Fosaprepitant dimeglumine freeze-dried powder injection and preparation method thereof
A technology of fosaprepitant dimeglumine and freeze-dried powder injection, which is applied in the field of fosaprepitant dimeglumine freeze-dried powder injection and its preparation, can solve the problem of affecting the filtration speed and filling accuracy, and affecting the medication Safety, production inconvenience and other issues, to achieve the effect of improving sterilization filtration speed and filling volume accuracy, ensuring drug stability, and avoiding foam generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
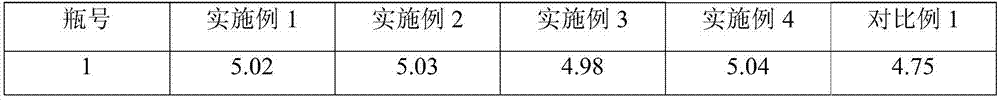
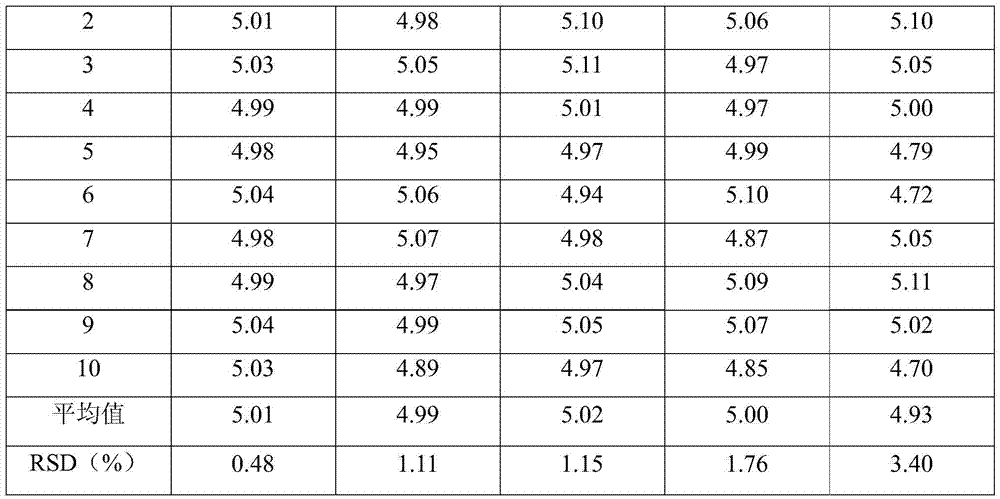
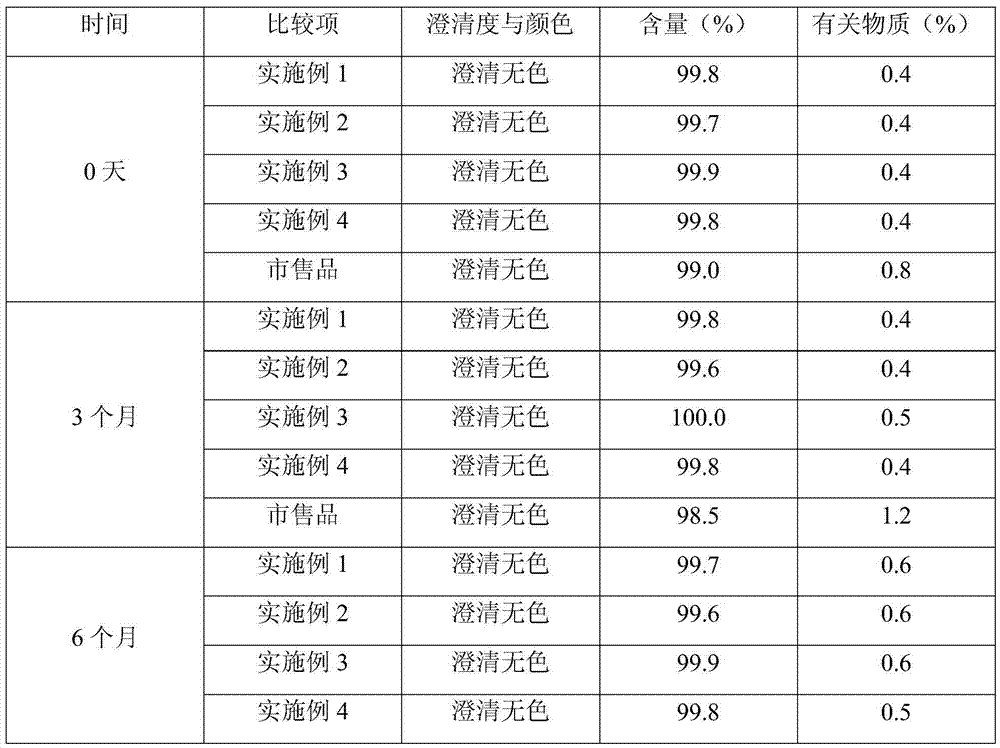
Embodiment 1
[0029] Fosaprepitant dimeglumine 200g Citric acid 5g Sodium citrate 5g Water for Injection Add to 5L
[0030] Preparation Process:
[0031] (1) 0.5L of tert-butanol is mixed with 2.5L of water for injection, heated to 60°C, and 200g of fosaprepitant dimeglumine is weighed and added thereto, and the stirring speed is set to 80r / min, and stirred until clarification;
[0032] (2) Weigh 5 g of citric acid and 5 g of sodium citrate, add 1.5 L of water for injection to dissolve, add 15 g of activated carbon for injection, heat to 50 ° C and stir for 15 minutes, decarbonize and filter to clarify;
[0033] (3) Mix the solutions (1) and (2), add water for injection to volume, cool to room temperature, sterilize, filter, fill, and freeze-dry in a freeze-drying machine. The freeze-drying curve is: the shelf is cooled to - 40°C and maintained for 4 hours to freeze the medicinal liquid; turn on the vacuum pump to make the vacuum in the freeze-drying box 20...
Embodiment 2
[0036] Fosaprepitant dimeglumine 200g Citric acid 7g Sodium citrate 8g Water for Injection Add to 5L
[0037] Preparation Process:
[0038] (1) 0.3L of tert-butanol is mixed with 3.0L of water for injection, heated to 50° C., and the recipe quantity Fosaprepitant dimeglumine is weighed and added therein, and the stirring speed is set to 60r / min, and stirred until clarification;
[0039] (2) Weigh 7g of citric acid and 8g of sodium citrate, add 1.5L water for injection to dissolve, add 15g of activated carbon for injection, heat to 55°C and stir for 15 minutes, decarbonize and filter to clarify;
[0040](3) Mix the solutions (1) and (2), add water for injection to volume, cool to room temperature, sterilize, filter, fill, and freeze-dry in a freeze-drying machine. The freeze-drying curve is: the shelf is cooled to - 45°C and hold for 4 hours to freeze the medicinal liquid; turn on the vacuum pump to make the vacuum in the freeze-drying box 60Pa...
Embodiment 3
[0043] Fosaprepitant dimeglumine 200g Citric acid 10g Sodium citrate 10g Water for Injection Add to 5L
[0044] Preparation Process:
[0045] (1) 0.4L of tert-butanol is mixed with 2.6L of water for injection, heated to 45°C, and the recipe quantity Fosaprepitant dimeglumine is weighed and added therein, and the stirring speed is set to 70r / min, and stirred until clarification;
[0046] (2) Weigh 10 g of citric acid and 10 g of sodium citrate, add 1.5 L of water for injection to dissolve, add 15 g of activated carbon for injection, heat to 60 ° C and stir for 15 minutes, decarbonize and filter to clarify;
[0047] (3) Mix the solutions (1) and (2), add water for injection to volume, cool to room temperature, sterilize, filter, fill, and freeze-dry in a freeze-drying machine. The freeze-drying curve is: the shelf is cooled to - 45 ℃ and maintain for 6 hours to make the liquid freeze solid; turn on the vacuum pump to make the vacuum in the freez...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com