Patents
Literature
108 results about "Aprepitant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
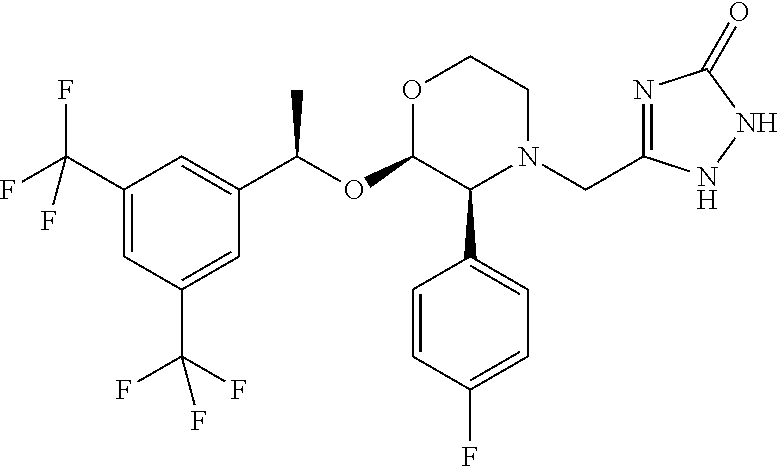
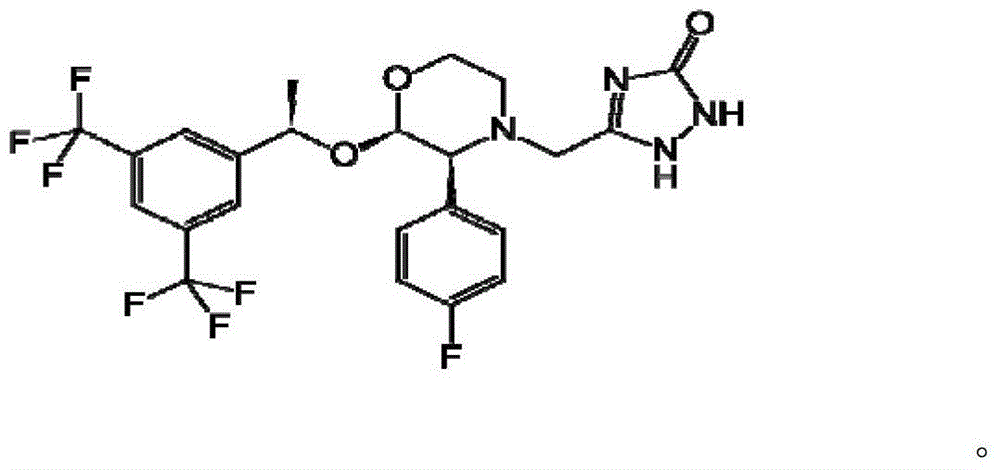
Aprepitant is used with other medications to help prevent nausea and vomiting caused by cancer drug treatment (chemotherapy). This medication is also used to prevent nausea and vomiting after surgery.
Aprepitant microemulsion for injection and preparation method thereof
InactiveCN102379845ARealize large-scale industrial productionLow costOrganic active ingredientsDigestive systemSocial benefitsOrganic solvent
The invention discloses an aprepitant microemulsion for injection. The aprepitant microemulsion consists of the following components in percentage by mass: 0.05 to 2 percent of aprepitant, 5 to 30 percent of oil for injection, 0.5 to 10 percent of emulsifier, 1 to 10 percent of co-emulsifier, 5 to 20 percent of protective agent and 60 to 80 percent of water for injection. Compared with the conventional oral aprepitant, the aprepitant microemulsion for the injection has the outstanding advantage that: the aprepitant is insoluble in the water and an organic solvent, in order to realize the aprepitant injection, the aprepitant microemulsion and aprepitant micro emulsion freeze-drying powder are prepared successfully and can be subjected to large-scale industrial production; and compared with a fosaprepitant dimethyl-meglumine injection, the aprepitant microemulsion has the advantages that: the cost is reduced greatly, the practicality is extremely high, and economic and social benefits are relatively good.
Owner:NANJING YOKO PHARMA GRP CO LTD
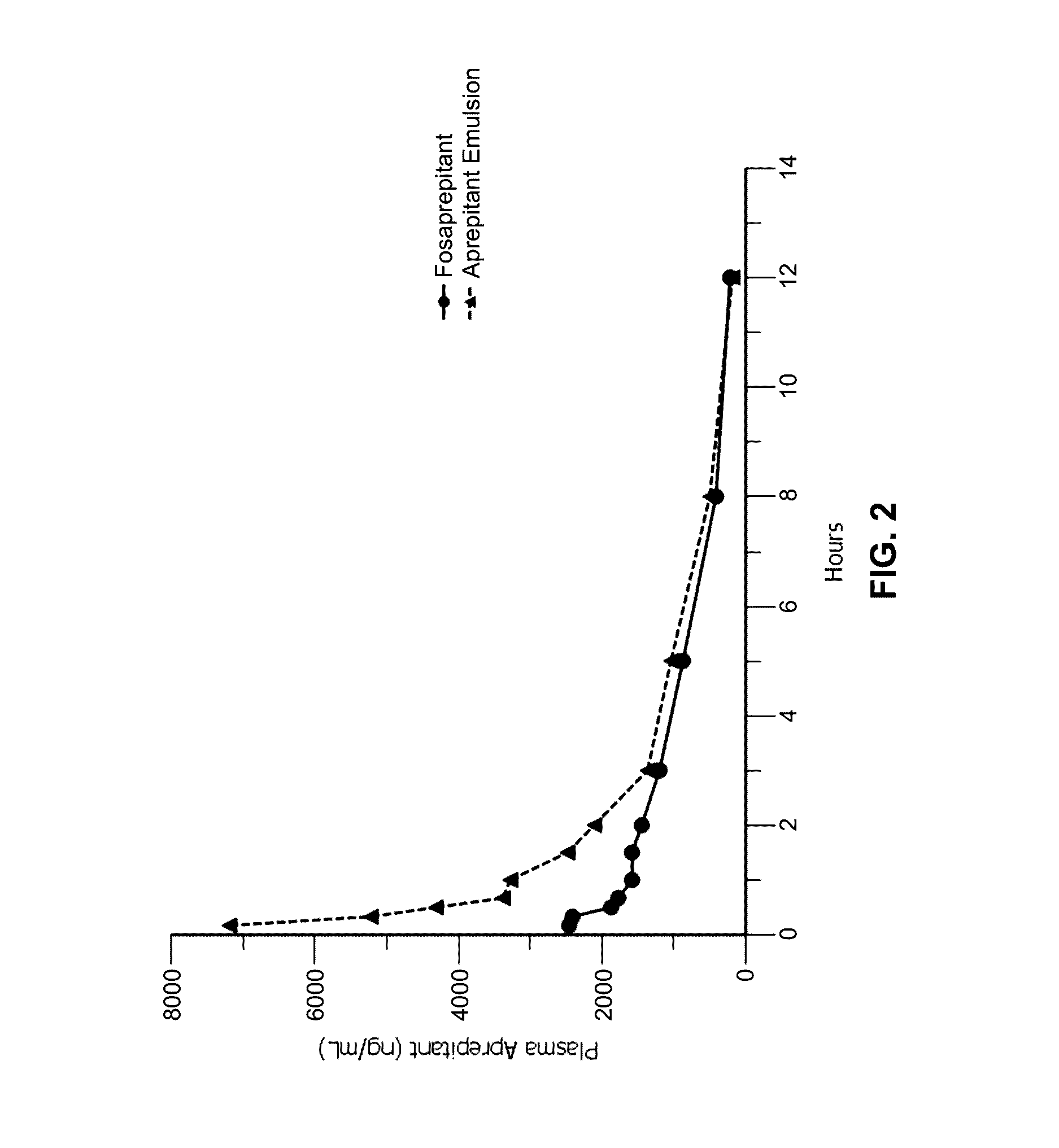
Aprepitant Injectable Formulations
InactiveUS20130317016A1Improve stabilityIncrease moisture contentOrganic active ingredientsDigestive systemHigh concentrationMedicine
An aqueous stable and ready-to-use formulation of aprepitant is prepared. Especially preferred formulations comprise a synergistic combination of a co-solvent and a surfactant and may further include a secondary co-solvent. Among other advantages of contemplated formulations, aprepitant is dissolved at high concentrations and remains dissolved and stable, even over prolonged periods of time.
Owner:INNOPHARMA
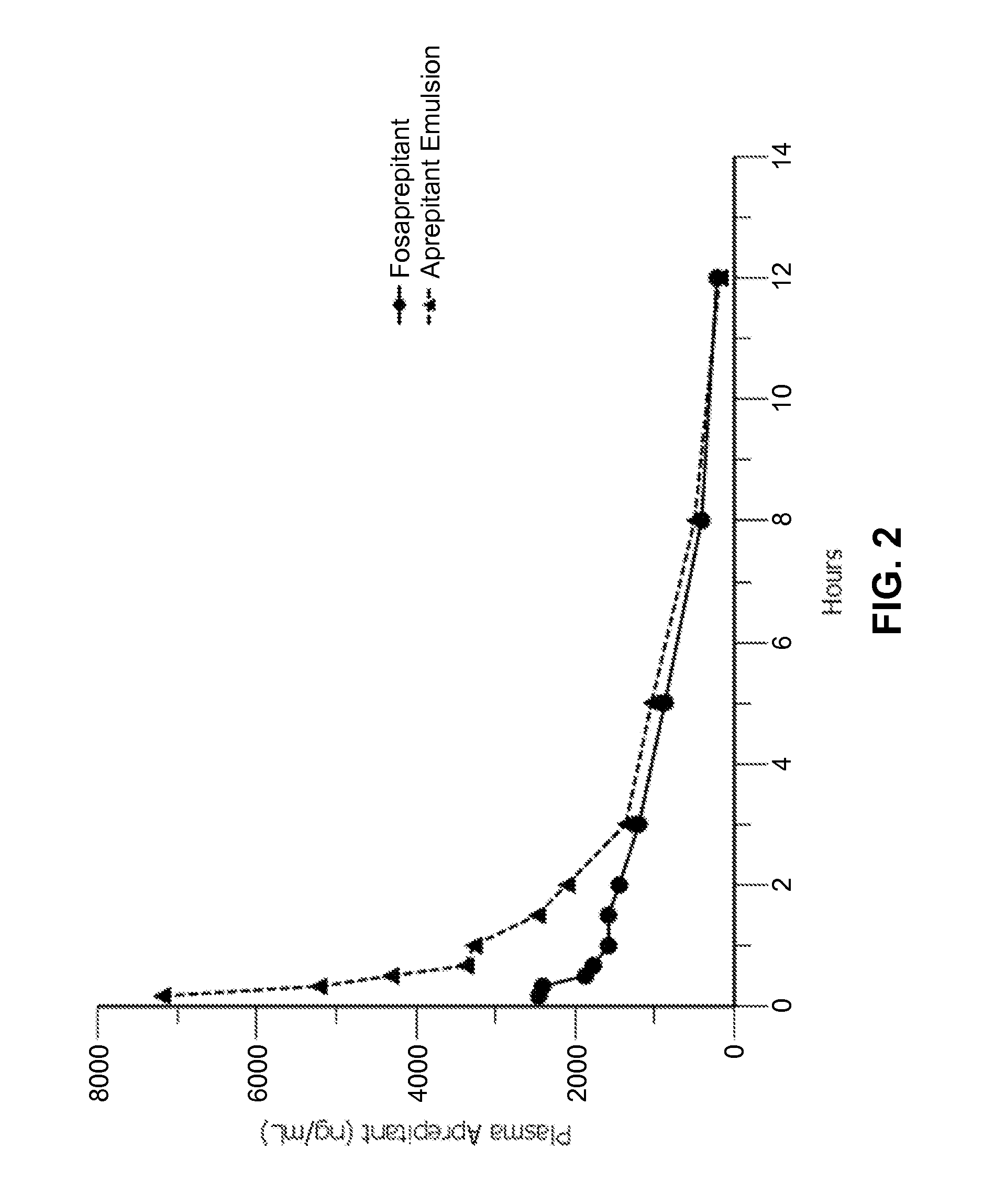
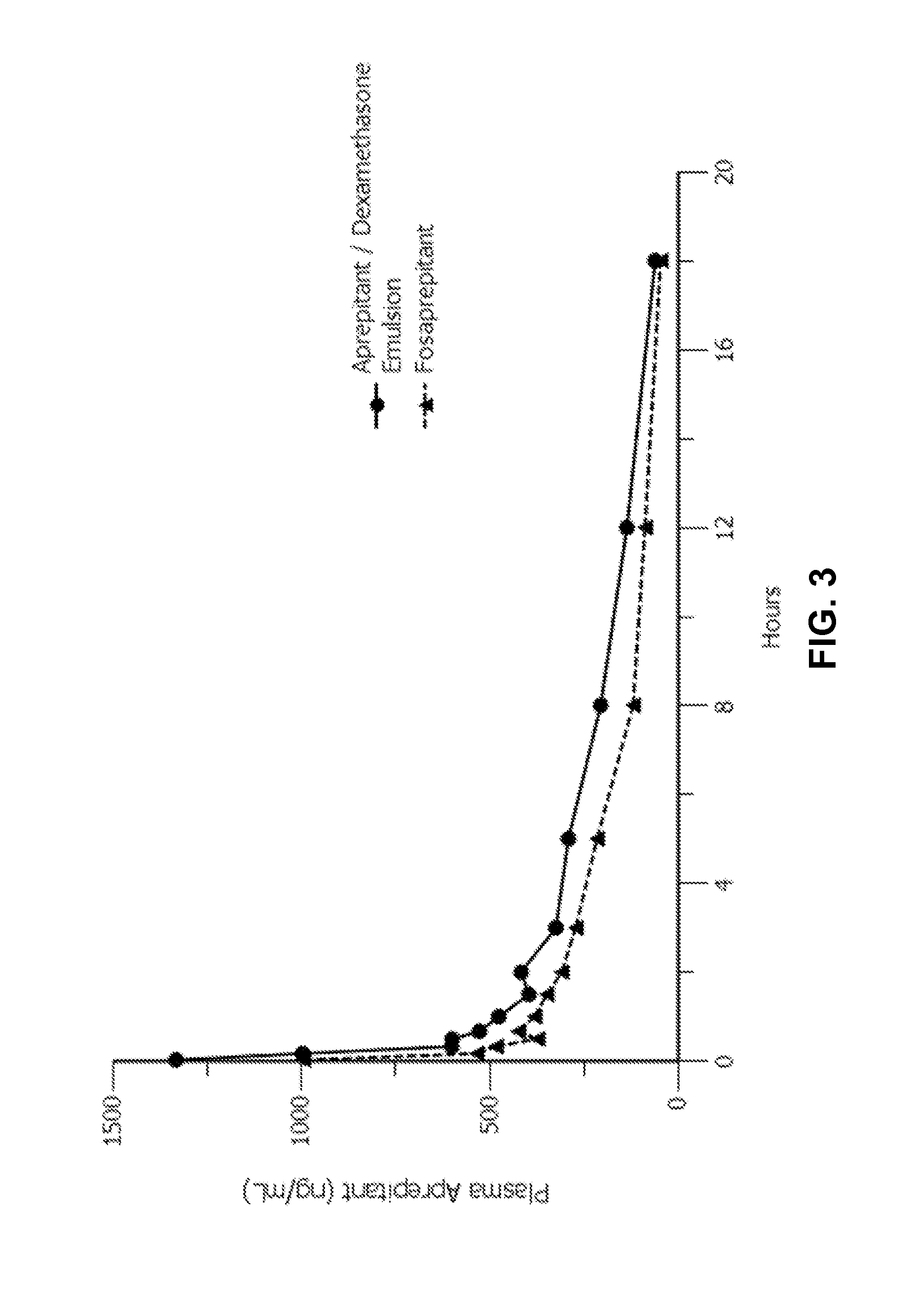
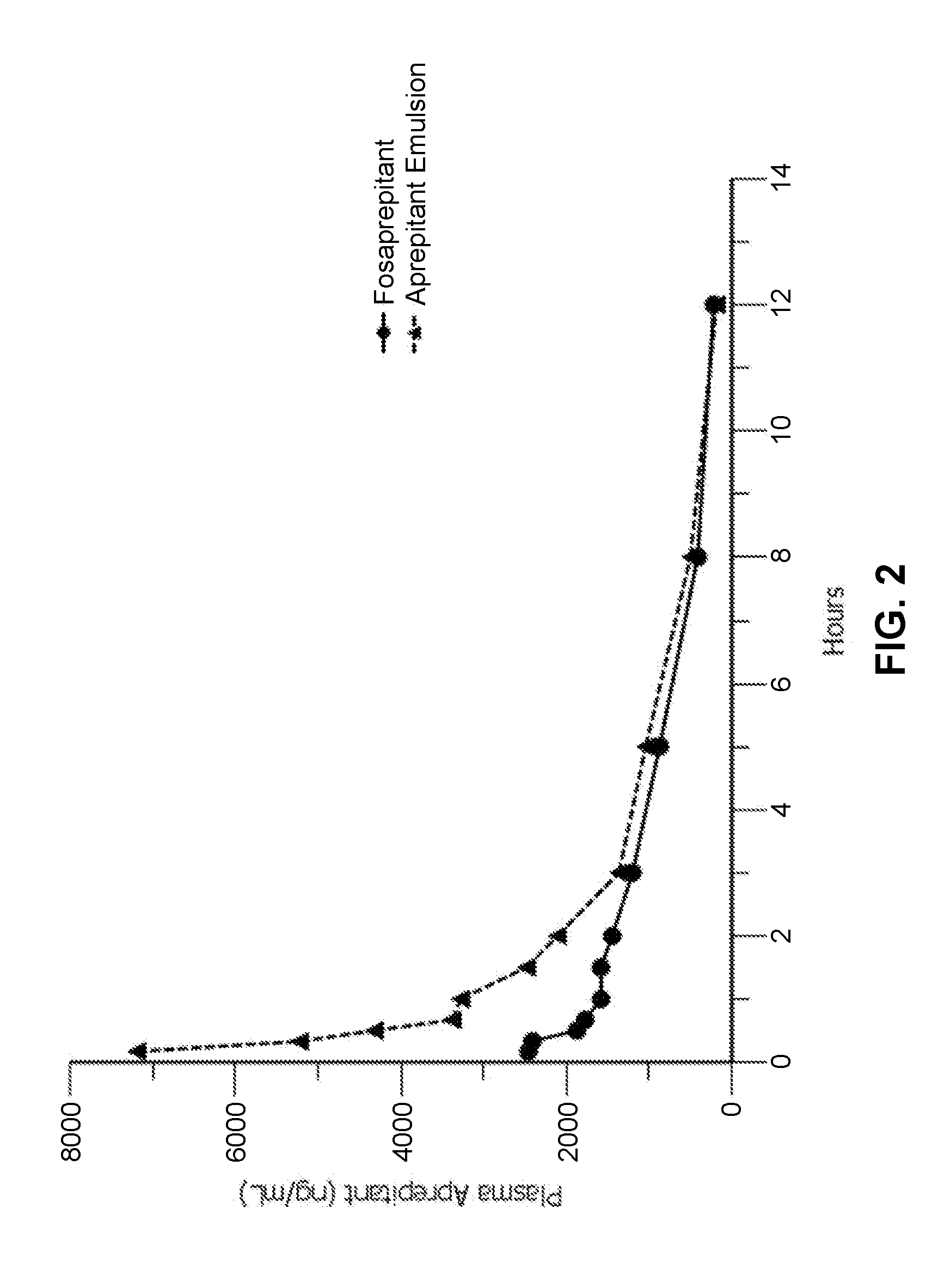
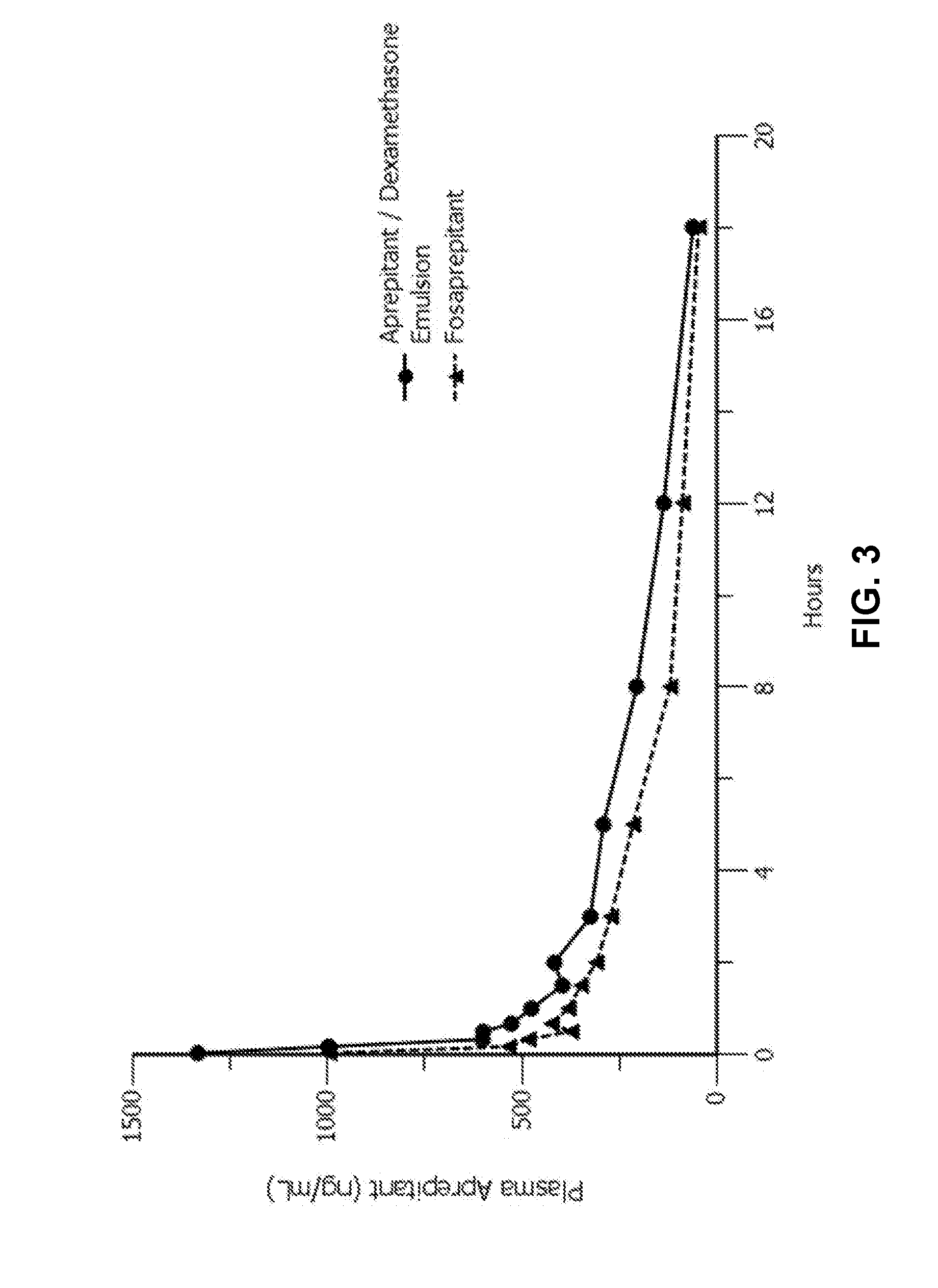
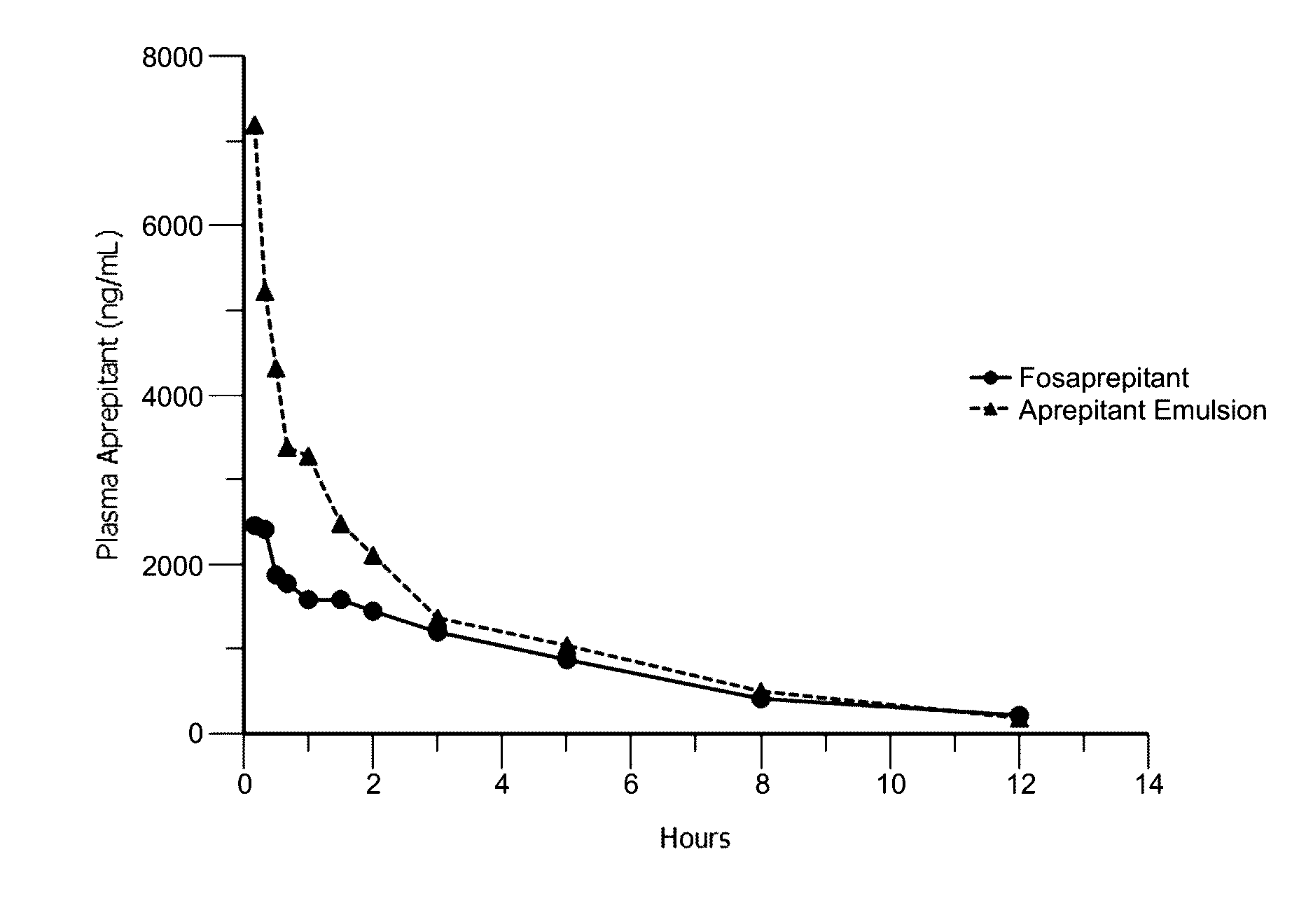
Emulsion formulations of aprepitant
ActiveUS20160082013A1Organic active ingredientsDigestive systemOral treatmentPharmaceutical formulation
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Emulsion formulations of aprepitant
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
Emulsion formulations of aprepitant
Disclosed herein are novel pharmaceutical formulations of aprepitant suitable for parenteral administration including intravenous administration. Also included are formulations including both aprepitant and dexamethasone sodium phosphate. The pharmaceutical formulations are stable oil-in-water emulsions for non-oral treatment of emesis and are particularly useful for treatment of subjects undergoing highly emetogenic cancer chemotherapy.
Owner:HERON THERAPEUTICS
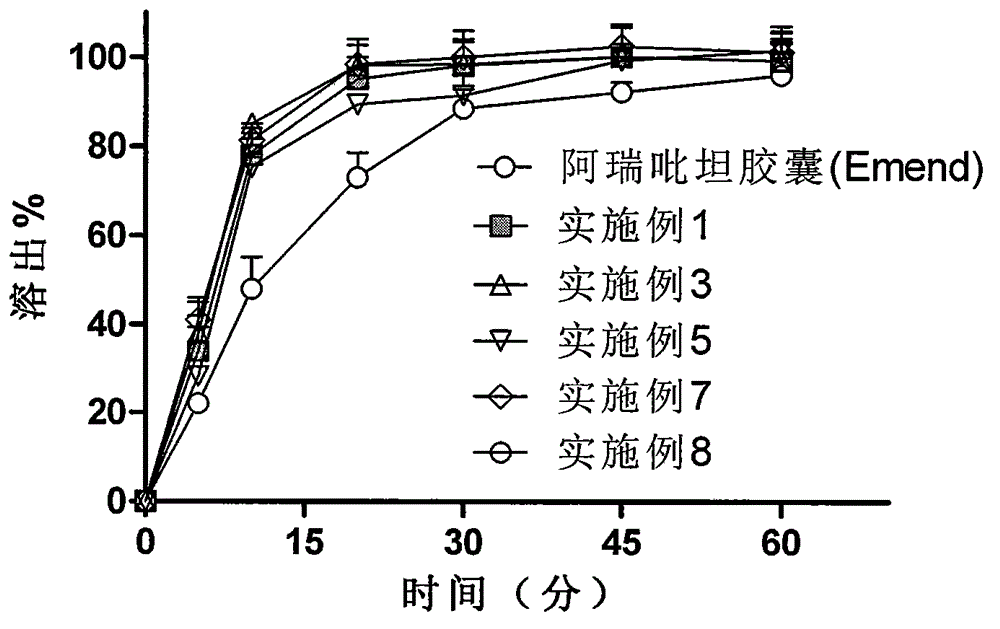
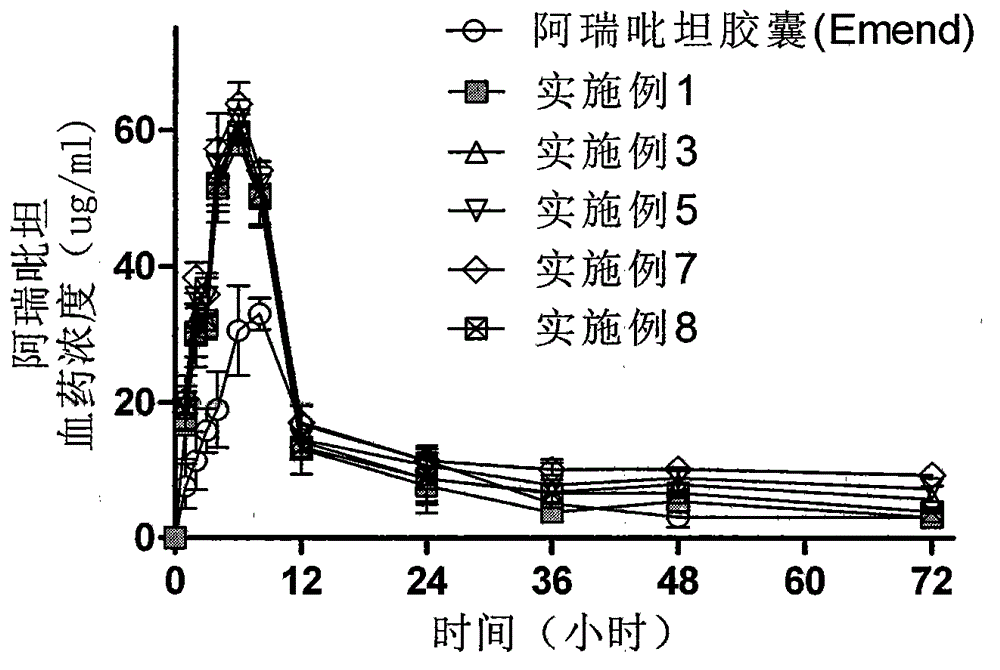
Aprepitant solid dispersion composition
ActiveCN102525880AGuaranteed stabilityImprove stabilityOrganic active ingredientsDigestive systemWater basedMass ratio
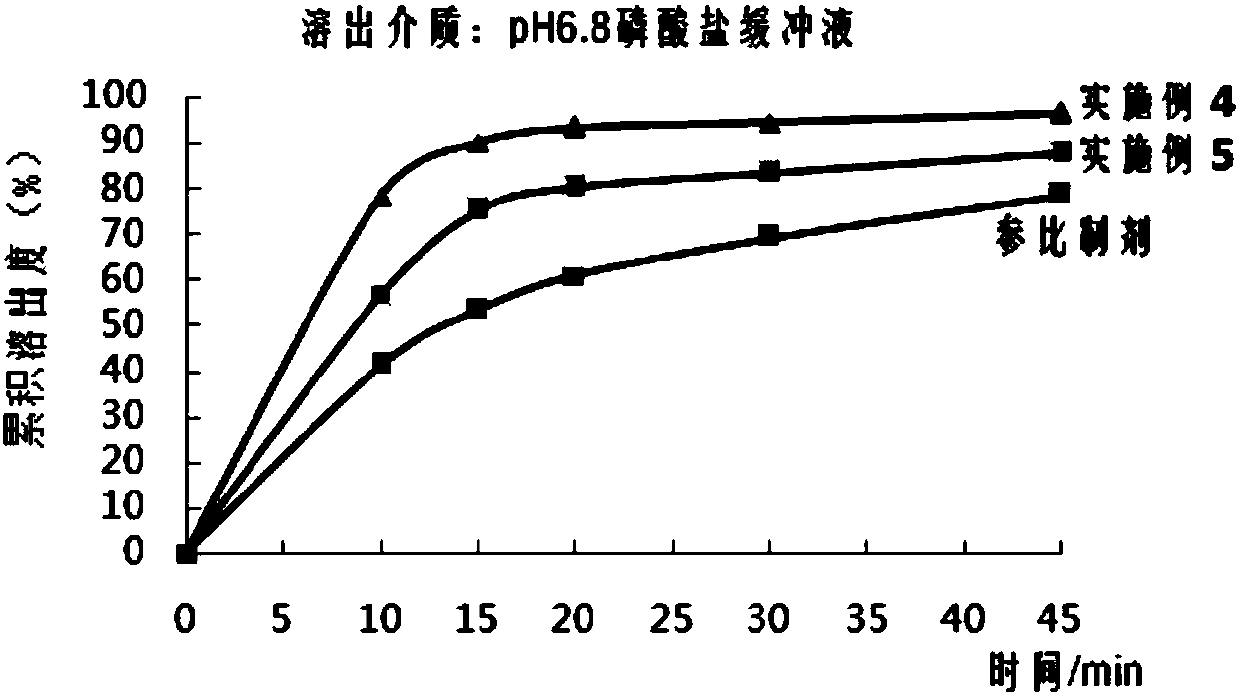
The invention belongs to the field of medicinal preparation, and particularly provides an aprepitant solid dispersion composition and a preparation method thereof as well as a medicinal composition prepared from the solid dispersion composition. The composition contains aprepitant and at least one water-soluble polymer, and the aprepitant is uniformly dispersed in the water-soluble polymer. The mass ratio of the aprepitant to the water-soluble polymer is 1:(0.5-10). The aprepitant solid dispersion composition provided by the invention is dissolved in a water-based medium at an unexpected high level in a relatively short time, and has the advantages of improved dissolution characteristic and obvious physical stability.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for preparing aprepitant solid dispersing composition
ActiveCN102525879AGuaranteed stabilityImprove stabilityOrganic active ingredientsDigestive systemWater basedWater soluble
The invention belongs to the field of medicament preparations, and in particular provides a method for preparing an aprepitant solid dispersing composition. The composition contains aprepitant and at least one water-soluble polymer. The method comprises the following steps of: crushing, mixing, melting and extruding the aprepitant and the water-soluble polymer in an extruder, wherein the heating temperature of the extruder is not below the Tg value of the water-soluble polymer or between 30 DEG C below the melting point and the melting point of the aprepitant; and cooling and curing the extruded product, and thus obtaining the solid dispersing composition. The aprepitant solid dispersing composition prepared by the method is dissolved in a water-based medium on an unexpected high level in relatively short time, and has improved dissolving characteristic and remarkable physical stability.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Stable injectable pharmaceutical composition of neurokinin 1 receptor antagonist and process for preparation thereof
InactiveUS20150165045A1Uniform and constant rateOvercome deficienciesOrganic active ingredientsBiocideNeurokinin-1 Receptor AntagonistsControlled release
The present invention relates to a controlled release sterile injectable formulation in the form of solution, suspension or sol-gel formulation for intramuscular or subcutaneous administration comprising a therapeutically effective amount of a neurokinin 1 receptor antagonist, in particular Aprepitant or Fosaprepitant or pharmaceutical acceptable salt, derivative or metabolite thereof. It also relates to a process for developing such formulations.
Owner:PHARMATHEN
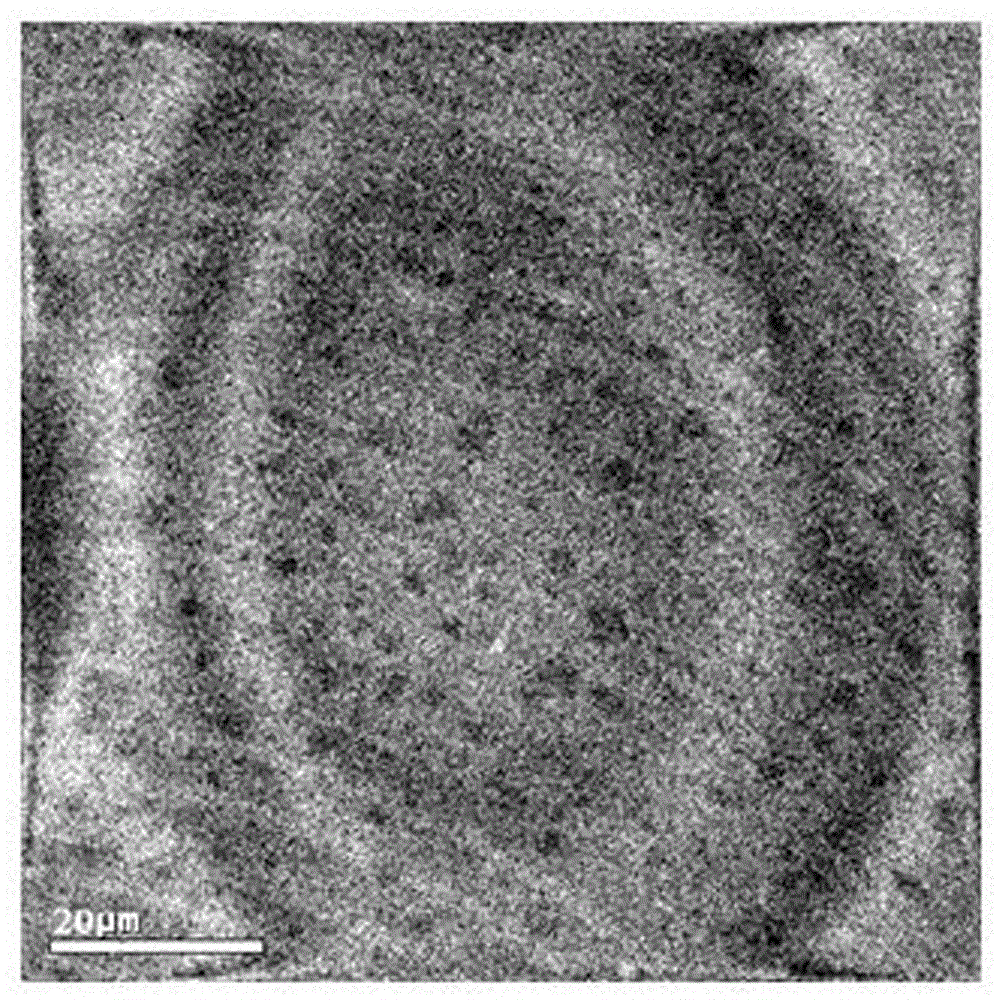
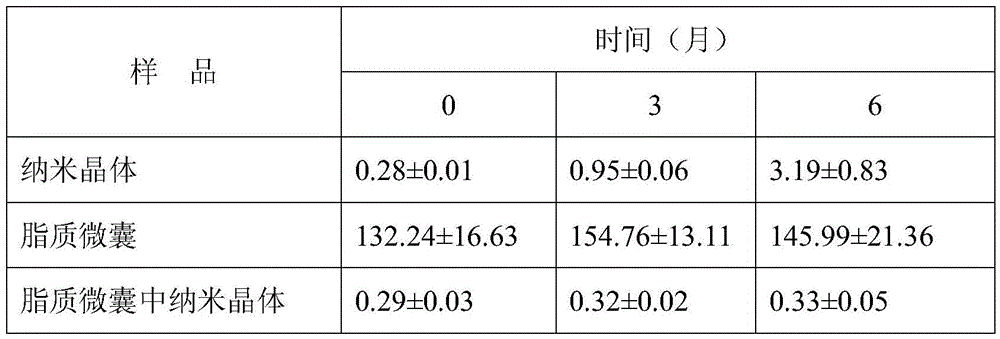
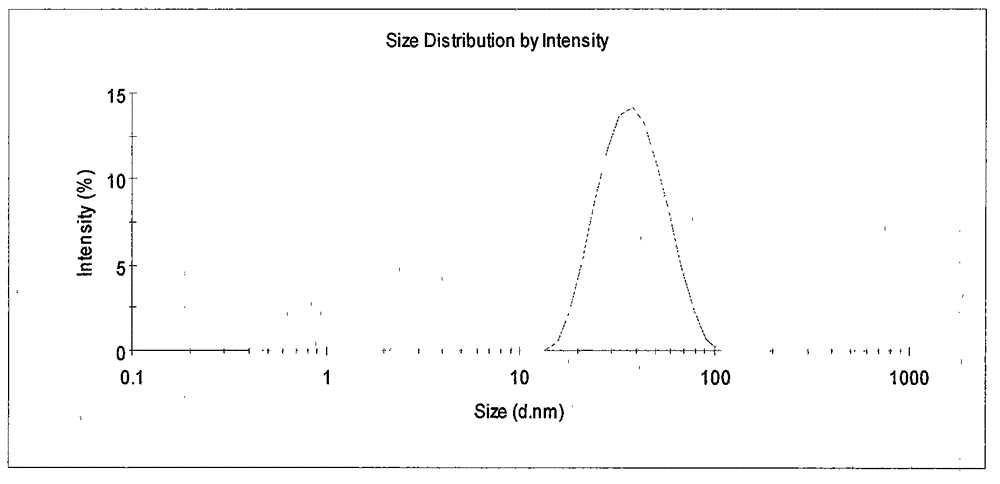
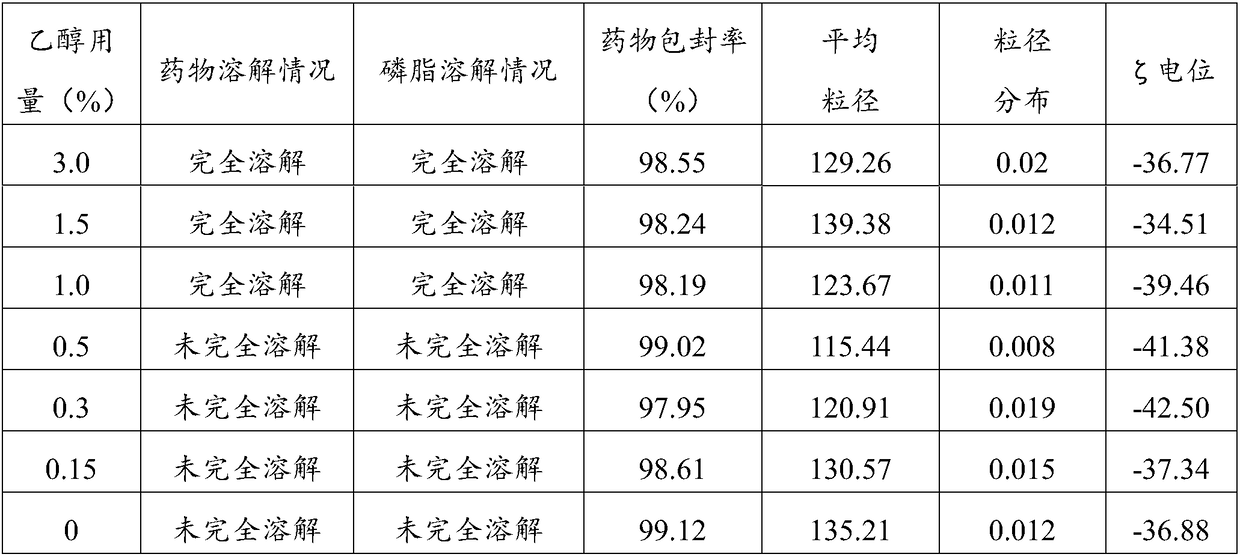
Loaded aprepitant nanocrystal lipid microcapsule and preparation method thereof
ActiveCN105456228AImprove stabilityMild preparation conditionsOrganic active ingredientsNervous disorderPhospholipidHigh pressure
The invention relates to a loaded aprepitant nanocrystal lipid microcapsule and a preparation method thereof, and belongs to the technical field of a medicine. The microcapsule is prepared by the method comprising the following steps: preparing an aprepitant nanocrystal from aprepitant by virtue of a grinding process, high-speed air jet pulverization or high-pressure homogenization, then mixing and emulsifying the aprepitant nanocrystal with phospholipid, removing a liquid phase and drying so as to obtain the aprepitant nanocrystal lipid microcapsule. The prepared loaded aprepitant nanocrystal lipid microcapsule can improve the stability of the aprepitant nanocrystal and the lipid loaded nanocrystal can increase the speed and the amount of being absorbed by gastrointestinal mucosa of a body, so that the effects of relatively high bioavailability and accelerated development of efficacy are achieved; and the preparation method of the loaded aprepitant nanocrystal lipid microcapsule is mild in condition, simple and controllable, low in preparation cost and is suitable for large-scale production.
Owner:FUREN PHARMA GROUP +2
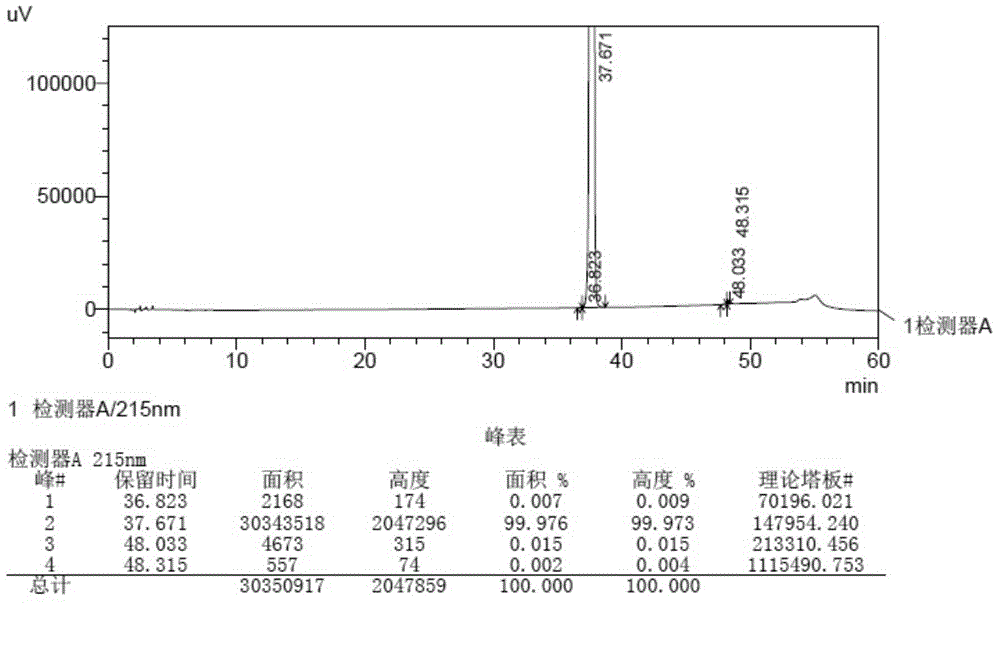
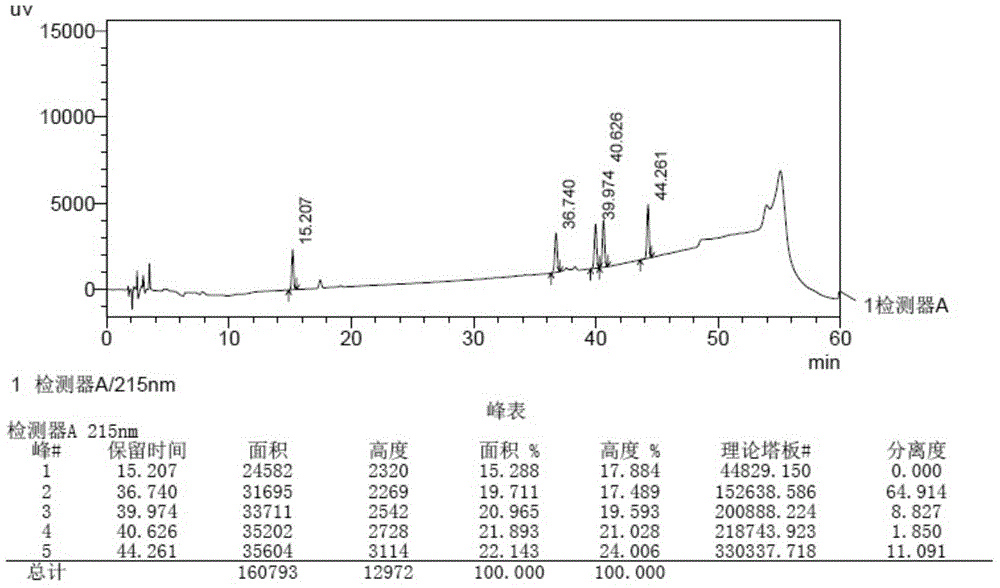
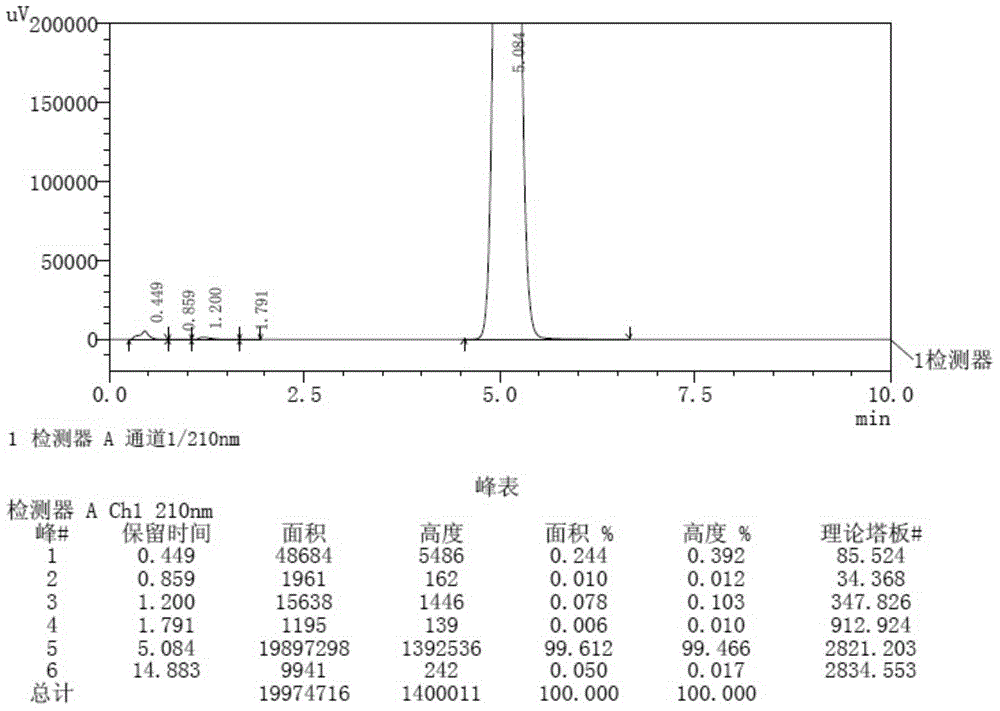
Method for detecting related substances in aprepitant by high performance liquid chromatography
The invention discloses a method for detecting related substances in aprepitant by high performance liquid chromatography. The method comprises the following steps: a. preparing a test solution; b. preparing a reference substance solution; c. respectively detecting the test solution and the reference substance solution by high performance liquid chromatography; and d. calculating the content of the related substances in a test article by peak area by virtue of an external standard method. By adopting a specific flowing phase and a gradient elution program, the related substances in aprepitant can be detected, the degree of separation of the main peak is high, the numerical value of a tailing factor of the main peak is small, and the degree of separation of impurity compounds is high. The related substances in aprepitant are relatively comprehensively detected and controlled, and the test result is relatively accurate and reliable. Furthermore, as no ion-pairing agent which poses great harm to the chromatographic column such as trifluoroacetic acid, lauryl sodium sulfate and the like is added, and according to the detection method disclosed by the invention, harm to the chromatographic column is slight.
Owner:CHENGDU BAIYU PHARMA CO LTD
Preparation of aprepitant
InactiveUS20110094321A1Easy to manageReduce impactOrganic chemistryMaterial analysisPolymer scienceAprepitant
Owner:DR REDDYS LAB LTD +1
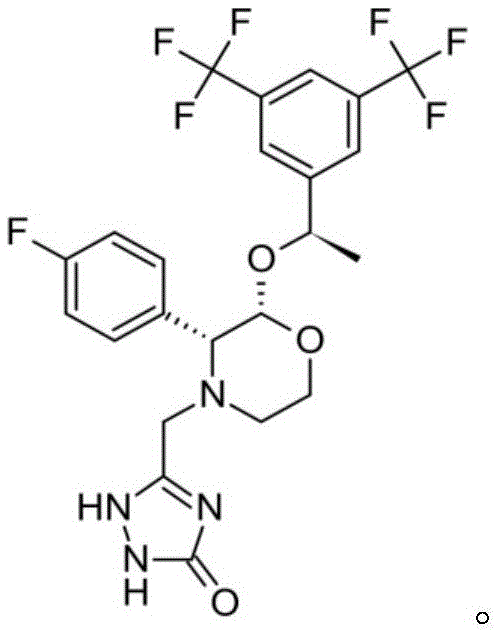
Aprepitant compound and its preparation method
ActiveCN104367551AOvercoming the problem of low solubilityImprove bioavailabilityOrganic active ingredientsPowder deliverySolubilitySuspending Agents
The invention provides an aprepitant compound. The compound comprises 30-70wt% of aprepitant, 20-60wt% of monosaccharide and 10-50wt% of a suspending agent. The monosaccharide in the compound is helpful for forming aprepitant nanocrystals; and the suspending agent can avoid local non-uniformity caused by wet grinding and settlement of a suspension in the spray drying process. The prepared aprepitant compound greatly improves the solubility of aprepitant, is helpful for improving the bioavailability of aprepitant, and has great clinical application values. The method of the invention is simple, and is suitable for industrial production.
Owner:SHANGHAI SUNTECH PHARMA +1
Aprepitant nanosuspension and preparation method thereof
ActiveCN103251556AOral bioavailability is lowRapid dissolutionOrganic active ingredientsDigestive systemBioavailabilityAprepitant
The invention belongs to the technical field of medicines, and discloses an aprepitant nanosuspension and a preparation method thereof. As aprepitant is small in solubility in water and lower in the oral bioavailability, popularization and use of aprepitant are limited. The aprepitant nanosuspension provided by the invention is characterized by comprising aprepitant, copovidone PVPS630 and povidone PVPK-90 by mass ratio of (4-10):(1-2):(1-2). The preparation method of the aprepitant nanosuspension is mild in condition and simple and controllable, and higher oral bioavailability of aprepitant is realized.
Owner:ZHEJIANG SUNDOC PHARMA SCI & TECH CO LTD
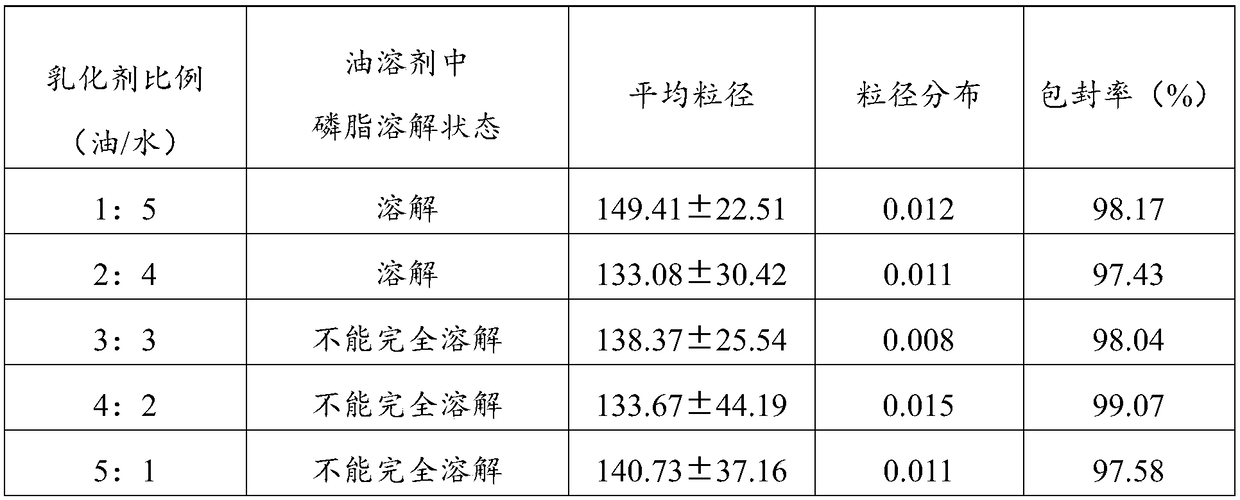
Aprepitant nano composition
InactiveCN105878250AImprove solubilityImprove bioavailabilityOrganic active ingredientsNervous disorderSolubilityOil phase
The invention discloses an aprepitant nano composition which is composed of aprepitant, an oil phase, an emulsifier, a co-emulsifier, a stabilizer, an auxiliary stabilizer and an optional solid adsorbent. The aprepitant nano composition significantly improves solubility, stability and bioavailability of the aprepitant.
Owner:北京乐嘉宝医药科技有限责任公司
Refining method of aprepitant key intermediate
The invention provides a refining and purifying method of aprepitant key intermediate (I); a product (I)-containing reaction liquid is obtained by Grignard addition reaction of a compound (II), the product (I)-containing reaction liquid is added into a mixture of an inorganic acid water solution and an organic solvent insoluble in water, after standing and layering, organic phase is washed successively with water and saturated salt water, and a white solid product is obtained by vacuum concentration to dry at a certain temperature. The refining method has low requirement to equipment, is in no need of control of the temperature in the quenching process, and is afe and convenient in operation, and the obtained intermediates (I) can be used for the preparation of aprepitant with higher purity, and is suitable for industrialized production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
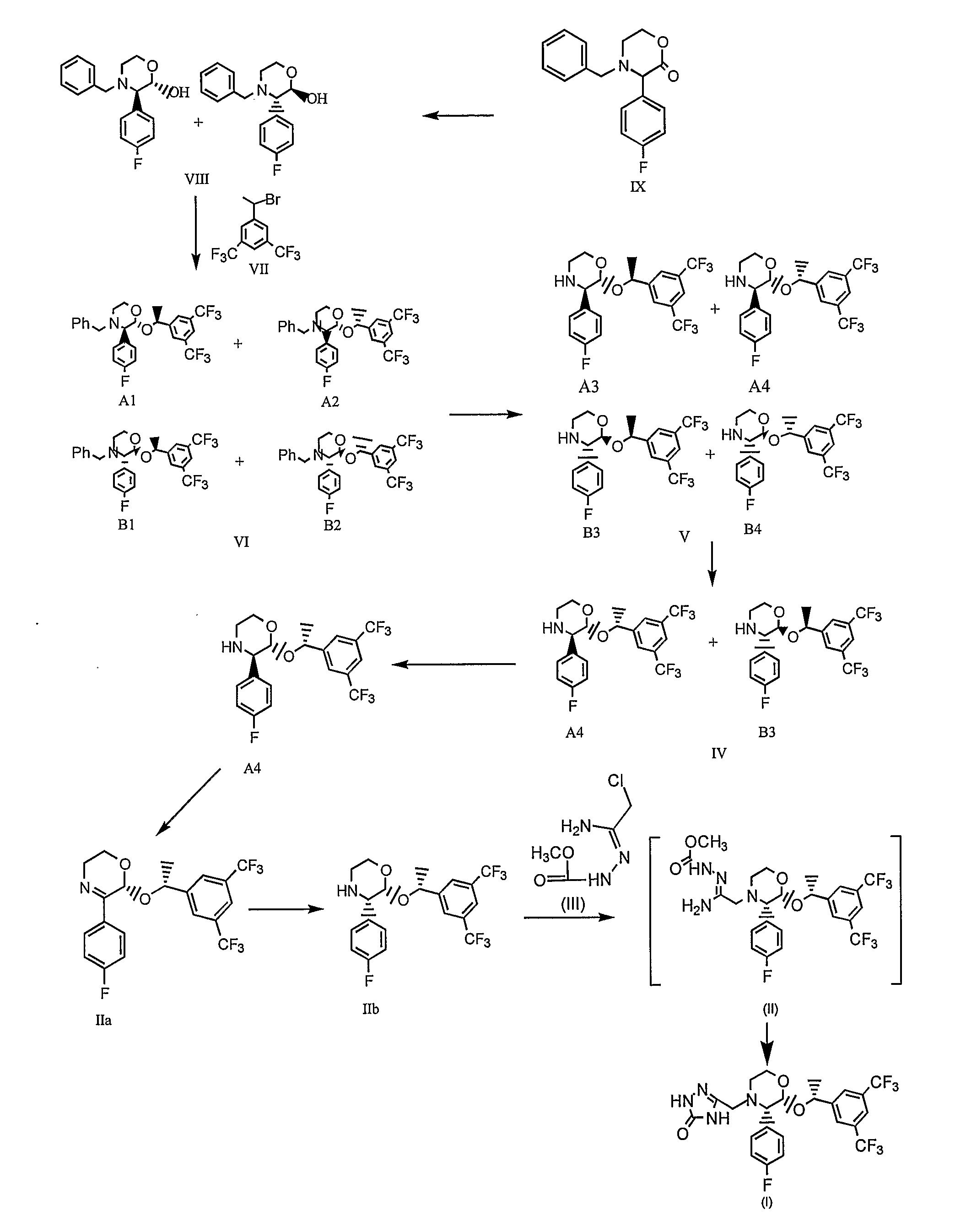
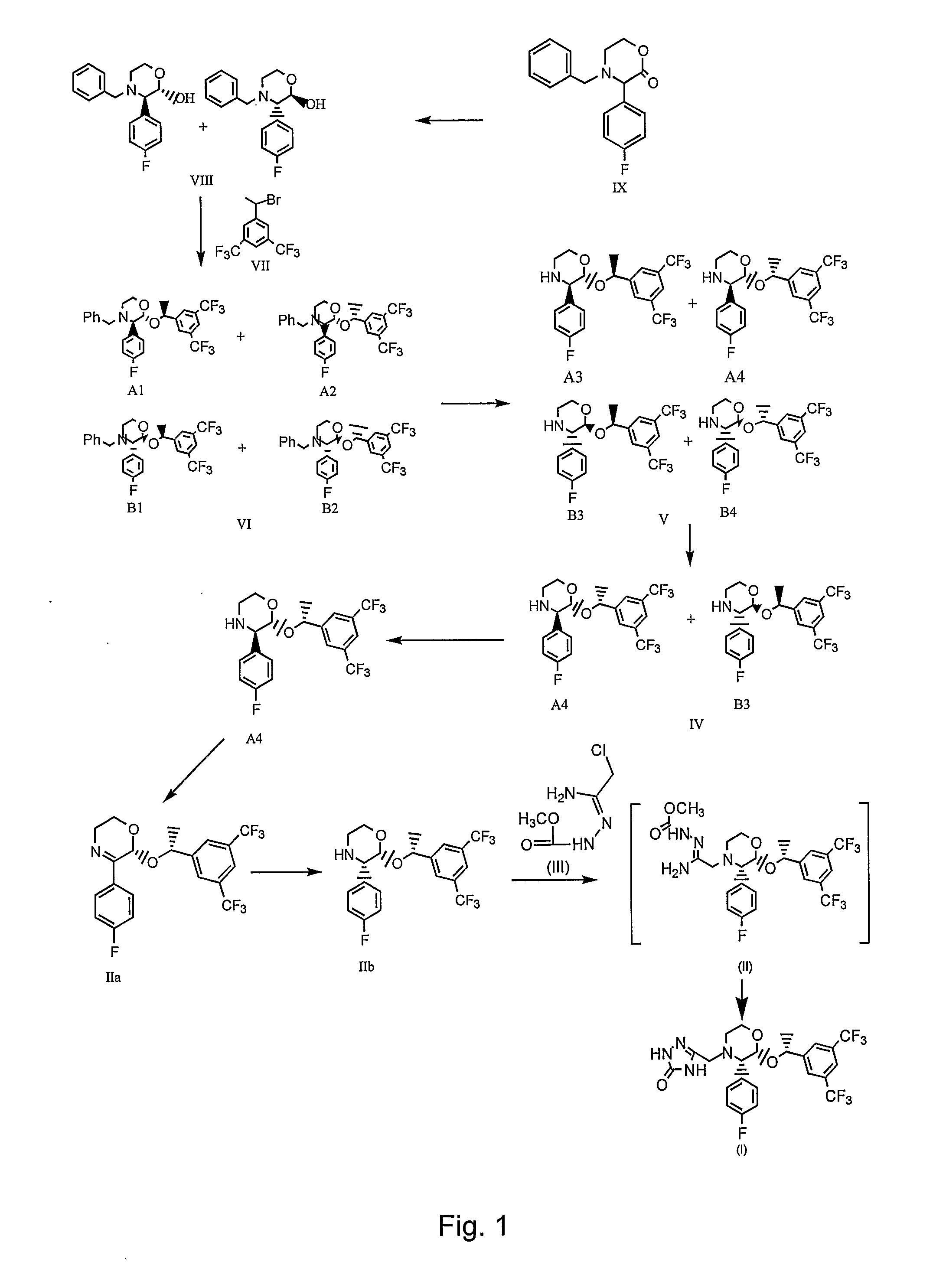
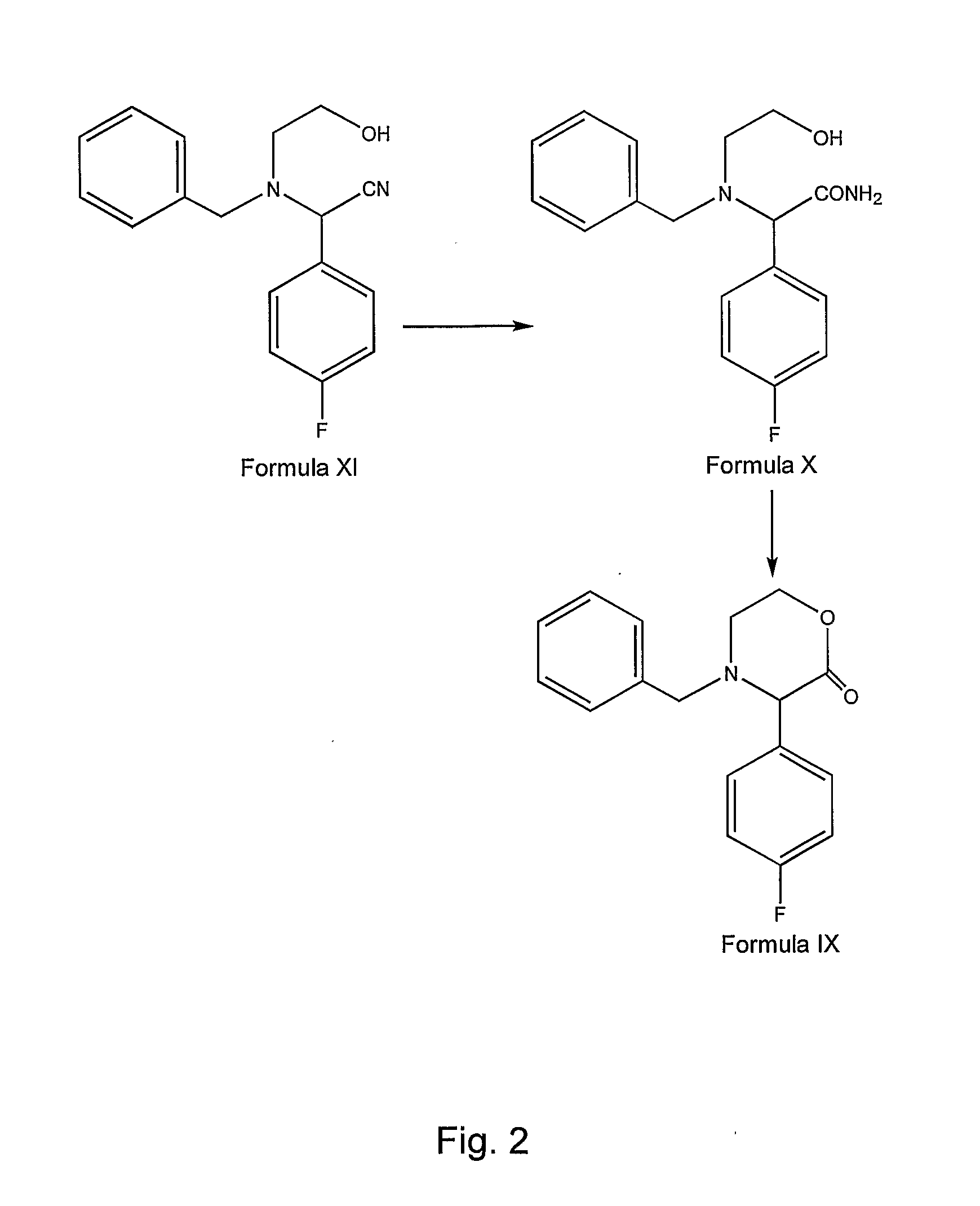
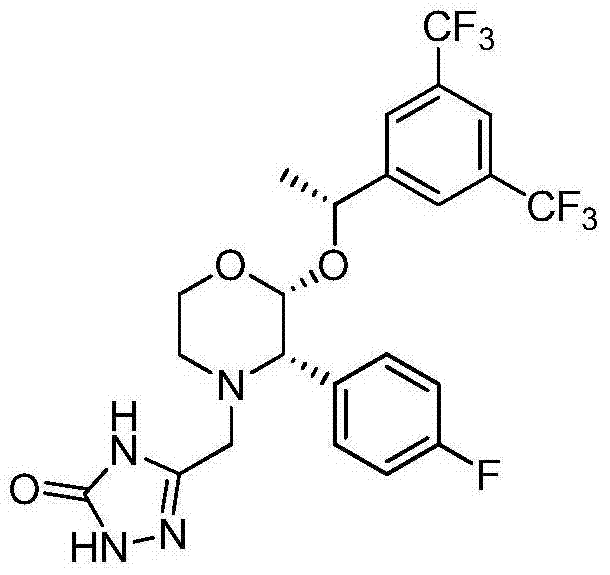
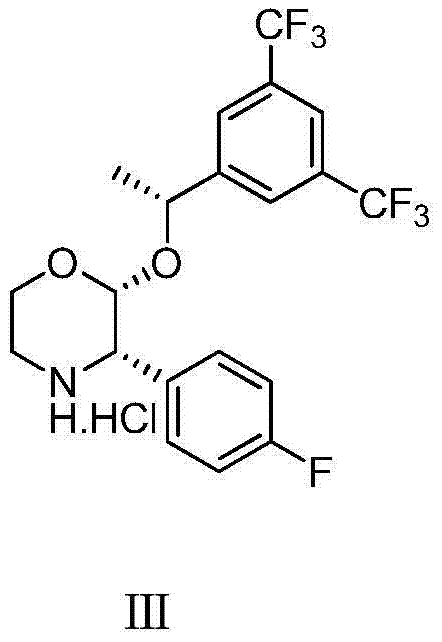
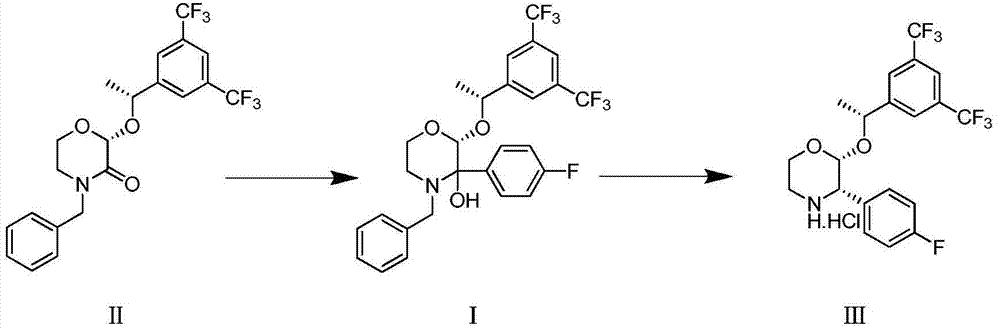
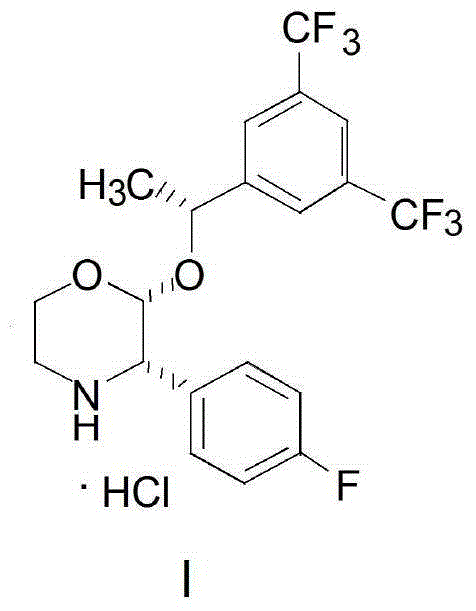
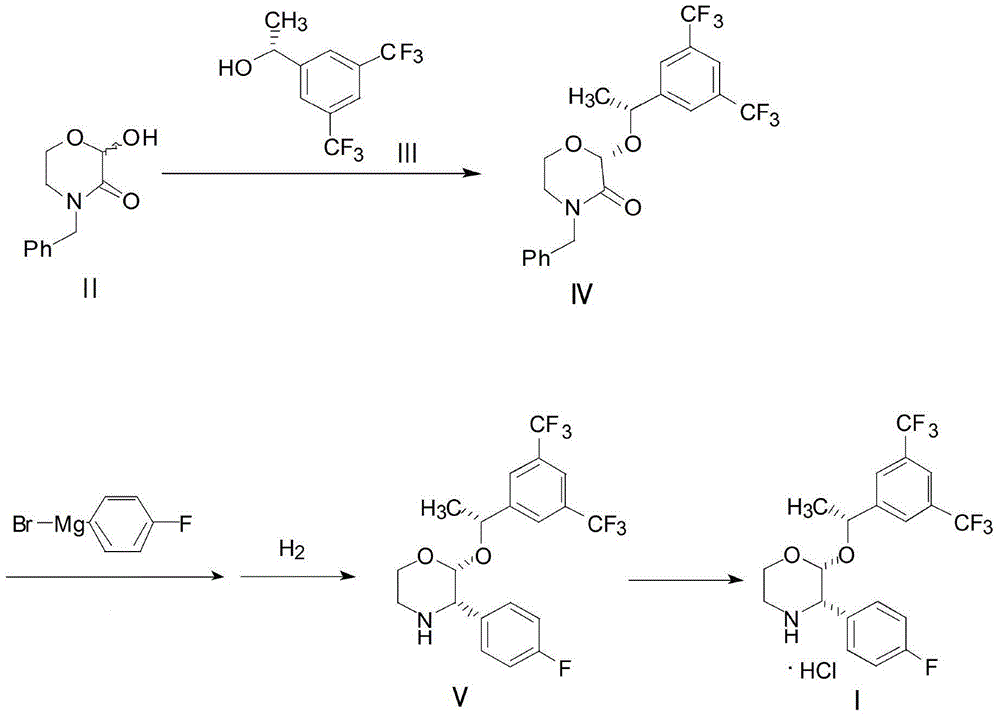
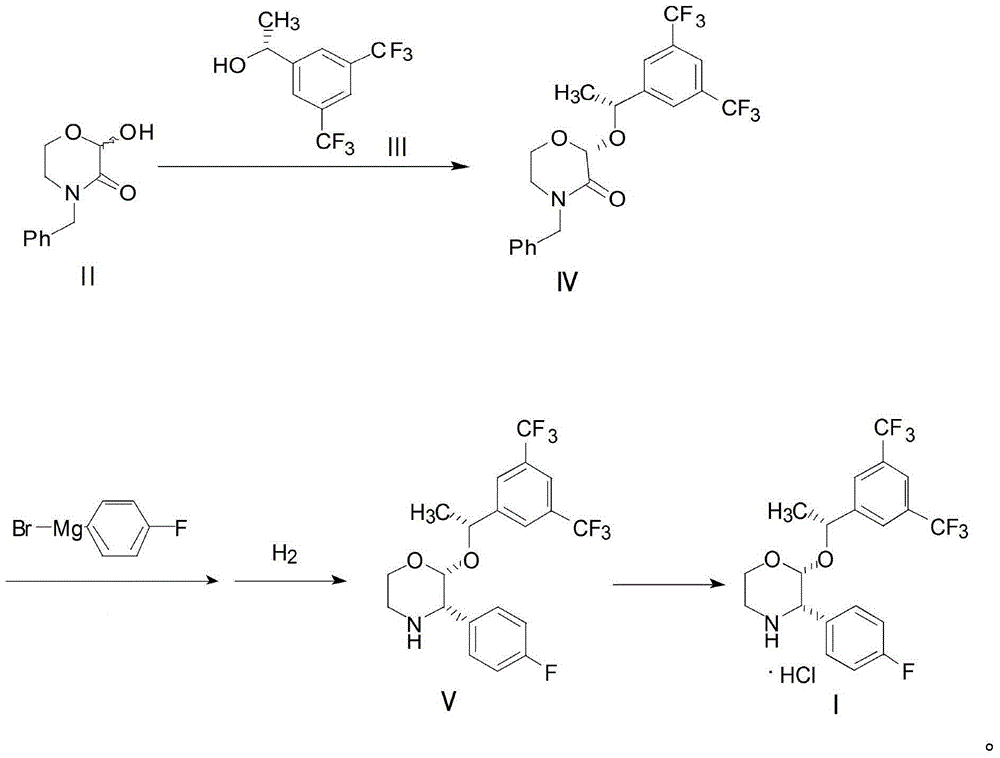
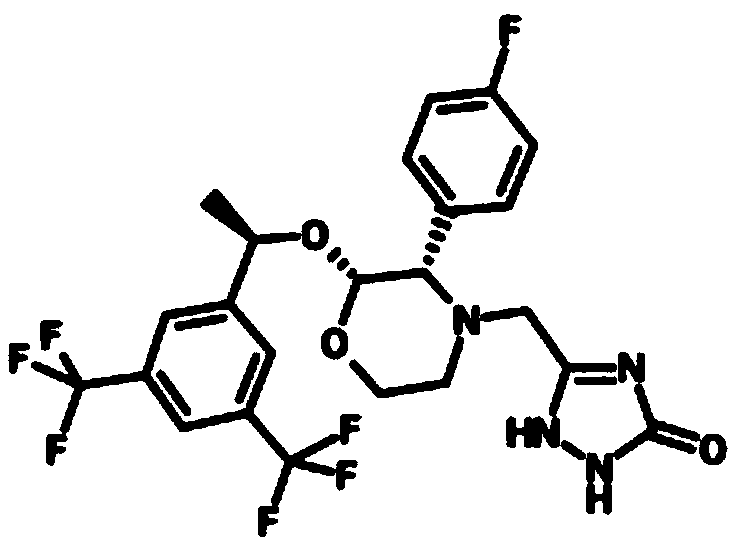
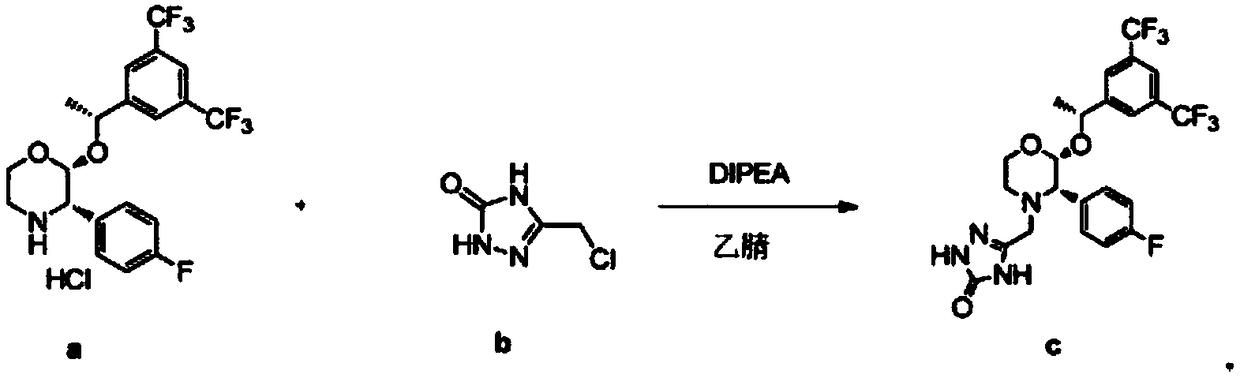
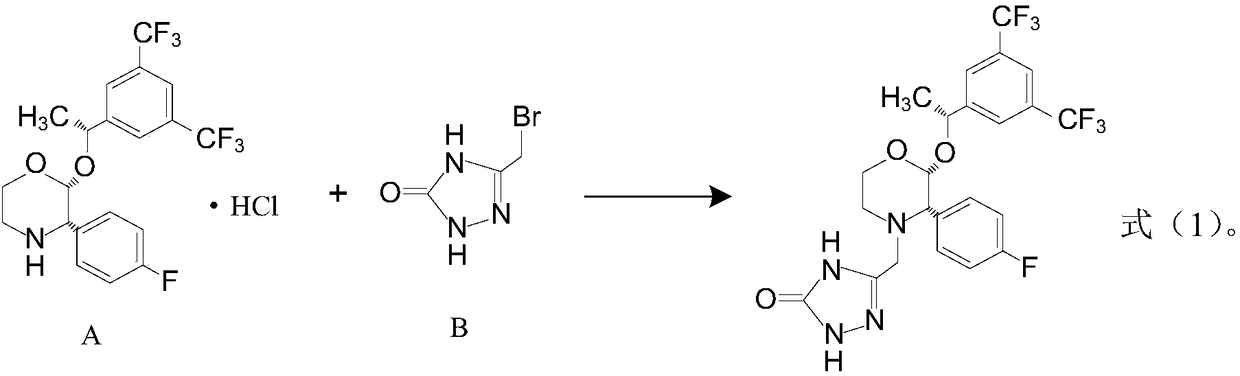
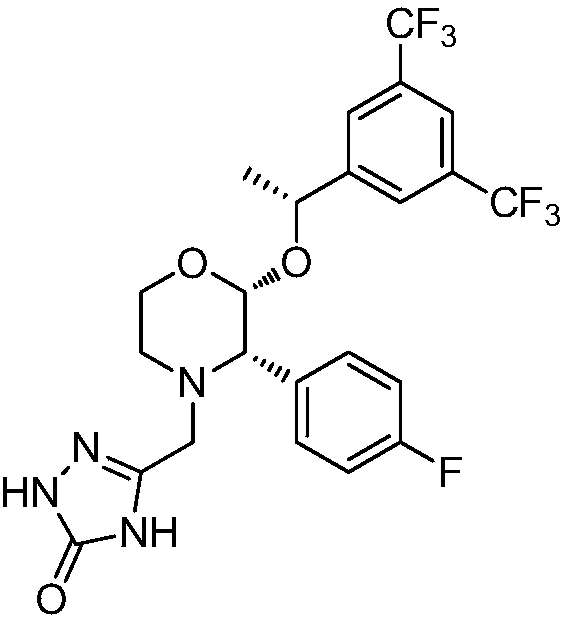
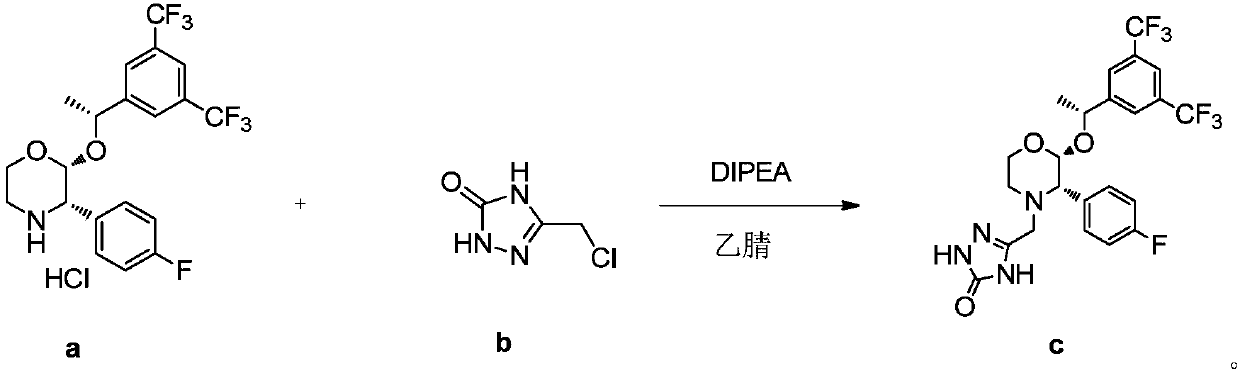
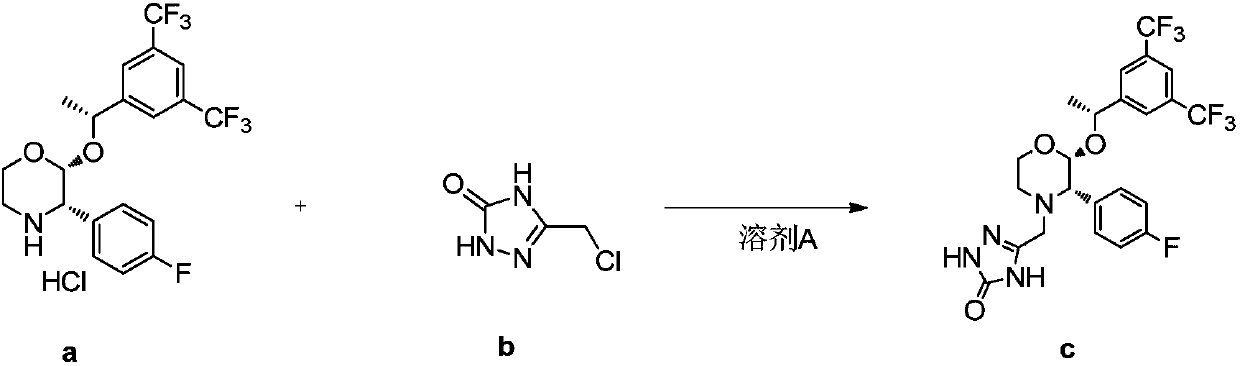
Preparation method of aprepitant intermediate
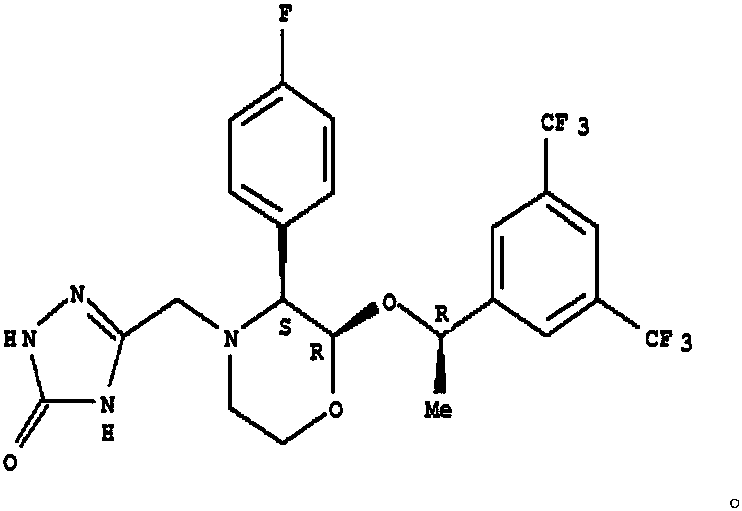
The invention discloses a method for synthesizing an aprepitant intermediate (2R, 3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethyoxyl]-3-(4-fluorophenyl)-morpholine hydrochloride I. The method comprises the following steps: performing a condensation reaction on the raw materials 4-benzyl-2-hydroxy-morpholine-3-one II and (R)-1-[3,5-bi(trifluoromethyl)phenyl]ethanol III in the presence of a catalyst to obtain a compound IV, adding a Grignard reagent to the obtained compound IV for having a Grignard reaction and converting the compound IV into a compound V under a reduction condition, and performing a hydrochlorination reaction on the obtained compound V to obtain the target compound I. The method is simple in preparation flow and suitable for industrial production, and has the advantages of high total recovery, convenient intermediate purification, high target product purity and the like.
Owner:武汉励合生物医药科技有限公司
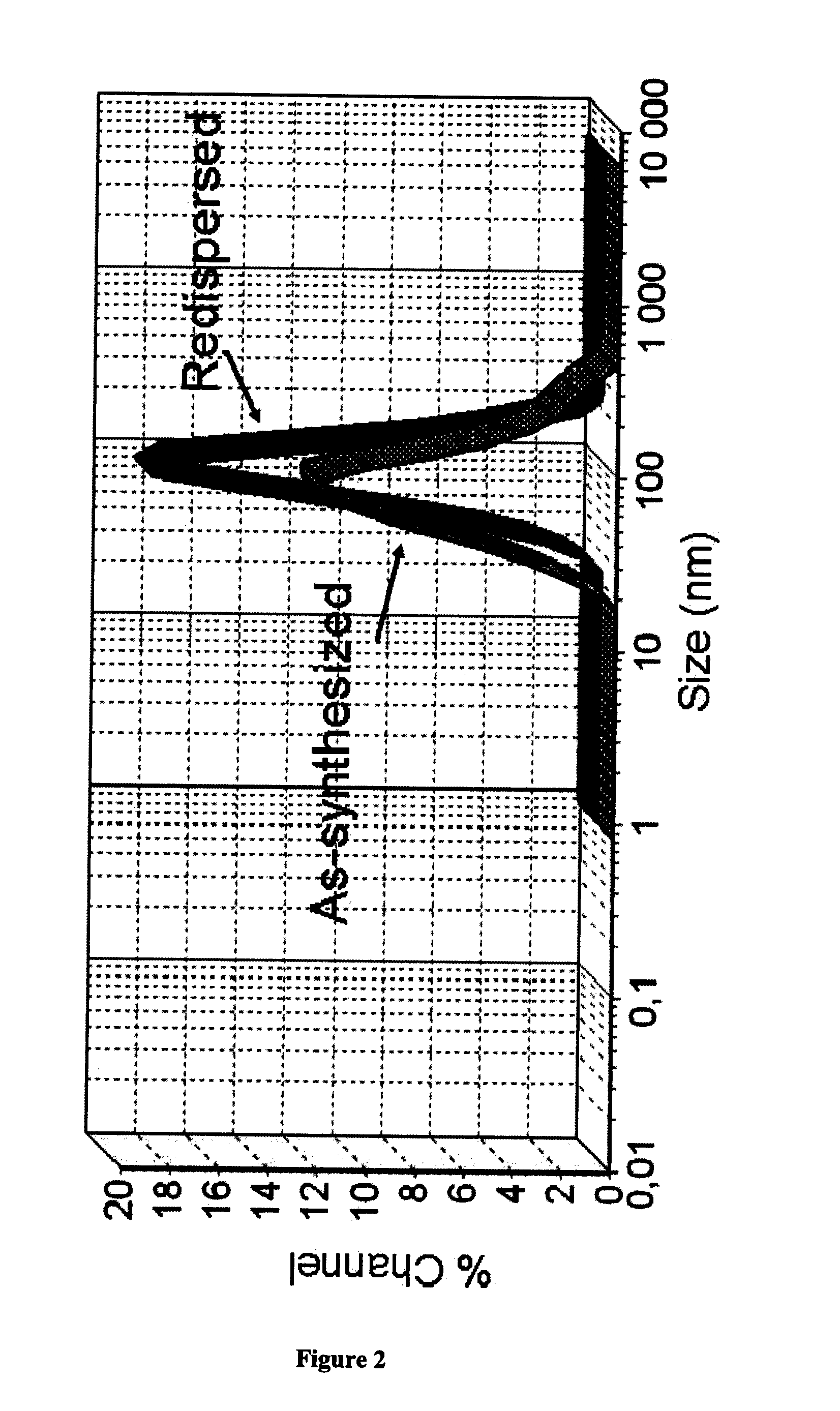
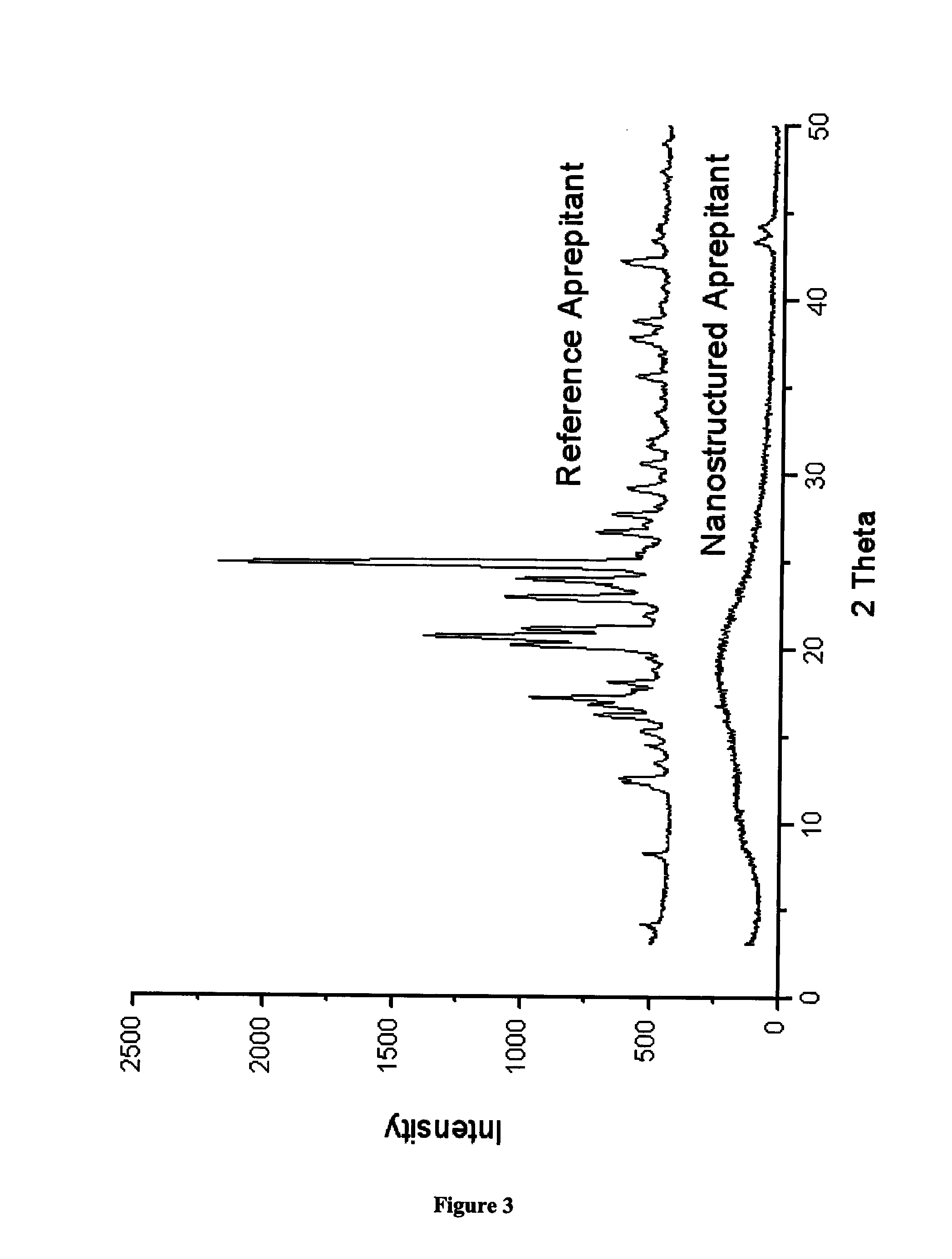
Nanostructured aprepitant compositions, process for the preparation thereof and pharmaceutical compositions containing them
InactiveUS20130209521A1Improve bioavailabilityReduce dosagePowder deliveryOrganic active ingredientsSolubilityChemical compound
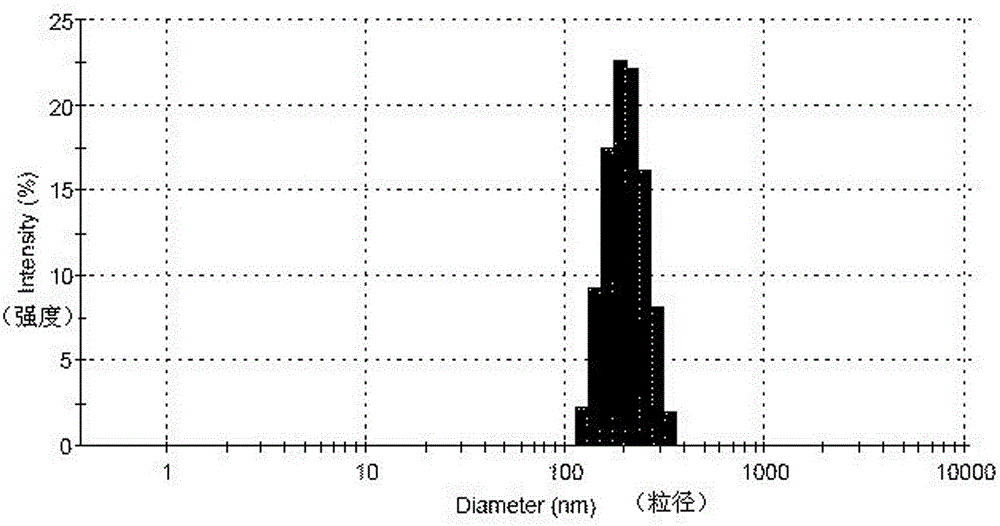
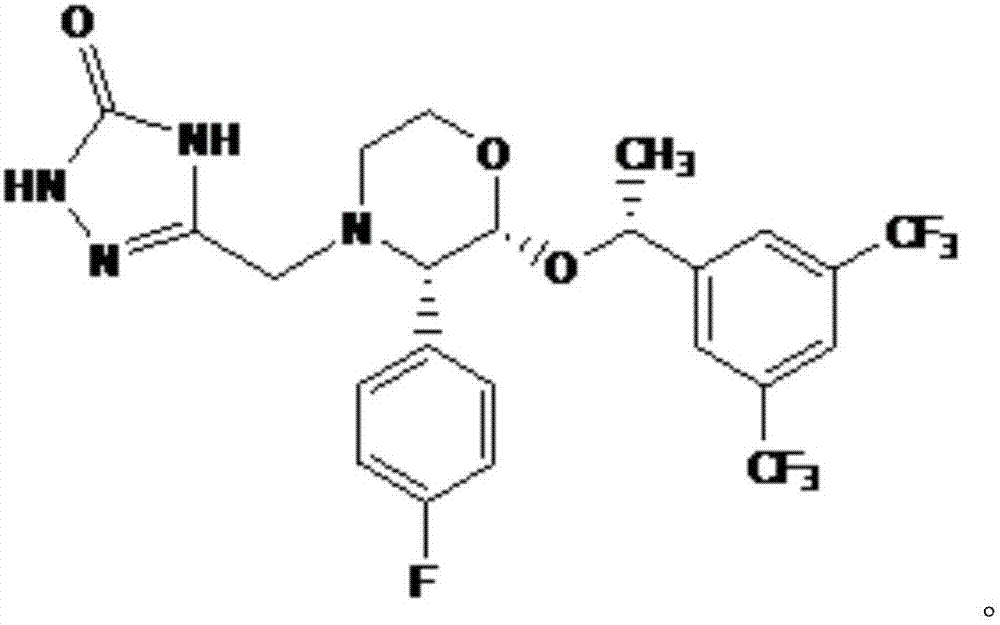
The present invention is directed to nanostructured Aprepitant compositions, process for the preparation thereof and pharmaceutical compositions containing them. The nanoparticles of Aprepitant according to the invention have an average particle size of less than about 200 nm. The stable nanostructured particles of the invention are presented by increased solubility, dissolution rate, permeability and bioequivalent or enhanced biological performance characterized by significantly decreased fed / fasted effect compared to the reference and marketed drug. Aprepitant is a chemical compound that belongs to a class of drugs called substance P antagonists (SPA). It mediates its effect by acting on neurokinin 1 receptor. Aprepitant is manufactured by Merck & Co. under the brand name Emend for prevention of acute and delayed chemotherapy-induced nausea and vomiting (CINV) and for prevention of postoperative nausea and vomiting.
Owner:NANOFORM CARDIOVASCULAR THERAPEUTICS
Aprepitant oral liquid formulations
A liquid pharmaceutical compositions comprising Aprepitant is preferably prepared as an oral suspension dosage form for the prevention and control of acute and delayed chemotherapy induced nausea and vomiting, and / or for prevention of postoperative nausea and vomiting.
Owner:INNOPHARMA
Aprepitant oral pharmaceutical preparation
InactiveCN105534987ADissolution rate is fastImprove bioavailabilityOrganic active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseHypromellose phthalate
The invention discloses an aprepitant oral pharmaceutical preparation. The aprepitant oral pharmaceutical preparation comprises 15wt%-25wt% of aprepitant, 45wt%-75wt% of hydroxypropyl methylcellulose phthalate / hydroxypropyl methylcellulose acetate succinate, 10wt%-25wt% of microcrystalline cellulose, lactose or mannitol, 2wt%-8wt% of low-substituted hydroxypropyl cellulose as well as croscarmellose sodium and / or crospovidone, 0-2wt% of silicon dioxide and / or talc and 0-2wt% of magnesium stearate. The pharmaceutical preparation can be prepared in a form of tablets or capsules andhas high stability and good bioavailability.
Owner:北京颐诺赛医药科技有限公司
Aprepitant emulsion formulationpreparation and preparation method thereof
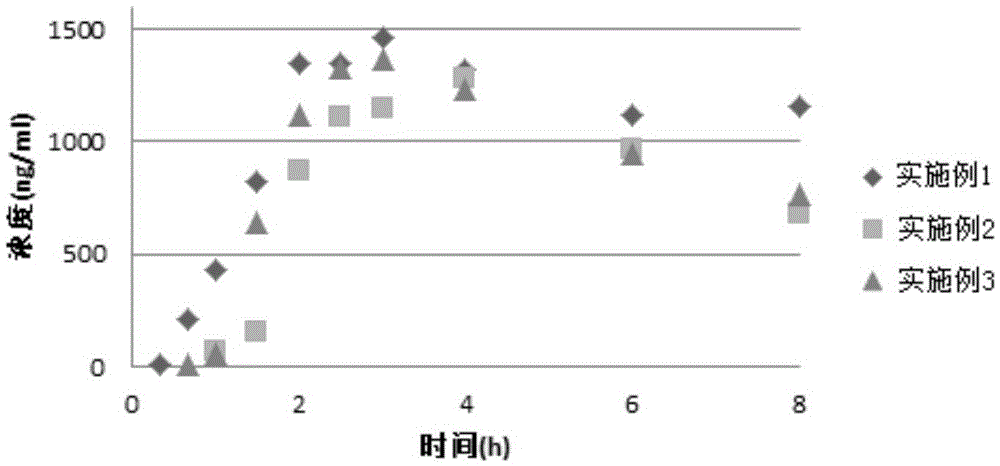
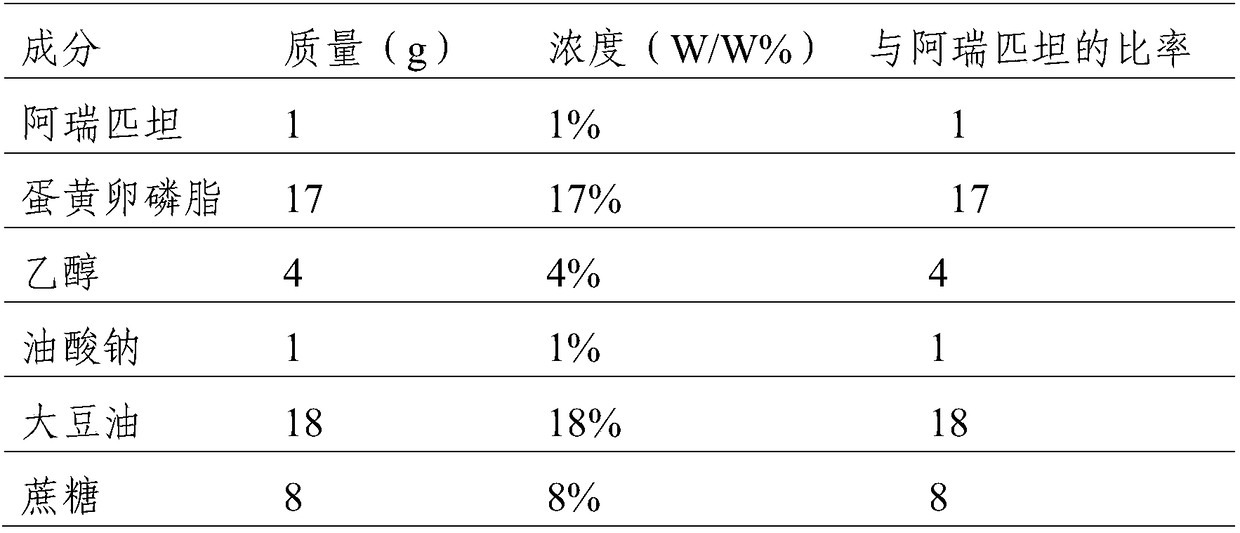
InactiveCN109010272ANot easy to produce sticky wallShort formation timeOrganic active ingredientsDigestive systemTreatment effectSucrose
The invention discloses an aprepitant emulsion formulationpreparation. The emulsion formulationpreparation is prepared from the following components in percentage by mass: 0.05-1.5% of aprepitant, 2-20% of soybean oil, 5-20% of egg yolk lecithin, 0.5-5% of ethanol, 0.05-2% of sodium oleate, 2-10% of sucrose, and 40-80% of injection water.According to the embodiment of the invention, sodium oleateis added into oil phase, sticky materials are not easily produced during mixing, the time of forming a colostrum formulationpreparation is shorter, and the preparation efficiency is high, and meanwhile, the emulsion formulation prepared during the preparation process provided by the invention is more stable and has a better treatment effect.
Owner:北京睿悦生物医药科技有限公司
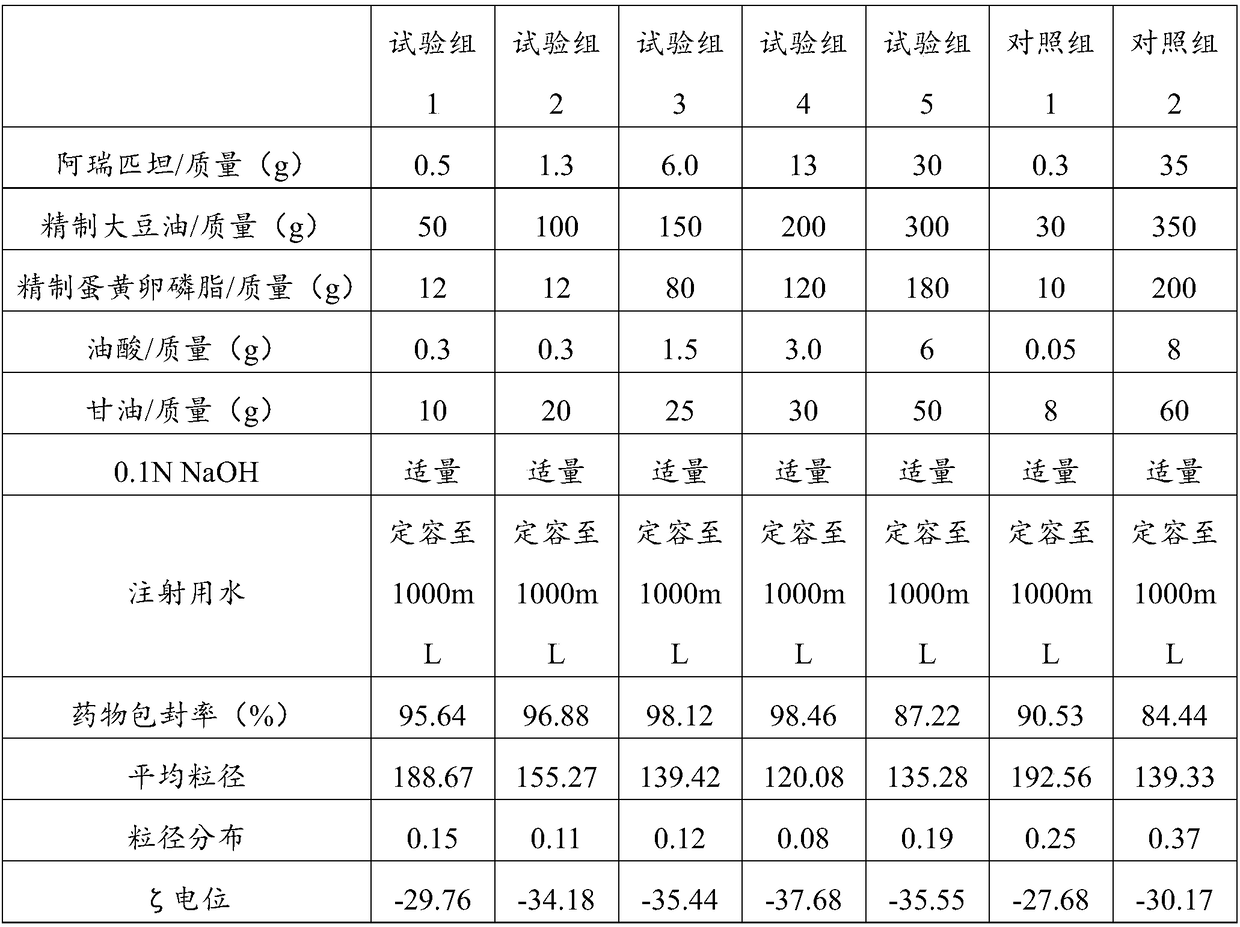
Aprepitant intravenous injection emulsion as well as preparation method and application thereof
PendingCN109364023AImprove liquidityNo hanging phenomenonOrganic active ingredientsDigestive systemHigh dosesMarketed products
The invention relates to aprepitant intravenous injection emulsion as well as a preparation method and an application thereof, and belongs to the technical field of pharmaceutics. The aprepitant intravenous injection emulsion is prepared from components in percentage by mass as follows: 0.05%-3% of aprepitant, 5%-30% of an oil phase solvent, 1.2%-18% of an emulsifier, 0.03%-0.6% of a stabilizer and 1%-5% of an isoosmotic adjusting agent, the injection emulsion further contains a pH regulating agent and the balance of water for injection, and pH value of the injection emulsion is 5.5-8.0. The aprepitant intravenous injection emulsion contains no low-carbon chain alcohol such as ethanol and the like, meanwhile, the quality requirement for a marketed product can be met by technological innovation and adjustment, and clinical use safety of the aprepitant intravenous injection emulsion is greatly improved; direct intravenous injection is utilized during clinical using, and dilution is not needed; the aprepitant intravenous injection emulsion can be used for treating acute and tardive nausea and vomiting caused by high-dose cis-platinum combined high-dose sensitizing cancer chemotherapy,and nausea and vomiting in early stage and middle stage of tumor chemotherapy are reduced.
Owner:GUANGZHOU HANFANG PHARMA
Preparation process of aprepitant
The invention discloses a preparation process of aprepitant. The process comprises the steps as follows: Step 1, a compound A and a compound B are firstly added to a mixed solvent of N,N-dimethylformamide and triethylamine to be stirred and dissolved to obtain a mixed solution; Step2, adding lithium diisopropylamide to the mixed solution in a nitrogen atmosphere and performing uniform mixing; Step3, dropwise adding a diethyl sulfate xylene solution under conditions of nitrogen shielding and stirring; Step 4, after addition, conducting a stirring reaction at 30-35 DEG C for 12-15 h under shielding of nitrogen; Step 5, separating and purifying a reaction product in Step 4 to obtain a final aprepitant product. By process improvement and formula optimization, the aprepitant synthesis processhas the characteristics of mild reaction condition, low cost and the like and is suitable for large-scale industrial production; the synthesized aprepitant product has low impurity content and high purity, and the yield of aprepitant is greatly increased.
Owner:成都晶富医药科技有限公司
Refining preparation process of high-purity aprepitant
Owner:GUANGXI ENANTIOTECH PHARM CO LTD
Novel preparation method for aprepitant capsule
InactiveCN107007568AImprove stabilityHigh purityOrganic active ingredientsDigestive systemCelluloseCombinatorial chemistry
The invention discloses a novel preparation method for an aprepitant capsule. The novel preparation method comprises the following steps: preparation of a hydroxy propyl cellulose solution, addition of the main drug aprepitant, grinding of a medium, preparation of a coating dispersion, bottom spraying of a coating, overall mixing and filling of a capsule. With the preparation method, the capsule is filled with aprepitant; the prepared aprepitant capsule has good stability, high purity and improved quality; and the preparation method is low in cost for raw materials and equipment and beneficial for large-scale production.
Owner:SICHUAN PHARMA
Amorphous Aprepitant Coprecipitates
InactiveUS20080214535A1Improve solubilityImprove bioavailabilityOrganic active ingredientsPowder deliverySolventAprepitant
A coprecipitate comprising amorphous aprepitant and a pharmaceutically acceptable carrier is prepared by rapidly removing solvent from a solution containing aprepitant and the carrier.
Owner:DR REDDYS LAB LTD +1
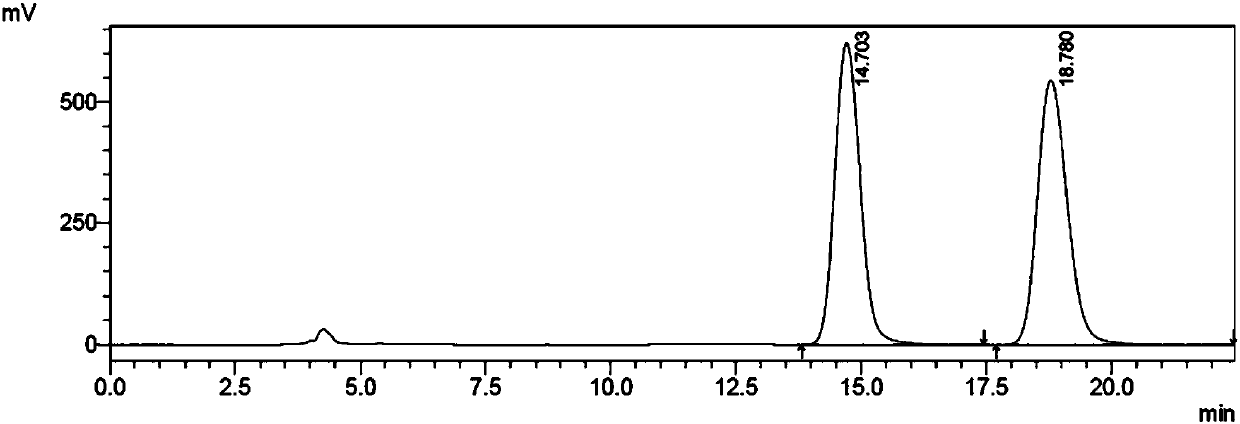
Aprepitant intermediate detection method
The invention discloses an aprepitant intermediate detection method. An aprepitant intermediate is (2R)-4-benzyl-2-[(1R)-1-[3,5,bis(trifluoromethyl)phenyl]ethoxy]morpholine-3-ketone. The detection method comprises the steps that detection is conducted through a high performance liquid chromatograph, and quantitative analysis is conducted through an area normalization method; the chromatographic conditions are that a chromatographic column is chiralcel OD-H, the sample size ranges from 15 microliters to 25 microliters, the flow velocity ranges from 0.7 ml / min to 0.9 ml / min, the column temperature ranges from 10 DEG C to 40 DEG C, the detection wavelength ranges from 212 nm to 218 nm, the mobile phase is prepared from n-hexane and isopropanol, the volume ratio of n-hexane to isopropanol is (40-60):50, and a detector is an ultraviolet detector. By means of the detection method, rapid and accurate determination on (2R)-4-benzyl-2-[(1R)-1-[3,5,bis(trifluoromethyl)phenyl]ethoxy]morpholine-3-ketone can be achieved, the sensitivity is very high, operation is easy, complete separation can be achieved, and the basis of research and development and quality detection can be provided for researching the compound.
Owner:ENANTIOTECH CORP
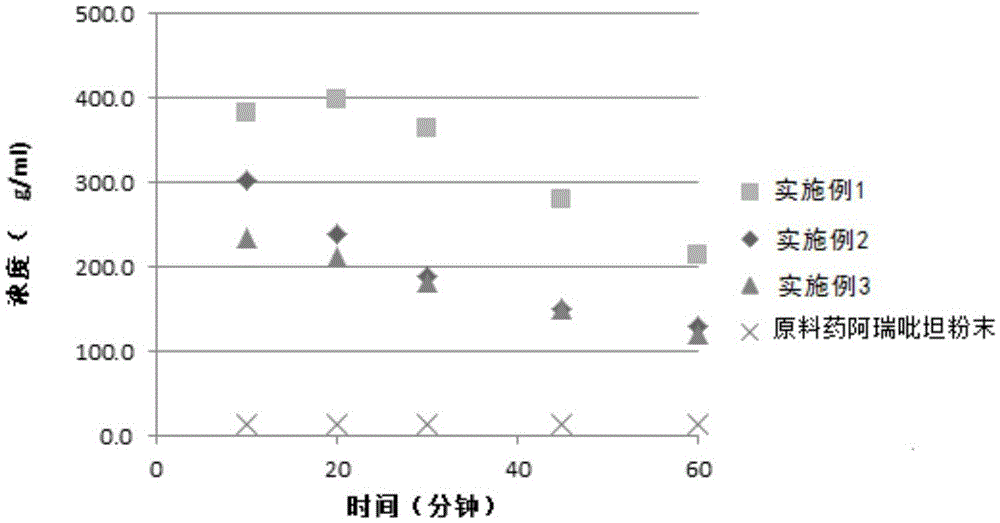
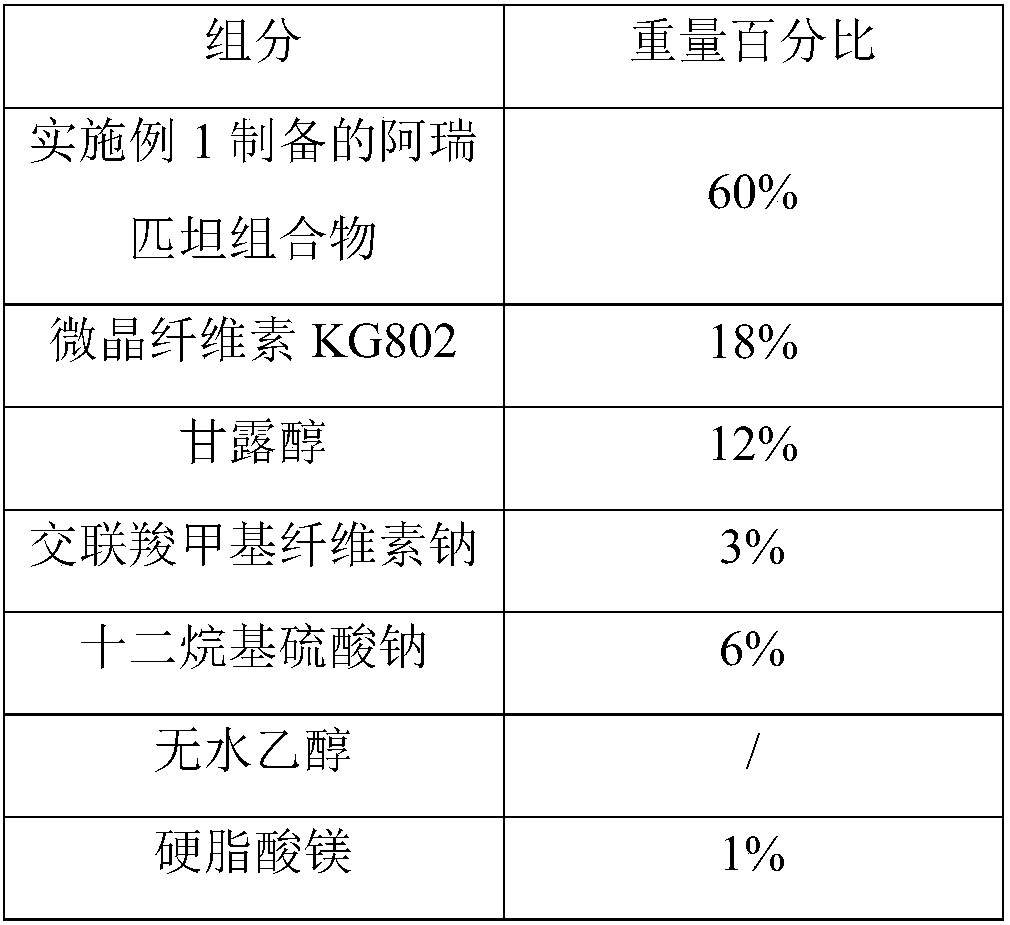
Preparation method of high stability aprepitant composition
ActiveCN108030924AGuaranteed physical stabilitySimple preparation processOrganic active ingredientsDigestive systemSolubilityBULK ACTIVE INGREDIENT
The invention provides a preparation method of a high stability aprepitant composition. The preparation method comprises the following steps: grinding aprepitant and an auxiliary material between -170DEG C and -100 DEG C for 0.5-3h, and then performing granulating. The preparation method can improve solubility of an insoluble medicine, namely aprepitant, and increase dissolving-out speed of a preparation. Compared with the existing aprepitant capsule, the prepared aprepitant capsule can ensure physical stability of active ingredients, and a preparation technology of the preparation is simple,appropriate in cost and applicable to industrial mass production.
Owner:CHENGDU BAIYU PHARMA CO LTD
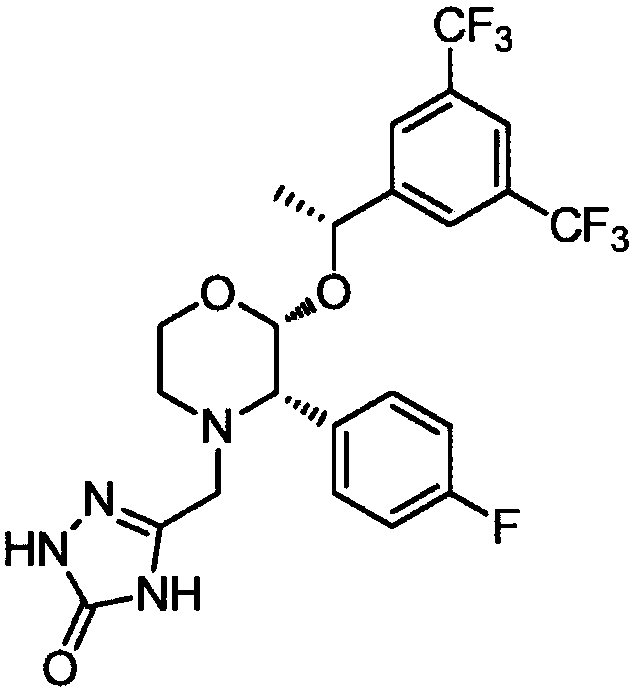
Chiral synthesis method of (R)-1-(3, 5-di (trifluoromethyl) phenyl] ethanol
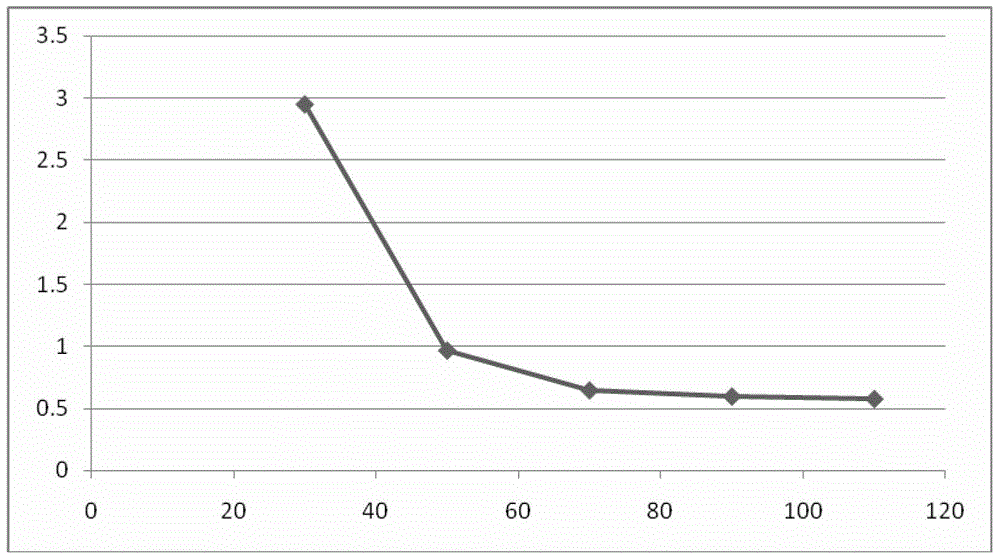
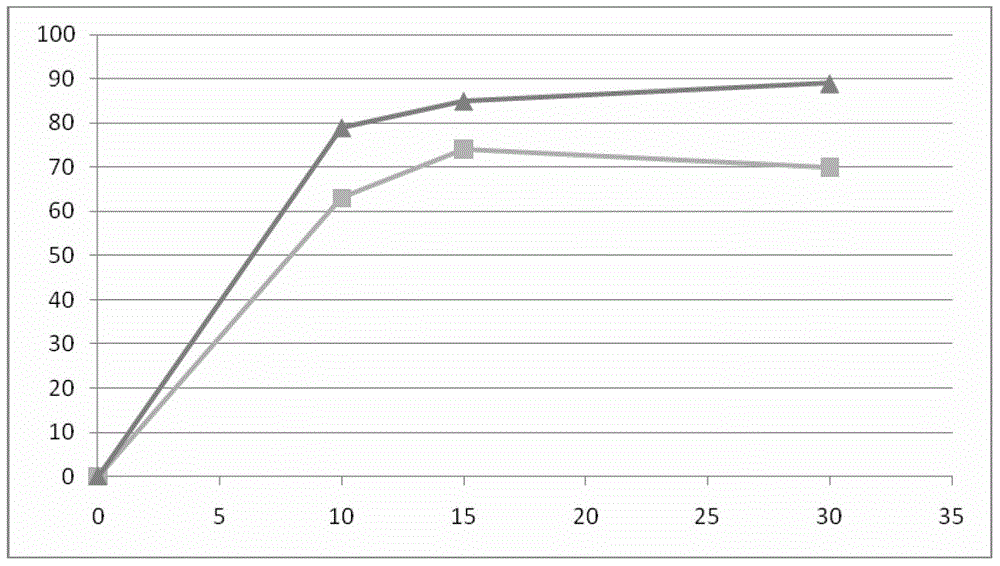
ActiveCN104513837ALow costEasy to operateOrganic compound preparationMicroorganism based processesChemical compoundSynthesis methods
The invention belongs to the technical field of chemistry, and in particular relates to a chiral synthesis method of (R)-1-(3, 5-di (trifluoromethyl) phenyl] ethanol, and the prepared compound is a key intermediate for the production of aprepitant. The technical scheme includes two reaction steps, namely a) biological catalytic reduction of formula I by use of bread yeast, and b) configuration reversion of byproduct formula III prepared by the step a) to obtain formula II, and the technical scheme can also include the preparation of immobilized yeast cells, namely bread yeast immobilization. The method is biological asymmetric reduction, has the advantages of low cost, simple operation, high yield, mild reaction conditions, great implementation value, and suitability for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
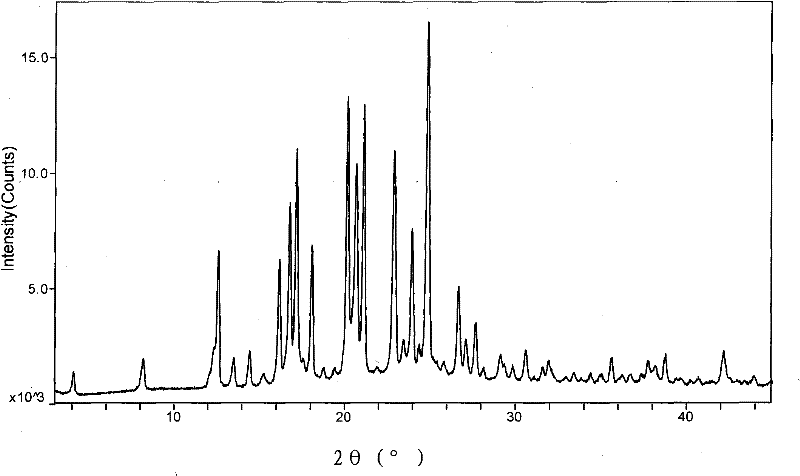
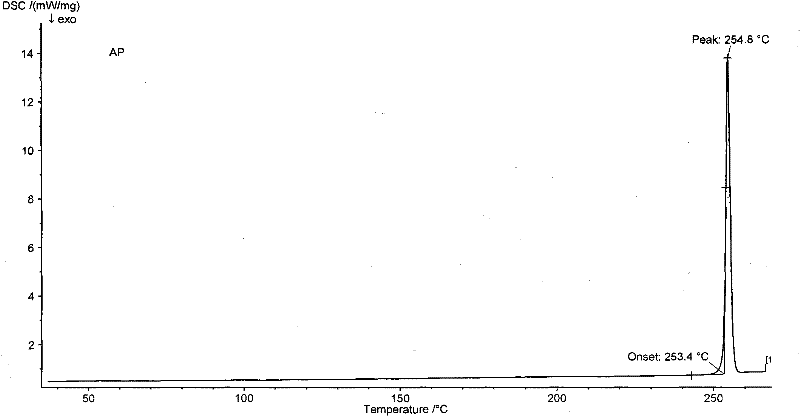
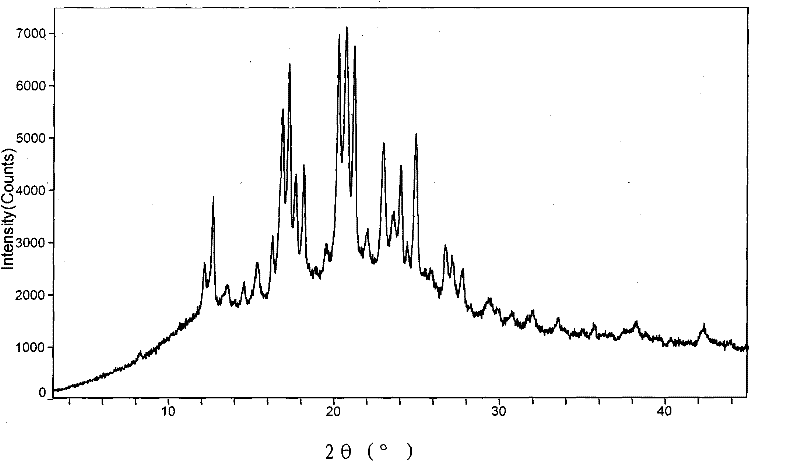
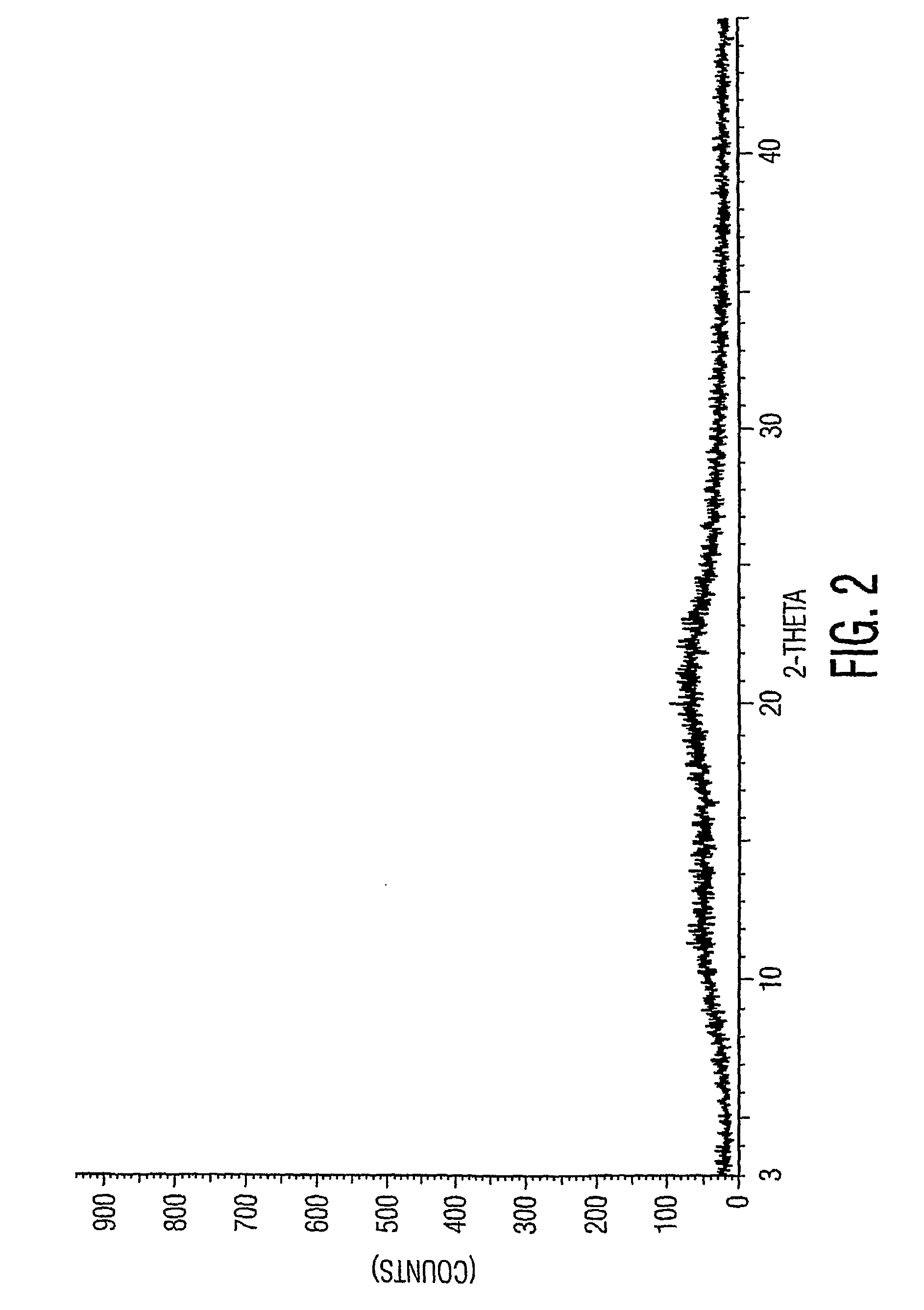
New neurokinin (NK-1) receptor antagonist crystal form and preparation method thereof
ActiveCN102850339AReduce manufacturing costEasy to operateOrganic chemistryDigestive systemX-rayLength wave
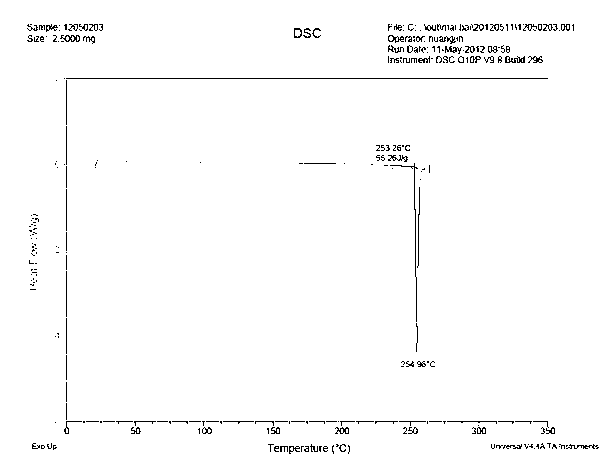
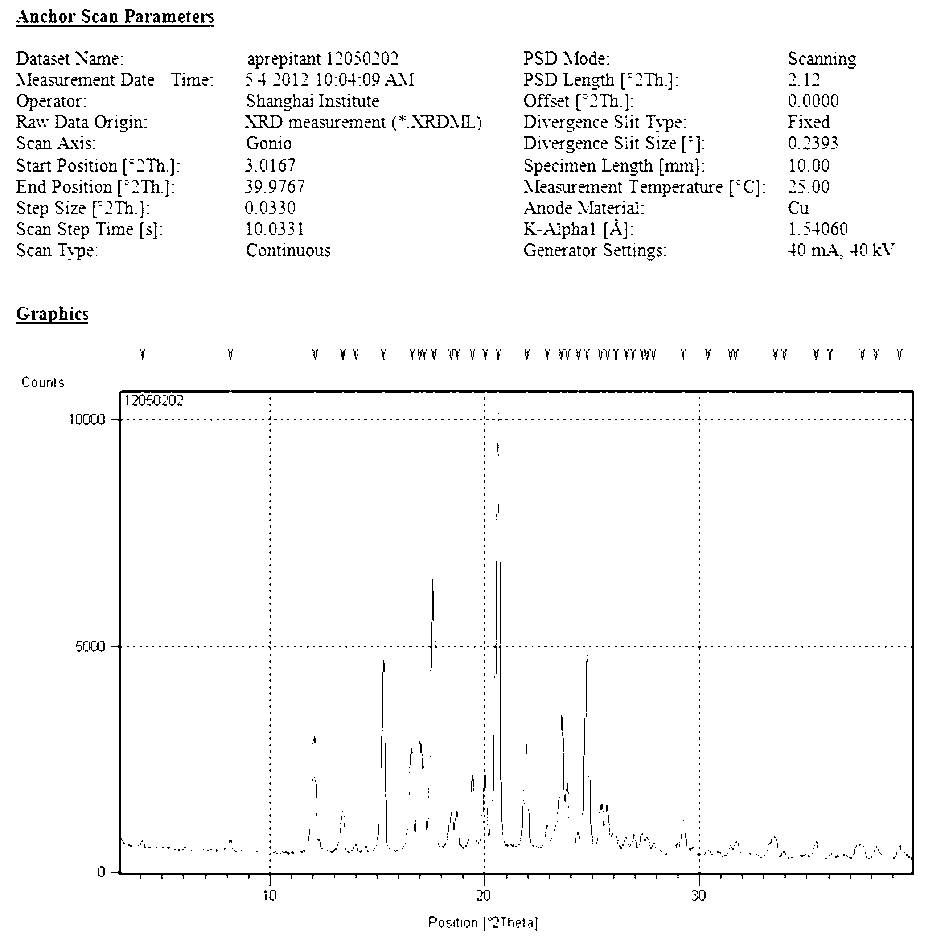
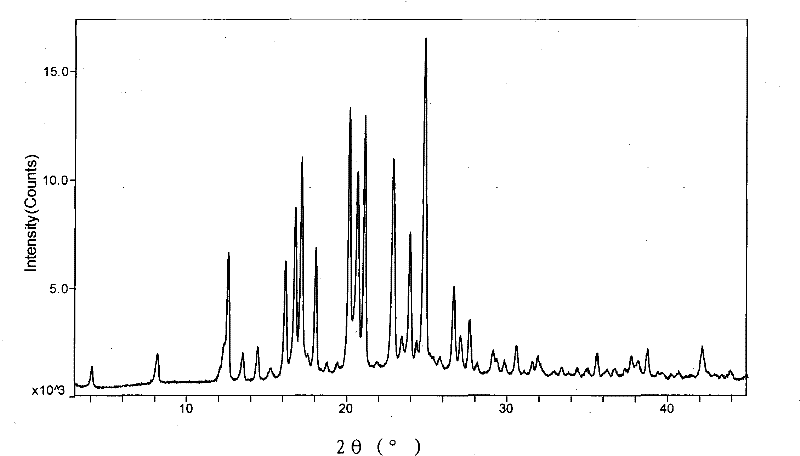
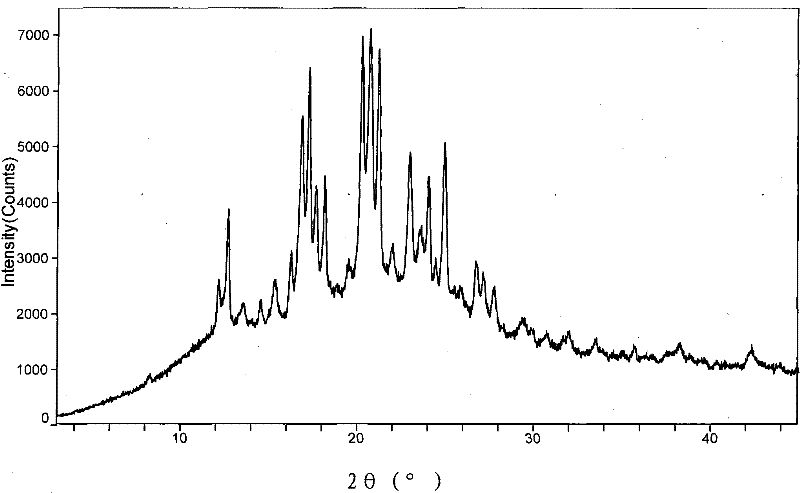
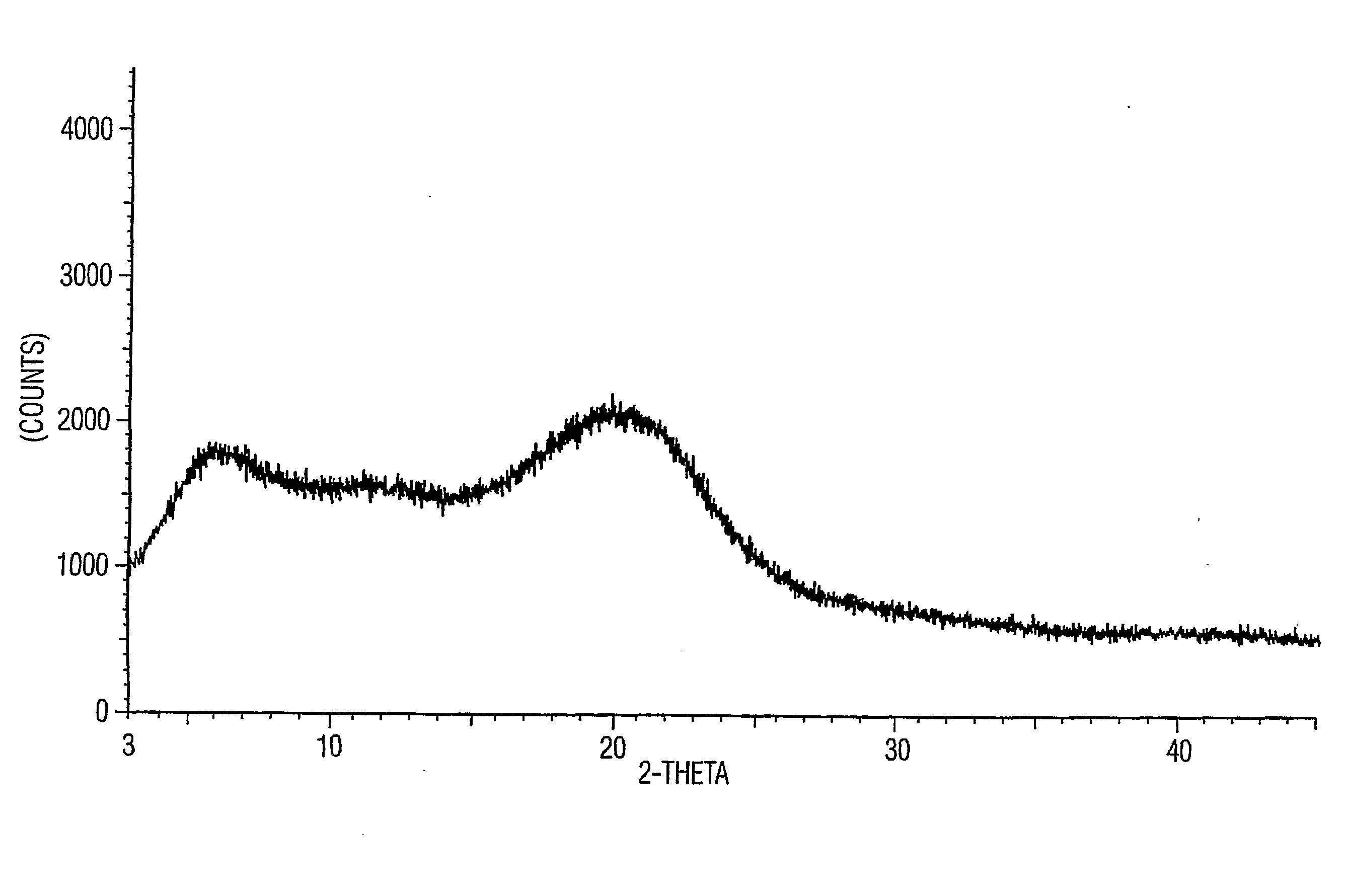
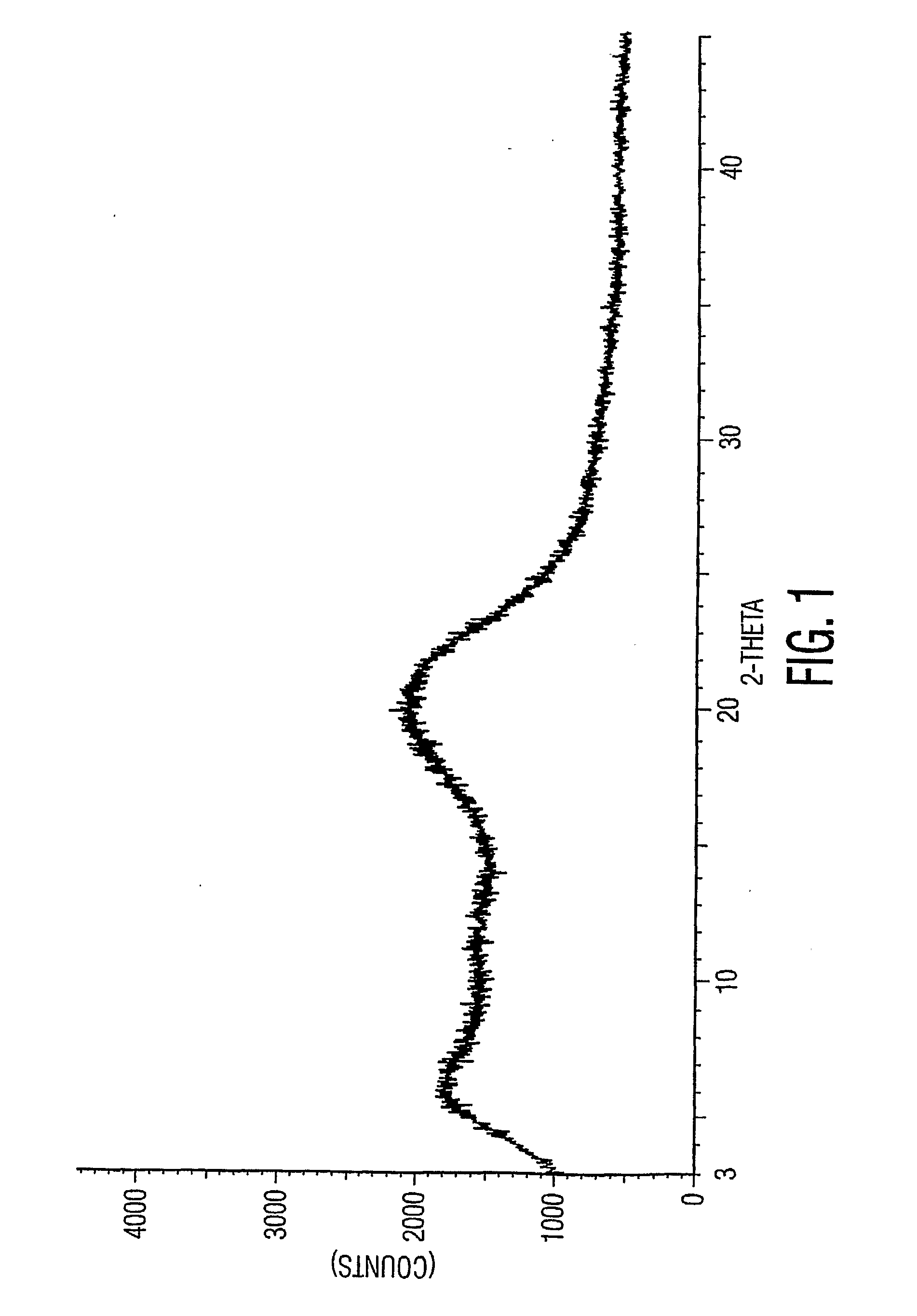
The invention provides a new neurokinin (NK-1) receptor antagonist aprepitant crystal form V. The new aprepitant crystal form V powder has characteristic X diffraction ray peaks at the wavelengths of 12.08 degrees + / -0.2, 15.31 degrees + / -0.2, 17.61 degrees + / -0.2, 20.62 degrees + / -0.2 and 24.76 degrees + / -0.2 2 theta in an X-ray diffraction pattern. The invention also provides a preparation method of the aprepitant new crystal form V.
Owner:上海云晟研新生物科技有限公司
Aprepitant capsules
ActiveCN104586814ADissolution rate is fastSimple processOrganic active ingredientsDigestive systemDiethylene glycol monoethyl etherDissolution
The invention belongs to the technical field of medicines, and specifically relates to aprepitant capsules. The aprepitant capsules contain aprepitant, hydroxy propyl cellulose, diethylene glycol monoethyl ether, fumed silica and other pharmaceutically acceptable filler, disintegrant and lubricant. A preparation method of the aprepitant capsules comprises the following steps: dissolving aprepitant in diethylene glycol monoethyl ether, adding hydroxy propyl cellulose, stirring to dissolve, adding fumed silica to adsorb, then uniformly mixing with the other pharmaceutically acceptable filler, disintegrant and lubricant, and directly filling capsules. Compared with the prior art, the aprepitant capsules disclosed by the invention are fast in medicine dissolution speed, simple in process, and free from a surfactant and a micronization treatment. An acceleration test result indicates that the prepared aprepitant capsules are high in dissolution rate.
Owner:SHANDONG NEWTIME PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Chiral synthesis method of (R)-1-(3, 5-di (trifluoromethyl) phenyl] ethanol Chiral synthesis method of (R)-1-(3, 5-di (trifluoromethyl) phenyl] ethanol](https://images-eureka.patsnap.com/patent_img/fa5211b6-69e7-48ab-a583-7f8833f3dff4/BDA0000392015700000021.PNG)
![Chiral synthesis method of (R)-1-(3, 5-di (trifluoromethyl) phenyl] ethanol Chiral synthesis method of (R)-1-(3, 5-di (trifluoromethyl) phenyl] ethanol](https://images-eureka.patsnap.com/patent_img/fa5211b6-69e7-48ab-a583-7f8833f3dff4/FDA0000392015690000011.PNG)