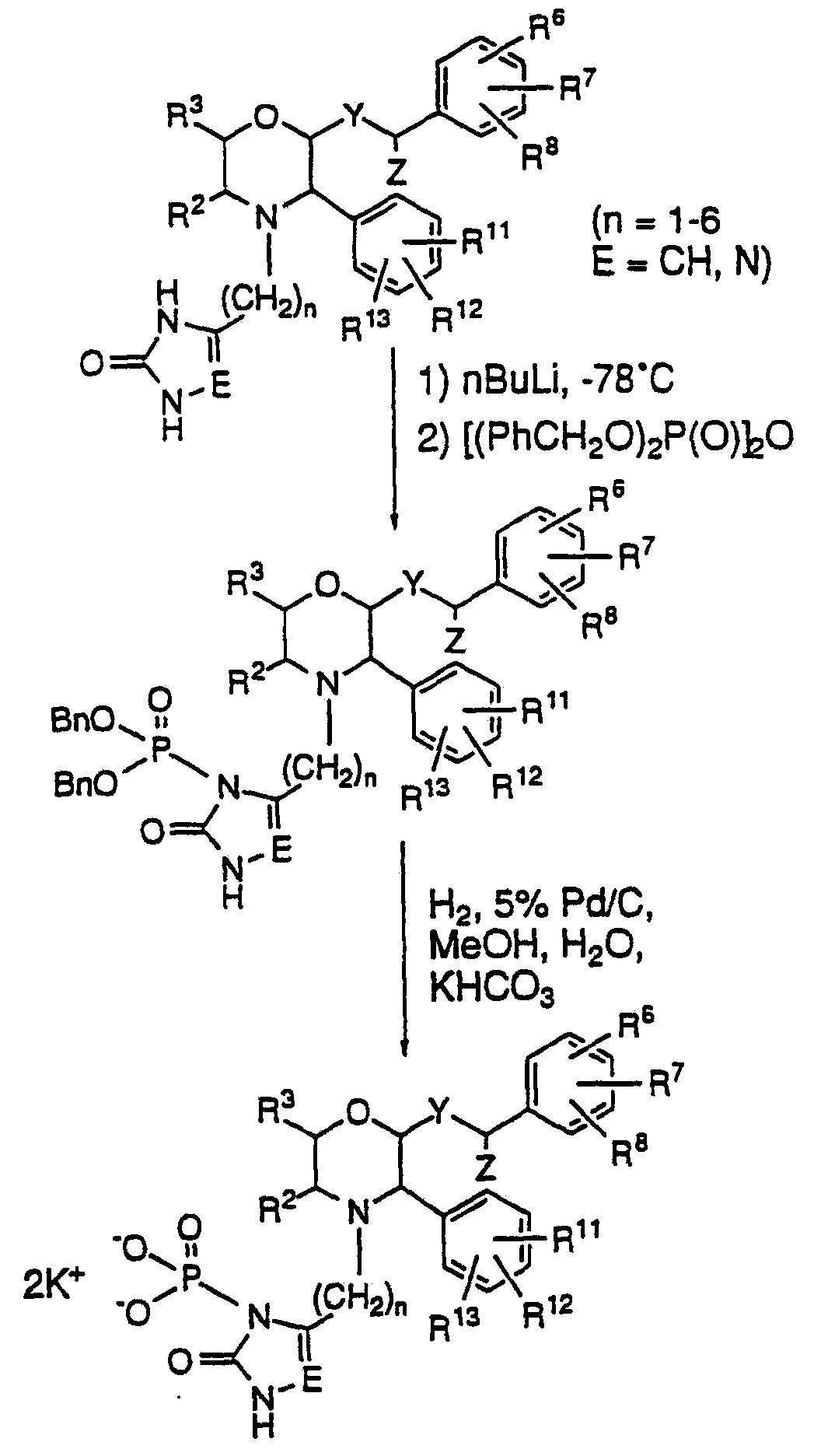
Fosaprepitant dimeglumine preparation method
A technology of fosaprepitant dimeglumine and benzhydryl group is applied in the field of preparing neurokinin-1 receptor antagonist fosaprepitant dimeglumine, which can solve the problem of difficult purification and removal and poor reaction yield. Ideal and other problems, to achieve the effect of enhancing regional selectivity and
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Step 1: Preparation of bis(diphenylmethyl)phosphite (compound VI)
[0038]
[0039] Add 68.7g of phosphorus trichloride (0.5mol) and 400mL of anhydrous toluene into a 1L three-necked flask, and cool down to -5 in an ice-salt bath under nitrogen protection. o C, slowly drop the mixed solution of 101.2g triethylamine (1.0mol) and 184.2g benzhydryl alcohol (1.0mol) in 300mL toluene, keep the temperature at 15 oBelow C, remove the ice bath after the dropwise addition, naturally rise to room temperature, and continue to stir for 2h. 300mL of water was dropped into the reaction solution, stirred at room temperature for 30min, the organic layer was separated, washed once with water (2*250mL) and saturated brine (250mL), dried over anhydrous magnesium sulfate, filtered with suction, and the filtrate was concentrated to dryness in vacuo to obtain 201g of shallow Yellow oil.
[0040] Step 2: Preparation of bis(benzhydryl)phosphoryl chloride (compound III)
[0041]
[00...
Embodiment 2
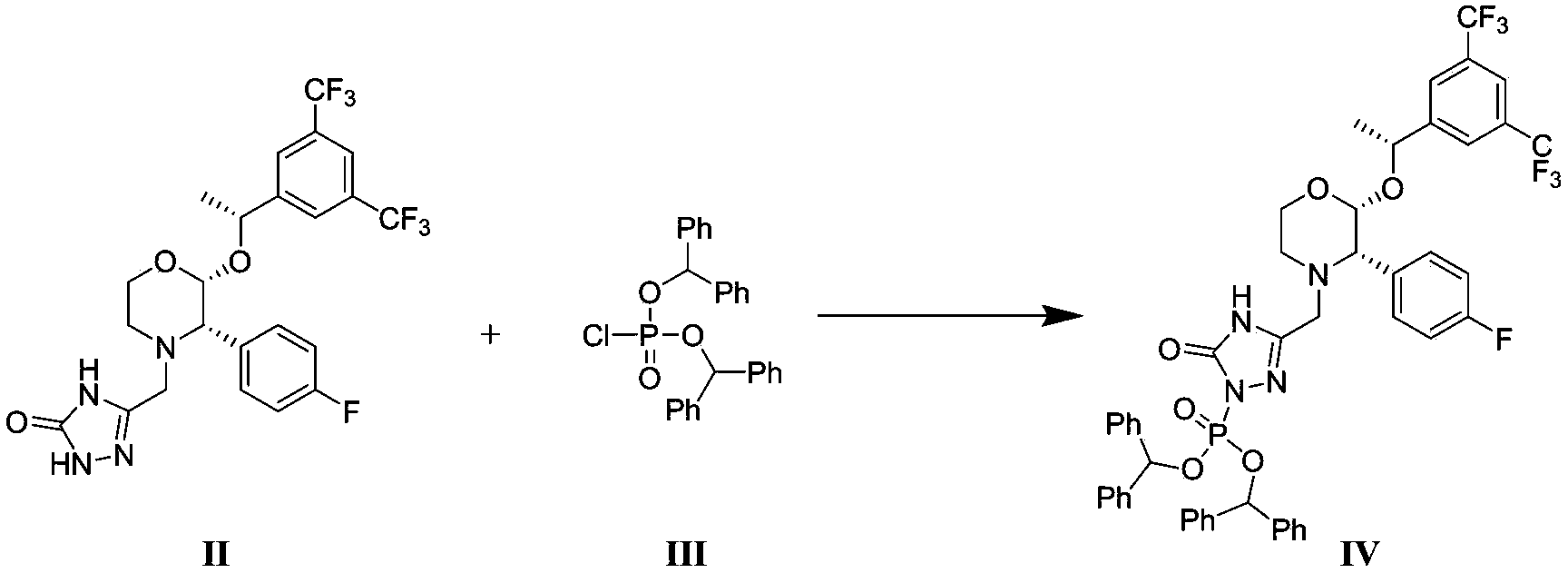
[0055] Step 1: {3-[[2(R)-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorobenzene base) morpholin-4-yl] methyl] -5-oxo-4,5-dihydro-1,2,4-triazol-1-yl} phosphate di(diphenylmethyl) ester (compound IV ) preparation
[0056]
[0057] Under nitrogen protection, add 15.0 g of compound II (28.1 mmol) into a 500 mL three-neck flask, add 120 mL of dioxane to dissolve it, and cool down to -5 o C, dropwise add 67mL of 1M bis(trimethylsilyl) sodium amide (67.0mmol), the temperature is controlled at 5 o Below C, stir for 30min after the dropwise addition, and then drop 12.9g of compound III (28.7mmol) in dioxane solution (80mL) into the bottle at a temperature of 5 o Below C, remove the ice-salt bath after the dropwise addition, naturally rise to room temperature, and continue to stir for 1h. Cool down in an ice bath, add 400 mL of saturated aqueous ammonium chloride dropwise to quench the reaction, extract the mixture with 400 mL of methyl tert-butyl ether, wash the or...
Embodiment 3
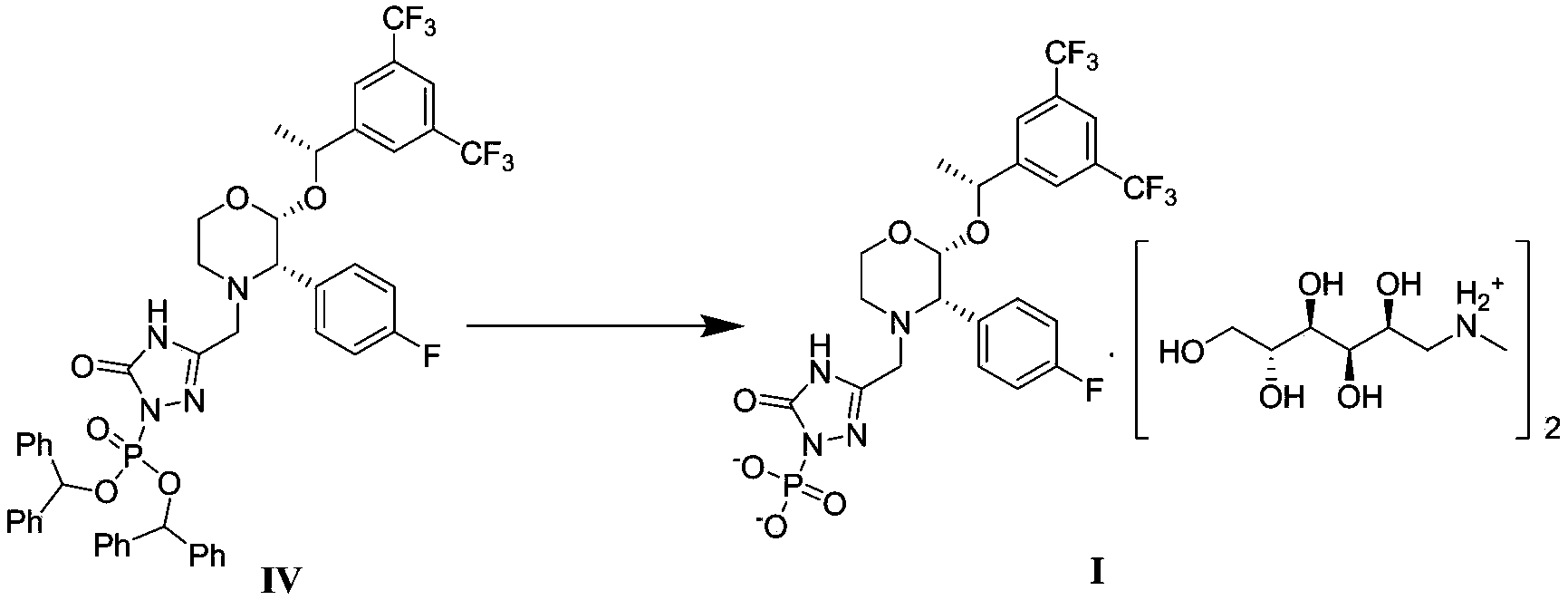
[0066] Step 1: {3-[[2(R)-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorobenzene base) morpholin-4-yl] methyl] -5-oxo-4,5-dihydro-1,2,4-triazol-1-yl} phosphate di(diphenylmethyl) ester (compound IV ) preparation
[0067]
[0068] Under nitrogen protection, add 15.0 g of compound II (28.1 mmol) into a 500 mL three-neck flask, add 120 mL of tetrahydrofuran to dissolve it, and cool down to -5 o C, dropwise add 73mL of 1M bis(trimethylsilyl) sodium amide (73.0mmol), the temperature is controlled at 5 o Below C, stir for 30min after the dropwise addition, then drop 12.9g of compound III (28.7mmol) in tetrahydrofuran solution (80mL) into the bottle, the temperature is at 5 o Below C, remove the ice-salt bath after the dropwise addition, naturally rise to room temperature, and continue to stir for 1h. Cool down in an ice bath, add 400 mL of saturated aqueous ammonium chloride dropwise to quench the reaction, extract the mixture with 400 mL of methyl tert-butyl eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com