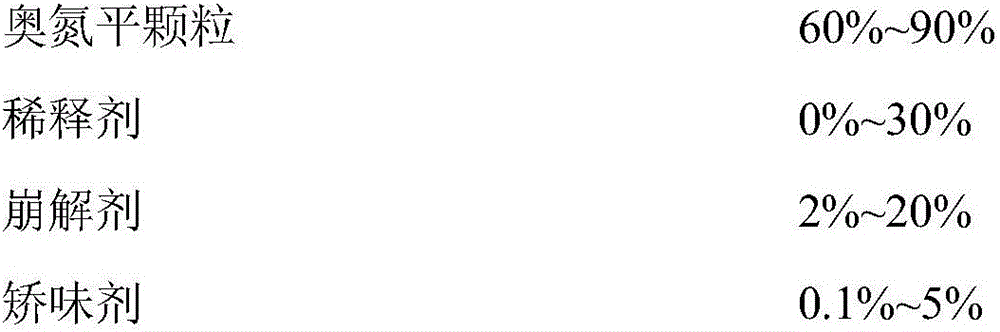Olanzapine orally disintegrating tablets and preparation method thereof
A technology of oral disintegrating tablets and olanzapine, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effect of good tablet formability, good friability and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
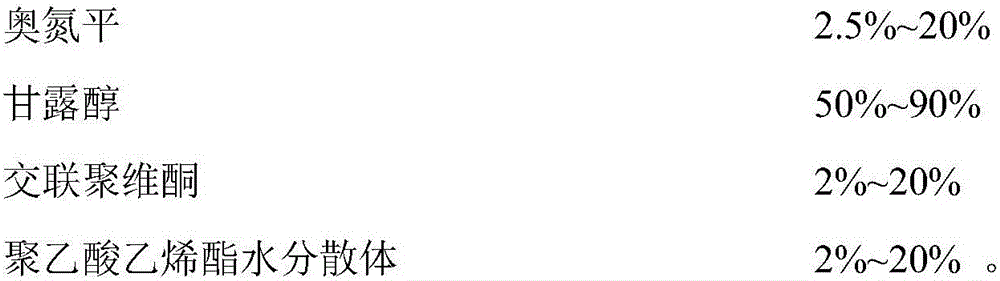
[0053]
[0054]Preparation: Dissolve polyvinyl acetate and povidone K30 in 15ml of purified water and stir for 5 minutes; add olanzapine and crospovidone to the above solution and stir evenly; put mannitol into a pellet coating machine, and add the above-mentioned Spray the drug solution onto the surface of mannitol, atomize and dry to obtain olanzapine granules; centrally control the content of olanzapine granules; pass low-substituted hydroxypropyl cellulose LH-11 through a 30-40 mesh sieve, weigh and add olanzapine Mix in the granules; add the prescribed amount of magnesium stearate to the above granules and mix evenly; compress orally disintegrating tablets of olanzapine with different specifications according to the content of olanzapine in the middle control.
[0055] Obtain the orally disintegrating tablet that is especially suitable for medicinal olanzapine with the method that following composition weight is substantially the same as embodiment 1:
Embodiment 2
[0057]
[0058] Preparation: Dissolve polyvinyl acetate and povidone K30 in 20ml of purified water and stir for 5 minutes; add olanzapine and crospovidone to the above solution and stir evenly; put mannitol into a pellet coating machine, and mix the above-mentioned Spray the drug solution onto the surface of mannitol, atomize and dry to obtain olanzapine granules; centrally control the content of olanzapine granules; pass low-substituted hydroxypropyl cellulose LH-11 through a 30-40 mesh sieve, weigh and add olanzapine granules Mix in the granules of Olanzapine; Add the prescribed amount of magnesium stearate to the granules and mix evenly; According to the controlled content of Olanzapine, compress Olanzapine orally disintegrating tablets of different specifications.
Embodiment 3
[0060]
[0061] Preparation: dissolve polyvinyl acetate and povidone K90 in 7ml of purified water and stir for 5 minutes; add olanzapine and crospovidone to the above solution and stir evenly; put mannitol into a pellet coating machine, and mix the above-mentioned The drug solution is sprayed onto the surface of mannitol, atomized and dried to obtain olanzapine granules; the content of olanzapine granules is controlled in the middle; aspartame is passed through a sieve of 80-100 mesh, and croscarmellose sodium is passed through a 30-mesh sieve 40-mesh sieve, weighed and added to the olanzapine granules and mixed evenly; added the prescribed amount of sodium stearate fumarate to the above-mentioned granules and mixed evenly; according to the content of olanzapine in the middle control, pressed different specifications of olanzapine Orally disintegrating tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com