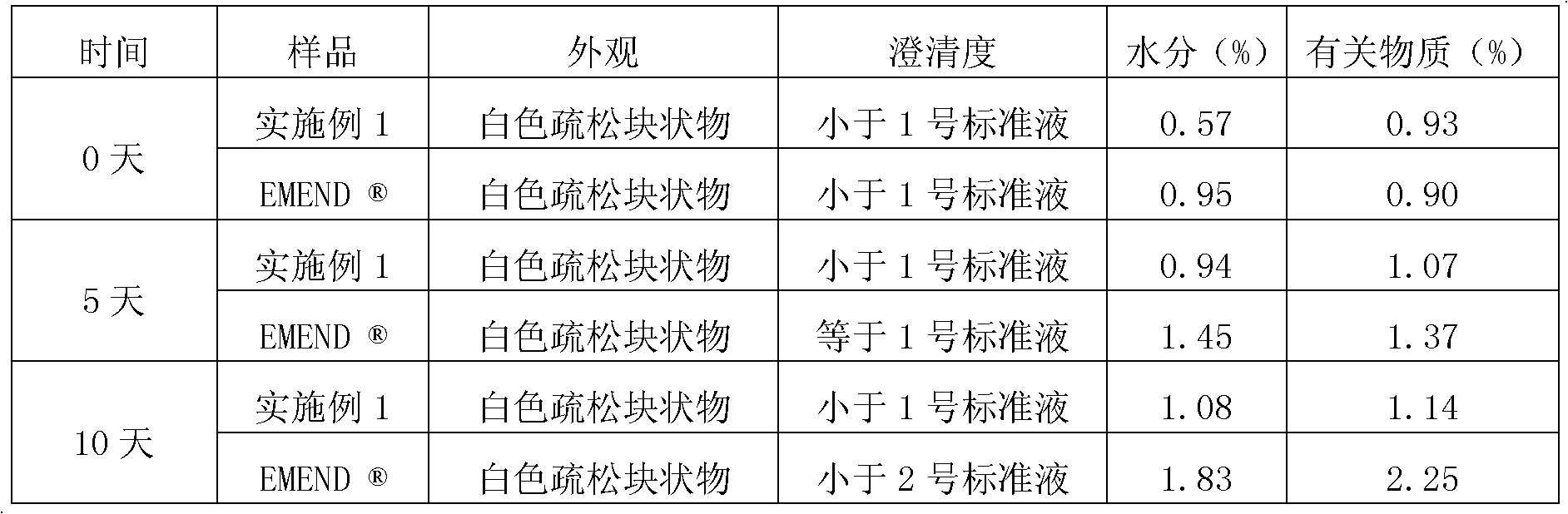
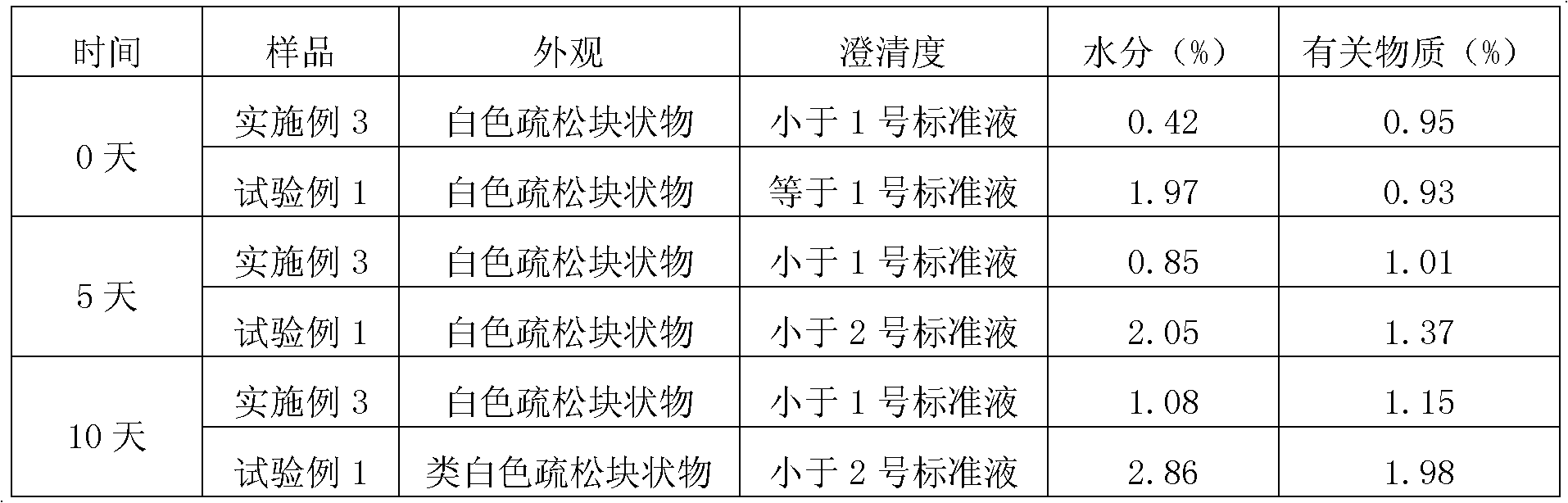
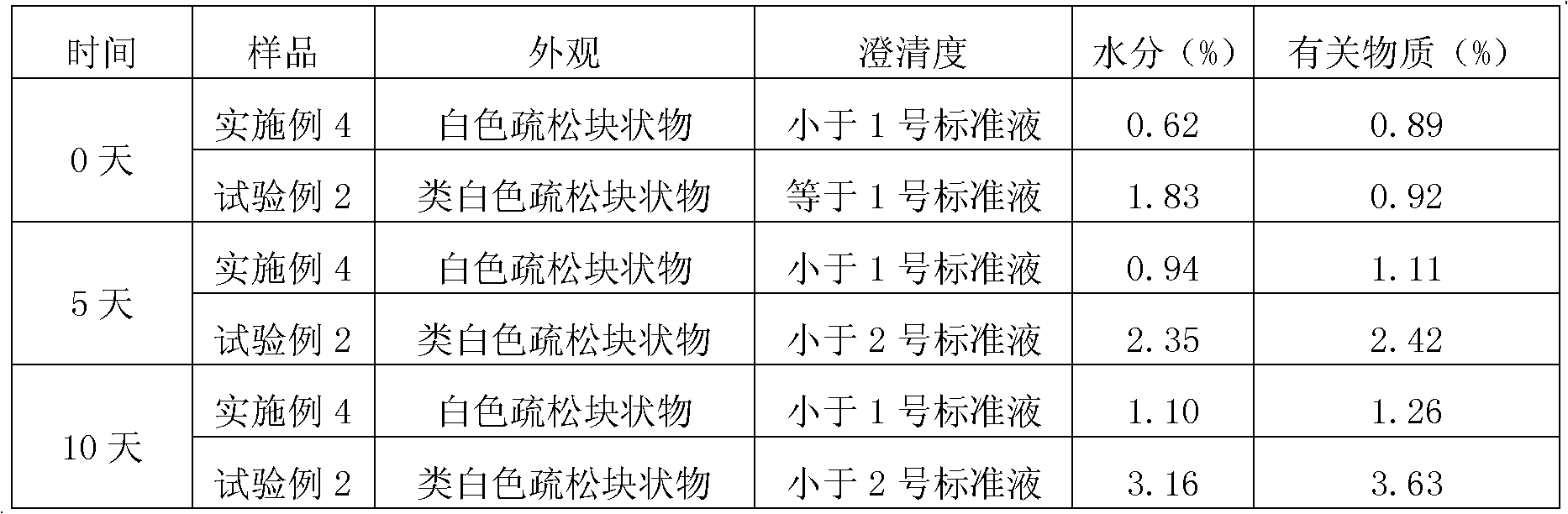
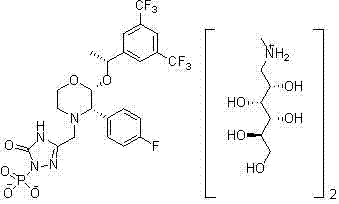
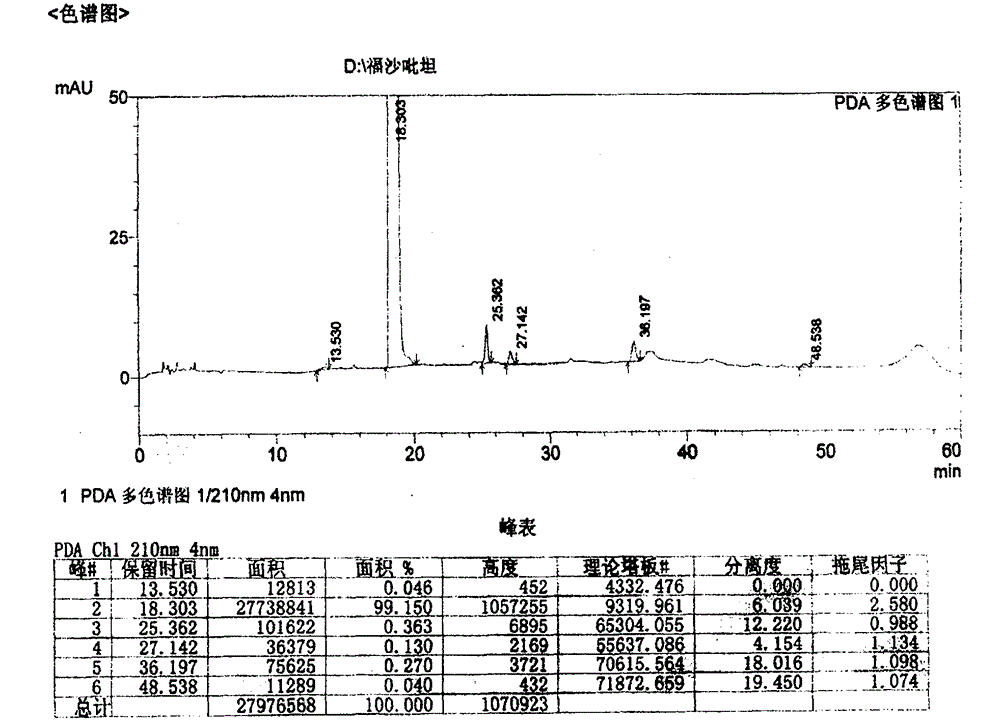
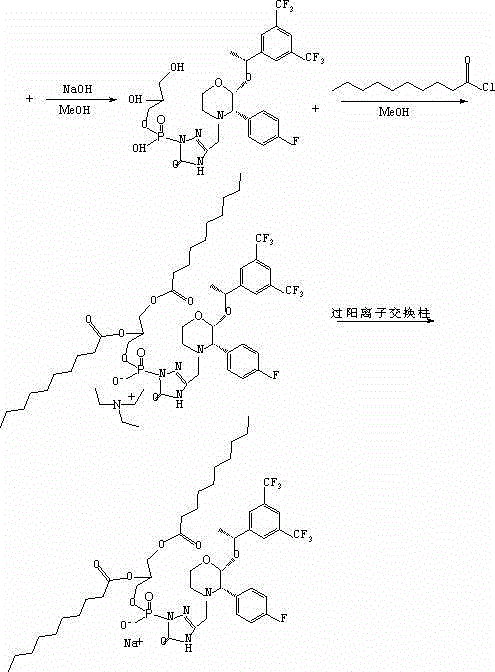
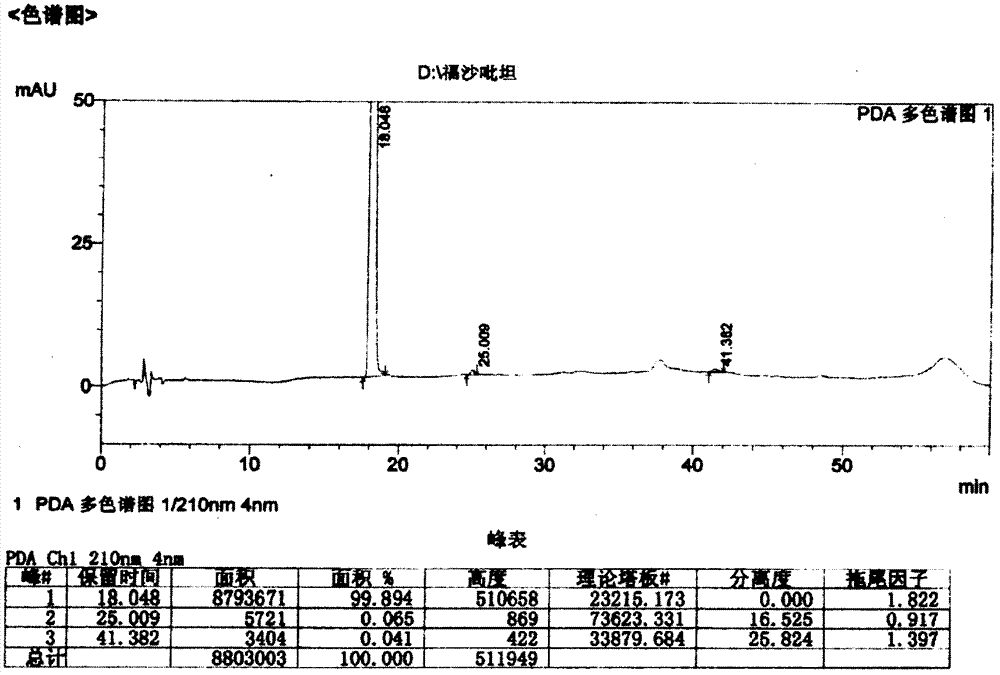
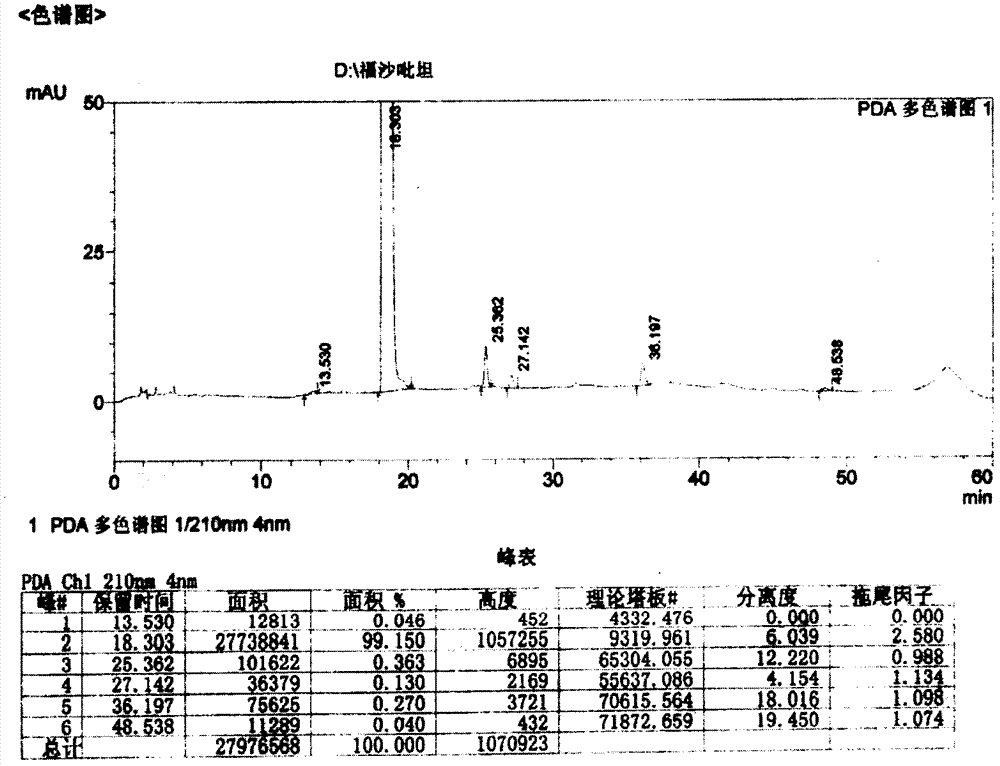
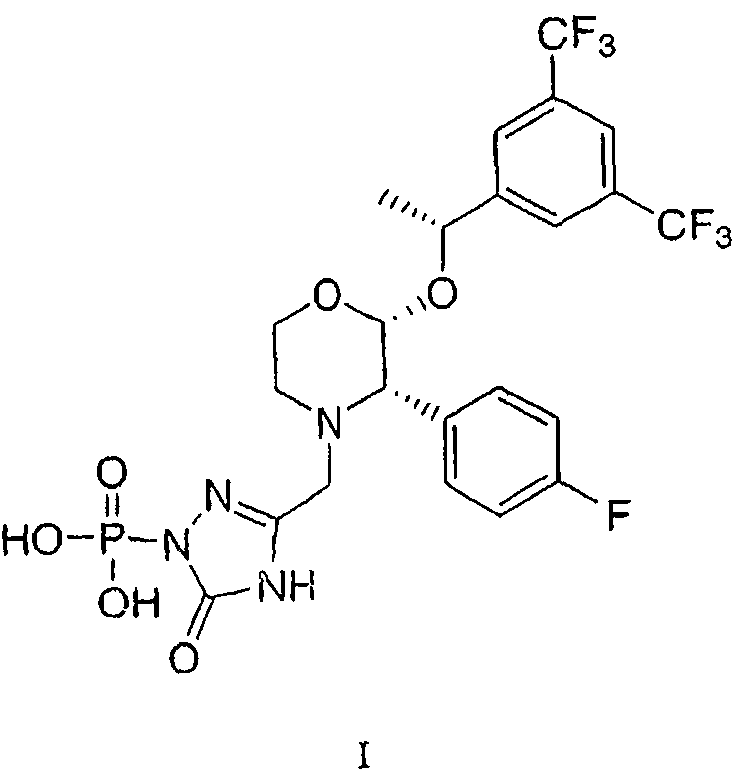
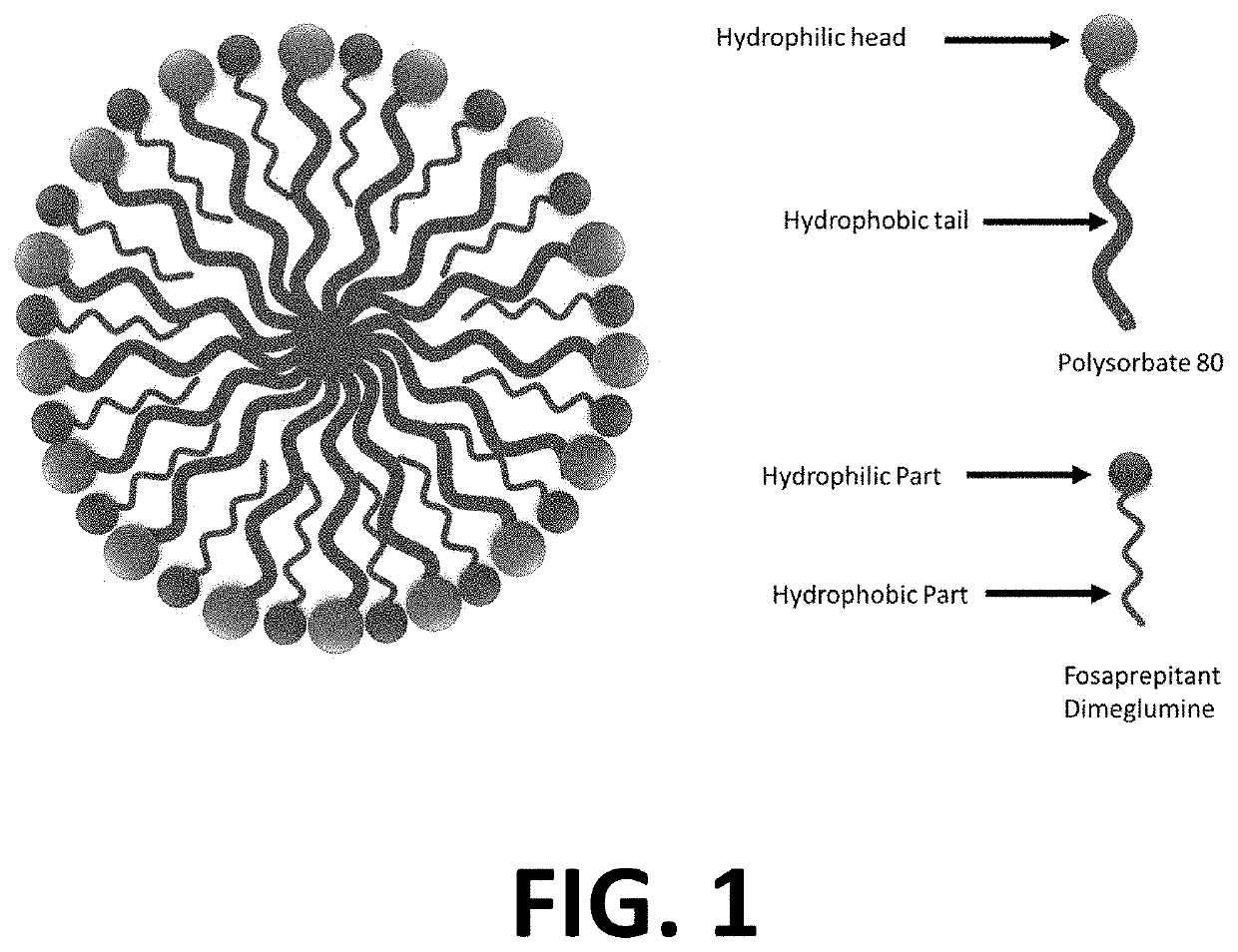
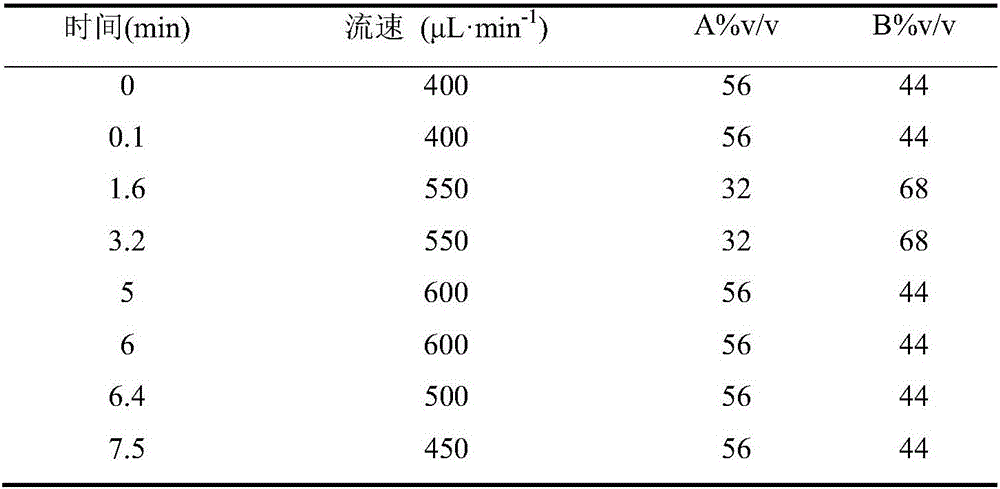
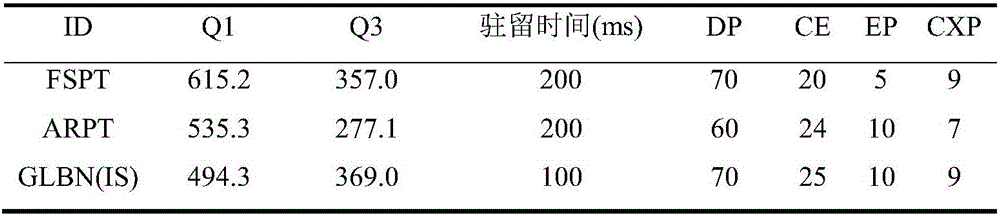
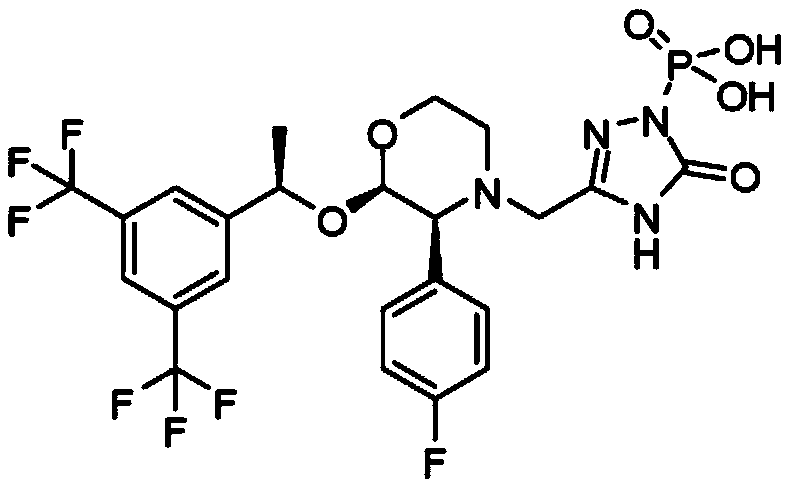
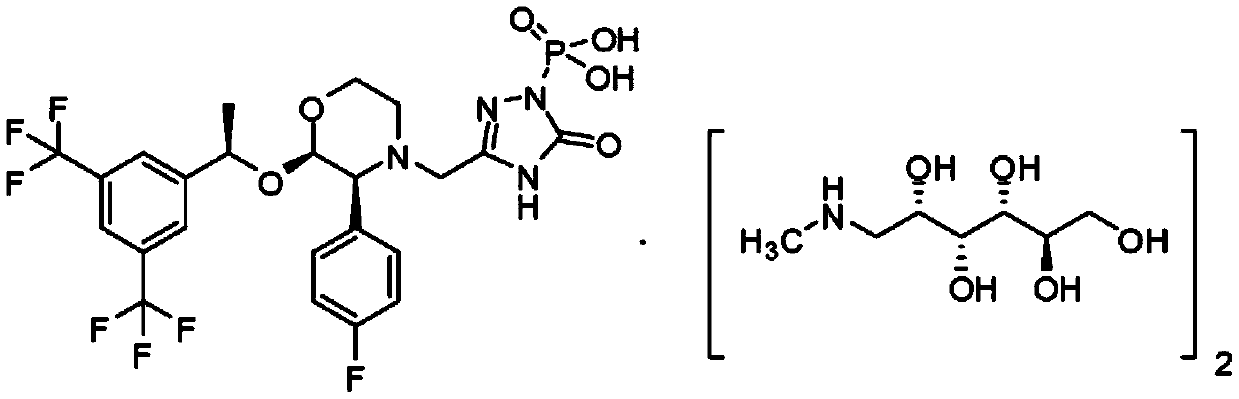
Patents
Literature
53 results about "Fosaprepitant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
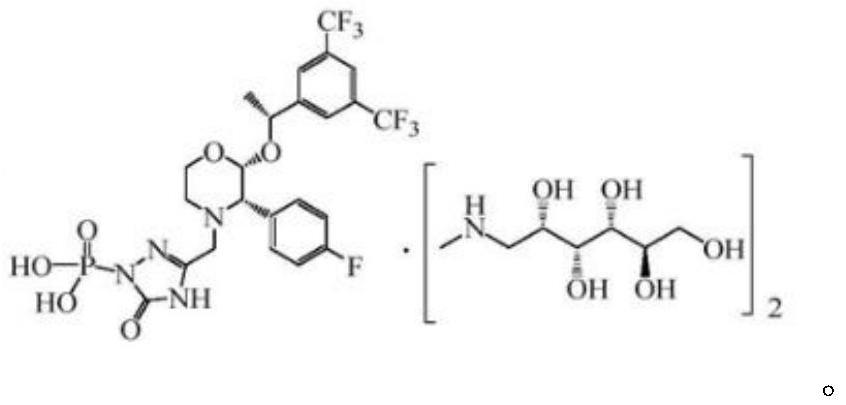
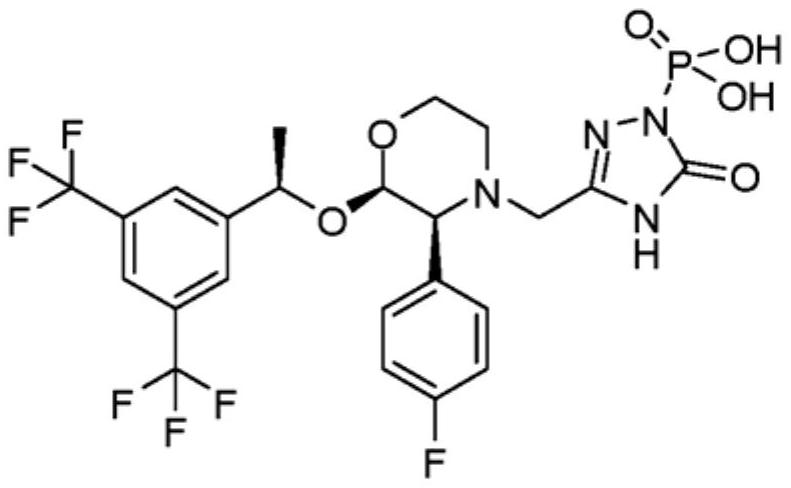
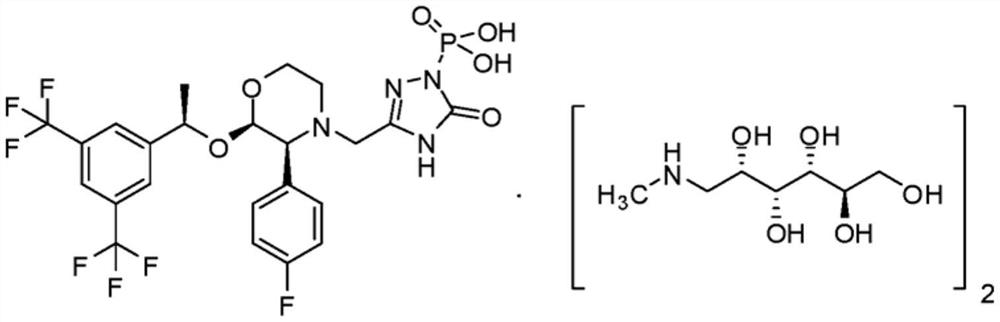
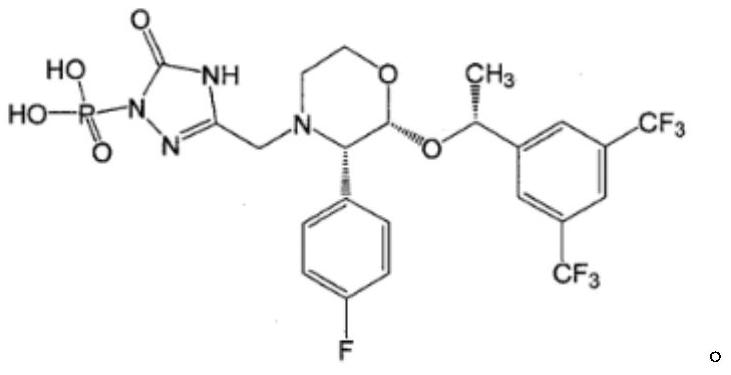
Fosaprepitant (Emend for Injection (US), Ivemend (EU)) is an antiemetic drug, administered intravenously. It is a prodrug of aprepitant. Fosaprepitant was developed by Merck & Co. and was approved by the United States Food and Drug Administration (FDA) on January 25, 2008 and by the European Medicines Agency (EMA) on January 11 of the same year.
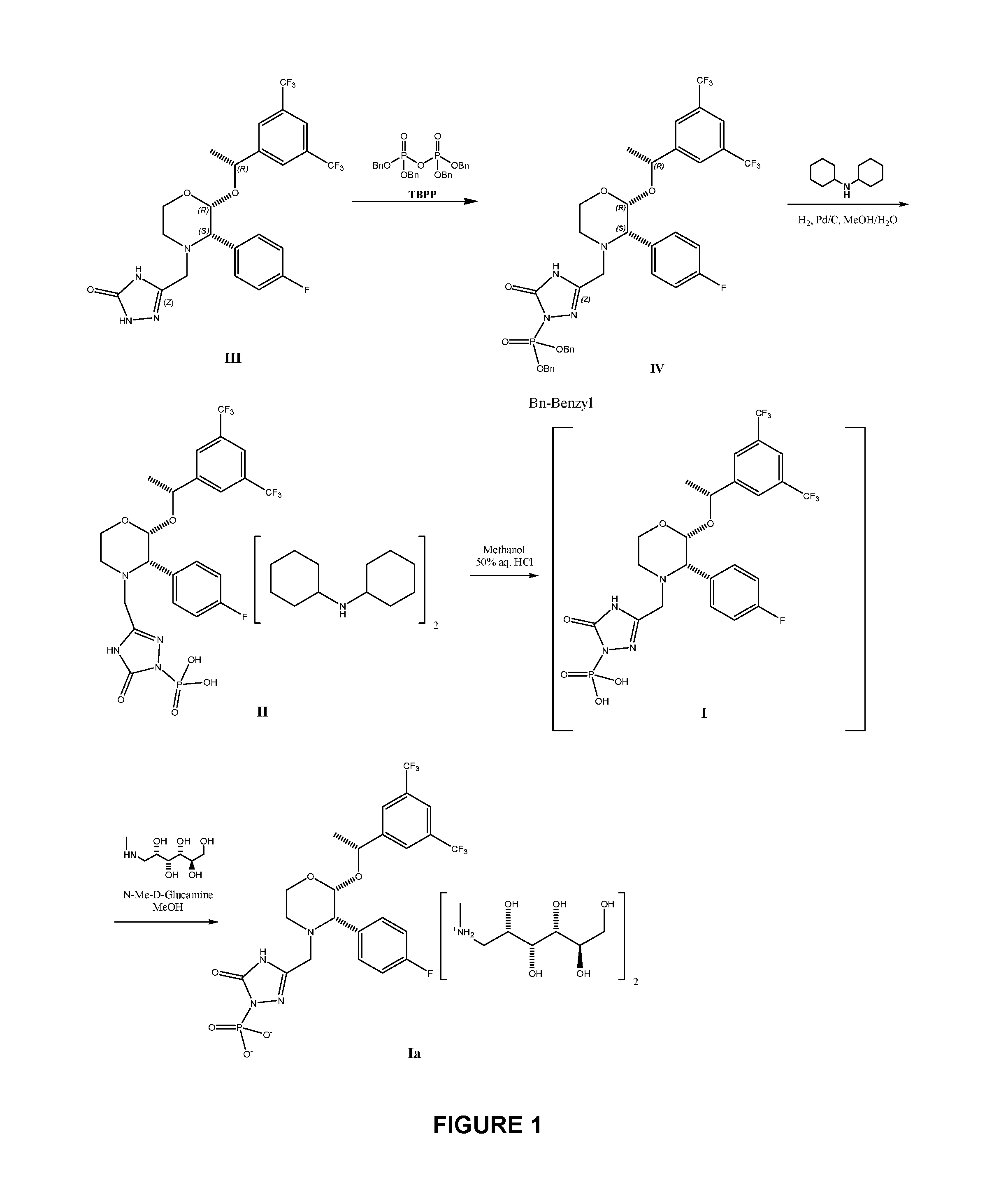
Sterile lyophilized preparation containing fosaprepitant, and preparation method thereof
ActiveCN102755296AEasy to useSimple processOrganic active ingredientsPowder deliveryTreatment effectArginine
Owner:QILU PHARMA
Method for reducing palladium residue in compound and preparation method of high-purity fosaprepitant dimeglumine by applying method
ActiveCN102838634AHigh purityCompliance with residue limit requirementsGroup 5/15 element organic compoundsFosaprepitant dimeglumineCombinatorial chemistry
The invention belongs to the field of medicines, and relate to a method for reducing palladium residue in a compound and a preparation method of high-purity fosaprepitant dimeglumine by applying the method. In the method, tributyl phosphane and triphenyl phosphine are used as palladium removing agent to treat compound solution. After palladium is removed by using the method, the high-purity fosaprepitant dimeglumine can be obtained through crystallization with poor solvent in one step. The residue of tributyl phosphane and triphenyl phosphine in a finished product is low, and the palladium residue limit is less than 1 ppm, therefore, the requirement of limit of palladium residue in crude drug for injection is satisfied, and the industrial production after magnification is further adapted.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of fosaprepitant dimeglumine
ActiveCN102558232AHigh purityHigh yieldOrganic compound preparationGroup 5/15 element organic compoundsCombinatorial chemistryFosaprepitant dimeglumine
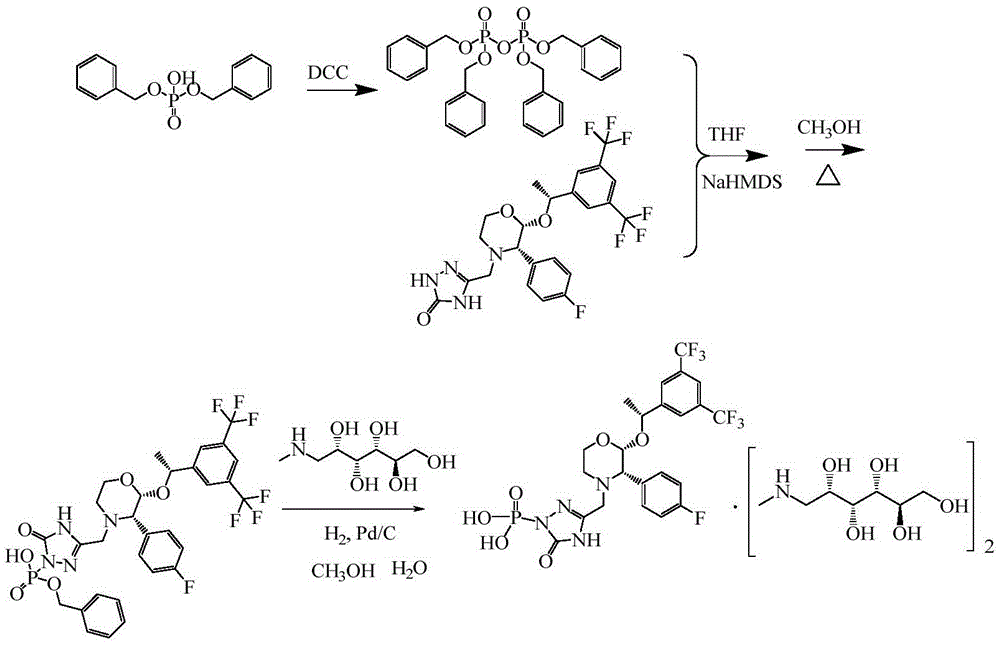
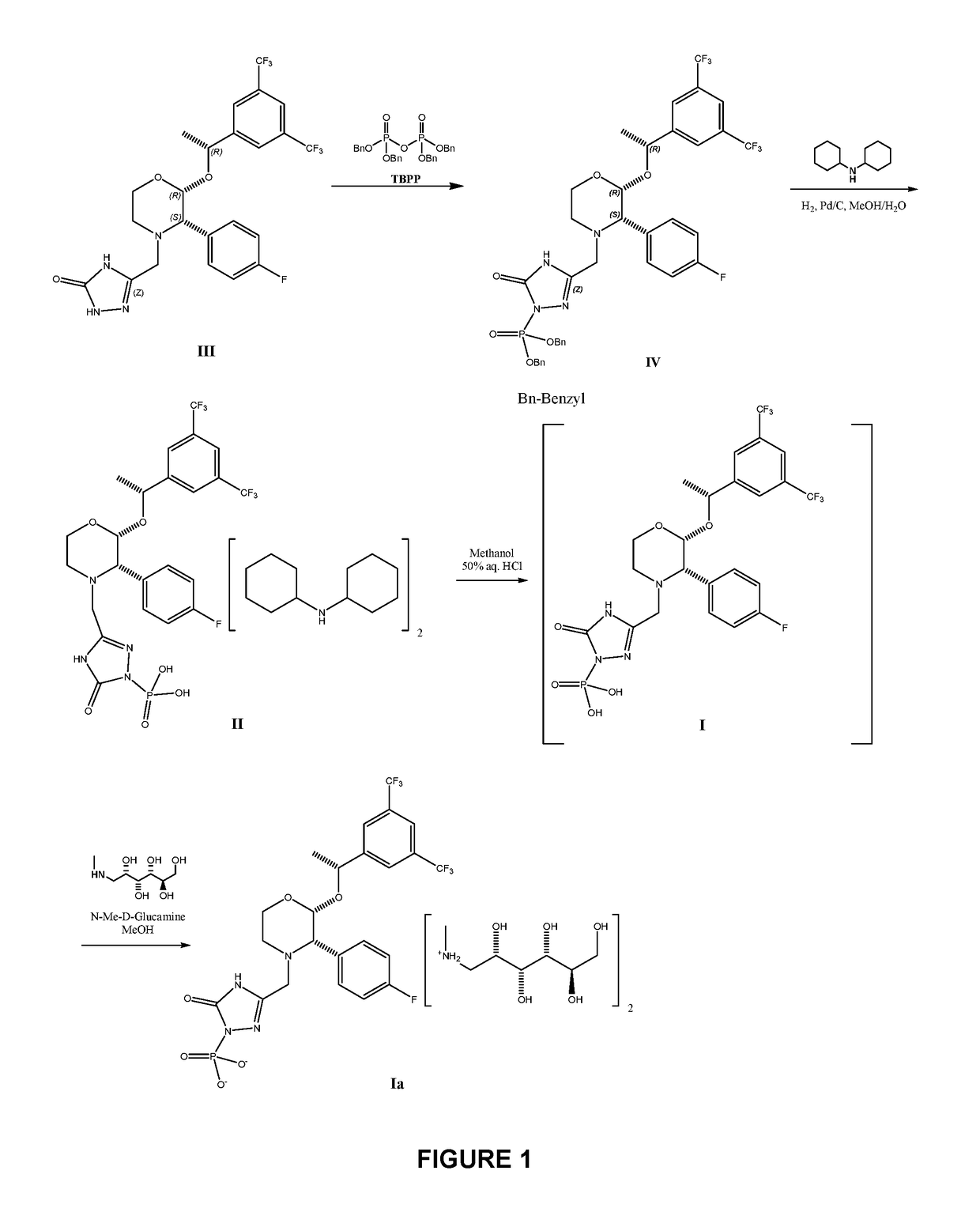
The invention provides a preparation method of fosaprepitant dimeglumine. The preparation method is characterized in that aprepitant is used as a raw material, under an alkaline condition, dibenzyl ester intermediate compound is obtained by phosphonylation, the intermediate compound is further hydrogenated and catalyzed to obtain fosaprepitant, the fosaprepitant is further reacted with N-methyl-D-glucamine, and finally the fosaprepitant dimeglumine is obtained. The preparation method has the advantages of short reaction cycle, simpleness in operation, low production cost and good product quality; the purity of the finished product is more than 99.5 percent, and the content of single impurity is less than 0.1 percent; and the preparation method is suitable for large-scale industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Fosaprepitant dimeglumine freeze-dried powder and preparation method thereof
InactiveCN103565760AShorten freeze-drying timeReduce manufacturing costPowder deliveryOrganic active ingredientsFreeze-dryingFosaprepitant dimeglumine
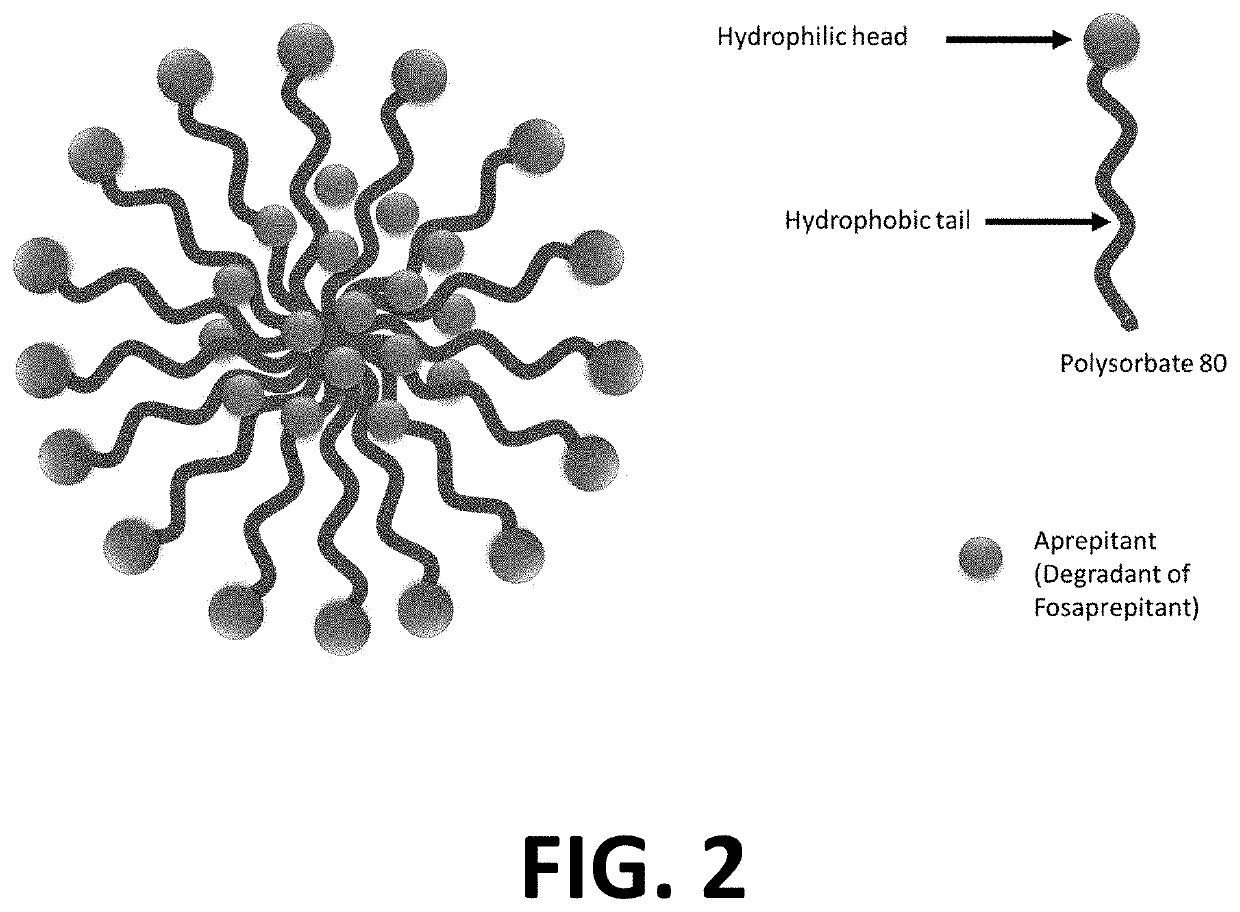
The invention discloses fosaprepitant dimeglumine freeze-dried powder and a preparation method thereof, and relates to the technical field of drugs and drug production. The freeze-dried powder injection contains fosaprepitant dimeglumine, lactose, polysorbate 80 and ethanol water solution. The freeze-dried powder adopts the ethanol water solution as freeze-dried preparation solvent, so that the freeze-dried time is largely shortened, the energy consumption is decreased, and the production cost is reduced.
Owner:NANJING CORE TECH CO LTD
New method for preparing fosaprepitant intermediate
ActiveCN102675369AHigh purityEasy to operateGroup 5/15 element organic compoundsOrganic solventAlcohol
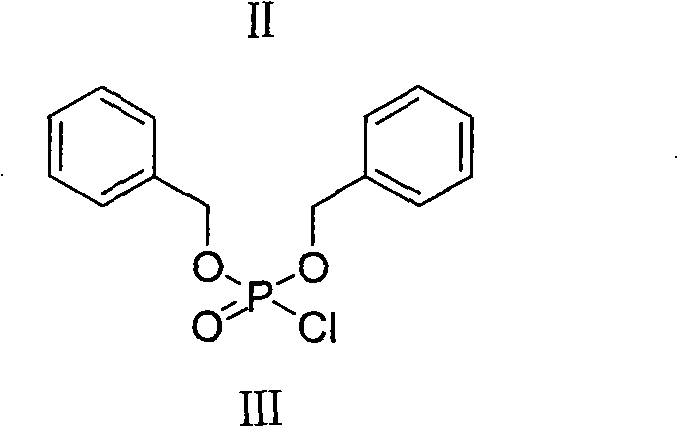
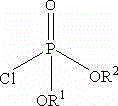
The invention relates to a new method for preparing fosaprepitant intermediate, namely, [3-[(2R)-[(1R)-1-(3, 5-bi (trifluoromethyl) phenyl] ethyoxyl]-3 (S)-(4-fluorophenyl) morpholine 4-group] methyl]-5-oxo-4, 5-dihydro-[1, 2, 4]-triazole-1-group] phosphonic acid-benzyl ester. The compound is an important medicine intermediate, and is used for preparing antiemetic drug fosaprepitant. The new method comprises the steps of: enabling newly prepared phosphorylation agent dibenzyl phosphorus oxychloride and aprepitant to have reaction in organic solvent under the action of steric hindrance alkali, treating the product with methyl alcohol to obtain the fosaprepitant intermediate. The method is simple in operation and low in cost, thus being more suitable for industrial production.
Owner:北京华众恩康医药技术有限公司
Refined palladium removing process for fosaprepitant
ActiveCN107176964AImprove efficiencyLow priceGroup 5/15 element organic compoundsFosaprepitant dimeglumineSilica gel
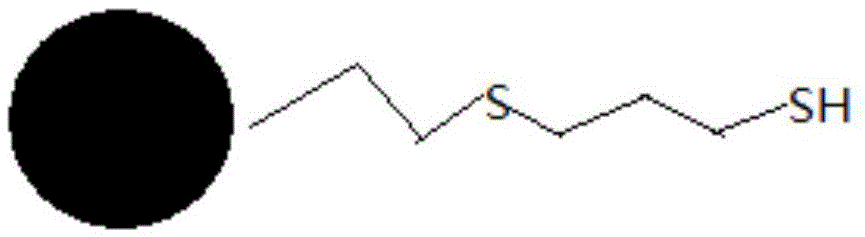
The invention provides a method of reducing the content of palladium in fosaprepitant dimeglumine. The method comprises the following steps: preparing a fosaprepitant dimeglumine solution; adding a sulfhydryl silica gel palladium removing agent, stirring and filtering and then carrying out vacuum distillation concentration; adding a proper amount of solvent into a concentrated solution containing the fosaprepitant for dissolving, and then dropwise adding a mixed solution into an antisolvent; standing, crystallizing and drying to obtain a finished product of fosaprepitant dimeglumine. The palladium removing method provided by the invention is higher in efficiency; the palladium residues in a finished product of the fosaprepitant can be reduced from an original high ppm value to below 5ppm, which conforms to the limit requirements of drugs for injection on the palladium content. Compared with an organic phosphorus reagent, a palladium removing agent provided by the invention has the advantages of safety and low toxicity; in addition, the palladium removing agent can be removed from the solution by simple filtering. The process has a significant cost advantage and is suitable for industrial production.
Owner:HANGZHOU JIUYUAN GENE ENG
Crystalline Fosaprepitant Dicyclohexylamine Salt And Its Preparation
ActiveUS20160355533A1Improve purification effectAllows preparationOrganic active ingredientsGroup 5/15 element organic compoundsFosaprepitant dimeglumineMedicinal chemistry
The present invention provides dicyclohexylamine salt of fosaprepitant (fosaprepitant DCHA), a process for preparing fosaprepitant DCHA, and a use of fosaprepitant DCHA in the preparation of pharmaceutically acceptable fosaprepitant dimeglumine with high purity. Fosaprepitant dimeglumine is prepared by treating fosaprepitant DCHA with an acid to form fosaprepitant, followed by adding N-methyl-D-glucamine to fosaprepitant.
Owner:NAVINTA
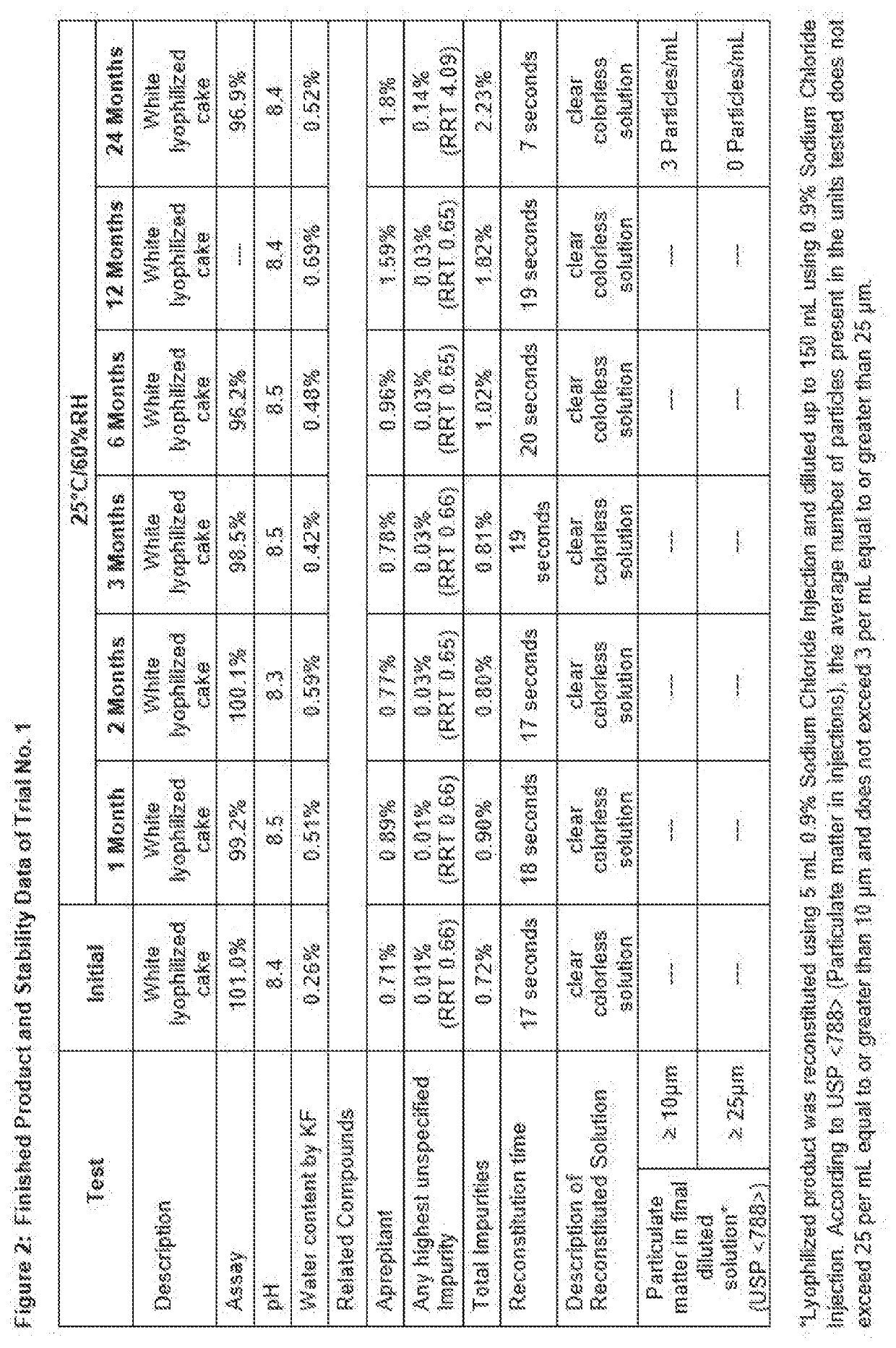
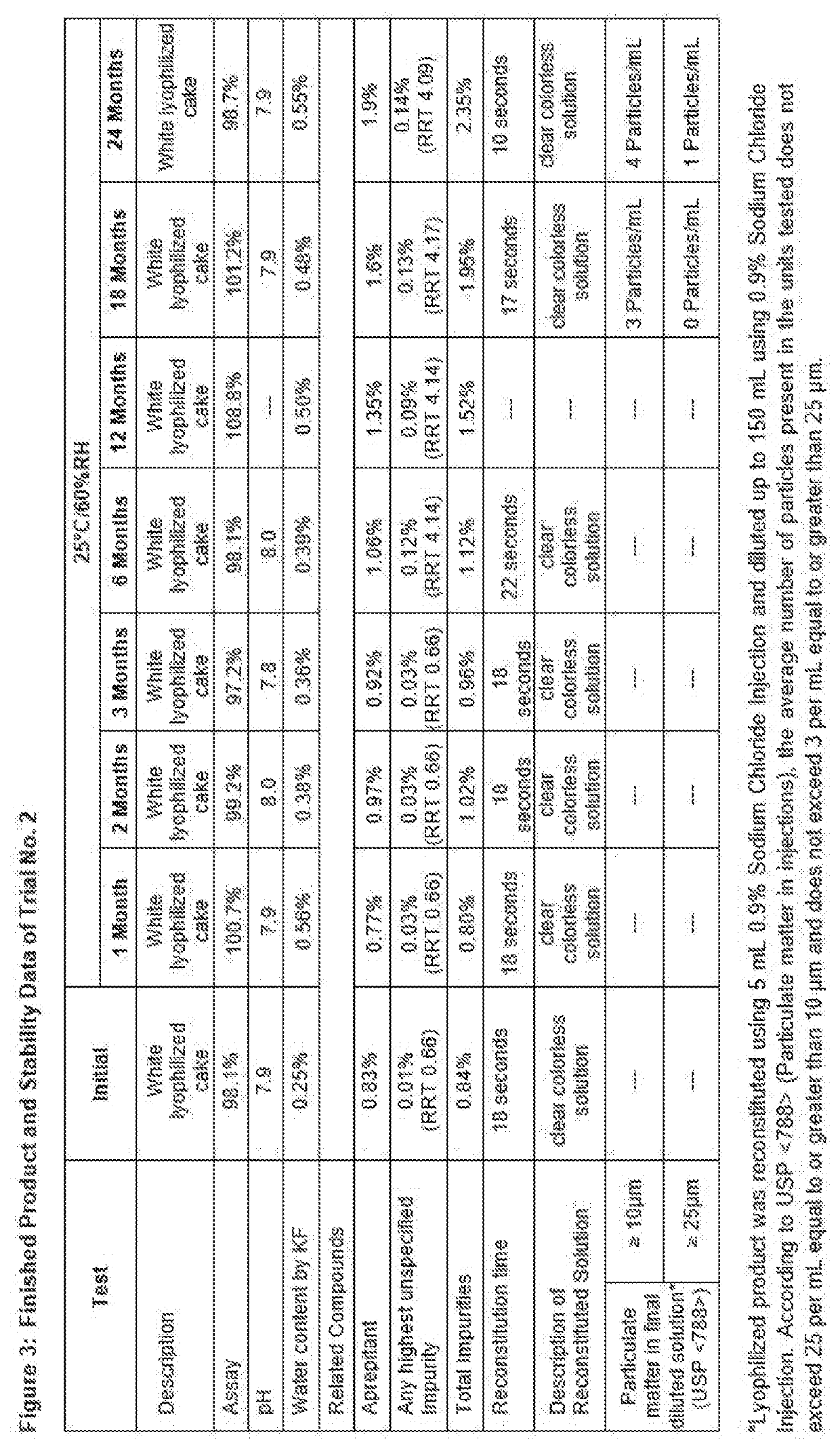
Process for preparing injectable fosaprepitant dimeglumine compositions having improved storage stability
ActiveUS20200237788A1Amount be controlOrganic active ingredientsPharmaceutical non-active ingredientsEngineeringFosaprepitant dimeglumine
Solid compositions having about 150 mg to about 245 mg Fosaprepitant that are stable after storage at about 25° C. for 6 months and processes for preparing solid Fosaprepitant compositions that are stable after long term storage at room temperature. The processes include freezing a Fosaprepitant solution in the primary packaging container at a first freezing temperature; applying vacuum at second temperature that is higher than the freezing temperature; fully stoppering the primary packaging container; and sealing the stoppered primary packaging container.
Owner:NAVINTA III INC
Application of borane-pyridine complex in preparation of NK-1 receptor antagonist
InactiveCN112300212AReduce usageImprove securityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhosphate
The invention provides an application of a borane-pyridine complex in preparation of an NK-1 receptor antagonist fosaprepitant dimeglumine. The application is characterized by comprising the followingsteps: step 1) catalyzing aprepitant dibenzyl phosphate under the action of the borane pyridine complex to prepare fosaprepitant; and 2) reacting fosaprepitant with N-methyl-D-glucosamine to generatefosaprepitant dimeglumine. The borane-pyridine complex is used as the catalyst, aprepitant dibenzyl phosphate can directly generate fosaprepitant, then the fosaprepitant dibenzyl phosphate reacts with N-methyl-D-glucosamine to generate fosaprepitant dimeglumine, the reaction is mild, and conversion of raw materials can be completed quickly at the temperature of about room temperature only by using a small amount of catalyst. The catalytic efficiency is high, the reaction conditions are mild and the yield is high. The high-purity and high-yield fosaprepitant can be obtained by recrystallizingthe reaction crude product with deionized water, the post-treatment is extremely simple, and the fosaprepitant dimeglumine is generated by reacting the reaction crude product with N-methyl-D-glucosamine so that the purity and the yield are high.
Owner:商河探荣新技术开发中心
New preparation method for fosaprepitant and pharmaceutically acceptable salt thereof
ActiveCN105254668AOvercome costsOvercome qualityGroup 5/15 element organic compoundsBulk chemical productionPhosphateChemistry
The present invention provides a new preparation method for fosaprepitant and a pharmaceutically acceptable salt thereof. The method comprises: adopting aprepitant dibenzyl phosphate as a raw material, carrying out catalytic hydrogenation in the absence of counter ions and supercritical fluids to prepare fosaprepitant, and forming the pharmaceutically acceptable salt with an alkali after separation or without separation. According to the present invention, the method has characteristics of good product quality, low production cost, simple operation and short reaction period, and is suitable for industrial production.
Owner:海南金泰药业有限公司
Refining method of fosaprepitant dimeglumine
ActiveCN102850399AReduce degradationReduce usageOrganic compound preparationGroup 5/15 element organic compoundsProcess engineeringFosaprepitant dimeglumine
The invention belongs to the field of medicine and relates to a refining method of fosaprepitant dimeglumine. In a refining process, single acetonitrile is used as an anti-solvent for carrying out crystallization treatment on products, and a mixed solvent used in the prior art is replaced. The purity of the obtained fosaprepitant dimeglumine raw material medicine is high, the solvent residue amount is fewer, particularly, the temperature and vacuum degree requirements in a drying process are low, and the refining method is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
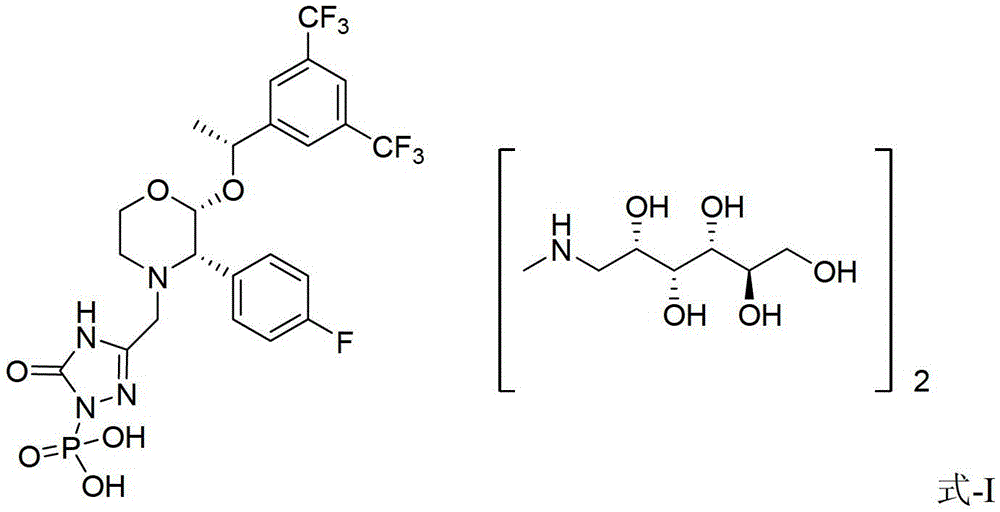
Fosaprepitant derivative, synthesis thereof, and use thereof in long acting preparation
InactiveCN106432337AOrganic active ingredientsGroup 5/15 element organic compoundsChemical compoundChemotherapy induced
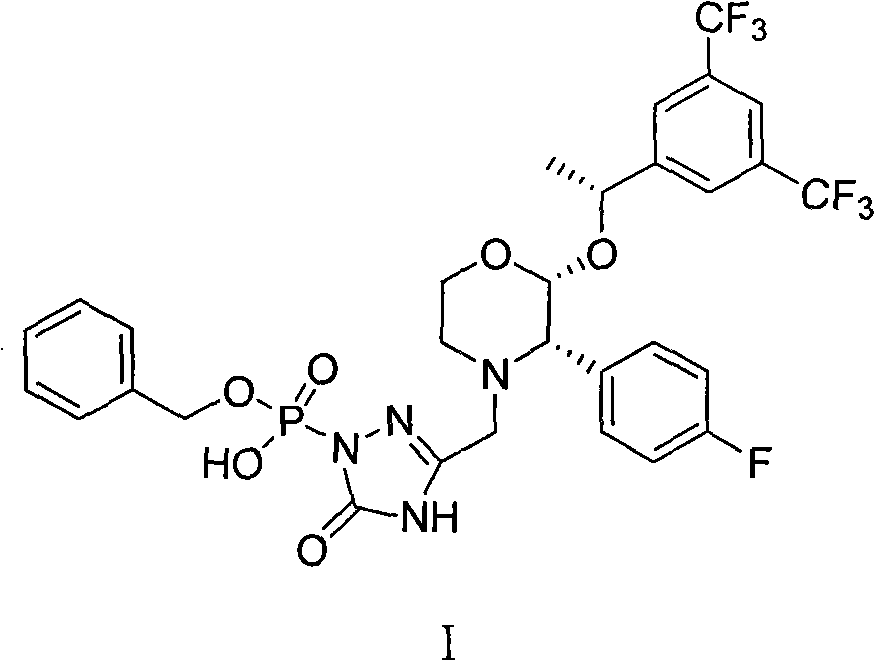
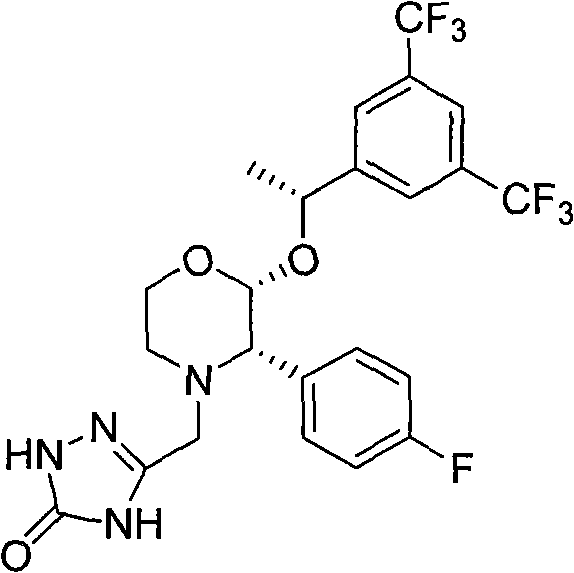
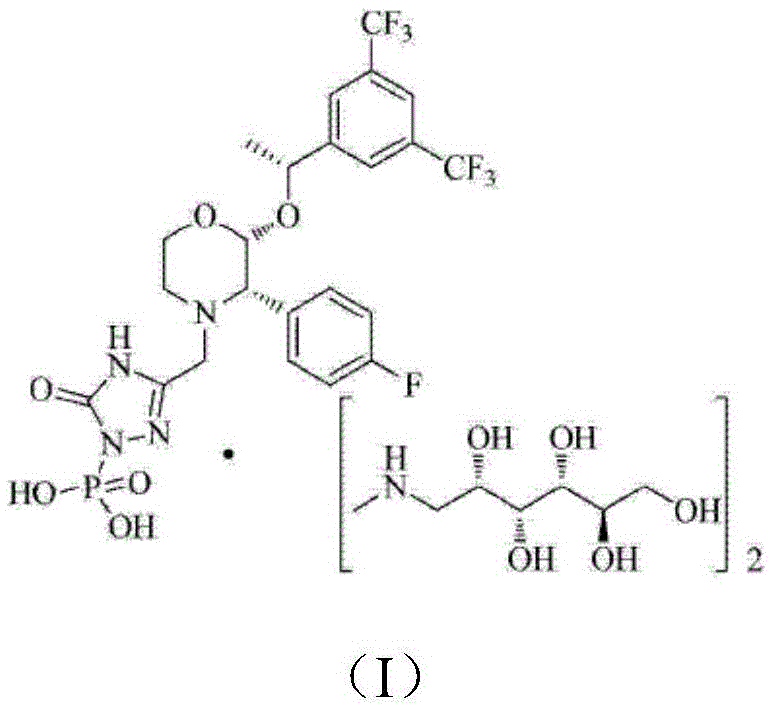
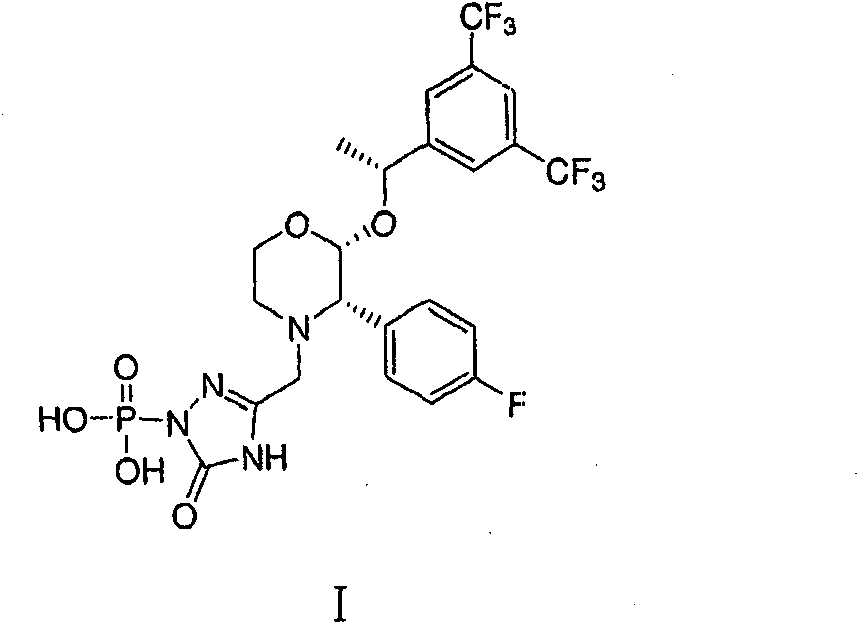
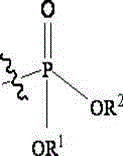
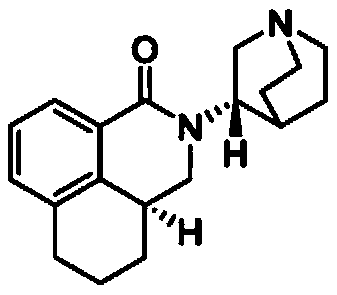
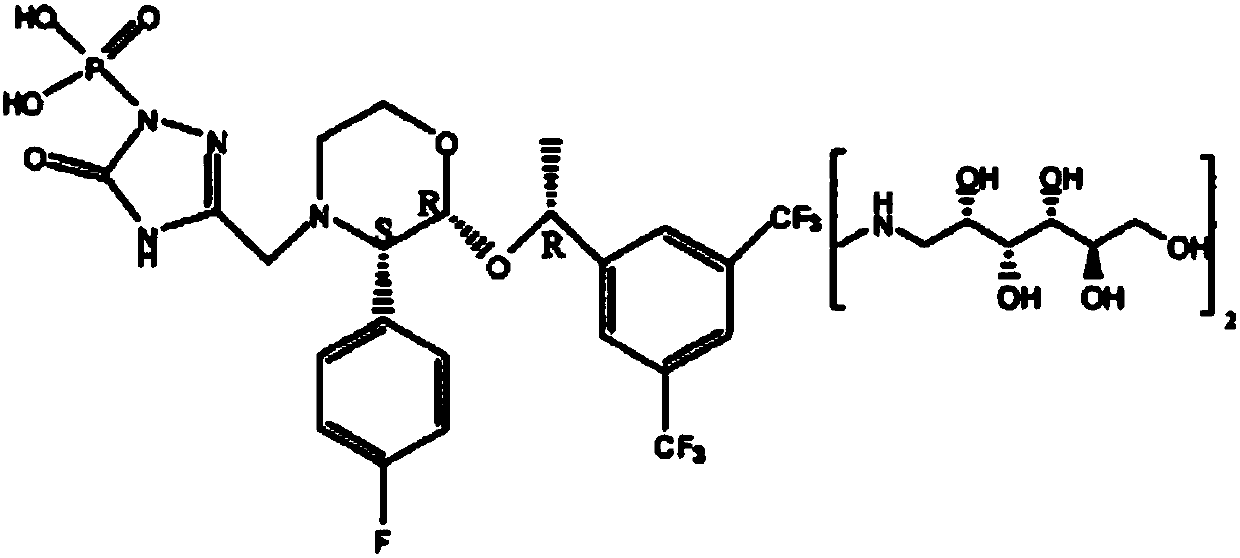
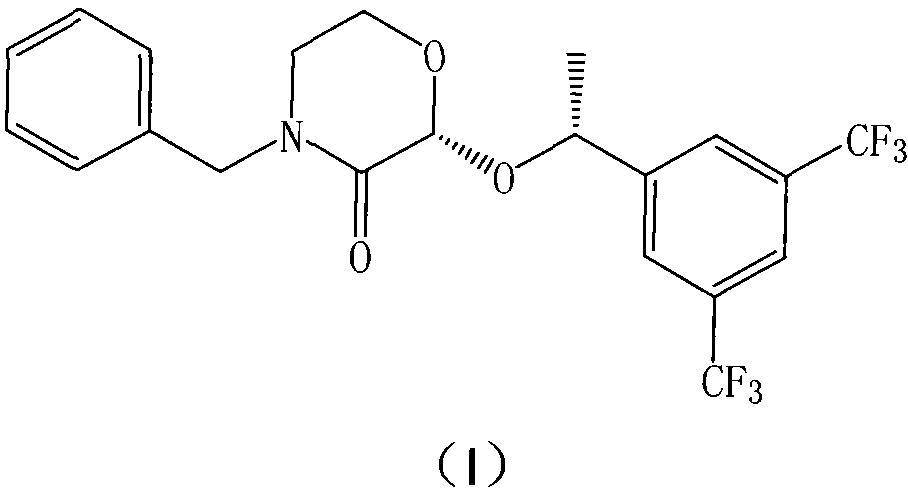
The invention relates to a Fosaprepitant derivative, a synthesis thereof, and a use thereof in a long acting preparation. The invention relates to a compound of formula (I), and a salt, an N-oxide, a quaternary ammonium and a stereoisomer thereof. R<1> to R<8> in the formula (I) are as defined in claims. The invention also relates to an intermediate for preparing the compound of formula (I), and a method for preparing the compound of formula (I). The invention further relates to a use of the compound of formula (I) as a drug especially used for preventing chemotherapy induced acute and late nausea and vomiting.
Owner:HC SYNTHETIC PHARMA CO LTD
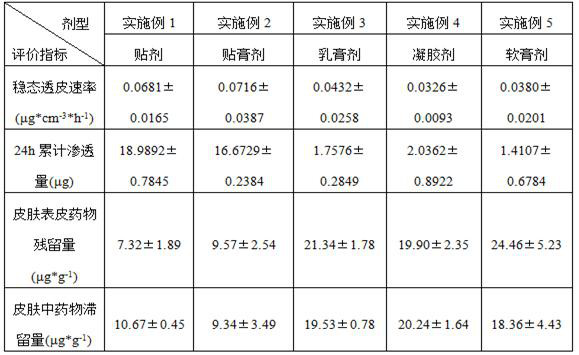
External preparation of neurokinin 1 receptor inhibitor and preparation method of external preparation
The invention discloses an external preparation of a series of neurokinin-1 receptors (NK1 receptors) inhibitors and a preparation method of the external preparation. The NK1 receptor inhibitors comprise, but are not limited to, aprepitant, fosaprepitant, rolapitant, netupitant or pharmaceutically acceptable salts thereof. The external preparation comprises, but is not limited to, ointment, cream, gel, tincture, lotion, aerosol, spray, liniment, a foaming agent, patch, emplastrum and the like. The external preparation of the NK1 receptor inhibitors can slowly deliver the medicine at a constant speed, and reduce the blood concentration fluctuation caused by dissolution and absorption of the oral medicine; the pertinence to local lesions can be improved, and the side effects of the whole body are reduced; and meanwhile, a choice with high compliance is provided for patients inconvenient to take orally, and the external preparation has important significance in relieving the pain of cancer patients clinically.
Owner:CHANGSHA JINGYI PHARM TECH CO LTD
A kind of injection composition containing fosaprepitant dimeglumine and preparation method thereof
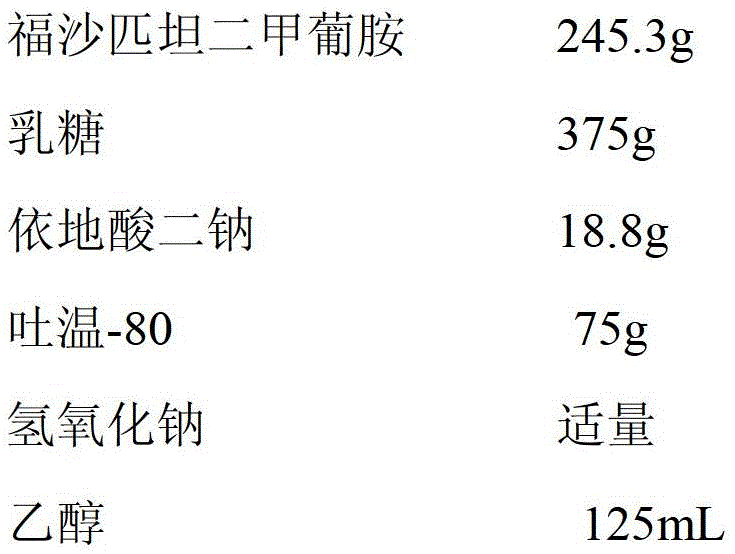
ActiveCN104042572BThe process is simple and easy to controlReduce energy consumptionPowder deliveryOrganic active ingredientsAlcoholFreeze-drying
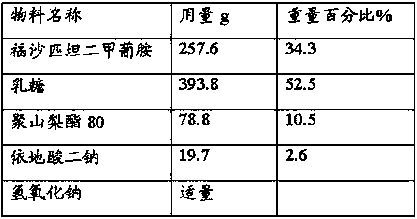
The invention provides a pharmaceutical composition containing fosaprepitant dimeglumine and a preparation method thereof. The pharmaceutical composition is composed of fosaprepitant dimeglumine, lactose, disodium edentate and tween-80, wherein a mass ratio among the fosaprepitant dimeglumine, the disodium edentate, the tween-80 and the lactose is 188-245.3:14.4-18.8:57.5-75:287.5-375. The composition is prepared in an aqueous containing alcohol in a freeze-drying manner. The preparation method is simple and controllable in processes and can reduce energy consumption. The pharmaceutical composition, prepared by the preparation method in the invention, is stable in quality and can ensure safety of clinical medication.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
A kind of preparation method of fosaprepitant and pharmaceutically acceptable salt thereof
The present invention provides a new preparation method for fosaprepitant and a pharmaceutically acceptable salt thereof. The method comprises: adopting aprepitant dibenzyl phosphate as a raw material, carrying out catalytic hydrogenation in the absence of counter ions and supercritical fluids to prepare fosaprepitant, and forming the pharmaceutically acceptable salt with an alkali after separation or without separation. According to the present invention, the method has characteristics of good product quality, low production cost, simple operation and short reaction period, and is suitable for industrial production.
Owner:海南金泰药业有限公司
Injectable Combination Products Of Fosaprepitant And 5-HT3 Blocker
InactiveUS20190358249A1Organic active ingredientsInorganic non-active ingredientsSalbutamolMethotrexate
Sterile pharmaceutical products of Fosaprepitant and a 5-HT3 blocker for parenteral administration including a container containing a formulation of Fosaprepitant or a pharmaceutically acceptable salt thereof and 5-HT3 blocker. The formulations may comprise surface-active agent and a pharmaceutically acceptable vehicle. The formulations may be lyophilized and diluted prior to administration or ready to use liquids or a pre-lyophilization solution.
Owner:NAVINTA III INC
Storage-stable ready-to-use injectable formulations of fosaprepitant dimeglumine
PendingUS20200316097A1Easy to manageDesirable safety profileOrganic active ingredientsPharmaceutical delivery mechanismFosaprepitant dimeglumineMedicinal chemistry
The present application provides a stable, ready-to-use fosaprepitant dimeglumine formulation which is easy to administer without need of any reconstitution step and has a desirable solubility, stability and safety profile. The concentration of the fosaprepitant dimeglumine in the liquid formulation is preferably less than about 80 mg / ml, or more preferably between about 20 mg / ml to about 60 mg / ml. In certain embodiments, the liquid formulation retains at least about 90% chemical stability of the fosaprepitant dimeglumine after storage for a commercially reasonable amount of time at a temperature between about 0° C. to about 40° C.
Owner:RK PHARMA SOLUTIONS LLC
A kind of freeze-dried preparation containing fosaprepitant and preparation method thereof
ActiveCN104971049BStable in natureLow hemolysis ratePowder deliveryOrganic active ingredientsValsartanHemolysis
The invention provides a freeze-dried preparation containing fosaprepitant and a preparation method of the freeze-dried preparation. The freeze-dried preparation contains an active ingredient and other carriers, wherein the active ingredient is an effective amount for treatment of fosaprepitant dimeglumine, and the other carriers include a solubilizing agent, a complexing agent and a freeze-drying excipient; before freeze-drying, an acidity regulator is used for regulating the pH value of a liquid medicine to be 6.5-9.5; and the solubilizing agent is selected from one or more of polyethylene glycol dodecahydroxyl lithium stearate, hydroxypropyl-beta-cyclodextrin and polyethylene glycol. The freeze-dried preparation containing fosaprepitant, provided by the invention, is stable in property and low in hemolysis rate, and improves the compliance of clinical medication of patients.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Formulations of fosaprepitant and aprepitant
ActiveCN109789154BOrganic active ingredientsPeptide/protein ingredientsPharmaceutical medicineAlbumin
Owner:ZHUHAI BEIHAI BIOTECH CO LTD
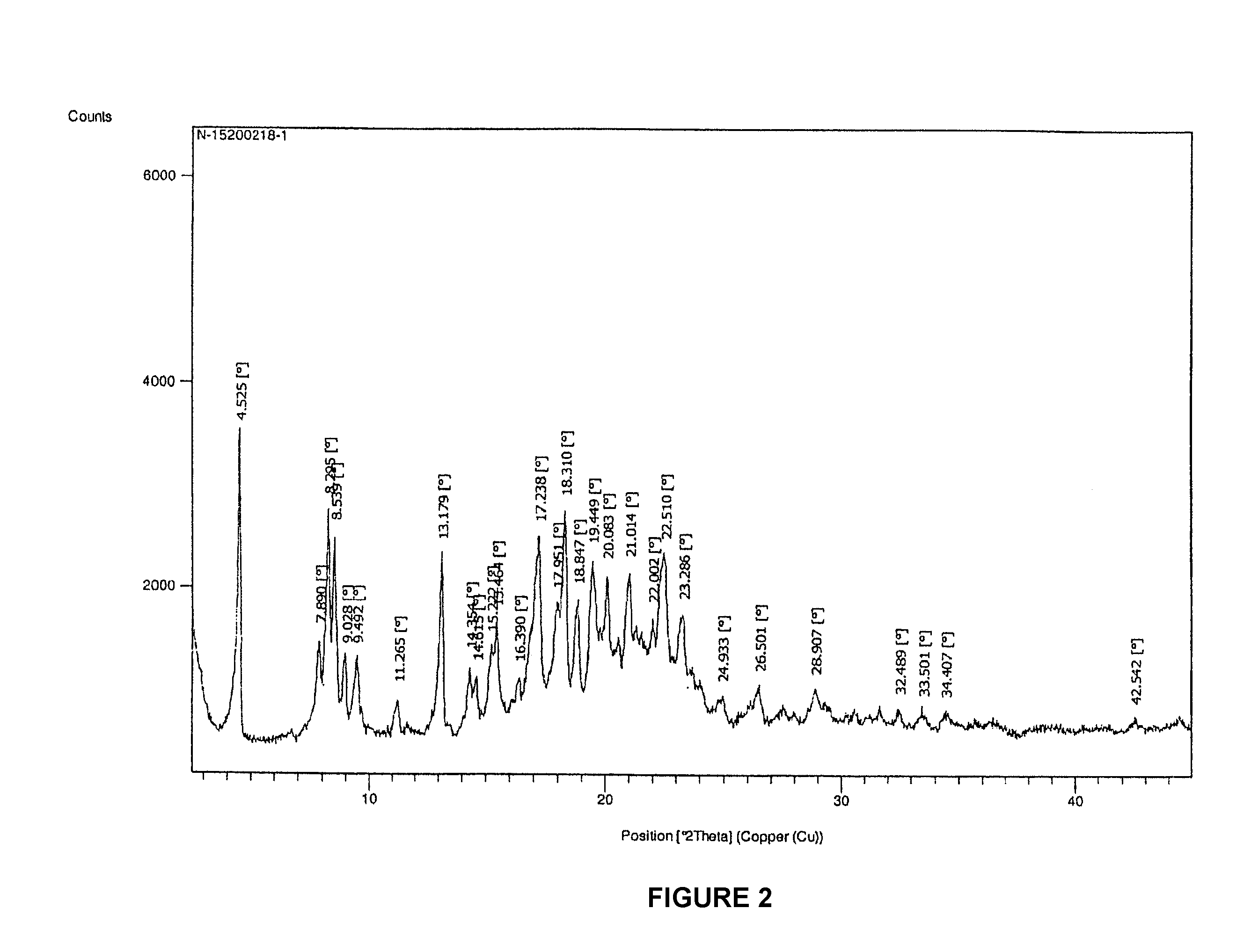
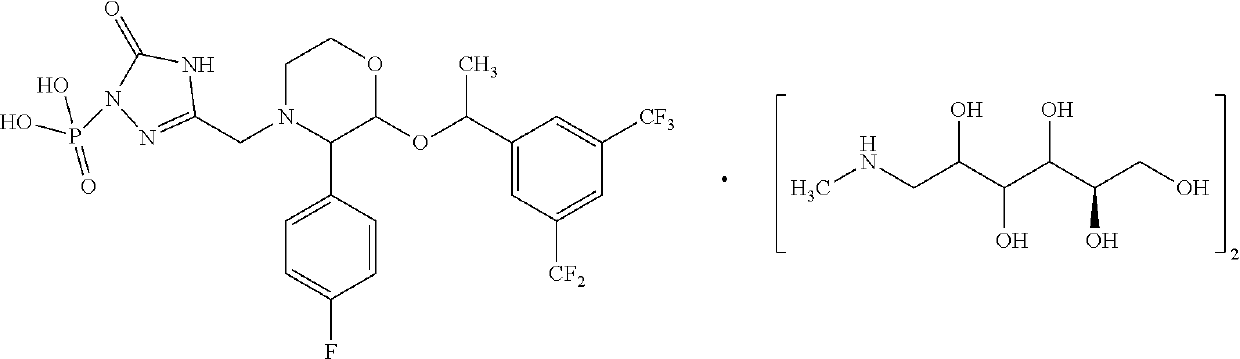
Fosaprepitant-meglumine crystal compound, preparation method thereof, and pharmaceutical composition
InactiveCN106565783ANot easy to absorb moistureGood hygroscopicityOrganic active ingredientsPowder deliveryX-rayIothalamate Meglumine
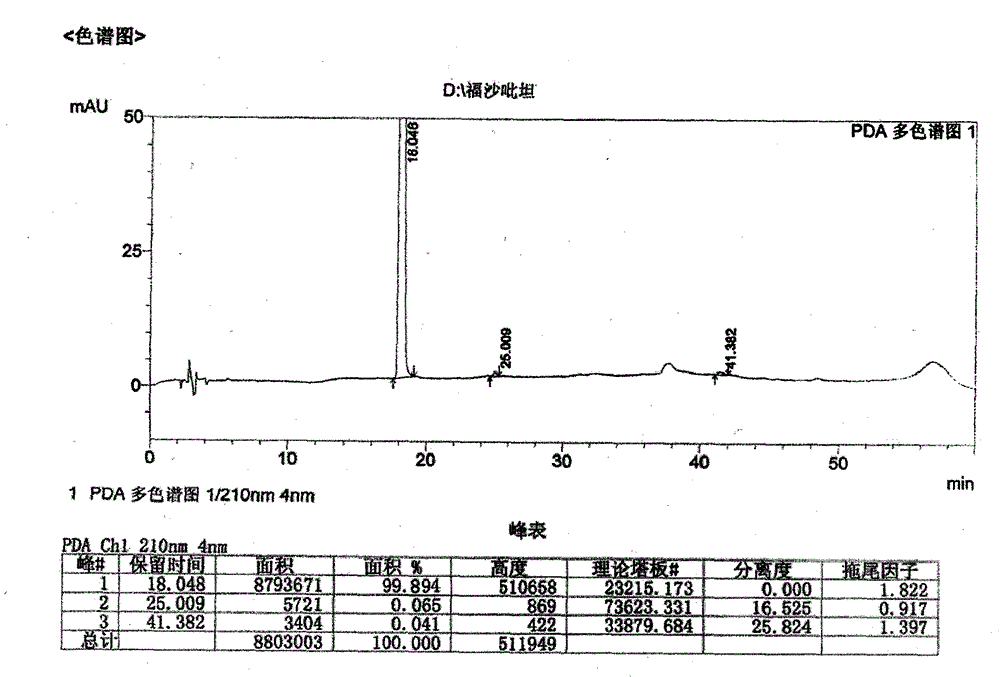
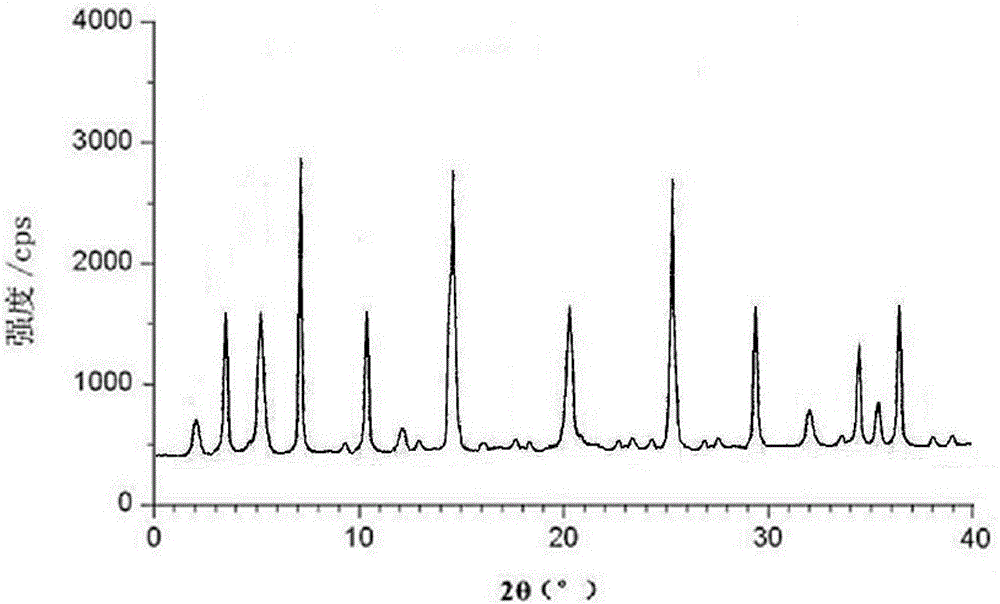
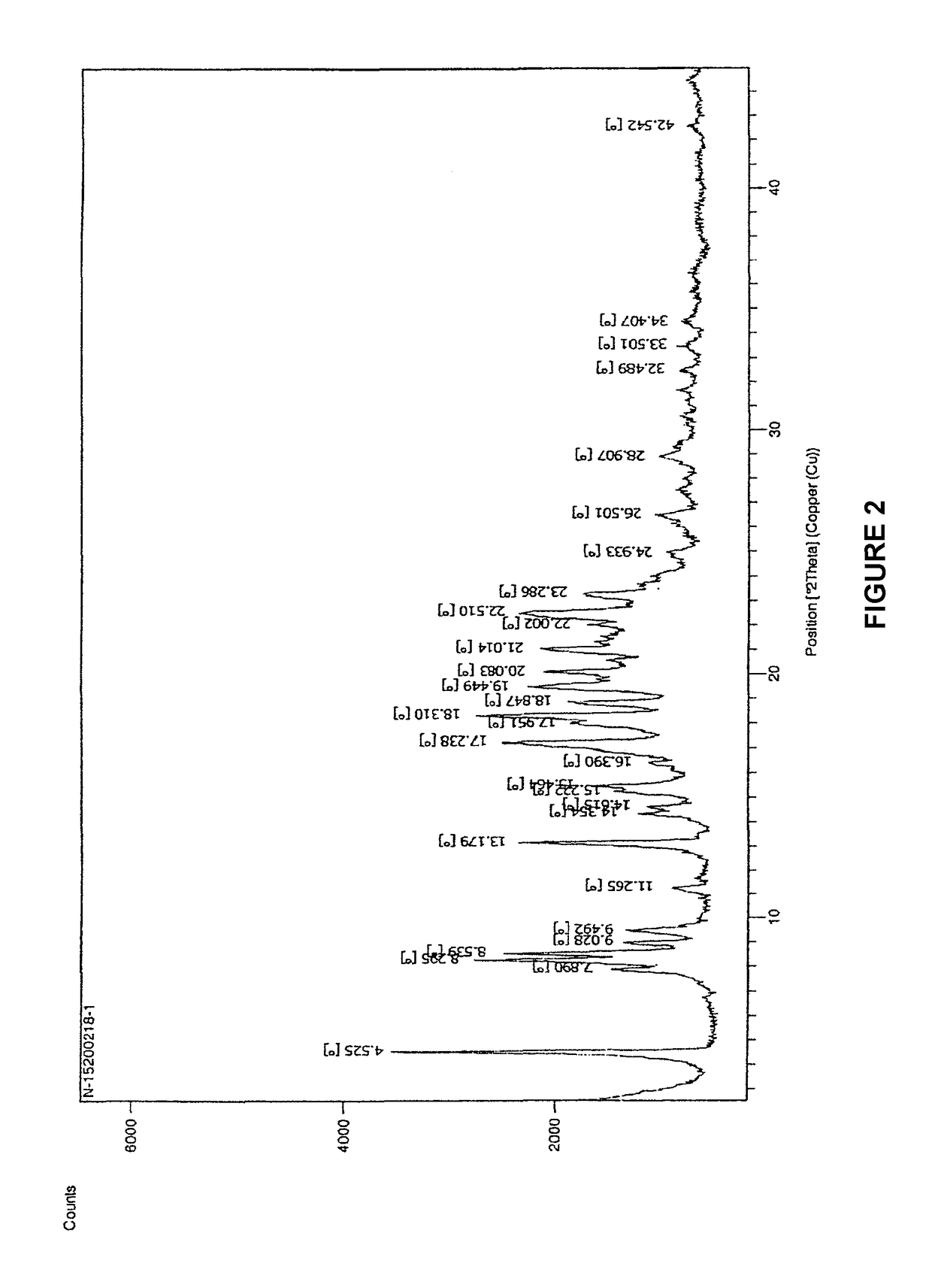
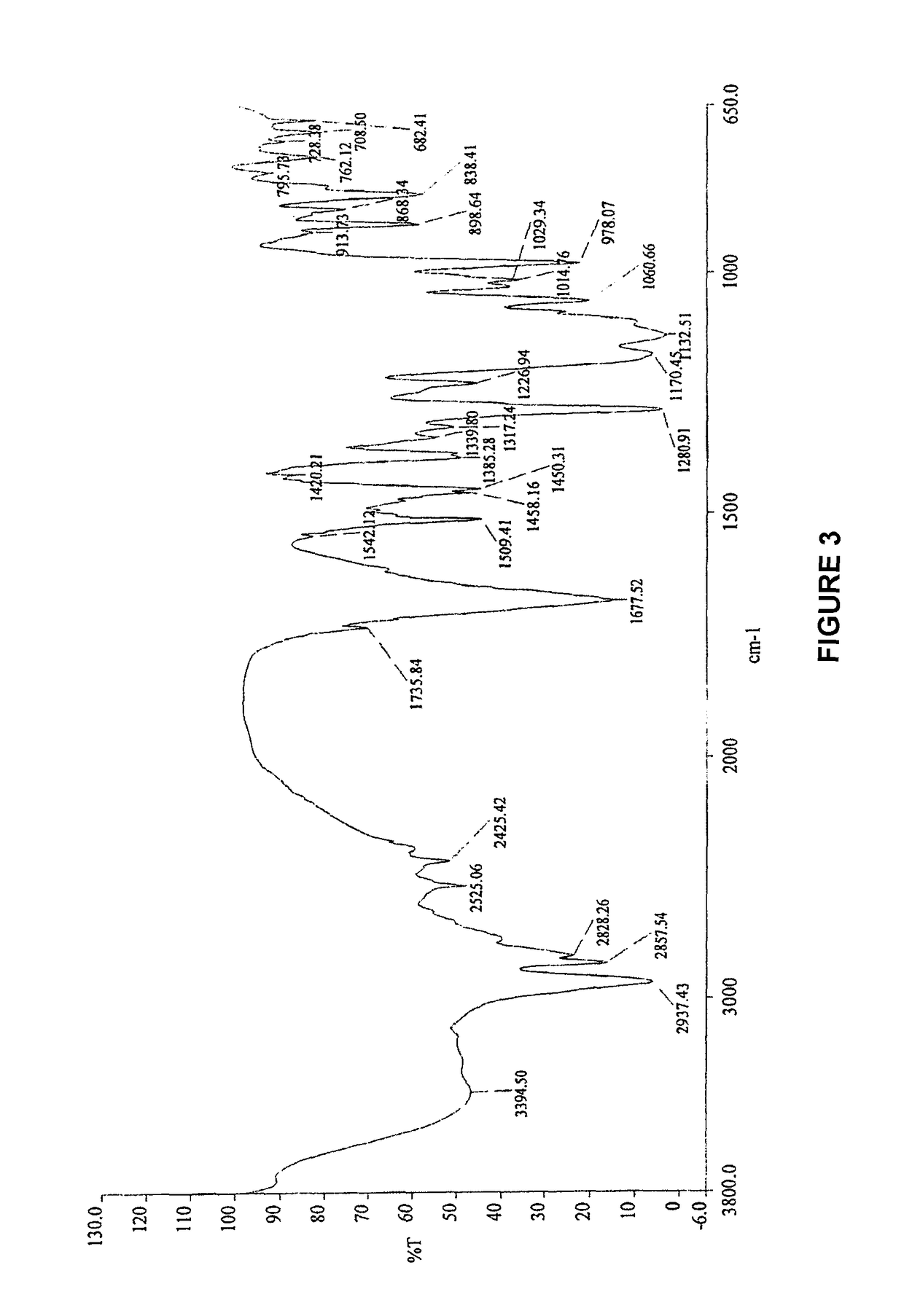
The invention relates to the field of medicines, and discloses a fosaprepitant-meglumine crystal compound, a preparation method thereof, and a pharmaceutical composition. The fosaprepitant-meglumine crystal compound is different from those available in the prior art, wherein Cu-K alpha rays are adopted for measurement to obtain an X-ray powder diffraction pattern as shown in FIG. 1. The fosaprepitant-meglumine crystal compound is good in stability, good in fluidity, not easy to absorb moisture, simple in preparation method and easy to operate. When the fosaprepitant-meglumine crystal compound is prepared as the pharmaceutical composition, the medication safety is greatly improved. Therefore, the fosaprepitant-meglumine crystal compound is very suitable for clinical application.
Owner:SHANDONG YUXIN PHARMA CO LTD
Method for simultaneously detecting fosaprepitant and aprepitant in plasma
InactiveCN105974016AHigh sensitivitySimple and fast operationComponent separationBlood plasmaPeak area
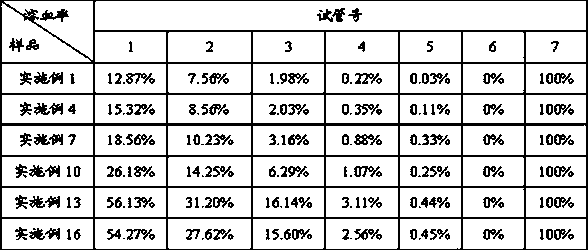
The invention discloses a method for simultaneously detecting fosaprepitant and aprepitant in plasma. The method comprises detecting a pretreated correction standard sample and a pretreated plasma sample through high performance liquid chromatography-tandem mass spectrometry, carrying out quantification through an internal standard method, building a standard curve equation through fosaprepitant and aprepitant correction standard sample concentrations as X coordinates and fosaprepitant and aprepitant peak area-to-internal standard peak area ratios as Y coordinates, introducing the plasma sample fosaprepitant and aprepitant peak area-to-internal standard peak area ratios into the standard curve equation and calculating fosaprepitant and aprepitant concentrations. The method is fast and accurate, has high sensitivity and strong specificity, is convenient for operation and is suitable for simultaneous determination of fosaprepitant and aprepitant concentrations of blood plasma.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Formulations of fosaprepitant and aprepitant
ActiveCN109789154AOrganic active ingredientsPeptide/protein ingredientsPharmaceutical medicineAlbumin
This document relates to a composition comprising fosaprepitant, or a pharmaceutically acceptable salt thereof, and human serum albumin, wherein the fosaprepitant, or a pharmaceutically acceptable salt thereof, and the human serum albumin in the composition have a ratio by weight from about 1:0.1 to about 1:500. This document also relates to a composition comprising aprepitant and human serum albumin, wherein the aprepitant and the human serum albumin in the composition have a ratio by weight from about 1:80 to about 1:1000. This document also relates to a composition comprising fosaprepitant,or a pharmaceutically acceptable salt thereof, aprepitant and human serum albumin.
Owner:ZHUHAI BEIHAI BIOTECH CO LTD
A kind of aseptic freeze-dried powder of fosaprepitant dimeglumine for injection and preparation process thereof
ActiveCN104414983BAvoid allergiesAvoid hemolysisOrganic active ingredientsPowder deliveryMedicineFosaprepitant dimeglumine
The invention particularly relates to a sterile freeze-dried fosaprepitant-dimeglumine powder for injection and a preparation process of the sterile freeze-dried fosaprepitant-dimeglumine powder. The sterile freeze-dried fosaprepitant-dimeglumine powder for injection comprises an active component, a solubilizing agent and a pH-value adjusting agent, wherein the active component is fosaprepitant dimeglumine. The preparation process of the sterile freeze-dried fosaprepitant-dimeglumine powder for injection is simple and feasible and is suitable for scale production. The sterile freeze-dried fosaprepitant-dimeglumine powder for injection has high quality and excellent stability and can be stored for a long time.
Owner:SHANDONG NEWTIME PHARMA
Fosaprepitant composition and preparation method thereof
The present application and its embodiments teach stable compositions of fosaprepitant or a pharmaceutically acceptable salt thereof with such compositions lacking polysorbate 80 and containing dual functional excipients of hydrolysis inhibition and solubility enhancement. Further described are methods of preparation of such compositions. Among other advantages of contemplated compositions, fosaprepitant hydrolysis degradation is kept low and the compositions maintain physically and chemically stable for prolonged period.
Owner:SPES PHARMA INC
Preparation method of fosaprepitant freeze-dried powder injection
InactiveCN109549932AProlong the action timeImprove solubilityOrganic active ingredientsPowder deliverySolubilityMicrosphere
The invention discloses a preparation method of a fosaprepitant freeze-dried powder injection. Firstly, fosaprepitant is loaded by PLGA, and then an S / O / W emulsified microsphere preparation is formedafter emulsification and freeze-drying. Emulsification can increase the solubility property and stability of microspheres, and also can improve the syringeability of the microspheres. After 6 weeks ofinjection with the preparation, the preparation with effective blood drug concentration can still be detected in rats, and the preparation prolongs the action time of the drug, reduces the frequencyof drug administration, improves the medication compliance of patients, and is of great significance.
Owner:SICHUAN PHARMA
Application of borane-pyridine complexe in preparation of pharmaceutical compound
InactiveCN112480172AReduce usageImprove securityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsPtru catalystPhosphate
The invention provides an application of a borane-pyridine complex in preparation of a pharmaceutical compound fosaprepitant. The application is characterized by comprising the following step: catalyzing aprepitant dibenzyl phosphate under the action of the borane-pyridine complex to prepare fosaprepitant. The borane-pyridine complex is used as the catalyst, aprepitant dibenzyl phosphate can directly generate fosaprepitant, the reaction is mild, the conversion of the raw materials can be quickly completed at the temperature of about room temperature only by using a small amount of catalyst, the catalytic efficiency is high, the reaction condition is mild, and the yield is high. The high-purity and high-yield fosaprepitant can be obtained by recrystallizing the reaction crude product by using deionized water, the post-treatment is extremely simple, and the deionized water is used as a solvent, so that the method is more economical and environment-friendly.
Owner:商河探荣新技术开发中心
Crystalline fosaprepitant dicyclohexylamine salt and its preparation
ActiveUS9850267B2Improve purification effectAllows preparationOrganic active ingredientsGroup 5/15 element organic compoundsFosaprepitant dimeglumineMedicinal chemistry
The present invention provides dicyclohexylamine salt of fosaprepitant (fosaprepitant DCHA), a process for preparing fosaprepitant DCHA, and a use of fosaprepitant DCHA in the preparation of pharmaceutically acceptable fosaprepitant dimeglumine with high purity. Fosaprepitant dimeglumine is prepared by treating fosaprepitant DCHA with an acid to form fosaprepitant, followed by adding N-methyl-D-glucamine to fosaprepitant.
Owner:NAVINTA
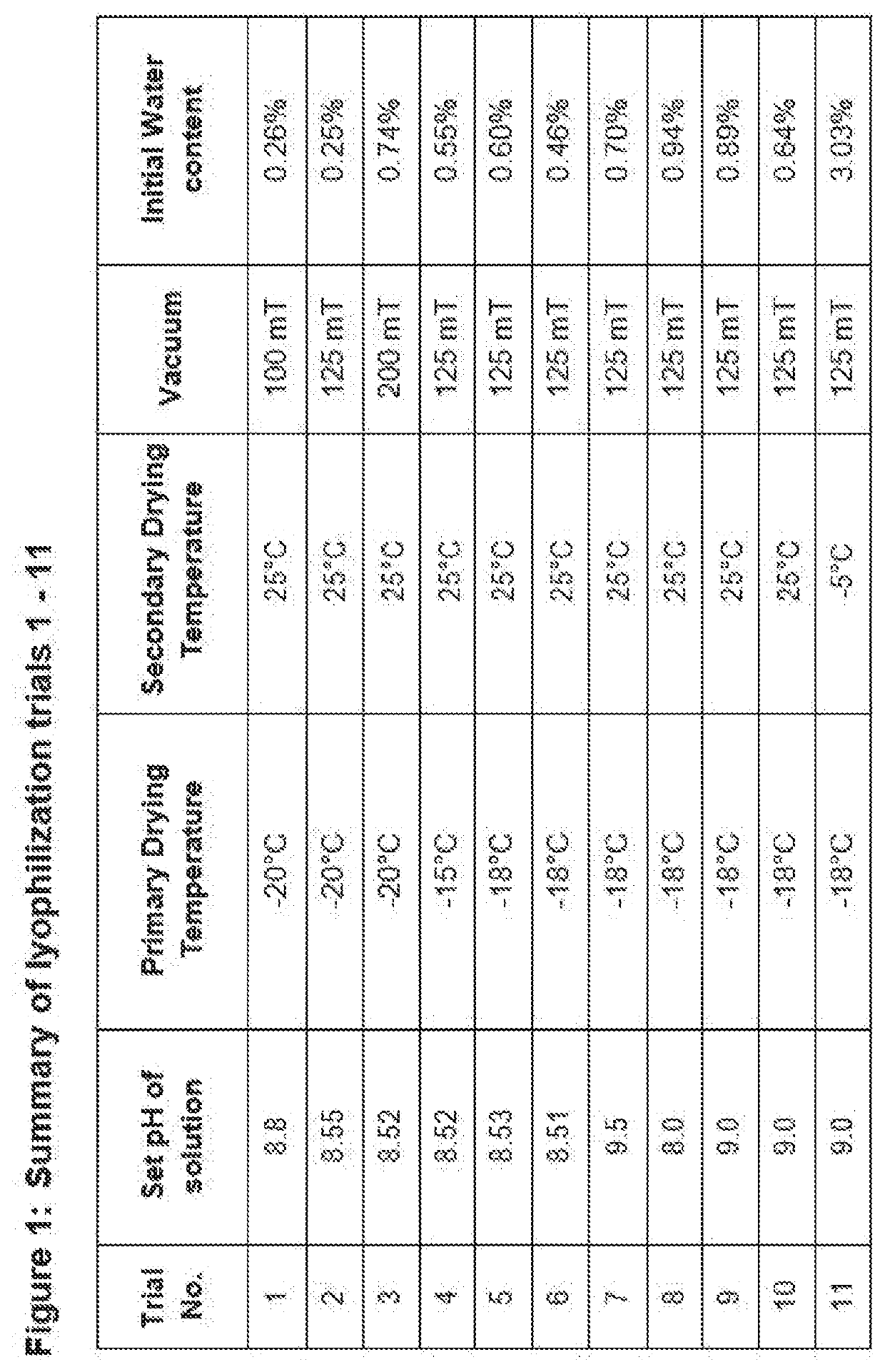
Freeze-drying preparation as well as preparation method and application thereof
ActiveCN114632065AReasonable ratioGood storage stabilityOrganic active ingredientsPowder deliverySide effectCurative effect
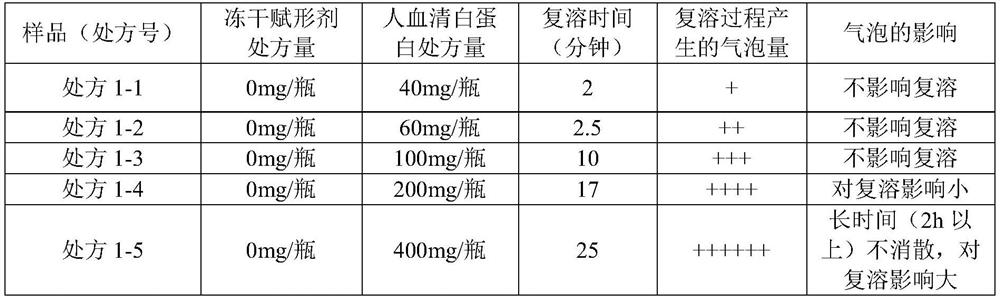
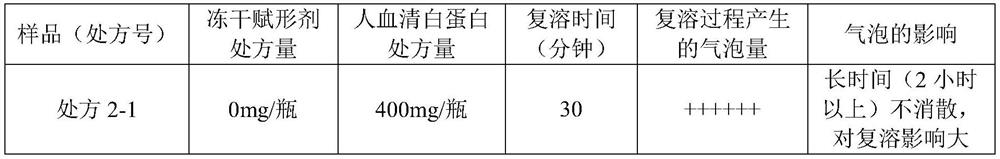
The invention relates to a freeze-dried preparation and a preparation method and application thereof, the freeze-dried preparation comprises fosaprepitant or pharmaceutically acceptable salt thereof and human serum albumin, and the weight ratio of fosaprepitant or pharmaceutically acceptable salt thereof to human serum albumin is 1: 0.2-1: 2. The freeze-dried preparation can further comprise palonosetron or a pharmaceutically acceptable salt of the palonosetron, a freeze-dried excipient and a pH value regulator. The freeze-drying preparation is short in redissolution time, good in storage stability, remarkable in curative effect and few in toxic and side effects, and the preparation method and the use method of the freeze-drying preparation are simple.
Owner:ZHUHAI BEIHAI BIOTECH CO LTD
Synthesis method of fosaprepitant dimeglumine
ActiveCN111662329AAvoid inactivationHigh activityGroup 5/15 element organic compoundsPalladium on carbonPtru catalyst
The invention discloses a synthesis method of fosaprepitant dimeglumine, and belongs to the technical field of medicines. The synthesis method of fosaprepitant dimeglumine mainly comprises the following steps: reacting tetrabenzyl pyrophosphate, adsorbing impurity tar, hydrogenating palladium mesoporous carbon, refining and the like. According to the invention, a one-pot reaction is adopted; a dibenzyl ester product is generated; sodium carbonate is added for quenching reaction, mesoporous carbon is added for adsorbing impurities and tar, palladium-carbon catalyst deactivation is avoided, thena tetrahydrofuran system is directly utilized for a reaction, palladium mesoporous carbon has high activity and can generate monobenzyl ester first and then remove groups through hydrogenation compared with a common route, steps are saved, and high yield is achieved.
Owner:LIANYUNGANG GUIKE PHARMA
A kind of preparation method of fosaprepitant intermediate
ActiveCN103012305BHigh yieldQuality improvementOrganic chemistryGlyoxylic acidCombinatorial chemistry
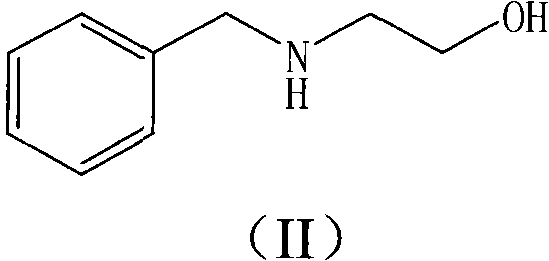
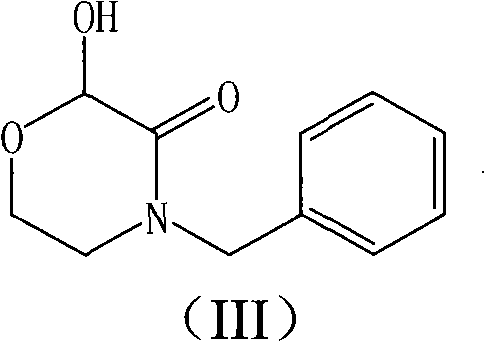
The invention relates to a method for preparing a fosaprepitant intermediate which is a compound shown in a formula (I). The method includes the steps of reacting a compound in a formula (II) and glyoxylic acid to generate a compound in a formula (III), reacting the compound in the formula (III) and (R)-1-(3,5-bi(trifluoromethyl) phenyl) ethanol, and then separating and purifying. According to the method, preparation steps are simple, the reaction yield is high, and the fosaprepitant intermediate is suitable for large-scale production of medical industry.
Owner:JIANGSU HANSOH PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com