Oral disintegrating acotiamide hydrochloride tablet and preparation method thereof
A technology of acotiamide hydrochloride and orally disintegrating tablets, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of poor taste, poor taste masking effect, and dissolution rate. Slow and other problems, to achieve the effect of easy storage and transportation, reduce friability, and improve dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] According to another aspect of the present invention, a kind of preparation method of above-mentioned acotiamide hydrochloride orally disintegrating tablet is provided, comprising the following steps:
[0055] 1) Acotiamide hydrochloride is carried out airflow pulverization to obtain micronized acotiamide hydrochloride, and the particle size is controlled to be D 90 not more than 10 μm,
[0056] 2) suspending the micronized acotiamide hydrochloride in the binder solution, and then spray-coating the suspension solution onto the blank pellet core to obtain unmasked acotiamide hydrochloride pellets,
[0057] 3) stirring and mixing the taste-masking coating material, porogen and ethanol solution to form a suspension dispersion to obtain a taste-masking coating liquid,
[0058] 4) Take the unmasked acotiamide hydrochloride pellets in step 2) and coat them with the taste-masking coating solution to obtain the acotiamide hydrochloride taste-masked pellets,
[0059] 5) Mix an...
Embodiment 1
[0064] Table 1 is the composition of acotiamide hydrochloride pellets
[0065] Table 1
[0066]
[0067]
[0068] The preparation method of above-mentioned acotiamide hydrochloride pellets is:
[0069] 1) Acotiamide hydrochloride is carried out jet milling, and the particle size is controlled to be D 90 not more than 10 μm,
[0070] 2) Suspend micronized acotiamide hydrochloride in the binder hydroxypropyl methylcellulose solution, and spray coat the hydroxypropyl methylcellulose solution suspended with acotiamide hydrochloride micropowder to On the blank ball core, the unmasked acotiamide hydrochloride pellets were prepared,
[0071] 3) stirring and mixing the taste-masking coating material, porogen and ethanol solution to form a suspension dispersion to obtain a taste-masking coating liquid,
[0072] 4) Coating the untaste-masked acotiamide hydrochloride pellets with a taste-masking coating solution to prepare the taste-masked acotiamide hydrochloride pellets.
Embodiment 2
[0074] Table 2 shows the orally disintegrating tablet compression of the acotiamide hydrochloride taste-masked pellets prepared in the above-mentioned Example 1 in different proportions.
[0075] Table 2
[0076]
[0077] Preparation method: first mix and sieve acotiamide hydrochloride taste-masked pellets with sucralose, essence, and silicon dioxide, then mix with crospovidone and mannitol, and press into tablets.
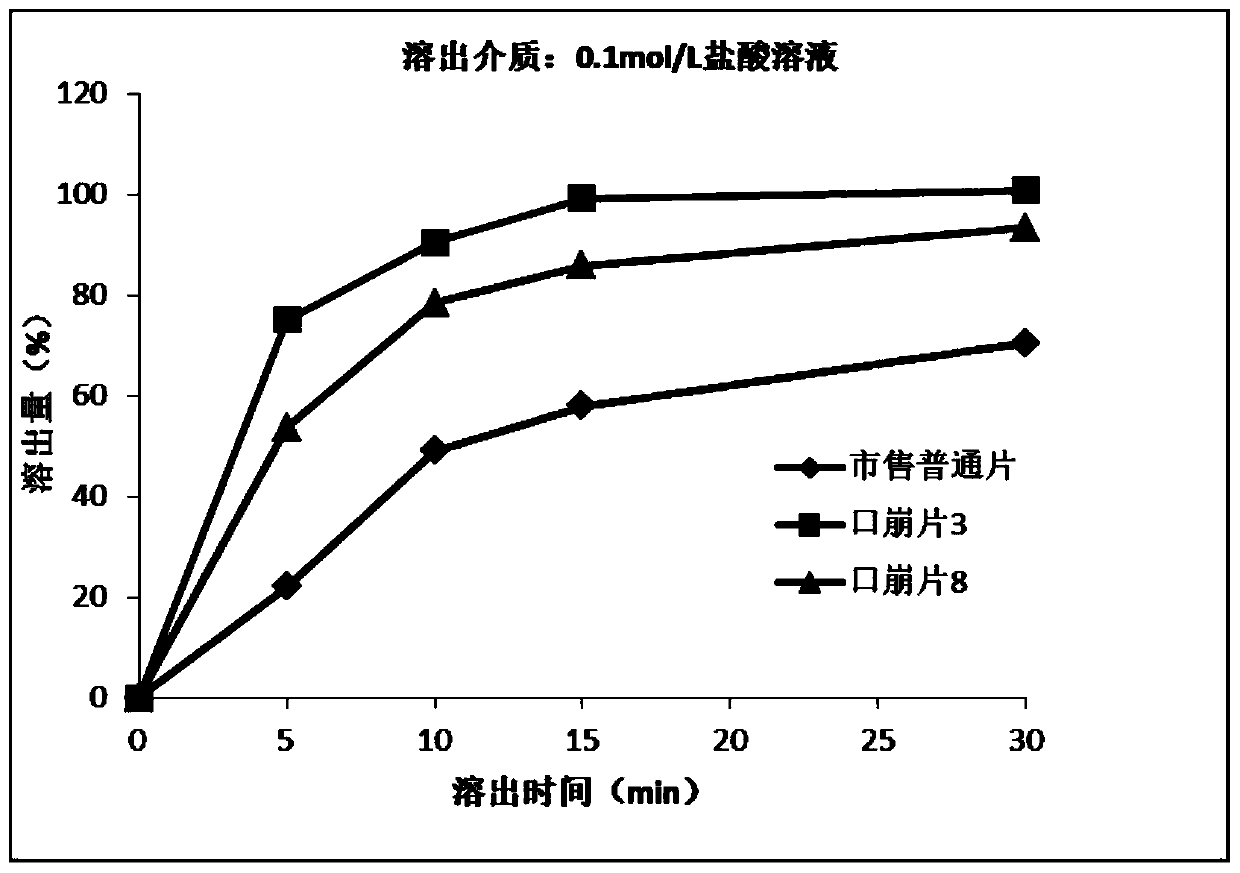
[0078] Results: The theoretical tablet weight of acotiamide hydrochloride orally disintegrating tablets prepared according to the above pellet ratios was not more than 500 mg, and the weight of the tablets was suitable and easy to take. The tablet weight of orally disintegrating tablet 2 to orally disintegrating tablet 6 is between 150 mg and 400 mg, and the tablet weight is very suitable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com