Olanzapine oral disintegration tablet and preparation method thereof
A technology of orally disintegrating tablets and olanzapine, applied in the field of pharmaceutical preparations, can solve the problems of small lumps falling, unsuitable for long-term storage, transportation and use, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
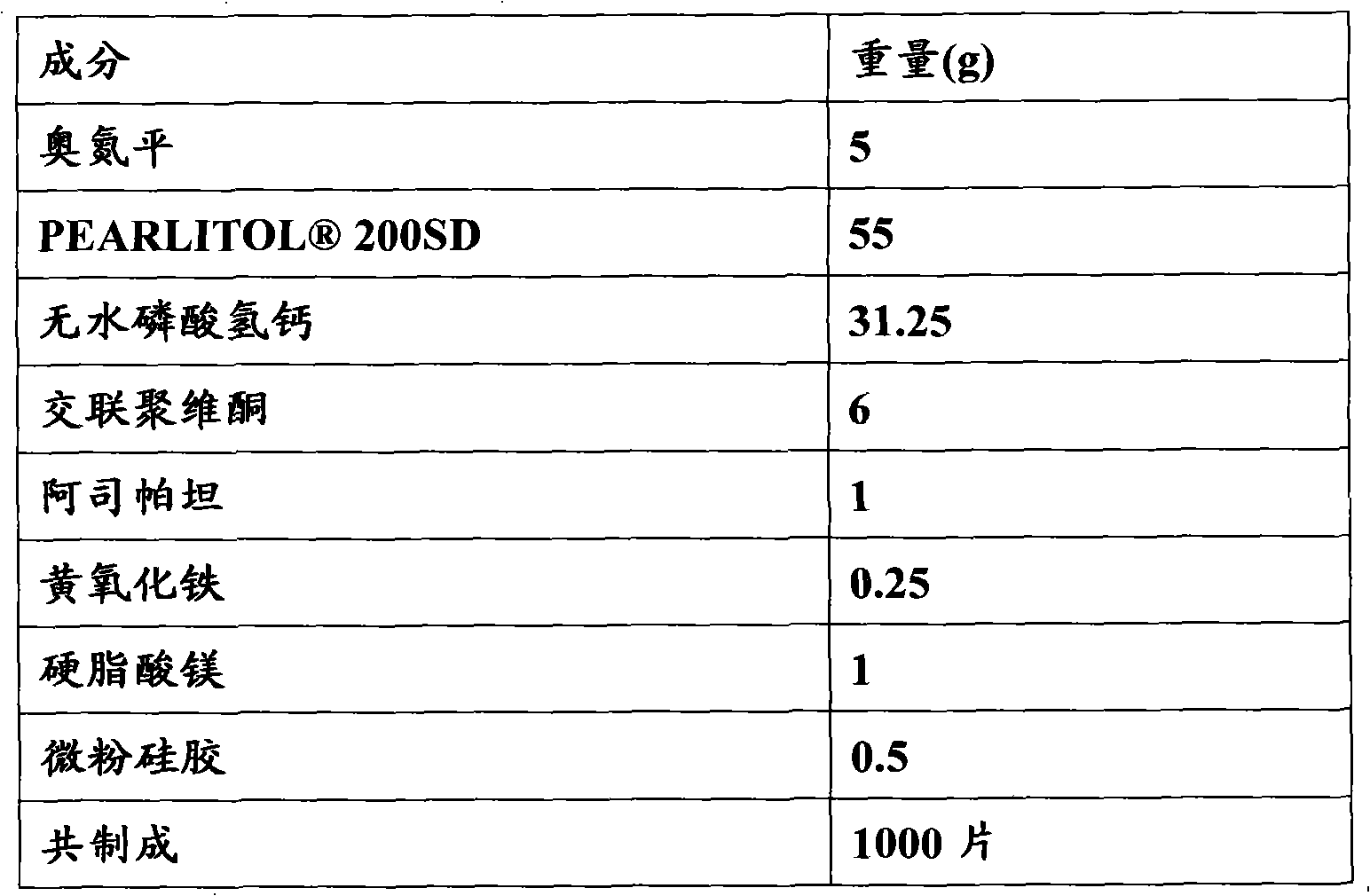
[0065] Embodiment 1: the preparation of olanzapine orally disintegrating tablet (5mg / tablet)
[0066] formula:
[0067]
[0068] making process:
[0069] 1) Take the prescribed amount of olanzapine and pass through a 120-mesh sieve, and anhydrous calcium hydrogen phosphate, aspartame, and crospovidone respectively pass through a 80-mesh sieve;
[0070] 2) Olanzapine, aspartame, yellow ferric oxide and mannitol are mixed uniformly in equal increments;
[0071] 3) Add anhydrous calcium hydrogen phosphate and crospovidone to the mixture in step 2, and mix well;
[0072] 4) Add magnesium stearate and micronized silica gel to the mixture obtained in step 3, and mix well. Select a suitable tablet press and punching die to compress the tablets, each tablet contains 5 mg of olanzapine, inspect, pack, and obtain the finished product.
Embodiment 2
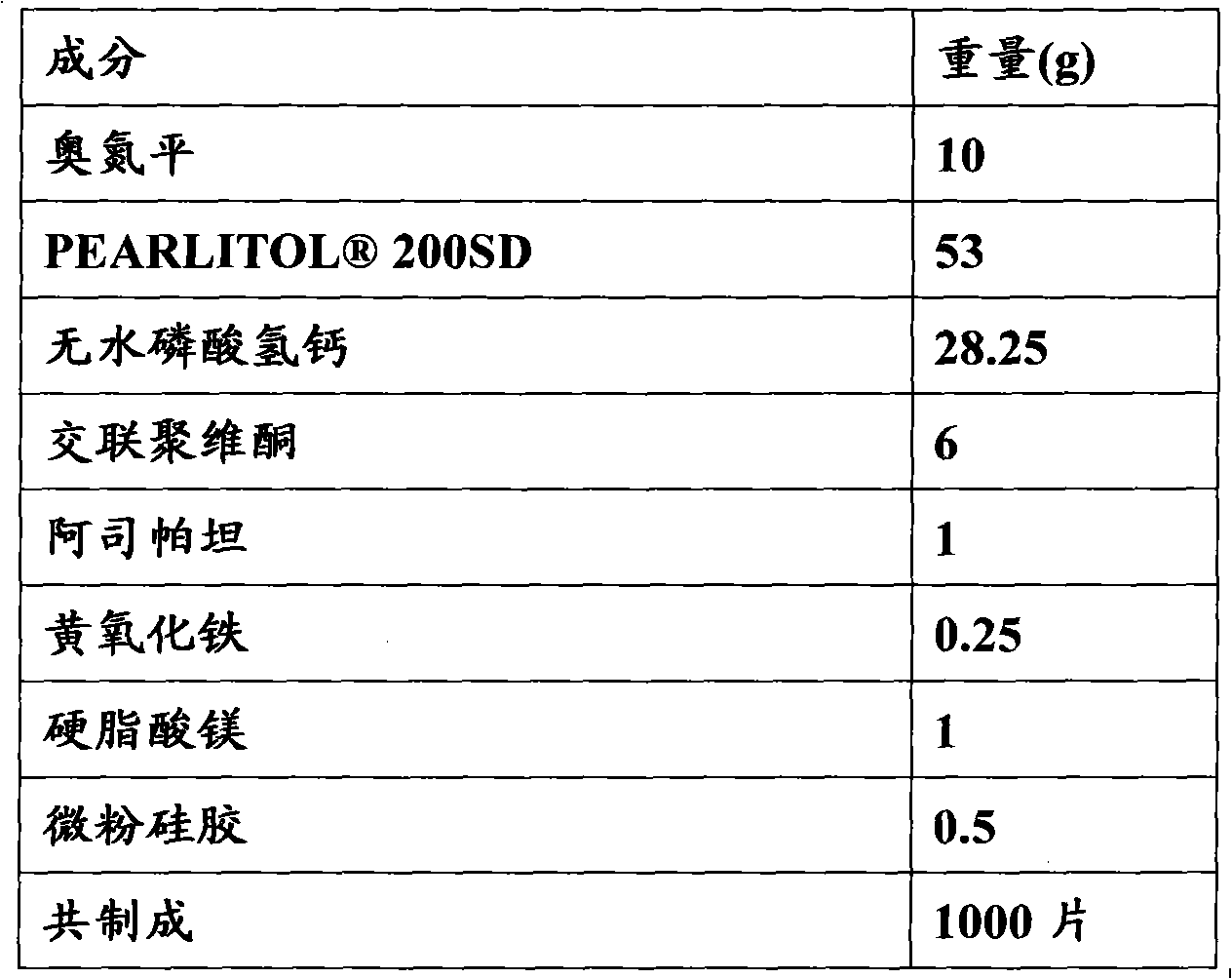
[0073] Embodiment 2: the preparation of olanzapine orally disintegrating tablet (10mg / tablet)
[0074] formula:
[0075]
[0076] making process:
[0077] 1) Take the prescribed amount of olanzapine and pass through a 120-mesh sieve, and anhydrous calcium hydrogen phosphate, aspartame, and crospovidone respectively pass through a 80-mesh sieve;
[0078] 2) Olanzapine, aspartame, yellow ferric oxide and mannitol are mixed uniformly in equal increments;
[0079] 3) Add anhydrous calcium hydrogen phosphate and crospovidone to the mixture in step 2, and mix well;
[0080] 4) Add magnesium stearate and micronized silica gel to the mixture obtained in step 3, and mix well. Select a suitable tablet press and punching die to compress the tablets, each tablet contains 10 mg of olanzapine, inspect, pack, and obtain the finished product.
Embodiment 3
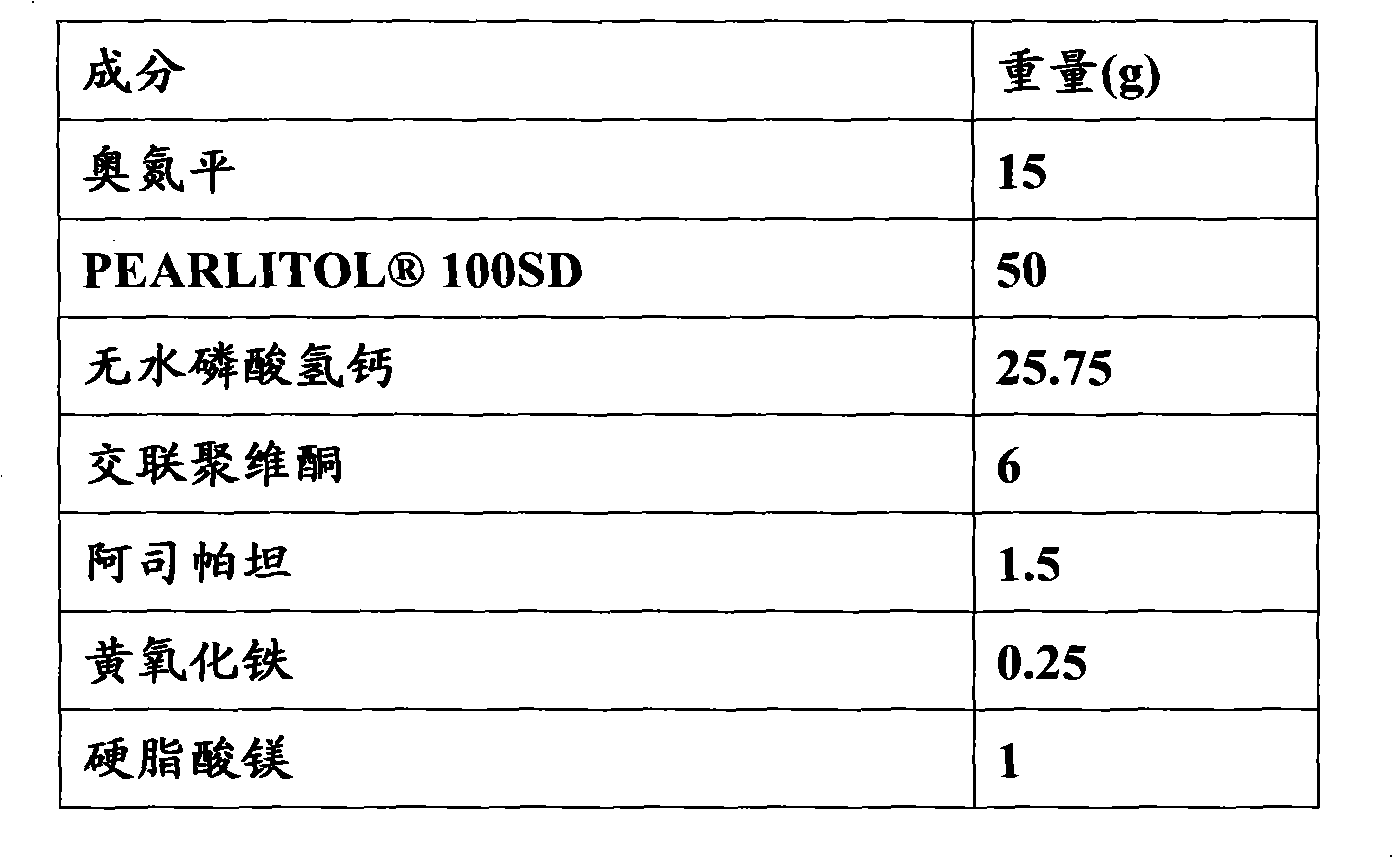
[0081] Embodiment 3: the preparation of olanzapine orally disintegrating tablet (15mg / tablet)
[0082] formula:
[0083]
[0084]
[0085] making process:
[0086] 1) Take the prescribed amount of olanzapine and pass through a 120-mesh sieve, and anhydrous calcium hydrogen phosphate, aspartame, and crospovidone respectively pass through a 80-mesh sieve;
[0087] 2) Olanzapine, aspartame, yellow ferric oxide and mannitol are mixed uniformly in equal increments;
[0088] 3) Add anhydrous calcium hydrogen phosphate and crospovidone to the mixture in step 2, and mix well;
[0089] 4) Add magnesium stearate and micronized silica gel to the mixture obtained in step 3, and mix well. Select a suitable tablet press and punching die to compress tablets, inspect and pack, and the finished product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com