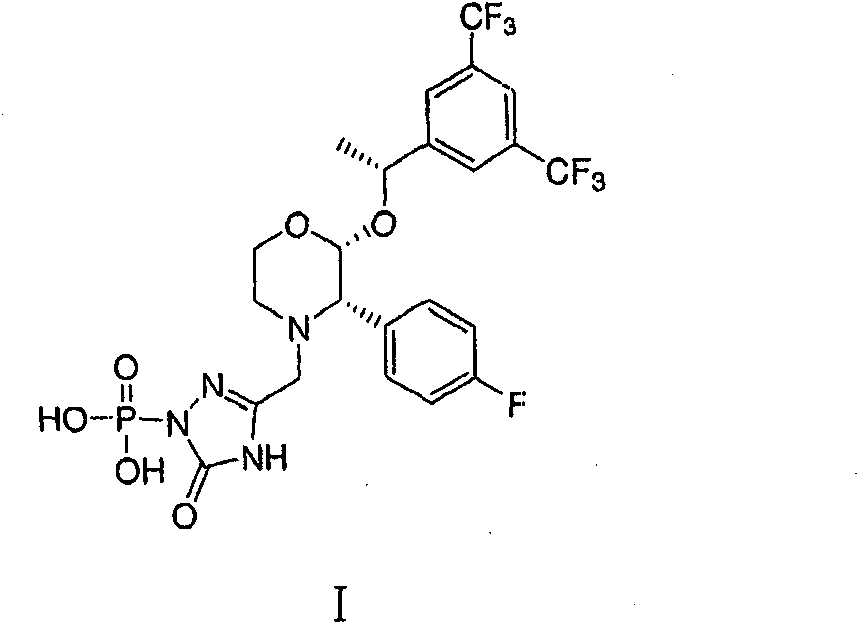
New preparation method for fosaprepitant and pharmaceutically acceptable salt thereof
A technology of medicinal salts and supercritical fluids, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, and bulk chemical production, and can solve high production costs, expensive precious metals, and waste of precious metals and other problems to achieve the effect of overcoming high production cost, good product quality and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
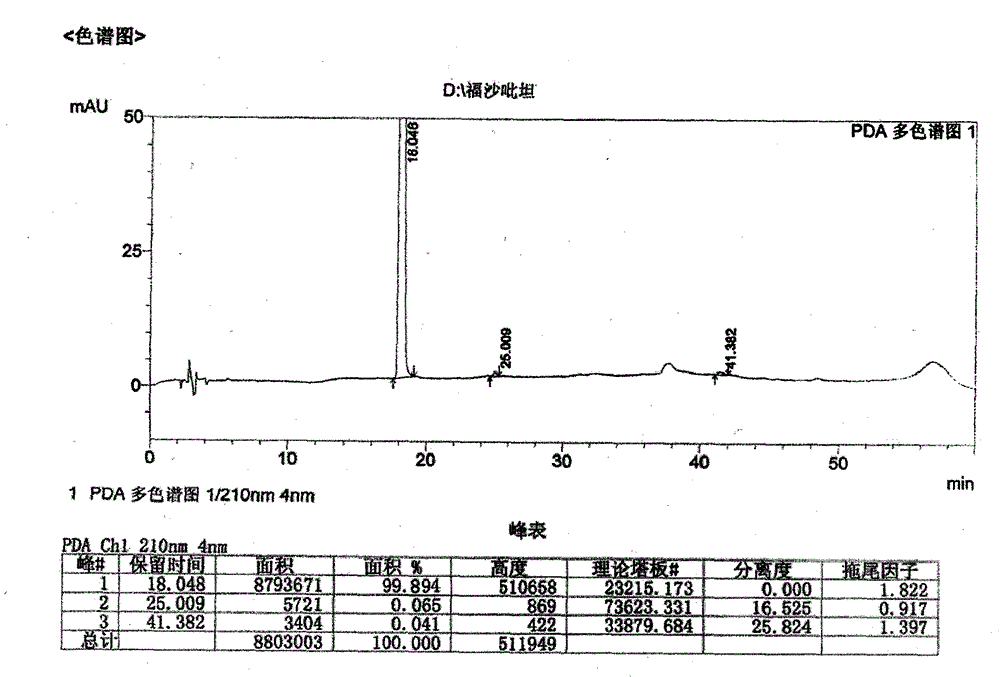
[0029] In a 500ml supercritical fluid reaction device, add 50.0g (0.063mol) of aprepitant dibenzyl phosphate and 0.5g of 5% palladium carbon, after replacing the air with carbon dioxide, first feed hydrogen to make the pressure reach 0.3-0.5MPa , and then feed carbon dioxide to make the total pressure reach 13-14MPa, control the temperature at 31-35° C. and stir the reaction for 1 hour. After removing the pressure, add 500ml of methanol, filter, add 24.6g (0.126mol) of N-methyl-D-glucosamine to the filtrate, stir at room temperature for 2 hours under nitrogen protection, drop the obtained solution into acetonitrile, and crystallize under stirring , press-filtered under the protection of nitrogen, and vacuum-dried at room temperature to obtain 55.4 g of fosaprepitant dimeglumine as a white solid, with a yield of 87.5%. Heavy metals are less than 10ppm, purity is 99.75%, defluorinated impurities are 0.03%, and other single impurities are less than 0.1%.
Embodiment 2
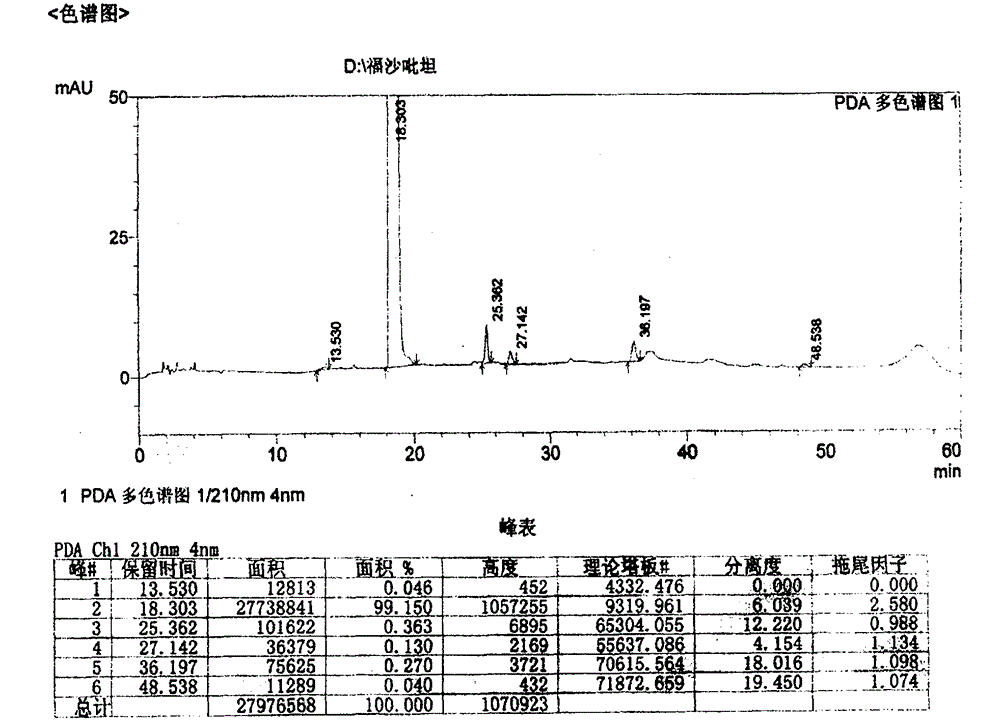
[0031] In a 2-liter supercritical fluid reaction device, add 200.0 g (0.252 mol) of aprepitant dibenzyl phosphate and 1.0 g of 10% palladium carbon. After replacing the air with carbon dioxide, first feed hydrogen to make the pressure reach 0.5-0.8 MPa, and then feed carbon dioxide to make the total pressure reach 16-20 MPa, control the temperature at 31-35° C. and stir for 1 hour. After removing the pressure, add 500ml of methanol, filter, add 98.4g (0.504mol) of N-methyl-D-glucosamine to the filtrate, stir at room temperature for 2 hours under the protection of nitrogen, drop the obtained solution into acetonitrile, and crystallize under stirring , press-filtered under the protection of nitrogen, and vacuum-dried at room temperature to obtain 227.8 g of fosaprepitant dimeglumine as a white solid, with a yield of 90.0%. The heavy metal is less than 10ppm, the purity is 99.73%, the defluorinated impurities are not detected, and the other single impurities are all less than 0.1...
Embodiment 3
[0033] In a 500ml supercritical fluid reaction device, add 50.0g (0.063mol) of aprepitant dibenzyl phosphate and 0.25g of 10% palladium carbon, after replacing the air with carbon dioxide, first feed hydrogen to make the pressure reach 0.3-0.5MPa , and then feed carbon dioxide to make the pressure reach 13-14MPa, control the temperature at 35-40°C and stir the reaction for 0.5 hours. After removing the pressure, add 500ml of methanol, filter, add 24.6g (0.126mol) of N-methyl-D-glucosamine to the filtrate, stir at room temperature under nitrogen protection for 1 hour, drop the obtained solution into acetonitrile, and crystallize under stirring , pressure filtration under nitrogen protection, and vacuum drying at room temperature to obtain 52.6 g of fosaprepitant dimeglumine as a white solid, with a yield of 83.1%. The heavy metal is less than 10ppm, the purity is 99.77%, the defluorinated impurities are not detected, and other single impurities are all less than 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com