Preparation method of fosaprepitant dimeglumine
A technology of glucosamine and methyl, which is applied in the field of preparation of nausea and vomiting and postoperative nausea and vomiting compounds, can solve the problems of low yield of final product, high amount of monobenzyl ester, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] step one
Embodiment 1
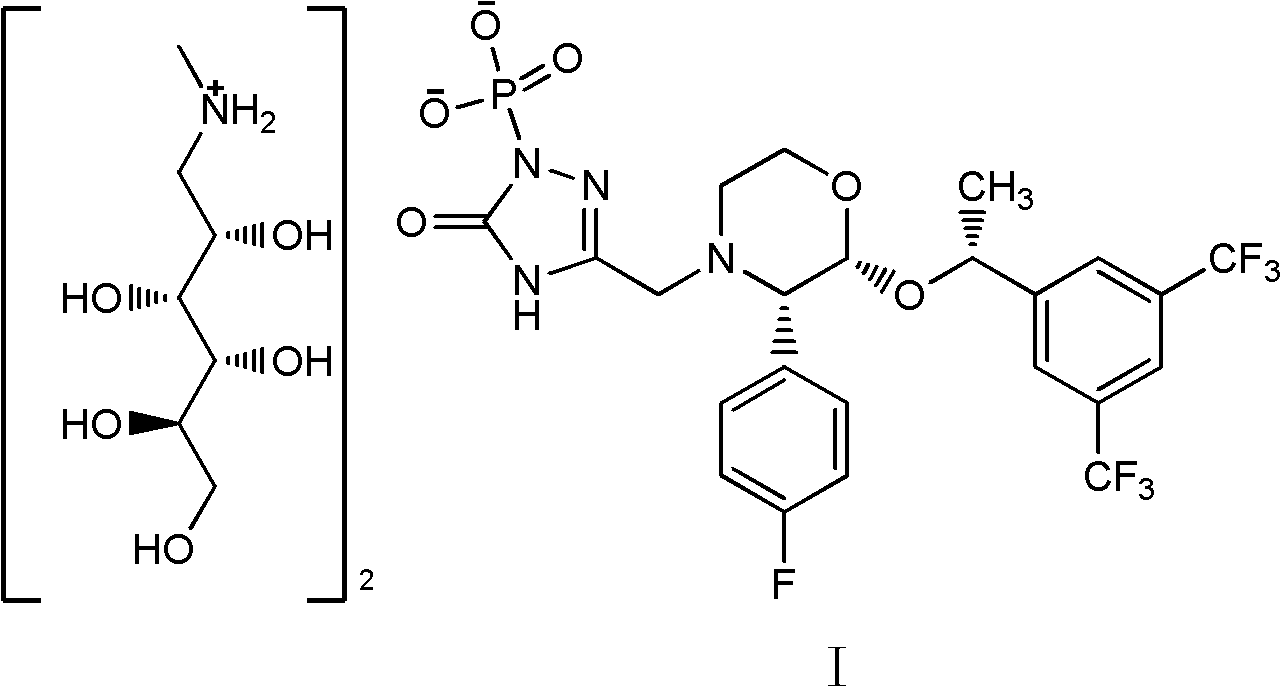
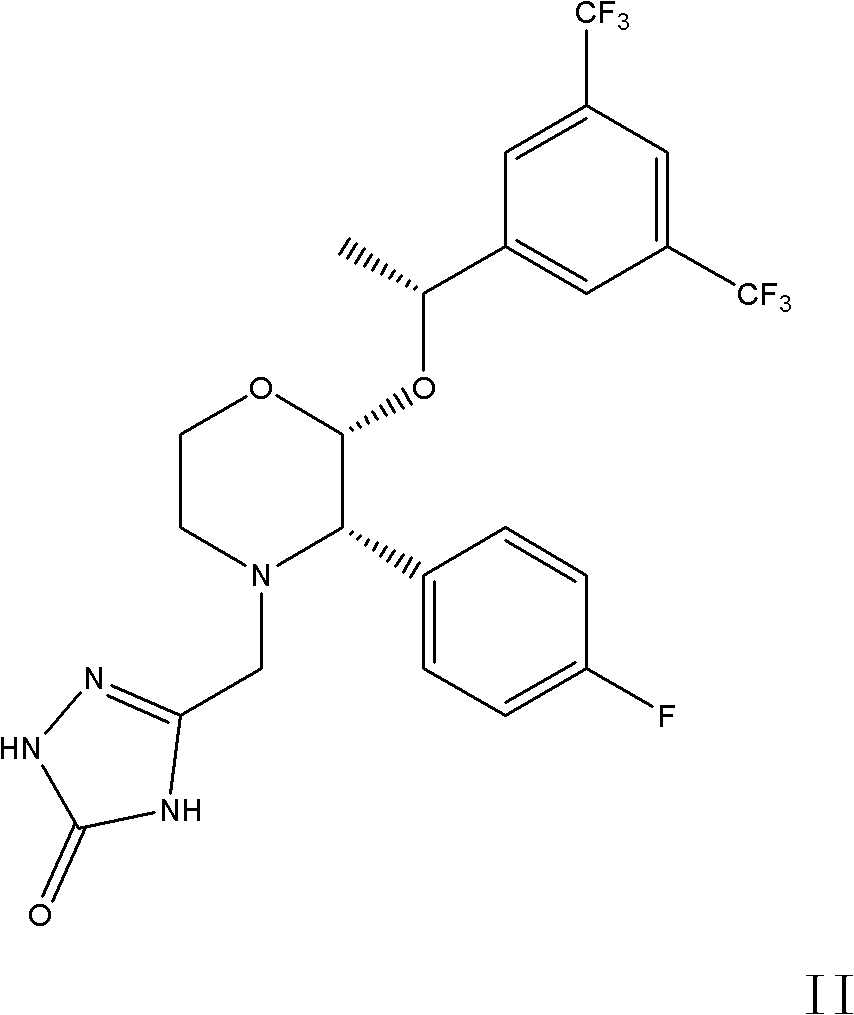
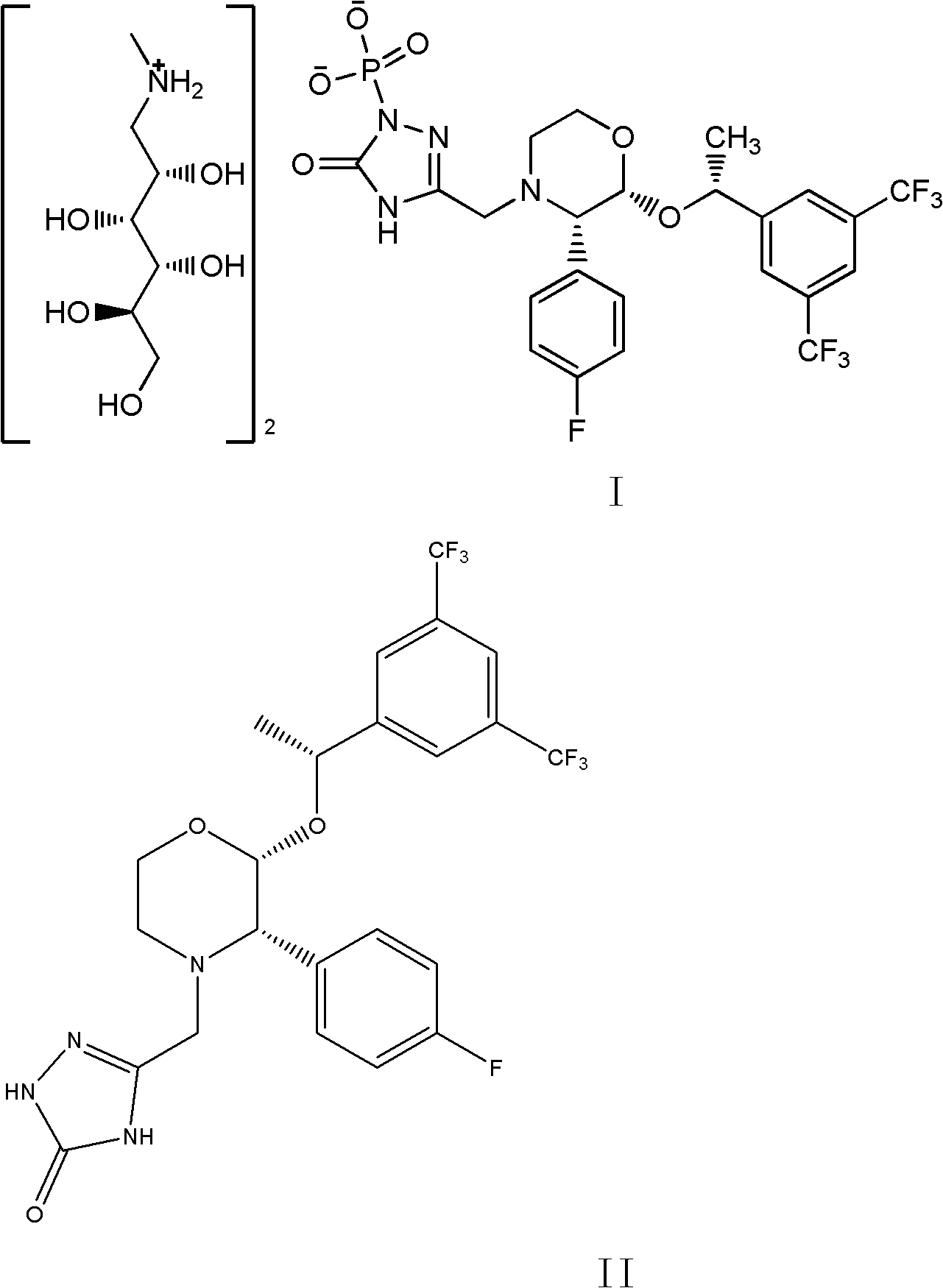
[0024] 5-[[2(R)-[1(R)-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl)-4 -Morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one (5.3g, 10mmol), tetrabenzyl pyrophosphate (7.0g, 13mmol) were dissolved in THF (60ml), cooled in an ice-water bath, was added dropwise with 2.0M sodium hexamethyldisilazane (NaHMDS, 12ml, 24mmol), and the reaction temperature was controlled at about -3°C. After dropping, stir for 2 hours until the reaction is complete, add methyl tert-butyl ether (150ml) and saturated sodium bicarbonate solution (150ml), separate the layers, saturated sodium bicarbonate solution (150ml), sodium bisulfate solution (150ml), water ( 150ml), concentrated to dryness, and dried to give 5.8g of white solid, yield 82.3%.
[0025] step two
[0026] The product (5.8g, 8.3mmol) and N-methyl-D-glucosamine (2.2g, 11.3mmol) obtained in the previous step were dissolved in methanol (25ml) / water (1ml), and 10% Pd / C (0.1 g), atmospheric pressure hydrogenation 4h until ...
Embodiment 2
[0031] step one
[0032] 5-[[2(R)-[1(R)-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl)-4 -Morpholinyl]methyl]-1,2-dihydro-3H-1,2,4-triazol-3-one (5.3g, 10mmol), tetrabenzyl pyrophosphate (7.0g, 13mmol) were dissolved in THF (60ml) was cooled in an ice-water bath, 2.0M NaHMDS (12ml, 24mmol) was added dropwise, and the reaction temperature was controlled at about -3°C. After dropping, stir for 2 hours until the reaction is complete, add methyl tert-butyl ether (150ml) and saturated sodium bicarbonate solution (150ml), separate the layers, saturated sodium bicarbonate solution (150ml), sodium bisulfate solution (150ml), water ( 150ml), concentrated to dryness, and dried to give 5.9g of white solid, yield 86.7%.
[0033] step two
[0034] The product (5.9g, 8.4mmol) and N-methyl-D-glucosamine (2.2g, 11.3mmol) obtained in the previous step were dissolved in methanol (25ml) / water (1ml), and 10% Pd / C (0.1 g), atmospheric pressure hydrogenation 4h until the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com