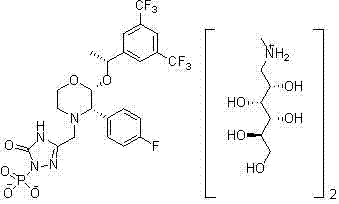
Preparation method of fosaprepitant dimeglumine
A technology for fosaprepitant dimeglumine and fosaprepitant, which is applied in the field of preparation of fosaprepitant dimeglumine, can solve the problems of being unsuitable for large-scale industrialized production, having a long reaction period and many production processes, and the like, Achieve the effect of shortening production cycle, low production cost and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 {3-[2(R)-[(1R)-1-[3, 5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl )morpholin-4-yl]methyl]-5-oxo-4,5-dihydro-[1,2,4]-triazol-1-yl}di-p-ethylbenzyl phosphate (intermediate ) preparation
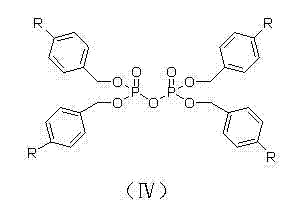
[0029] Add 12g (22.4mmol) of aprepitant (22.4mmol), 21.9g (33.6mmol) of tetra-p-ethylbenzyl pyrophosphate and 150mL of THF into a 250mL three-necked flask, stir to dissolve, cool the temperature to 0~5°C in an ice-salt bath, and divide 4.3 g (44.8 mmol) of sodium tert-butoxide was added in batches, and the temperature was controlled below 5° C. during the addition, and monitored by TLC until the reaction was completed. Pour the reaction solution into 150 mL of saturated NaHCO 3 Then add 150mL of ether to the solution, separate the layers, wash the organic phase with 100mL of purified water until the pH is neutral, and concentrate to dryness under reduced pressure to obtain 16.2g of the product with a purity of 99.63% and a yield of 85.0%.
Embodiment 2
[0030] Example 2 {3-[2(R)-[(1R)-1-[3, 5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl )morpholin-4-yl]methyl]-5-oxo-4,5-dihydro-[1,2,4]-triazol-1-yl}di-p-methylbenzyl phosphate (intermediate ) preparation
[0031] Add 12g (22.4mmol) of aprepitant, 20.0g (3.36mmol) of tetra-p-methylbenzyl pyrophosphate and 165mL of THF into a 250mL three-neck flask, stir to dissolve, cool the temperature to 0~5°C in an ice-salt bath, and add methanol Sodium 2.4g (44.8mmol), the temperature was controlled below 5°C during the addition process, and monitored by TLC until the reaction was completed. Pour the reaction solution into 120 mL of saturated NaHCO 3 Then add 180mL ether to the solution, separate the layers, wash the organic phase with 120mL of purified water twice until the pH is neutral, and concentrate to dryness under reduced pressure to obtain 16.1g of the product with a purity of 99.70% and a yield of 85.2%.
Embodiment 3
[0032] Example 3 {3-[2(R)-[(1R)-1-[3, 5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-fluorophenyl )morpholin-4-yl]methyl]-5-oxo-4,5-dihydro-[1,2,4]-triazol-1-yl}di-p-chlorobenzyl phosphate (intermediate) preparation of
[0033] Add 12g (22.4mmol) of aprepitant, 22.7g (33.6mmol) of tetra-p-chlorobenzyl pyrophosphate and 150mL of THF into a 250mL three-neck flask, stir to dissolve, cool the temperature in an ice-salt bath to 0~5°C, and add in batches 4.9 g (44.8 mmol) of sodium tert-amylate, the temperature was controlled below 5°C during the addition process, and monitored by TLC until the reaction was completed. Pour the reaction solution into 150 mL of saturated NaHCO 3 Then add 150mL ether to the solution, separate the layers, wash the organic phase with 150mL of purified water twice until the pH is neutral, and concentrate to dryness under reduced pressure to obtain 16.8g of the product with a purity of 99.55% and a yield of 87.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com