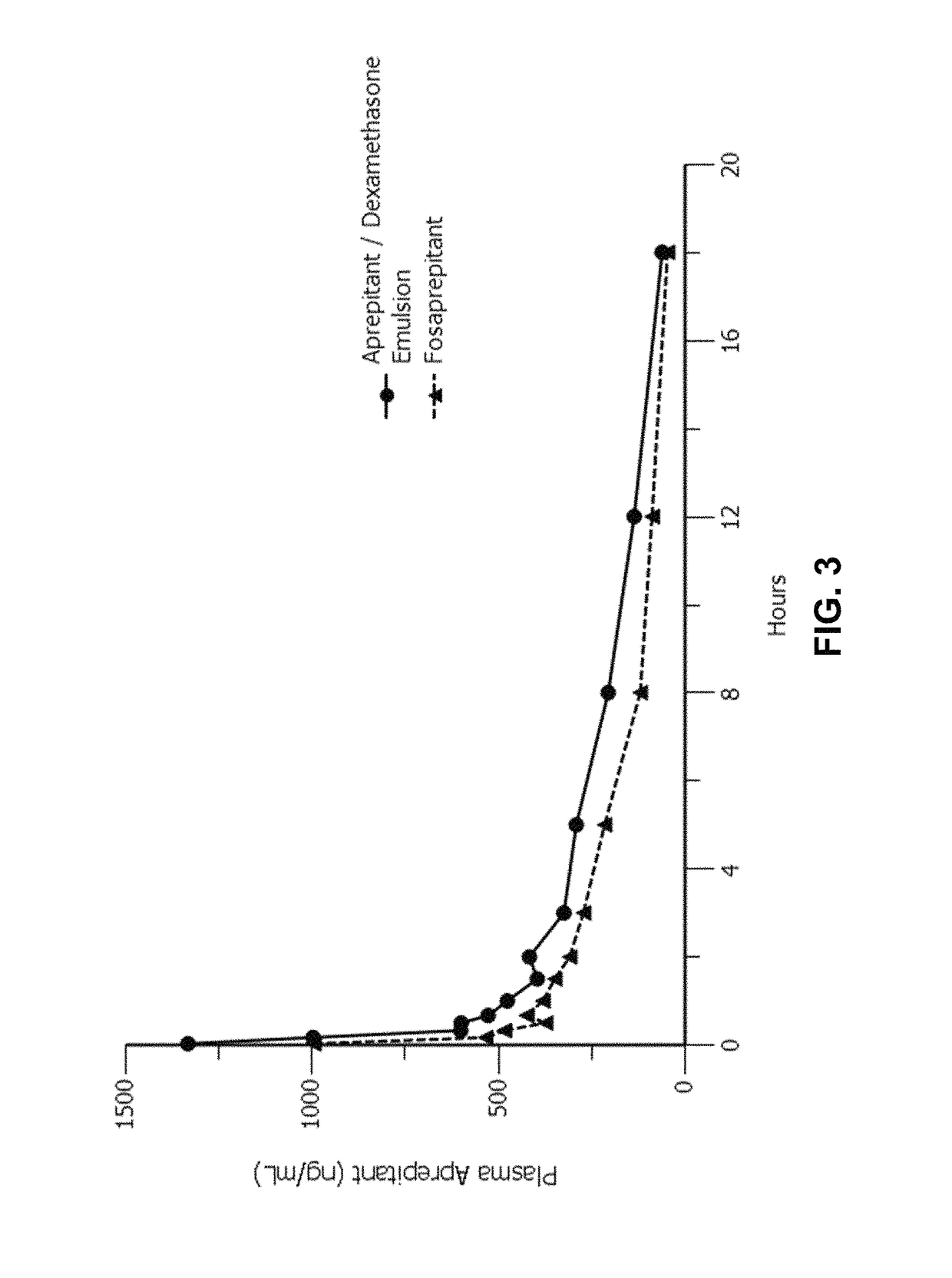
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Preparation method of hexadecadrol sodium phosphate freeze-dried powder injection
ActiveCN101703484AFreeze fastImprove stabilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLSodium phosphates
The invention provides a preparation method of hexadecadrol sodium phosphate freeze-dried powder injection, which comprises the following steps: mannitol is dissolved, added with active carbon, boiled and filtered, added with the hexadecadrol sodium phosphate and prepared to a liquid medicine; the liquid medicine is treated by prefreezing, primary freeze drying and secondary freeze drying; and then hydraulic pressure adding stopper, rolling cap and packaging are carried out. The method has the advantages that the method improves the liquid medicine preparation and freeze-drying technology, does not affect the content of the hexadecadrol sodium phosphate, can increase the single batch output, simultaneously shortens the freeze-drying time, increases the product stability, saves the energy, increases the production and reduces the production cost.
Owner:MAANSHAN BBCA PHARMA
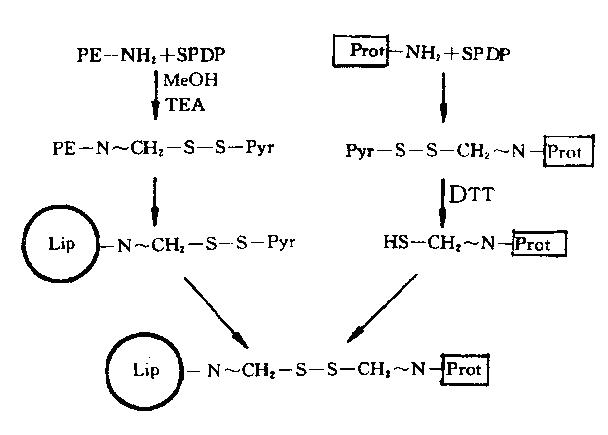
Pulmonic targeting immuno nano liposome and preparation method thereof
InactiveCN102370620AAchieve targeted deliveryIncreased DXM peak concentrationOrganic active ingredientsRespiratory disorderDiseaseAntiendomysial antibodies
The invention, belonging to the technical field of medicament, relates to an immuno liposome with pulmonic target activity and a preparation method thereof. The immuno liposome disclosed herein comprises pulmonic surfactant protein (SP) A polyclonal antibody, actie pharmaceutical ingredients and a nano liposome, wherein, dexamethasone sodium phosphate (DXM) and other glucocorticoids are used as the actie pharmaceutical ingredients, the nano liposome is used as a carrier, and the SP-A polyclonal antibody is used as a specific pulmonic targeted agent. The immuno nano liposome disclosed herein has the advantages of definite pulmonic target activity, and efficient and stable realization of the targeted conveying of the actie pharmaceutical ingredients to lung, so that maximum concentration ofDXM in the lung is increased by 5.08 times compared with common medicines, and the area under curve when 12 hours after injecting the medicine is increased by 40.21 times. According to the invention,the dosage of glucocorticoids can be reduced, the curative effect on lung diseases can be improved, and systematic side effect can be reduced at the same time. The invention has new values for clinicapplication.
Owner:SHANGHAI PULMONARY HOSPITAL AFFILIATED TO TONGJI UNIV
Emulsion formulations of aprepitant
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Emulsion formulations of an nk-1 receptor antagonist and uses thereof
Disclosed herein are novel pharmaceutical formulations of a neurokinin-1 (NK-1) receptor antagonist suitable for parenteral administration including intravenous administration. Also included are formulations including both the NK-1 receptor antagonist and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Emulsion formulations of aprepitant
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Compound sulfamonomethoxine sodium injection and preparation method thereof
InactiveCN101385704AImprove the immunityLong local irritationOrganic active ingredientsAntipyreticSulfamonomethoxineTrimethoprim
The invention relates to a compound sulfamonomethoxine sodium injection for veterinary use and a preparation method thereof, and belongs to the technical field of medicines for veterinary use. The raw material components of the compound sulfamonomethoxine sodium injection for veterinary use comprise sulfamonomethoxine sodium, dexamethasone sodium phosphate, trimethoprim, imidocarb, astragalus polysaccharide, organic solvent and water for injection. As a special compound preparation for veterinary use, the injection has remarkable efficacy on strengthening resistance of livestock and poultry, and treating mixed infection and severe diseases caused by a plurality of pathogenic bacteria and viruses. The injection has the advantages of convenient use, short treatment course, low drug resistance and the like.
Owner:陈建波
Grafting fruit tree notch consolidant and preparation method thereof
InactiveCN105767025APromote healingRestore aestheticsBiocidePlant growth regulatorsVitamin CGibberellin
The invention discloses a grafting fruit tree notch consolidant and a preparation method thereof.The consolidant is prepared from 0.001% to 0.01% of 2,4-D, 0.001% to 0.01% of gibberellins, 2.5% to 5.5% of cytex, 8% to 10% of rapin, 1.5% to 4.5% of milkvetch root extracting solution, 2.5% to 4.8% of vitamin C, 0.002% to 0.004% of dexamethasone sodium phosphate, 3.5% to 5.5% of potassium sorbate, 0.08% to 0.22% of thiophanate methyl, 0.08% to 0.20% of animal serum, 0.001% to 0.003% of alpha-pimacol, 0.01% to 0.015% of 45% ethyl alcohol and the balance aquae sterilisata.The milkvetch root extracting solution is prepared from milkvetch roots through the methods of soaking to be soft, decocting, squeezing, soaking and juice taking and distilling; 2,4-D, gibberellins and alpha-pimacol are dissolved with ethyl alcohol; the raw materials are taken according to the volume percent and mixed to be prepared into the consolidant.Compared with an existing consolidant, the consolidant can fast heal a wound, a good healing effect is achieved for the wound surface of a notch of a stock in the grafting process, the healing time can be obviously shortened, and the risk of wound surface infection can be reduced.
Owner:刘发元
Compound tylosin injection for animals and preparation method thereof
InactiveCN101822688APromote absorptionProlong the action timeAntibacterial agentsTetracycline active ingredientsTrimethoprimDoxycycline hydrochloride
The invention relates to compound tylosin injection for animals and a preparation method thereof. The injection consists of tylosin, doxycycline hydrochloride, trimethoprim, aminophylline, dexamethasone sodium phosphate, organic solvent and injection water. As a dedicated compound preparation for animals, the injection has special effect for treating respiratory tract infection caused by livestock and poultry sensitive bacteria and mycoplasmosis of pigs and poultry. The method has the advantages of convenient use, short course of treatment, low drug resistance and the like.
Owner:陈建波
Multiple-effect instillation liquid for trees and use method thereof
ActiveCN102718595ASolve the problem that cannot be directly absorbed by plantsEffective moisture balanceFertilising methodsCultivating equipmentsVitamin CInjury mouth
The invention relates to the technical field of landscaping plantation and especially relates to a multiple-effect instillation liquid for trees and a use method thereof. The multiple-effect instillation liquid for trees comprises: coconut, radix astragali and ephedra, Chinese ephedra root, dextrose, coenzyme A, triphosadenine, vitamin B1, vitamin C, dexamethasone sodium phosphate, cytex, brassin, potassium sorbate, boric acid, manganese sulfate, zinc sulfate, an auxin and an antiseptic. The multiple-effect instillation liquid for trees can realize the one-time supply of nutrients to a nursery stock, balancing of moisture of a nursery stock, resistance of water evaporation, fast healing of wounds of a wounded root system, promotion of early rooting, and prevention of infection.
Owner:LINGNAN LANDSCAPE
Topical ophthalmic or otic solution formulations containing moxifloxacin hydrochloride and dexamethasone phosphate
Topical ophthalmic and otic solution compositions of moxifloxacin and dexamethasone phosphate are disclosed.
Owner:NOVARTIS AG
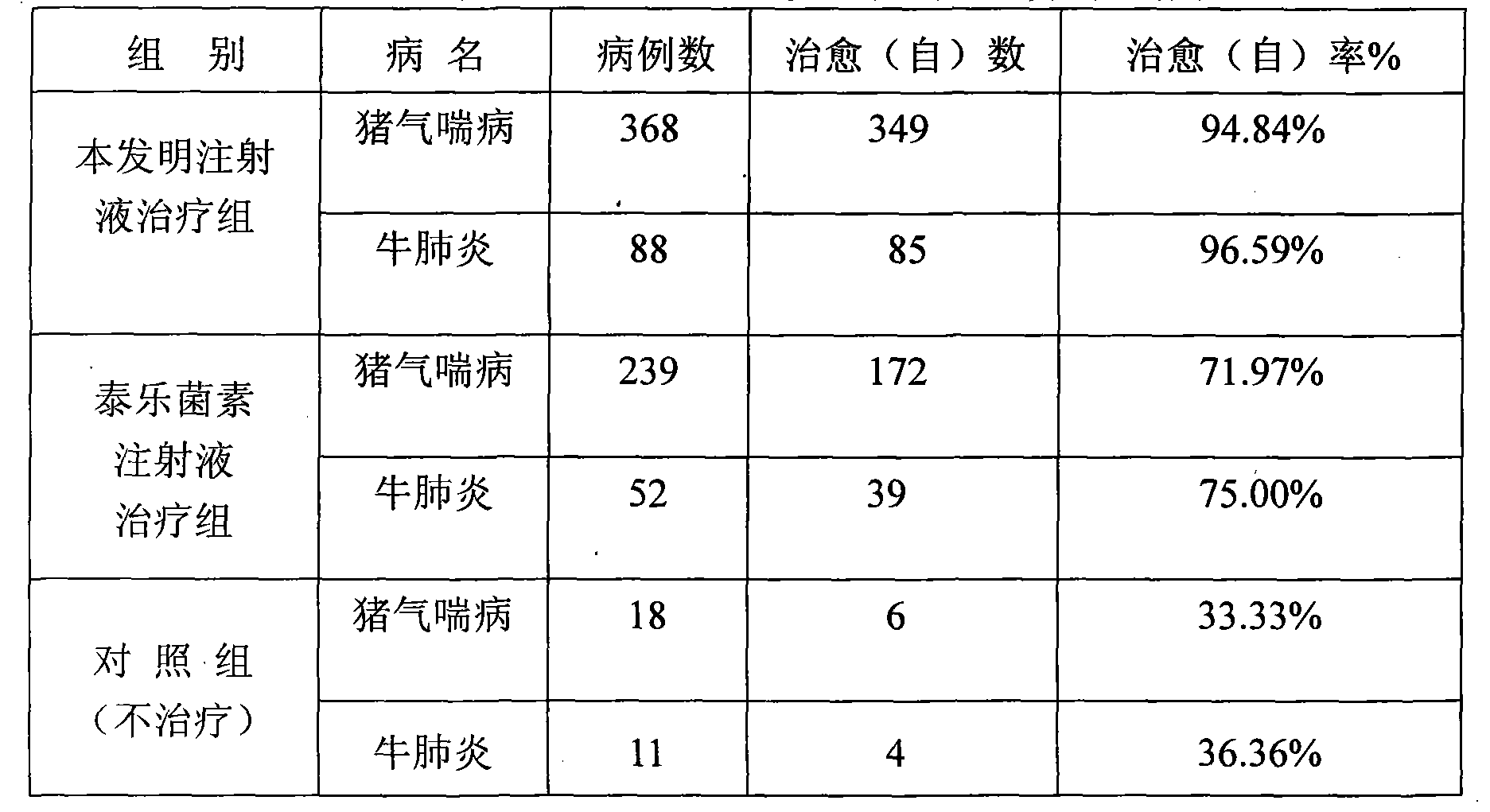
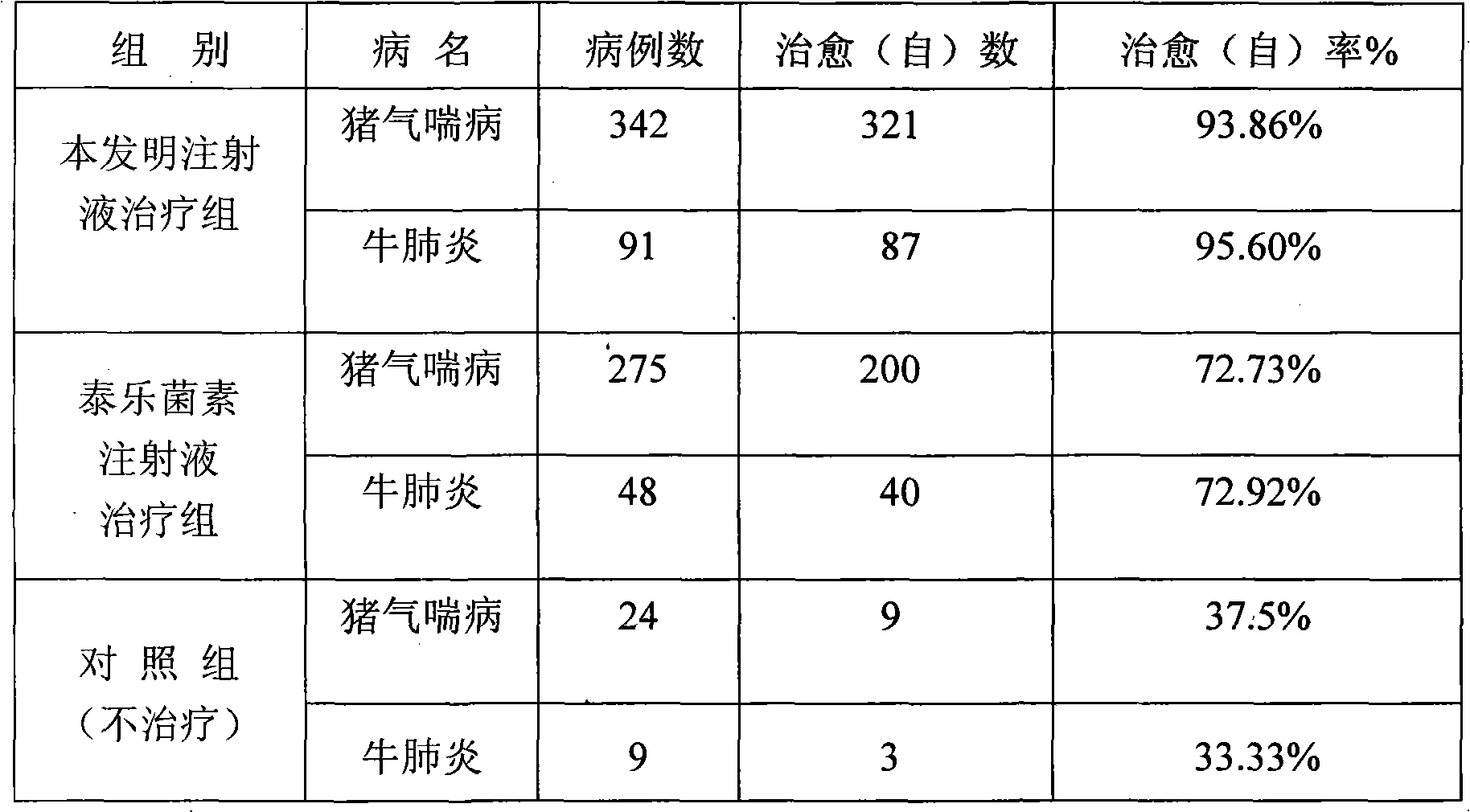
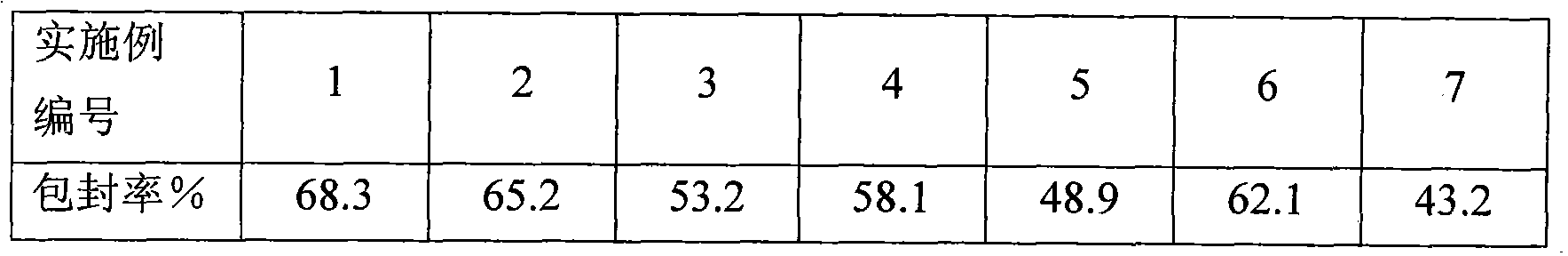
Dexamethasone sodium phosphate lipidosome injection
InactiveCN101926769AGood curative effectReduce dosageOrganic active ingredientsAntipyreticPh bufferingActive component
The invention relates to a lipidosome injection with dexamethasone sodium phosphate as an active component, which comprises dexamethasone sodium phosphate lipidosomes and one or more medical auxiliary materials for injection, wherein the liposome encapsulation rate of the active component is 40-70 percent, and the lipidosome injection comprises 0.025-0.5 percent of the dexamethasone sodium phosphate as the active component, 0.125-5 percent of phospholipid, 0.002-1 percent of lipophilic additive, a pH buffering agent with the pH value kept at 7-7.5 and the balance of water for injection.
Owner:TIANJIN JINYAO GRP
Veterinary compound sulfadiazine sodium injection and preparation method thereof
InactiveCN101829124AImprove antibacterial propertiesLittle side effectsAntibacterial agentsPharmaceutical delivery mechanismFLUNIXIN MEGLUMINETrimethoprim
The invention relates to a veterinary compound sulfadiazine sodium injection and a preparation method thereof. The veterinary compound sulfadiazine sodium injection comprises sulfadiazine sodium, enrofloxacin, flunixin meglumine, trimethoprim, dexamethasone sodium phosphate, an organic solvent and water for injection. The injection serving as a veterinary special compound preparation has a special treating effect on various infectious diseases, mixed infections and systemic infections of the digestive system, the respiratory system, the urinary system and the skin soft tissue caused by sensitive bacteria and mycoplasmas of oxen, swines, poultries, canines, cats and aquatic animals The injection has the advantages of convenient use, short period of treatment, low medicament resistance and the like.
Owner:陈建波
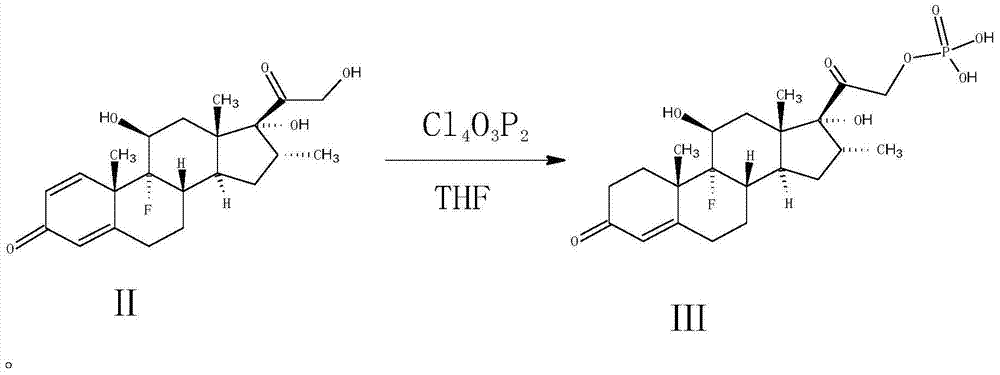
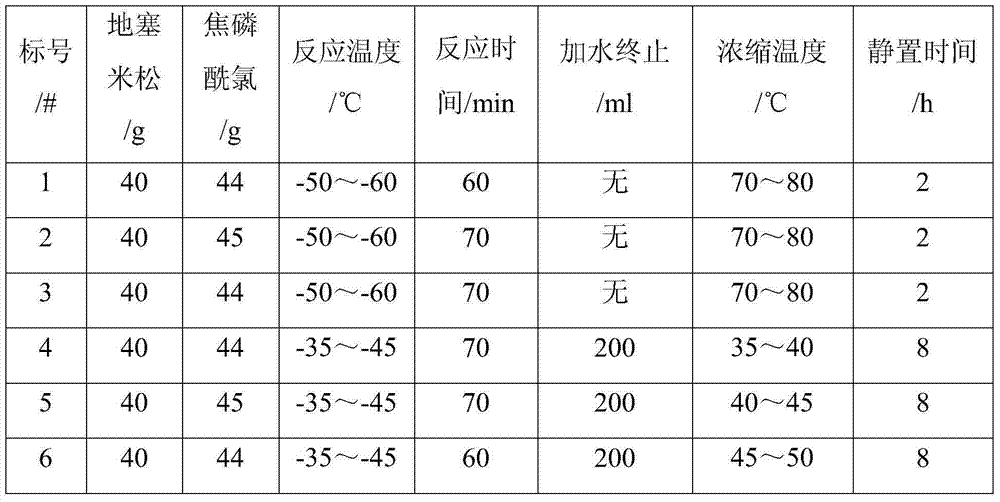
Improved preparation method of dexamethasone sodium phosphate intermediate
The invention provides an improved preparation method of a dexamethasone sodium phosphate intermediate. The improved preparation method of the dexamethasone sodium phosphate intermediate comprises the following steps: (a) in tetrahydrofuran, with dexamethasone and pyrophosphoryl chloride as raw materials, carrying out a reaction at the temperature ranging from minus 35 DEG C to minus 45 DEG C, so that dexamethasone phosphate is obtained; (b) adding purified water, carrying out hydraulic analysis after a termination reaction is finished, and then adding sodium hydrogen carbonate for salifying; (c) filtering, and carrying out reduced pressure concentration at the temperature of 30-45 DEG C until tetrahydrofuran is not contained in filtrate; (d) adding an organic solvent for extraction; and (e) filtering, carrying out reduced pressure concentration at the temperature of 30-45 DEG C until the organic solvent is not contained in the filtrate, adding acid for acidification, stirring for 6-8 hours, filtering, and carrying out vacuum drying, so that dexamethasone phosphate is obtained. The improved preparation method of the dexamethasone sodium phosphate intermediate has the advantages that utilization rate of raw materials and purity and yield of a product are improved, so that production cost is reduced; and the improved preparation method of the dexamethasone sodium phosphate intermediate has a wide industrial application prospect.
Owner:SHANGHAI NEW HUALIAN PHARMA
Dexamethasone sodium phosphate freeze-dried powder injection
ActiveCN103371980ALarge specific surface areaHigh speedOrganic active ingredientsPowder deliveryFreeze-dryingBottle
The invention relates to a dexamethasone sodium phosphate freeze-dried powder injection. Every bottle contains 1-20mg of dexamethasone sodium phosphate, 20-150mg of excipient and pH regulator. The components in the formula are dissolved in a solvent and freeze-dried to obtain the powder injection. The invention is characterized in that the solvent is ethanol-containing water for injection, and the liquor prepared by dissolving the components in the formula in the solvent contains 5-10 vol% of ethanol.
Owner:TIANJIN JINYAO GRP
Emulsion formulations of an NK-1 receptor antagonist and uses thereof
Disclosed herein are novel pharmaceutical formulations of a neurokinin-1 (NK-1) receptor antagonist suitable for parenteral administration including intravenous administration. Also included are formulations including both the NK-1 receptor antagonist and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Injection for treating porcine mixed infection
ActiveCN102188436AImprove antibacterial propertiesGood antiviral effectAntibacterial agentsOrganic active ingredientsTrimethoprimSulfamonomethoxine
The invention discloses injection for treating porcine mixed infection. Each 100 liters of injection contains 10 to 15 kilograms of sulfamonomethoxine sodium, 2 to 5 kilograms of ofloxacin, 2 to 4 kilograms of diclofenac sodium, 1 to 2 kilograms of baicalin, 2 to 4 kilograms of trimethoprim, 0.1 to 0.2 kilogram of dexamethasone sodium phosphate, 0.1 to 0.2 kilogram of sodium thiosulfate, 3 to 6 liters of ethanolamine, 3 to 6 liters of phenyl carbinol, 30 to 60 liters of propylene glycol and the balance of water for injection. Compared with the conventional compound preparation for treating the porcine mixed infection, the injection effectively treats the porcine mixed infection and is convenient to use.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Compound ofloxacin injection for animals
InactiveCN101433518AImprove survival rateIncrease weightAntibacterial agentsSalicyclic acid active ingredientsAtropine sulfateSodium salicylate
The invention discloses compound ofloxacin injection for livestock, and belongs to medicine preparation containing organic effective components, or anti-infective medicament for the livestock. The injection of each 1, 000 liters is prepared by the following raw materials: 100 to 400 kilograms of sodium salicylate, 5 to 110 kilograms of ofloxacin, 10 to 150 kilograms of mequindox, 1 to 20 kilograms of atropine sulfate, 1 to 20 kilograms of dexamethasone sodium phosphate, and the balance being water for injection. The injection adopts combination of medicaments such as the ofloxacin, the atropine sulfate, the mequindox, and the like, can enhance curative effect, treats both symptoms and root causes, can effectively relieve stress reaction of sick livestock body, can be widely applied to treating pig scour, improve livability of pigs, and increase weight of the pigs.
Owner:TIANJIN SHENGJI GRP CO LTD
Preparation method for dexamethasone sodium phosphate
InactiveCN105348358ASimple stepsRaw materials are easy to getSteroidsDexamethasone acetateReaction temperature
The invention relates to a preparation method for dexamethasone sodium phosphate. The preparation method comprises the following steps: a ring-opening reaction is carried out, namely, dexamethasone acetate epoxide is employed as an initial raw material, HF and DMF are added, a reaction is performed for 3h, a ring-opening reaction is carried out and a dexamethasone acetate solution is prepared; recrystallization is carried out, namely, methanol is added in the dexamethasone acetate solution, recrystallization is carried out, and dexamethasone acetate is prepared; base catalysis hydrolysis is carried out, namely, dexamethasone acetate is added in Na2CO3 and methanol, a reaction is carried out for 10min, dexamethasone is prepared; pyrophosphoryl chlorine esterification is carried out, namely, dexamethasone prepared in the third step is reacted with pyrophosphoryl chlorine and THF, and dexamethasone phosphate ester is prepared; a neutralization salt forming reaction is carried out, namely, the dexamethasone phosphate ester obtainedin the fourth step is reacted with NaOH and methanol at a reaction temperature of 20 DEG C-30 DEG C for 1h, and dexamethasone sodium phosphate is prepared. The steps are simple, raw materials are easily available, the reaction conditions are mild, the method is suitable for industrial production, and the cost is low.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Preparation method of superparamagnetism conductive nano gamma-ferric oxide/ polyaniline-dexamethasone sodium phosphate
InactiveCN102000342AGood biocompatibilityImprove conductivityOrganic active ingredientsAntipyreticClasmatocyteMalignant lymphoma
The invention discloses a preparation method of superparamagnetism conductive nano gamma-ferric oxide / polyaniline-dexamethasone sodium phosphate, relating to the technical field of synthesis of superparamagnetism conductive nano gamma-ferric oxide / polyaniline-dexamethasone sodium phosphate. The superparamagnetism conductive nano gamma-ferric oxide / polyaniline-dexamethasone sodium phosphate is prepared by a doping method. The product of the invention can better suspend in water, still has favorable electrochemical activity and electric conduction magnetoconductivity within the pH range of human body environment, and can directionally reach diseased tissues under the regulation and control of an external magnetic field without damaging normal clasmatocyte. The targeted medicine is hopefully applied to treating acute leukemia, malignant lymphoma, connective tissue diseases, rheumatoid arthritis and the like. In addition, the method of the invention is simple, convenient, economical and favourable for industrial production and application.
Owner:YANGZHOU UNIV
Compound doxycycline hydrochloride injection for animals and preparation method thereof
InactiveCN101380301AReduce the impactProof of StabilityAntipyreticTetracycline active ingredientsTrimethoprimDoxycycline hydrochloride
The invention relates to a compound doxycycline hydrochloride injection for veterinary use and a preparation method thereof. The raw materials comprise doxycycline hydrochloride, thiamphenicol, dexamethasone sodium phosphate, trimethoprim, diclofenac sodium, organic solvent and water for injection. As a special compound preparation for veterinary use, the injection has obvious curative effects on infectious diseases caused by gram-positive bacteria, gram-negative bacteria and mycoplasma, quickly takes effects after the injection is injected into livestock with the diseases, and has special efficacy on mixed infection and severe diseases caused by various pathogenic bacteria and viruses. After intramascular for animals, the injection is easily absorbed, takes effect for a long time and causes little local stimulation. The injection has the advantages of convenient use, short treatment course, low drug resistance, and the like.
Owner:陈建波
Dexamethasone sodium phosphate powder injection and production method thereof
InactiveCN102309455ASimple preparation processOvercomes the disadvantage of requiring up to 30% or more propylene glycol as a co-solventPowder deliveryOrganic active ingredientsOrganic solventBottle
The invention discloses a dexamethasone sodium phosphate powder injection and a production method thereof. The method for preparing the dexamethasone sodium phosphate powder injection comprises the following steps of: 1) dissolving dexamethasone sodium phosphate in an organic solvent to obtain a medicinal solution (1); and 2) performing sterilization and pyrogen removal on the medicinal solution (1), directly filling into an injection bottle, removing the organic solvent from the filled injection bottle by adopting a reduced-pressure drying method, capping and packaging, and recovering the organic solvent at the same time.
Owner:TIANJIN JINYAO GRP
Glucocorticoid-loaded nanoparticles for prevention of corneal allograft rejection and neovascularization
ActiveUS20170157147A1Severe side effectEasy to manageOrganic active ingredientsSenses disorderGlucocorticoidTransplant rejection
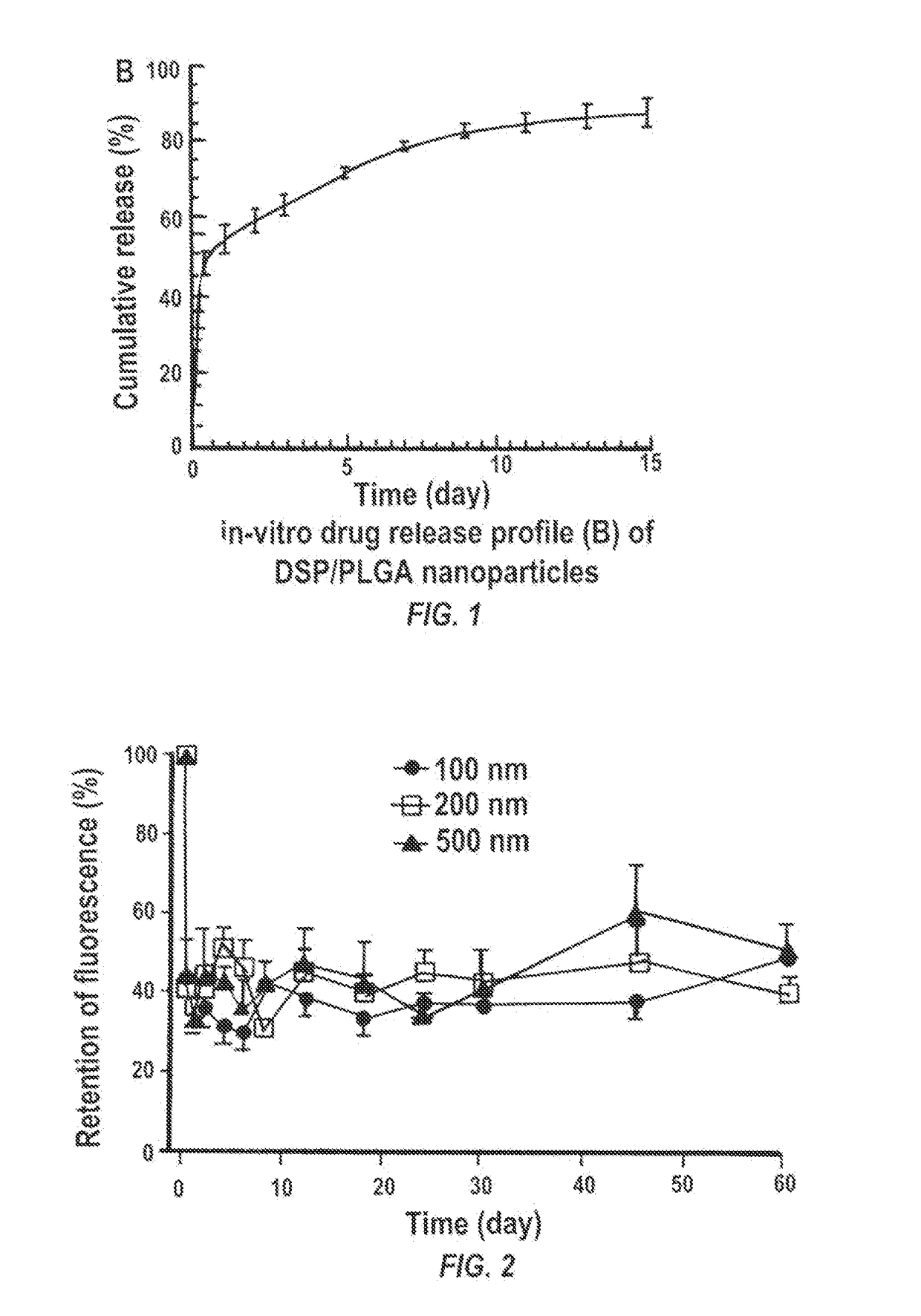
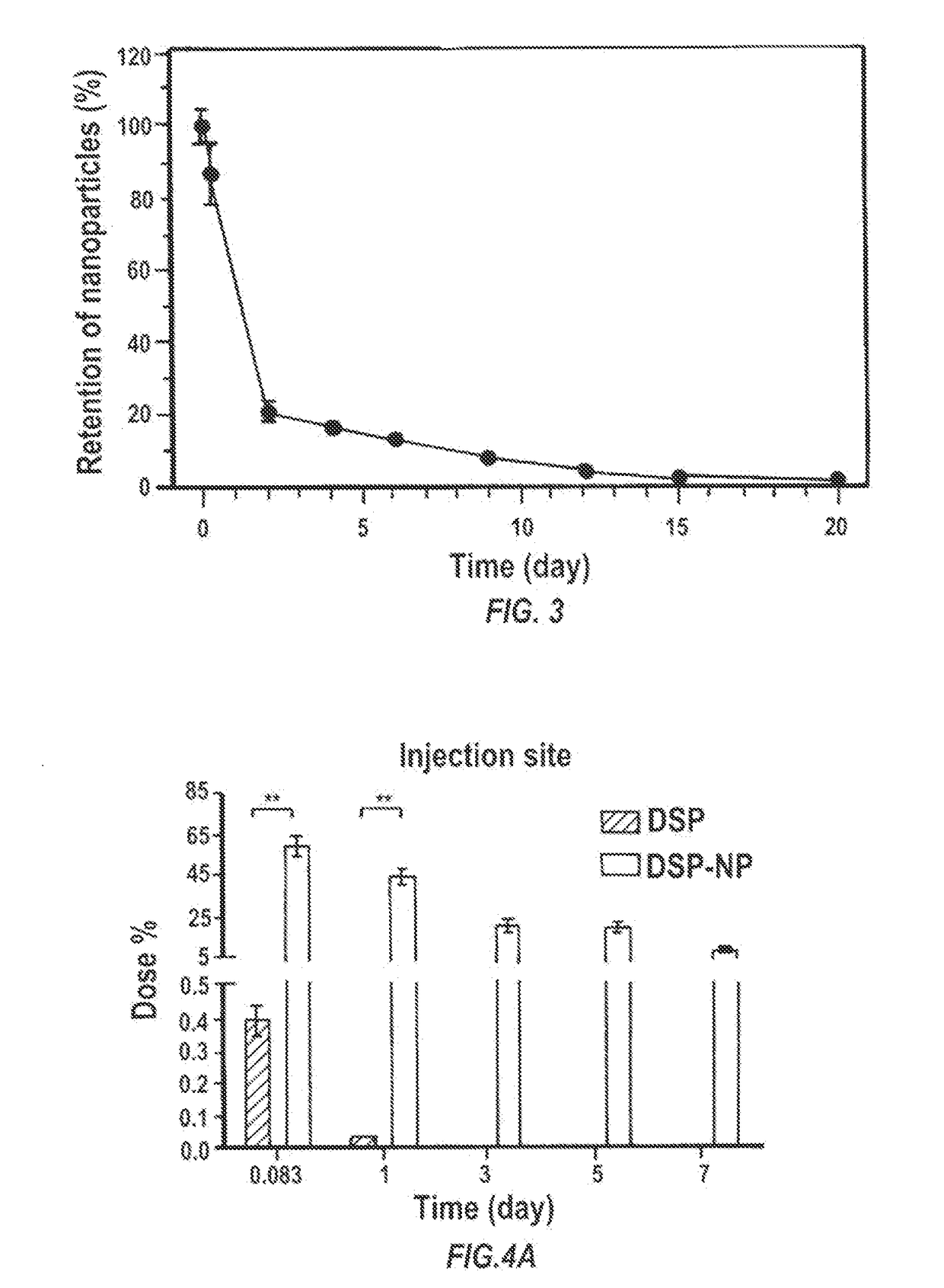
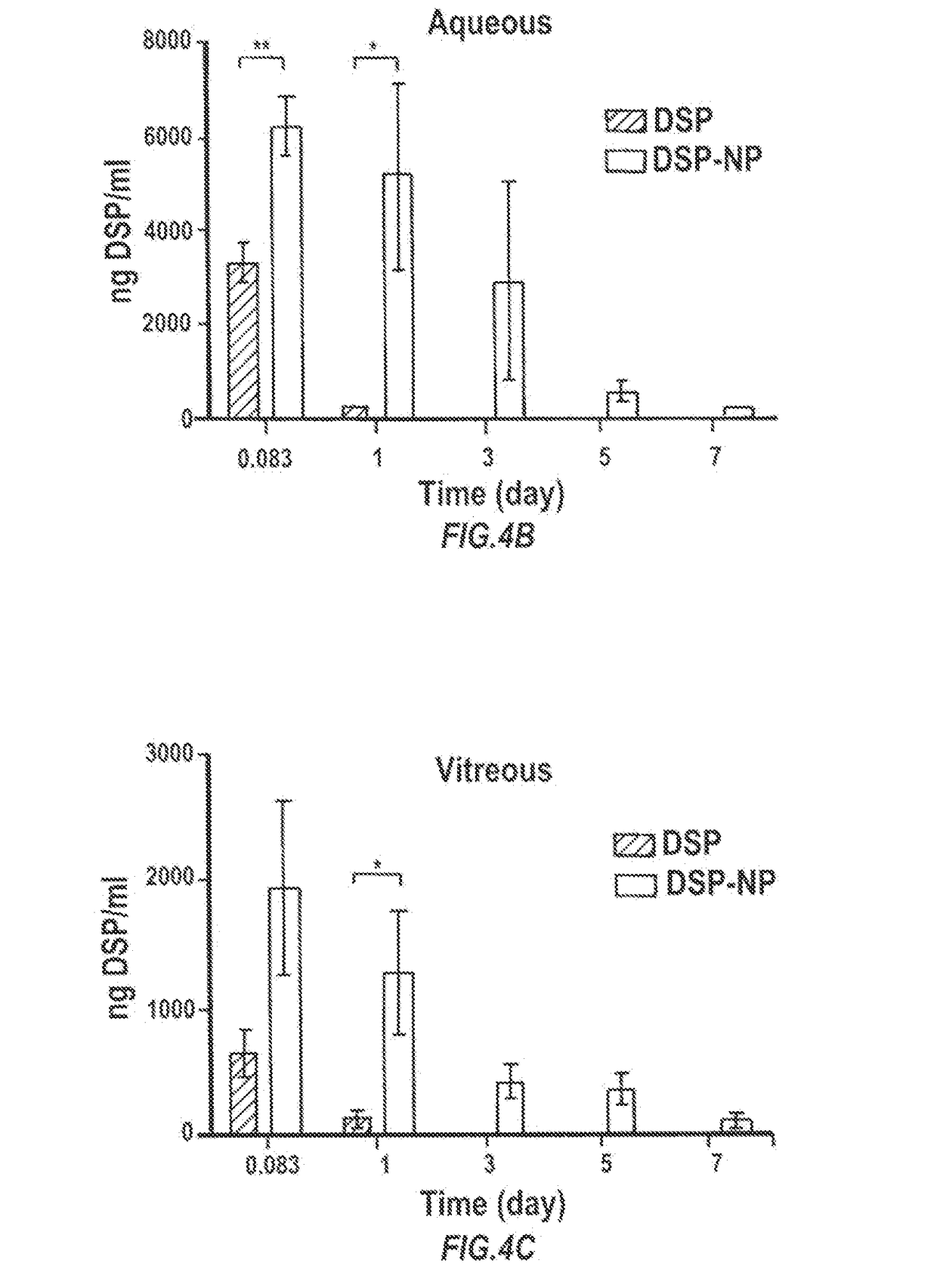
Particles encapsulating a glucocorticoid such as dexamethasone sodium phosphate (DSP) into a matrix such as biodegradable poly(lactic-coglycolic acid) (PLGA) which is densely coated with hydrophilic polymer such as PEG or PLURONIC® F127, exhibit sustained release of DSP for up to 7 days in vitro. These nanoparticles can be used to prevent corneal graft rejection or corneal neovascularization.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compound ofloxacin injection for animals for treating postnatal infection and preparation method thereof
InactiveCN101612158ADelay drug resistanceIncreased sensitivityAntibacterial agentsDigestive systemIntestinal structureDisease
The invention discloses a compound ofloxacin injection for animals for treating postnatal infection and a preparation method thereof. The invention aims to provide a compound ofloxacin injection for the animals which has quick response on treating the postnatal infection of livestock, is effective and efficient and can treat both principal and secondary aspects of diseases, and a preparation method thereof. Each 100L of injection comprises the following components: 0.5 to 10kg of ofloxacin, 3 to 15 of billion units of kanamycin sulfate, 0.06kg of dexamethasone sodium phosphate, 0.02 to 0.1kg of bethanechol chloride, 1,000ml of hydrochloric acid, and the balance of water for injection. The injection has double functions of bacteria inhibition and sterilization by using the ofloxacin in combination with the kanamycin sulfate, reduces the probability that bacteria produce the drug resistance, and increases the sensitivity of the bacteria; in addition, the dexamethasone sodium phosphate has anti-inflammatory, detoxicating and antiallergic functions; and the bethanechol chloride stimulates the intestines and stomach as well as uterine smooth muscle to creep. The product treats both principal and secondary aspects of diseases on the postnatal infection of dams and complicating diseases thereof, and can accelerate the postpartum recovery of the dams.
Owner:天津市海纳德动物药业有限公司
Medicine for treating coryza and its preparation method
InactiveCN101380328AAvoid surgical sequelaeRespiratory disorderHeterocyclic compound active ingredientsMillion UnitsPenicillin K
A drug for treating rhinitis and a preparation method thereof relate to an improved drug for treating the rhinitis. The invention provides the drug for treating the rhinitis and the preparation method thereof. The drug contains 1.6 million units of penicillin sodium salt, 6-9mg of hydrocortisone and 4-7mg of dexamethasone sodium phosphate according to weight proportion. The preparation method is characterized by comprising the following steps: (1) 0.8 million units of the penicillin sodium salt and 6-9mg of the hydrocortisone are mixed and dissolved; (2) 0.8 million units of the penicillin sodium salt and 4-7mg of the dexamethasone sodium phosphate are mixed and dissolved; and (3) the two mixed solutions are mixed to prepare the drug for treating the rhinitis.
Owner:李想
Synergetic compound analgin injection and preparation method thereof
ActiveCN103083338AImprove heat dissipation abilityReduce manufacturing costOrganic active ingredientsAntipyreticEthylene diamineMedicine
The invention relates to a synergetic compound analgin injection and a preparation method thereof. The injection comprises the following components by weight in 100 milliliters: 10 to 30g of analgin, 2 to 4g of antioxidant, 50 to 150mg of dexamethasone sodium phosphate, 0.05g of EDTA (ethylene diamine tetraacetic acid)-Na2, injection water filled until the amount reaches 100ml, and proper pH regulating agent, wherein the usage of the pH regulating agent is sufficient to regulate the pH value of the system to be between 5.5 and 6.5. The preparation method comprises the following steps of adding the EDTA-Na2, the antioxidant and the analgin into the injection water, stirring until the EDTA-Na2, the antioxidant and the analgin are completely dissolved to prepare liquid A; dissolving the dexamethasone sodium phosphate with trace injection water to prepare liquid B; adding the liquid B into the liquid A, uniformly stirring, continuously stirring for 20 minutes, regulating the pH value to be between 5.5 and 6.5, and fixing the volume to 100ml; filtering medicinal liquid, filling the filtrate into a bottle with a filling and sealing machine; and sterilizing for 30 minutes at a temperature of 115 DEG C and at a pressure of 0.2MPa, and cooling and outputting to obtain a finished product. The dexamethasone sodium phosphate is added into the injection reasonably and compatibly, so that the antipyretic effect is reinforced, and the function of anti-inflammation and anti-allergy are given at the same time. The synergetic compound analgin injection is wide in drug effect and convenient to use.
Owner:金河牧星(重庆)生物科技有限公司
Topical ophthalmic or otic solution formulations containing moxifloxacin hydrochloride and dexamethasone phosphate
Owner:NOVARTIS AG
Injection for treating paratyphoid of dogs and preparation method thereof
InactiveCN101590012AInfection controlReduce chance of drug resistanceAntibacterial agentsDigestive systemGlucocorticoidSulfamonomethoxine
The invention discloses an injection for treating the paratyphoid of dogs and a preparation method thereof, which aim to provide an injection which has quick effect and can reduce the drug resistant probability of pathogenic bacteria, treat both symptoms and root causes and quicken the recovery, and a preparation method which has simple process and is easy to realize. Per 100L of the injection comprises 2-15kg of sulfamonomethoxine sodium, 1-10kg of lincomycin hydrochloride, 0.05-0.3kg of atropine sulfate, 0.06kg of dexamethasone sodium phosphate, 0.2kg of sodium bisulfite, 0.01kg of EDTA-2Na and the balance of water. The injection adopts antibacterial drugs, antibiotics, glucocorticoids for diminishing inflammation and detoxifying and atropine sulfate for controlling the diarrhea of the dogs and reducing dehydration, and a compound preparation prepared by reasonably proportioning the dosages of the components has the function of double anti-bacteria, can reduce the drug resistant probability of salmonella typhimuria, control the infection of other gram negative bacteria and positive bacteria and accelerate the recovery and has high cure rate.
Owner:TIANJIN SHENGJI GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com