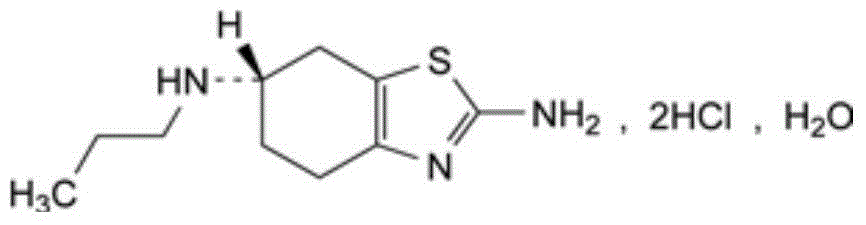
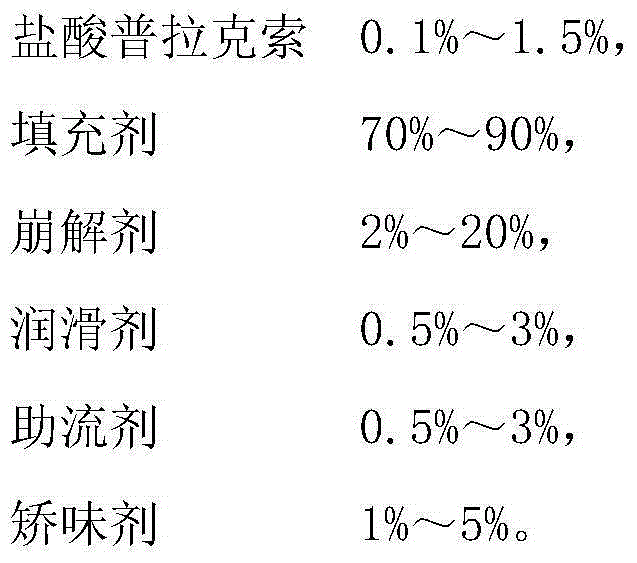
Medicine composition containing pramipexole dihydrochloride and preparation method thereof
A technology of pramipexole hydrochloride and its composition, which is applied in the field of pharmaceutical preparations, and can solve the problems of inability to meet the requirements of preparations, poor compatibility of pramipexole hydrochloride, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] The preparation of embodiment 1 pramipexole hydrochloride orally disintegrating tablets
[0103] Based on 1000 dosage units, and each dosage unit contains 0.125mg of pramipexole hydrochloride, its prescription is
[0104] ingredient name
Dosage (g)
pramipexole hydrochloride
0.125
50
35
10
1.5
Micropowder silica gel
1.5
1
Co-made
1000 pieces
[0105] Preparation Process:
[0106] (1) Pass lactose, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, micronized silica gel and aspartame through an 80-mesh sieve, and pramipexole hydrochloride through a 200-mesh sieve;
[0107] (2) Mix all materials evenly. When mixing, pramipexole hydrochloride and lactose are mixed in equal increments;
[0108] (3) Microcrystalline cellulose, croscarmellose sodium, micropowder...
Embodiment 2
[0110] The preparation of embodiment 2 pramipexole hydrochloride orally disintegrating tablets
[0111] Based on 1000 dosage units, and each dosage unit contains 0.25mg of pramipexole hydrochloride, its prescription is as follows:
[0112] ingredient name
Dosage (g)
pramipexole hydrochloride
0.25
50
microcrystalline cellulose
30
[0113] Croscarmellose Sodium
5
Low-substituted hydroxypropyl cellulose
10
1.5
Micropowder silica gel
1.5
1
Co-made
1000 pieces
[0114] Preparation Process:
[0115] (1) Pass mannitol, microcrystalline cellulose, croscarmellose sodium, low-substituted hydroxypropyl cellulose, magnesium stearate, micronized silica gel and aspartame through an 80-mesh sieve, and pramipexole hydrochloride Pass through 200 mesh sieve;
[0116] (2) Mix all materials evenly. When mixing, pramipexole hydr...
Embodiment 3
[0119] The preparation of embodiment 3 pramipexole hydrochloride orally disintegrating tablets
[0120] Based on 1000 dosage units, and each dosage unit contains 0.5mg of pramipexole hydrochloride, its prescription is as follows:
[0121] ingredient name
Dosage (g)
pramipexole hydrochloride
0.5
50
30
Crospovidone
5
Low-substituted hydroxypropyl cellulose
10
Magnesium stearate
1.5
Micropowder silica gel
1.5
stevia
1
Co-made
1000 pieces
[0122] Preparation Process:
[0123] (1) respectively passing mannitol, microcrystalline cellulose, crospovidone, low-substituted hydroxypropyl cellulose, magnesium stearate, micronized silica gel and stevioside through a 80-mesh sieve, and pramipexole hydrochloride through a 200-mesh sieve;
[0124] (2) Mix all materials evenly. When mixing, pramipexole hydrochloride and mannitol are mixe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com