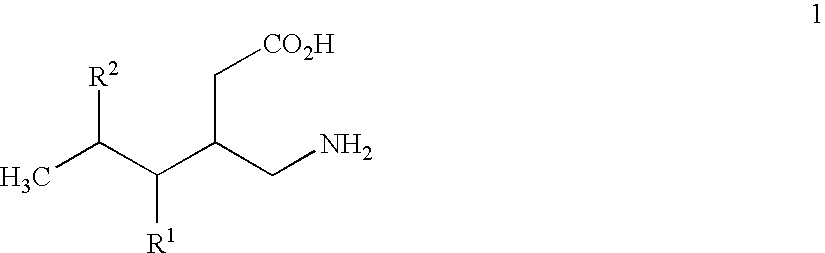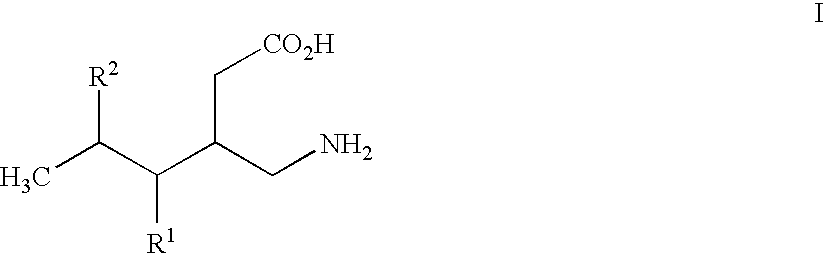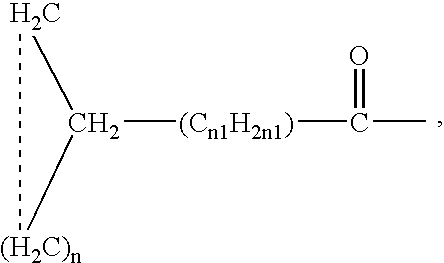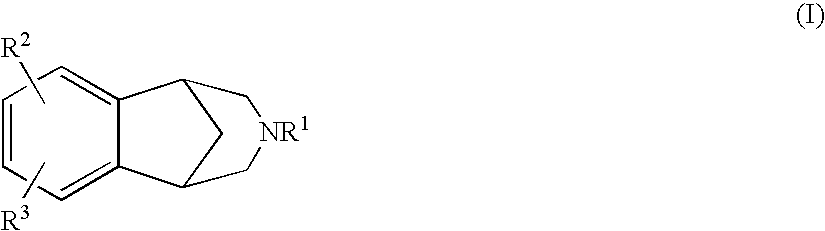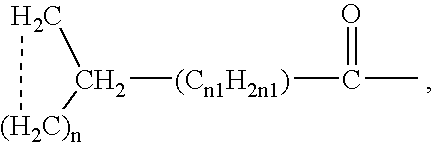Patents
Literature
81 results about "Restless legs syndrome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nervous disorder that causes an uncontrollable urge to move legs.
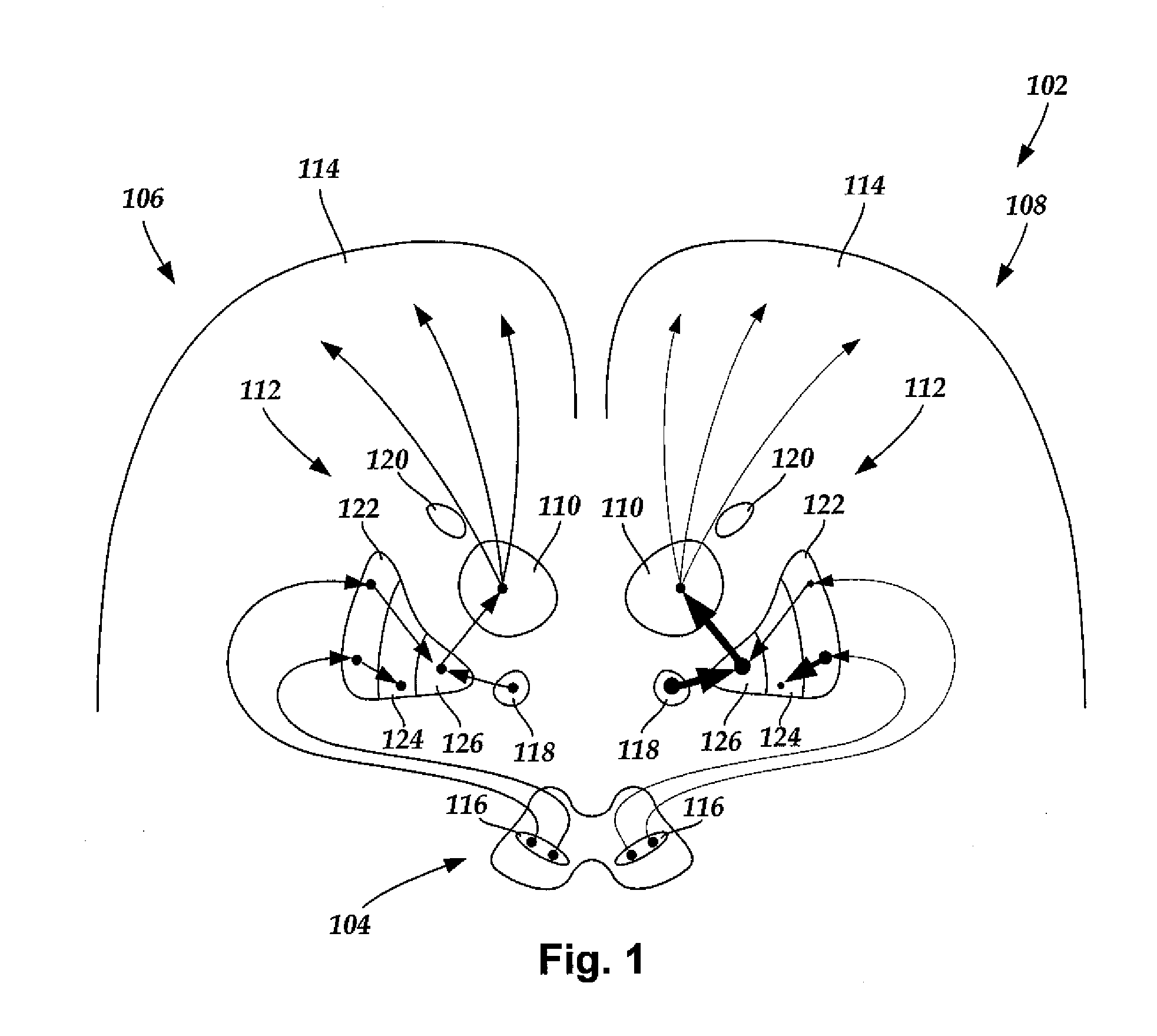
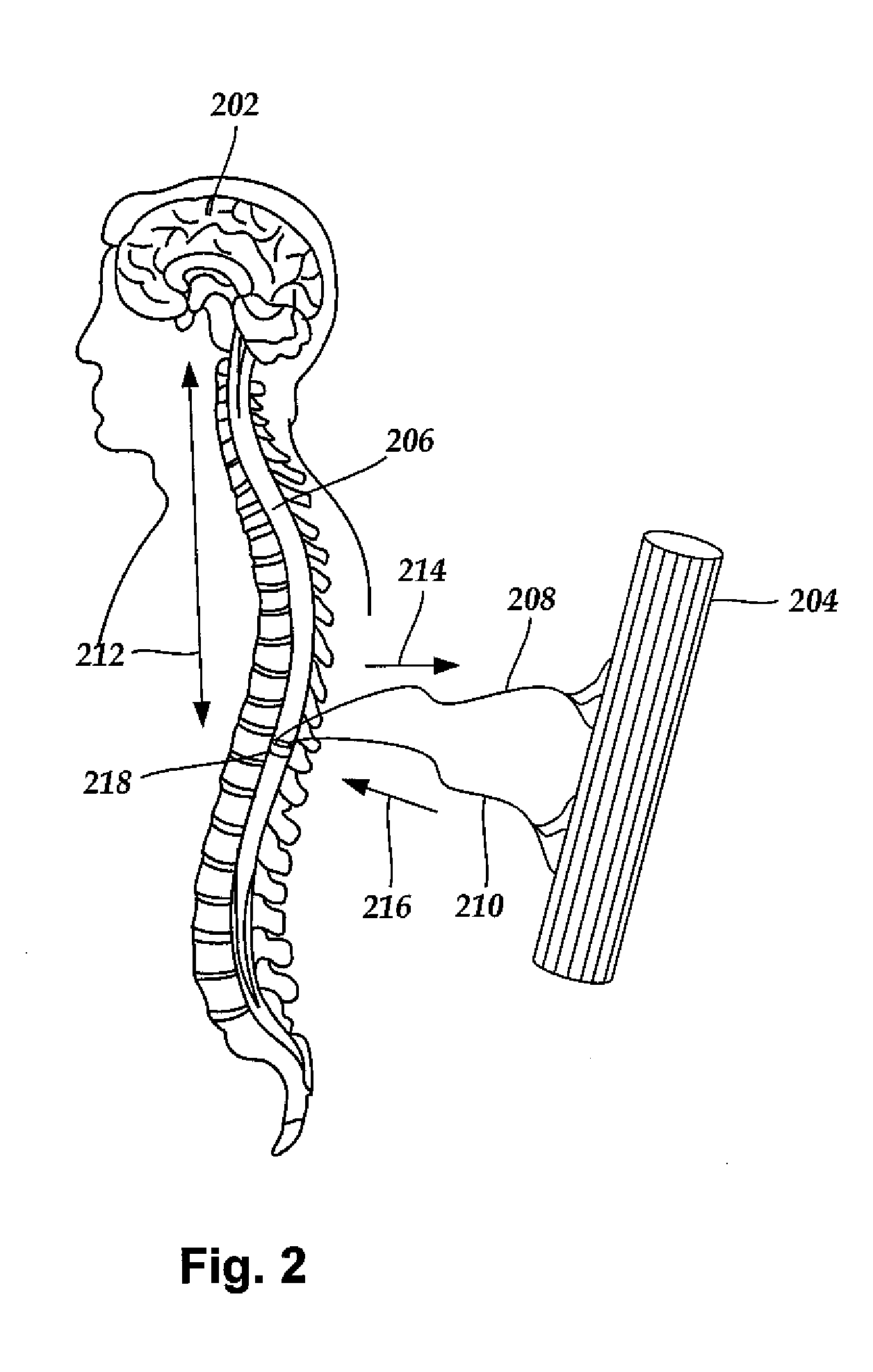
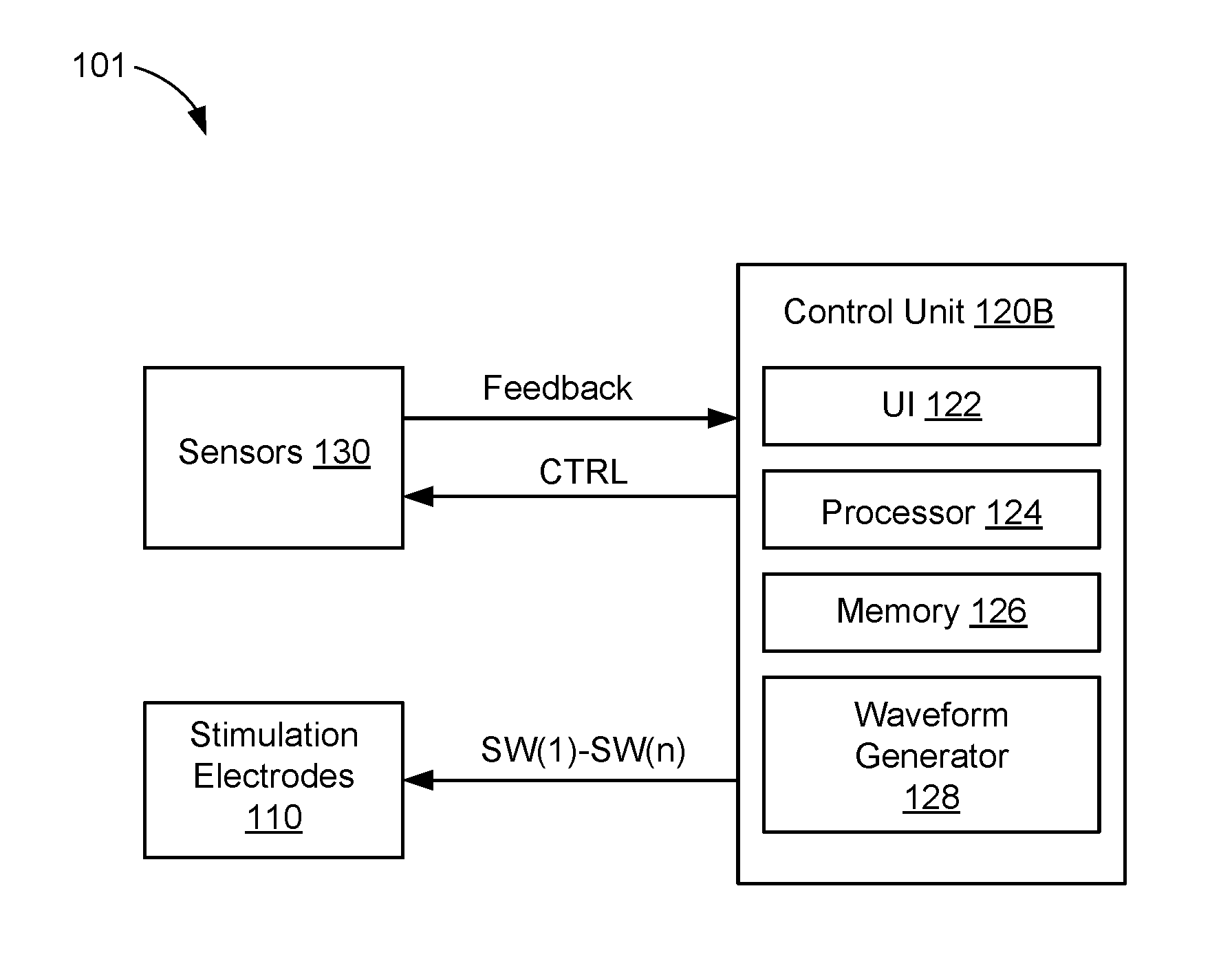
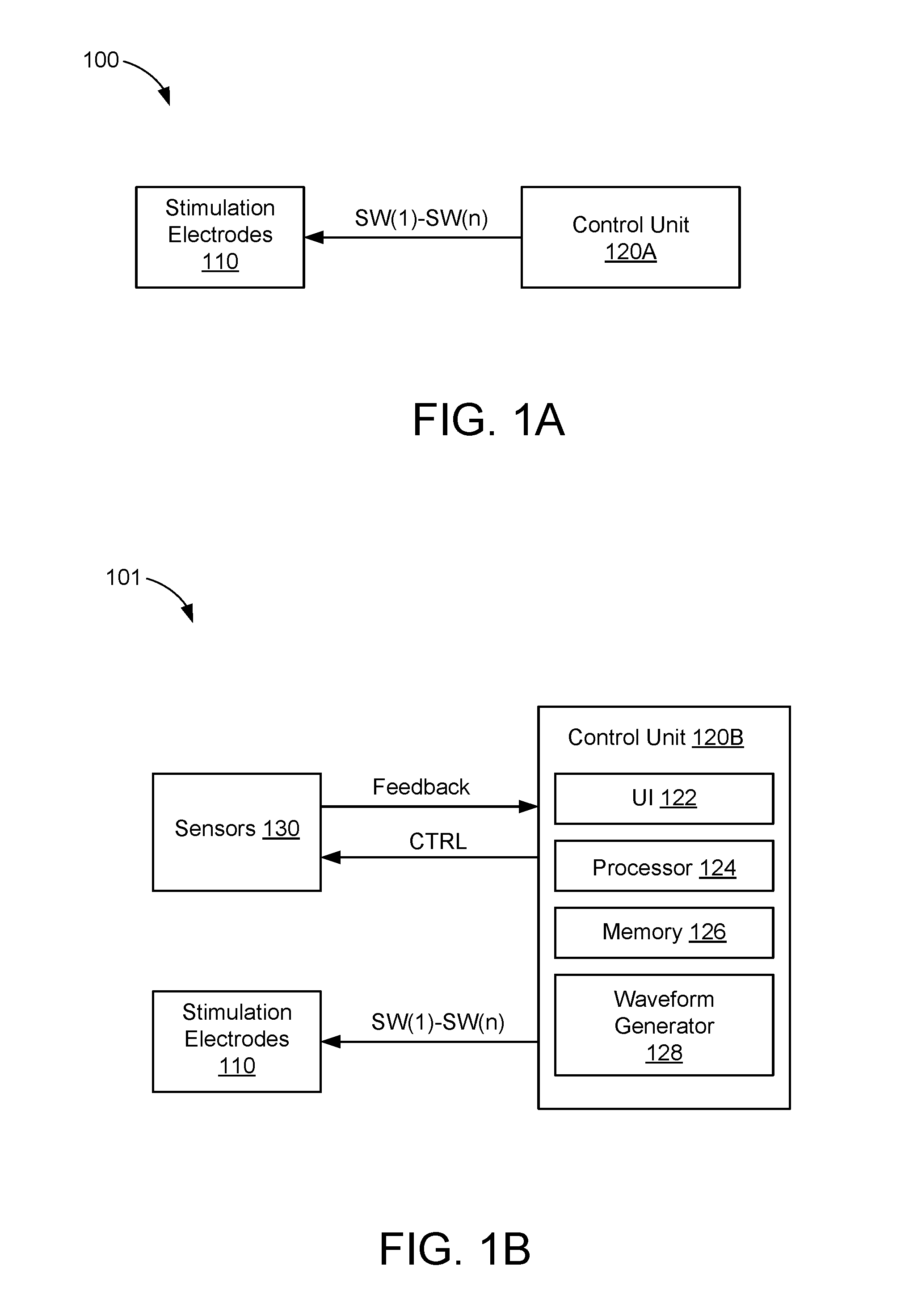
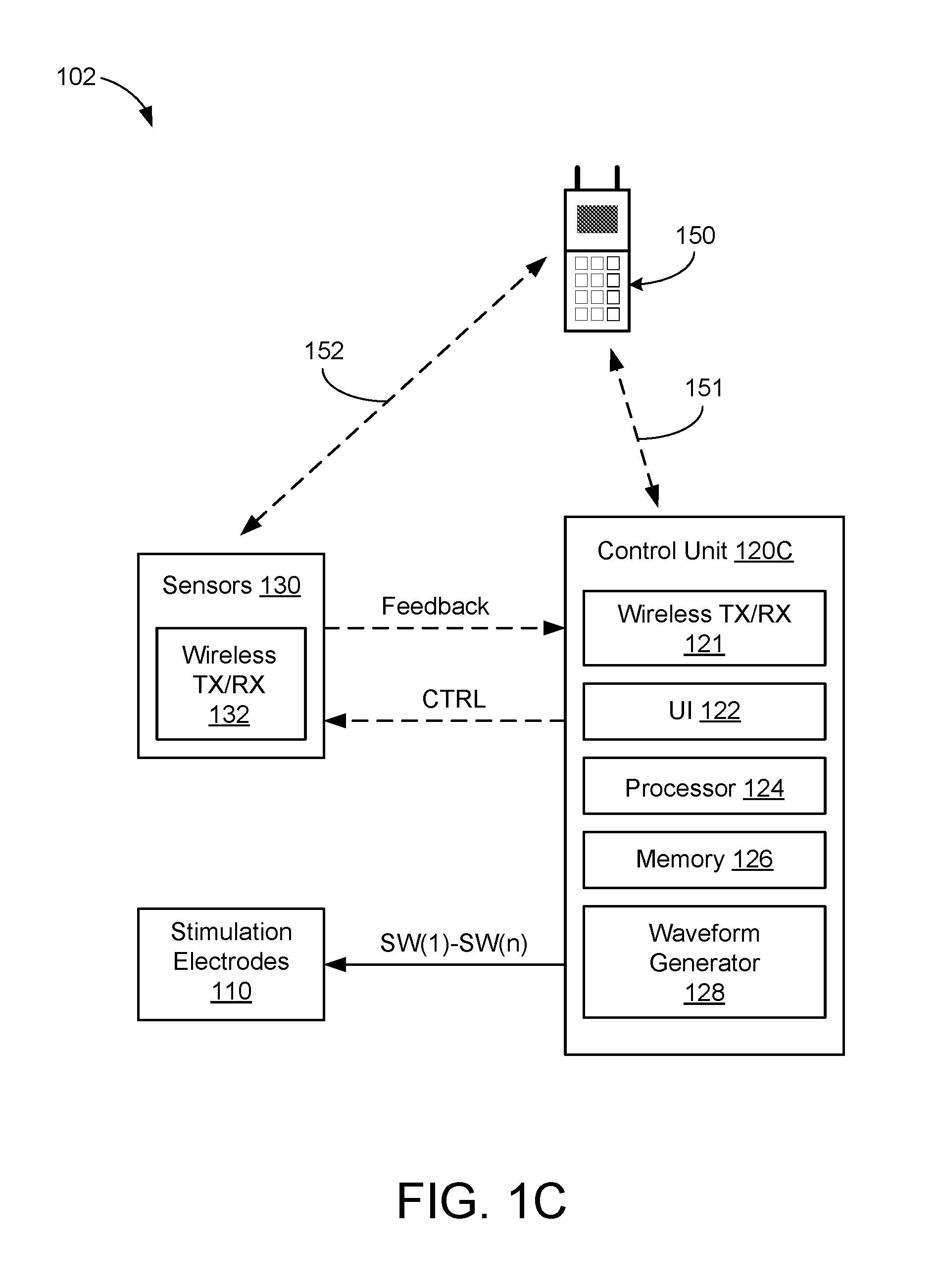
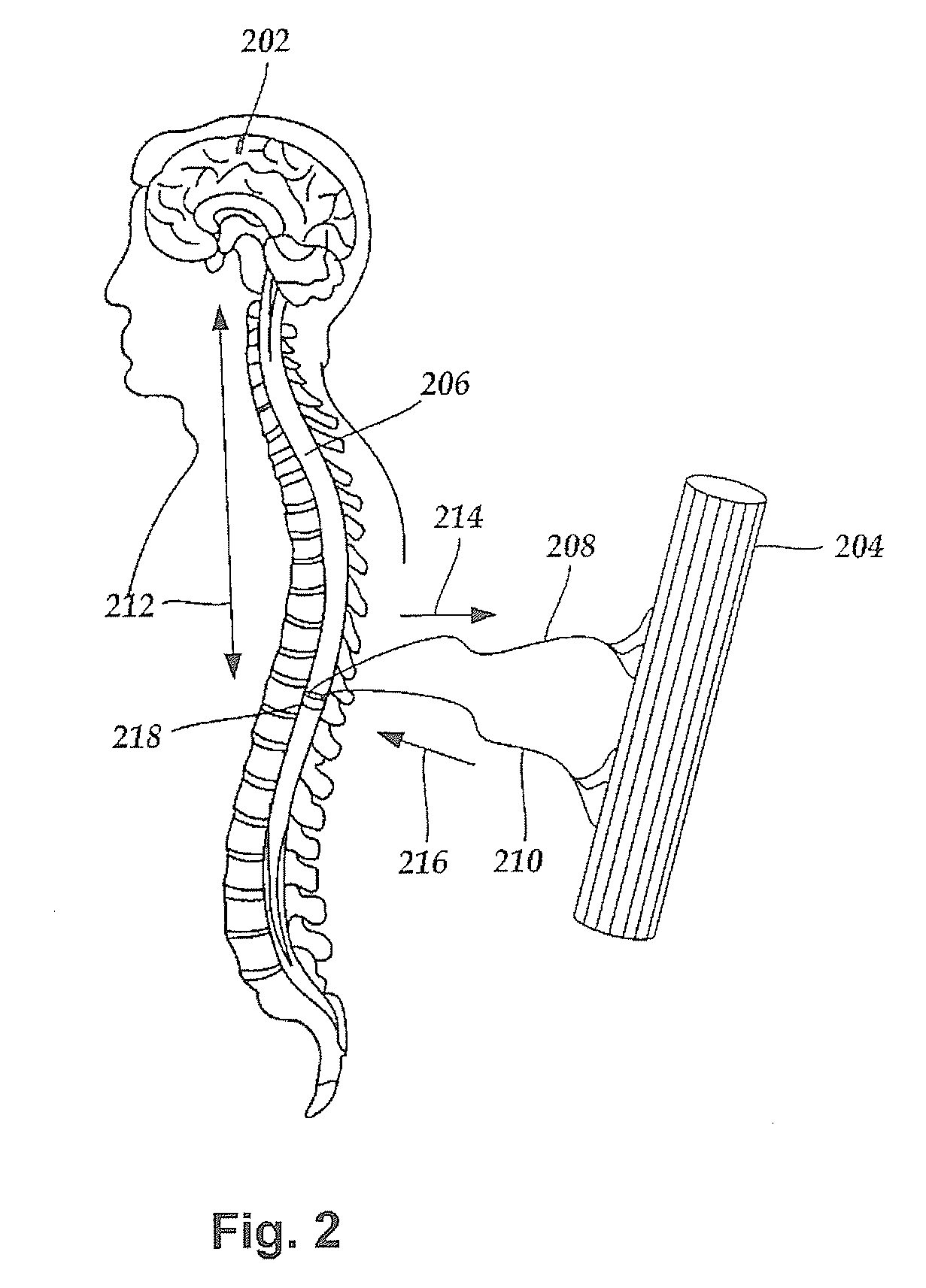
Systems and Methods for Treating Essential Tremor or Restless Leg Syndrome Using Spinal Cord Stimulation
InactiveUS20100023103A1Reduce, alleviate, or eliminate at least one adverse effectSpinal electrodesEssential tremorMedicine
A method for treating essential tremor or restless leg syndrome using spinal cord stimulation includes implanting a lead near a spinal cord of a patient. The lead includes a plurality of electrodes disposed on a distal end of the lead and electrically coupled to at least one contact terminal disposed on a proximal end of the lead. Electrical signals are provided from a control module coupled to the lead to stimulate a portion of the spinal cord of the patient using at least one of the electrodes. The electrical signals reduce, alleviate, or eliminate at least one adverse effect of essential tremor or restless leg syndrome.
Owner:BOSTON SCI NEUROMODULATION CORP
Method for Treating a Restless Limb Disorder
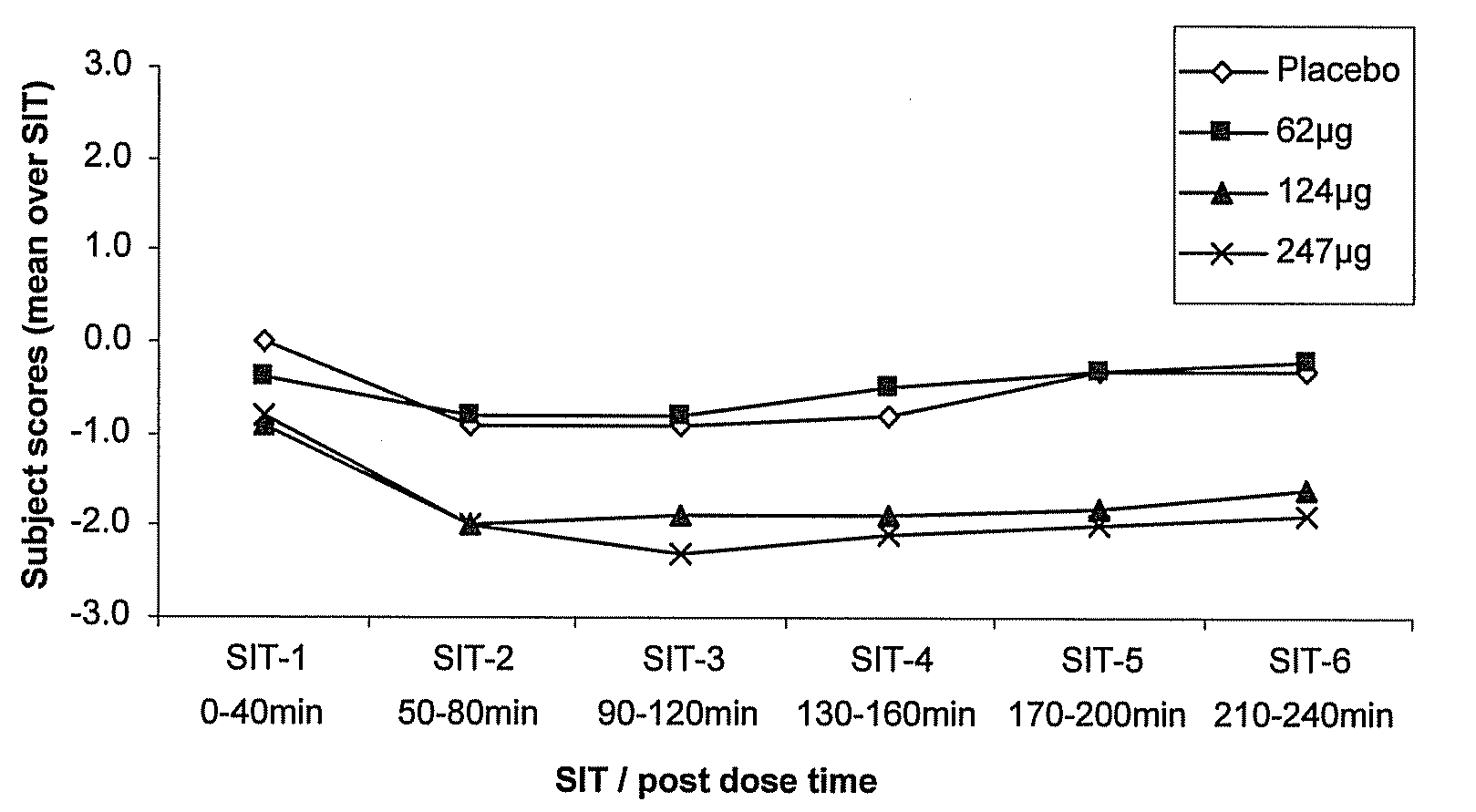
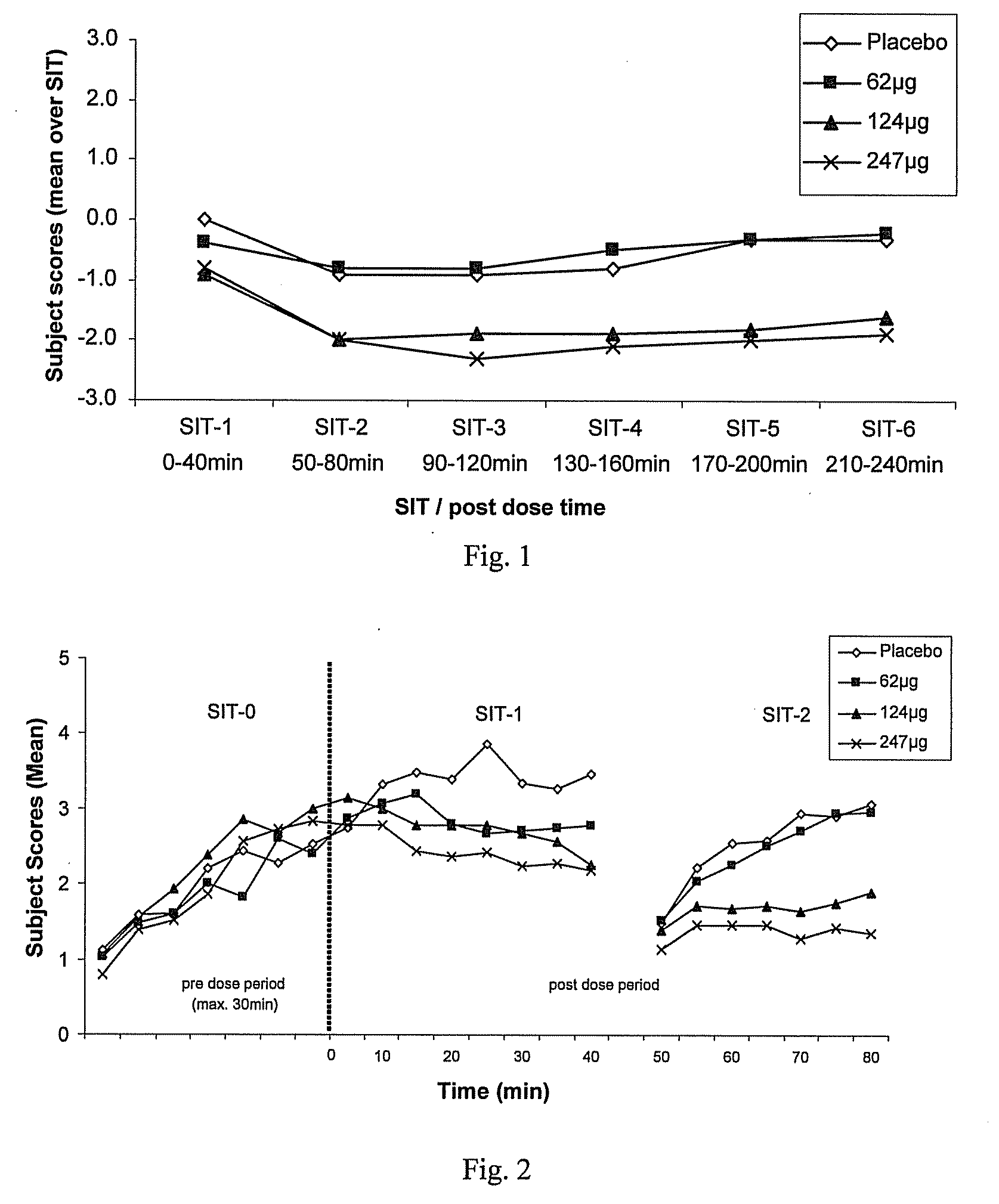
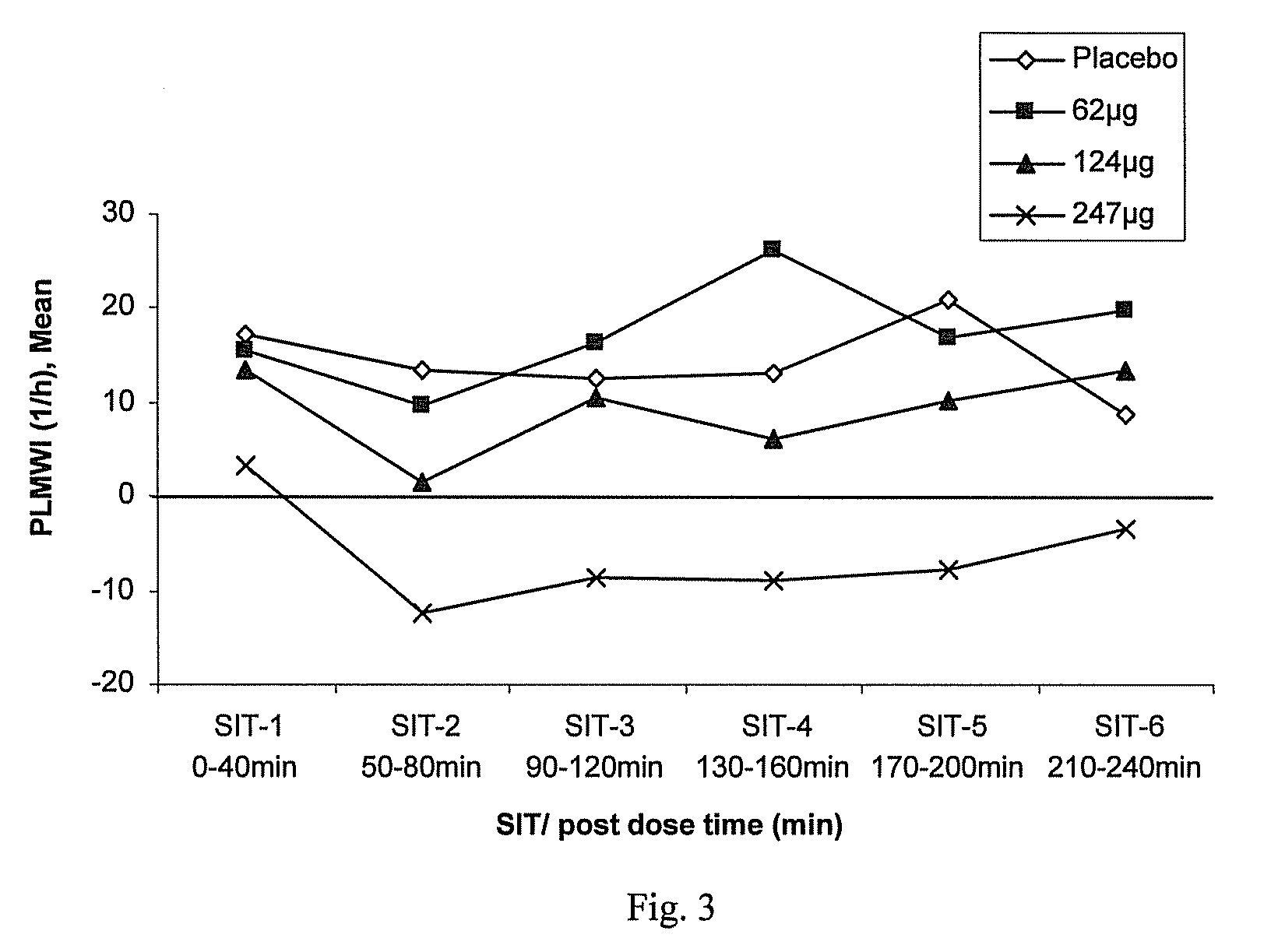
A method for treating a restless limb disorder such as restless legs syndrome in a subject comprises administering, transmucosally in the oronasopharyngeal chamber of the subject, one or more doses of rotigotine or a pharmaceutically acceptable salt, prodrug or metabolite thereof, wherein each such dose comprises an amount effective to reduce occurrence and / or severity of one or more symptoms of the disorder, but wherein the total of all such doses in a 24-hour period does not exceed about 450μg rotigotine free base equivalent.
Owner:UCB SA
Drug treatment for restless leg syndrome
A method for the treatment of Restless Leg Syndrome (RLS), which comprises administering an alpha2-agonist and a second agent selected from the group consisting of the dopamine agonists, opioids, benzodiazepines and the combination of L-DOPA plus a decarboxylase inhibitor.
Owner:BRECHT HANS MICHAEL
Sublingual films
ActiveUS8414922B2Alleviating dyskinesiaEffectively alleviatedBiocideNervous disorderSexual functioningDopamine
The invention features sublingual film formulations of dopamine agonists and methods of treating Parkinson's disease, tremors, restless leg syndrome, sexual dysfunction, and depressive disorders therewith.
Owner:SUNOVION PHARMA INC
Methods for treating restless legs syndrome
ActiveUS8623913B2Symptoms improvedAmeliorating and eliminating symptomBiocideNervous disorderCarbamateAnesthesia
The invention is directed to a method of treating restless legs syndrome in a subject, comprising administering a therapeutically effective amount of a carbamate compound, or pharmaceutically acceptable salt or amide thereof.
Owner:BIOPHARM
Use of rasagilline for the treatment of restless legs syndrome
ActiveUS20070232700A1Effective treatmentRelieve symptomsBiocideNervous disorderN-propargylPediatrics
Disclosed are methods for the treatment of Restless Legs Syndrome comprising administering an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Alpha-Aminoamide Derivatives Useful In The Treatment Of Restless Legs Syndrome And Addictive Disorders
Owner:NEWRON PHARMACEUTICALA SPA
Devices and methods for treating restless leg syndrome
An exemplary system for generating a counter-stimulation in a patient suffering from RLS includes a device configured and arranged to generate a counter-stimulation in a patient suffering from RLS, the counter-stimulation of an amplitude, intensity, and time duration lower than that which would wake the patient and higher than that sufficient to relieve RLS, or sufficient to relieve RLS symptoms and allow the patient to return to sleep, a controller configured and arranged to drive the counter-stimulation generation device, the controller being in communication with the counter-stimulation device, and a base configured and arranged to hold the counter-stimulation generation device adjacent to a patient, the counter-stimulation device attached to the base. An exemplary method of treating RLS includes selecting a patient experiencing RLS, and stimulating a portion of the patient at an amplitude, intensity, and duration sufficient to act as a counter-stimulation to RLS.
Owner:SENSORY NEUROSTIMULATION INC
Methods and compositions for administration of iron for the treatment of restless leg syndrome
InactiveUS20060116349A1Safe and efficicacious deliveryFaster labile iron releaseOrganic active ingredientsBiocideAnesthesiaRestless legs syndrome
Owner:LUITPOLD PHARMA INC
Sublingual films
ActiveUS20120195955A1Alleviating dyskinesiaEffectively alleviatedBiocideNervous disorderDiseaseSexual dysfunction
The invention features sublingual film formulations of dopamine agonists and methods of treating Parkinson's disease, tremors, restless leg syndrome, sexual dysfunction, and depressive disorders therewith.
Owner:SUNOVION PHARMA INC
Treatment of restless legs syndrome
A method for the treatment of restless legs syndrome in a patient using a combination of a COMT-inhibitor, a decarboxylase inhibitor and a dopamine precursor.
Owner:ORION CORP
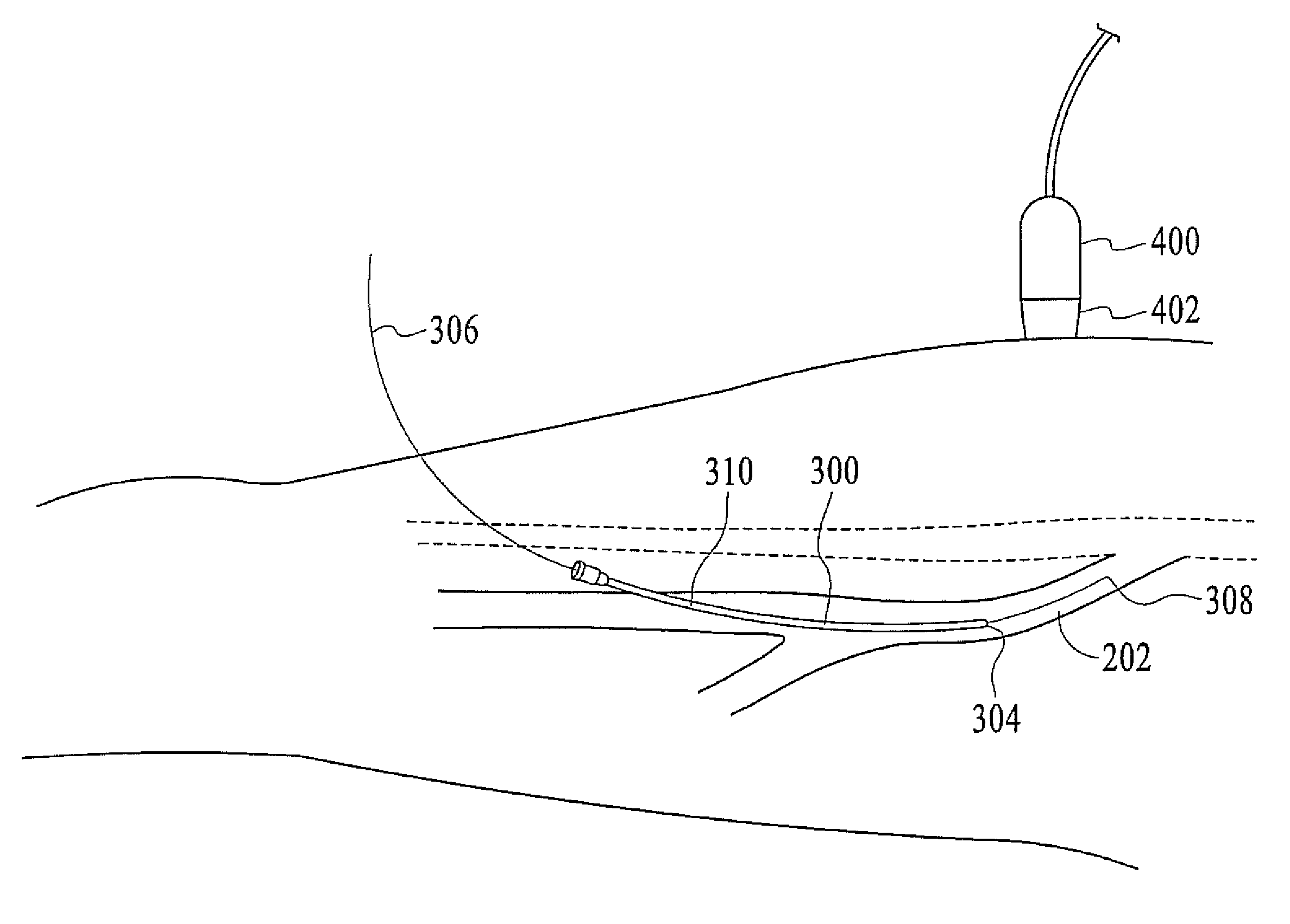
Restless leg syndrome treatment
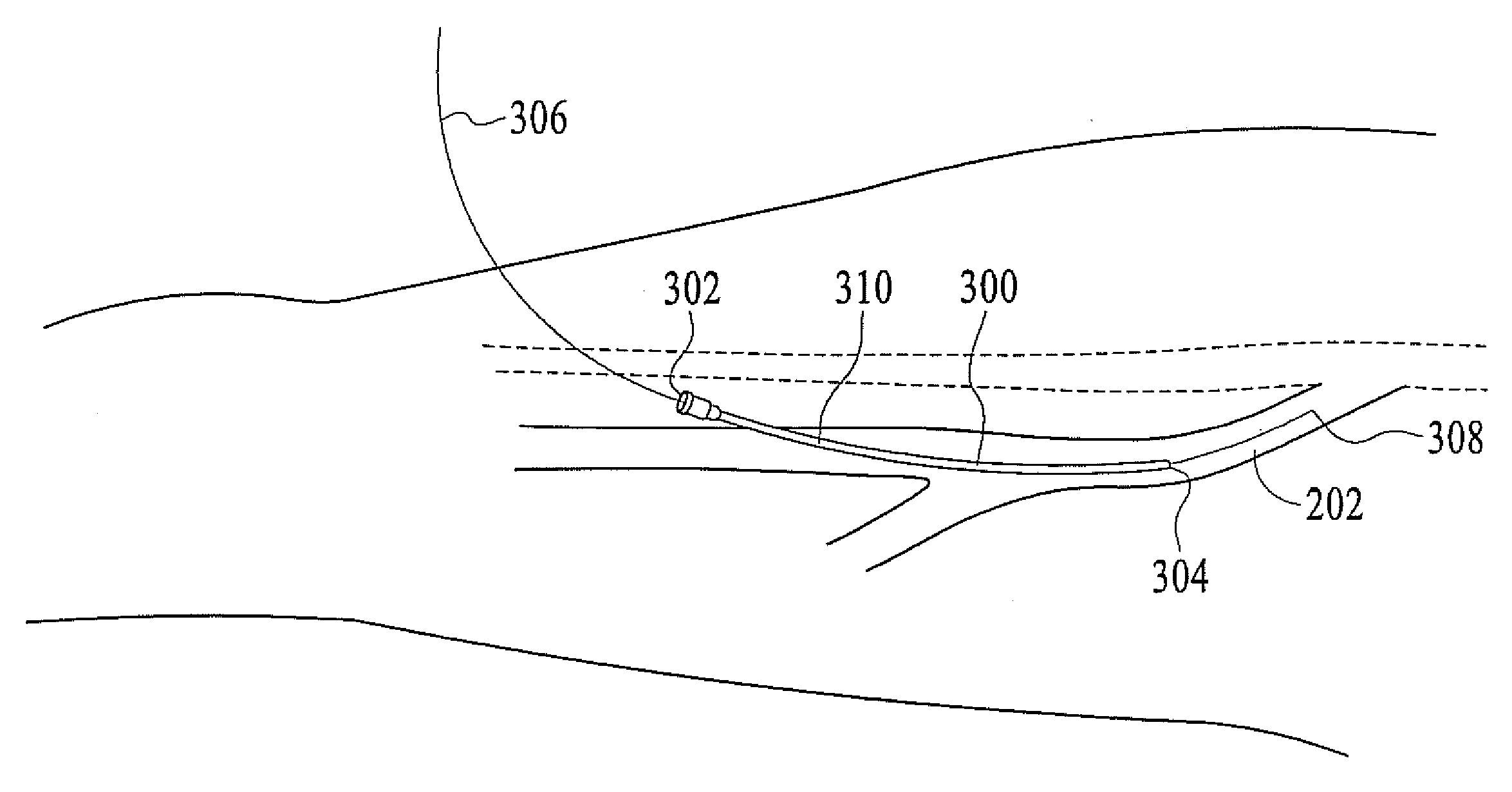
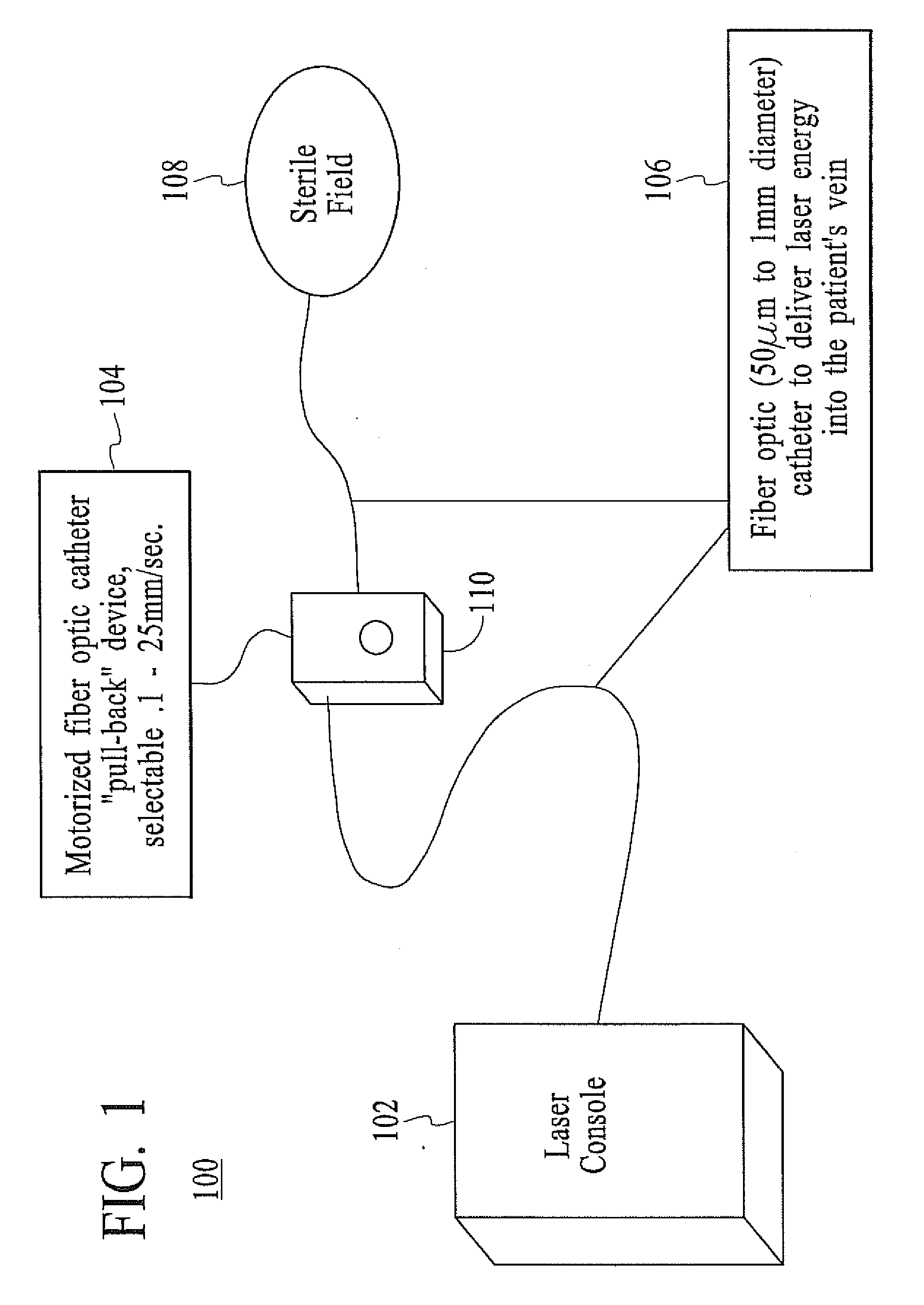
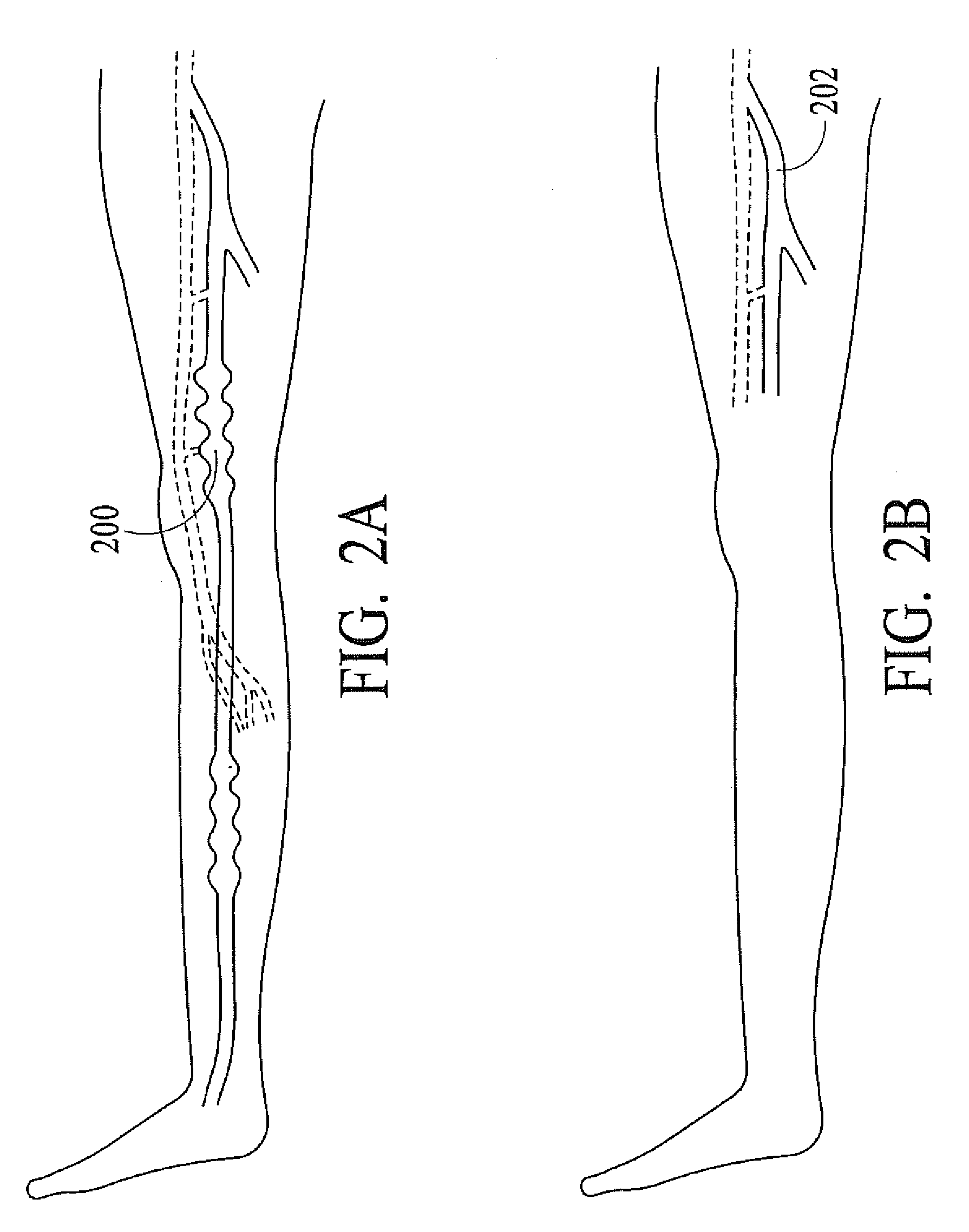
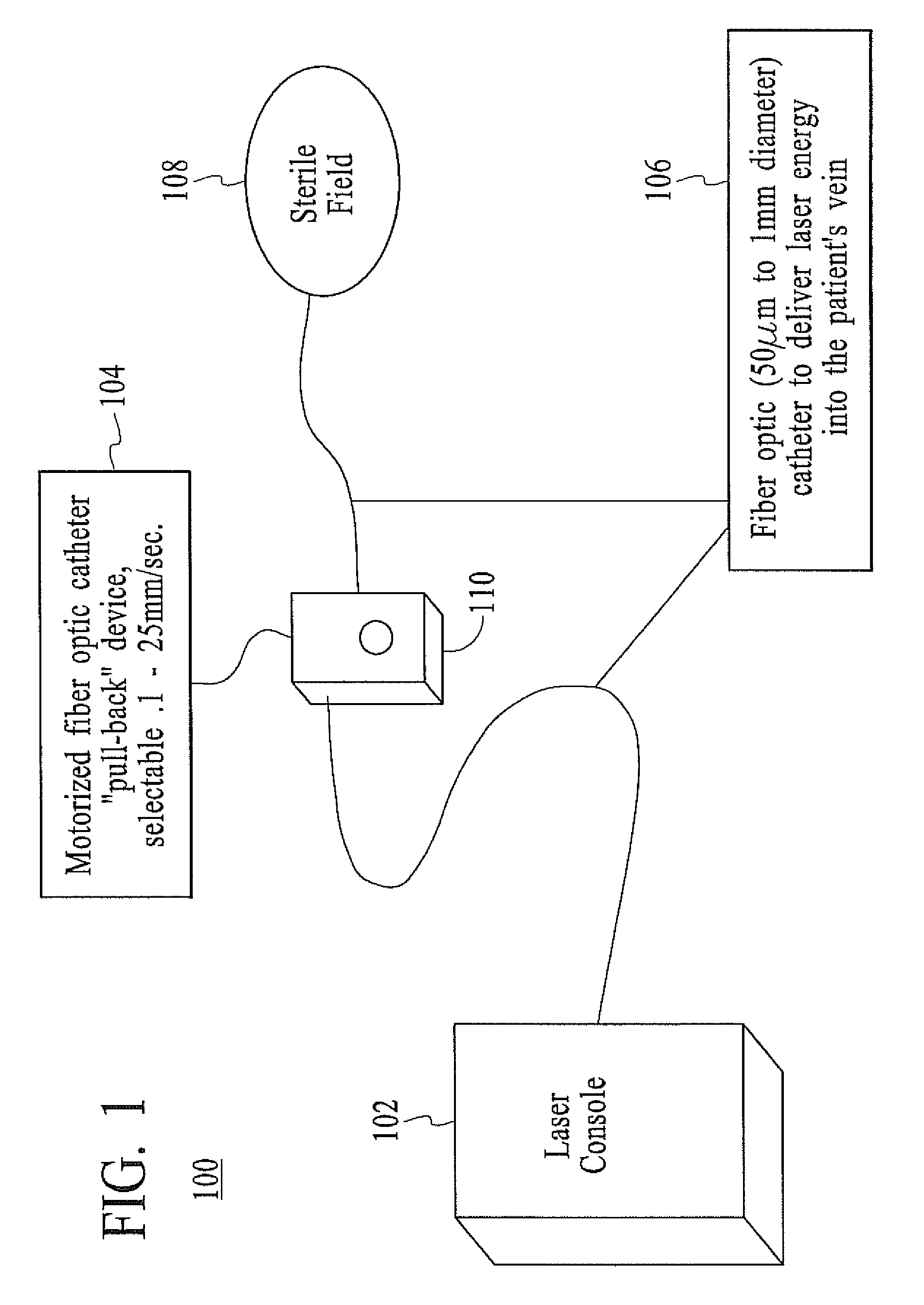
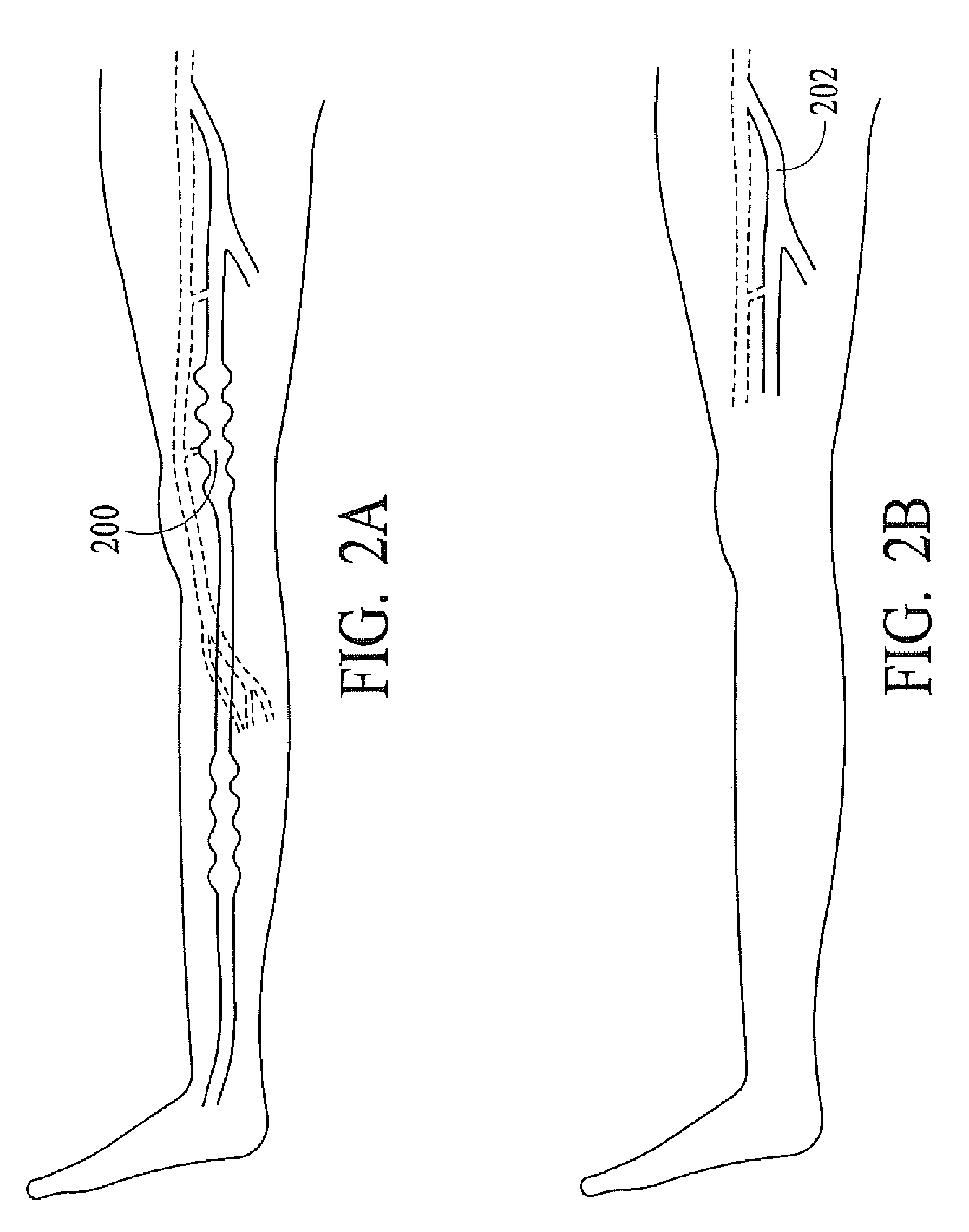
InactiveUS20080071333A1Good shortGood long-term resultDiagnosticsSurgical instrument detailsVeinCatheter
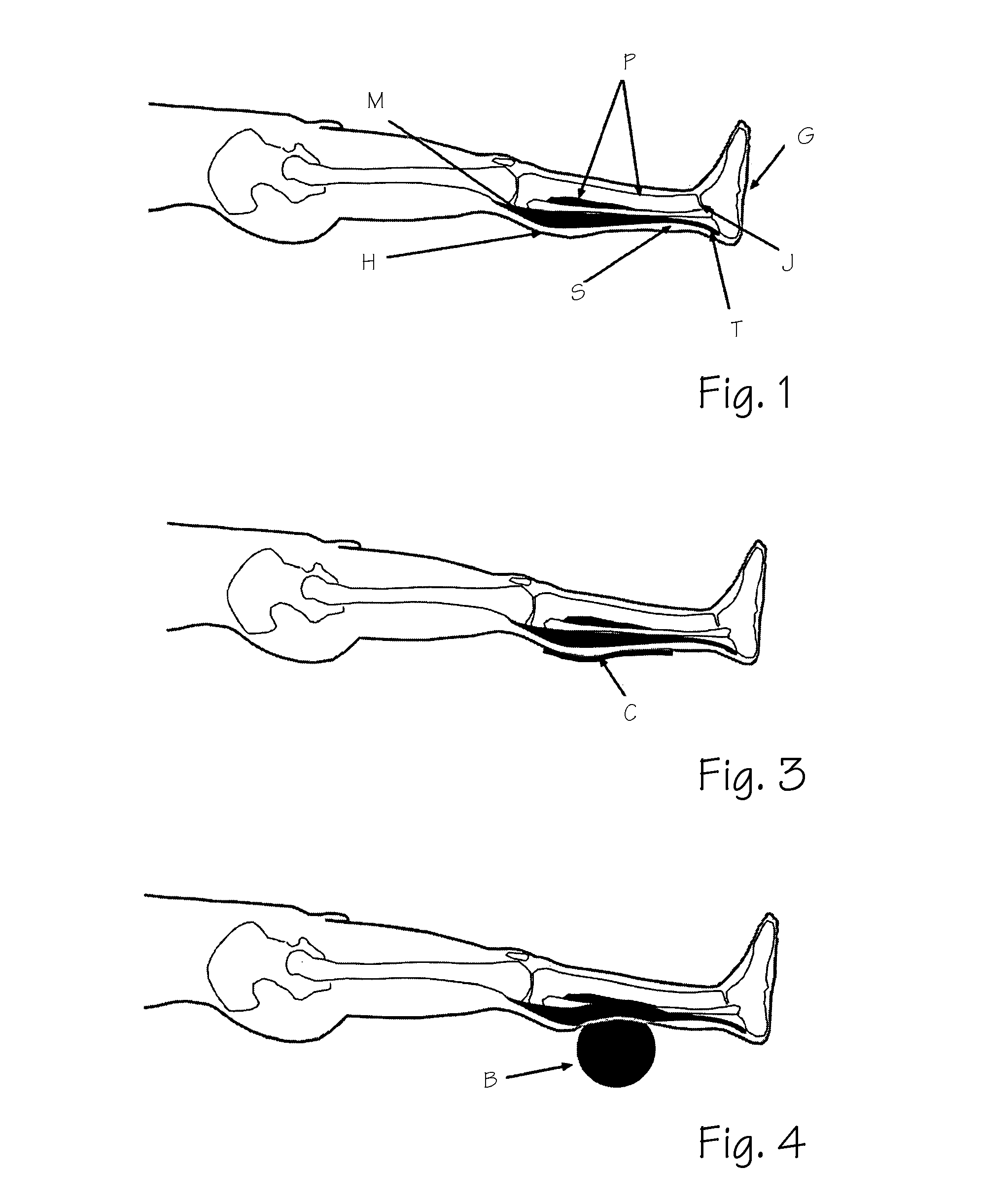
The present invention describes a method of treating restless leg syndrome by eliminating venous reflux in an underlying vein. The malfunctioning vein can be removed or ablated by inserting a catheter into the vein that transmits sufficient energy to coagulate or ablate the lining of the vein causing it to permanently close, eliminating the source of venous reflux and the symptom of restless leg syndrome.
Owner:NEW STAR LASERS
Ketamine treatment of restless legs syndrome
The present invention is directed to a method for treating symptoms associated with Restless Legs Syndrome (RLS). The method includes administering to a warm-blooded animal in need of such treatment a dose of ketamine sufficient to alleviate symptoms associated with RLS.
Owner:TEMPLE UNIVERSITY
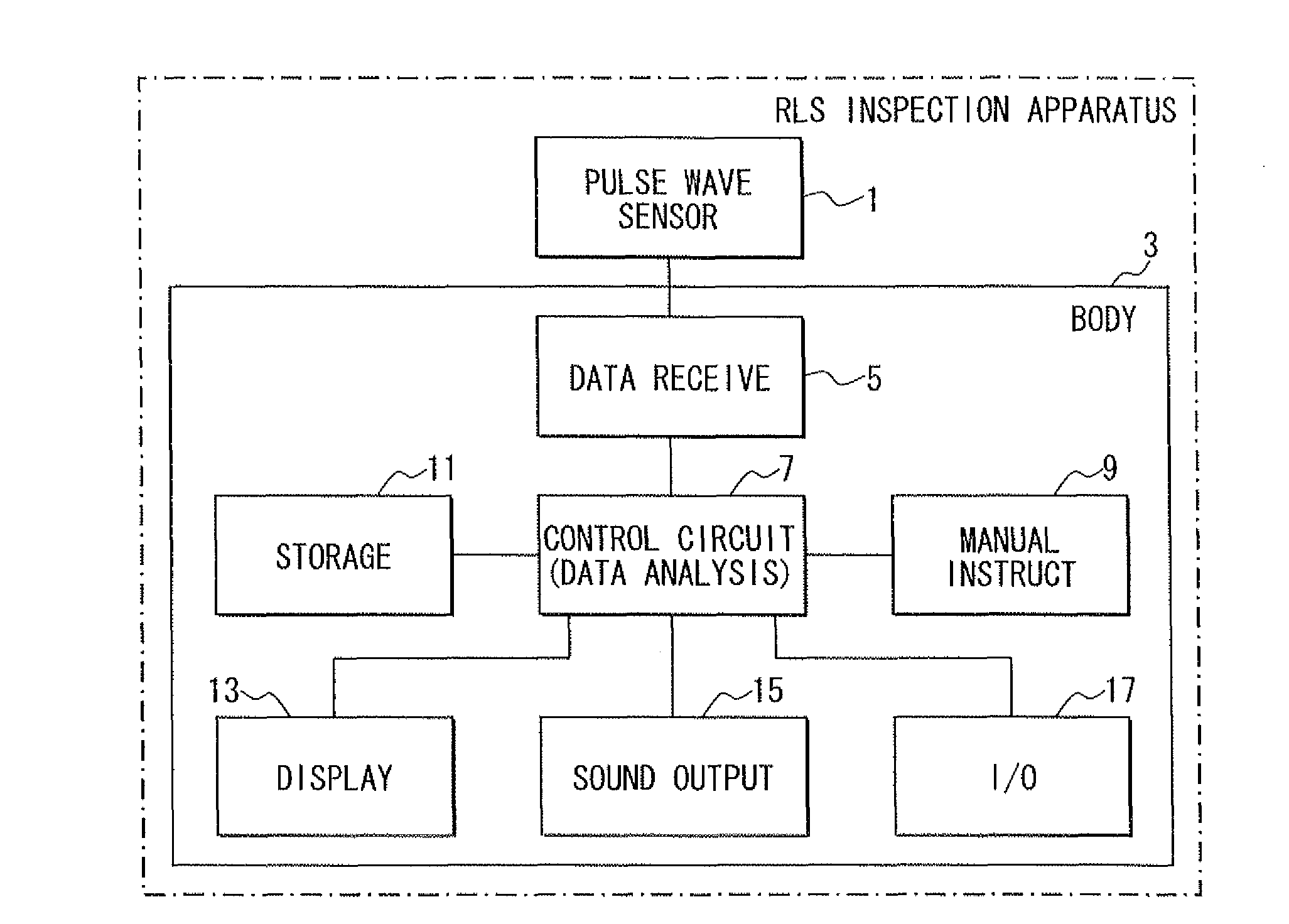
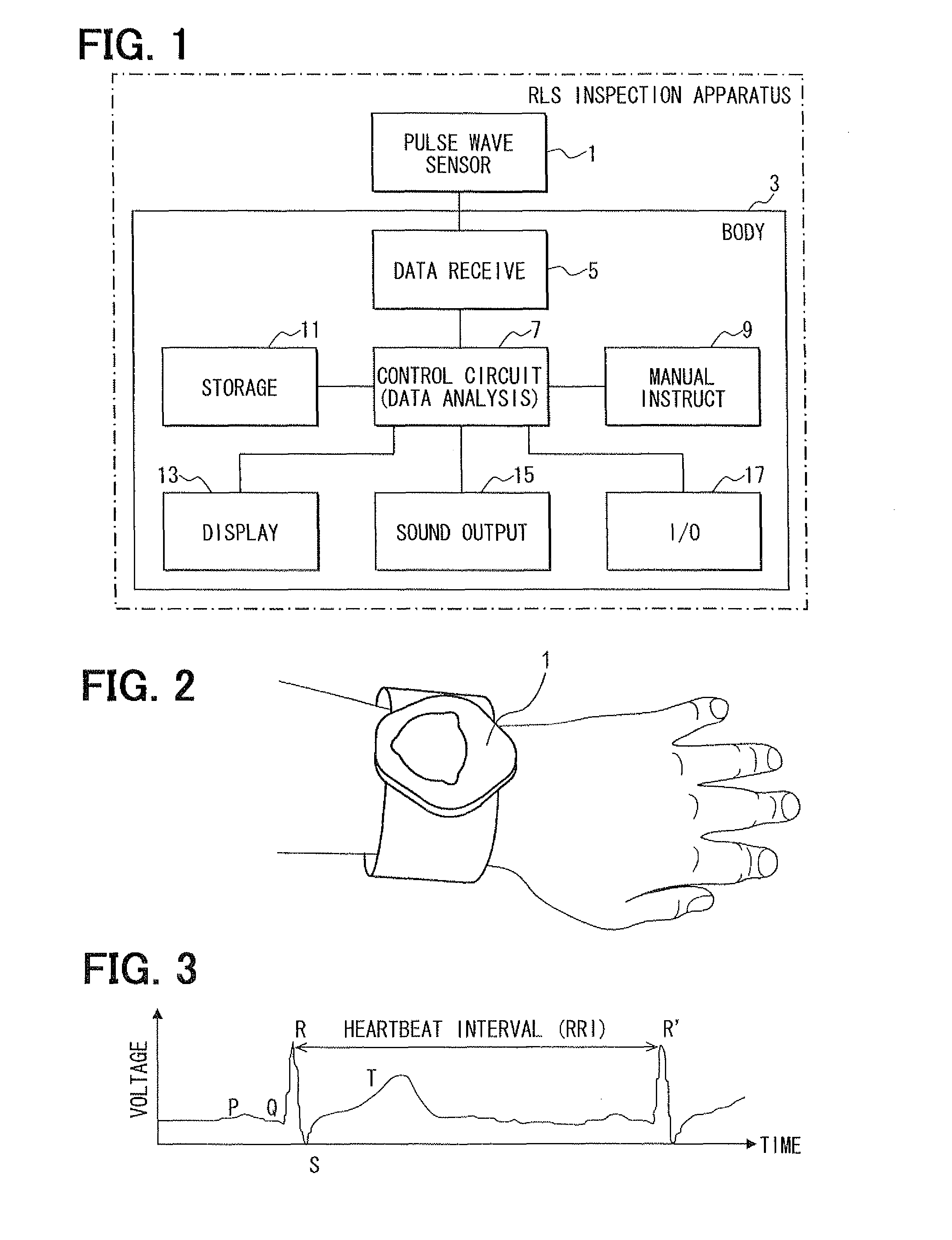
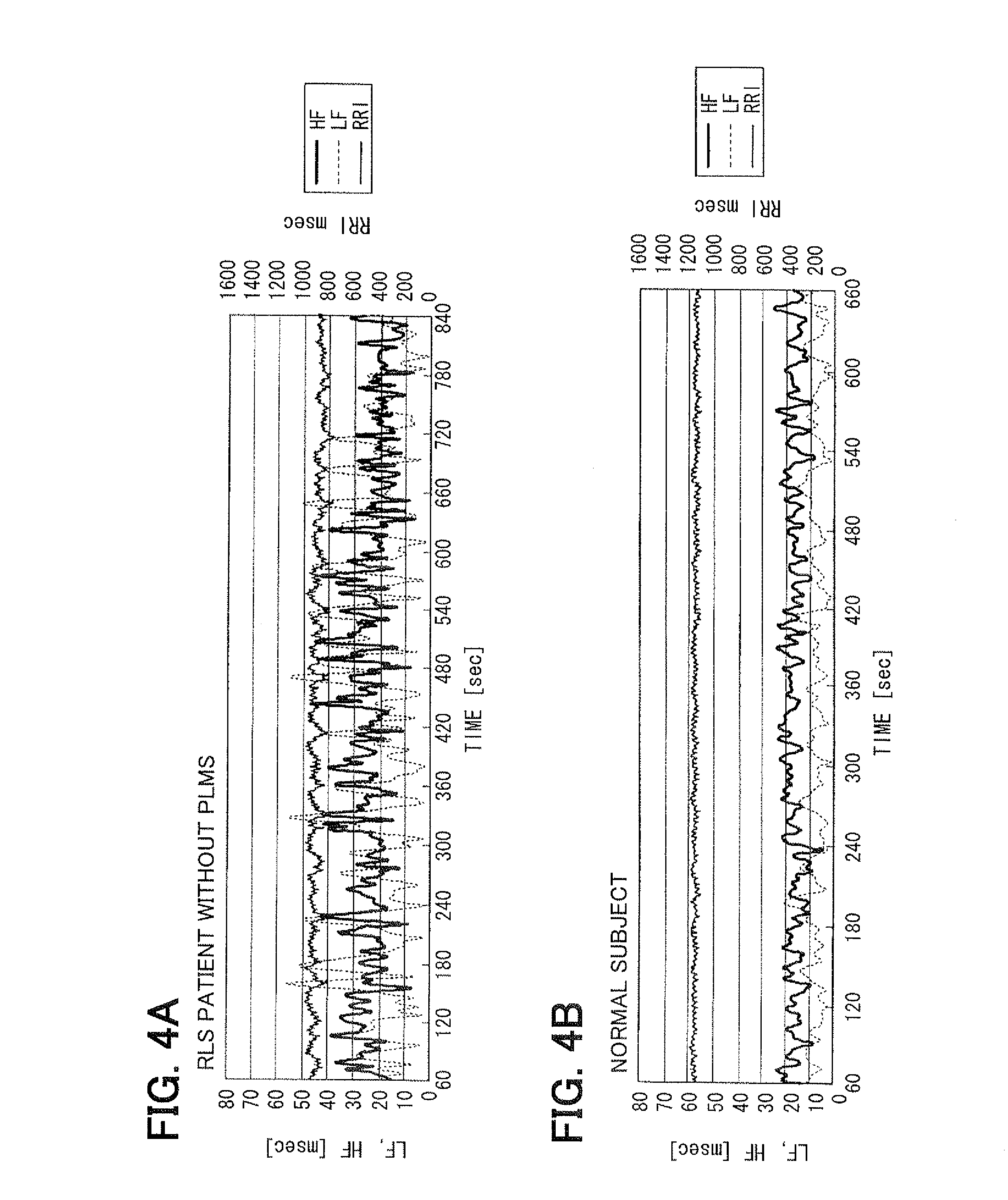
Living body inspection apparatus, and relevant method and program product
InactiveUS20100228139A1Simple technologyCatheterDiagnostic recording/measuringBiological bodyPR interval
In a living body inspection apparatus to inspect RLS (Restless Legs Syndrome), a pulse interval is obtained from a pulse wave signal, thereby performing a frequency analysis of the obtained pulse interval using COM. From a result of the frequency analysis, the low frequency components ranging from 0.04 to 0.15 Hz and the high frequency components ranging from 0.15 to 0.4 Hz are extracted. As an index posterior to an age amendment, low frequency components (LF) / high frequency components (HF) is obtained, for instance. It is then determined whether LF / HF is equal to or greater than a predetermined determination value indicating RLS. For example, it is determined whether LF / HF is equal to or greater than 0.65, which suspects RLS. It is determined whether a signal is accurately calculated which indicates an activity of autonomic nerve. A state of RLS is determined using LF / HF.
Owner:DENSO CORP +1
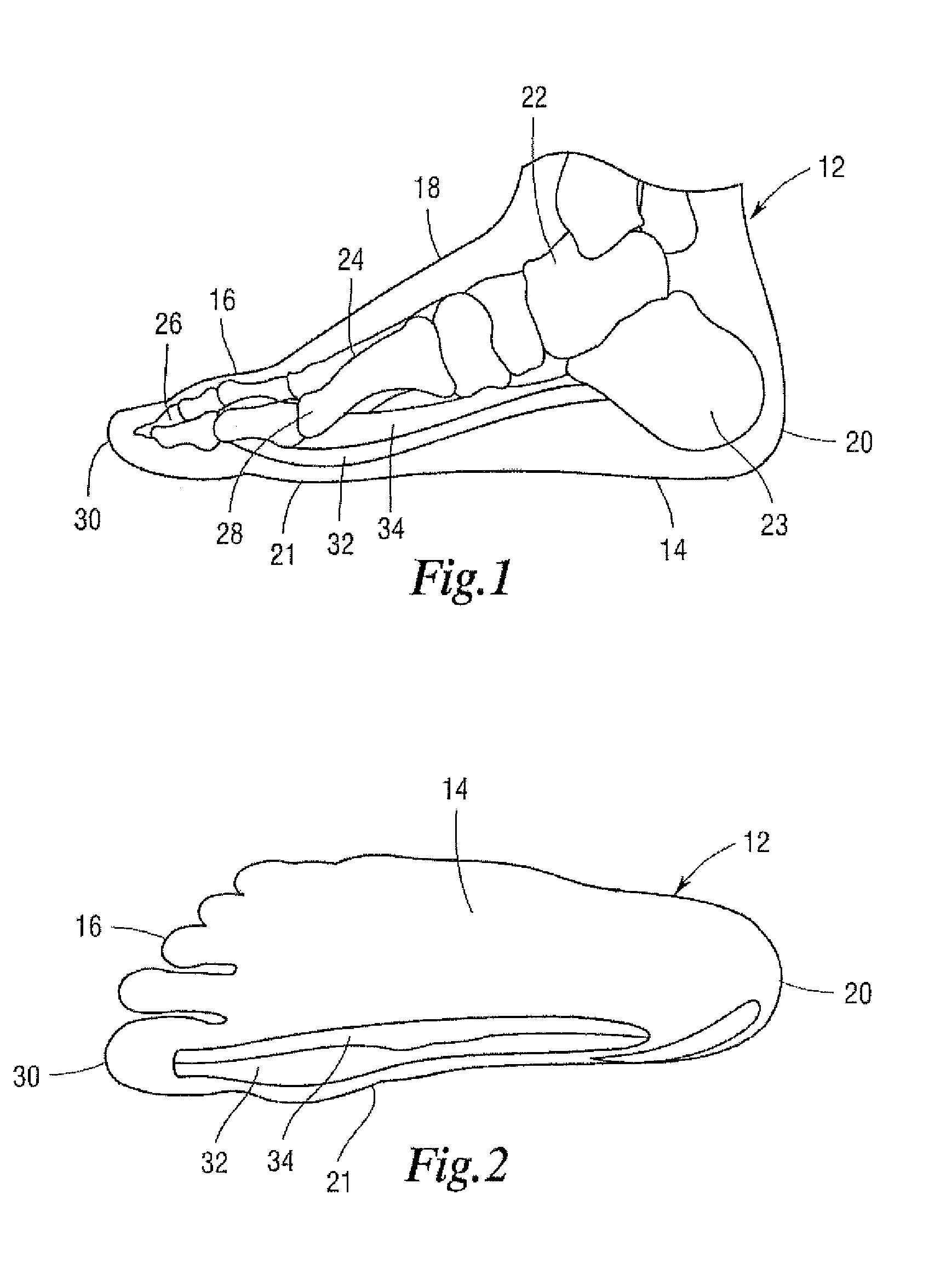
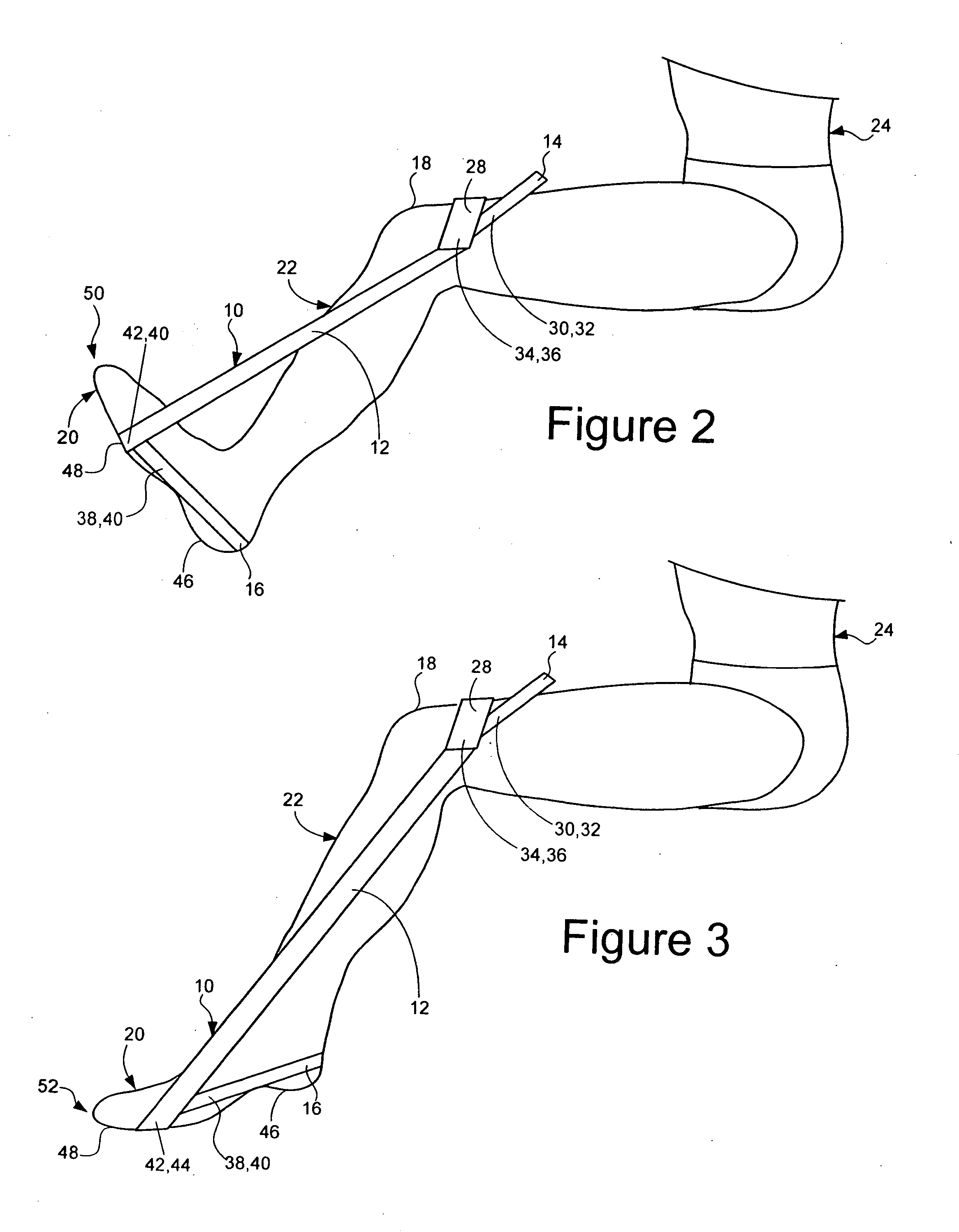
Device to Alleviate the Symptoms of Restless Leg Syndrome, Restless Arms Syndrome, and Foot and Leg Cramps
InactiveUS20100049111A1Relieve symptomsAlleviate foot and leg crampsSolesInsertsMuscle groupLeg cramps
A flexible foot relief pad that is wrapped about and secured to the individual's foot in order to relieve the symptoms of restless leg syndrome, restless arms, and foot and leg cramps includes a pliable cloth, wrap having a foot engaging portion joined to a securing portion by a fold, with the foot engaging portion enclosing a cavity for holding therein a layered raised pressure application pad that applies pressure to select areas of the inner side and sole of the foot with the layered pad configured so that a portion of the pad extends transverse to the sole of the foot and a raised portion extends along the inner side of the foot for applying pressure to the specific muscle groups involved in restless leg syndrome. The flexible foot relief pad also includes adjustable securement members that wrap around and encompass both the foot and the relief pad for securing the pad to the foot, and the point of attachment for each securement member is adjustable thereby providing for the even application of pressure against the specific areas of the sole of the foot or for varying the amount of pressure applied to such areas on the sole of the foot.
Owner:SORG MARY
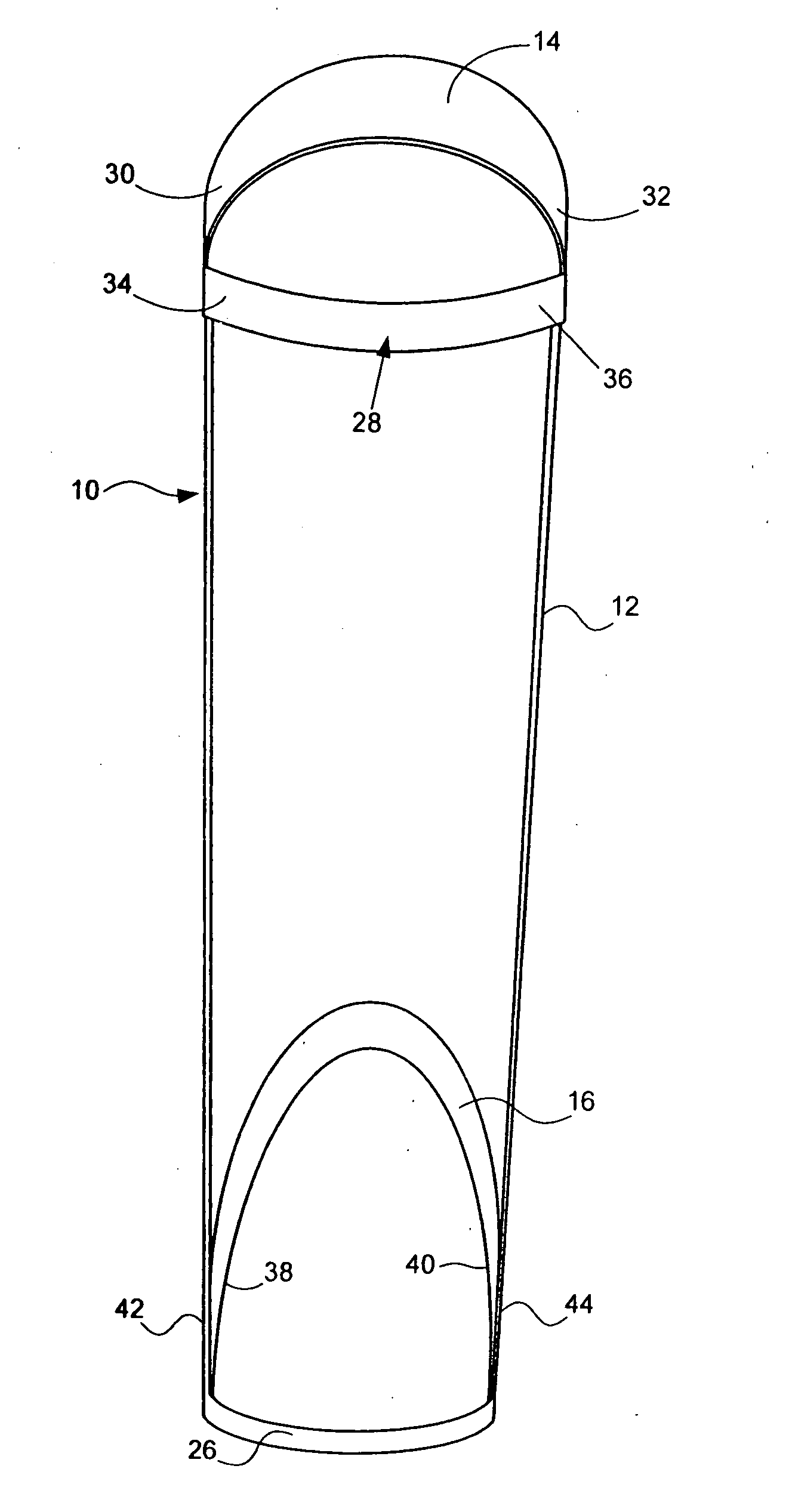
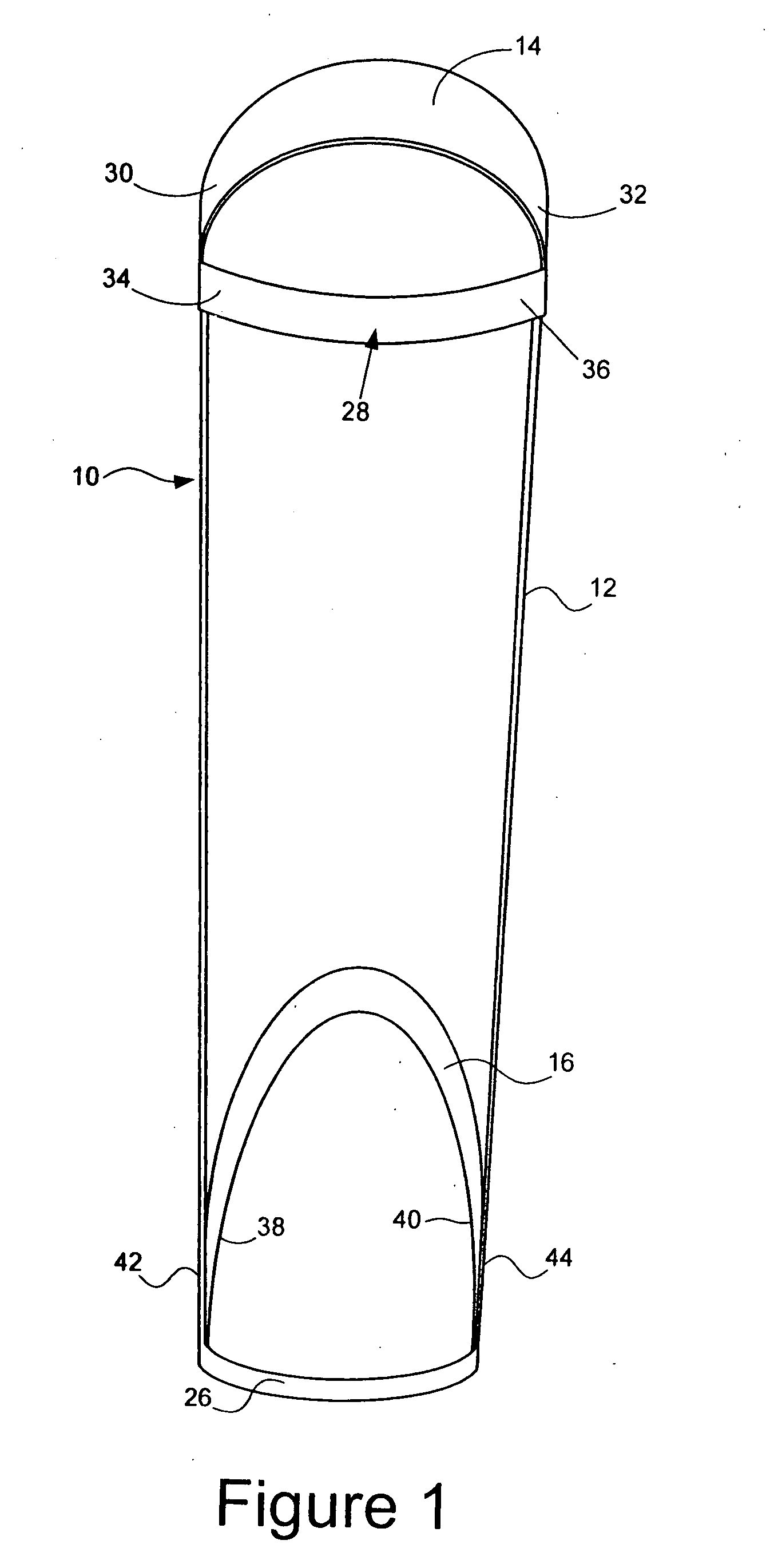
Method of alleviating symptoms of restless legs syndrome
InactiveUS20080039303A1Resilient force resistorsPhysical medicine and rehabilitationRestless legs syndrome
A method of alleviating symptoms of restless legs syndrome, includes (a) fitting an apparatus to a leg of a person afflicted with the symptoms of restless legs syndrome during a period rest, where the apparatus includes means for retarding movement of a foot of the leg of the person as the foot moves from a first position towards a second position; and (b) before the period of rest, alternately moving the foot between the first position and the second position, where the apparatus retards movement of the foot as the foot moves from the first position towards the second position.
Owner:RODNEY EDWARD WILCOCKS
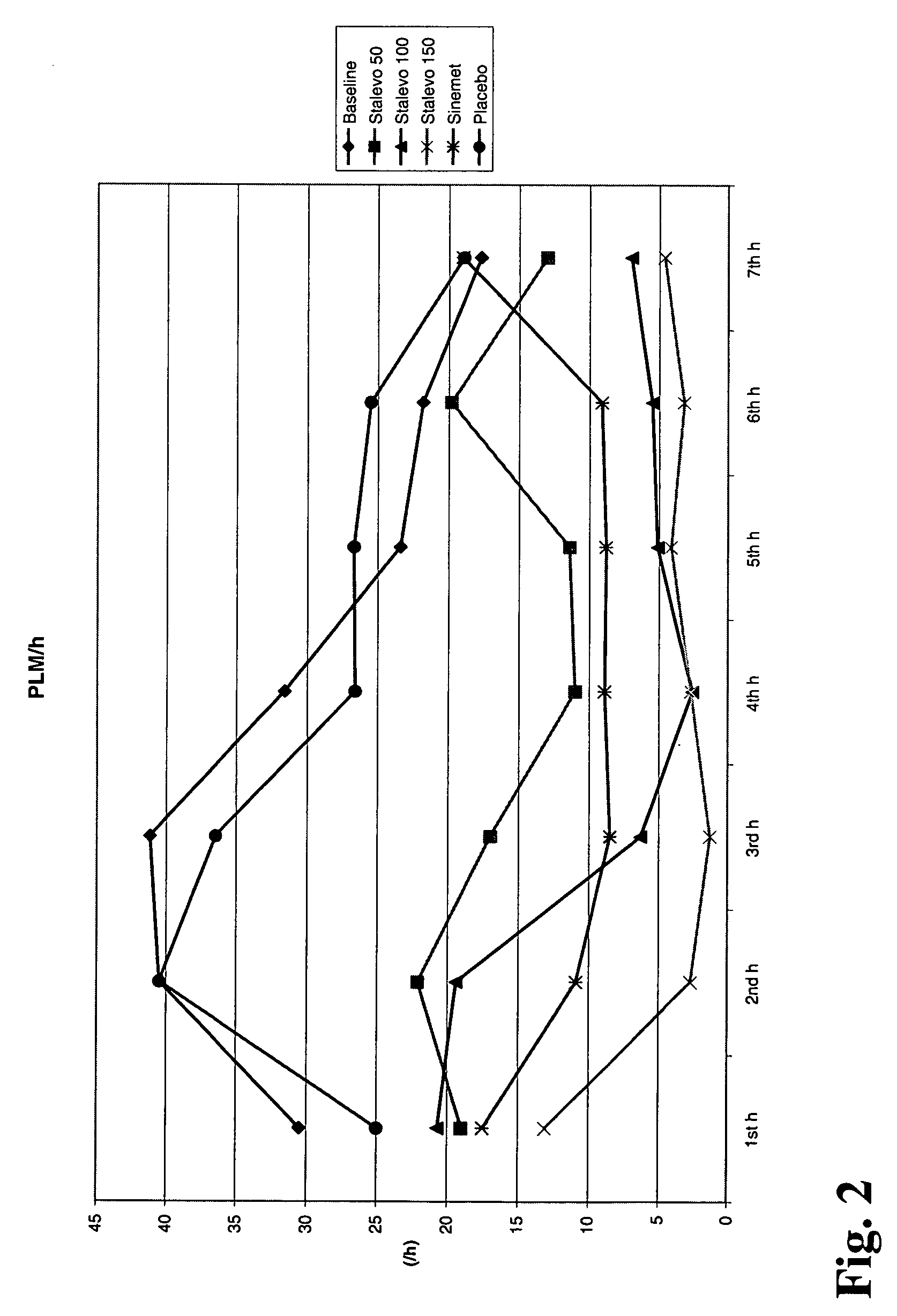
Adenosine A2a receptor antagonists for the treatment of extra-pyramidal syndrome and other movement disorders
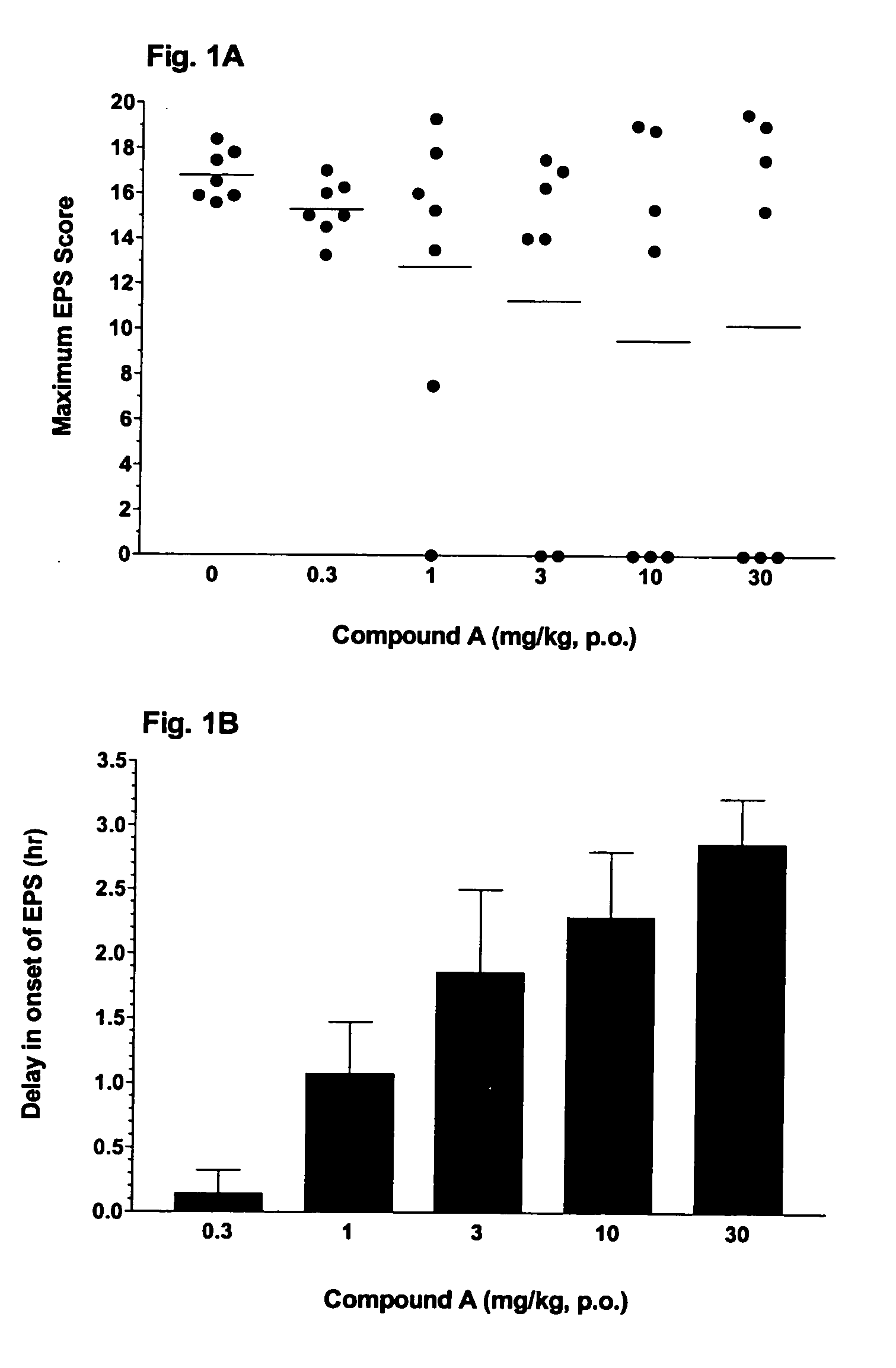
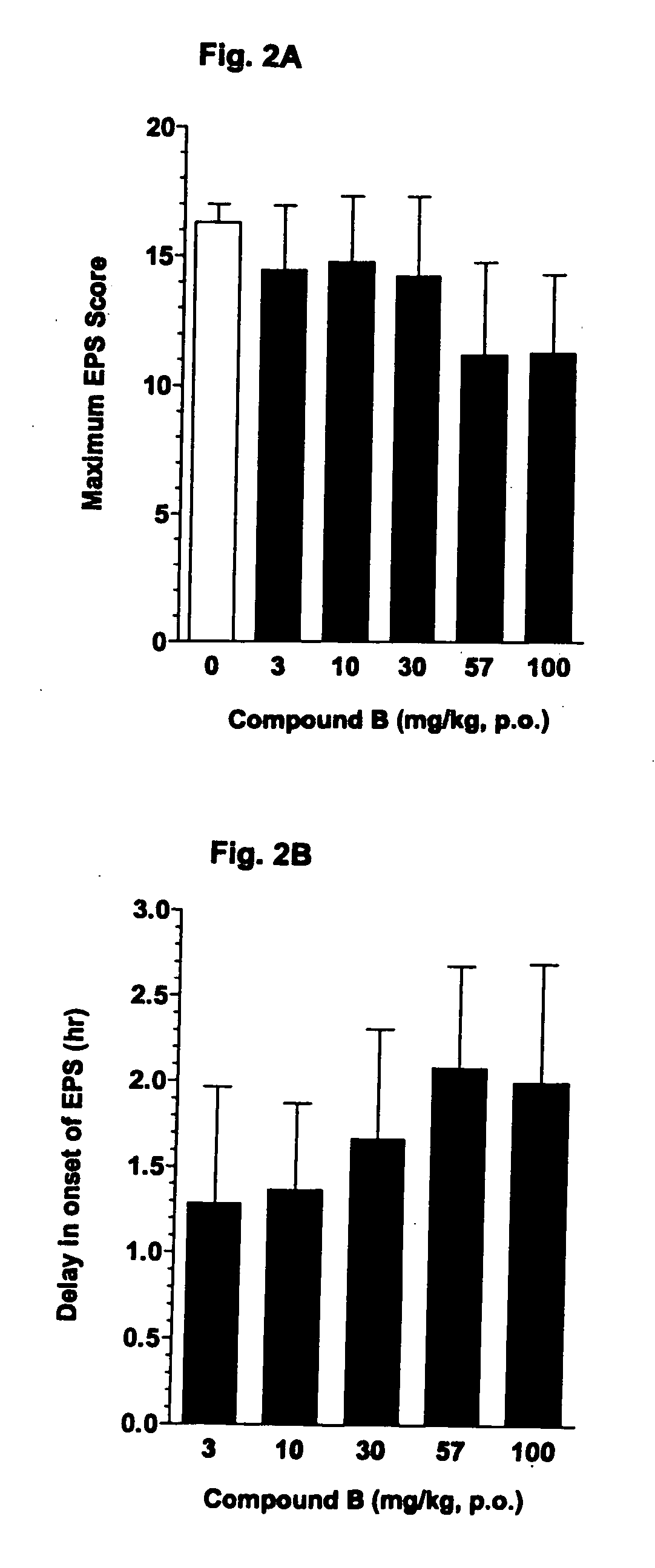
There is disclosed a method for the treatment or prevention of Extra Pyramidal syndrome (EPS), dystonia, restless legs syndrome (RLS) or periodic leg movement in sleep (PLMS) comprising the administration of an adenosine A2a receptor antagonist, alone or in combination with other agents useful for treating EPS, dystonia, RLS or PLMS; also claimed are pharmaceutical compositions consisting of an adenosine A2a receptor antagonist in combination with an antipsychotic agent, an anticonvulsant agent, lithium or an opioid.
Owner:SCHERING CORP
Method and apparatus for treating restless legs syndrome
InactiveUS20160354604A1Reduces and/or prevents on the onset of one or more RLS symptomsExternal electrodesArtificial respirationFemoral nerveElectrical stimulations
Owner:INVICTA MEDICAL
Restless leg syndrome treatment
The present invention describes a method of treating restless leg syndrome by eliminating venous reflux in an underlying vein. The malfunctioning vein can be removed or ablated by inserting a catheter into the vein that transmits sufficient energy to coagulate or ablate the lining of the vein causing it to permanently close, eliminating the source of venous reflux and the symptom of restless leg syndrome.
Owner:NEW STAR LASERS
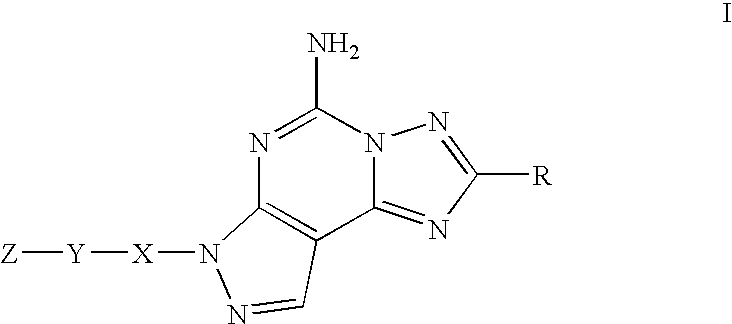
7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine
The compound having the structural formula I or a pharmaceutically acceptable salt thereof, is disclosed, as well as its use in the treatment of central nervous system diseases, in particular Parkinson's disease, Extra Pyramidal Syndrome, restless legs syndrome and attention deficit hyperactivity disorder, pharmaceutical compositions comprising it, and combinations with other agents.
Owner:MERCK SHARP & DOHME LLC
Systems and methods for treating essential tremor or restless leg syndrome using spinal cord stimulation
InactiveUS20150066105A1Reduce, alleviate, or eliminate at least one adverse effectSpinal electrodesExternal electrodesEssential tremorMedicine
A method for treating essential tremor or restless leg syndrome using spinal cord stimulation includes implanting a lead near a spinal cord of a patient. The lead includes a plurality of electrodes disposed on a distal end of the lead and electrically coupled to at least one contact terminal disposed on a proximal end of the lead. Electrical signals are provided from a control module coupled to the lead to stimulate a portion of the spinal cord of the patient using at least one of the electrodes. The electrical signals reduce, alleviate, or eliminate at least one adverse effect of essential tremor or restless leg syndrome.
Owner:BOSTON SCI NEUROMODULATION CORP
7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine
Owner:MERCK SHARP & DOHME LLC
Remedies for psychoneurosis
A therapeutic drug for psychoneurotic disorders, which is useful for therapies of psychoneurotic disorders, especially restless legs syndrome is disclosed. The therapeutic drug for psychoneurotic disorders according to the present invention comprises as an effective ingredient an opioid κ receptor agonist compound (excluding pentazocine) such as (−)-17-(cyclopropylmethyl)-3,14β-dihydroxy-4,5α-epoxy-6β[N-methyl-trans-3-(3-furyl)acrylamide]morphinan hydrochloric acid salt.
Owner:TORAY IND INC
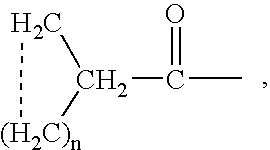
Alpha2delta ligands for fibromyalgia and other disorders
InactiveUS7164034B2Increasing slow wave sleepBiocidePeptide/protein ingredientsDiseaseOctanoic Acids
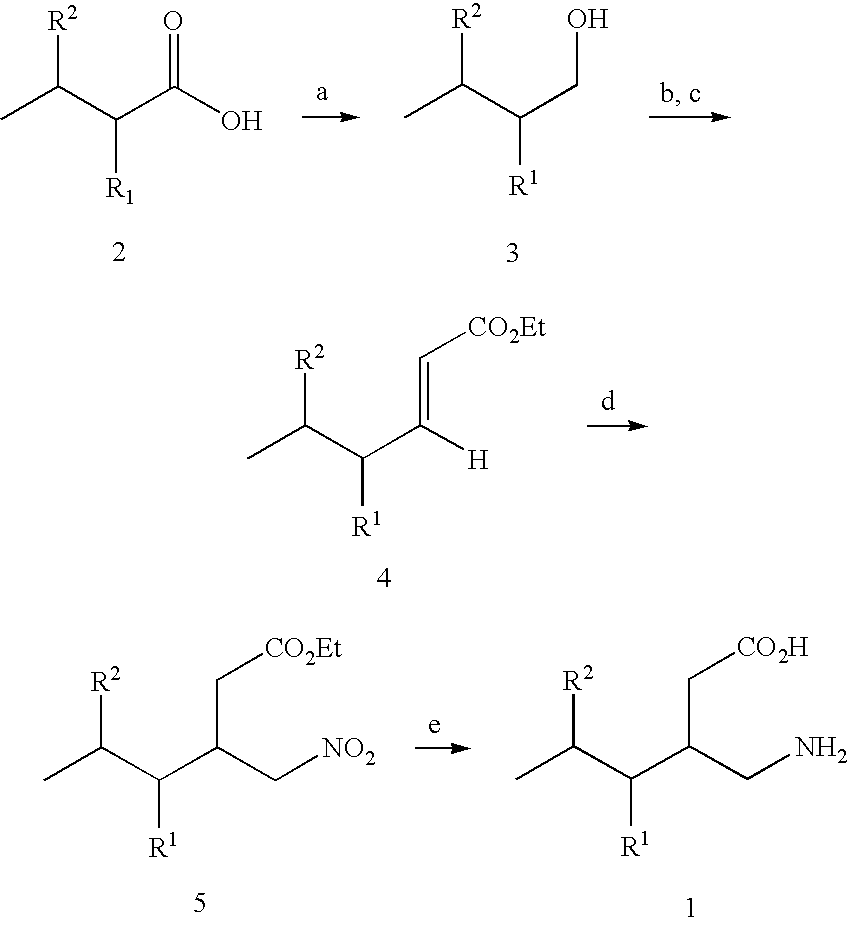
This invention relates to a method of treating a disorder selected from OCD, agoraphobia, agoraphobia without history of panic disorder, specific phobia, social phobia, PTSD, restless legs syndrome, premenstrual dysphoric disorder, hot flashes, and fibromyalgia by administering a compound of the formula 1or a pharmaceutically acceptable salt thereof, wherein:R1 is hydrogen, straight or branched alkyl of from 1 to 6 carbon atoms or phenyl; andR2 is straight or branched alkyl of from 4 to 8 carbon atoms, straight or branched alkenyl of from 2 to 8 carbon atoms, cycloalkyl of from 3 to 7 carbon atoms, alkoxy of from 1 to 6 carbon atoms, -alkylcycloalkyl, -alkylalkoxy, -alkyl OH, -alkylphenyl, -alkylphenoxy, or -substituted phenyl. The invention also relates to a method of treating the above disorders by administering the compound (3S,5R)-3-Aminomethyl-5-methyl-octanoic acid.
Owner:PFIZER INC
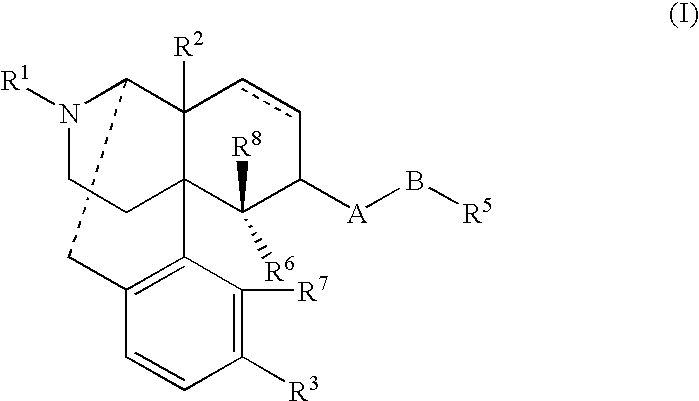
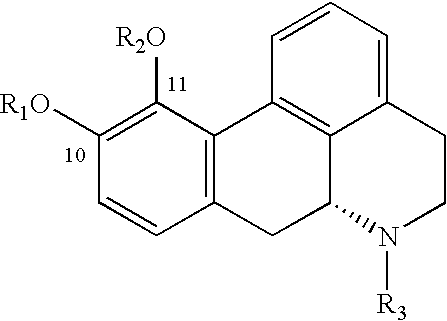
Aporphine esters and their use in therapy
InactiveUS20050143408A1Improve bioavailabilityLong duration of actionBiocideNervous disorderMedicineSexual dysfunction
New aporphine derivatives are disclosed which have formula (I) and the physiologically acceptable salts thereof. Said derivatives may be used for the treatment of Parkinson's disease, hemicrania, restless legs syndrome (RLS), sexual dysfunction in men and women, hyperprolactemia and psychotic disorders, and / or evaluation of Parkinson's disease. Processes for the preparation of such derivatives are also disclosed.
Owner:AXON BIOCHEMICALS BV
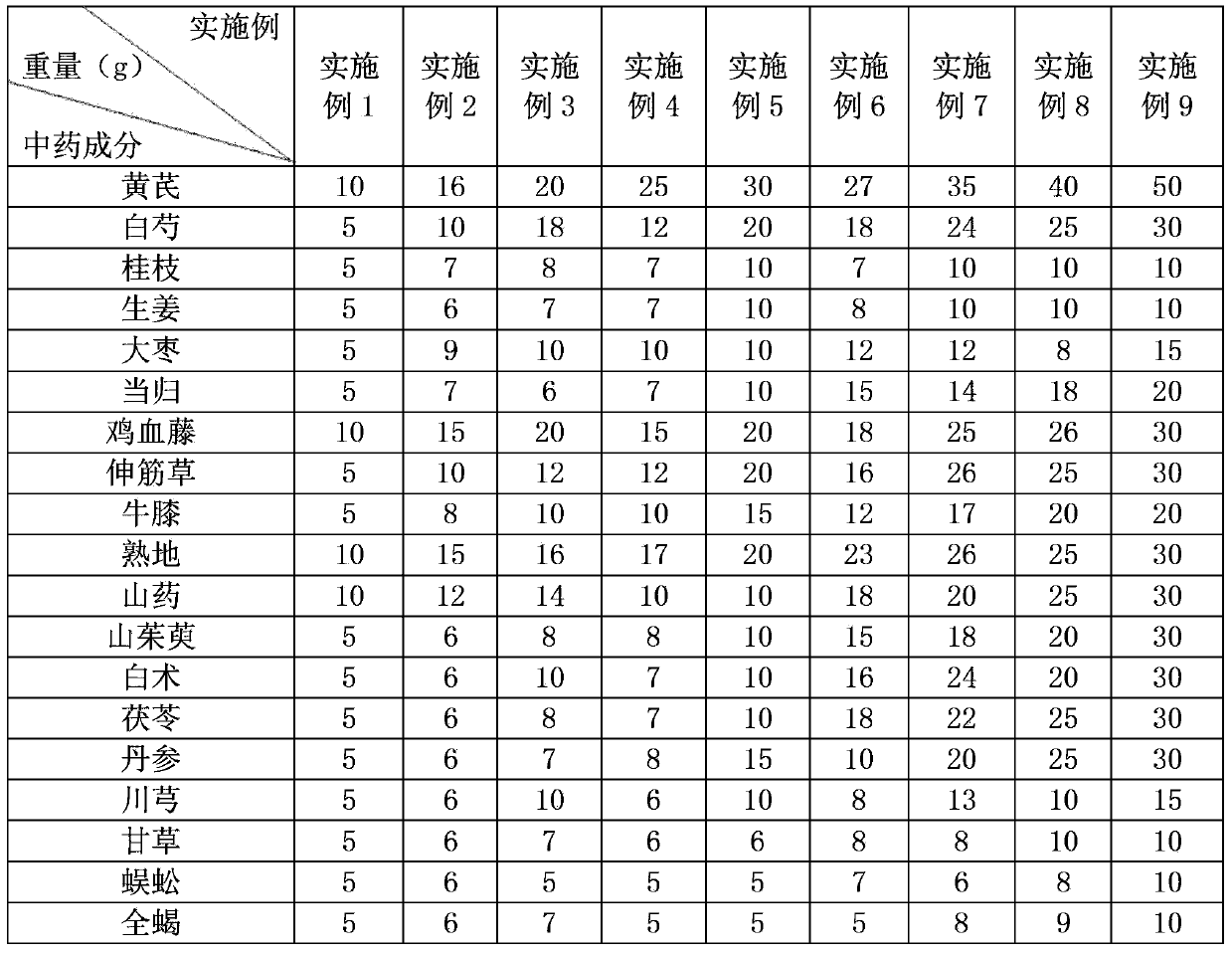
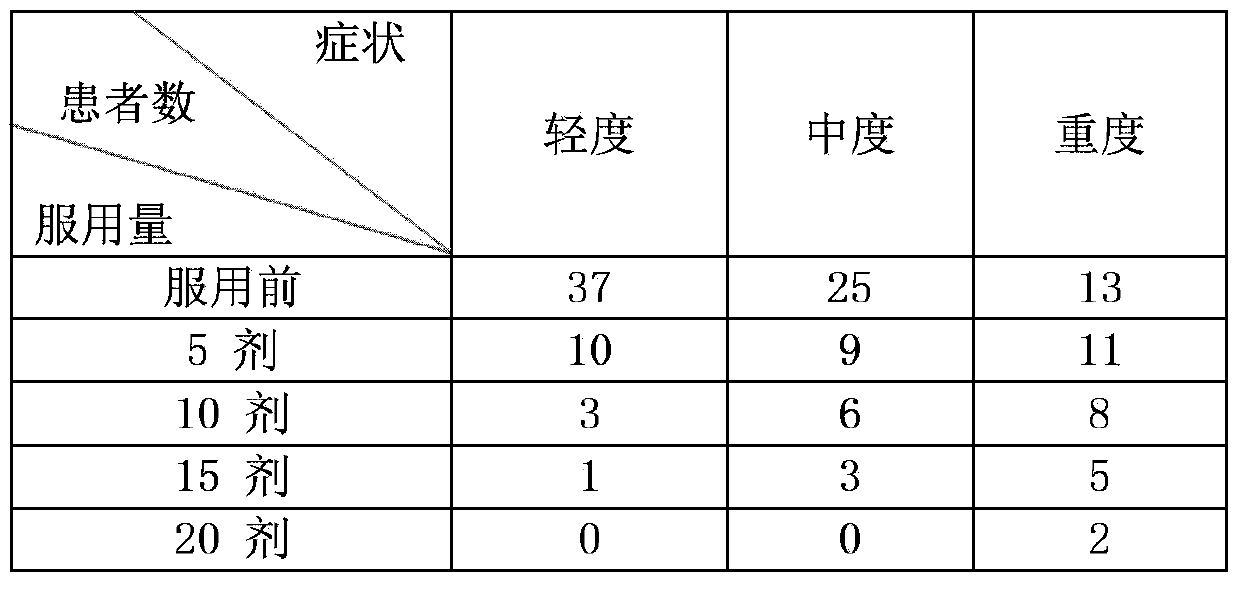
Traditional Chinese medicine composition for treating restless legs syndrome
ActiveCN103463586AShort course of treatmentHigh cure rateNervous disorderAnthropod material medical ingredientsTreatment effectLycopodium clavatum
The invention discloses a traditional Chinese medicine composition for treating restless legs syndrome and belongs to the technical field of traditional Chinese medicine compositions. The traditional Chinese medicine composition comprises the following components in parts by weight: 10-50 parts of astragalus, 5-30 parts of white paeony root, 5-10 parts of cassia twig, 5-10 parts of ginger, 5-15 parts of jujube, 5-20 parts of angelica, 10-30 parts of suberect spatholobus stem, 5-30 parts of lycopodium clavatum, 5-20 parts of radix achyranthis bidentatae, 10-30 parts of prepared rhizome of rehmannia, 10-30 parts of rhizoma dioscoreae, 5-30 parts of cornus officinalis, 5-30 parts of atractylis ovata, 5-30 parts of poria cocos, 5-30 parts of salvia miltiorrhizae, 5-15 parts of szechuan lovage rhizome, 5-10 parts of radix glycyrrhizae, 5-10 parts of centipede and 5-10 parts of scorpion. The traditional Chinese medicine composition provided by the invention has a remarkable pharmacological effect on restless legs syndrome; on the whole, the traditional Chinese medicine composition provided by the invention has the advantages of short treatment course, high cure rate (above 95%), remarkable curative effect and low cost in treating restless legs syndrome.
Owner:YINGSHAN COUNTY PEOPLES HOSPITAL +1
Non-Contact Sonic Treatment for Restless Legs Syndrome
InactiveUS20160030280A1Improve sleep qualityLow frequency beatChiropractic devicesVibration massageMedicineRestless legs syndrome
Owner:SENSORY NEUROSTIMULATION
Multi-Day Patch for the Transdermal Administration of Rotigotine
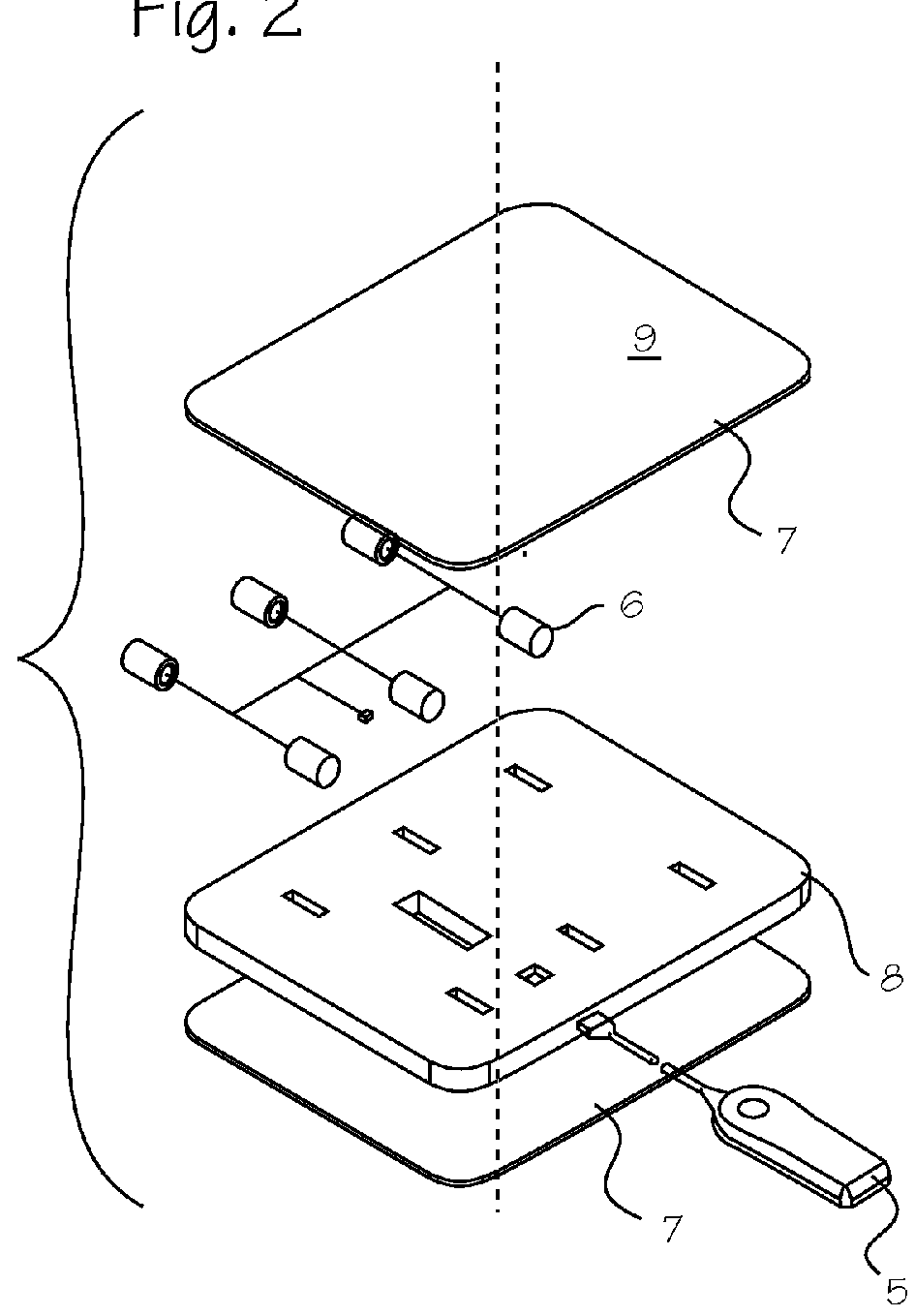
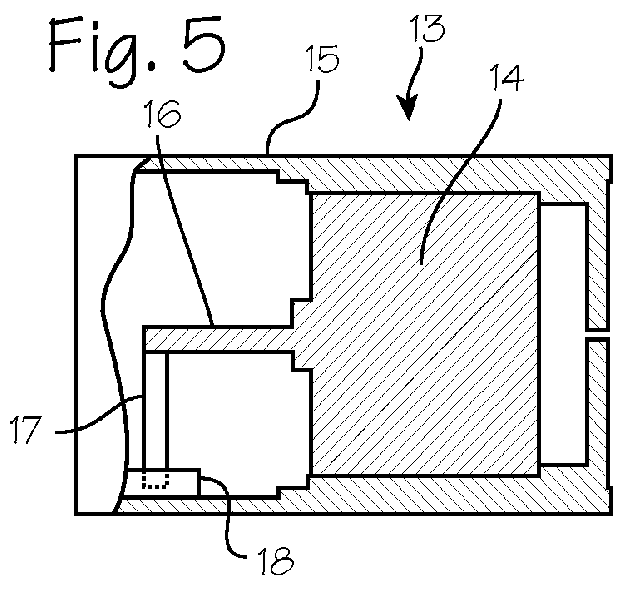
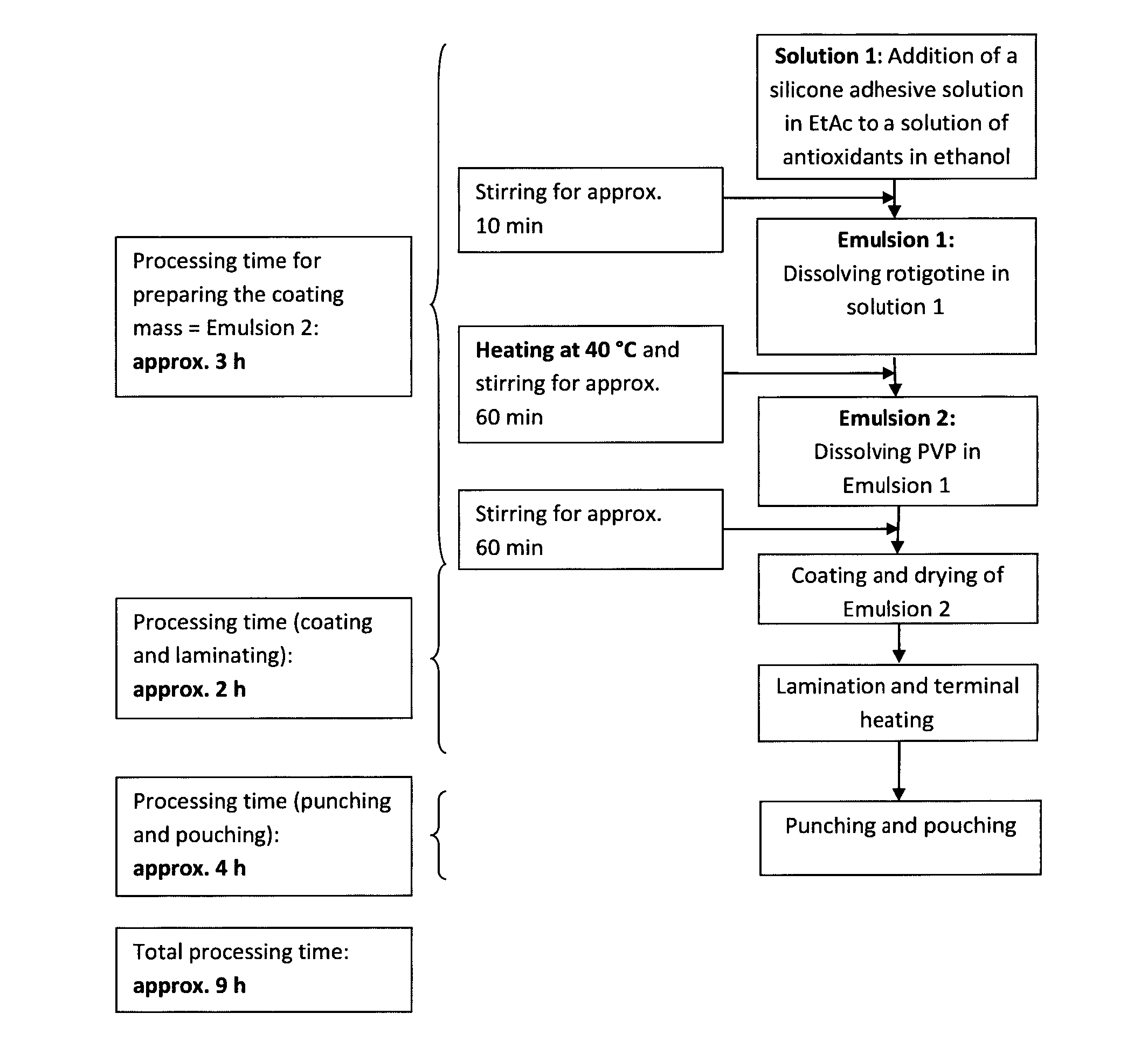
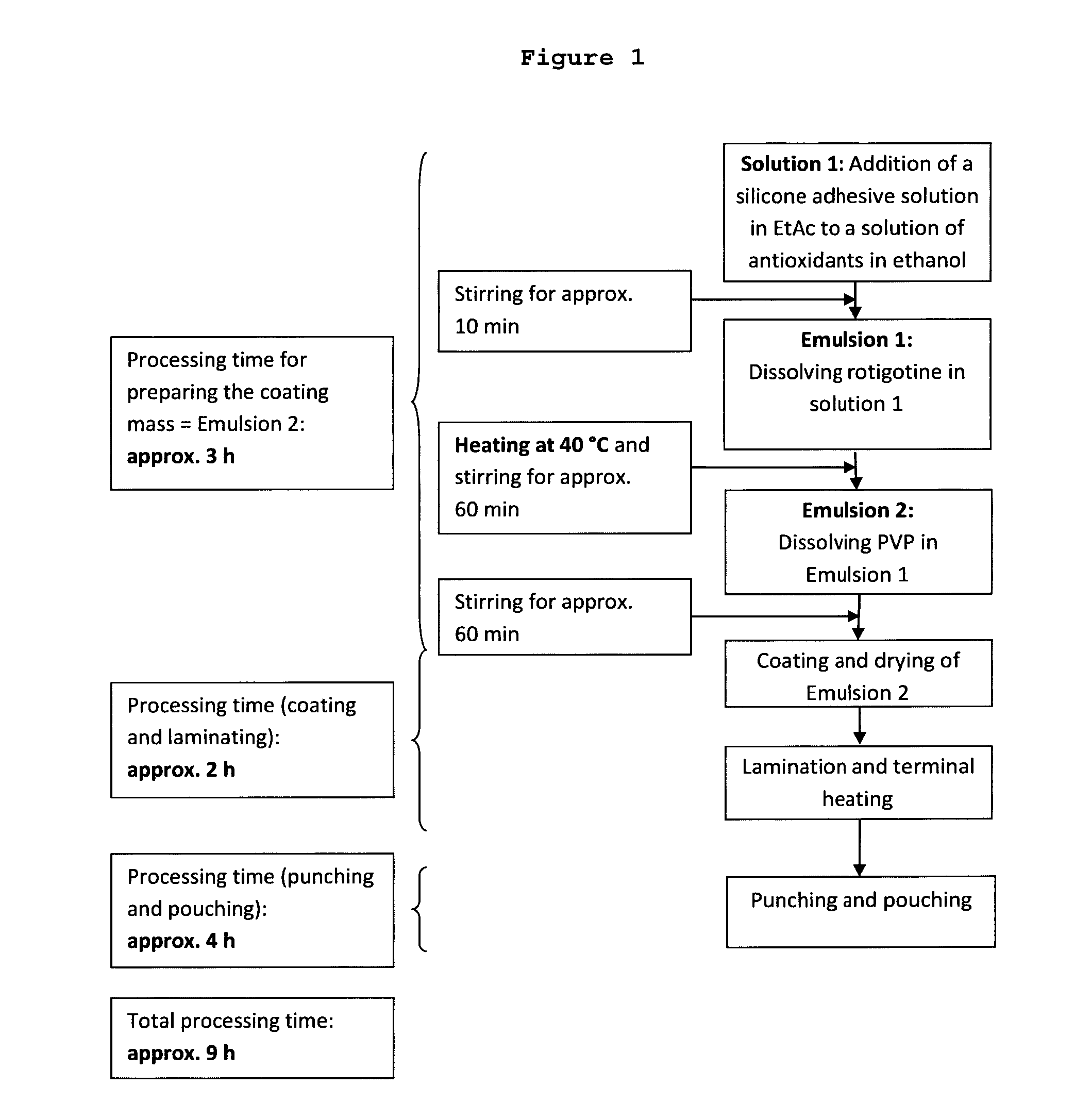
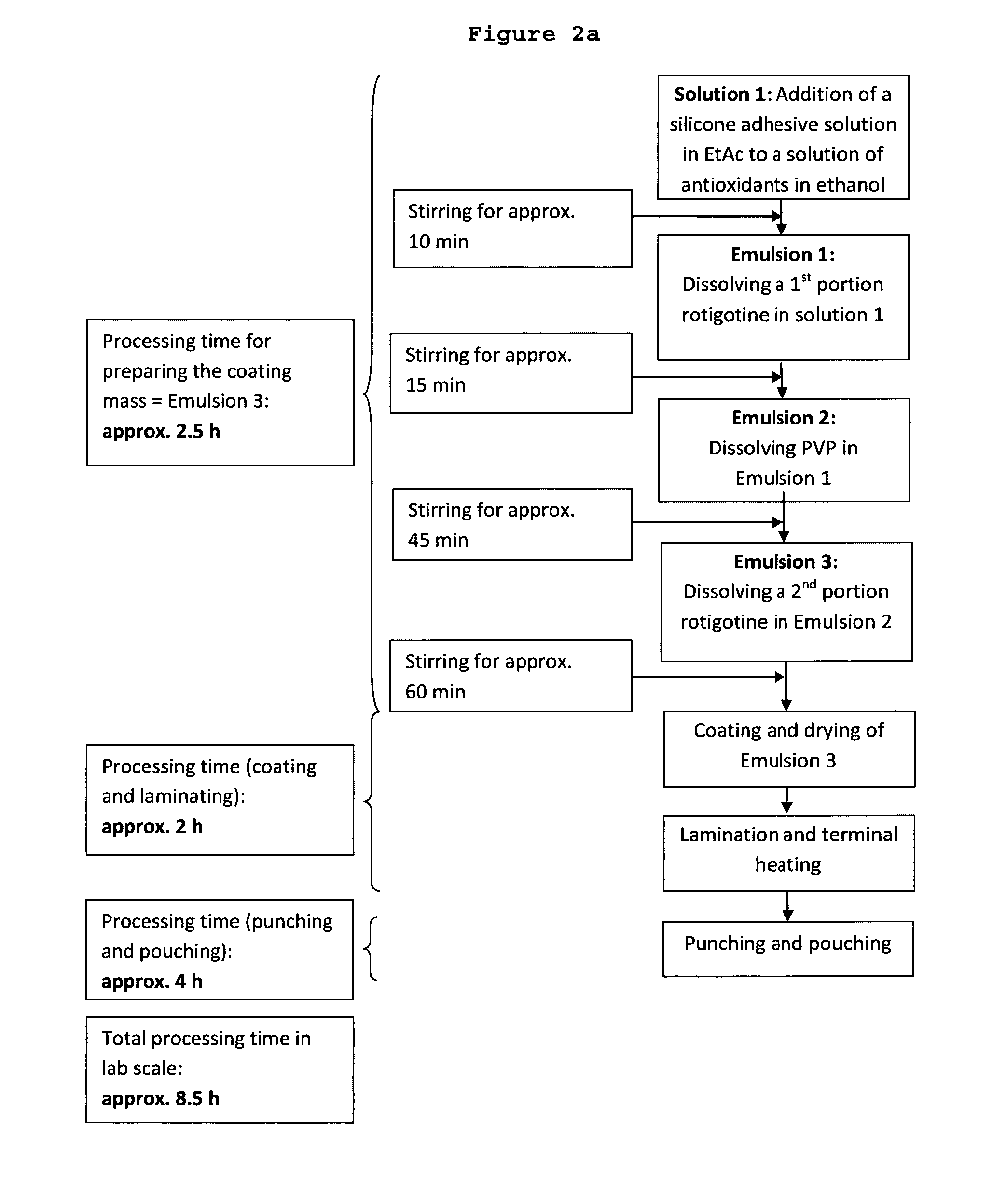
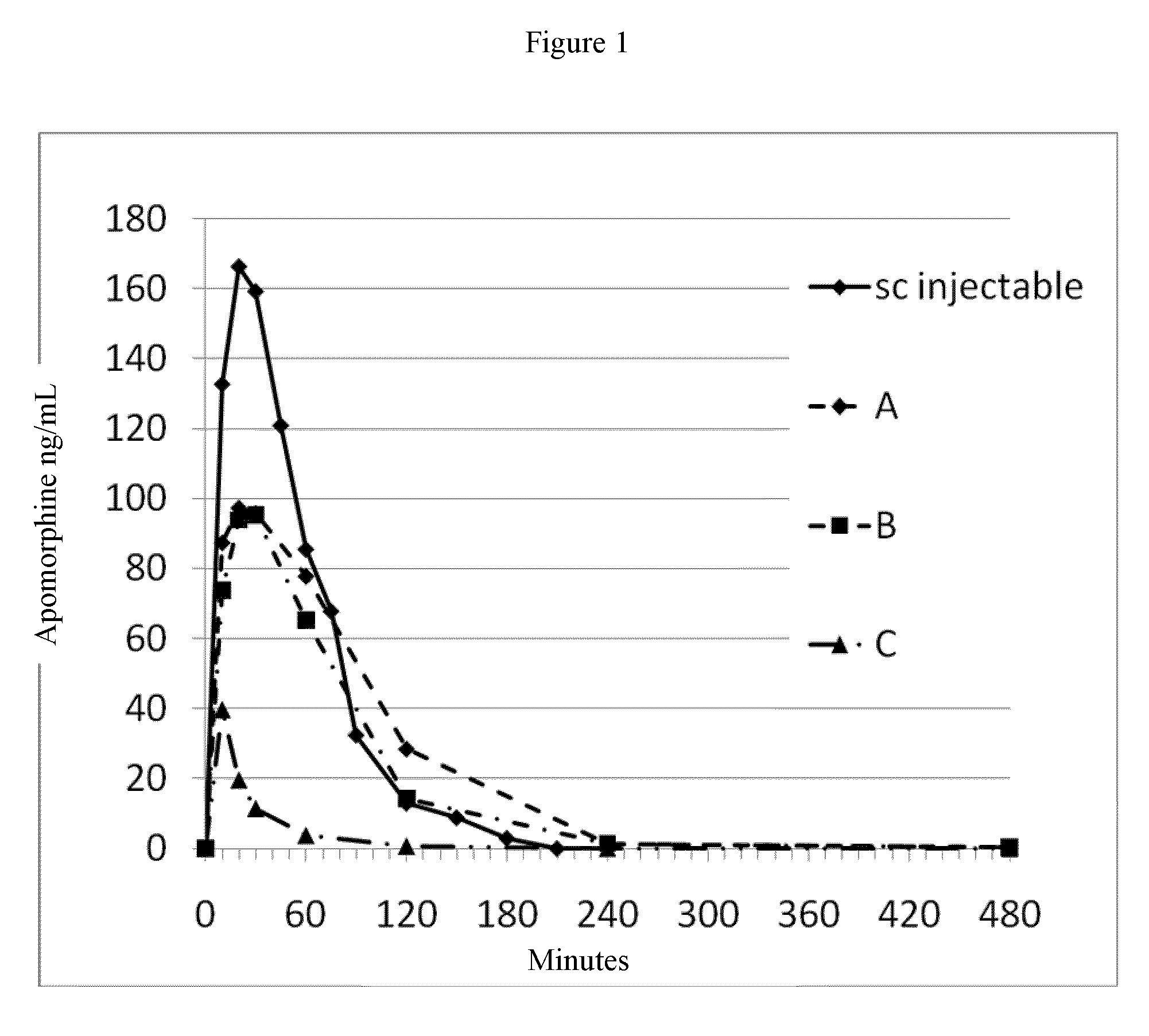
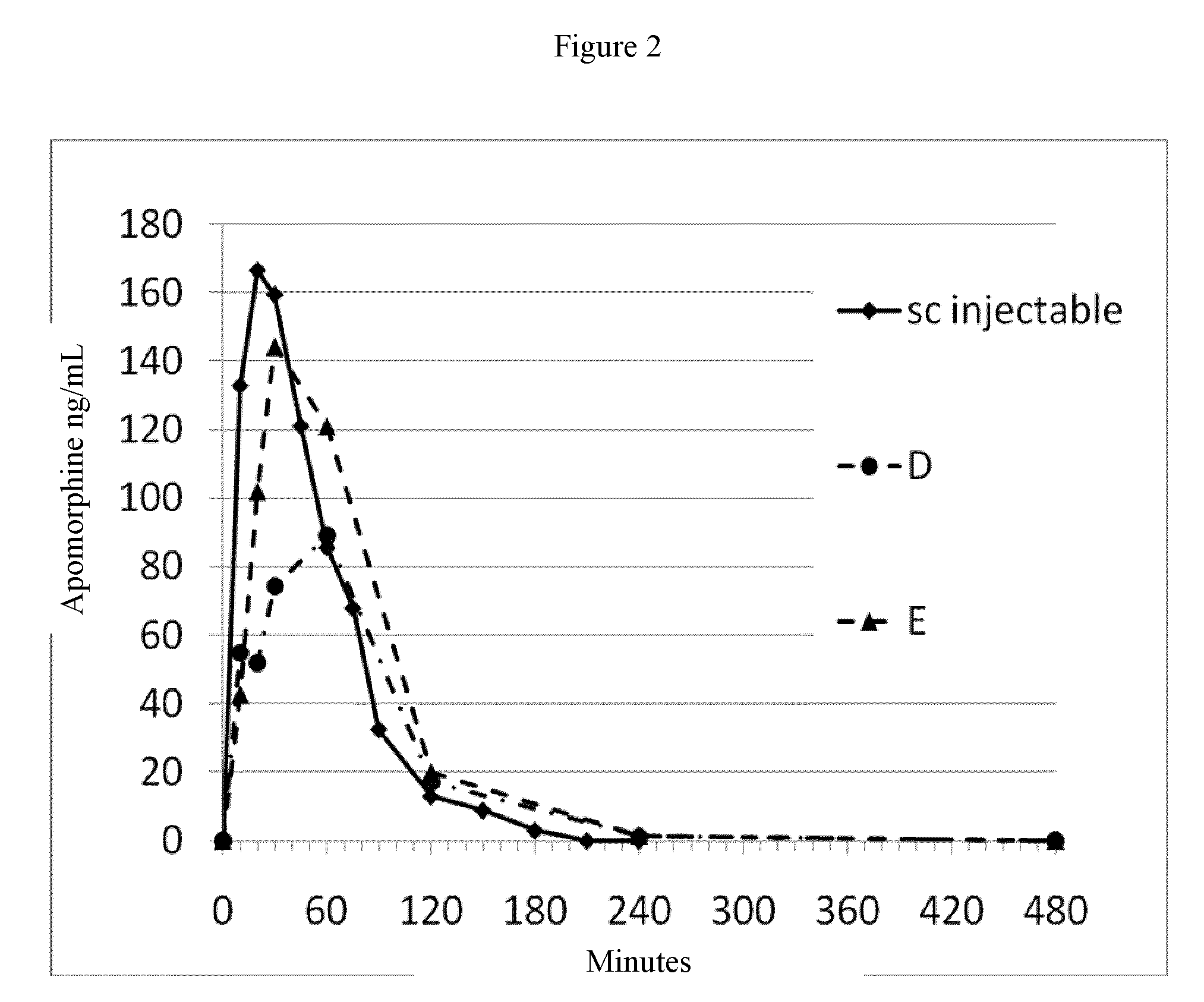
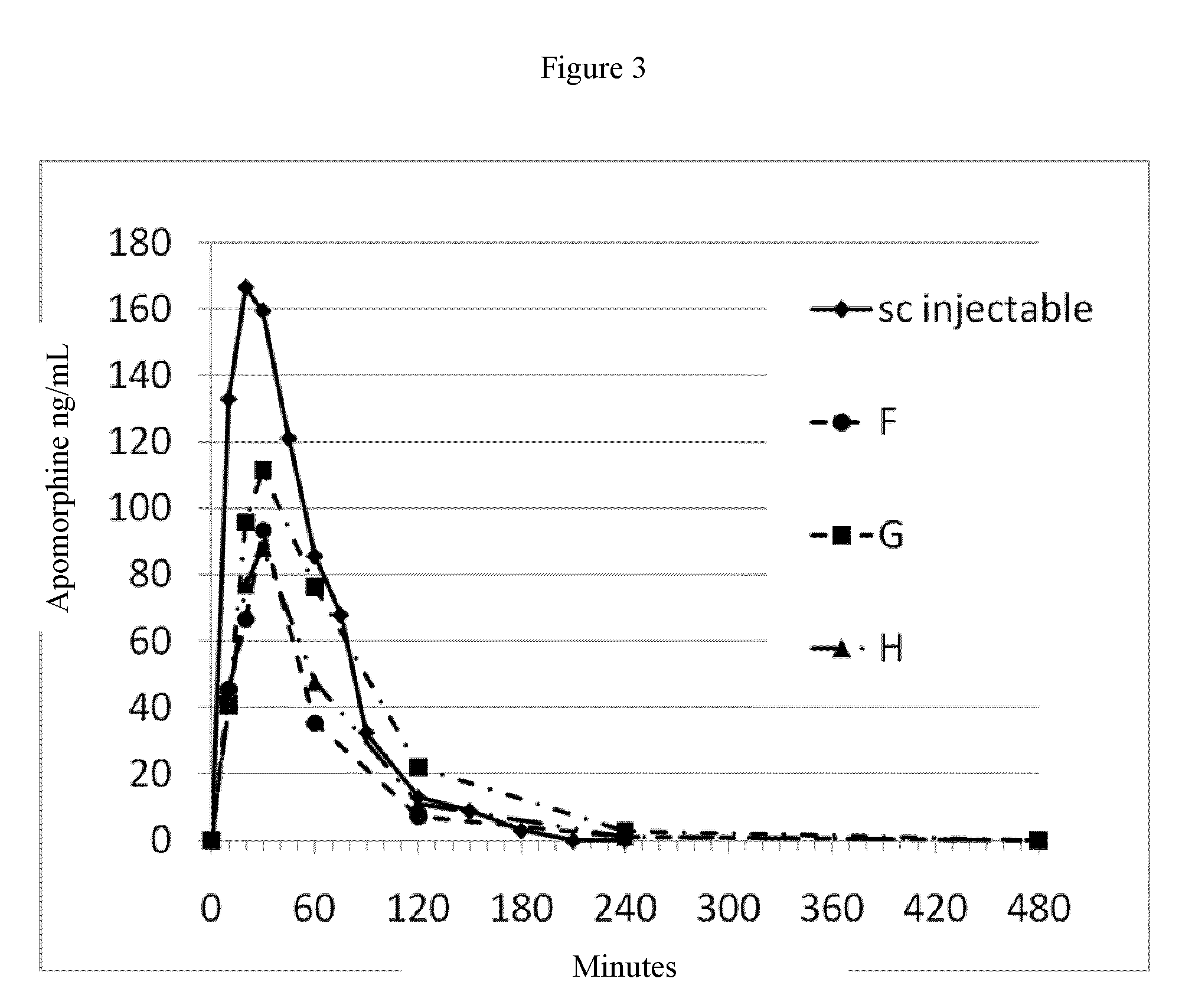
ActiveUS20150290142A1Easily and cost-effectively be preparedReduce application frequencyOrganic active ingredientsNervous disorderSolvent basedCognitive diseases
The present invention relates to a transdermal therapeutic system, comprising (a) a backing layer, (b) a solvent-based self-adhesive matrix layer containing rotigotine as active ingredient, and (c) a release liner, wherein the self-adhesive matrix layer has a coating weight of about 75-400 g / m2 and comprises a reservoir layer containing about 9-25 wt.-% rotigotine based on the weight of the reservoir layer, a kit comprising two transdermal therapeutic systems of the present invention as well as a method for the preparation of the transdermal therapeutic system of the present invention. In addition, the present invention relates to a transdermal therapeutic system comprising rotigotine as active ingredient for use in the treatment of patients suffering from Parkinson's disease, Parkinson's plus syndrome, depression, fibromyalgia and the restless-legs syndrome and for use in the treatment or prevention of dopaminergic neuron loss or cognitive disorders by transdermal administration of rotigotine once or twice weekly, wherein the transdermal therapeutic system comprises a backing layer, a solvent-based rotigotine containing self-adhesive matrix layer as well as a release liner and is adapted to allow for the transdermal administration of therapeutically effective amounts of rotigotine for at least 3 days.
Owner:LTS LOHMANN THERAPIE-SYST AG
Nicotinic acetylcholine receptor antagonists in the treatment of restless legs syndrome
InactiveUS20050250806A1BiocideDrug compositionsPharmaceutical drugNicotinic Acetylcholine Receptor Agonist
This invention relates to the use of nicotinic acetylcholine receptor agonists for the treatment of restless legs syndrome (RLS). The invention further relates to the use of a nicotinic acetylcholine receptor agonist in the manufacture of a medicament for the treatment of RLS. The present invention also relates to a pharmaceutical composition for the treatment of RLS containing a nicotinic acetylcholine receptor agonist.
Owner:PFIZER INC
Aporphine esters and their use in therapy
InactiveUS7238705B2Improve bioavailabilityLong duration of actionBiocideNervous disorderDiseaseSexual dysfunction
New aporphine derivatives are disclosed which have formula (I) and the physiologically acceptable salts thereof. Said derivatives may be used for the treatment of Parkinson's disease, hemicrania, restless legs syndrome (RLS), sexual dysfunction in men and women, hyperprolactemia and psychotic disorders, and / or evaluation of Parkinson's disease. Processes for the preparation of such derivatives are also disclosed.
Owner:AXON BIOCHEMICALS BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine 7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine](https://images-eureka.patsnap.com/patent_img/c4eebf24-e2e2-4443-9b85-e599b5d04af7/US20070072867A1-20070329-C00001.png)
![7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine 7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine](https://images-eureka.patsnap.com/patent_img/c4eebf24-e2e2-4443-9b85-e599b5d04af7/US20070072867A1-20070329-C00002.png)
![7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine 7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-YL)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-c]-pyrimidin-5amine](https://images-eureka.patsnap.com/patent_img/c4eebf24-e2e2-4443-9b85-e599b5d04af7/US20070072867A1-20070329-C00003.png)
![7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine 7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine](https://images-eureka.patsnap.com/patent_img/e1a3c287-9418-4ffe-9578-52d2e65162a3/US07572802-20090811-C00001.png)
![7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine 7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine](https://images-eureka.patsnap.com/patent_img/e1a3c287-9418-4ffe-9578-52d2e65162a3/US07572802-20090811-C00002.png)
![7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine 7-[2-[4-(6-fluoro-3-methyl-1,2-benzisoxazol-5-yl)-1-piperazinyl]ethyl]-2-(1-propynyl)-7H-pyrazolo-[4,3-e]-[1,2,4]-triazolo-[1,5-C]-pyrimidin-5-amine](https://images-eureka.patsnap.com/patent_img/e1a3c287-9418-4ffe-9578-52d2e65162a3/US07572802-20090811-C00003.png)