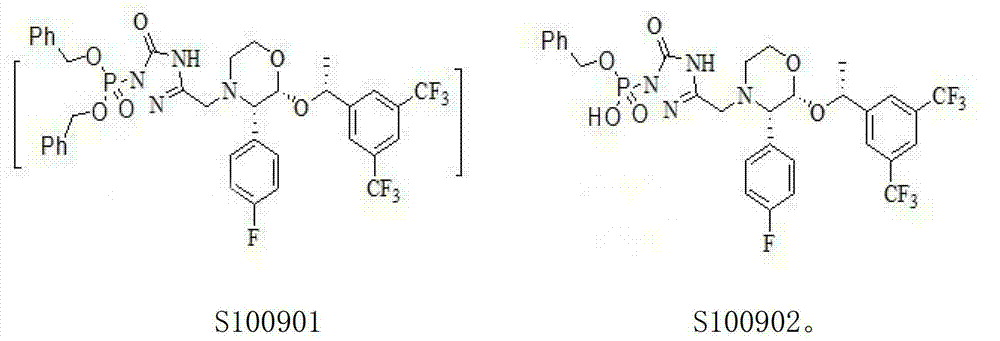
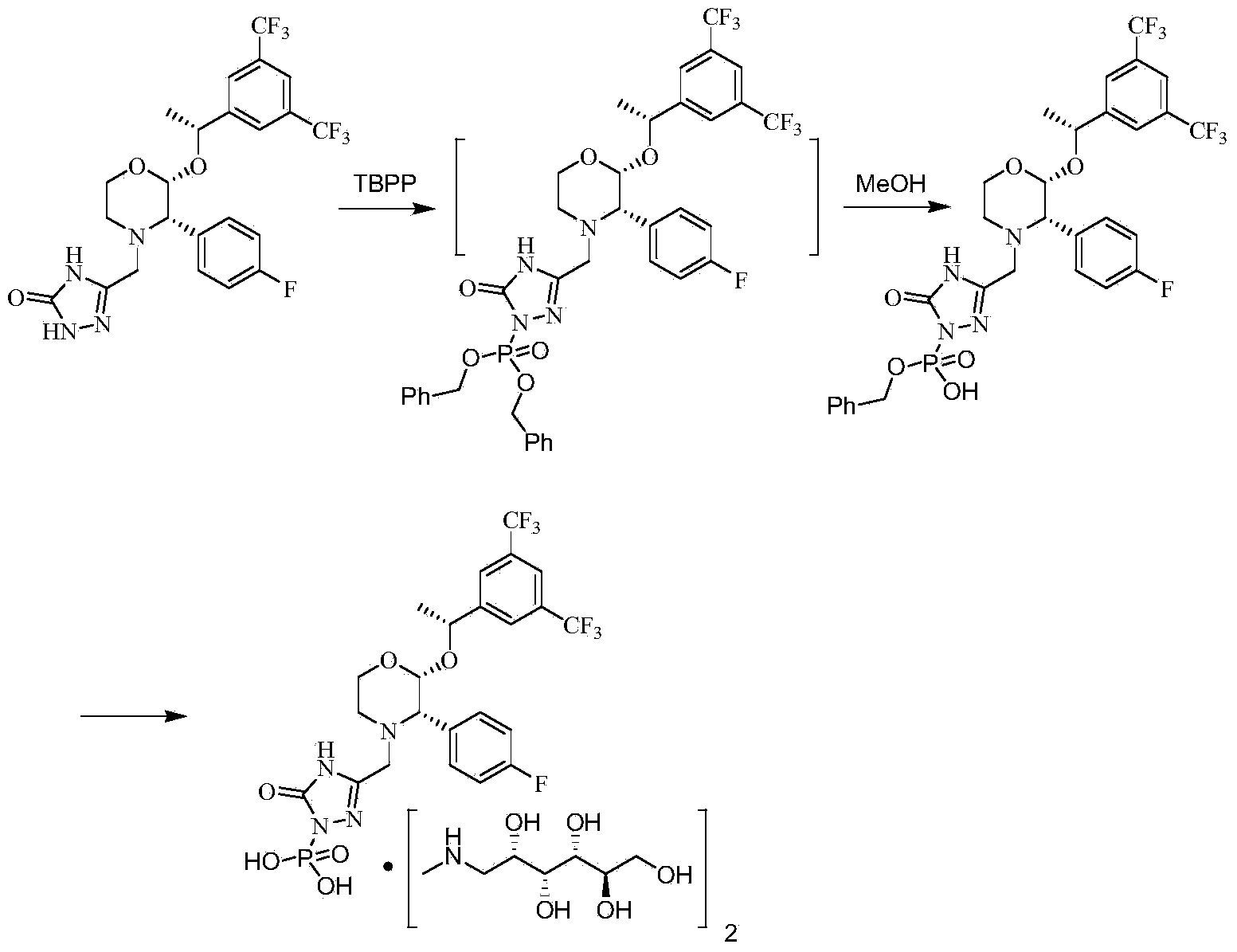
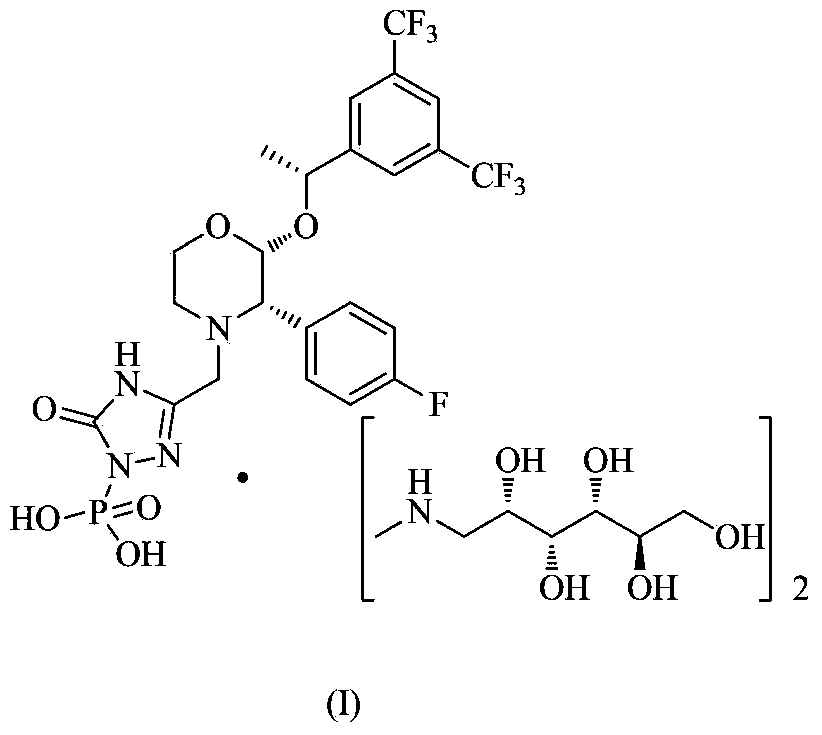
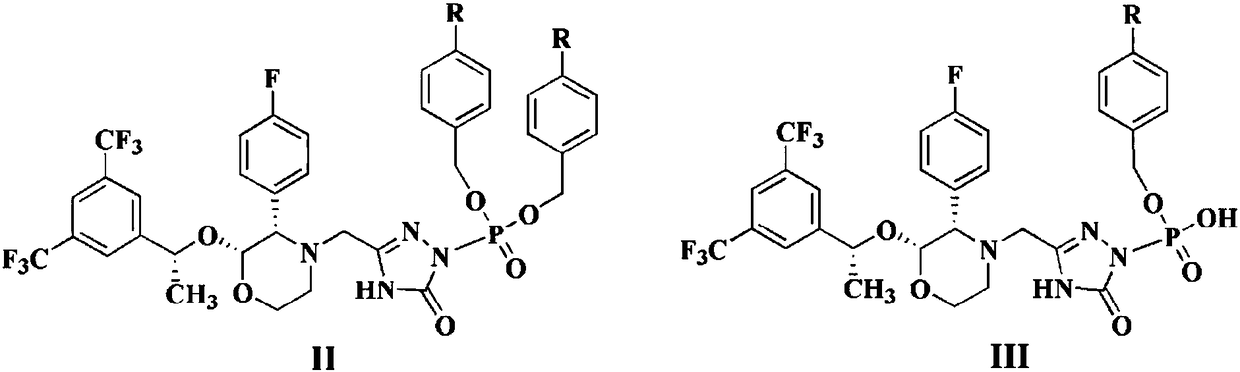
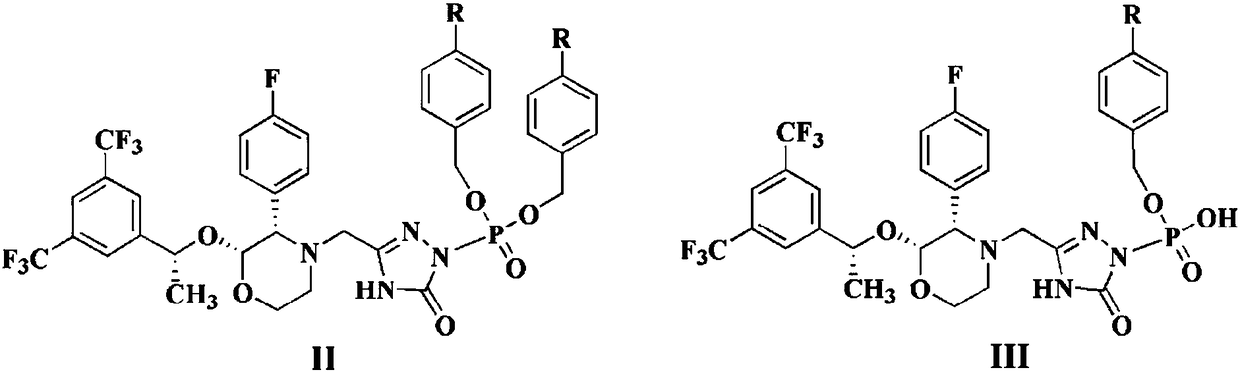
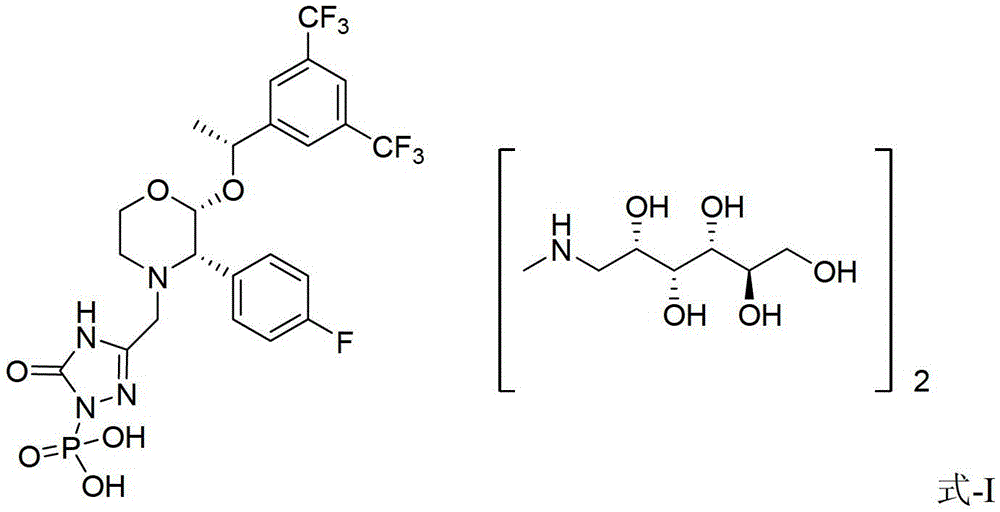
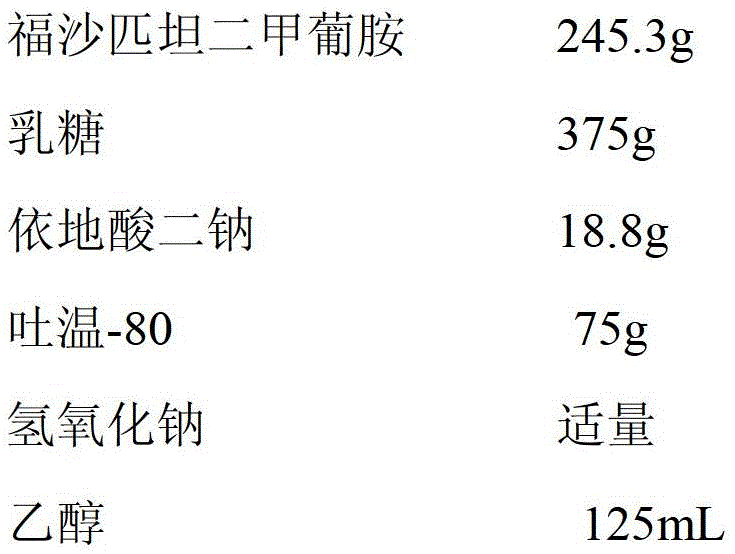
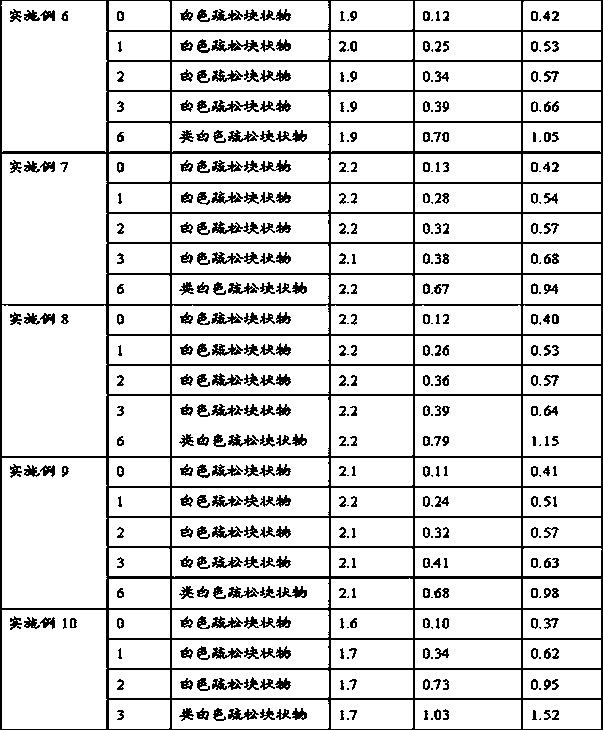
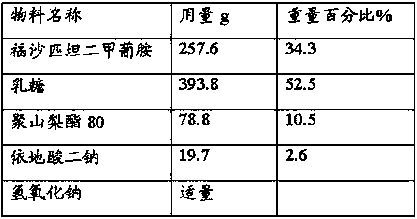
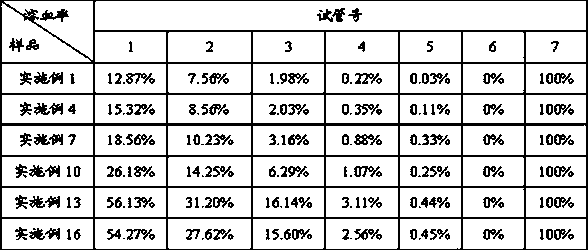
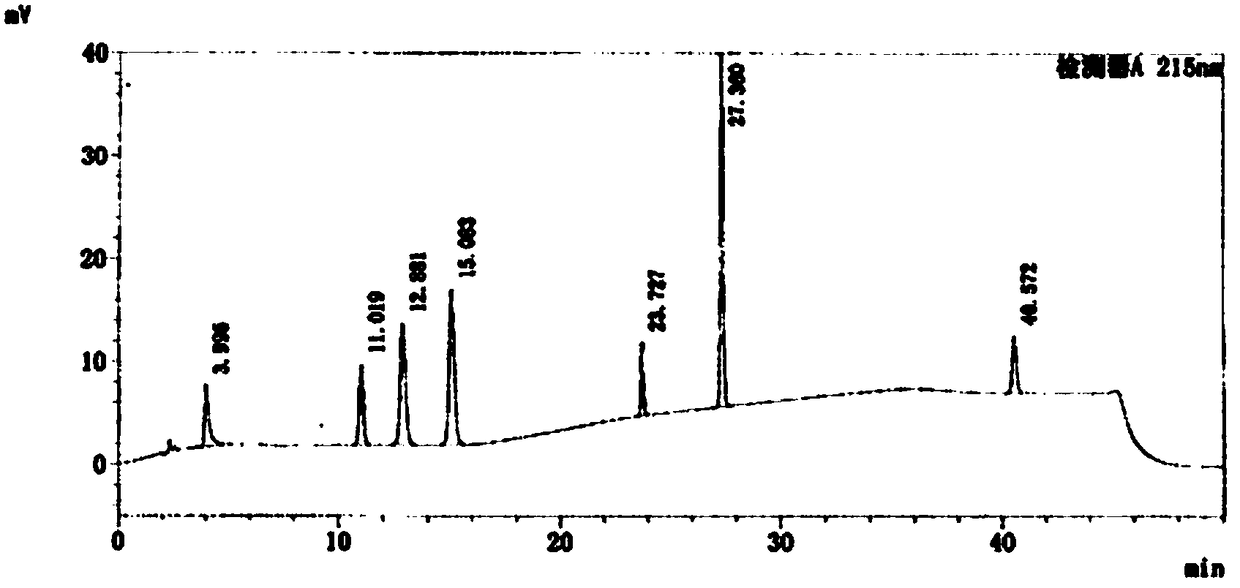
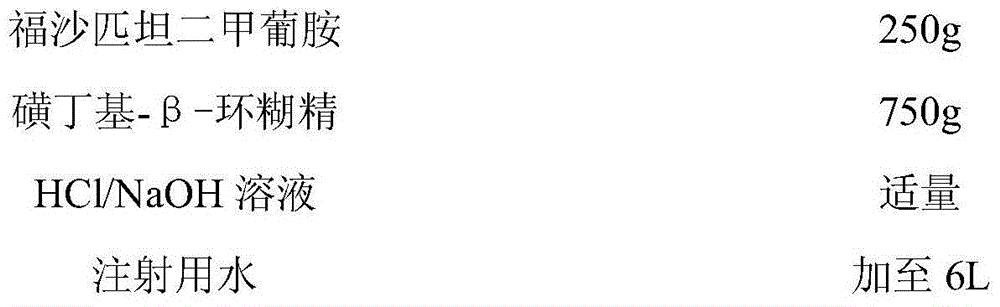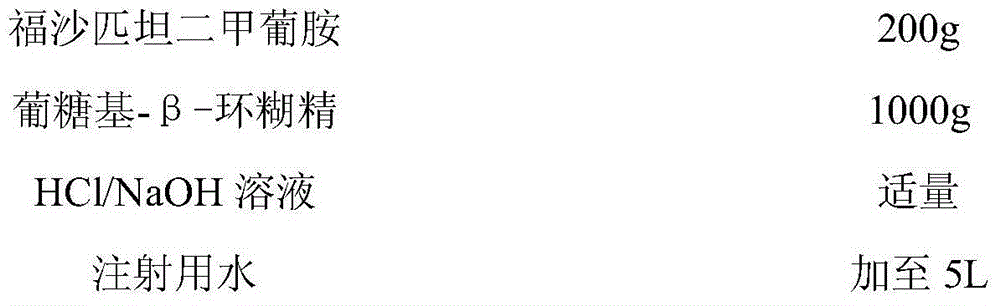Patents
Literature
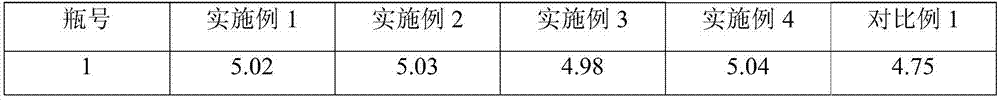
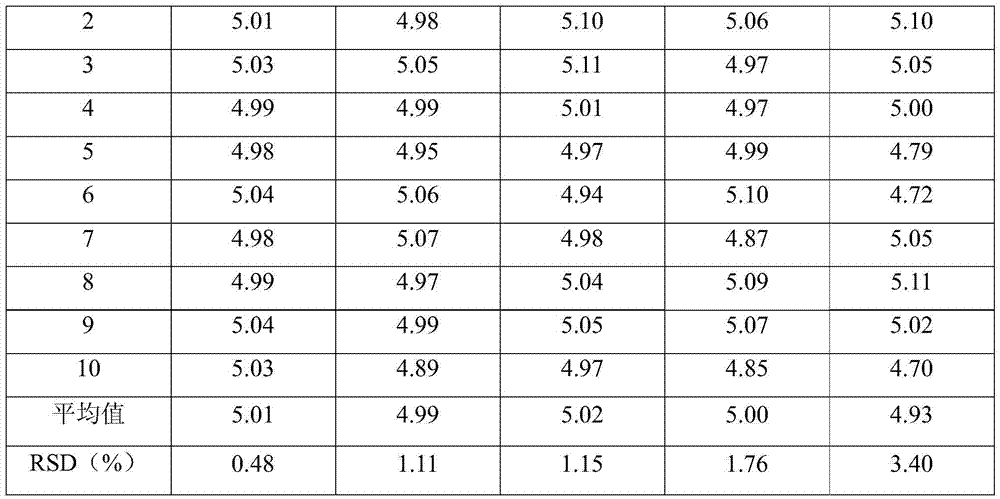
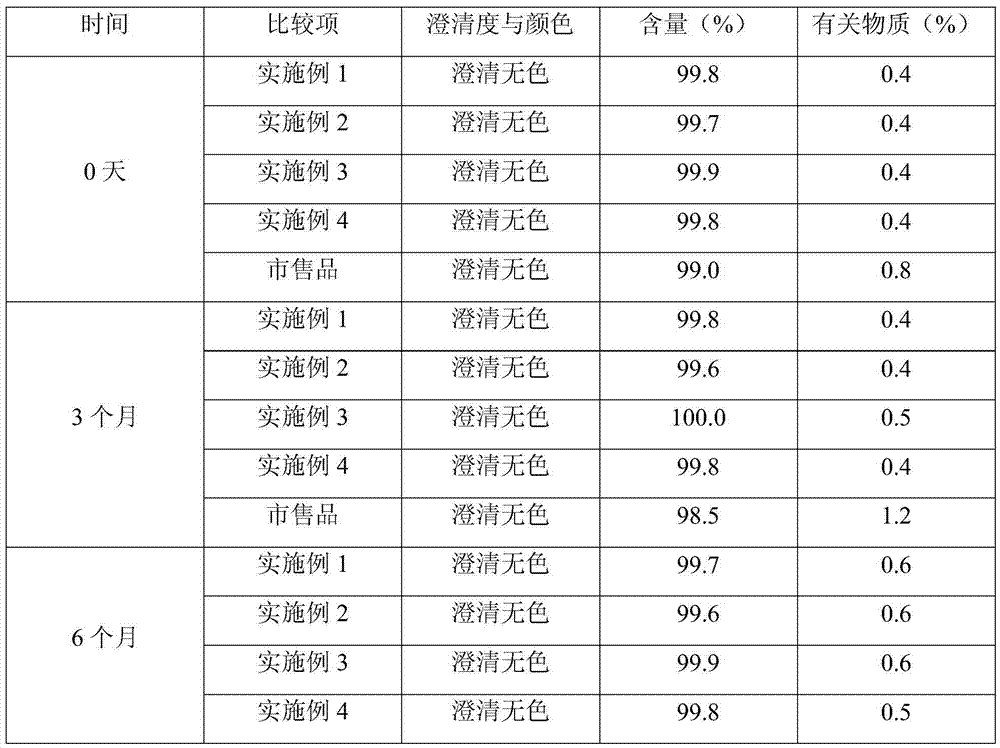
53 results about "Fosaprepitant dimeglumine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
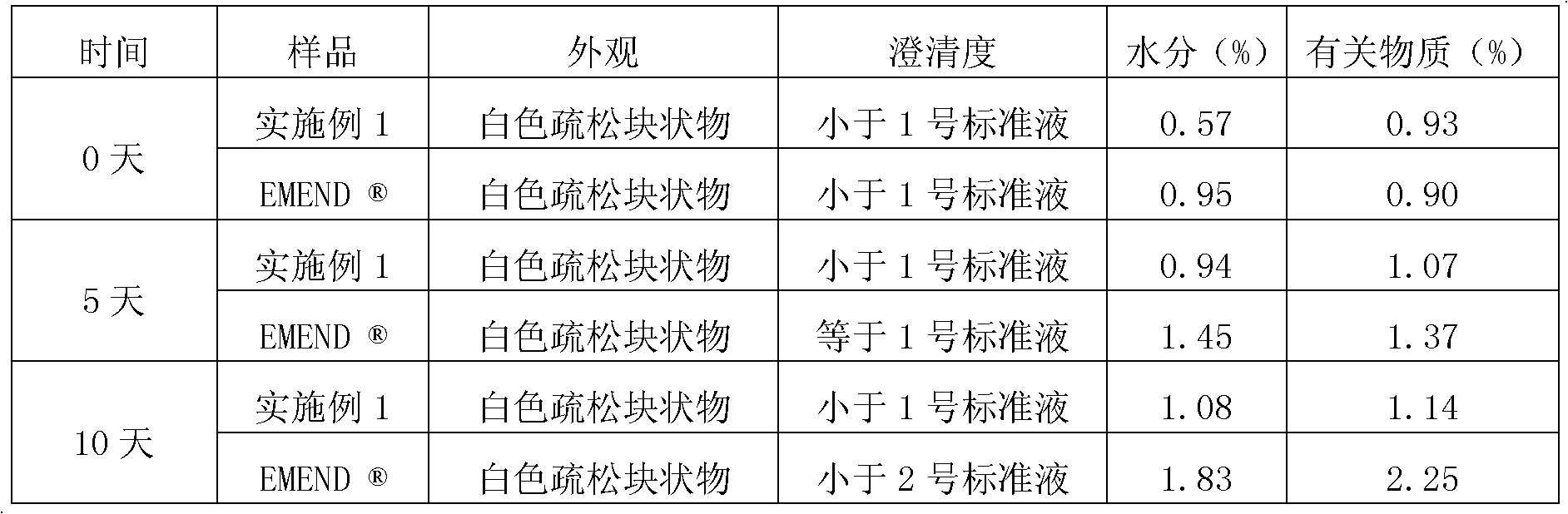
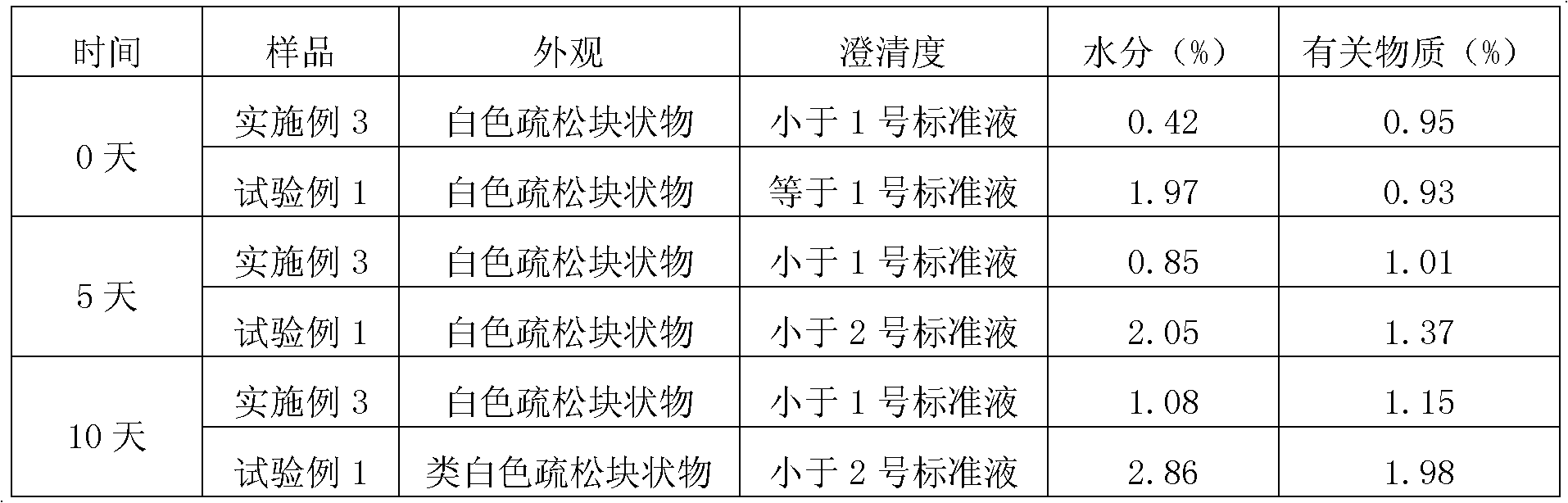
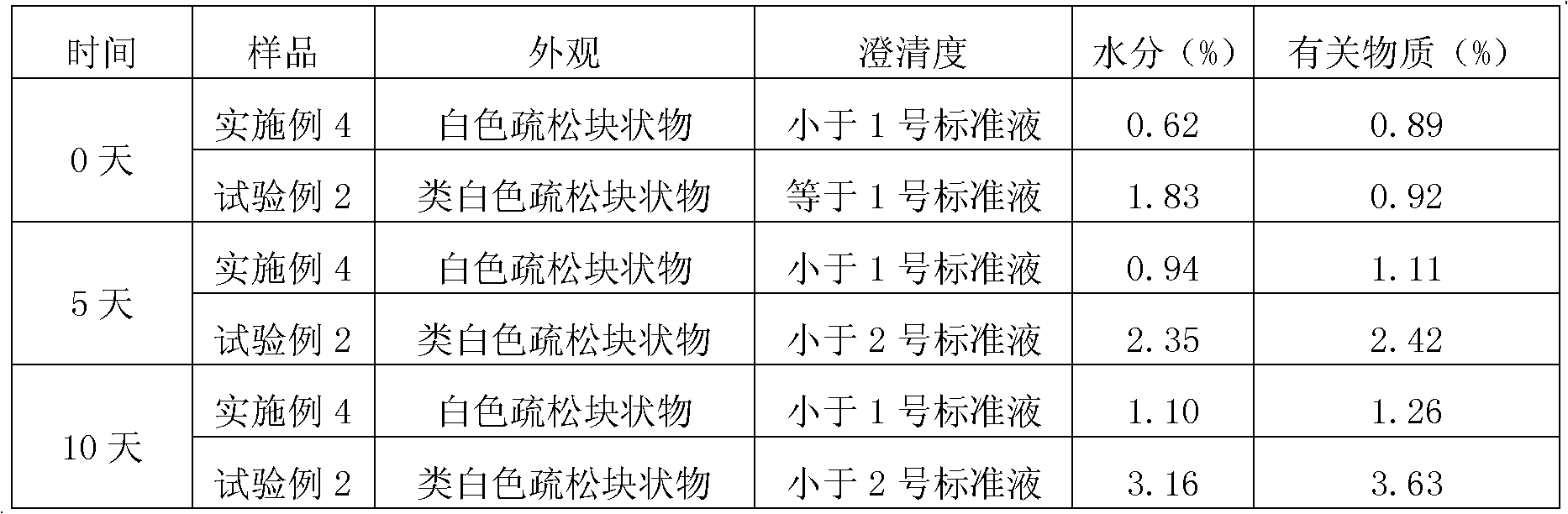
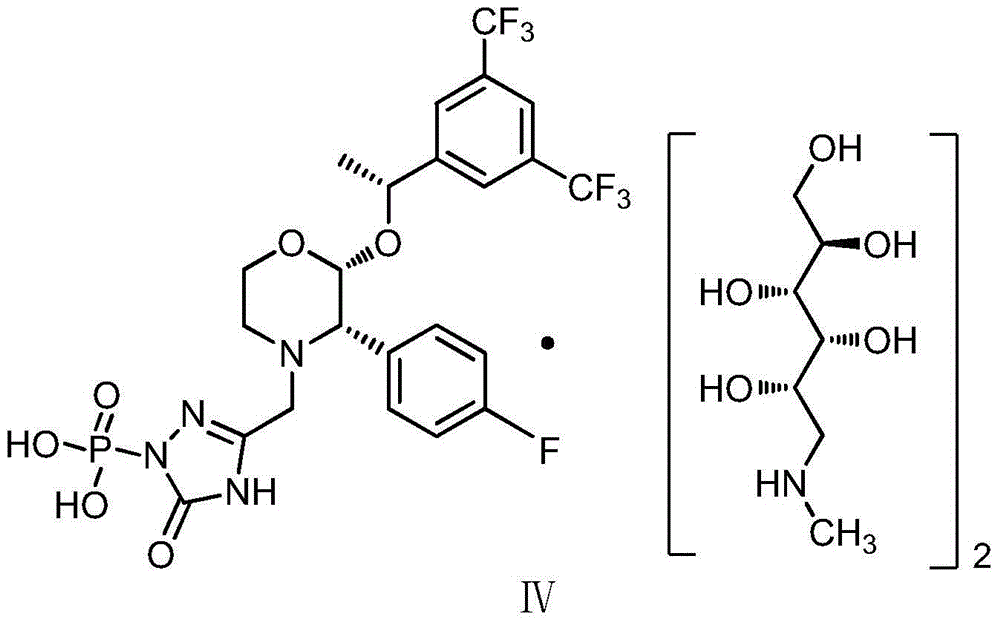
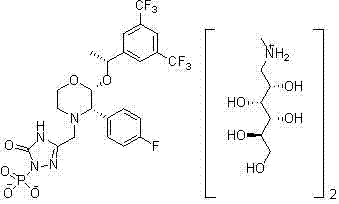
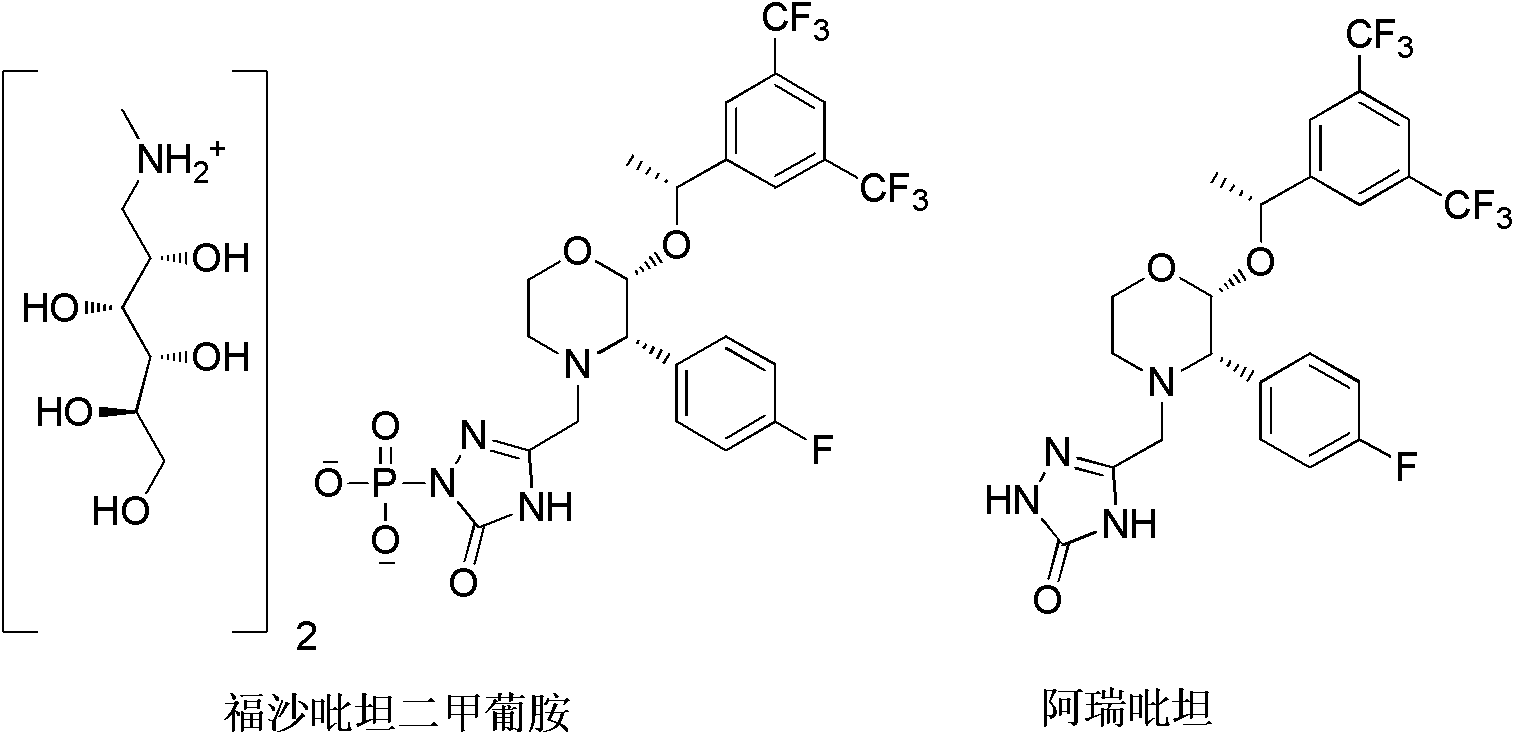
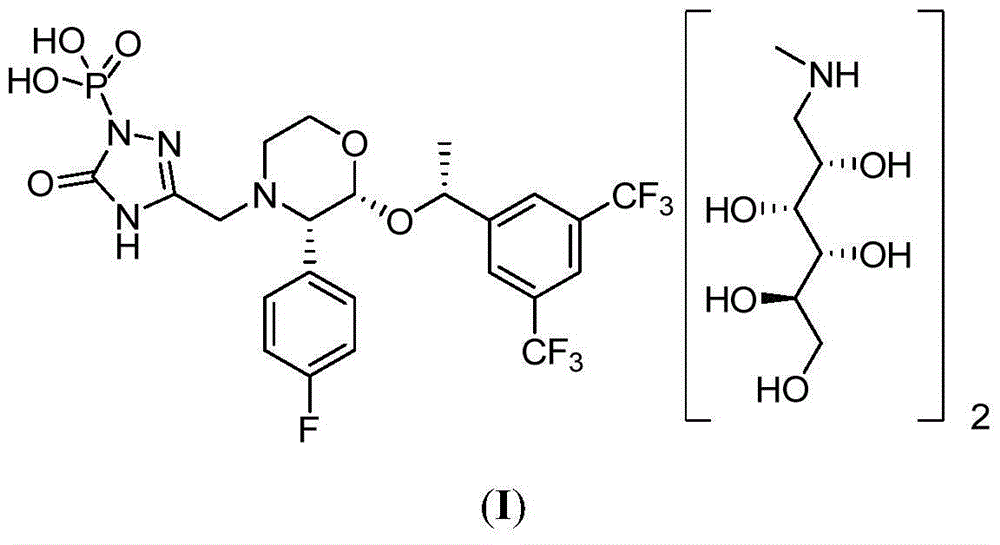
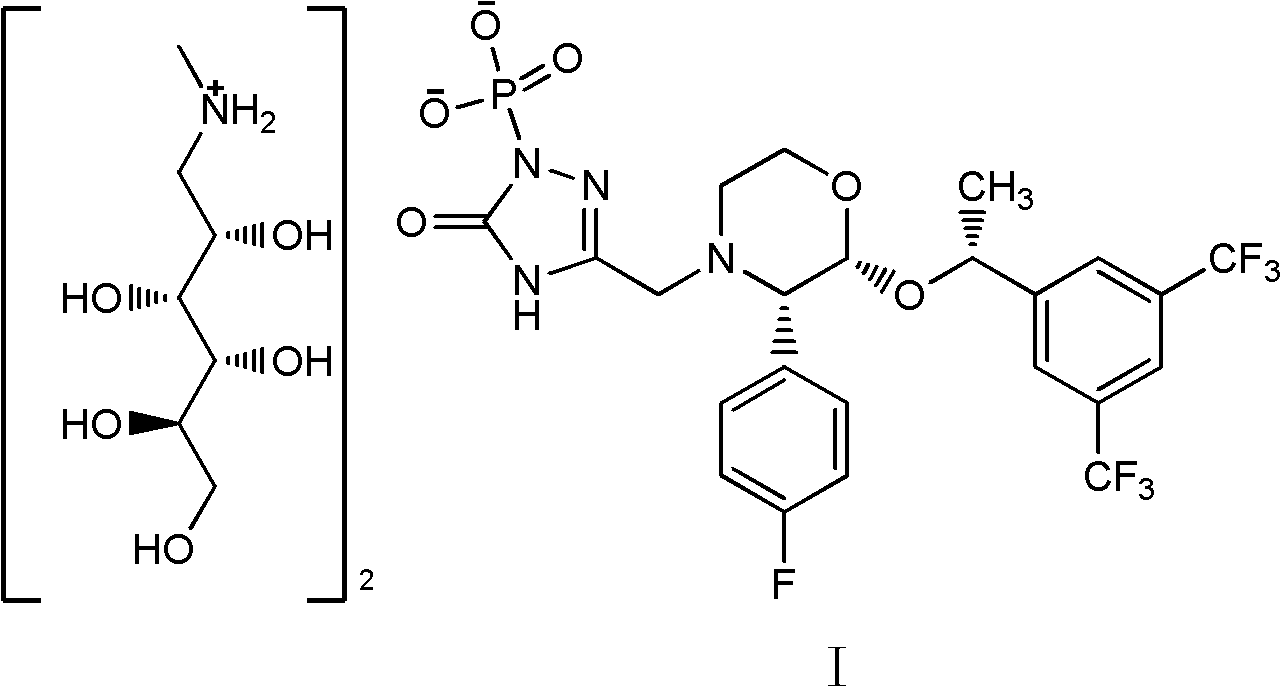
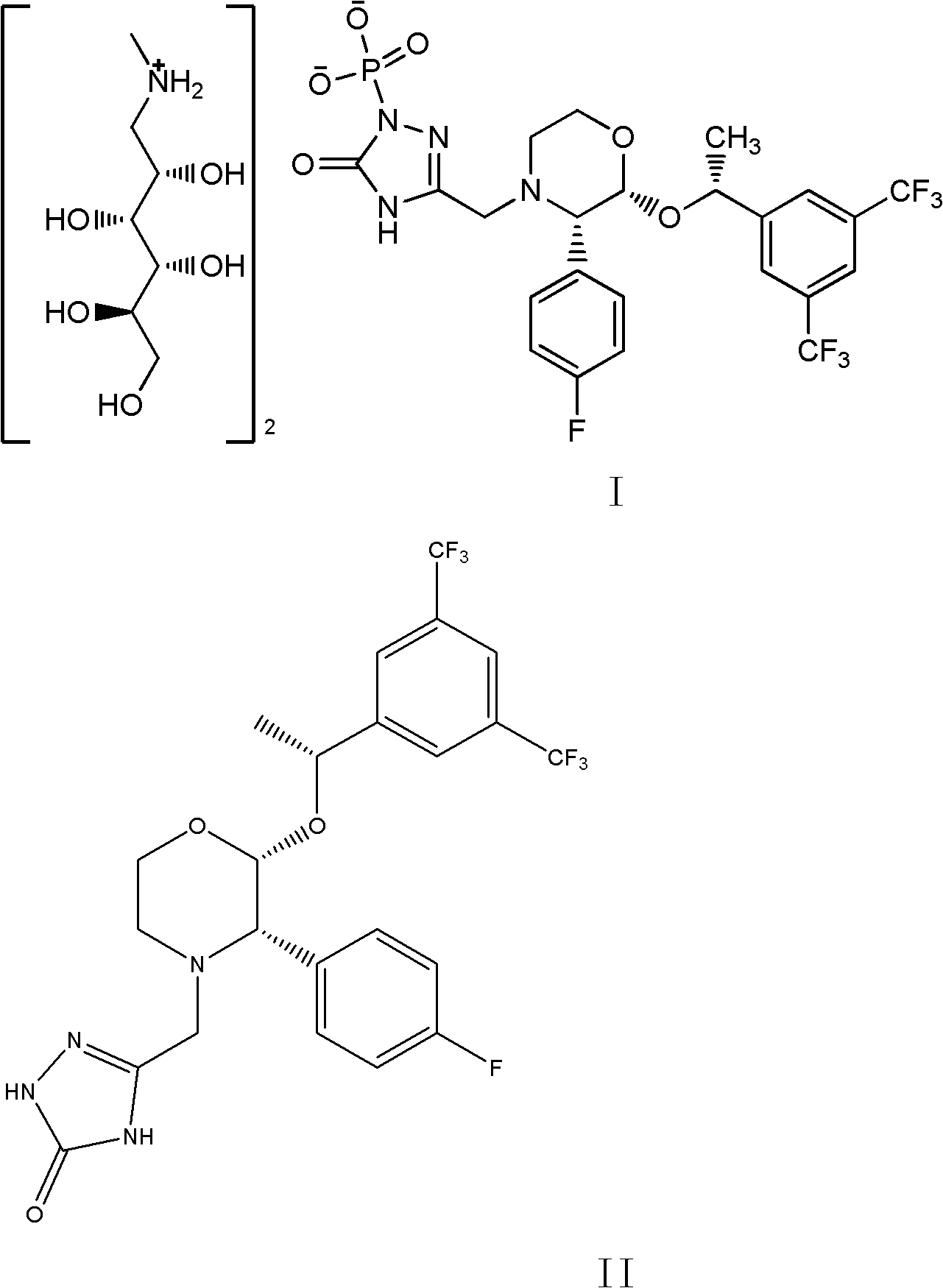
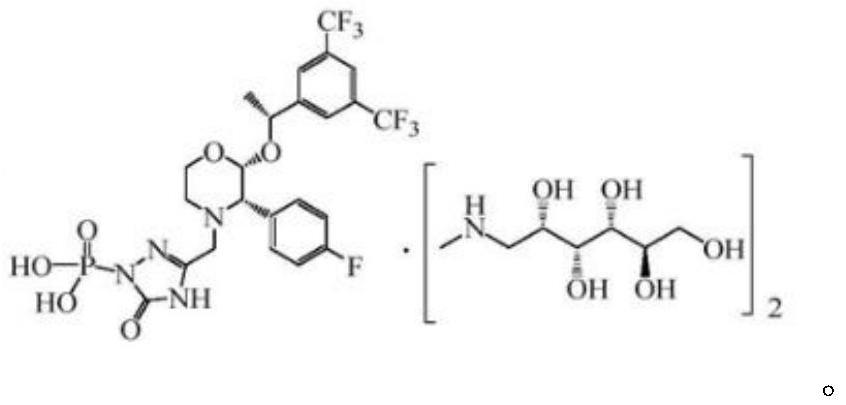
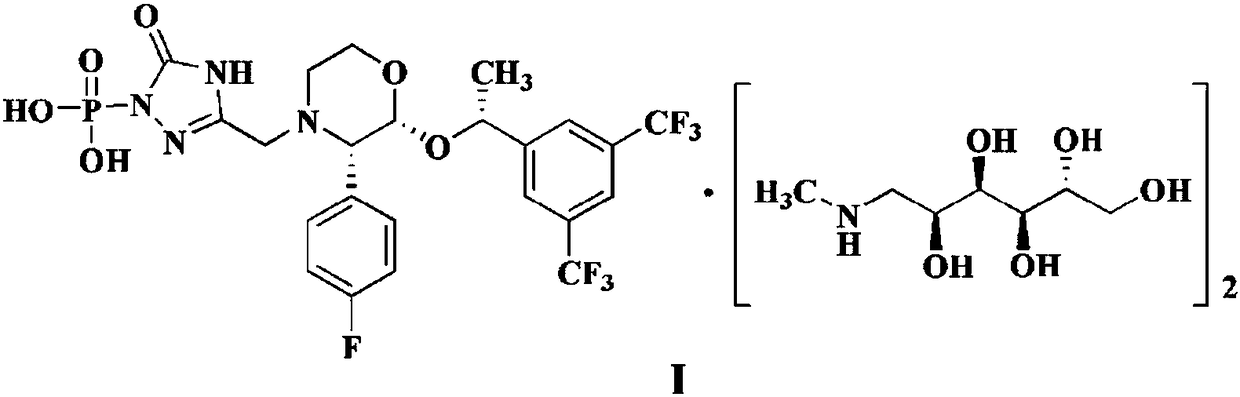
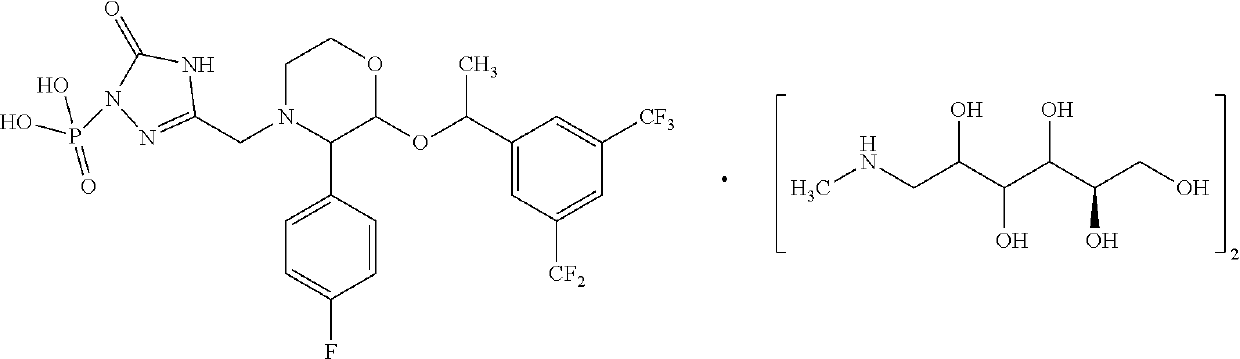
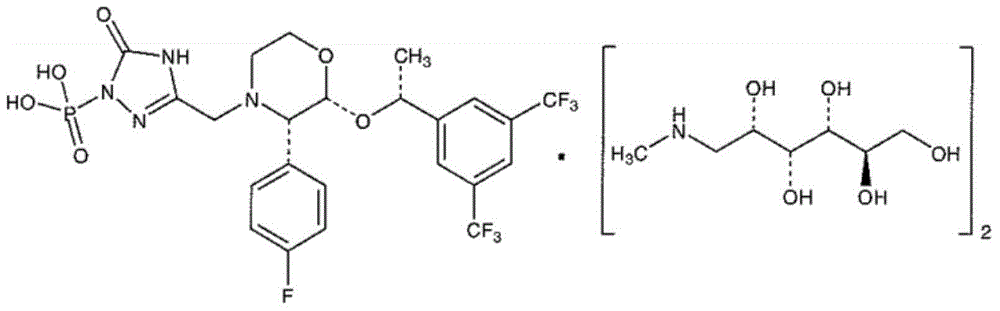
EMEND (fosaprepitant) for injection is a sterile, lyophilized formulation containing fosaprepitant dimeglumine, a prodrug of aprepitant, a substance P/neurokinin-1 (NK1) receptor antagonist, an antiemetic agent, chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl) ...
Sterile lyophilized preparation containing fosaprepitant, and preparation method thereof
ActiveCN102755296AEasy to useSimple processOrganic active ingredientsPowder deliveryTreatment effectArginine
Owner:QILU PHARMA
Preparation method of fosaprepitant dimeglumine
ActiveCN104650142AReduce usageAddressing Safety Concerns in Hydrogenation ReactionsOrganic compound preparationGroup 5/15 element organic compoundsFosaprepitant dimeglumineMethyl group
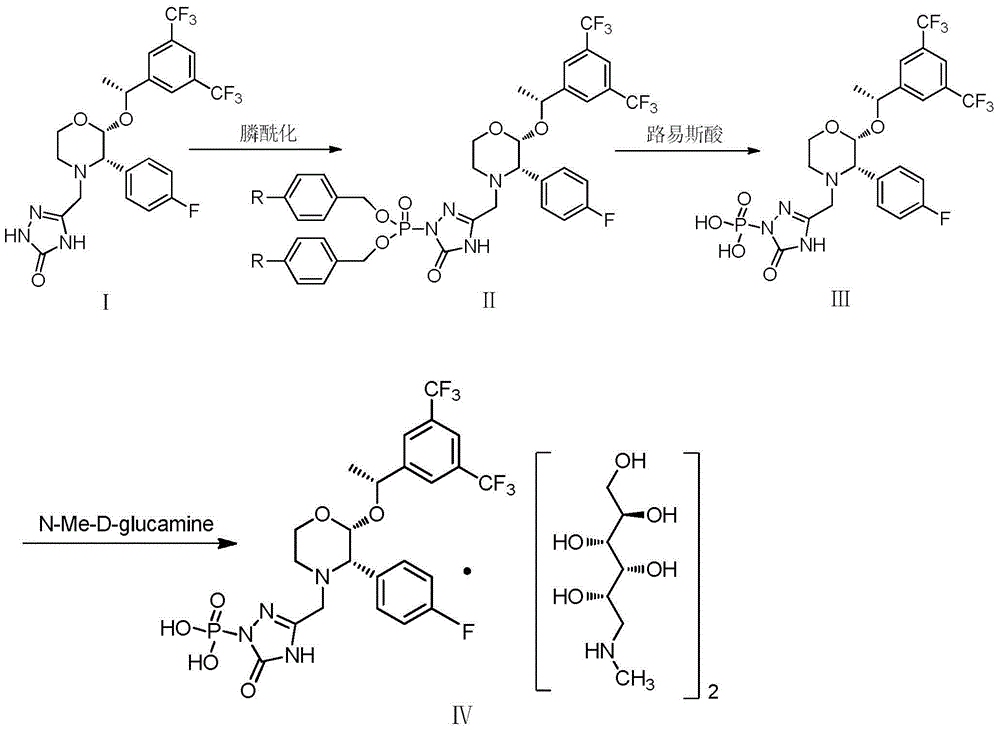
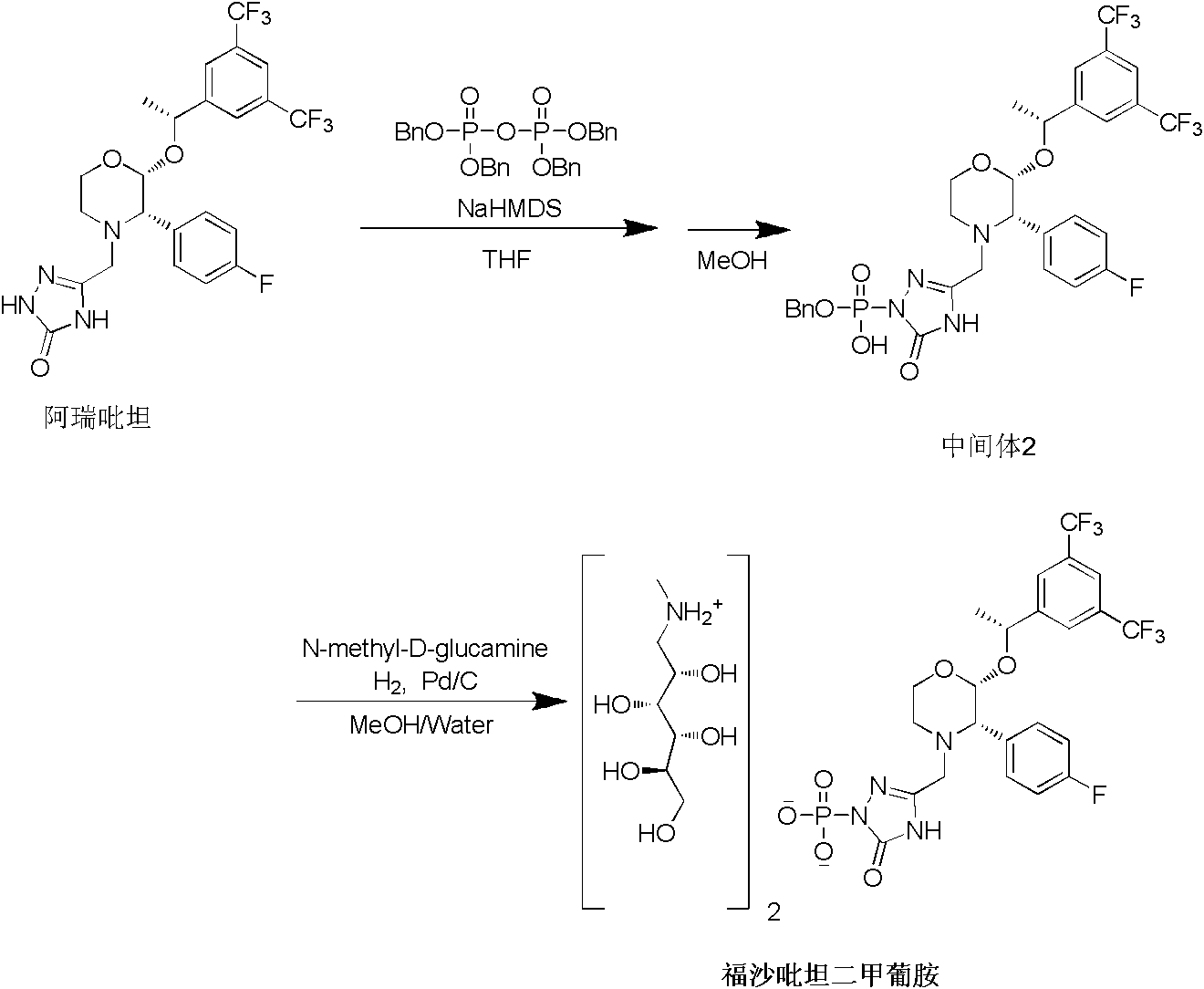
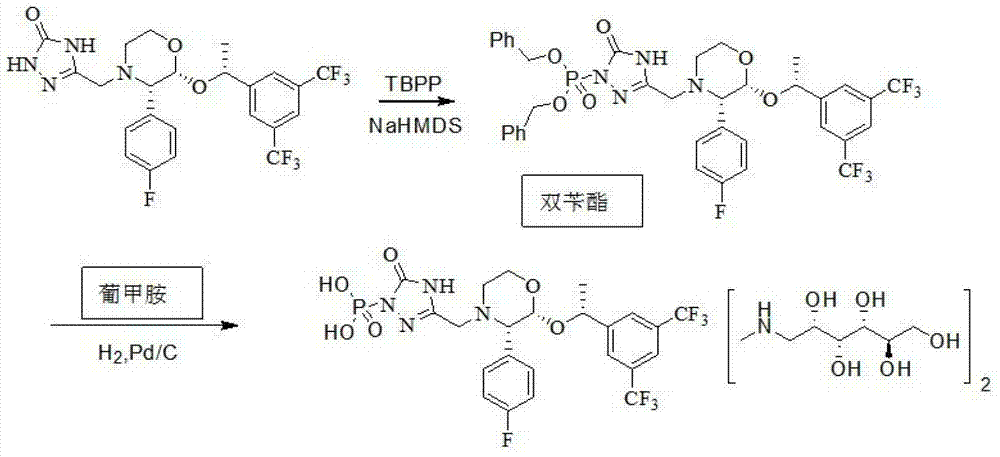
The invention provides a preparation method of fosaprepitant dimeglumine. The preparation method comprises the following steps: by taking aprepitant as a raw material, carrying out phosphorylation on the raw material under an alkaline condition to obtain a dibenzyl ester intermediate; further hydrolyzing the intermediate to obtain fosaprepitant; and further reacting with N-methyl-D-glucosamine to obtain the fosaprepitant dimeglumine. The method provided by the invention is simple to operate and mild in reaction condition, and avoids pressurization and v, so that the method is safe and effective and suitable for industrial production on a large scale.
Owner:LUNAN BETTER PHARMA
Method for reducing palladium residue in compound and preparation method of high-purity fosaprepitant dimeglumine by applying method
ActiveCN102838634AHigh purityCompliance with residue limit requirementsGroup 5/15 element organic compoundsFosaprepitant dimeglumineCombinatorial chemistry
The invention belongs to the field of medicines, and relate to a method for reducing palladium residue in a compound and a preparation method of high-purity fosaprepitant dimeglumine by applying the method. In the method, tributyl phosphane and triphenyl phosphine are used as palladium removing agent to treat compound solution. After palladium is removed by using the method, the high-purity fosaprepitant dimeglumine can be obtained through crystallization with poor solvent in one step. The residue of tributyl phosphane and triphenyl phosphine in a finished product is low, and the palladium residue limit is less than 1 ppm, therefore, the requirement of limit of palladium residue in crude drug for injection is satisfied, and the industrial production after magnification is further adapted.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of fosaprepitant dimeglumine freeze-dried preparation for injection
InactiveCN102166199AProduct looseQuality improvementPowder deliveryOrganic active ingredientsPorosityFreeze-drying
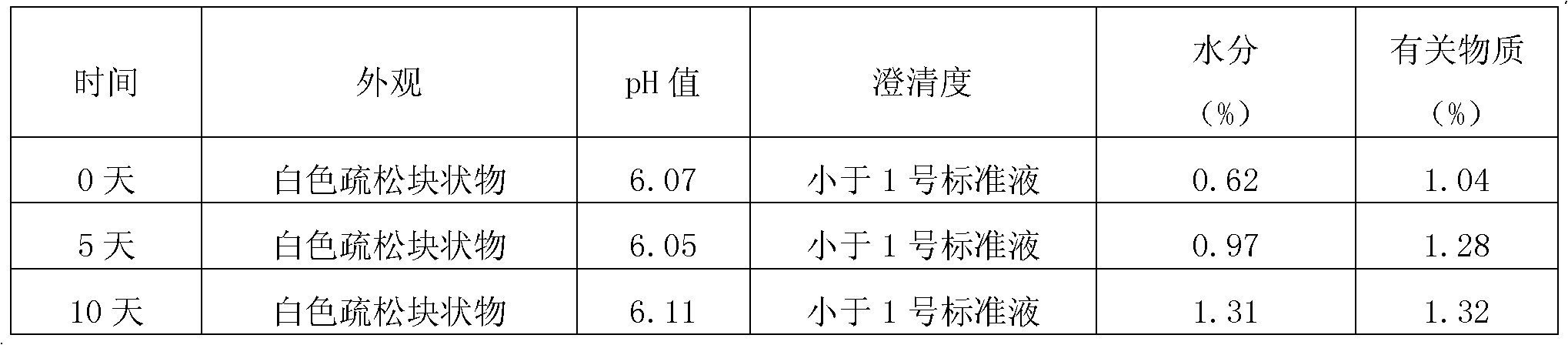
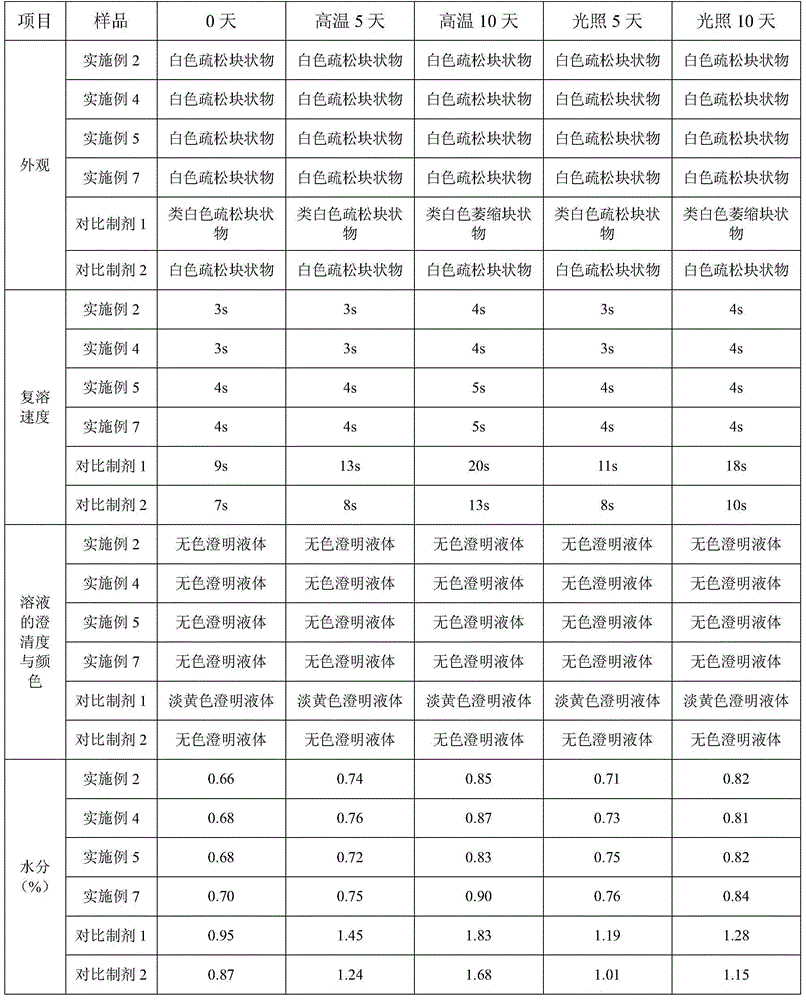
A fosaprepitant dimeglumine freeze-dried preparation prepared by adopting the invention has the characteristics of porosity, stable quality, high redissolution speed, good clarity and the like. The invention provides a preparation method of a fosaprepitant dimeglumine freeze-dried preparation for injection, which comprises the following steps: (A) pre-freezing: firstly, cooling a fosaprepitant dimeglumine injection to (-12)-(-14) DEG C, and freezing through temperature oscillation; and then, cooling to (-32)-(-35) DEG C, and oscillating and freezing for 42-62 minutes; (B) sublimating: vacuumizing in a box body until the air pressure is 12-17 Pa, heating a product to (-35)-(-37) DEG C, and preserving the temperature for 8-10 hours; and intermittently injecting nitrogen gas, oscillating for 1-1.5 hours by taking air pressure (17-22 Pa) in the box body as an amplitude, heating the product to (-25)-(-29) DEG C, and preserving the temperature for 15-18 hours; and (C) drying: gradually heating the medicament to 38-42 DEG C, and preserving the temperature for 1-2 hours.
Owner:WUHAN LEADPHARM TECH CO LTD
Preparation method of fosaprepitant dimeglumine
ActiveCN102558232AHigh purityHigh yieldOrganic compound preparationGroup 5/15 element organic compoundsCombinatorial chemistryFosaprepitant dimeglumine
The invention provides a preparation method of fosaprepitant dimeglumine. The preparation method is characterized in that aprepitant is used as a raw material, under an alkaline condition, dibenzyl ester intermediate compound is obtained by phosphonylation, the intermediate compound is further hydrogenated and catalyzed to obtain fosaprepitant, the fosaprepitant is further reacted with N-methyl-D-glucamine, and finally the fosaprepitant dimeglumine is obtained. The preparation method has the advantages of short reaction cycle, simpleness in operation, low production cost and good product quality; the purity of the finished product is more than 99.5 percent, and the content of single impurity is less than 0.1 percent; and the preparation method is suitable for large-scale industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Fosaprepitant dimeglumine injection composition and preparation method thereof
InactiveCN103156813ALow costNo side effectsPowder deliveryOrganic active ingredientsFormularyDisodium Edetate
The invention relates to the field of medicament preparation, and particularly relates to a fosaprepitant dimeglumine injection composition and a preparation method thereof. The pharmaceutical active ingredients of the fosaprepitant dimeglumine injection composition include the following components in parts by weight: 180-250 parts of fosaprepitant dimeglumine, 12.0-20.0 parts of disodium edetate, 50.0-80.0 parts of polysorbate-80, 250-400 parts of lactose and 2000-3000 parts of water for injection. The preparation method comprises the following steps: dissolving, preparing, adsorbing, pre-freezing, performing sublimation drying and mounting an injection piston. The fosaprepitant dimeglumine injection composition provided by the invention is prepared from the low low-cost auxiliary materials according to a self-developed formula, has the same medicament effect in comparison with the existing fosaprepitant medicament available in America, is low in medicament cost and has no side effect basically; and the lactose used as excipient resists infection and is easy to absorb, so that favorable pharmaceutical effect can be achieved, and crushing of the medicament during storage can be avoided.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Sterile freeze-dried fosaprepitant-dimeglumine powder for injection and preparation process of sterile freeze-dried fosaprepitant-dimeglumine powder
ActiveCN104414983ALow hemolyticImprove solubilityOrganic active ingredientsPowder deliveryFosaprepitant dimeglumineBiomedical engineering
The invention particularly relates to a sterile freeze-dried fosaprepitant-dimeglumine powder for injection and a preparation process of the sterile freeze-dried fosaprepitant-dimeglumine powder. The sterile freeze-dried fosaprepitant-dimeglumine powder for injection comprises an active component, a solubilizing agent and a pH-value adjusting agent, wherein the active component is fosaprepitant dimeglumine. The preparation process of the sterile freeze-dried fosaprepitant-dimeglumine powder for injection is simple and feasible and is suitable for scale production. The sterile freeze-dried fosaprepitant-dimeglumine powder for injection has high quality and excellent stability and can be stored for a long time.
Owner:SHANDONG NEWTIME PHARMA
Fosaprepitant dimeglumine preparation method
ActiveCN104098604AImprove stabilityHigh regional selectivityOrganic compound preparationGroup 5/15 element organic compoundsDiphenylmethanePhosphorylation
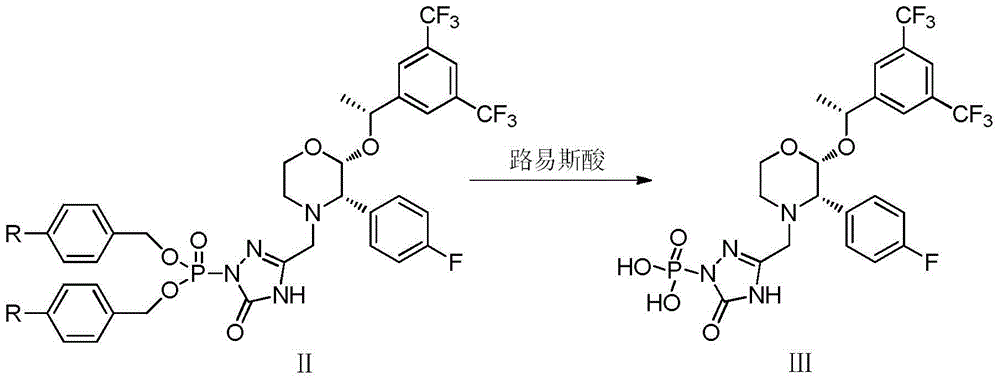
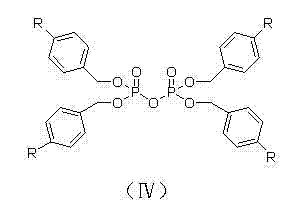
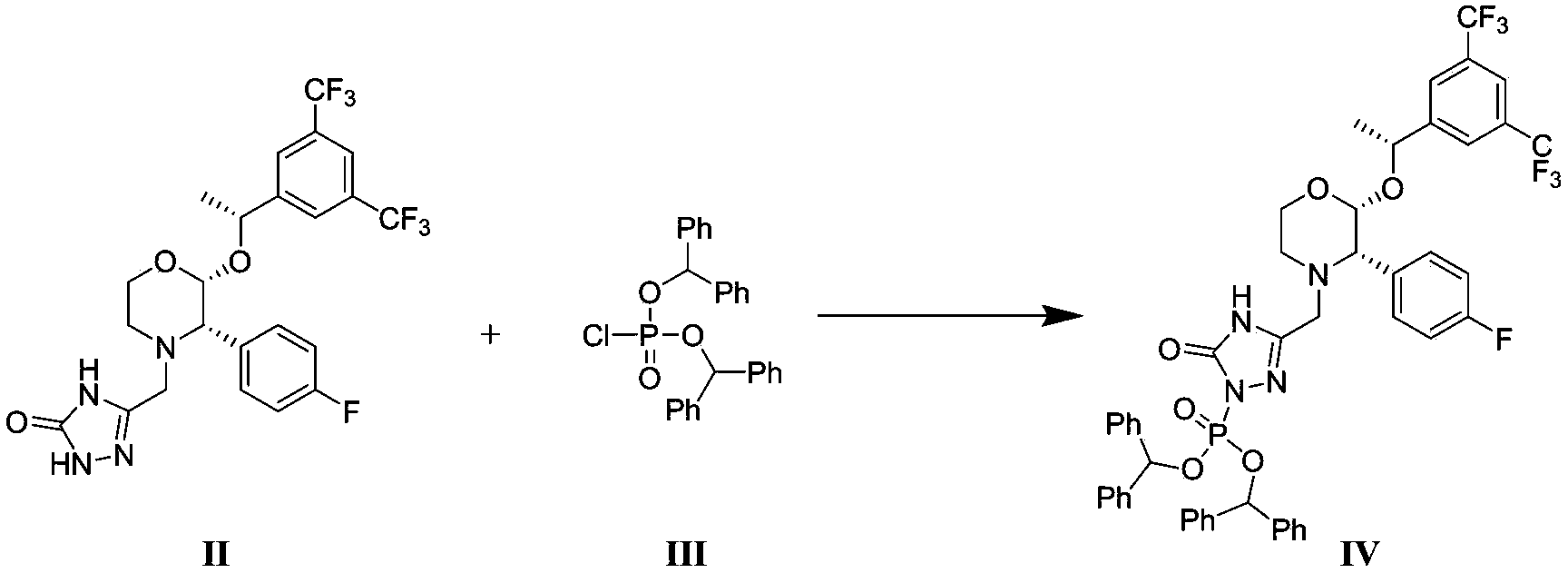
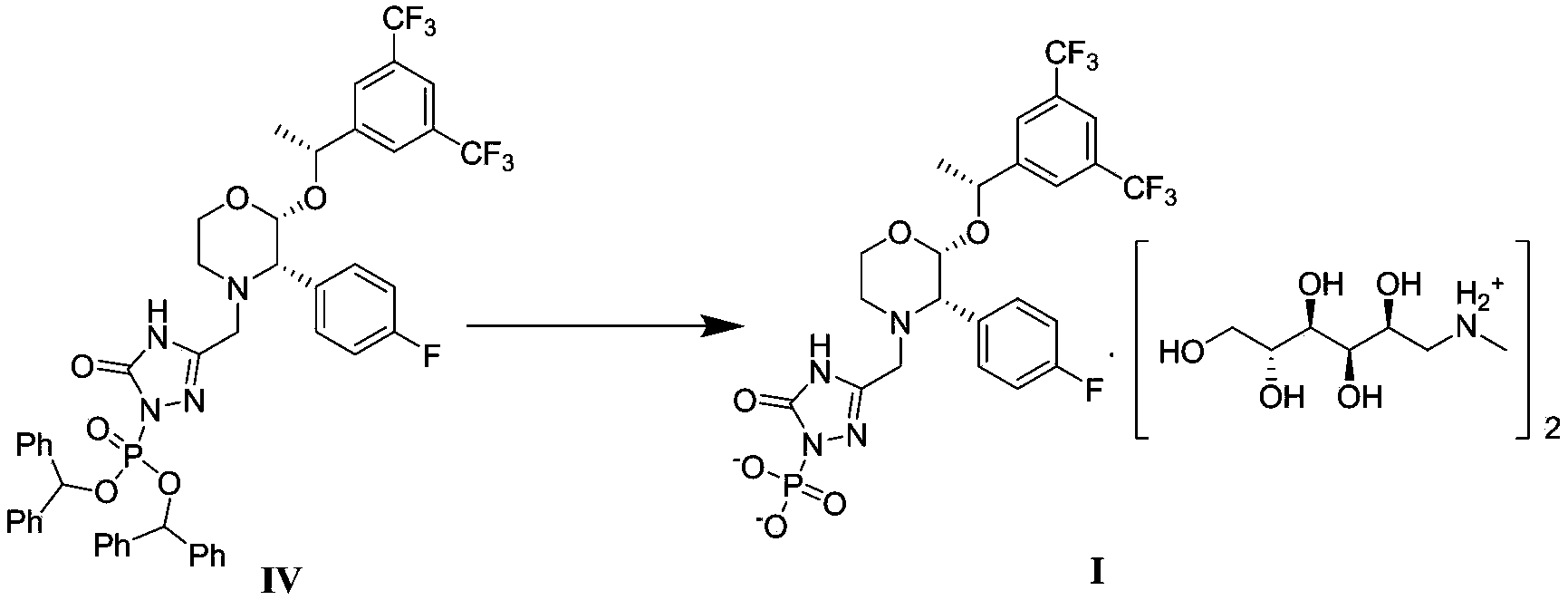
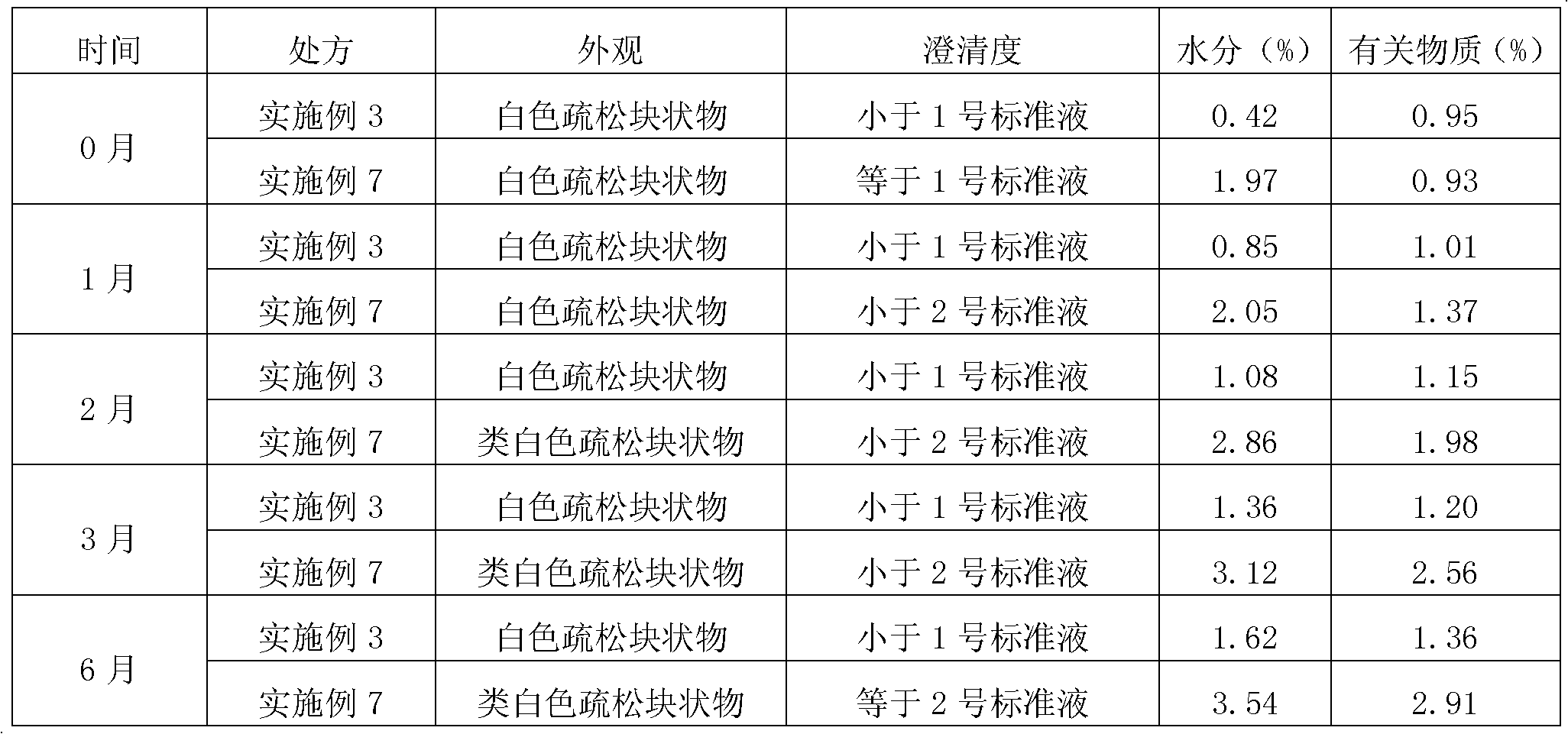
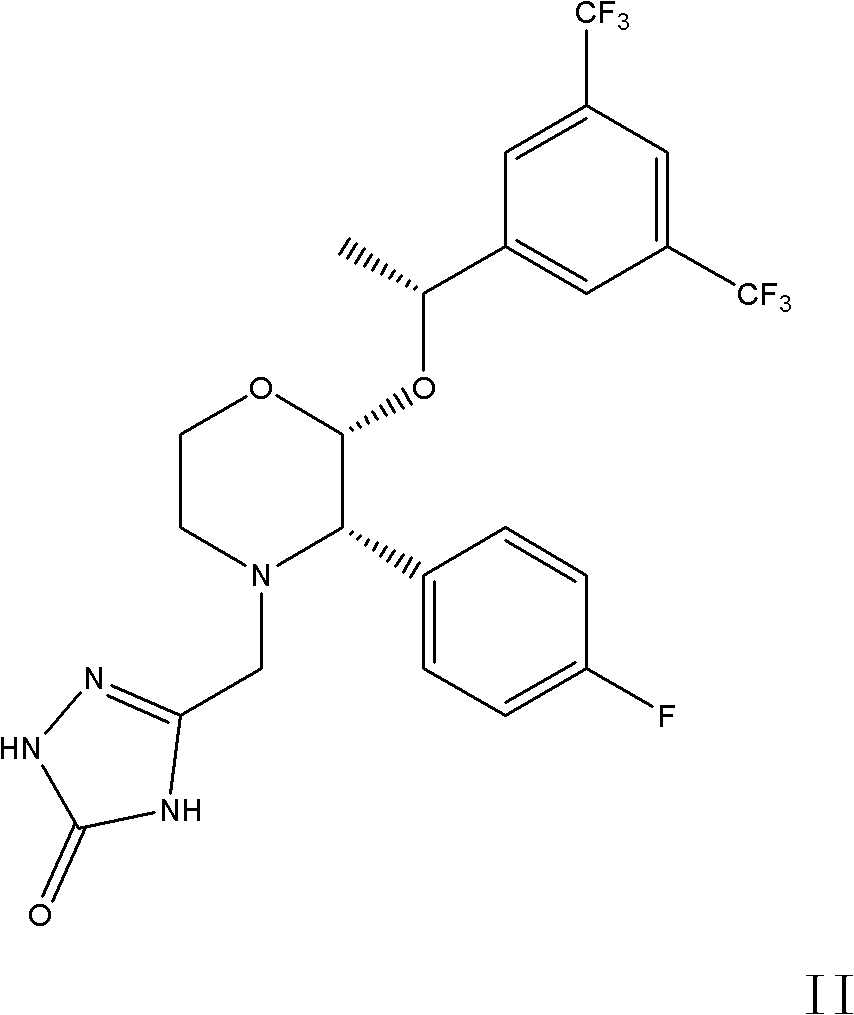
The invention discloses a fosaprepitant dimeglumine preparation method, fosaprepitant dimeglumine has a structure shown as the formula I, and the route of synthesis of the method comprises the following two steps: (1) reacting compound di(benzhydryl) phosphoryl chloride (shown as the formula III) with aprepitant (shown as the formula II) under the action of a steric hindrance strong alkali to produce a new phosphorylation product intermediate (shown as the IV); (2) removing protecting group diphenylmethane of the new compound by catalytic reduction to obtain fosaprepitant and simultaneously to obtain a target product by salifying with N-methyl-D - glucosamine. A phosphorylation reagent used by the method has two higher steric hindrance protecting groups, due to the space steric hindrance effect, the regional selectivity and intermediate stability of the first step reaction can be enhanced, the intermediate stability can be enhanced, and the reaction yield can be improved.
Owner:INST OF BIOPHARM OF SHANDONG PROVINCE
Fosaprepitant dimeglumine freeze-dried powder and preparation method thereof
InactiveCN103565760AShorten freeze-drying timeReduce manufacturing costPowder deliveryOrganic active ingredientsFreeze-dryingFosaprepitant dimeglumine
The invention discloses fosaprepitant dimeglumine freeze-dried powder and a preparation method thereof, and relates to the technical field of drugs and drug production. The freeze-dried powder injection contains fosaprepitant dimeglumine, lactose, polysorbate 80 and ethanol water solution. The freeze-dried powder adopts the ethanol water solution as freeze-dried preparation solvent, so that the freeze-dried time is largely shortened, the energy consumption is decreased, and the production cost is reduced.
Owner:NANJING CORE TECH CO LTD
Preparation method for fosaprepitant dimeglumine
InactiveCN103183708ASimple and fast operationMild conditionsGroup 5/15 element organic compoundsTrimethylsilylPhenyl phosphate
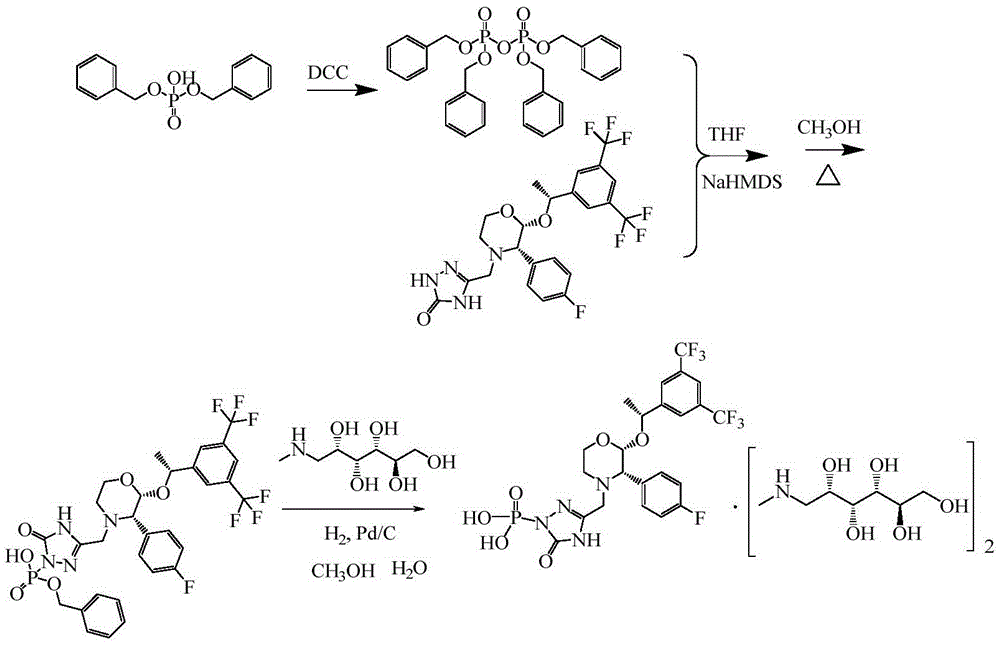
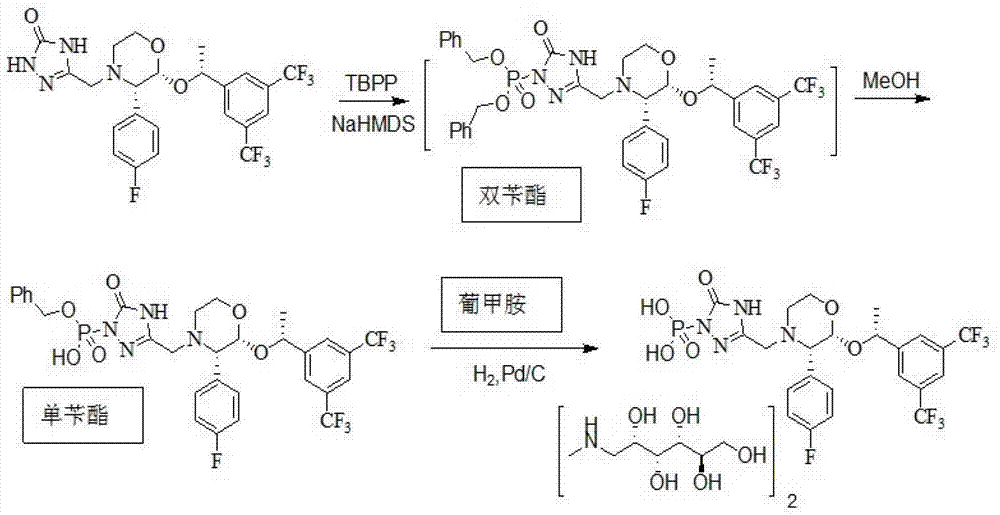
The invention provides a preparation method for fosaprepitant dimeglumine. The method comprises the steps of condensation reacting aprepitant and tetrabenzyl pyrophosphate in tetrahydrofuran in presence of bis(trimethylsilyl)sodamide to obtain bis-O-phenyl phosphate; then temperature reacting with methanol to obtain mono-O-phenyl phosphate; and finally obtaining the fosaprepitant dimeglumine through a hydrogenation reaction. The method is simple in operations, mild in conditions, low in cost and high in yield, is suitable for industrialized production and has relatively large application value.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Refined palladium removing process for fosaprepitant
ActiveCN107176964AImprove efficiencyLow priceGroup 5/15 element organic compoundsFosaprepitant dimeglumineSilica gel
The invention provides a method of reducing the content of palladium in fosaprepitant dimeglumine. The method comprises the following steps: preparing a fosaprepitant dimeglumine solution; adding a sulfhydryl silica gel palladium removing agent, stirring and filtering and then carrying out vacuum distillation concentration; adding a proper amount of solvent into a concentrated solution containing the fosaprepitant for dissolving, and then dropwise adding a mixed solution into an antisolvent; standing, crystallizing and drying to obtain a finished product of fosaprepitant dimeglumine. The palladium removing method provided by the invention is higher in efficiency; the palladium residues in a finished product of the fosaprepitant can be reduced from an original high ppm value to below 5ppm, which conforms to the limit requirements of drugs for injection on the palladium content. Compared with an organic phosphorus reagent, a palladium removing agent provided by the invention has the advantages of safety and low toxicity; in addition, the palladium removing agent can be removed from the solution by simple filtering. The process has a significant cost advantage and is suitable for industrial production.
Owner:HANGZHOU JIUYUAN GENE ENG
Compound preparation containing fosaprepitant and palonosetron hydrochloride, and preparation method thereof
InactiveCN102755338AMeet clinical needsImprove compliancePowder deliveryOrganic active ingredientsPatient complianceFosaprepitant dimeglumine
The invention provides a sterile lyophilized compound preparation containing fosaprepitant and palonosetron hydrochloride, and a preparation method thereof. According to the invention, effective dosages of fosaprepitant dimeglumine and palonosetron hydrochloride are adopted as effective components, and auxiliary materials such as a solubilizer, a complexing agent, an excipient, and an acidity adjusting agent are contained in the preparation. The preparation is filtered, sterilized, sub-packaged, and lyophilized. When in use, the sterile lyophilized compound preparation provided by the invention is added into a solvent, such that injection solution with an appropriate concentration is prepared and can be used. With one time of medication, clinical demands of two treatment medicines can be satisfied, such that patient compliance is improved. The preparation method of the compound preparation is simple, and the method is suitable for industrialized productions.
Owner:QILU PHARMA
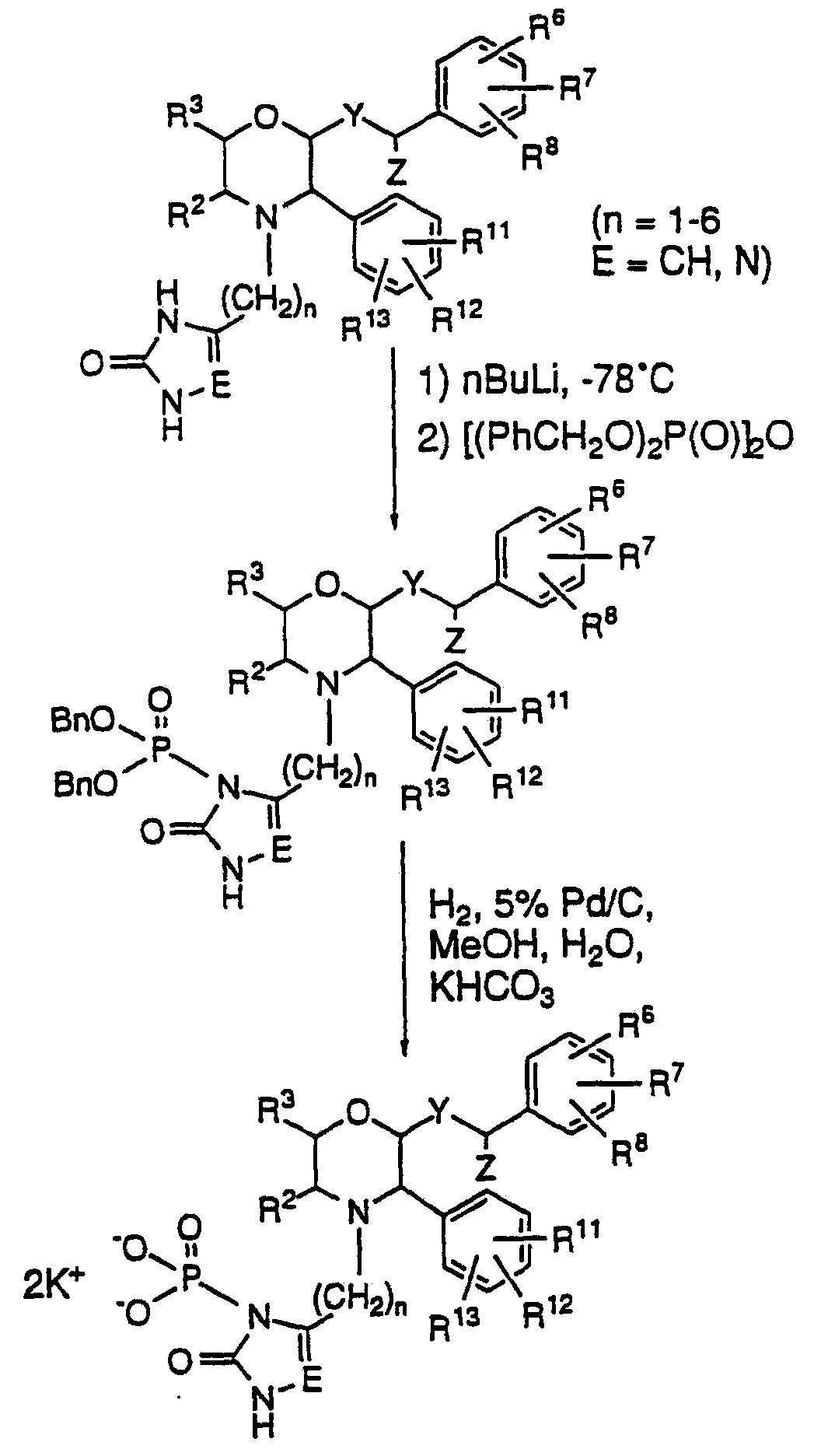
Crystalline Fosaprepitant Dicyclohexylamine Salt And Its Preparation
ActiveUS20160355533A1Improve purification effectAllows preparationOrganic active ingredientsGroup 5/15 element organic compoundsFosaprepitant dimeglumineMedicinal chemistry
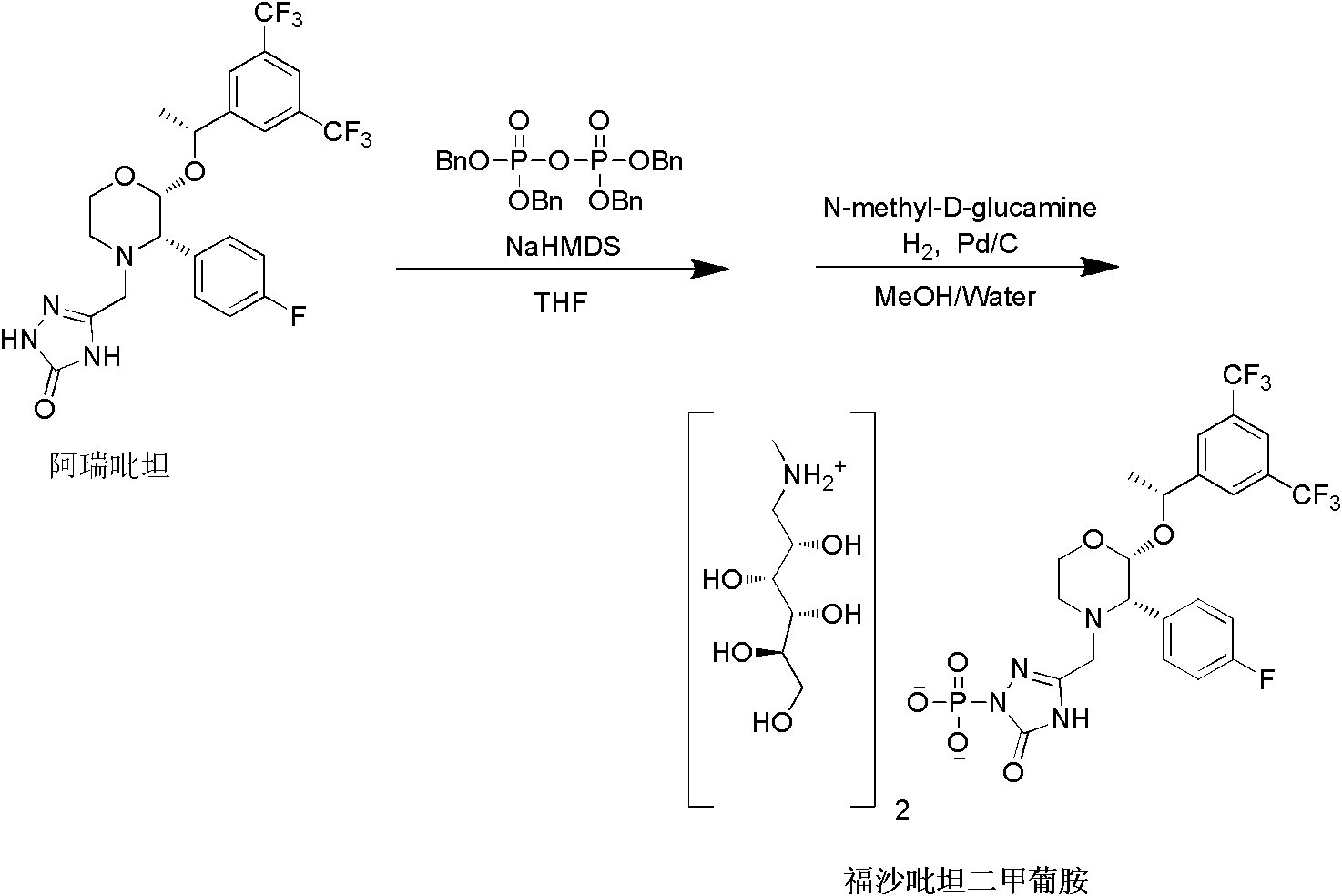
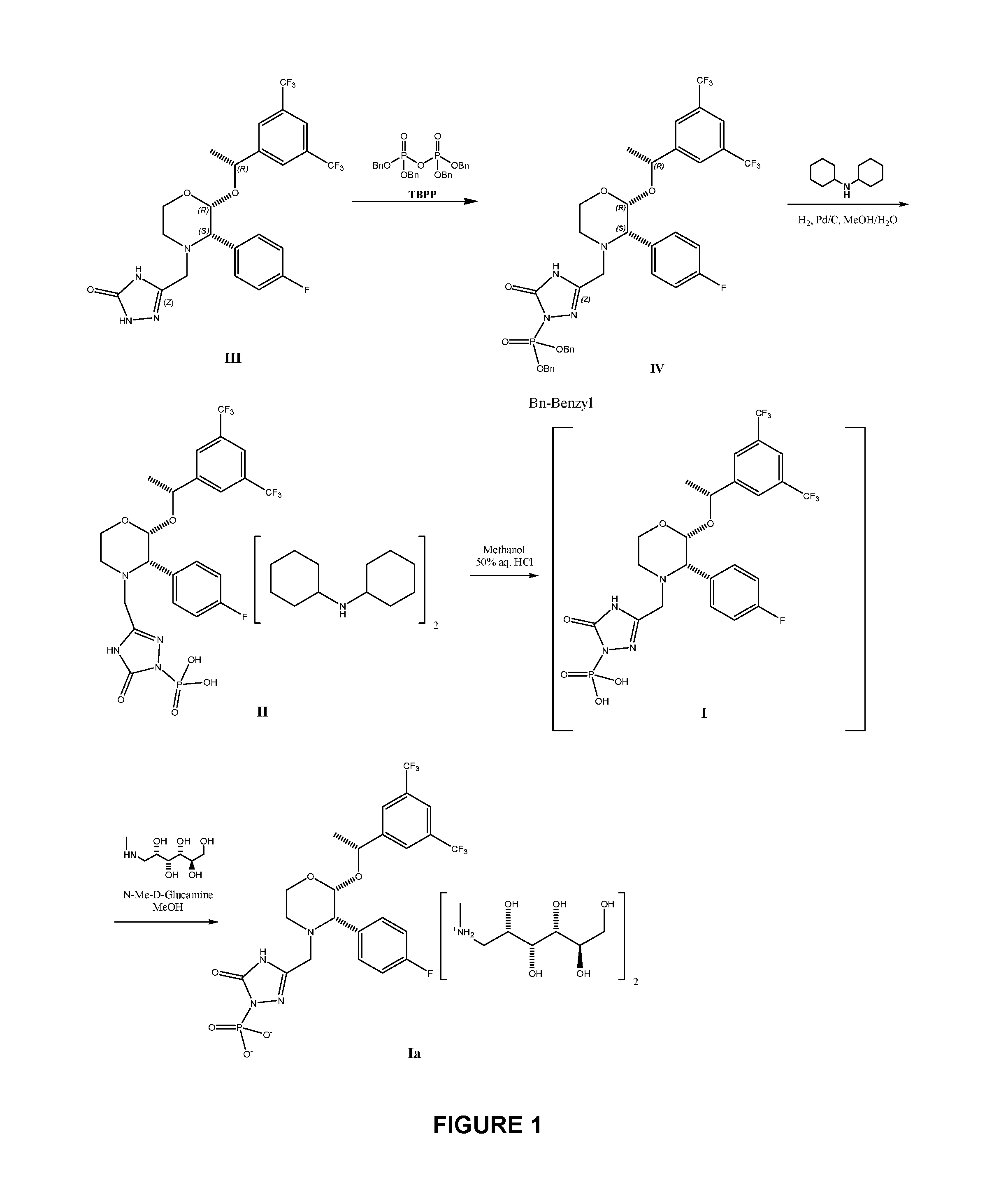
The present invention provides dicyclohexylamine salt of fosaprepitant (fosaprepitant DCHA), a process for preparing fosaprepitant DCHA, and a use of fosaprepitant DCHA in the preparation of pharmaceutically acceptable fosaprepitant dimeglumine with high purity. Fosaprepitant dimeglumine is prepared by treating fosaprepitant DCHA with an acid to form fosaprepitant, followed by adding N-methyl-D-glucamine to fosaprepitant.
Owner:NAVINTA
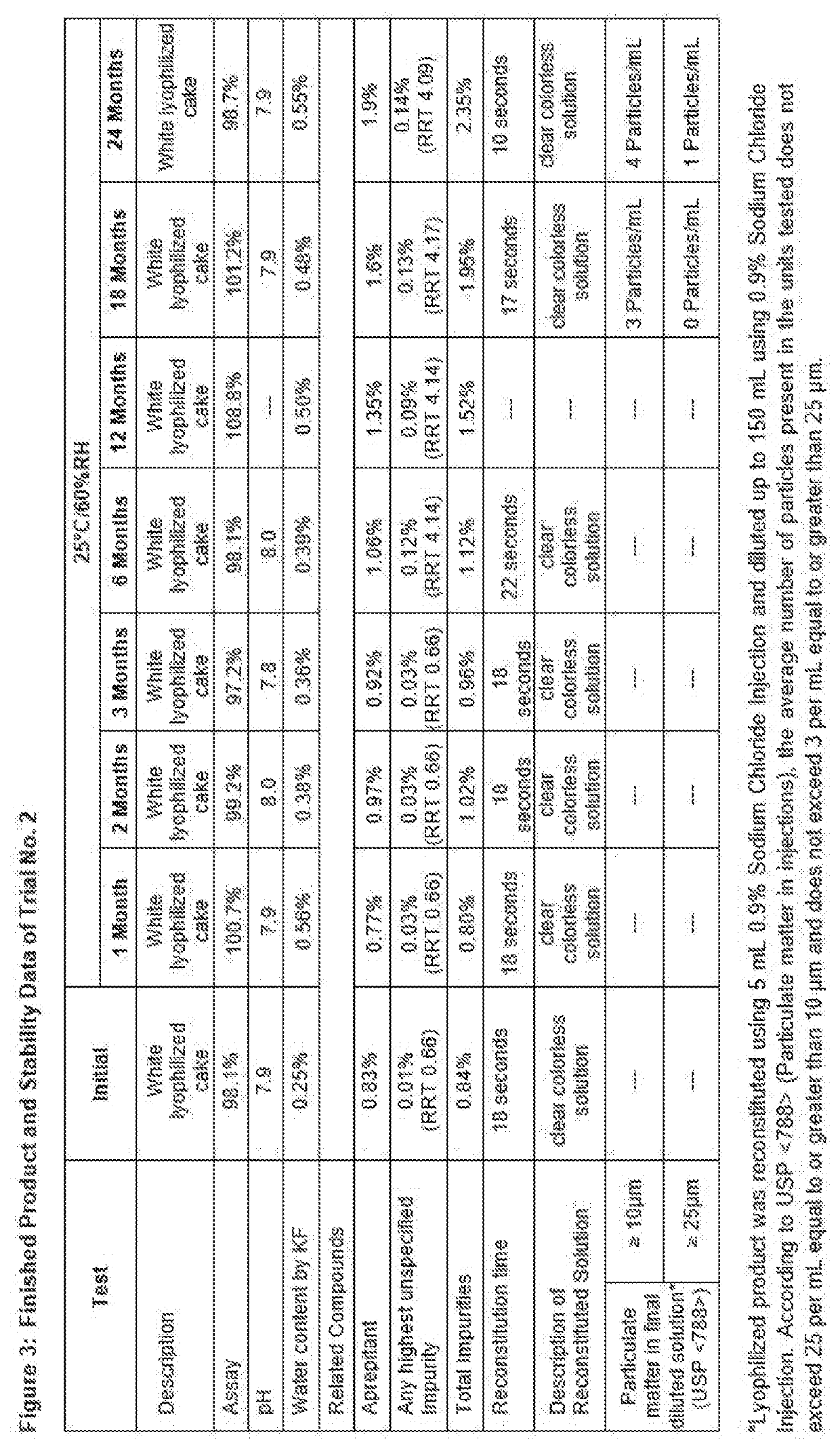
Process for preparing injectable fosaprepitant dimeglumine compositions having improved storage stability
ActiveUS20200237788A1Amount be controlOrganic active ingredientsPharmaceutical non-active ingredientsEngineeringFosaprepitant dimeglumine
Solid compositions having about 150 mg to about 245 mg Fosaprepitant that are stable after storage at about 25° C. for 6 months and processes for preparing solid Fosaprepitant compositions that are stable after long term storage at room temperature. The processes include freezing a Fosaprepitant solution in the primary packaging container at a first freezing temperature; applying vacuum at second temperature that is higher than the freezing temperature; fully stoppering the primary packaging container; and sealing the stoppered primary packaging container.
Owner:NAVINTA III INC
Fosaprepitant dimeglumine composition for injection
InactiveCN103301139AImprove immunityAvoid instabilityPowder deliveryOrganic active ingredientsSide effectTreatment effect
The invention provides a fosaprepitant dimeglumine composition for injection, and relates to the technical field of medicine manufacturing. The main medicine of the composition comprises fosaprepitant dimeglumine and melatonin, wherein the melatonin comprises a quick release part and a cyclodextrin-included slow release part. According to the fosaprepitant dimeglumine composition for injection provided by the invention, the therapeutic effect of fosaprepitant dimeglumine is improved, instability caused by oral administration of MT (Melatonin) is avoided and MT is quick to distribute and eliminate and the like, and the first pass effect of MT is reduced. The dosage of fosaprepitant dimeglumine is reduced. The design of dosage combining quick release and slow release is in accordance with secretion characteristic of MT, so that the problem of half-life period of MT is solved and the bioavailability of the product is improved. The composition has the synergistic effect to treat CINV (Chemotherapy Induced Nausea And Vomiting) by fosaprepitant dimeglumine, and fosaprepitant dimeglumine and melatonin are combined to not only treat CINV so as to improve the therapeutic effect of fosaprepitant dimeglumine to CINV and shorten the course of treatment, but also reduce the use level of fosaprepitant dimeglumine, reduce the side effect of fosaprepitant dimeglumine and improve the immunity of a human body. The stress response of organism can be effectively reduced by maintaining melatonin in a certain concentration in blood of a human body to the benefit of treatment of CINV.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Refining method of fosaprepitant dimeglumine
InactiveCN104650141AReduce degradation productsResidue reductionOrganic compound preparationGroup 5/15 element organic compoundsEtherFosaprepitant dimeglumine
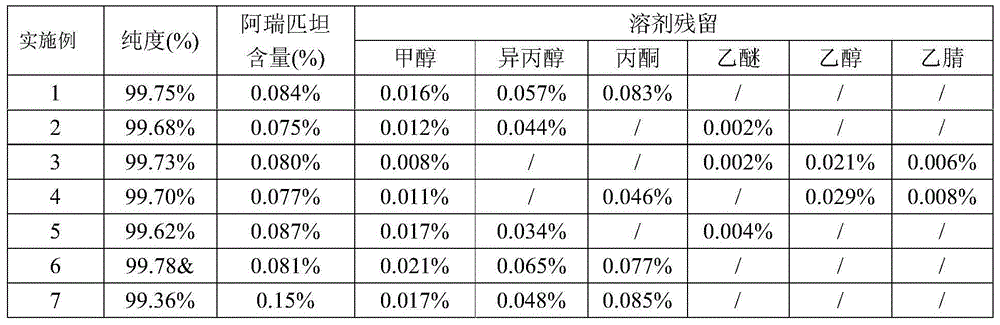
The invention belongs to the field of chemicals and specifically relates to a refining method of a fosaprepitant dimeglumine crude drug. During refining process, acetone or ether is used to wash a product, and a high-toxicity acetonitrile solvent used in the prior art is replaced. Purity of the obtained fosaprepitant dimeglumine crude drug is high; solvent residue content is low; drying temperature is reduced; and drying time is shortened. The refining method provided by the invention is suitable for industrial production.
Owner:SHANGHAI HUILUN BIOLOGICAL TECH CO LTD
Preparation method of fosaprepitant dimeglumine
ActiveCN102977142AQuality improvementEase of mass productionOrganic compound preparationGroup 5/15 element organic compoundsFosaprepitant dimegluminePyrophosphate
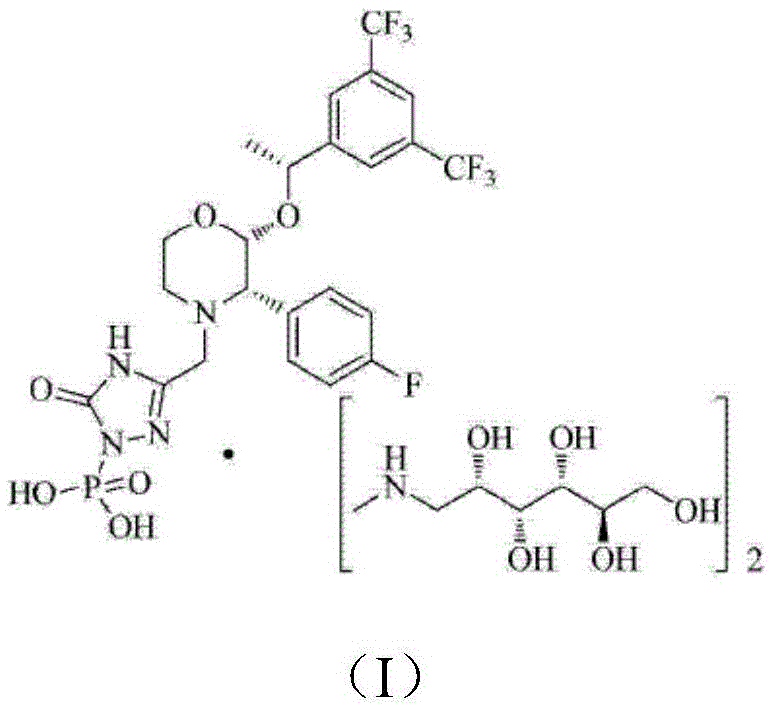
The invention relates to a preparation method of fosaprepitant dimeglumine. Fosaprepitant dimeglumine is a compound shown in the formula I. The preparation method is characterized in that a compound shown in the formula II reacts with tetrabenzyl pyrophosphate in the presence of a steric hindrance alkali; and reaction products undergo a hydrogenation-reduction reaction in the presence of N-methyl-D-glucosamine to produce the compound shown in the formula I. The preparation method has simple processes, a high reaction yield, few by-products, and controllable reaction conditions, and is suitable for industrial large-scale production of medicines.
Owner:JIANGSU HANSOH PHARMA CO LTD
Application of borane-pyridine complex in preparation of NK-1 receptor antagonist
InactiveCN112300212AReduce usageImprove securityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhosphate
The invention provides an application of a borane-pyridine complex in preparation of an NK-1 receptor antagonist fosaprepitant dimeglumine. The application is characterized by comprising the followingsteps: step 1) catalyzing aprepitant dibenzyl phosphate under the action of the borane pyridine complex to prepare fosaprepitant; and 2) reacting fosaprepitant with N-methyl-D-glucosamine to generatefosaprepitant dimeglumine. The borane-pyridine complex is used as the catalyst, aprepitant dibenzyl phosphate can directly generate fosaprepitant, then the fosaprepitant dibenzyl phosphate reacts with N-methyl-D-glucosamine to generate fosaprepitant dimeglumine, the reaction is mild, and conversion of raw materials can be completed quickly at the temperature of about room temperature only by using a small amount of catalyst. The catalytic efficiency is high, the reaction conditions are mild and the yield is high. The high-purity and high-yield fosaprepitant can be obtained by recrystallizingthe reaction crude product with deionized water, the post-treatment is extremely simple, and the fosaprepitant dimeglumine is generated by reacting the reaction crude product with N-methyl-D-glucosamine so that the purity and the yield are high.
Owner:商河探荣新技术开发中心
Synthesis method of fosaprepitant dimeglumine
InactiveCN103204878ARaw materials are easy to getThe reaction conditions are mild and controllableOrganic compound preparationGroup 5/15 element organic compoundsSynthesis methodsPyrophosphate
The invention relates to the field of medicaments, and in particular provides a synthesis method of fosaprepitant dimeglumine. According to the method of the invention, aprepitant is employed as initial material to be reacted with tetrabenzyl pyrophosphate in anhydrous tetrahydrofuran with sodium hexamethyldisilazide as a base to obtain dibenzyl ester intermediate, a benzyl group of the dibenzyl ester is removed in anhydrous methanol to generate single-benzyl ester intermediate, which is hydrogenated to remove a remaining benzyl group, and salified with meglumine to obtain fosaprepitant dimeglumine. The method comprises three major steps: (1) preparing intermediate S100902; (2) preparing crude fosaprepitant dimeglumine; and (3) refining crude fosaprepitant dimeglumine. The synthesis method is featured as abundant available raw materials, moderate and controllable reaction conditions, simple process, high yield and is suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Method for controlling palladium residue in fosaprepitant dimeglumine
InactiveCN104230991AImprove adsorption capacityHigh purityGroup 5/15 element organic compoundsOrganic solventAlcohol
The invention provides a method for controlling a palladium residue in fosaprepitant dimeglumine. The fosaprepitant dimeglumine has a structure as shown in the formula (I), and the content of palladium is 0-150ppm. The method is characterized by comprising the following steps: 1) dissolving fosaprepitant dimeglumine crude product into an alcohol solution, stirring and dissolving; and 2) adding a palladium removing agent, stirring, filtering and dropwise adding filtrate into a mixed solvent of absolute ethyl alcohol and anhydrous acetonitrile, filtering, and drying in vacuum, so as to obtain a fosaprepitant dimeglumine sample. Compared with the prior art, the method provided by the invention can be used for producing high-purity product, wherein the palladium residue limit of the product is smaller than 1ppm and accords with the requirement of medicines for injection on the palladium residue limit. The method disclosed by the invention specifically comprises the steps of directly dissolving the crude product; adding the palladium removing agent; stirring and filtering; and dropwise adding the filtrate into an organic solvent, filtering and drying, thus obtaining a pure product. The method is simple and convenient to operate, is good in palladium removal effect, uses cheap and easily available raw material, and is suitable for being put into industrial production.
Owner:TAIZHOU EOC PHARMA CO LTD
Refining method of fosaprepitant dimeglumine
ActiveCN102850399AReduce degradationReduce usageOrganic compound preparationGroup 5/15 element organic compoundsProcess engineeringFosaprepitant dimeglumine
The invention belongs to the field of medicine and relates to a refining method of fosaprepitant dimeglumine. In a refining process, single acetonitrile is used as an anti-solvent for carrying out crystallization treatment on products, and a mixed solvent used in the prior art is replaced. The purity of the obtained fosaprepitant dimeglumine raw material medicine is high, the solvent residue amount is fewer, particularly, the temperature and vacuum degree requirements in a drying process are low, and the refining method is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Fosaprepitant dimeglumine freeze-dried powder injection and preparation method thereof
ActiveCN106943358ALittle impact on securitySpeed up filteringOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The present invention relates to a fosaprepitant dimeglumine freeze-dried powder injection and a preparation method thereof, wherein the fosaprepitant dimeglumine freeze-dried powder injection comprises an active component fosaprepitant dimeglumine and a stabilizer so as to avoid the use of Tween 80 and lactose and improve the product safety. According to the present invention, during the preparation process, the solubility of fosaprepitant dimeglumine is increased by using the tert butyl alcohol-water cosolvent, such that the concentration required by the preparation is achieved so as to meet the production requirement; and the generation of foams is avoided during the preparation process, such that the sterilization and filtration rate and the filling accuracy are improved.
Owner:SHANDONG NEWTIME PHARMA
Preparation method of fosaprepitant dimeglumine pharmaceutical salt
ActiveCN108948080AOvercome the shortcomings of poor security and severely limited production scaleReduce usageOrganic compound preparationGroup 5/15 element organic compoundsHydrogenSilanes
The invention belongs to the technical field of medicine chemistry, and particularly relates to a novel preparation method of fosaprepitant dimeglumine. According to the preparation method, triethyl silane is used for replacing hydrogen, and high-pressure equipment can be prevented from being used, so that the safety is improved, and the obtained product is good in appearance, high in purity and suitable for industrial mass production.
Owner:QILU PHARMA
A kind of injection composition containing fosaprepitant dimeglumine and preparation method thereof
ActiveCN104042572BThe process is simple and easy to controlReduce energy consumptionPowder deliveryOrganic active ingredientsAlcoholFreeze-drying
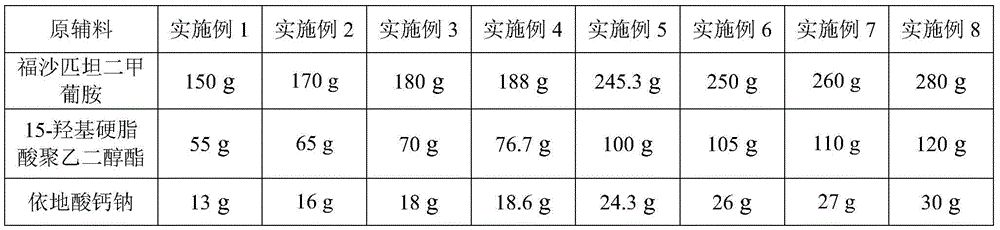
The invention provides a pharmaceutical composition containing fosaprepitant dimeglumine and a preparation method thereof. The pharmaceutical composition is composed of fosaprepitant dimeglumine, lactose, disodium edentate and tween-80, wherein a mass ratio among the fosaprepitant dimeglumine, the disodium edentate, the tween-80 and the lactose is 188-245.3:14.4-18.8:57.5-75:287.5-375. The composition is prepared in an aqueous containing alcohol in a freeze-drying manner. The preparation method is simple and controllable in processes and can reduce energy consumption. The pharmaceutical composition, prepared by the preparation method in the invention, is stable in quality and can ensure safety of clinical medication.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Storage-stable ready-to-use injectable formulations of fosaprepitant dimeglumine
PendingUS20200316097A1Easy to manageDesirable safety profileOrganic active ingredientsPharmaceutical delivery mechanismFosaprepitant dimeglumineMedicinal chemistry
The present application provides a stable, ready-to-use fosaprepitant dimeglumine formulation which is easy to administer without need of any reconstitution step and has a desirable solubility, stability and safety profile. The concentration of the fosaprepitant dimeglumine in the liquid formulation is preferably less than about 80 mg / ml, or more preferably between about 20 mg / ml to about 60 mg / ml. In certain embodiments, the liquid formulation retains at least about 90% chemical stability of the fosaprepitant dimeglumine after storage for a commercially reasonable amount of time at a temperature between about 0° C. to about 40° C.
Owner:RK PHARMA SOLUTIONS LLC
Fosaprepitant dimeglumine composition for injection and preparation method thereof
ActiveCN104414980AEliminate security risksImprove securityOrganic active ingredientsPowder deliveryPolyethylene glycolHydroxystearic Acid
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
A kind of freeze-dried preparation containing fosaprepitant and preparation method thereof
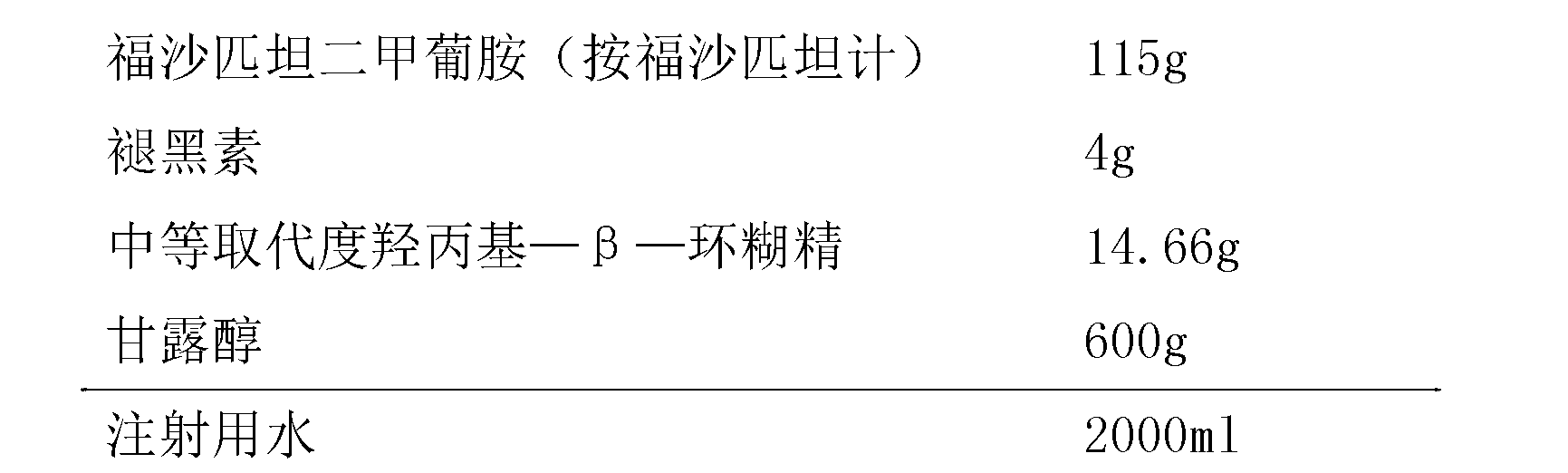
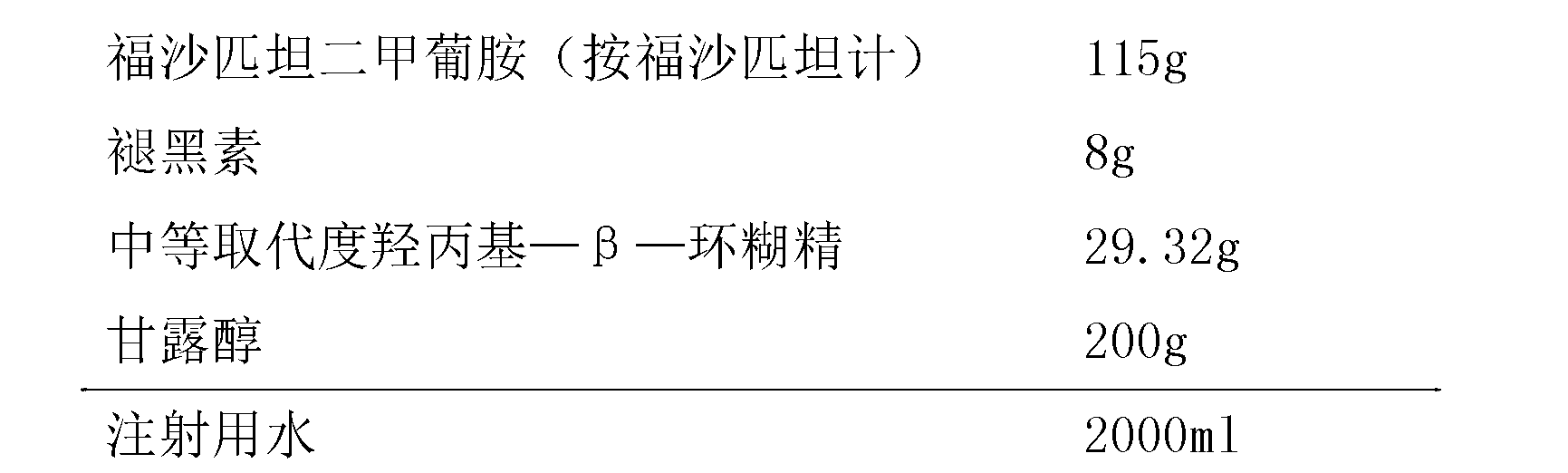
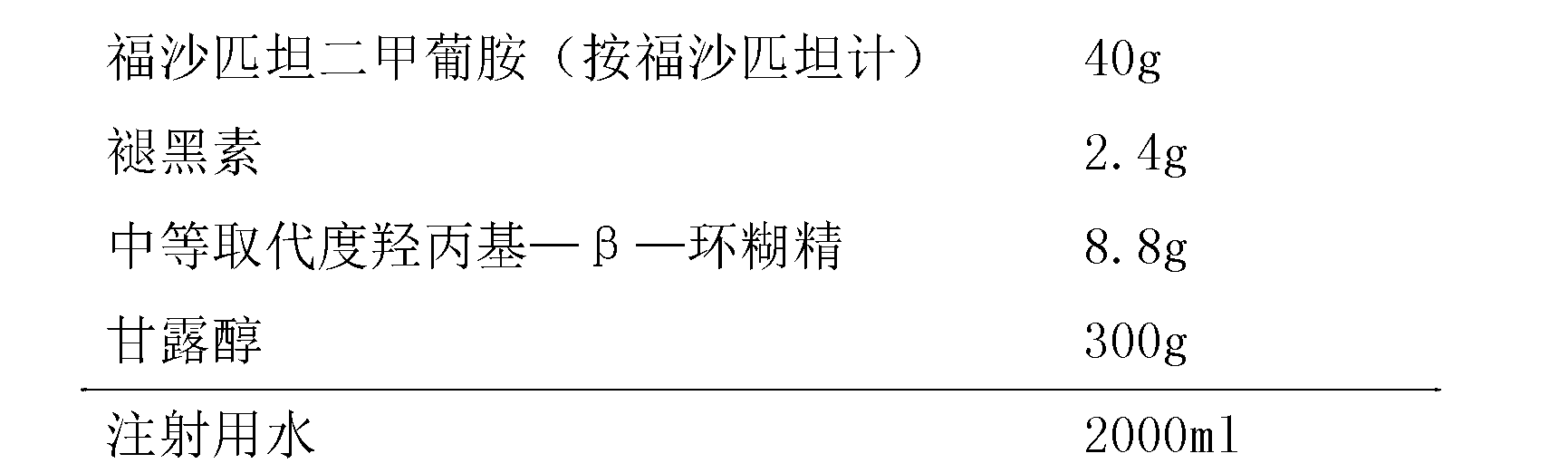
ActiveCN104971049BStable in natureLow hemolysis ratePowder deliveryOrganic active ingredientsValsartanHemolysis
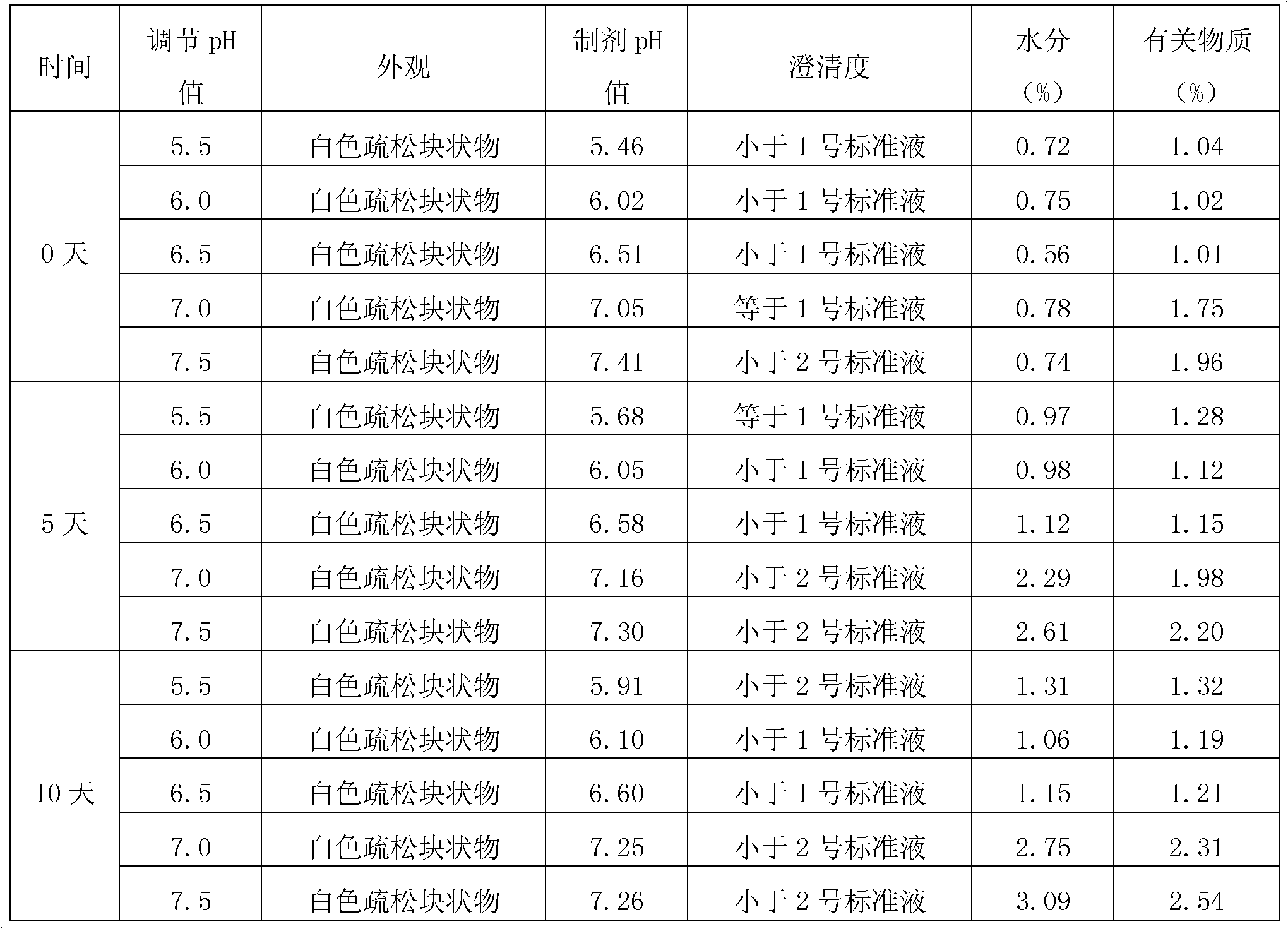
The invention provides a freeze-dried preparation containing fosaprepitant and a preparation method of the freeze-dried preparation. The freeze-dried preparation contains an active ingredient and other carriers, wherein the active ingredient is an effective amount for treatment of fosaprepitant dimeglumine, and the other carriers include a solubilizing agent, a complexing agent and a freeze-drying excipient; before freeze-drying, an acidity regulator is used for regulating the pH value of a liquid medicine to be 6.5-9.5; and the solubilizing agent is selected from one or more of polyethylene glycol dodecahydroxyl lithium stearate, hydroxypropyl-beta-cyclodextrin and polyethylene glycol. The freeze-dried preparation containing fosaprepitant, provided by the invention, is stable in property and low in hemolysis rate, and improves the compliance of clinical medication of patients.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method of fosaprepitant dimeglumine freeze-dried preparation for injection
InactiveCN102166199BProduct looseQuality improvementOrganic active ingredientsPowder deliveryPorosityFreeze-drying
A fosaprepitant dimeglumine freeze-dried preparation prepared by adopting the invention has the characteristics of porosity, stable quality, high redissolution speed, good clarity and the like. The invention provides a preparation method of a fosaprepitant dimeglumine freeze-dried preparation for injection, which comprises the following steps: (A) pre-freezing: firstly, cooling a fosaprepitant dimeglumine injection to (-12)-(-14) DEG C, and freezing through temperature oscillation; and then, cooling to (-32)-(-35) DEG C, and oscillating and freezing for 42-62 minutes; (B) sublimating: vacuumizing in a box body until the air pressure is 12-17 Pa, heating a product to (-35)-(-37) DEG C, and preserving the temperature for 8-10 hours; and intermittently injecting nitrogen gas, oscillating for1-1.5 hours by taking air pressure (17-22 Pa) in the box body as an amplitude, heating the product to (-25)-(-29) DEG C, and preserving the temperature for 15-18 hours; and (C) drying: gradually heating the medicament to 38-42 DEG C, and preserving the temperature for 1-2 hours.
Owner:WUHAN LEADPHARM TECH CO LTD
Detection methods of fosaprepitant dimeglumine raw material or preparation and impurity in fosaprepitant dimeglumine raw material or preparation
Owner:CHENGDU BAIYU PHARMA CO LTD
A kind of aseptic freeze-dried powder of fosaprepitant dimeglumine for injection and preparation process thereof
ActiveCN104414983BAvoid allergiesAvoid hemolysisOrganic active ingredientsPowder deliveryMedicineFosaprepitant dimeglumine
The invention particularly relates to a sterile freeze-dried fosaprepitant-dimeglumine powder for injection and a preparation process of the sterile freeze-dried fosaprepitant-dimeglumine powder. The sterile freeze-dried fosaprepitant-dimeglumine powder for injection comprises an active component, a solubilizing agent and a pH-value adjusting agent, wherein the active component is fosaprepitant dimeglumine. The preparation process of the sterile freeze-dried fosaprepitant-dimeglumine powder for injection is simple and feasible and is suitable for scale production. The sterile freeze-dried fosaprepitant-dimeglumine powder for injection has high quality and excellent stability and can be stored for a long time.
Owner:SHANDONG NEWTIME PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com