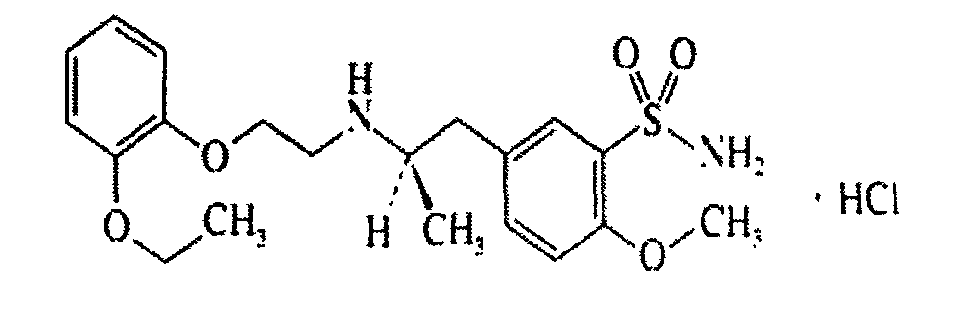
Orally disintegrating sustained-release preparation containing tamsulosin hydrochloride and preparation method thereof
A tamsulosin hydrochloride, slow-release preparation technology, applied in the field of medicine, can solve the problem of uneven content, achieve the effects of short coating time, ensure uniform content, and good slow-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
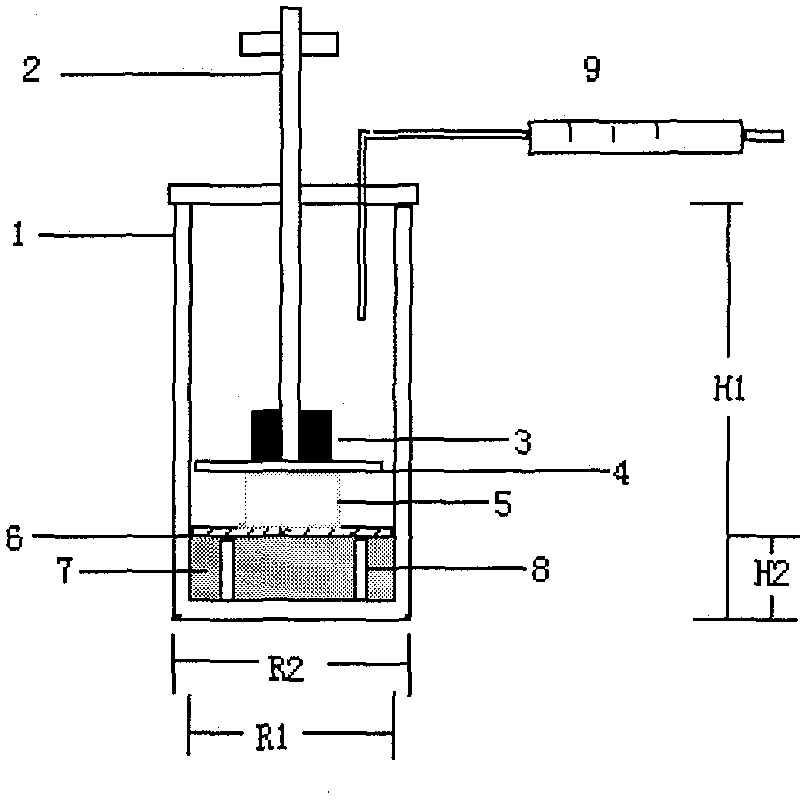
Image
Examples
Embodiment 1
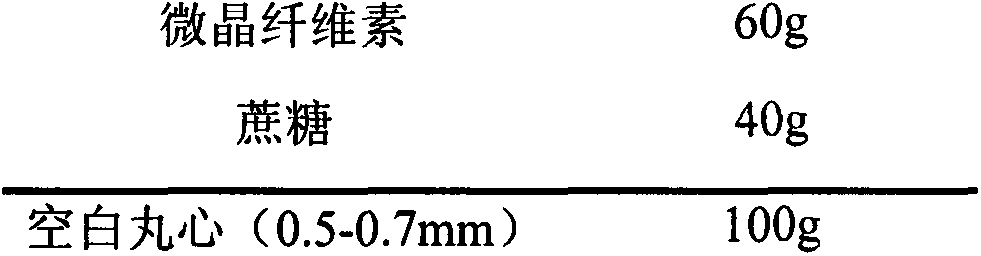
[0023] prescription
[0024]
[0025] The preparation process takes the prescription amount of microcrystalline cellulose and sucrose, mixes them evenly, adds an appropriate amount of water to make a soft material, extrudes a spheronizer to prepare pellets, and passes through a 20-mesh and 30-mesh sieve after drying to obtain a blank core (0.5-0.7mm);
[0026] Coating prescription
[0027]
[0028]
[0029] The preparation process configures the coating liquid according to the prescription, weighs 100g of the blank core, puts it into a multifunctional fluidized bed, coats 10g with a 5% drug-containing layer (main content is 0.2g / 100g), and dries for 5min with 12.5% uterine Odd NE30D coating 25g, get sustained-release pills with medicine:
[0030] Oral collapse prescription
[0031]
[0032] Preparation process: Weigh the prescribed amount of medicated pellets, mannitol, lactose hydrate, and powdered sugar, and mix them with a three-dimensional mixer. After the ...
Embodiment 2
[0035] prescription
[0036]
[0037] The preparation process takes the prescription amount of microcrystalline cellulose and sucrose, mixes them evenly, adds an appropriate amount of water to make a soft material, extrudes a spheronizer to prepare pellets, and passes through a 20-mesh and 30-mesh sieve after drying to obtain a blank core (0.5-0.7mm);
[0038] Coating prescription
[0039]
[0040]
[0041] The preparation process configures the coating solution according to the prescription, weighs 100g of the blank core, puts it into a multi-functional fluidized bed, coats 15g with a 5% drug-containing layer (the main content is 0.2g / 100g), and dries for 5min with 12.5% uterine Odd NE30D coating 25g, get sustained-release pills with medicine:
[0042] Orally Disintegrating Tablet Prescription
[0043]
[0044] Preparation process: Weigh the prescribed amount of medicated pellets, mannitol, lactose hydrate, and microcrystalline cellulose, and mix them evenly w...
Embodiment 3
[0047] prescription
[0048]
[0049] The preparation process takes the prescription amount of microcrystalline cellulose and sucrose, mixes them evenly, adds an appropriate amount of water to make a soft material, extrudes a spheronizer to prepare pellets, and passes through a 20-mesh and 30-mesh sieve after drying to obtain a blank core (0.5-0.7mm);
[0050] Prescription with drug layer
[0051]
[0052]
[0053] The preparation process configures the coating solution according to the prescription, weighs 100g of the blank core, and puts it into In the chemical bed, 5% drug-containing layer (the main content is 0.2g / 100g) is coated with 15g, dried for 5min, NE30D coated 30g, get sustained release pills with medicine
[0054] Orally Disintegrating Tablet Prescription
[0055]
[0056] Preparation process: Weigh the prescribed amount of pills with medicine, mannitol, lactose hydrate, Vegetables, mixed with a three-dimensional mixer, mixed with the prescripti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com