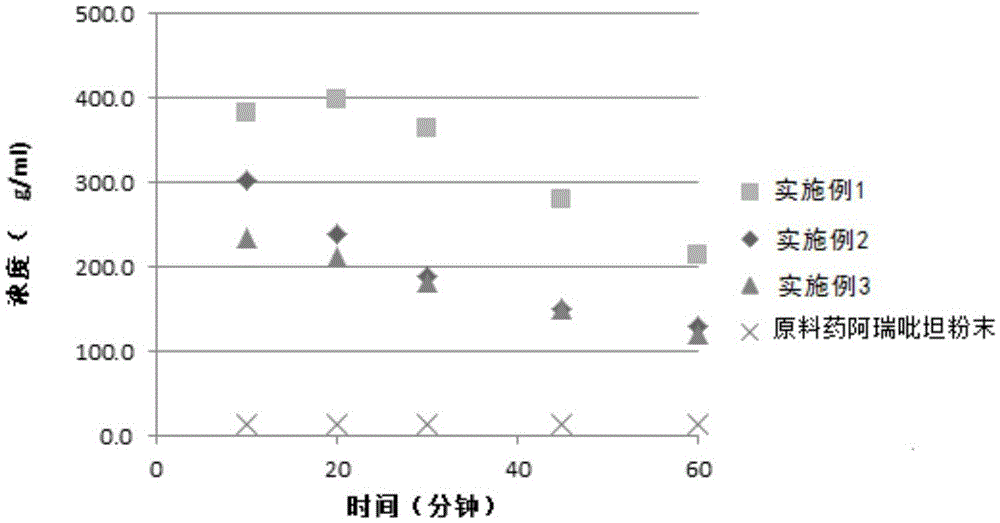
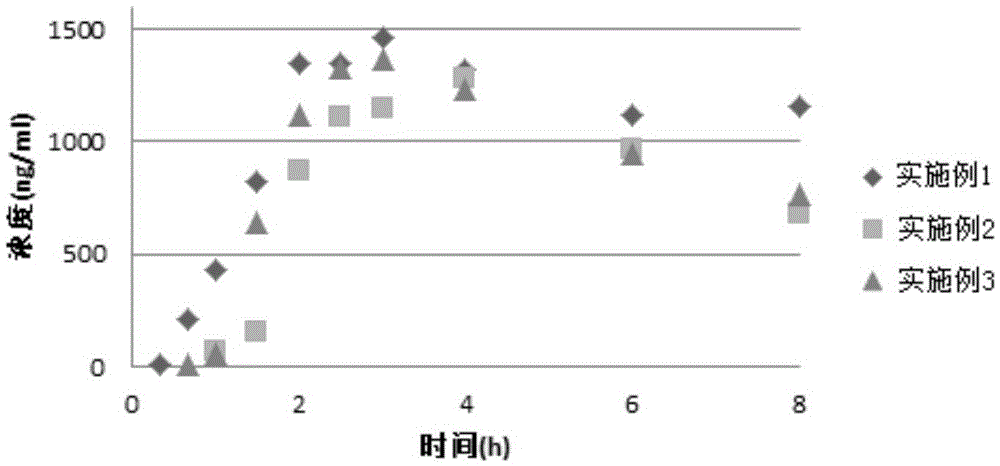
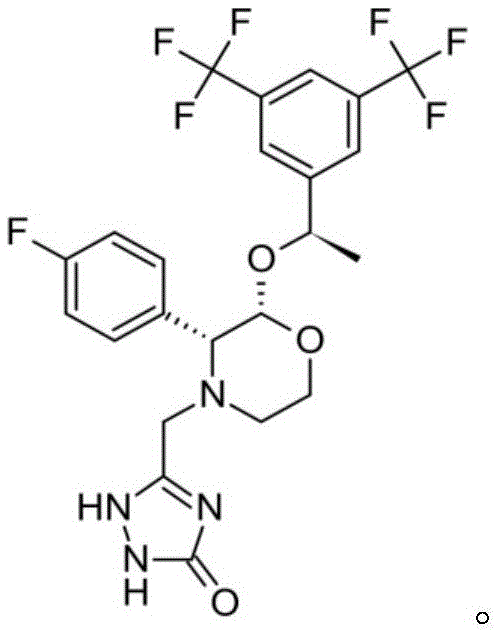
Aprepitant oral pharmaceutical preparation
A technology for aprepitant and pharmaceutical preparations, applied in the field of oral pharmaceutical preparations of aprepitant, can solve the problems of debris pollution of grinding machines, restrict the popularization and use of aprepitant, and high cost, achieve high stability, fill Market blank, the effect of improving the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] An oral tablet of Aprepitant, the formula is as follows:
[0034]
[0035] Dissolve aprepitant and hypromellose acetate succinate in absolute ethanol, dry under reduced pressure, and pass the dried product through a 150-mesh sieve to obtain a solid dispersion for use;
[0036] (2) Pass component C to component F through a 150 mesh sieve for use;
[0037] (3) Weigh the sieved solid dispersion, add lactose, croscarmellose sodium, silicon dioxide and magnesium stearate to it in sequence, mix well, and press into tablets.
Embodiment 2
[0039] An oral tablet of Aprepitant, the formula is as follows:
[0040]
[0041] Dissolve aprepitant and hypromellose phthalate in absolute ethanol, dry under reduced pressure, and pass the dried product through a 150-mesh sieve to obtain a solid dispersion for use;
[0042] (2) Pass component C to component F through a 150 mesh sieve for use;
[0043] (3) Weigh the sieved solid dispersion, add lactose, crospovidone, talc and magnesium stearate to it in sequence, mix well, and press into tablets.
Embodiment 3
[0045] An oral capsule of Aprepitant, the formula is as follows:
[0046]
[0047]
[0048] Dissolve aprepitant and hypromellose phthalate in absolute ethanol, dry under reduced pressure, and pass the dried product through a 150-mesh sieve to obtain a solid dispersion for use;
[0049] (2) Pass component C to component F through a 150 mesh sieve for use;
[0050] (3) Weigh the sieved solid dispersion, add microcrystalline cellulose, low-substituted hydroxypropyl cellulose and magnesium stearate to it in sequence, mix them evenly, and put them into HPMC capsule shells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com