Sterile lyophilized preparation containing fosaprepitant, and preparation method thereof
A freeze-dried preparation, fosaprepitant dimeglumine technology, applied in the field of medicine, can solve the problems affecting the quality of life of patients, reducing treatment compliance, potential safety hazards, etc., and achieves accelerated experiments with good stability, safe use, and avoidance of The effect of security risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
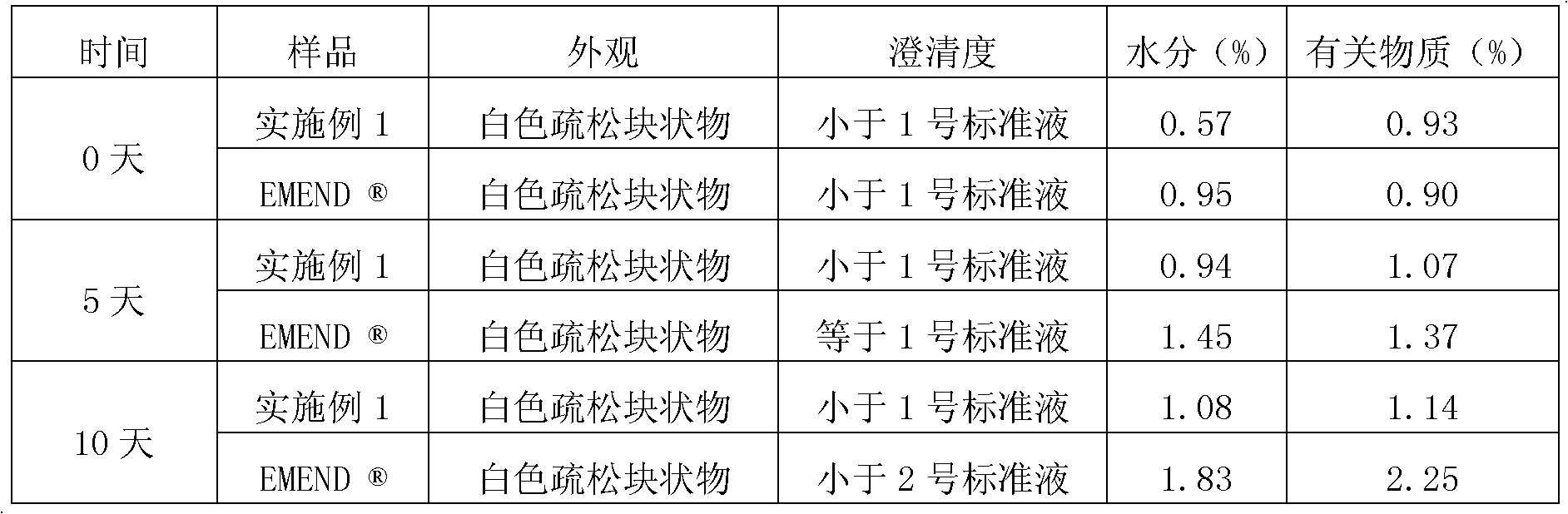
Embodiment 1
[0055] Embodiment 1. prepare 1000 bottles of sterile lyophilized preparations containing 115mg fosaprepitant per bottle
[0056] formula:
[0057] Fosaprepitant dimeglumine 188g, polysorbate-80 57.5g, edetate disodium 14.4g, mannitol 287.5g, sodium hydroxide / hydrochloric acid (acidity regulator), add water for injection to a total of 4000mL.
[0058] Preparation Process:
[0059] 1) Liquid preparation: Weigh according to the formula, stir and dissolve 188g of fosaprepitant dimeglumine, 57.5g of polysorbate-80, 14.4g of edetate disodium, and 287.5g of mannitol in an appropriate amount of sterile water for injection Add sterile water for injection to 4000mL, adjust the pH value to 9.2 with 2mol / L sodium hydroxide solution and 0.1mol / L hydrochloric acid, then pass the liquid medicine through a sterile microporous filter membrane with a pore size of 0.22 microns, and pack 1000 Bottles are half-stoppered with halogenated butyl rubber and placed in a freeze dryer. Bacteria and end...
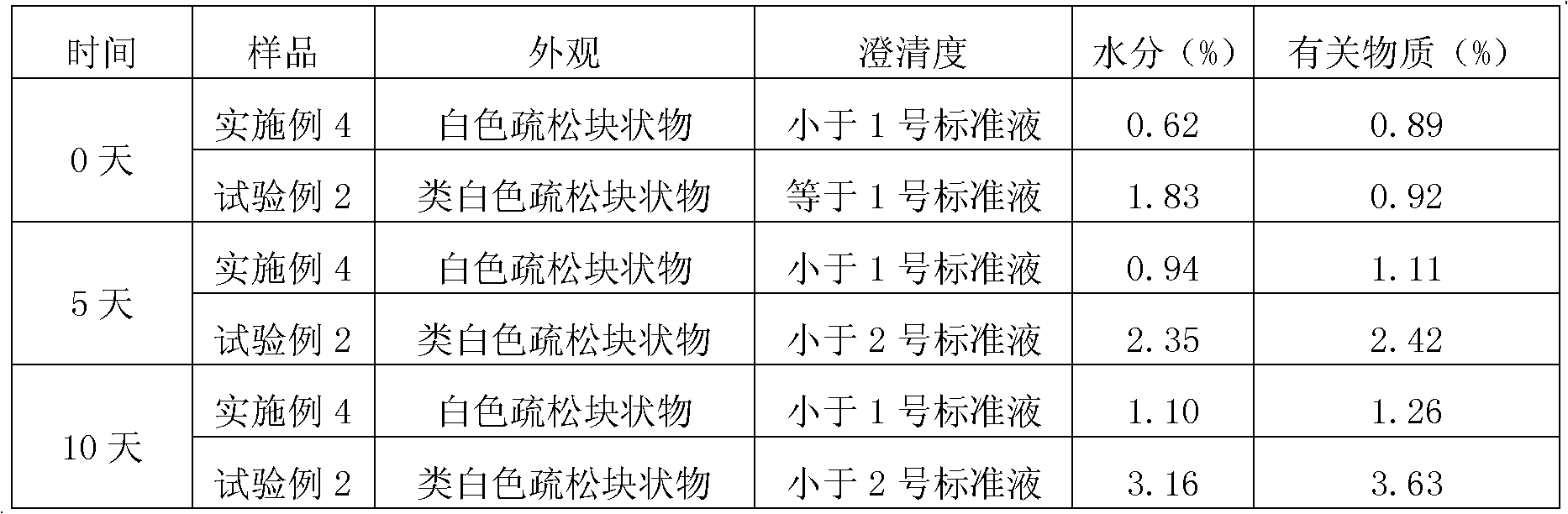
Embodiment 2
[0068] Embodiment 2. prepare 1000 bottles of sterile freeze-dried preparations containing 120mg fosaprepitant per bottle
[0069] formula:
[0070] Fosaprepitant dimeglumine 196.2g, polysorbate-20 65g, edetate disodium 16.5g, glucose 325g, sodium bicarbonate, add water for injection to a total of 5000mL.
[0071] Preparation Process:
[0072] 1) Liquid preparation: Weigh according to the formula, stir and dissolve 196.2 g of fosaprepitant dimeglumine, 65 g of polysorbate-20, 16.5 g of edetate disodium, and 325 g of glucose in an appropriate amount of sterile water for injection, Add sterile water for injection to 5000mL, adjust the pH value to 8.3 with 1mol / L sodium bicarbonate, then pass the liquid medicine through a sterile microporous filter membrane with a pore size of 0.22 microns, pack into 1000 bottles, and half stopper with halogenated butyl rubber Then put it into the freeze dryer, and the bacteria and endotoxins are strictly controlled during the whole process;
...
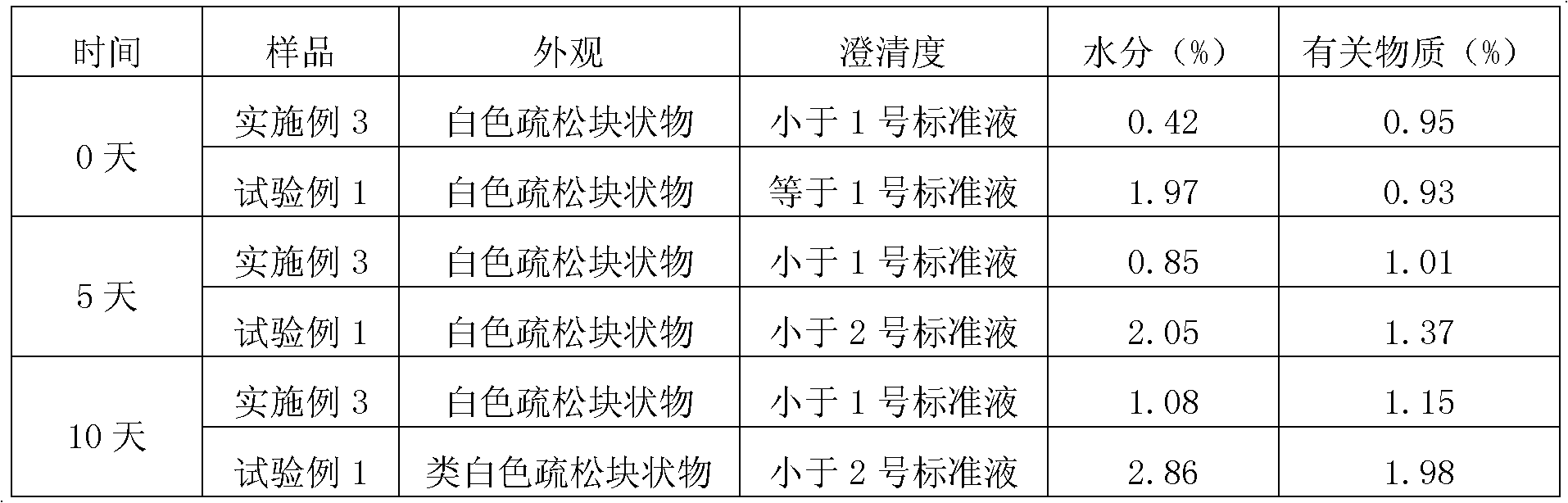
Embodiment 3
[0081] Embodiment 3. prepare 1000 bottles of sterile freeze-dried preparations containing 130mg fosaprepitant per bottle
[0082] formula:
[0083] Fosaprepitant dimeglumine 212.6g, polyethylene glycol 70g, triethanolamine 17.2g, sorbitol 332g, sodium hydroxide / hydrochloric acid, add water for injection to a total of 5000mL.
[0084] Preparation Process:
[0085] 1) Liquid preparation: Weigh according to the formula, stir and dissolve 212.6g of fosaprepitant dimeglumine, 70g of polyethylene glycol, 17.2g of triethanolamine, and 332g of sorbitol in an appropriate amount of sterile water for injection, add sterile Water for injection to 5000mL, adjust the pH value to 7.2 with 2mol / L sodium hydroxide solution / 0.1mol / L hydrochloric acid, then pass the liquid medicine through a sterile microporous filter membrane with a pore size of 0.22 microns, pack into 1000 bottles, half-stoppered and halogenated Put the butyl rubber stopper into the freeze dryer, and the bacteria and endotox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com