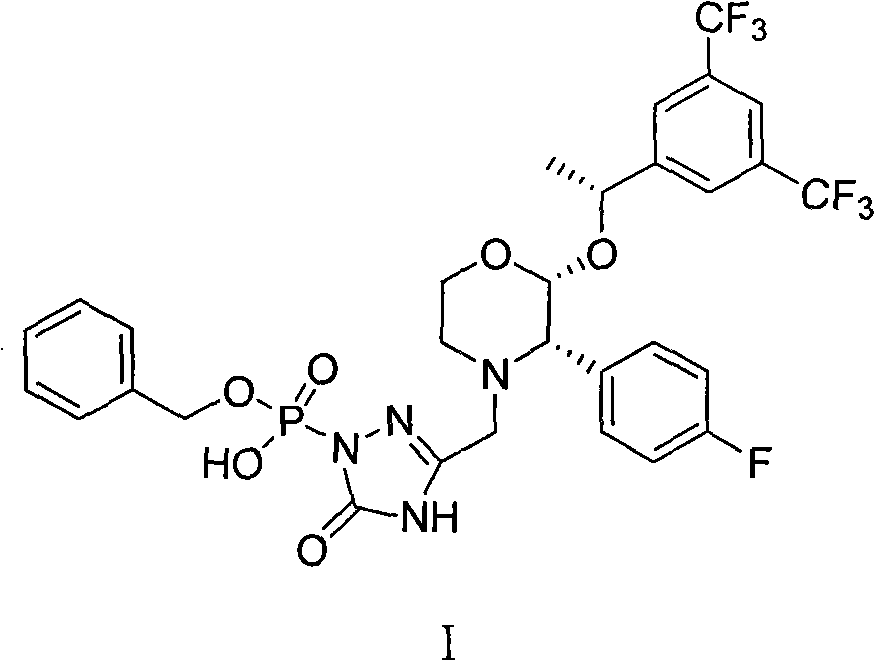
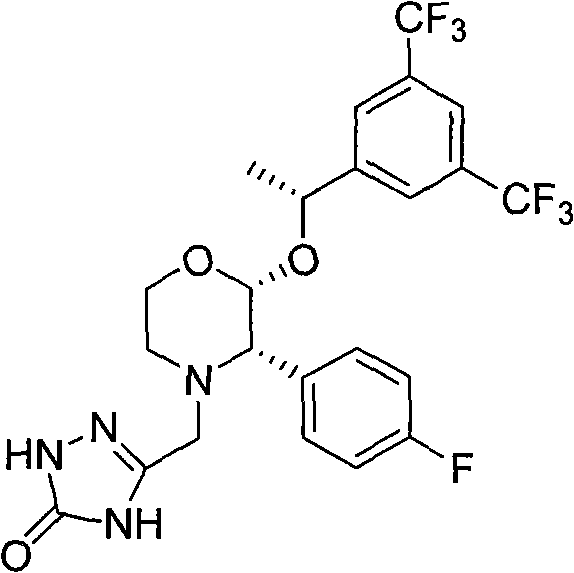
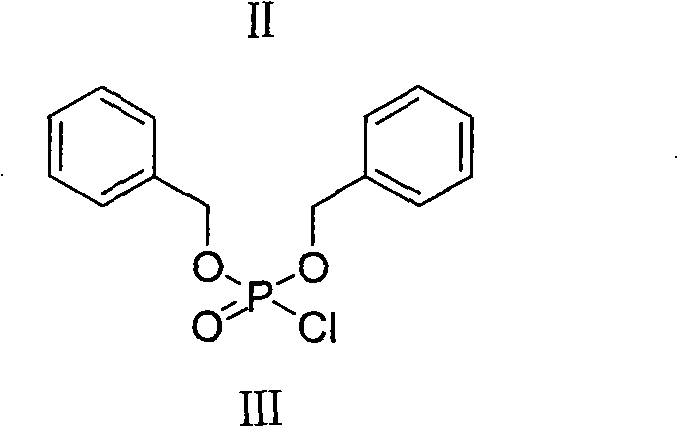
New method for preparing fosaprepitant intermediate
A new method and compound technology, applied in the chemical field, can solve the problems of complex purification process and unfavorable large-scale production, and achieve the effect of reducing cost and simplifying operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of Dibenzylphosphoryl Chloride
[0025]
[0026] Put a 100ml three-necked bottle, equipped with a thermometer, magnetic stirring, N 2 . in N 2 Under protection, 10 g (36 mmol) of dibenzyl phosphate and 25 ml of toluene were first added to the bottle. Control the temperature of the reaction mixture below 25°C, and slowly add 3.5ml (43mmol) of sulfuryl chloride dropwise. After all the addition was completed, the temperature was maintained and stirring was continued for 30 minutes. The reaction solution was transferred to a 100ml dry single-necked bottle, and the solvent was evaporated under reduced pressure. 10ml of toluene was added to the residue again, and then evaporated under reduced pressure. This process was repeated three times to ensure that the residue did not contain sulfonyl chloride. The residue was dissolved in 5ml of tetrahydrofuran and used directly in the next reaction.
[0027] [3-[(2R)-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]...
Embodiment 2
[0031] Preparation of Dibenzylphosphoryl Chloride
[0032] Put a 100ml three-necked bottle, equipped with a thermometer, magnetic stirring, N 2 . in N 2 Under protection, 10 g (36 mmol) of dibenzyl phosphate and 25 ml of toluene were first added to the bottle. Control the temperature of the reaction mixture below 25°C, and slowly add 3.5ml (43mmol) of sulfuryl chloride dropwise. After all the addition was completed, the temperature was maintained and stirring was continued for 30 minutes. The reaction solution was transferred to a 100ml dry single-necked bottle, and the solvent was evaporated under reduced pressure. 10ml of toluene was added to the residue again, and then evaporated under reduced pressure. This process was repeated three times to ensure that the residue did not contain sulfonyl chloride. The residue was dissolved in 5ml of tetrahydrofuran and used directly in the next reaction.
[0033] [3-[(2R)-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-flu...
Embodiment 3
[0036] Preparation of Dibenzylphosphoryl Chloride
[0037] Put a 100ml three-necked bottle, equipped with a thermometer, magnetic stirring, N 2 . in N 2 Under protection, 10 g (36 mmol) of dibenzyl phosphate and 25 ml of toluene were first added to the bottle. Control the temperature of the reaction mixture below 25°C, and slowly add 3.5ml (43mmol) of sulfuryl chloride dropwise. After all the addition was completed, the temperature was maintained and stirring was continued for 30 minutes. The reaction solution was transferred to a 100ml dry single-necked bottle, and the solvent was evaporated under reduced pressure. 10ml of toluene was added to the residue again, and then evaporated under reduced pressure. This process was repeated three times to ensure that the residue did not contain sulfonyl chloride. The residue was dissolved in 5ml of tetrahydrofuran and used directly in the next reaction.
[0038] [3-[(2R)-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3(S)-(4-flu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com