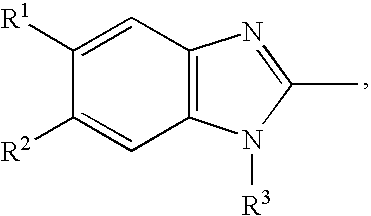
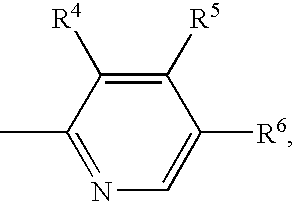
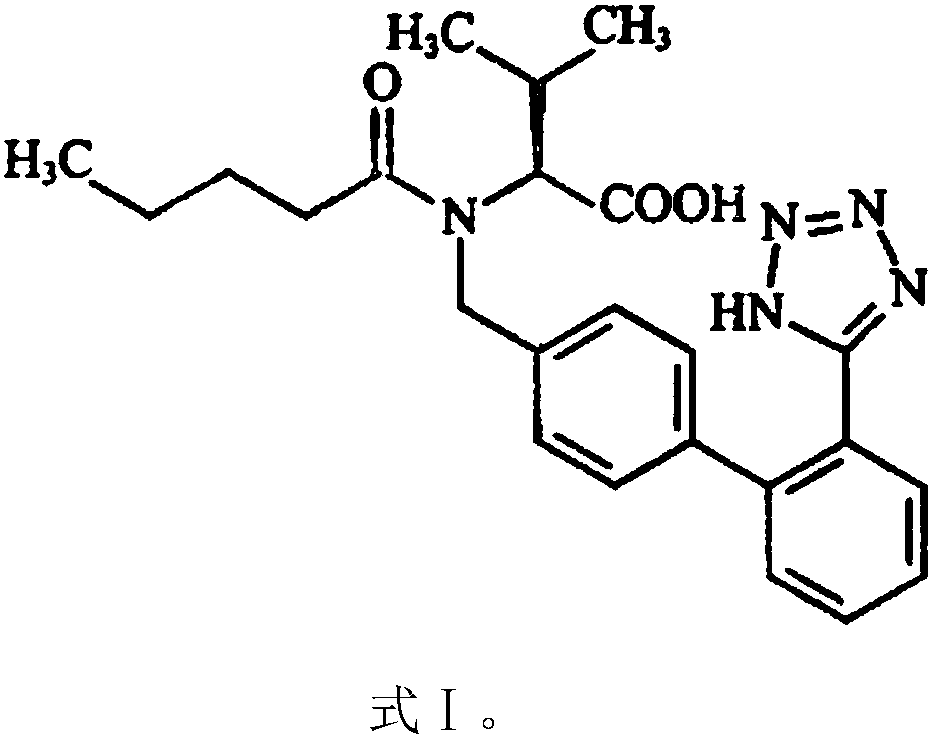
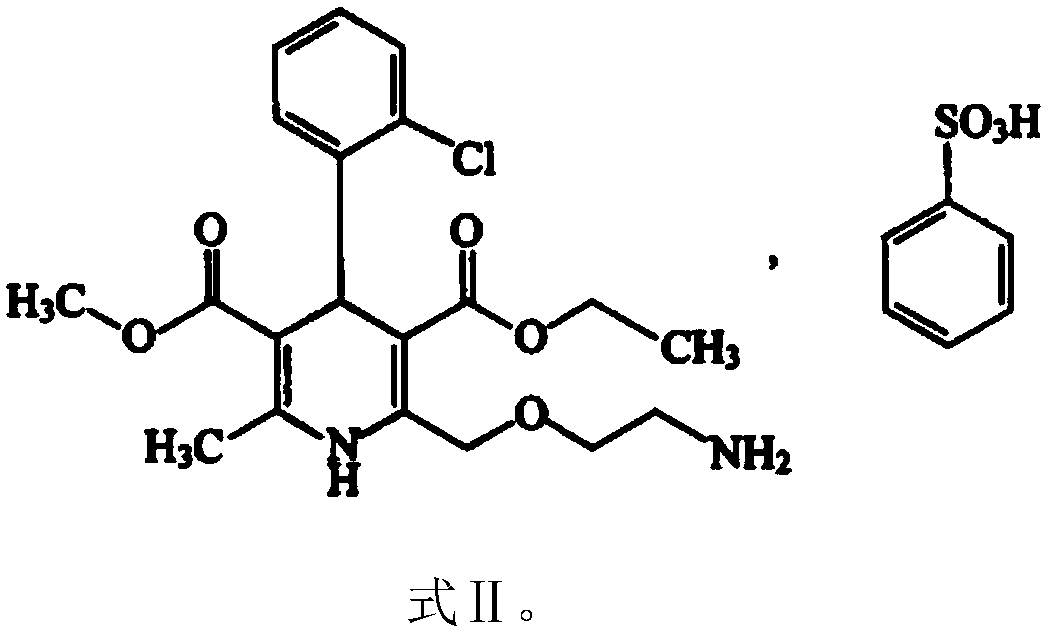
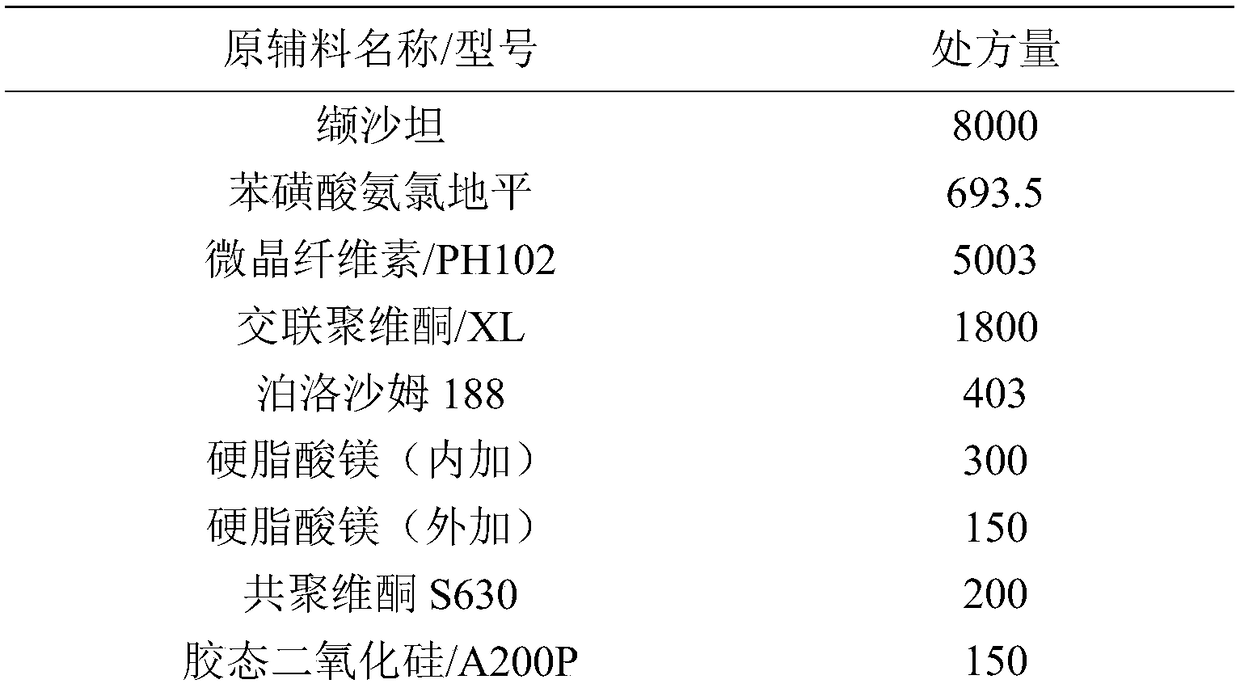
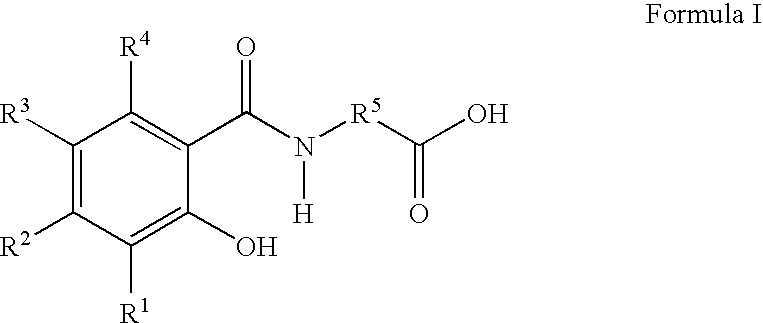
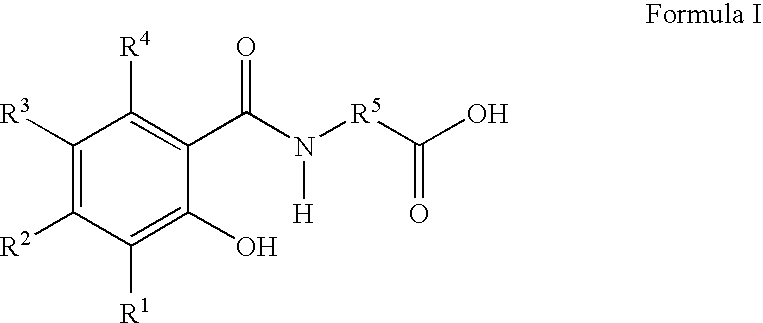
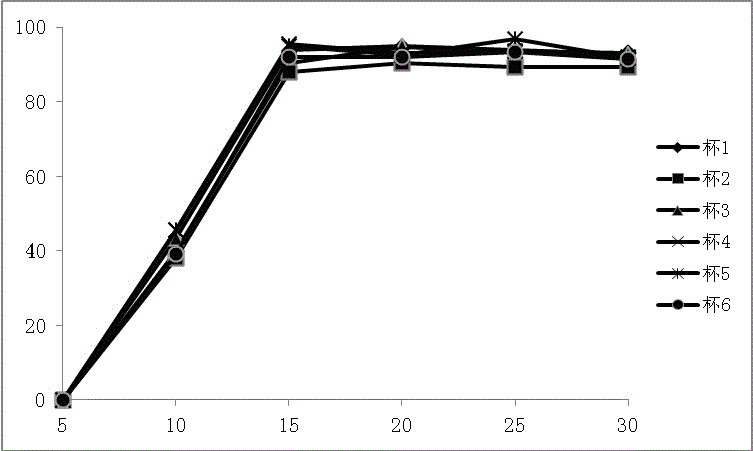
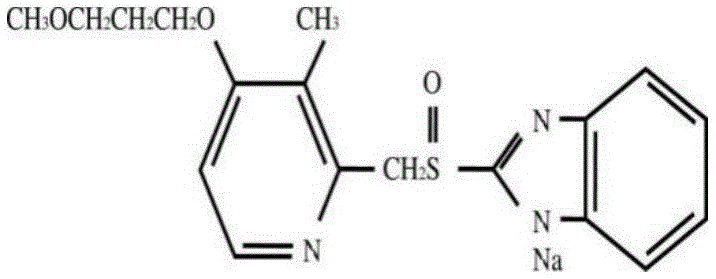
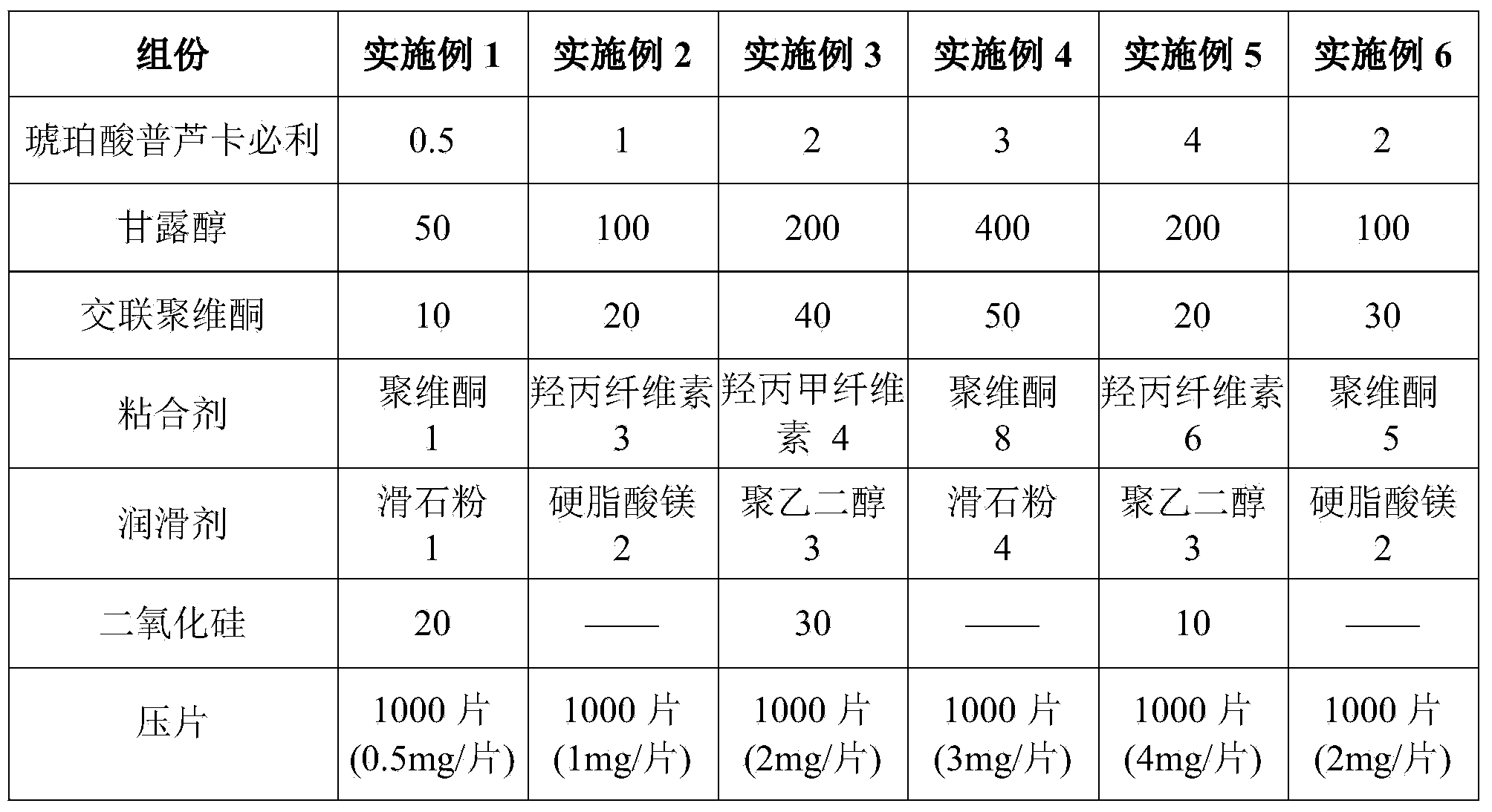
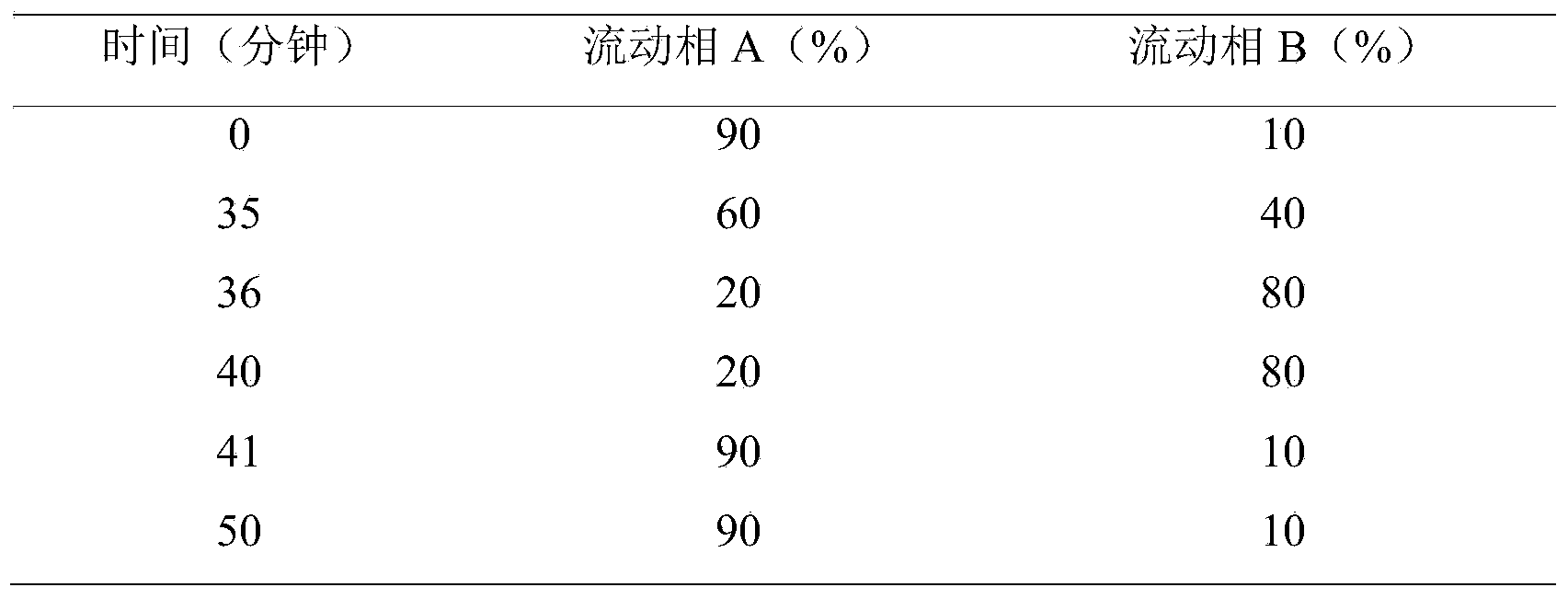
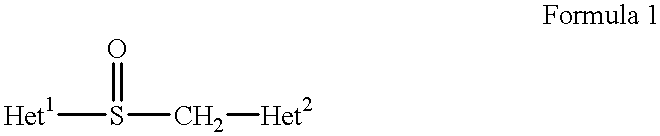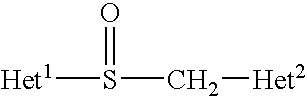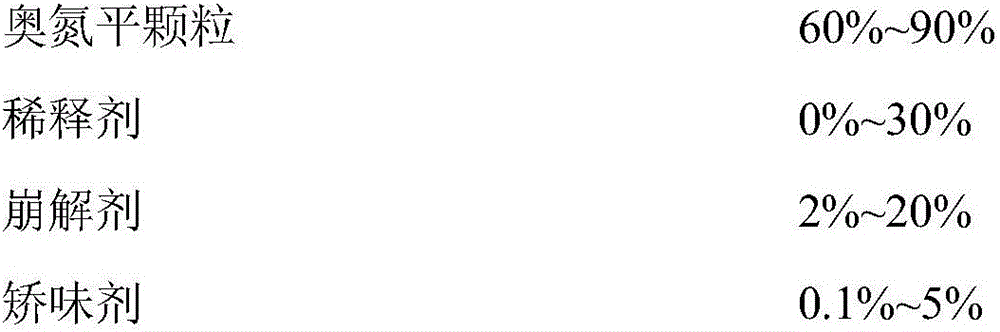Patents
Literature
167 results about "Crospovidones" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
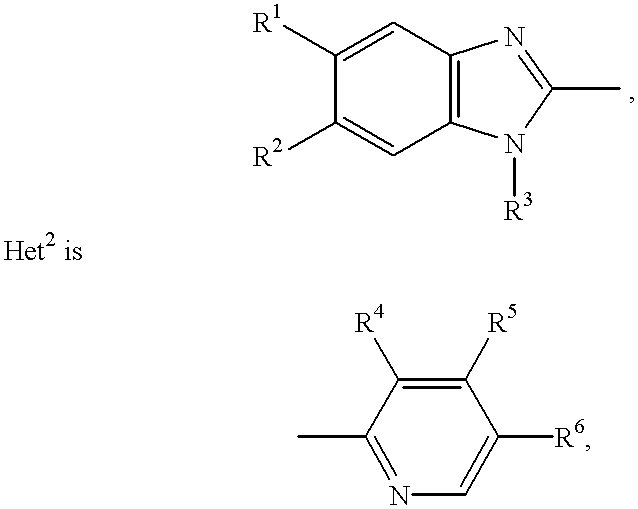
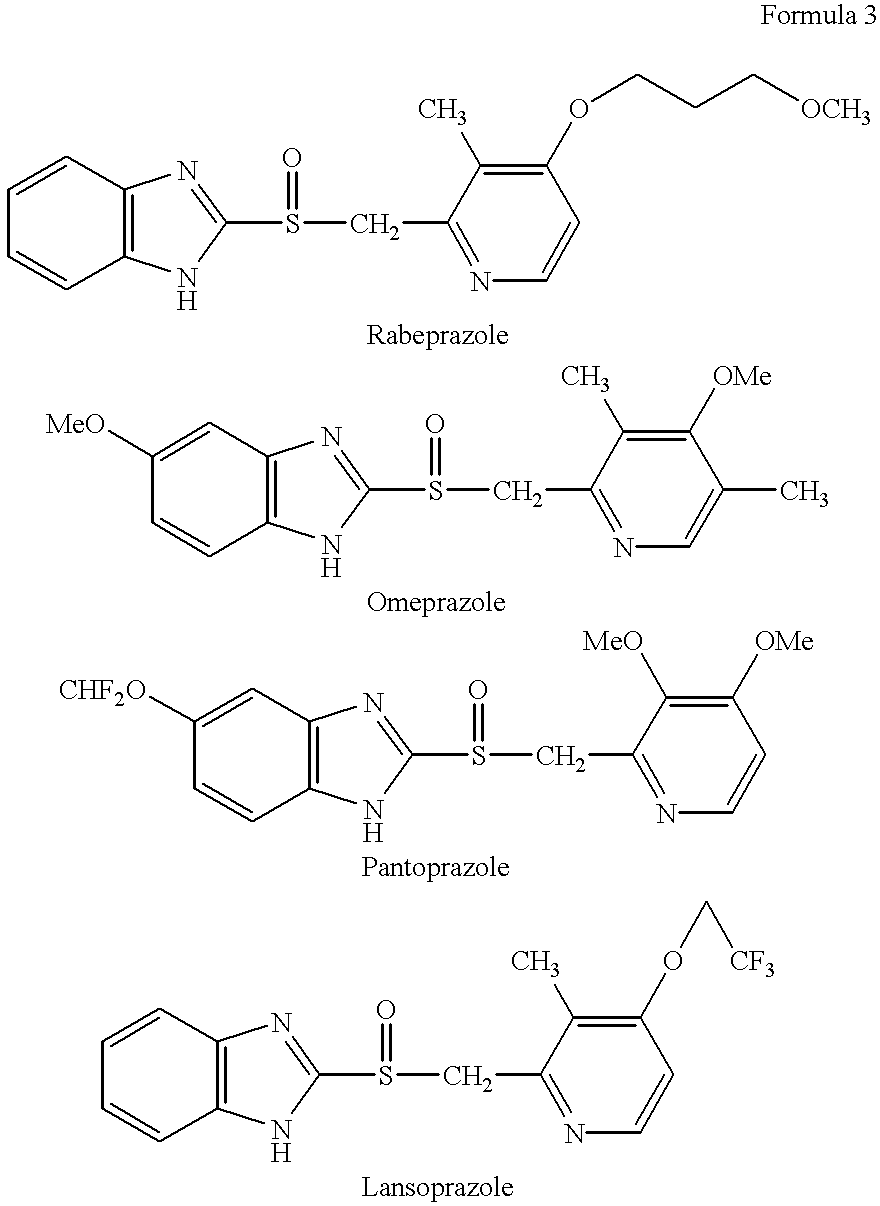
Stabilized compositions containing benzimidazole-type compounds
InactiveUS20020039597A1Improve stabilityLess color changeDigestive systemPill deliveryCrospovidonesMetallole
The present invention provides a chemically stable pharmaceutical preparation of a benzimidazole type compound. That is, the present invention relates to a composition comprising at least one substance selected from sodium carbonate, potassium carbonate, sodium hydroxide, potassium hydroxide, aminoalkyl methaacrylate copolymer E, arginine aspartate, hydroxypropyl cellulose and crospovidone incorporated into a benzimidazole type compound or an alkali metal salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Pharmaceutical compositions for the oral delivery of pharmacologically active agents
InactiveUS7049283B2Improve oral bioavailabilityBiocidePeptide/protein ingredientsCrospovidonesActive agent
Solid pharmaceutical compositions suitable for the oral delivery of pharmacologically active agents, e.g. peptides, comprising a therapeutically-effective amount of a pharmacologically active agent; a crospovidone or povidone; and a delivery agent for said pharmacologically active agent are disclosed. The compositions provide excellent oral bioavailability of pharmacologically active agents, particularly calcitonin.
Owner:NOVARTIS AG
Ondansetron orally disintegrating tablets
InactiveUS7390503B1Safe and effective absorptionImprove bioavailabilityPowder deliveryPill deliveryWater dispersibleOrally disintegrating tablet
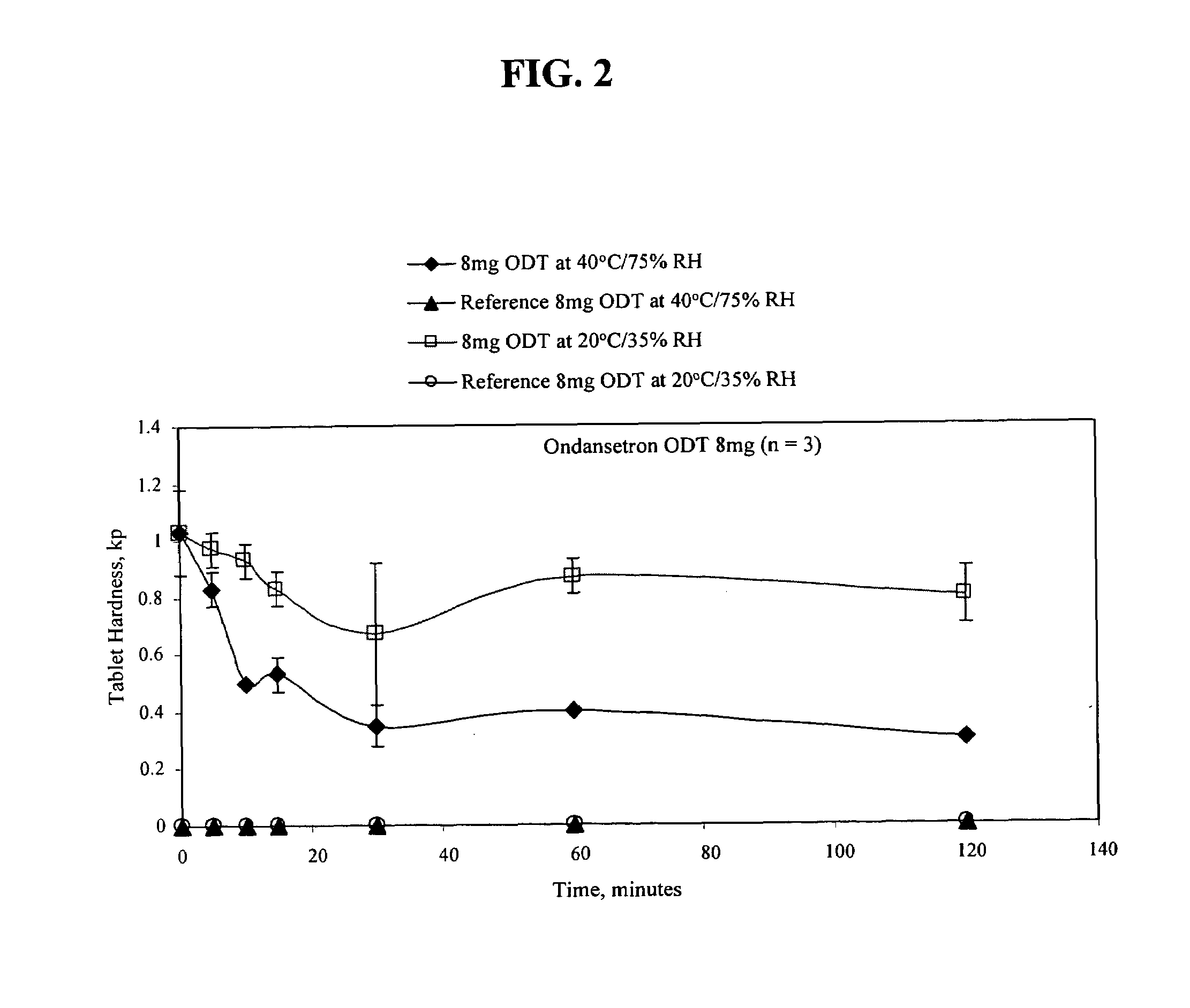
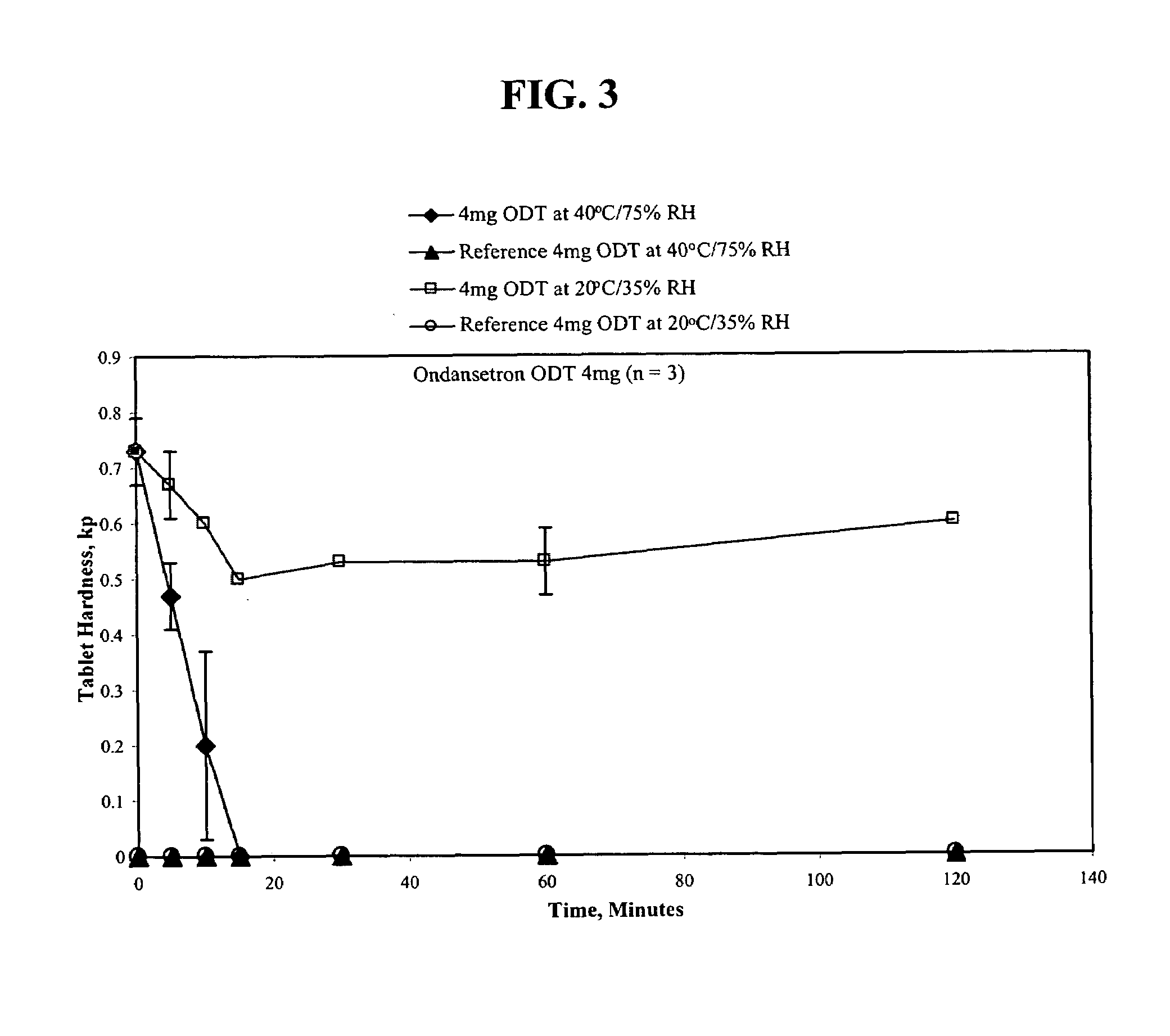
An ondansetron solid orally disintegrating dosage form for oral administration having at least one first water-dispersible component or water-insoluble cellulose derivative, a component having a —CHOH functional group, a disintegrating agent and at least one lubricant is provided. The dosage form can comprise ondansetron, a hydrophilic polymer such as microcrystalline cellulose, a component having a —CHOH functional group such as mannitol or xylitol and a disintegrating agent such as crospovidone. The lubricant may be a mixture of magnesium stearate, sodium stearyl fumarate and colloidal silicon dioxide. The present invention provides a non-effervescent tablet comprising the ondansetron dosage form. Another aspect of the invention is the treatment of emesis such as nausea and vomiting caused by cancer chemotherapy and radiation by the administration of the ondansetron formulation of the present composition. Finally, a process of forming an ondansetron disintegrating tablet using the ondansetron dosage form is disclosed.
Owner:BARR LAB
Stabilized composition comprising a benzimidazole type compound
The present invention provides a chemically stable pharmaceutical preparation of a benzimidazole type compound. That is, the present invention relates to a composition comprising at least one substance selected from sodium carbonate, potassium carbonate, sodium hydroxide, potassium hydroxide, aminoalkyl methaacrylate copolymer E, arginine aspartate, hydroxypropyl cellulose and crospovidone incorporated into a benzimidazole type compound or an alkali metal salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
ActiveCN102558154AImprove medication safetyImprove stabilityOrganic active ingredientsOrganic chemistryLansoprazoleCrospovidones
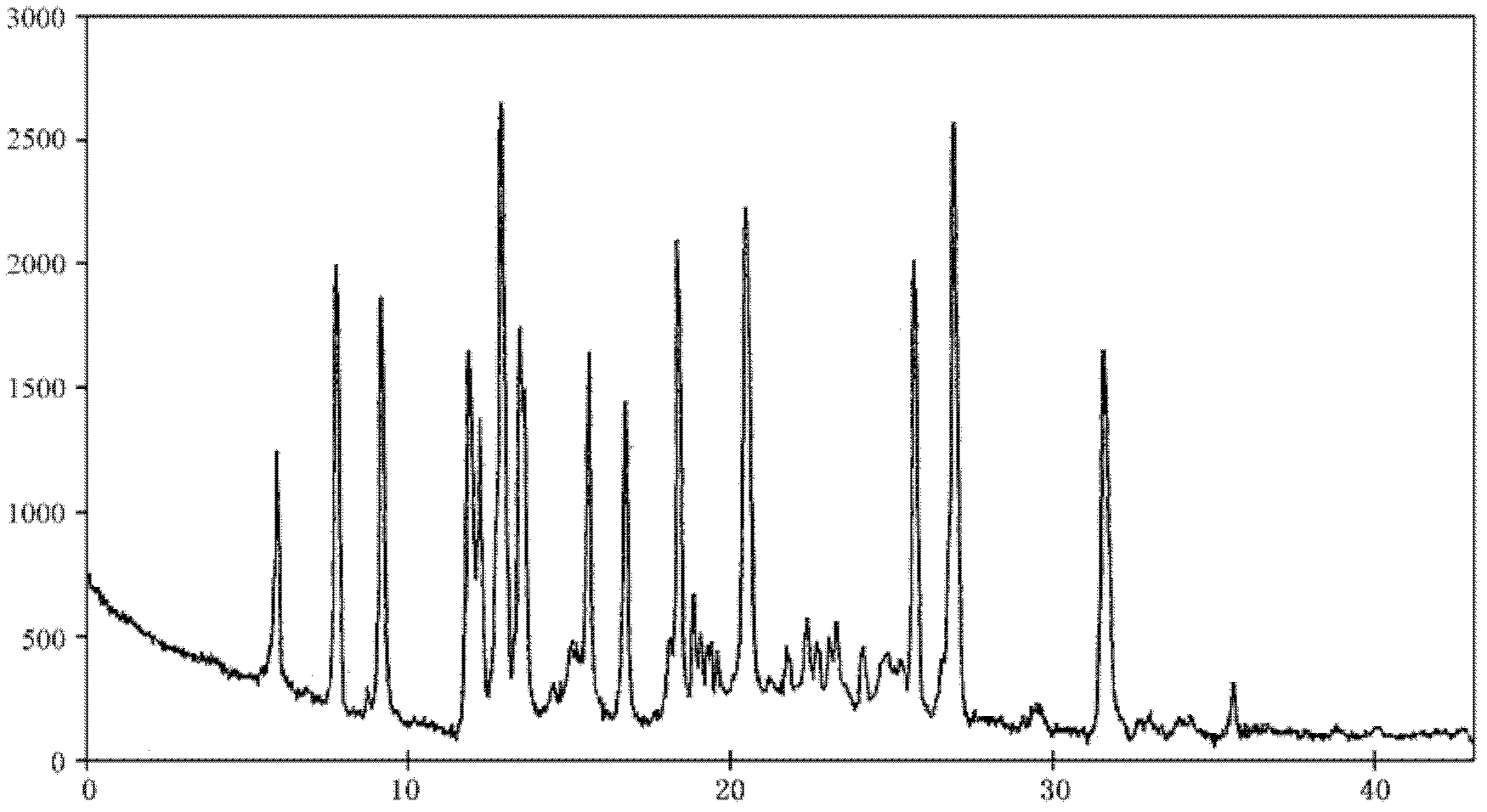
The invention relates to a lansoprazole crystalline compound. An X-ray powder diffraction pattern represented by a diffraction angle of 2 theta + / - 0.2 DEG displays feature diffraction peaks at the positions of 5.8 DEG, 7.5 DEG, 9.1 DEG, 11.8 DEG, 12.1 DEG, 12.8 DEG, 13.3 DEG, 15.6 DEG, 16.7 DEG, 18.3 DEG, 20.4 DEG, 25.7 DEG, 26.8 DEG and 31.5 DEG. The invention also relates to a lansoprazole enteric capsule containing the lansoprazole crystalline compound. The lansoprazole enteric capsule comprises 20 to 60 parts of l crystalline compound, 90 to 140 parts of microcrystalline cellulose, 1.5 to 3.5 parts of disodium hydrogen phosphate, 2 to 5 parts of anhydrous sodium sulphite, 1 to 10 parts of crospovidone, 0.8 to 4.2 parts of lauryl sodium sulfate, 2 to 8 parts of povidone K30 and 1 to 3 parts of magnesium stearate.
Owner:HAINAN JINRUI PHARMA CO LTD
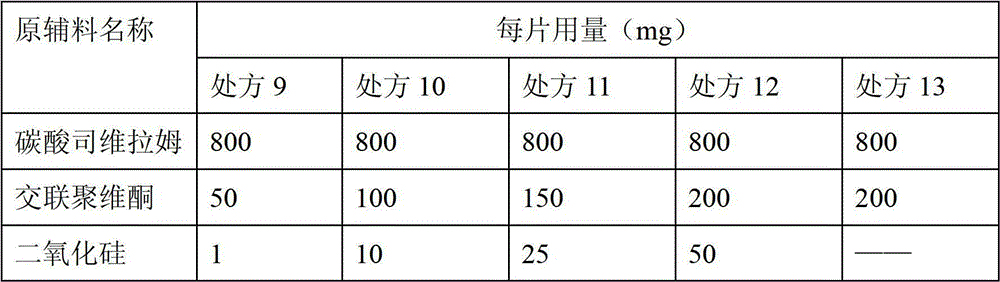
Sevelamer carbonate medical tablet composition and preparation method thereof
The invention discloses a sevelamer carbonate medical tablet composition and a preparation method thereof. The tablet composition comprises the following components in parts by weight: 60-95 parts of sevelamer carbonate, 5-30 parts of crospovidone and 0.1-10.0 parts of silicon dioxide. The preparation method of the tablet composition comprises the following steps: a. sevelamer carbonate is mixed with crospovidone; b. the mixture obtained in step a is pelletized; c. silicon dioxide is mixed with the particles produced in step b to form tablets; d. the tablets obtained is film-coated by water-soluble coating materials. The tablet composition provided by the invention is characterized in that the formability is good, the rigidity is high, the disintegration is quick, and the disintegration is less affected by the rigidity.
Owner:NANJING LIFENERGY R & D +1
Spirulina tablets and preparation method thereof
ActiveCN101999713AImprove metabolismHigh yieldFood shapingFood preparationHigh absorptionAcrylic resin
Owner:YUNNAN PHYTOPHARML
Inclusion method of medicament containing volatile component
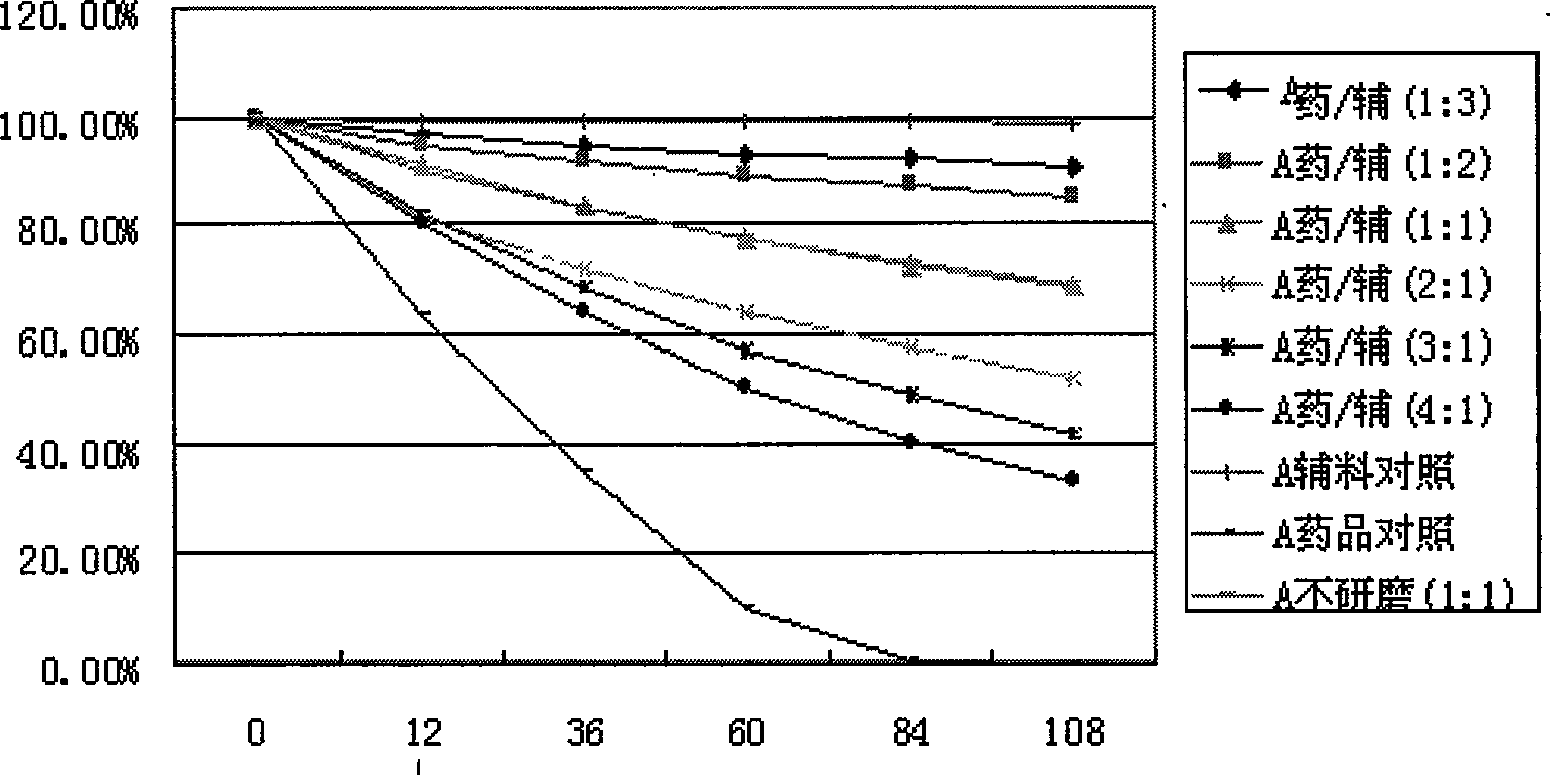
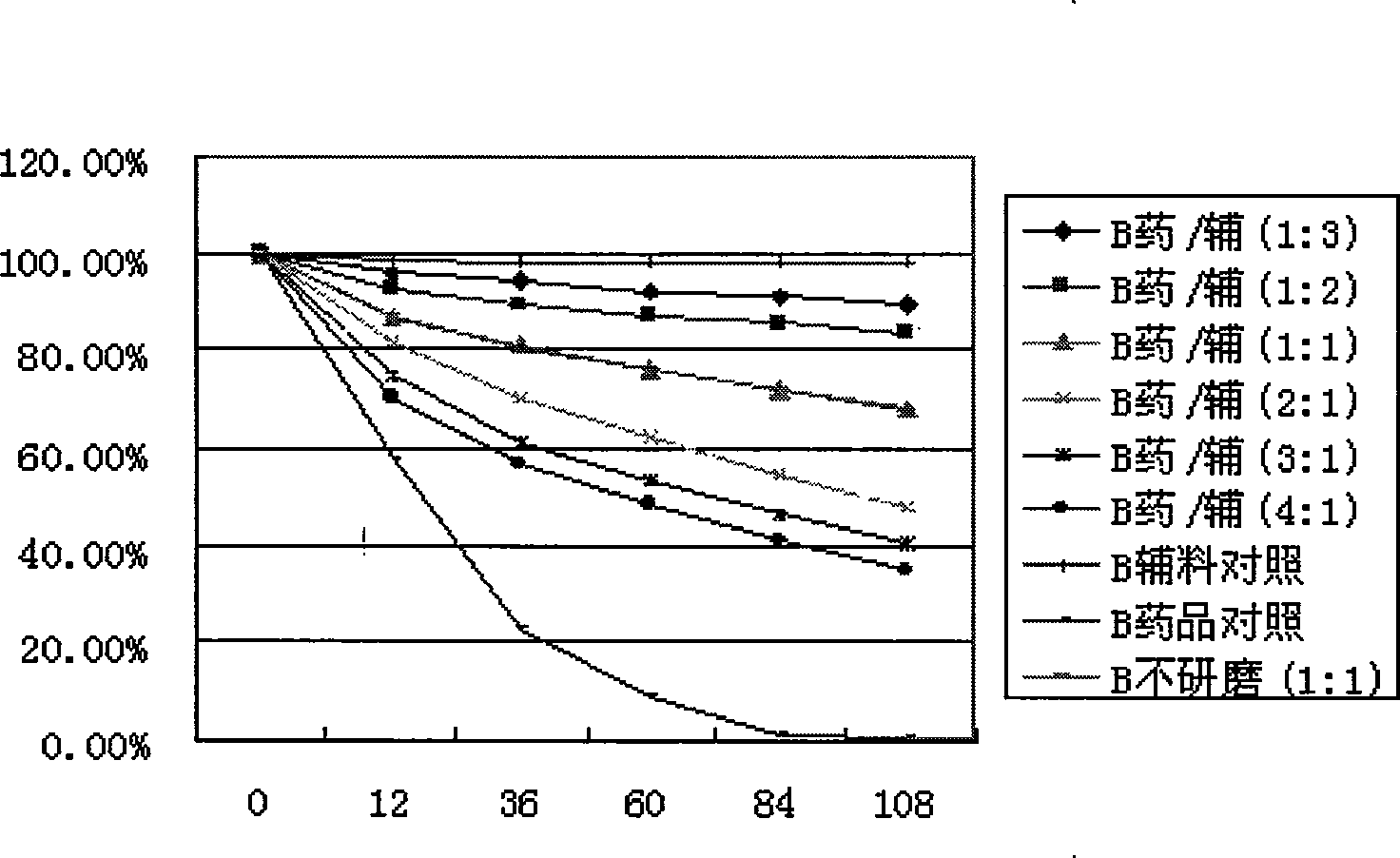
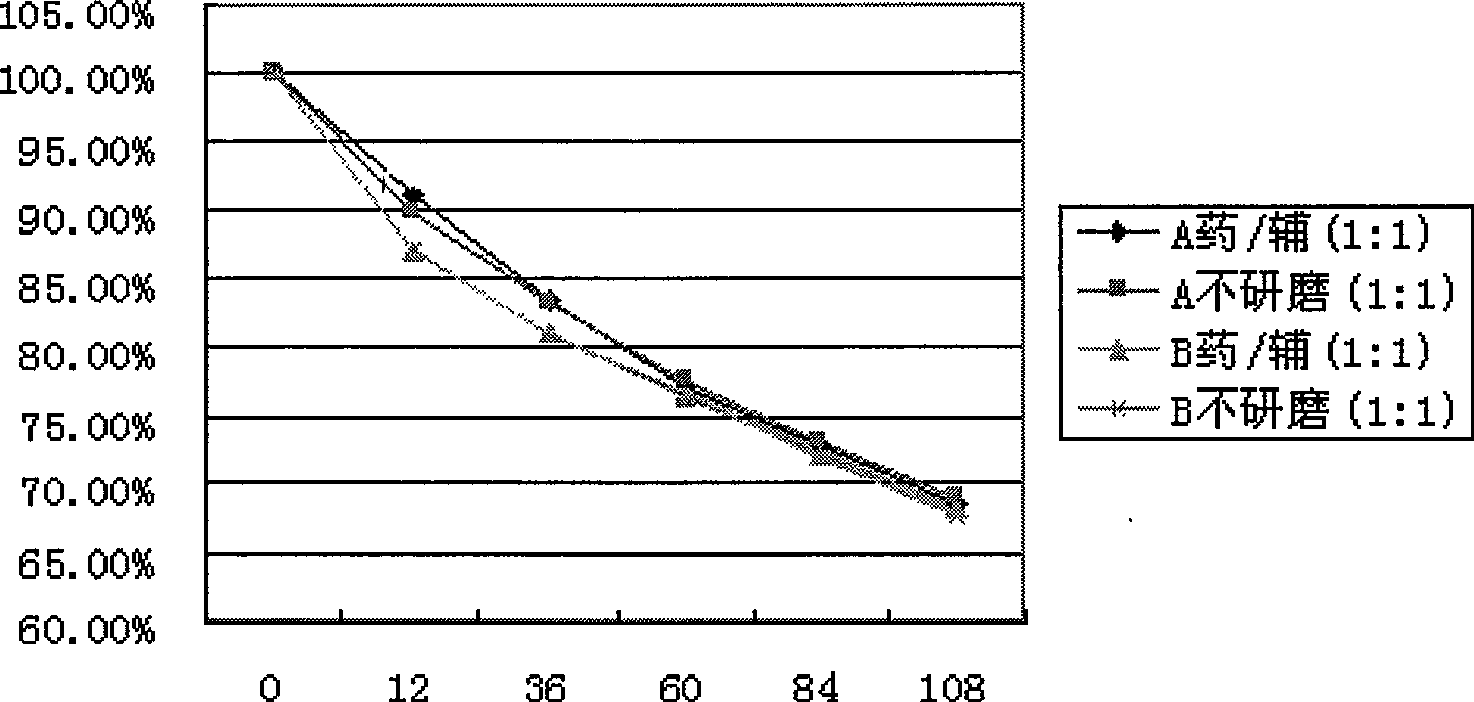
The invention discloses an inclusion method for enhancing the stability of drug containing volatile components. Crospovidone is added during the inclusion process of the drug containing the volatile component. The proportions of the drugs and the crospovidone are 4:1, 3:1, 2:1, 1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7 or 1:8. Materials can be ground during the inclusion process, and regular organic solvent such as ethanol, aether and the like can also be added. The proportions of the drug and the organic solvents are 2:1, 1:1 or 5:1, wherein the drugs mainly includes volatile traditional Chinese medicine in common use, such as borneol, menthol and the like. The invention, by experiments, proves that the crospovidone can reduce the volatile quantity of model drugs such as the borneol and the menthol, and the action can be promoted by certain mixing method and adding proper solvent.
Owner:PREMIER SPECIALTY CHEM
Valsartan amlodipine capsule and preparation method thereof
InactiveCN102028686ASimple production processFast absorptionPharmaceutical non-active ingredientsCapsule deliveryCrospovidonesValsartan
The invention relates to a capsule containing valsartan and amlodipine besylate, which is prepared from high-dose valsartan and amlodipine besylate, disintegrating agent crospovidone, filling agent microcrystalline cellulose and lubricating agent silicondioxide. The problem that the compound preparation contianing a high dose of valsartan and amlodipine besylate is not easy to disintegrate and dissolve is solved by adopting the conventional process and equipment of powder mixing and direct capsule filling without using special equipment and the bilayer tablet process.
Owner:林海平
Methods for stabilizing benzimidazole compounds
The present invention provides a method for stabilizing an oral solid formulation containing a benzimidazole-based compound or a physiologically acceptable salt thereof. That is, it provides a method for stabilizing a benzimidazole-based compound or a physiologically acceptable salt thereof, comprising incorporating 1) a crospovidone or a crospovidone and 2) sodium hydroxide and / or potassium hydroxide to a benzimidazole-based compound or a physiologically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Coated tablets
InactiveUS20060257494A1Improve bioavailabilityReduce the differencePowder deliveryBiocideCelluloseMicroparticle
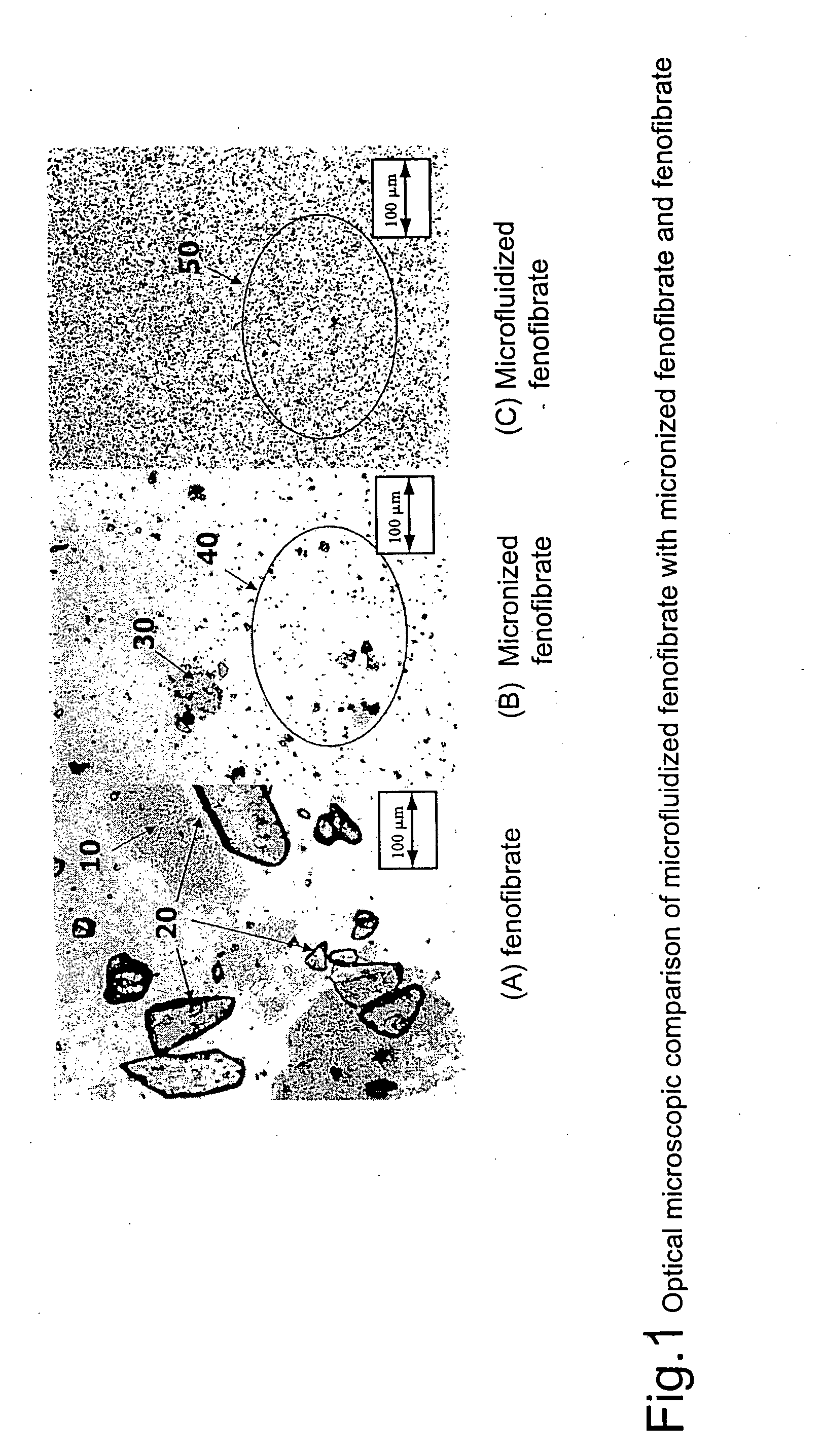
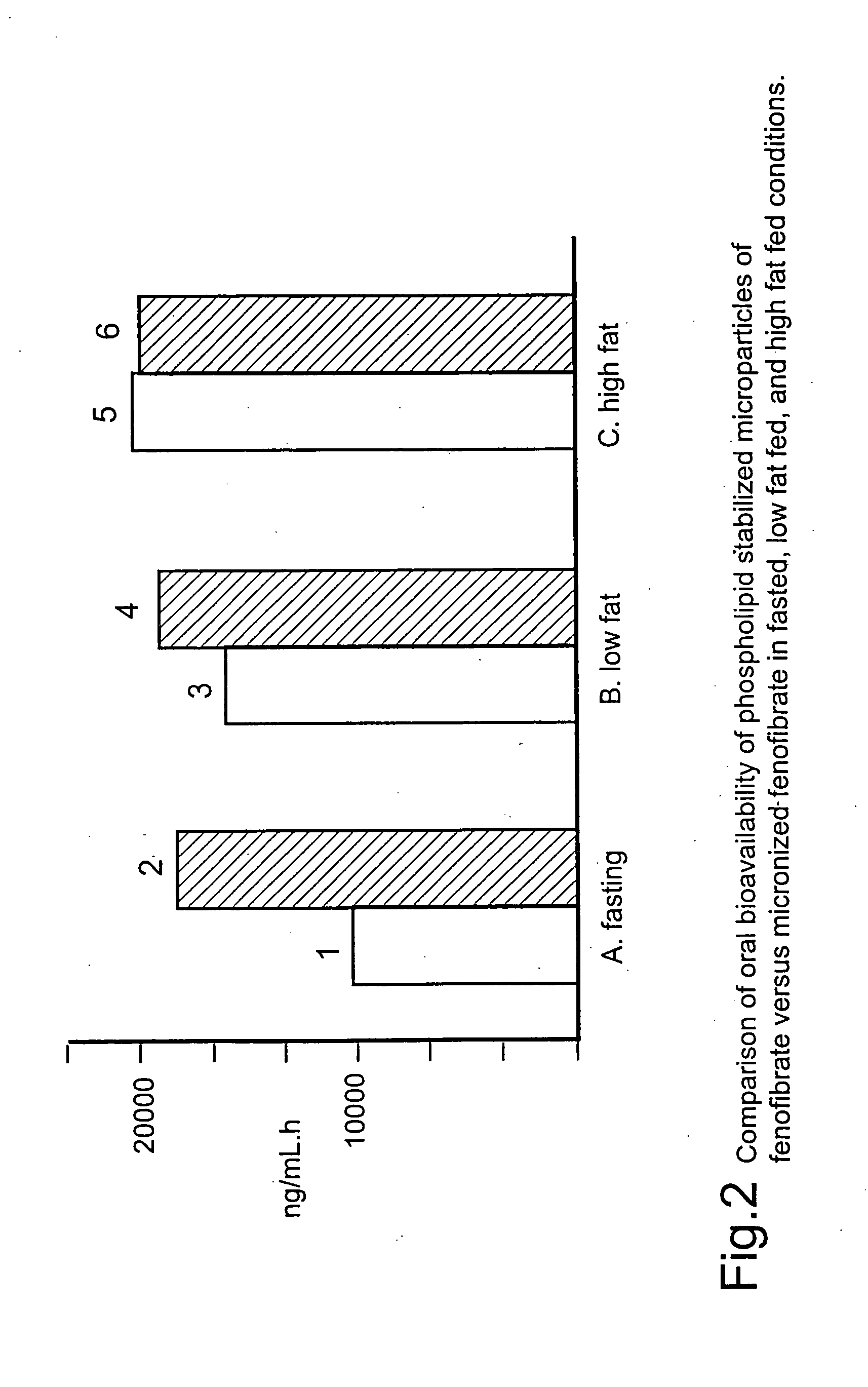
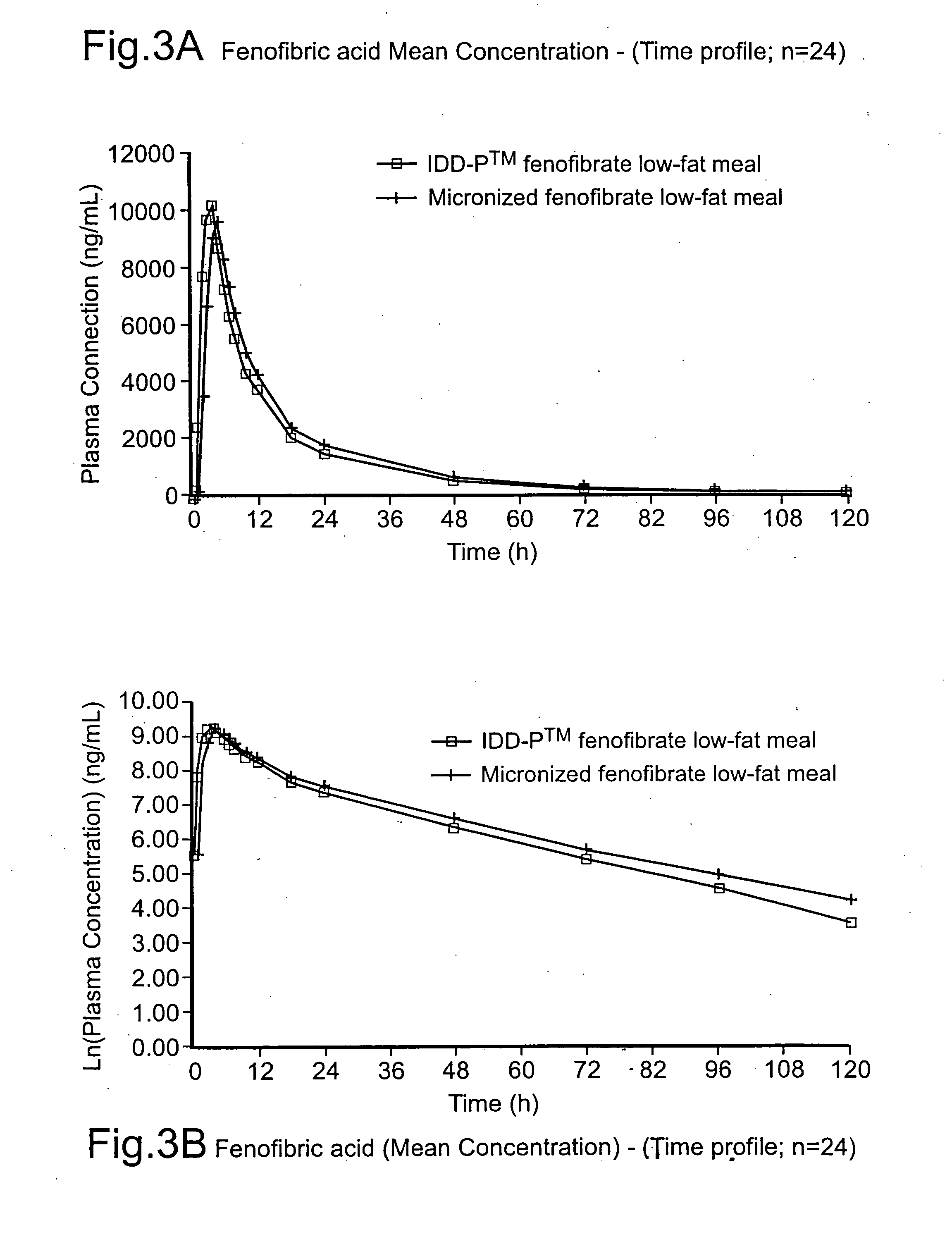
Disclosed is a pharmaceutically acceptable oral dosage form comprising fenofibrate, phospholipid, a buffer salt, a water-soluble bulking agent selected from maltodextrin, mannitol, and combinations thereof, a cellulosic additive, beads or crystals of a pharmaceutically acceptable water-soluble excipient support material, a polyvinylpyrrolidone or crospovidone, croscarmellose sodium, granular mannitol, sodium dodecyl sulfate, silicon dioxide, and a stearate, wherein the fenofibrate is in the form of microparticles, and wherein at least a portion of the phospholipid is coated on the surfaces of the fenofibrate microparticles, the phospholipid coated microparticles are embedded in a matrix comprising the water-soluble bulking agent, phospholipid that is not coated on the microparticles, the buffer salt and the cellulosic additive, and the matrix is coated on up to 100% of the surfaces of the beads or crystals of the excipient support material.
Owner:JAGOTEC AG
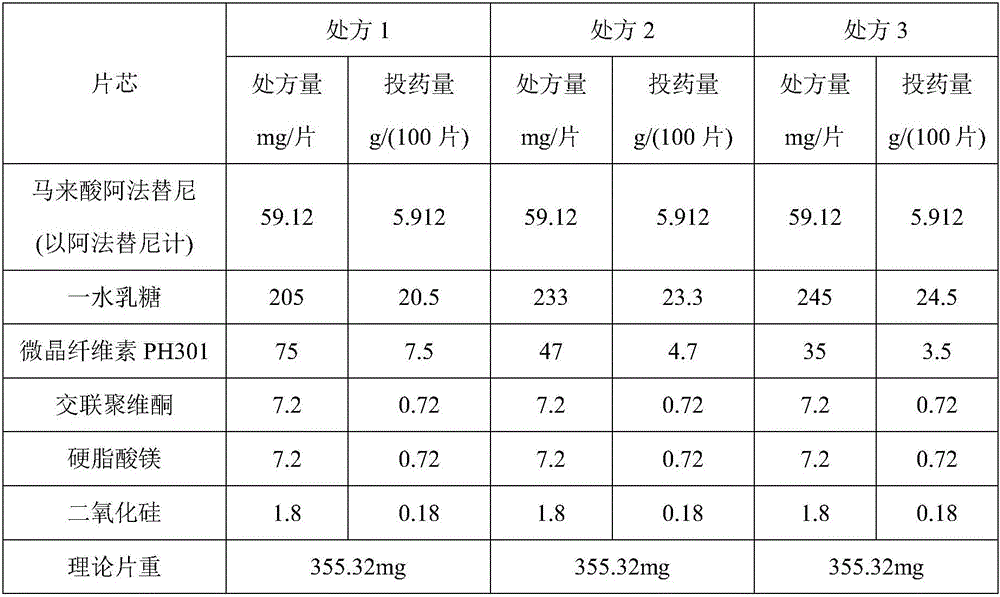
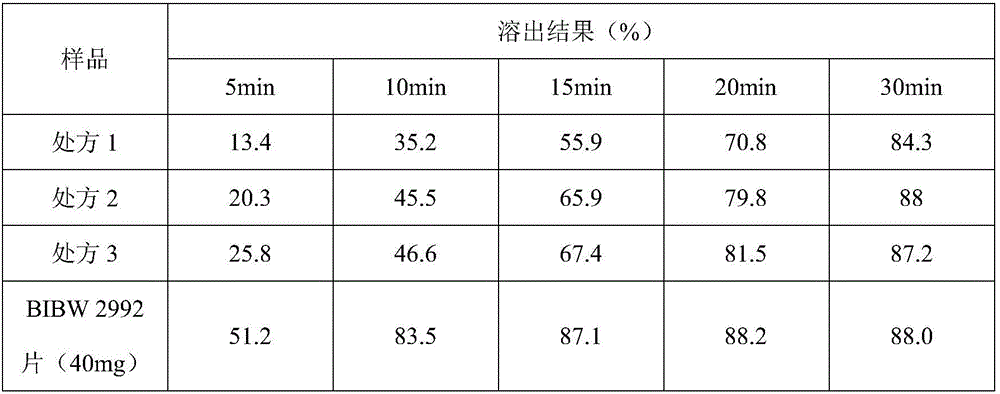
Afatinib dimaleate tablet and preparation method thereof
InactiveCN106074427AInhibition of segregationAvoid unevennessOrganic active ingredientsPharmaceutical non-active ingredientsCrospovidonesLACTOSE MONOHYDRATE
The invention provides an afatinib dimaleate tablet and a preparation method thereof. The afatinib dimaleate tablet is composed of a table core and a film coating wrapping the exterior of the table core and prepared by taking lactose monohydrate and microcrystalline cellulose PH301 as filling agents, taking crospovidone as a disintegrating agent, taking silicon dioxide as a flow aid and taking magnesium stearate as a lubricating agent. The preparation method has the advantages that the tablet is prepared by adopting a dry granulation technique, the fluidity can be improved, separation of all the components can be prevented to avoid the condition that the tablet components are not uniform, the bulk density is regulated, and the dissolving property and uniform pressure transmission in tablet production are improved.
Owner:NANJING CHENGONG PHARM CO LTD
Imatinib mesylate tablet and preparation method thereof
InactiveCN103222965APromote dissolutionDissolution completeOrganic active ingredientsPharmaceutical non-active ingredientsImatinib mesylateCrospovidones
The invention discloses an imatinib mesylate tablet and a preparation method thereof. The imatinib mesylate tablet comprises 8-30% of crospovidone and 8 to 40% of silica. The preparation method comprises the following steps of carrying out granulation of imatinib mesylate and a waterless organic solvent, drying the granules, uniformly mixing the granules, crospovidone, silica, a filler and a lubricant, and carrying out tabletting. The preparation method solves the problem of a slow dissolution rate of a preparation obtained by the prior art.
Owner:QINGDAO UNIV
Oral disintegrating tablet
InactiveUS20100098756A1Good effectAppropriate strengthBiocidePharmaceutical non-active ingredientsCrospovidonesD-mannitol
An oral disintegrating tablet containing (1) D-mannitol, (2) an active ingredient, (3) one or more disintegrating agents selected from the group consisting of crospovidone and carmellose, and (4) one or more lubricants selected from the group consisting of sodium stearyl fumarate and sucrose esters of fatty acids. The oral disintegrating tablet of the present invention has some excellent properties of (1) allowing easy production in a common facility without necessitating a specialized pharmaceutical technique, (2) having an appropriate strength that does not breakdown in the process of distribution, (3) having a fast disintegrating ability in the oral cavity, and (4) also having excellent ingestion feel such as greatly reduced bitterness or gritty feel; therefore, the tablet can be suitably used as a dosage form that is suitable for aged individuals, children, and seriously ill patients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Valsartan amlodipine tablet and preparation method thereof
ActiveCN108785267AImprove solubilityExtended stayPharmaceutical non-active ingredientsCoatingsSolubilityValsartan
The invention discloses a valsartan amlodipine tablet and a preparation method thereof. The valsartan amlodipine tablet comprises the following substances as raw materials in parts by weight: 80 partsof valsartan, 5 parts of amlodipine besylate (counted by amlodipine), 50-100 parts of microcrystalline cellulose PH102, 8-30 parts of crospovidone XL, 0.1-10 parts of poloxamer 188, 1-5 parts of magnesium stearate, 1- 3 parts of copovidone S630, 1-5 parts of colloidal silicon dioxide and 4-10 parts of a film coating premix (gastric). Through addition of the poloxamer 188 into a medicinal auxiliary material of the valsartan amlodipine tablet, the solubility of the valsartan is improved and the retention time thereof in the gastrointestinal tract is prolonged, so that absorption is improved, and thus the bioavailability of the valsartan amlodipine tablet is improved; through addition of the copovidone S630 into a coating solution, the solubility and the bioavailability of the valsartan canbe also improved, and thus the bioavailability of the valsartan amlodipine tablet is further improved. The preparation method of the valsartan amlodipine tablet is stable, and is suitable for large-scale preparation.
Owner:BEIJING BAIAO PHARMA
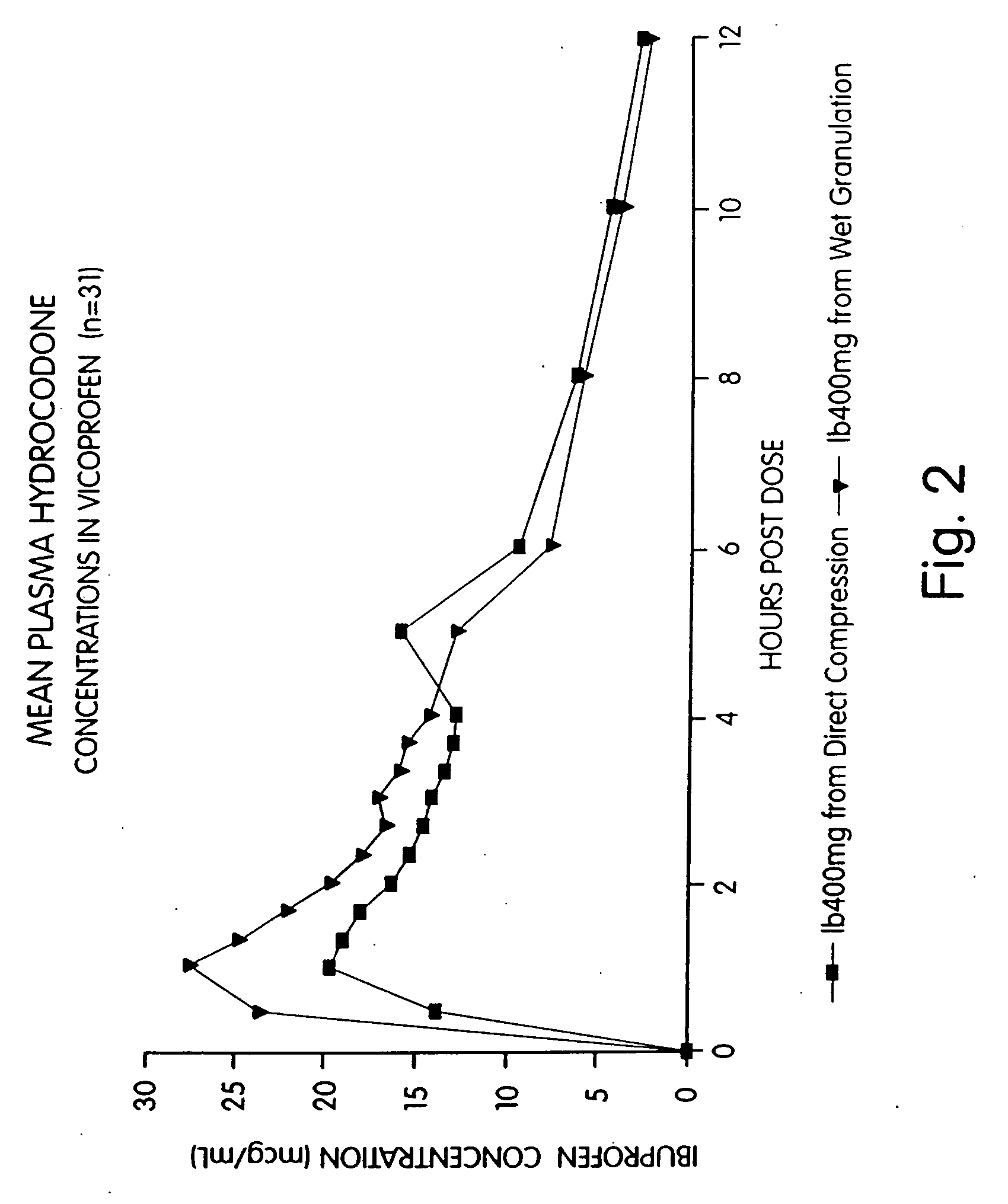
Ibuprofen and narcotic analgesic compositions
Provided herein are compositions and methods of making compositions of ibuprofen in combination with a narcotic analgesic. Specifically provided is a pharmaceutical tablet composition comprising ibuprofen; a narcotic analgesic; colloidal silicon dioxide; a filler selected from the group consisting of microcrystalline cellulose and powdered cellulose; a disintegrant selected from the group consisting of croscarmellose sodium, crospovidone, and sodium starch glycolate; a binder consisting of an akylhydroxy methylcellulose; a starch; and a lubricant. Also provided herein is a method of preparing a pharmaceutical tablet composition comprising: (a) Granulating ibuprofen, a narcotic analgesic, a first glidant, a first disintegrant, a binder, and starch to form granules wherein said granulating step comprises a wet granulation process; (b) blending the granules with extra-granular material comprised of a second glidant, a second disintegrant, a filler and starch to form a blend of granules and extra-granular material; and (c) compressing the blend into a tablet.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Anti-inflammatory tablet and preparation method thereof
ActiveCN105168624AThe active ingredients are fully extractedExtract completelyAntipyreticAnalgesicsCrospovidonesTreatment effect
The invention discloses an anti-inflammatory tablet. The anti-inflammatory tablet comprises dandelion, purpleflower violet, wild chrysanthemum flowers and baical skullcap roots. A preparation method includes grinding 1 / 2 of the baical skullcap roots into fine powder; grinding 1 / 3 of the dandelion and 1 / 3 of the wild chrysanthemum flowers into fine powder; adding compound enzyme into remaining 1 / 2 of the baical skullcap roots for enzymolysis and decocting to obtain clear paste A; subjecting remaining 2 / 3 of the dandelion to enzymolysis by compound enzyme and decocting to obtain extract B; obtaining volatile oil C from the purpleflower violet; decocting purpleflower violet decoction dregs with water to obtain filtrate D; decocting remaining 2 / 3 of the wild chrysanthemum flowers with water and blending the obtained wild chrysanthemum flower decoction with the filtrate D to obtain clear paste E; stirring the clear paste A, the extract B, the volatile oil C and the clear paste E uniformly, adding the fine powder of the baical skullcap roots, the dandelion and the wild chrysanthemum flowers for mixing uniformly, adding crospovidone and croscarmellose sodium for mixing uniformly and tabletting. The anti-inflammatory tablet is more complete in technological extraction, higher in content and stability and shorter in disintegration time, and adverse effect of disintegration slowness on treatment effect is avoided.
Owner:韩志强 +1
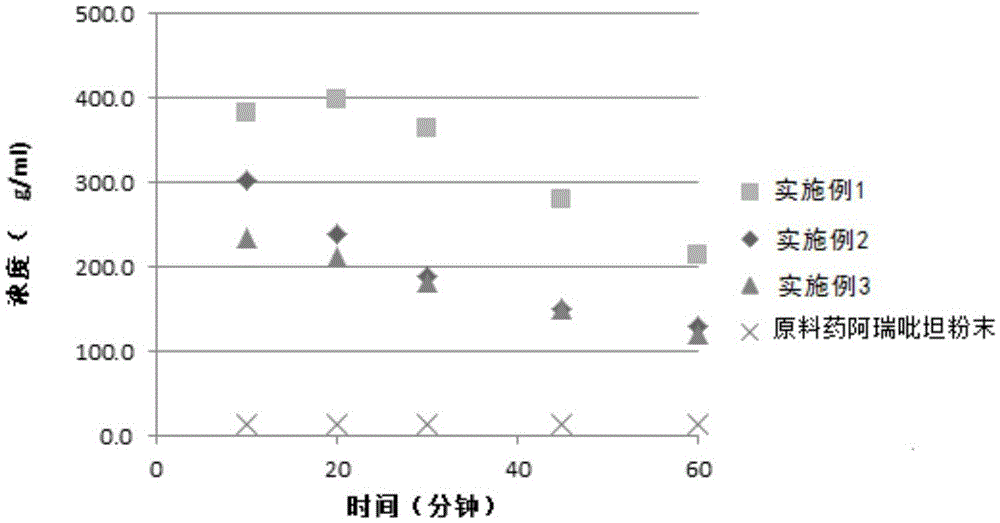
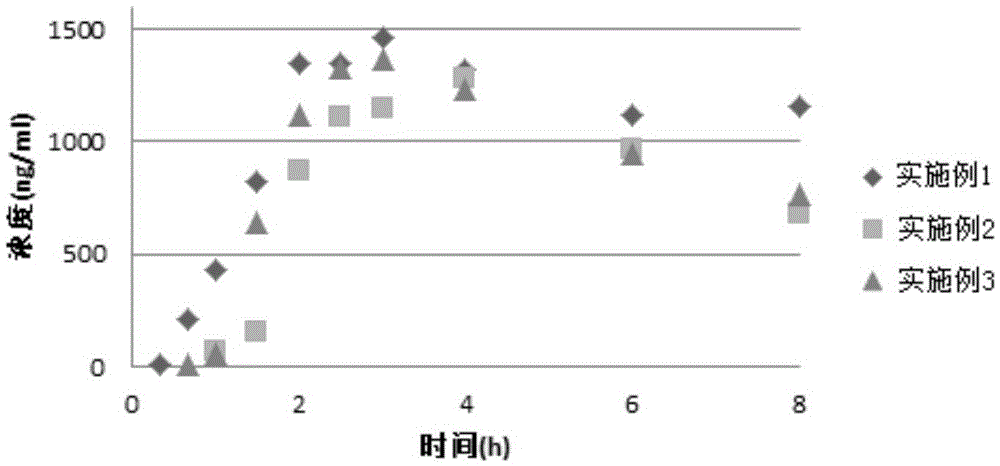
Aprepitant oral pharmaceutical preparation
InactiveCN105534987ADissolution rate is fastImprove bioavailabilityOrganic active ingredientsDigestive systemLow-substituted hydroxypropylcelluloseHypromellose phthalate
The invention discloses an aprepitant oral pharmaceutical preparation. The aprepitant oral pharmaceutical preparation comprises 15wt%-25wt% of aprepitant, 45wt%-75wt% of hydroxypropyl methylcellulose phthalate / hydroxypropyl methylcellulose acetate succinate, 10wt%-25wt% of microcrystalline cellulose, lactose or mannitol, 2wt%-8wt% of low-substituted hydroxypropyl cellulose as well as croscarmellose sodium and / or crospovidone, 0-2wt% of silicon dioxide and / or talc and 0-2wt% of magnesium stearate. The pharmaceutical preparation can be prepared in a form of tablets or capsules andhas high stability and good bioavailability.
Owner:北京颐诺赛医药科技有限公司
Orally dosed pharmaceutical compositions comprising a delivery agent in micronized form
ActiveUS20080234179A1Improve oral bioavailabilityPeptide/protein ingredientsSkeletal disorderCrospovidonesOral medication
Solid pharmaceutical compositions and methods of their use suitable for the oral delivery of pharmacologically active agents, e.g. peptides, comprising a therapeutically-effective amount of a pharmacologically active agent; a crospovidone or povidone; and a delivery agent for said pharmacologically active agent are disclosed. The compositions utilize micronized forms of the delivery agent which provides enhanced bioavailability of pharmacologically active agents, particularly calcitonin.
Owner:NOVO NORDISK AS
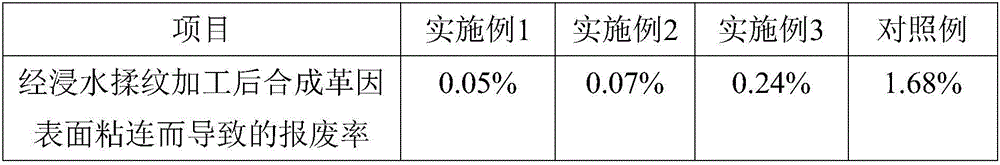
Waterborne anti-sticking agent applicable to synthetic leather
The invention discloses a waterborne anti-sticking agent applicable to synthetic leather and relates to the technical field of synthetic leather functional auxiliaries. The waterborne anti-sticking agent is prepared by, by weight, 15-20 parts of oxidized polyethylene wax-hydrolytic polymaleic anhydride grafted copolymer, 5-10 parts of water-soluble modified crospovidone, 3-5 parts of volcanic ash, 3-5 parts of croscarmellose, 2-3 parts of ultrafine ceramic powder, 2-3 parts of carnauba wax, 1-2 parts of asbestos powder, 0.5-1 part of polyaluminum chloride, 0.5-1 part of nano titanium dioxide, 0.2-0.3 part of molecular sieve raw powder, 0.05-0.1 part of yttrium oxide and 150-200 parts of water. The waterborne anti-sticking agent has the advantages that the waterborne anti-sticking agent is added into a soaking box for post-processing the synthetic leather, sticking of the synthetic leather during rolling drying and subsequent grain kneading after soaking is prevented, the surface quality of the synthetic leather after the grain kneading is increased, and surface damage of the synthetic leather is avoided.
Owner:南平慧薇网知识产权营运有限公司
Rebeprazole sodium enteric-coated tablet and preparation process thereof
InactiveCN105640915AImprove acid resistanceImprove bioavailabilityOrganic active ingredientsDigestive systemFiller ExcipientHypromellose phthalate
A rebeprazole sodium enteric-coated tablet comprises, by weight, 65-125 parts of tablet core, 10-40 parts of isolation layer and 80-170 parts of enteric-coated layer, wherein filler is one or more of microcrystalline cellulose, lactose and mannitol, stabilizer is one or more of magnesium oxide and anhydrous sodium carbonate, disintegrating agent is one or more of polyvinyl pyrrolidone, cross-linking sodium carboxymethyl cellulose and lauryl sodium sulfate, lubricating agent is one or two of talcum powder and magnesium stearate, binder is the anhydrous ethanol solution of crospovidone or the aqueous solution of hydroxypropyl methylcellulose, the isolation layer is one or two of magnesium oxide and ethyl cellulose, and the enteric-coated layer is one or more of hydroxypropyl methylcellulose phthalic acid ester, enteric-coated acrylic resin, cellulose acetate phthalic ester, talcum powder and 93F19255 type coating agent. A preparation process of the rebeprazole sodium enteric-coated tablet is simple, low in equipment requirement, high in yield, and controllable in product quality. The dissolution rate of the rebeprazole sodium enteric-coated tablet is highly consistent with the release behavior of originally-developed drugs, and the rebeprazole sodium enteric-coated tablet is good in stability.
Owner:吉林修正药业新药开发有限公司
Prucalopride succinate tablet composition
ActiveCN104069080AReasonable prescriptionNo Maillard reactionOrganic active ingredientsDigestive systemCrospovidonesMaillard reaction
The invention provides a prucalopride succinate tablet composition and a preparation method thereof. The prucalopride succinate tablet contains prucalopride succinate, mannitol, crospovidone and the like. The prucalopride succinate tablet has the advantages that a Maillard reaction is not generated; the tablet can be prepared via a wet granulation method; the tablet is high in stability and the like.
Owner:HEBEI RENHE YIKANG PHARMA
Ramelteon sublingual tablet and preparation method thereof
InactiveCN110433142AGreat tasteGrind evenlyOrganic active ingredientsNervous disorderCross-linkSucrose
The invention belongs to the field of pharmaceutical preparations, and relates to ramelteon sublingual tablets and a preparation method thereof. The sublingual tablet contains effective amounts of ramelteon, a filler, a disintegrant, a lubricant, and a flavoring agent, and the proportion of a main drug is 0.1 to 0.5%; the filler is selected from one or a combination of mannitol, lactose, sucrose and xylitol; the disintegrant is selected from one or the combination of crospovidone, cross linked sodium carboxymethyl cellulose, and low-substituted hydroxypropyl cellulose; and the lubricant is selected from one or a combination of magnesium stearate, aerosil, and sodium stearyl fumarate; and the flavoring agent is mint flavor. The invention also provides the preparation method of the ramelteonsublingual tablets, that is, ramelteon and the filler are ground and mixed by a ball mill, and mixed with the disintegrating agent, the lubricant, and the flavoring agent, and the materials are pressed to prepare tablets. The ramelteon sublingual tablet of the invention can avoid the first pass effect of the liver and improve the bioavailability.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Solid oral dosage forms of lamivudine
The present invention relates to the oral solid pharmaceutical composition comprising lamivudine or a pharmaceutically acceptable salt thereof with isomalt as a filler. The present invention also relate to the combination of lamivudine and other Anti-HIV agents. Thus, for example, the present invention provides a stable tablet formulation comprising lamivudine, isomalt, crospovidone, calcium stearate and opadry white.
Owner:HETERO RES FOUND
Olanzapine orally disintegrating tablets and preparation method thereof
ActiveCN106265559ARapid disintegration in vitroImprove stabilityOrganic active ingredientsNervous disorderCrospovidonesPolyvinyl acetate
The invention relates to olanzapine orally disintegrating tablets and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The novel pharmaceutical composition is prepared from olanzapine granules and an additive, wherein the olanzapine granules are prepared from olanzapine, mannitol, crospovidone and a water disperse system of polyvinyl acetate through granulation. The invention further relates to the olanzapine granules and the preparation method of the olanzapine orally disintegrating tablets.
Owner:JIANGSU HANSOH PHARMA CO LTD
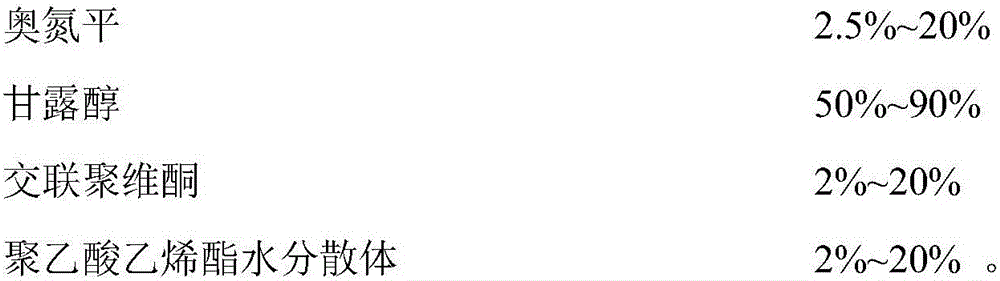
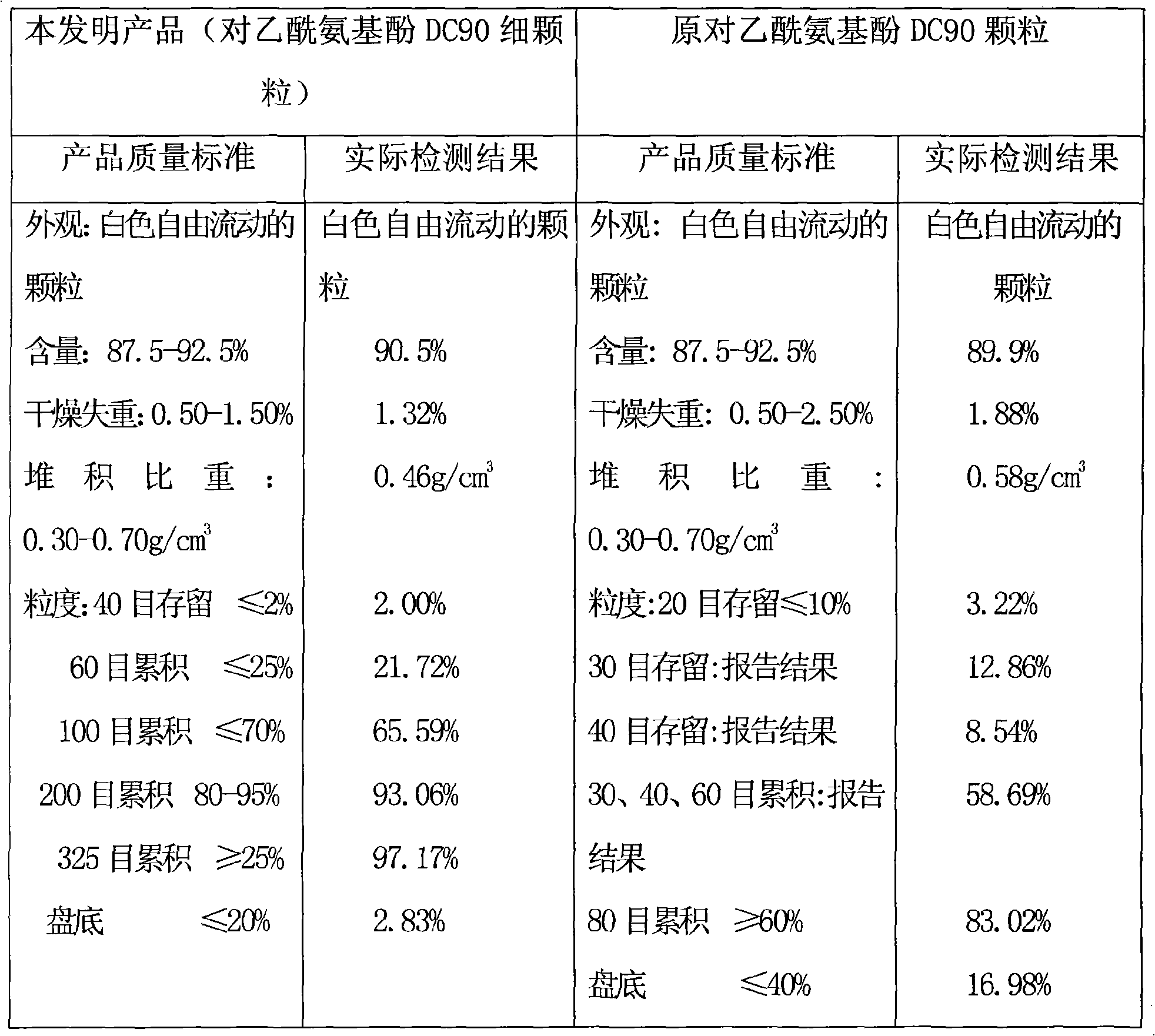
Acetaminopher DC90 fine particle and preparation method thereof
ActiveCN101342144AFine and uniform particlesWell mixedOrganic active ingredientsPowder deliveryCrospovidonesPrill
The invention discloses paracetanol DC90 fine particles and a preparation method thereof. The paracetanol DC90 comprises following materials by weight ratio: 90 percent of paracetanol, 3.50 percent of cornstarch, 3.50 percent of pre gelatinized starch, 2.00 percent of povidone, 0.5 percent of crospovidone and 0.5 percent of stearic acid. Firstly, the paracetanol and the cornstarch are mixed evenly, then the pre gelatinized starch and the povidone are made into starch slurry to be used as bond which is evenly sprayed on the mixture of the fluidied paracetanol and cornstarch, fluidized bed granulation is carried out in the inlet air temperature, the wet particles are dried under the temperature of 80 DEG C and then are cooled at the temperature of 50 DEG C till water conforms to the requirement, the sizes of the particles are adjusted, the stearic acid and the crospovidone are added after the particle sizes are adjusted to be mixed evenly to obtain the paracetanol DC90 fine particles. The invention adopts the above technical proposal to inspect the product, and the particles are fine and uniform and have favorable fluidity, thereby being well and evenly mixed with other small quantities of active components powder.
Owner:ANQIU LUAN PHARMA
Infantile domperidone orally disintegrating tablet and preparation method thereof
ActiveCN105560199AGreat tasteAddressing AdherenceOrganic active ingredientsDigestive systemCrospovidonesOrally disintegrating tablet
The invention discloses an infantile domperidone orally disintegrating tablet and a preparation method thereof. The orally disintegrating tablet is prepared from the following raw materials in parts by mass: 10 to 30 parts of domperidone, 10 to 30 parts of fatty glyceride, 5 to 10 parts of tween 80, 900 to 2700 parts of white sugar, 2 to 5 parts of crospovidone, 10 to 30 parts of mannitol, 0.01 to 0.05 part of essence, 0.2 to 0.5 part of magnesium stearate and 0.2 to 0.5 part of aerosil. According to the infantile domperidone orally disintegrating tablet and the preparation method thereof, an orally disintegrating tablet medicament is prepared by using the domperidone as an effective component of the medicament and through an innovative process. By using the domperidone orally disintegrating tablet prepared by the invention, the problems that the dissolution rate of the medicament is low, the medicament has a bitter taste, and the like, are solved successfully; the infantile domperidone orally disintegrating tablet has a favorable effect on infantile functional dyspepsia.
Owner:SHANDONG SBOND PHARMA +1
Synthesis method of crospovidone
The invention relates to a synthesis method and especially relates to a synthesis method of crospovidone and belongs to the field of medicinal auxiliary materials. The synthesis method includes the steps of: dissolving anhydrous sodium sulfate, anhydrous disodium hydrogen phosphate and polyvinylpyrrolidone K30 in purified water in a water-bath heating manner; adding N-vinyl pyrrolidone and filling the reaction system with nitrogen gas; and when the temperature of the solution is increased to 50-80 DEG C, adding a hydrogen peroxide solution and divinylbenzene to perform a polymerization reaction for 3-9.5 h. The synthesis method employs safe and cheap hydrogen peroxide as a catalyst, wherein the polymerization is initiated by means of free radicals generated during a redox reduction of the hydrogen peroxide which is then decomposed into water and oxygen, so that the method is safe and toxic-free.
Owner:贵州省欣紫鸿药用辅料有限公司
High-strength composite packaging material and preparation method thereof
The invention discloses a high-strength composite packaging material and a preparation method thereof. The high-strength composite packaging material comprises the following raw materials in parts by weight: 20 to 45 parts of chlorinated butyl rubber, 15 to 30 parts of polybutadiene, 10 to 20 parts of poly(butylene succinate), 3 to 7 parts of polycaprolactone, 14 to 20 parts of nano-carbon fibres, 3 to 6 parts of trimethylolpropane, 1 to 3 parts of silicone oil, 5 to 8 parts of nano-graphite powder, 2 to 8 parts of zinc oxide, 12 to 20 parts of polyvinyl alcohol, 7 to 15 parts of polyvinylidene chloride, 3 to 12 parts of acrylic acid, 3 to 6 parts of magnesium hydrate, 4 to 12 parts of succinimide, 3 to 8 parts of propylene glycol monomethyl ether, 3 to 7 parts of sodium melamine phosphate, 3 to 6 parts of a wear-resistant agent, 1 to 5 parts of crospovidone and 3 to 8 parts of tetramethoxysilane. Compared with the prior art, the packaging material disclosed by the invention has the advantages of high strength, long service life, simple preparation method and the like, so that requirements on industrial production can be met better.
Owner:桐城市人和包装有限公司
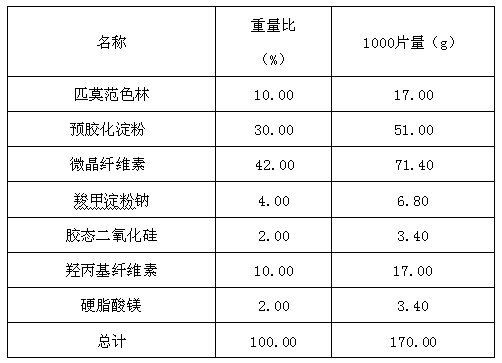
Pimavanserin tablet and preparation method thereof
InactiveCN109568278ADissolution stabilityReduce static electricityOrganic active ingredientsNervous disorderLow-substituted hydroxypropylcellulosePolyethylene glycol
The invention belongs to the field of pharmaceutic preparation, and particularly relates to a pimavanserin tablet and a preparation method thereof. The pimavanserin tablet is composed of active components of pimavanserin, colloidal silicon dioxide, polyethylene glycol, a filling agent selected from dextrin, corn starch, pregelatinized starch, cellulose microciystalline, mannitol and lactose, a disintegrating agent selected from carboxymethyl starch sodium, croscarmellose sodium, low-substituted hydroxypropyl cellulose and crospovidone, and a lubricating agent selected from magnesium stearate,talcum powder, and sodium stearyl fumarate. The pimavanserin tablet has good stability, and can significantly improve drug dissolution and bioavailability.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com