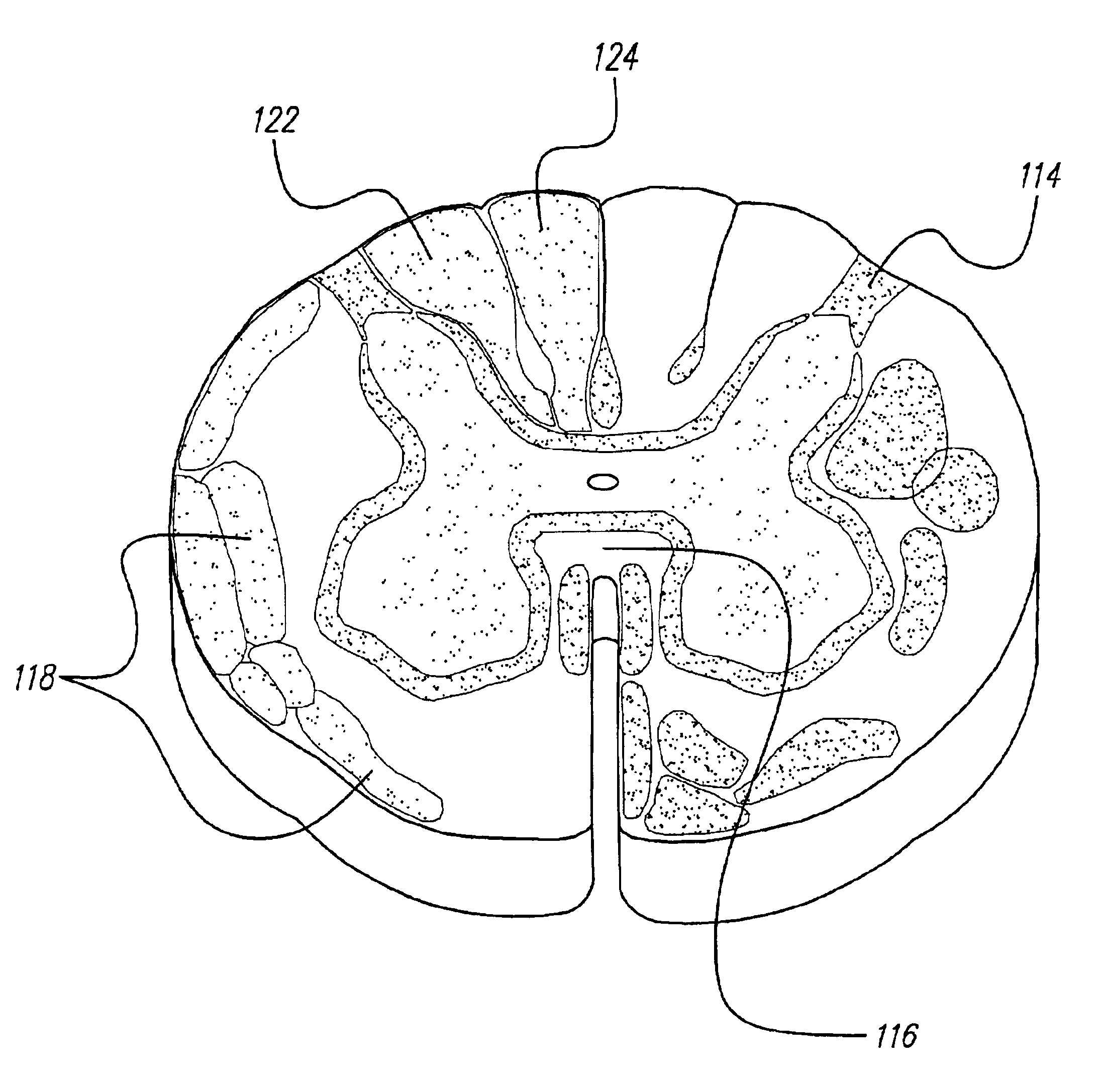
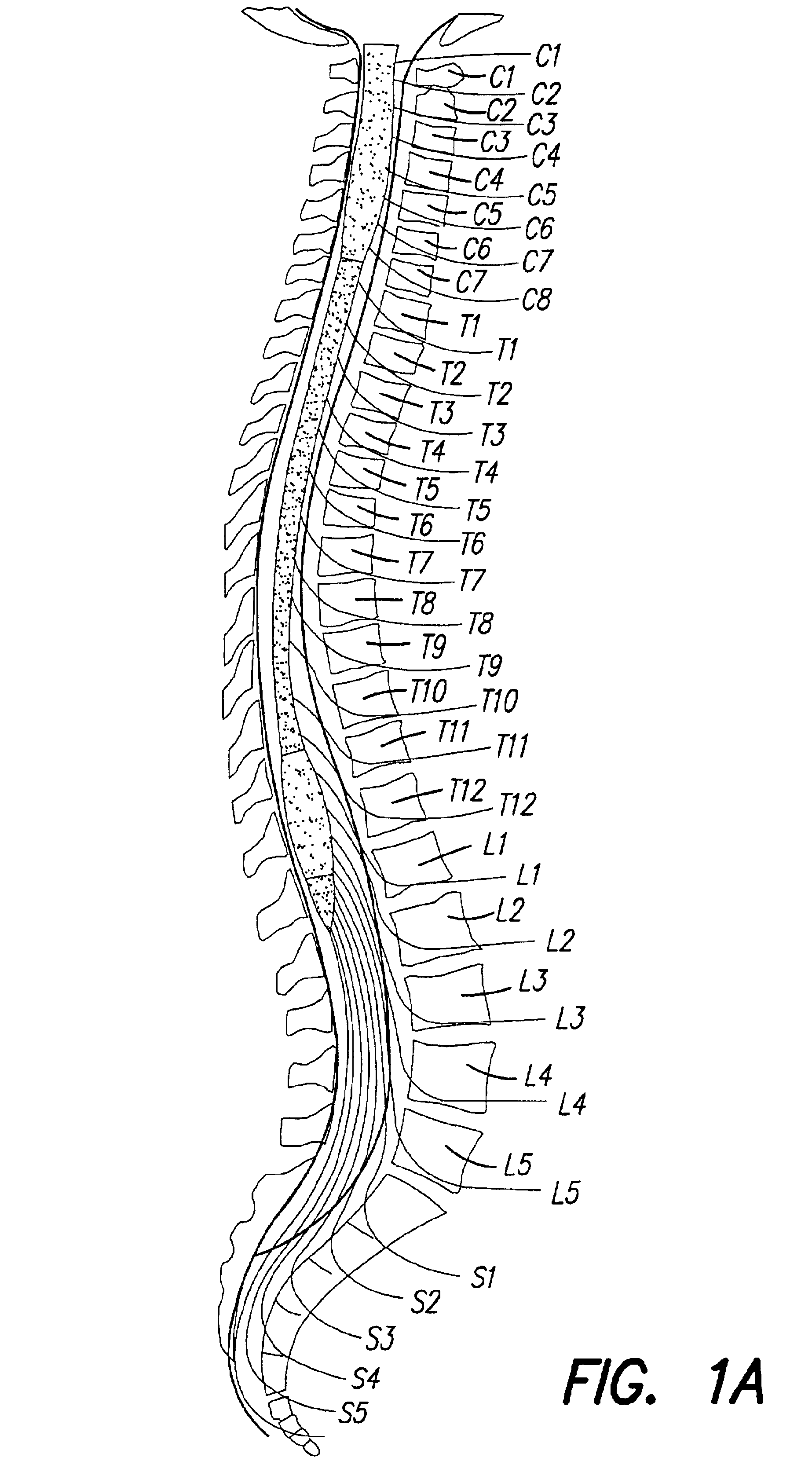
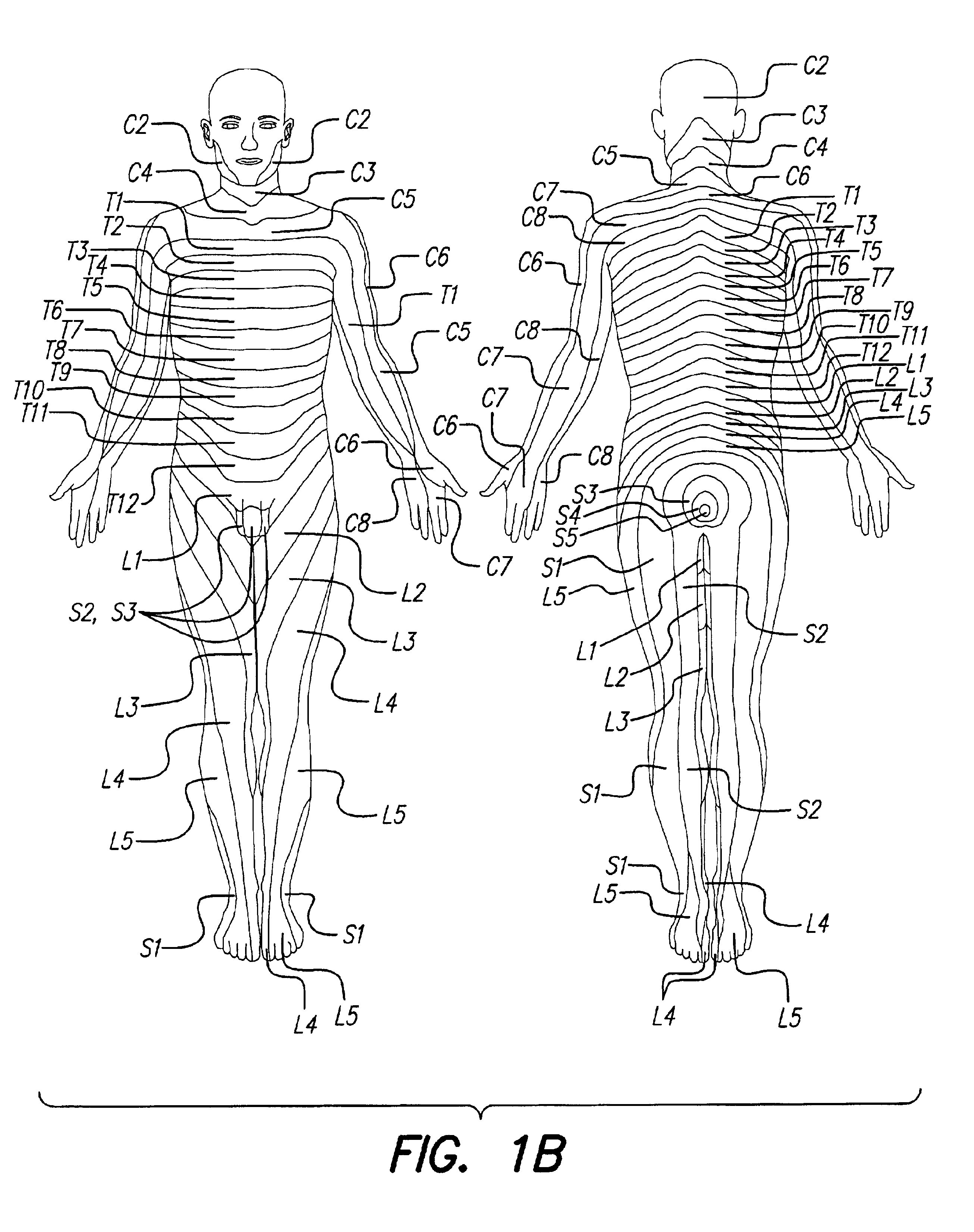
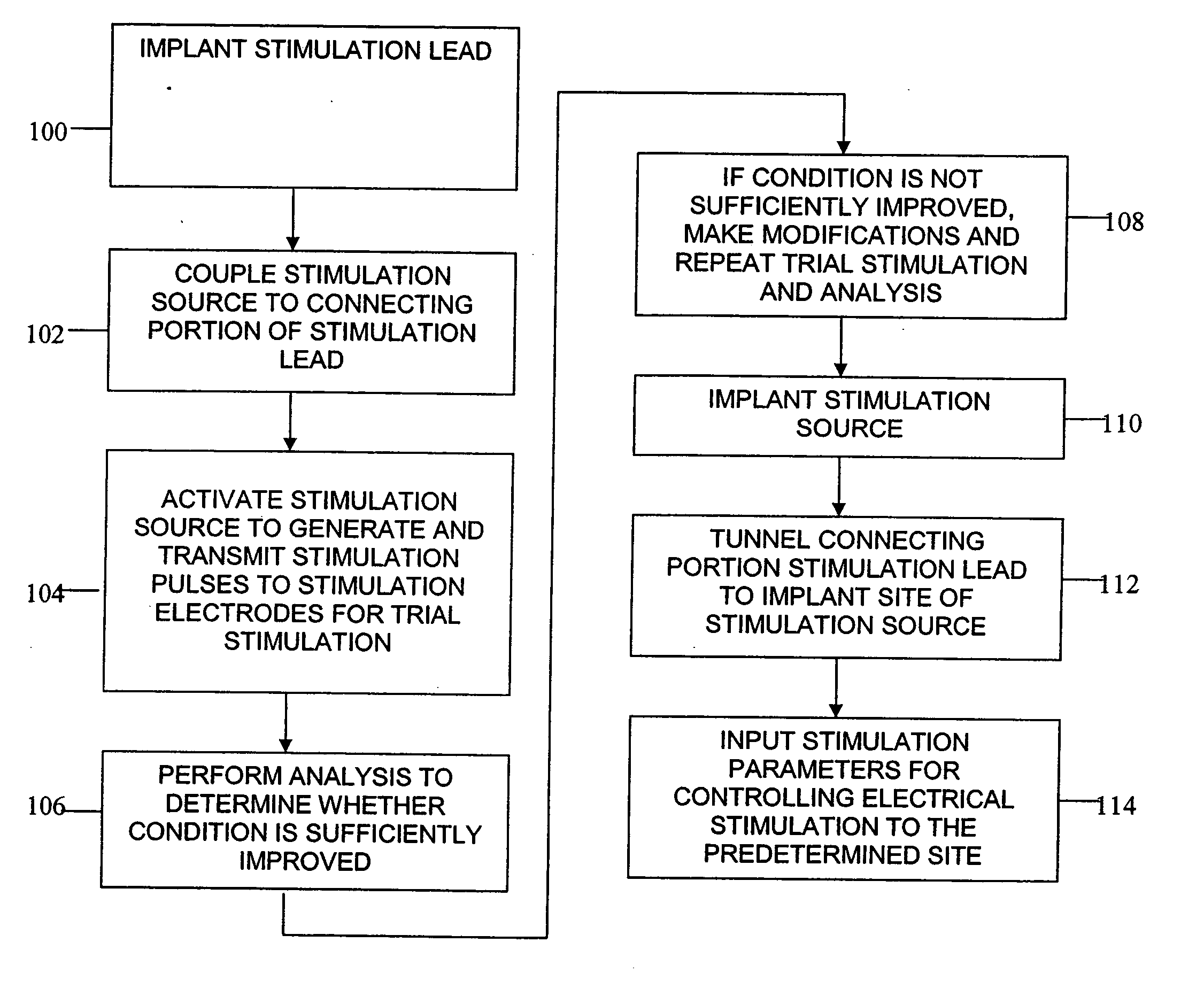
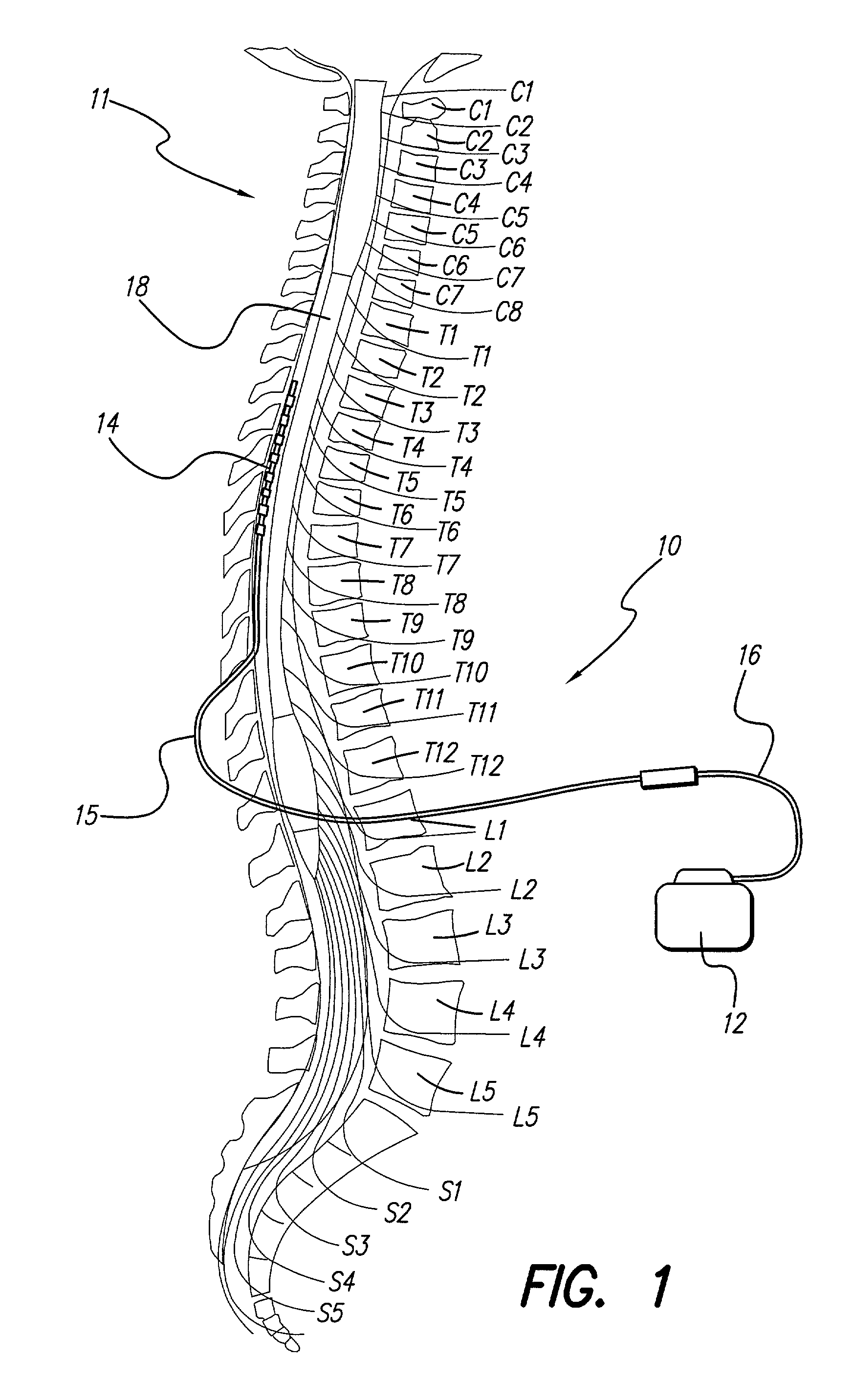
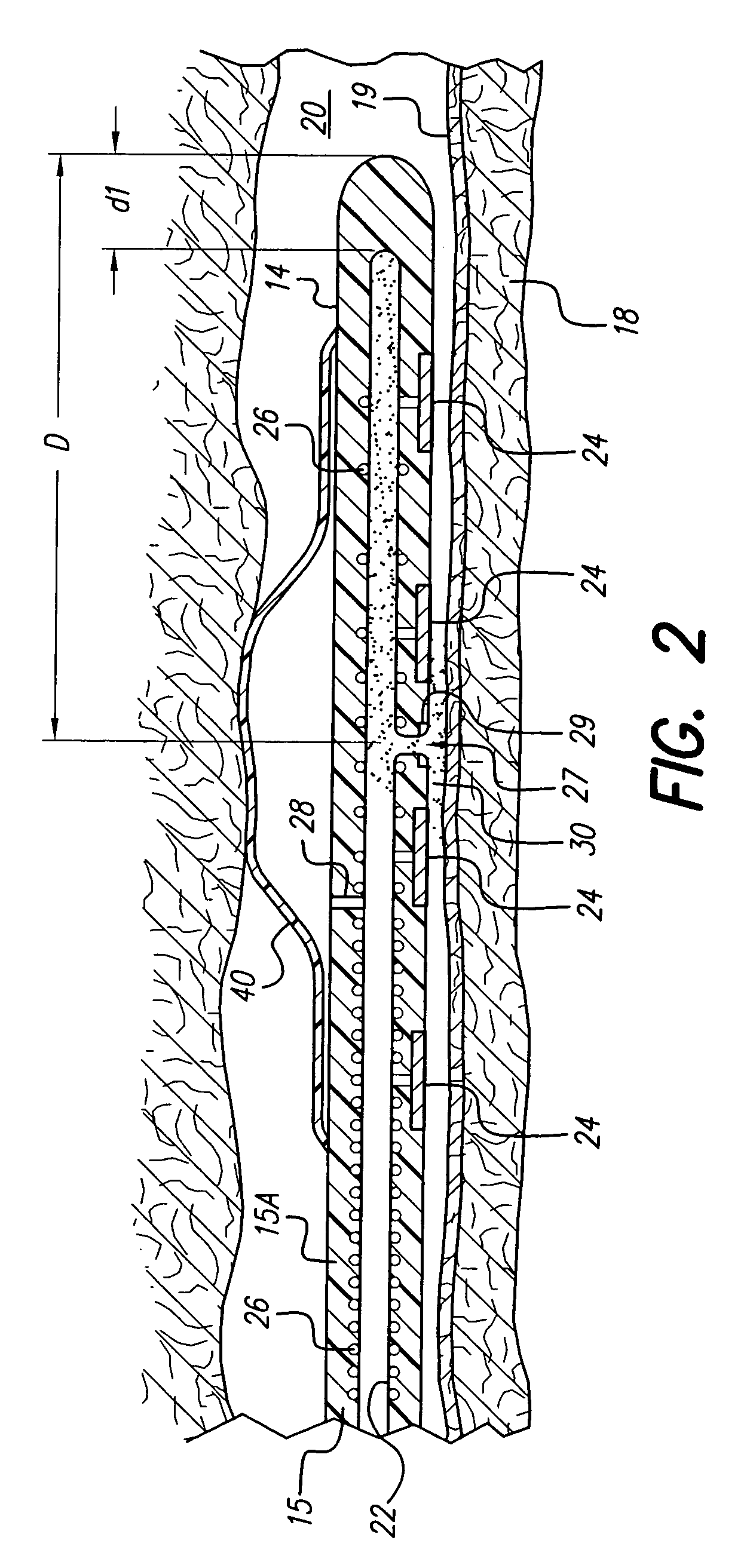
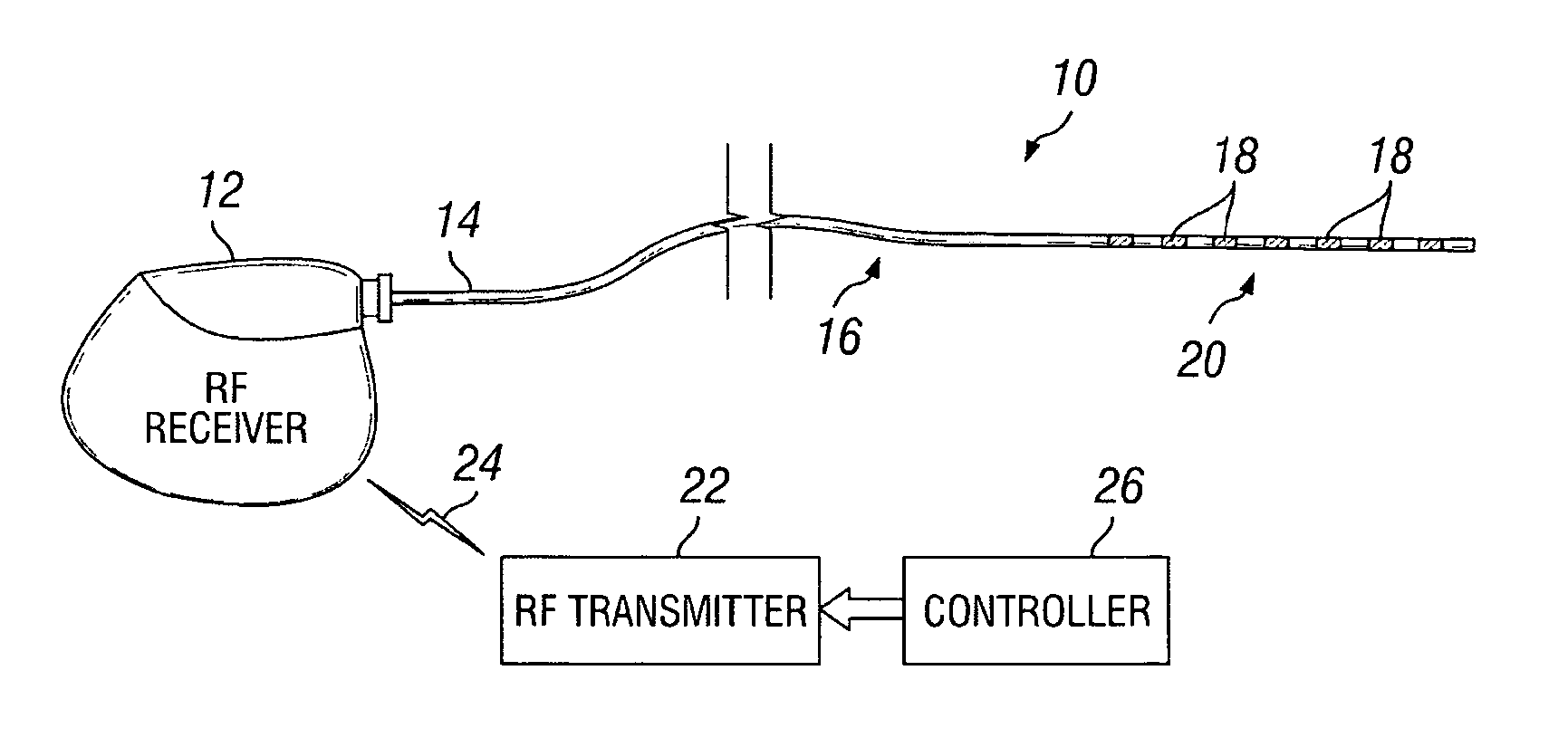
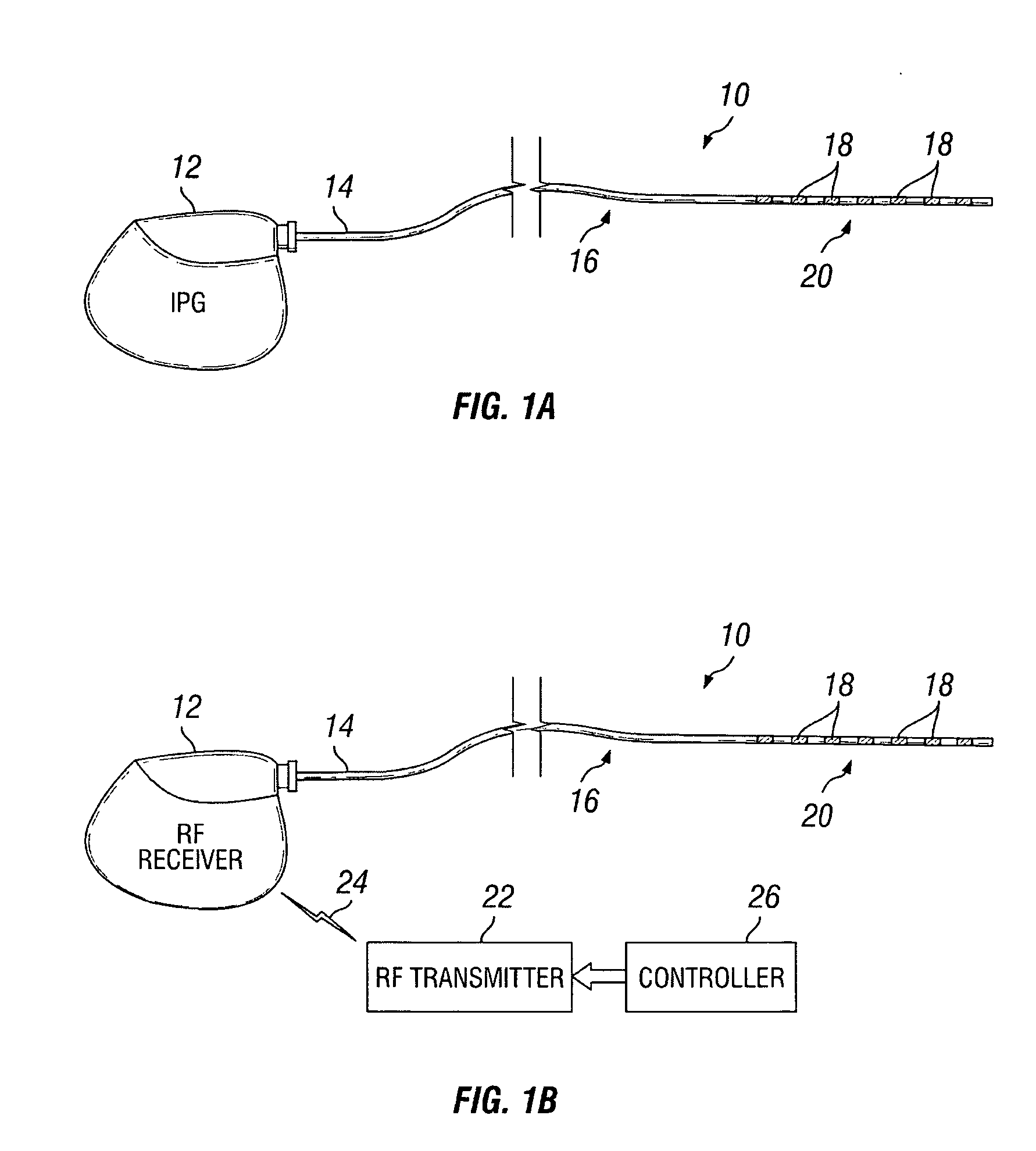
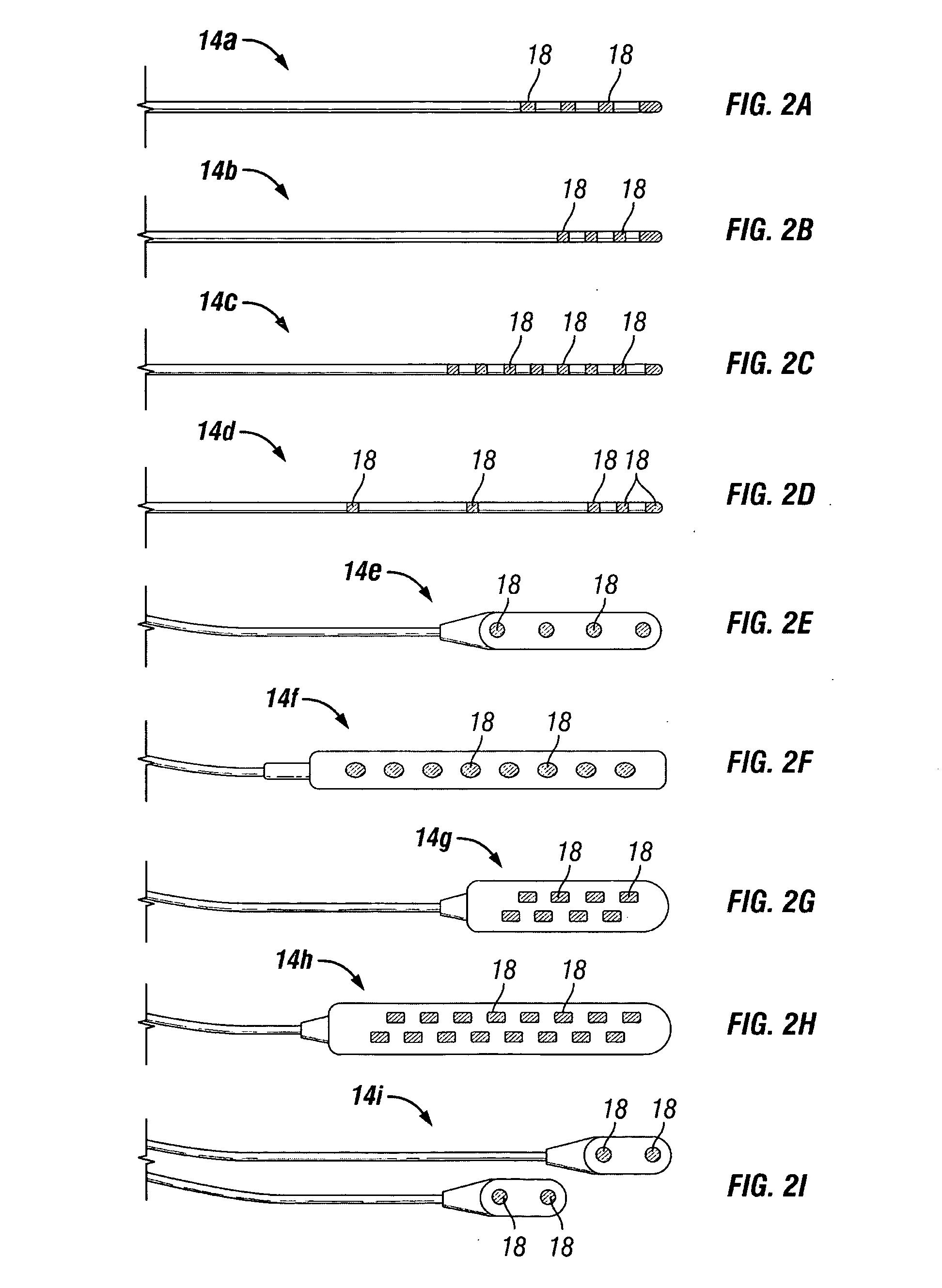
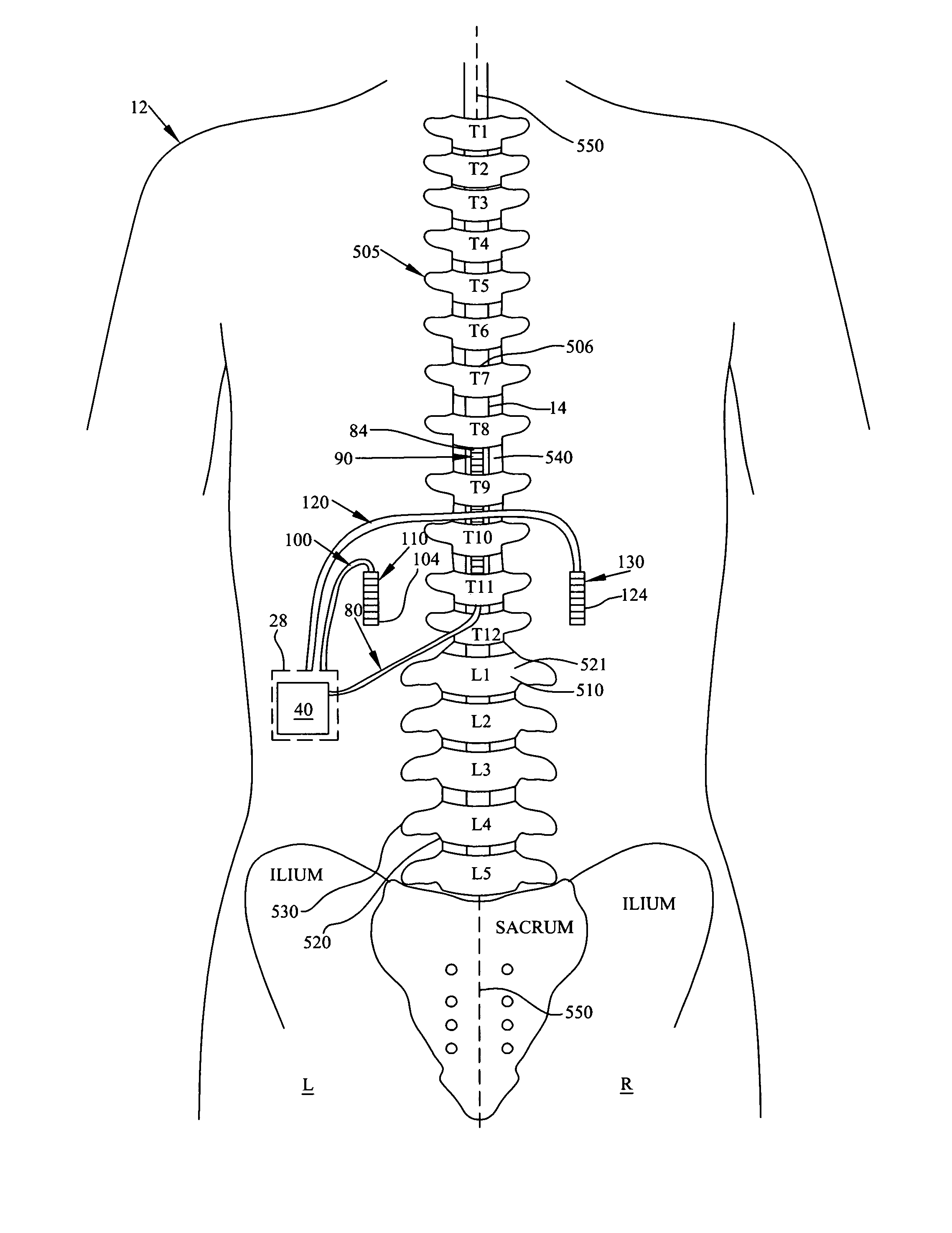
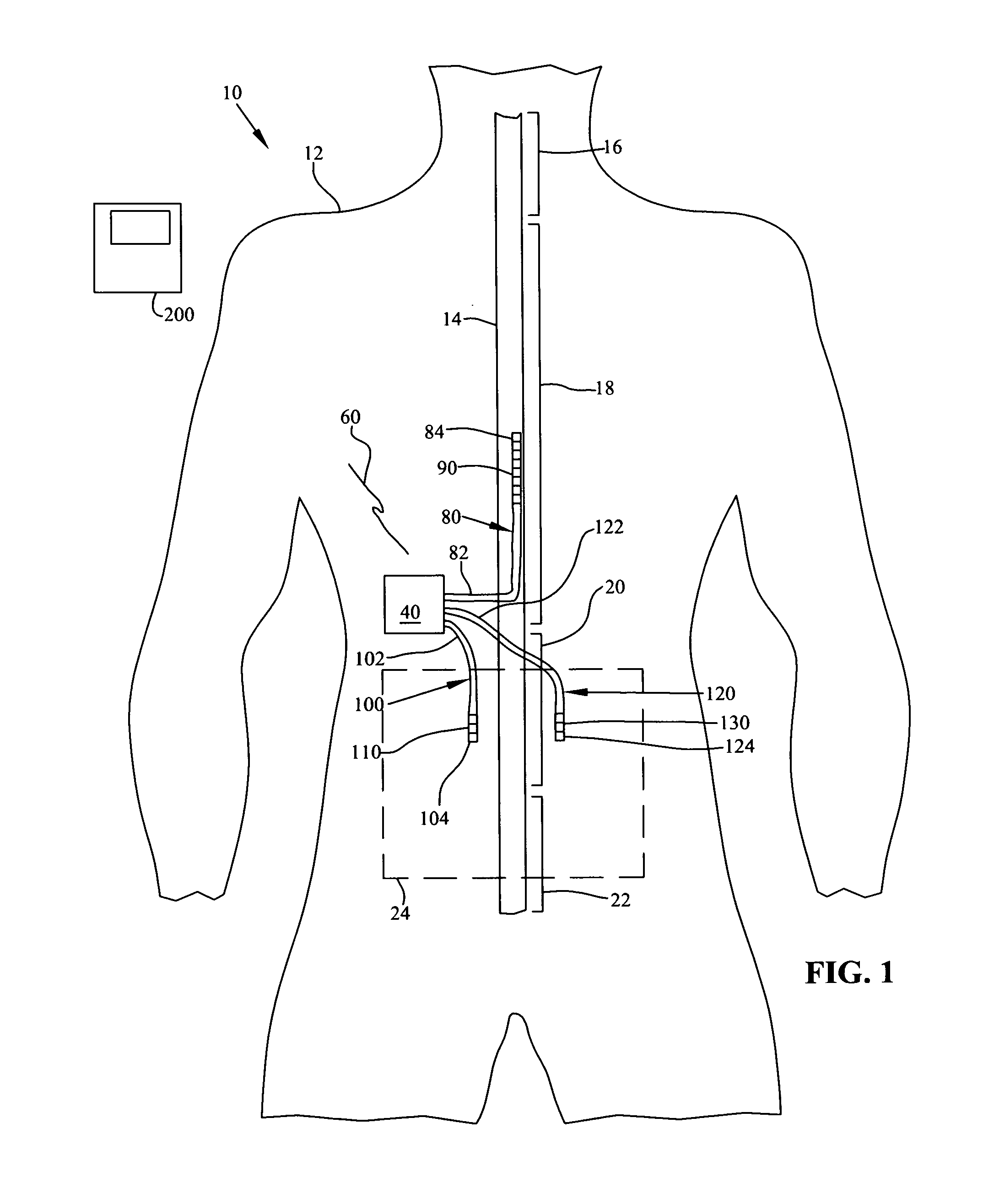
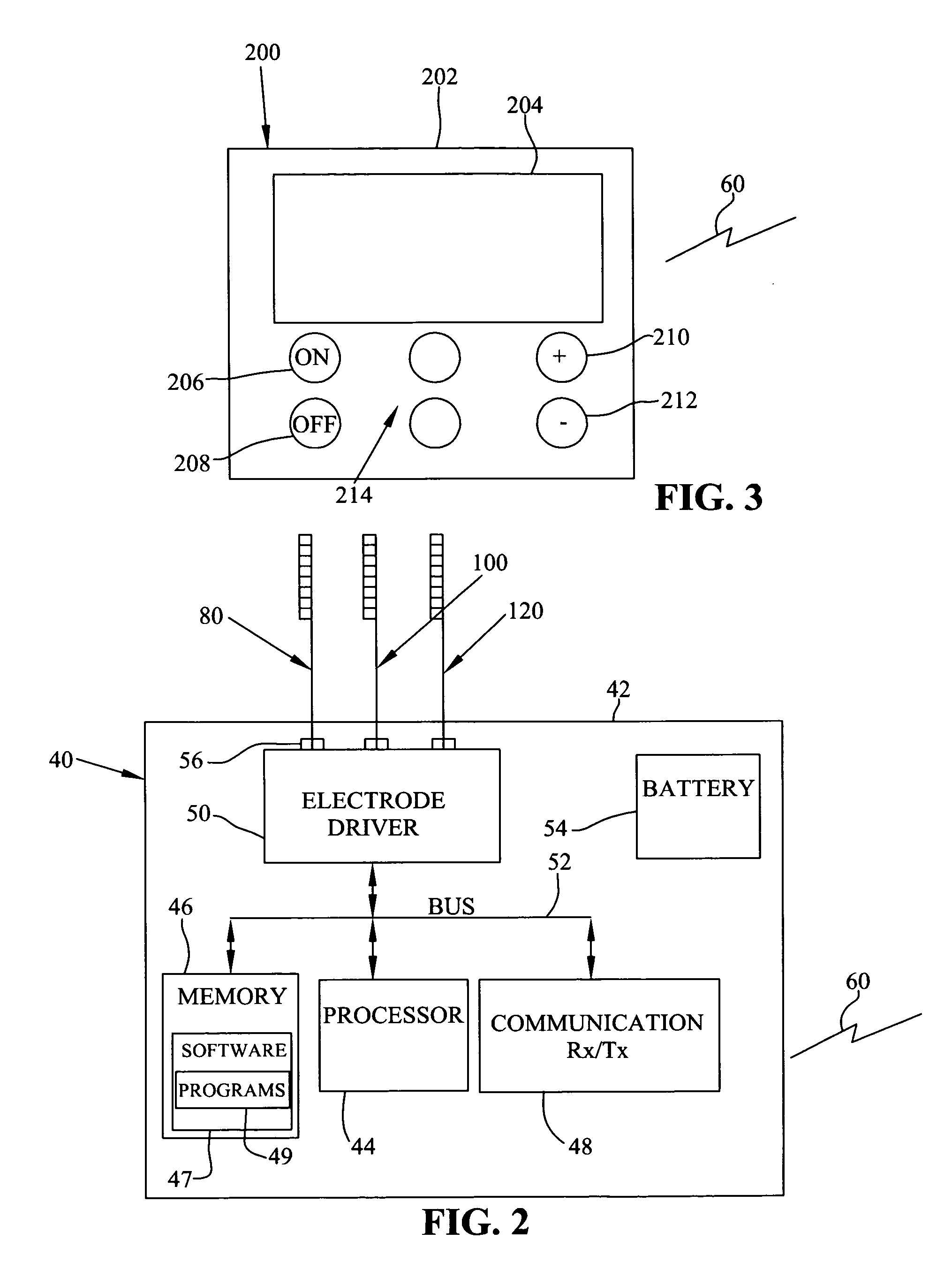
Patents
Literature
198 results about "Spinal cord stimulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
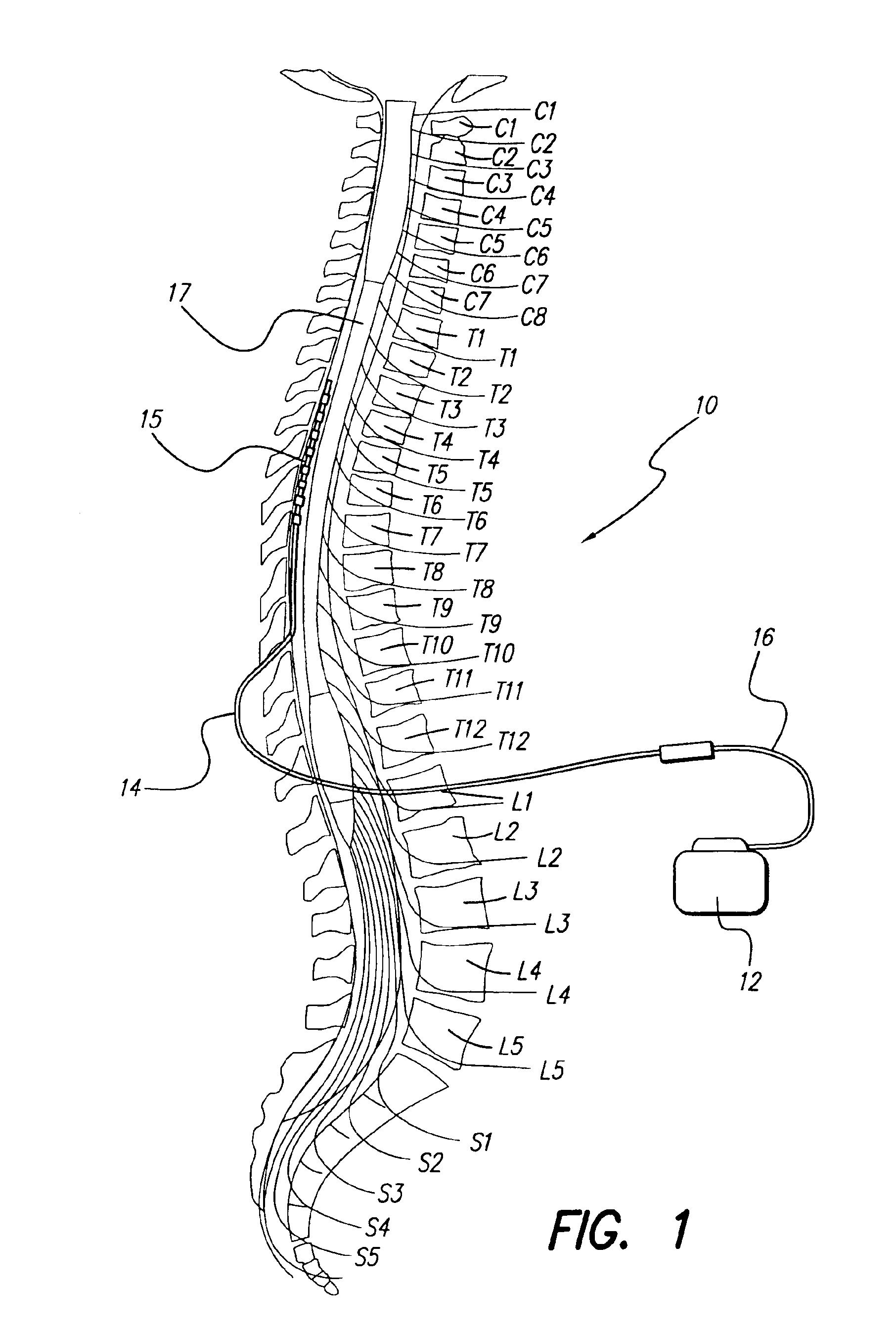
Spinal cord stimulation (SCS) delivers mild electrical stimulation to nerves along the spinal column, modifying or blocking nerve activity in a non-medicinal way to minimize the sensation of pain reaching the brain. Spinal cord stimulation was first used to treat pain in 1967.
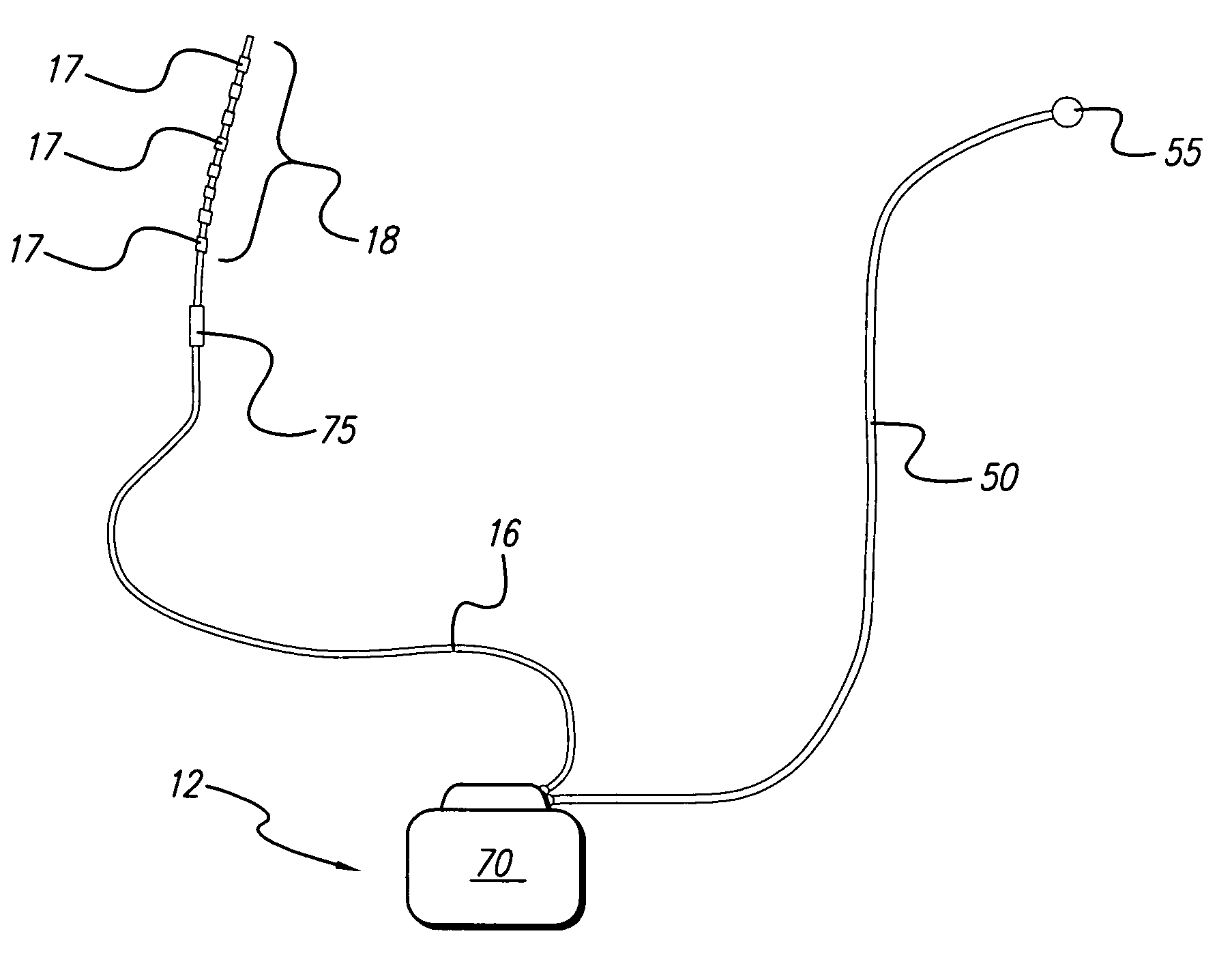
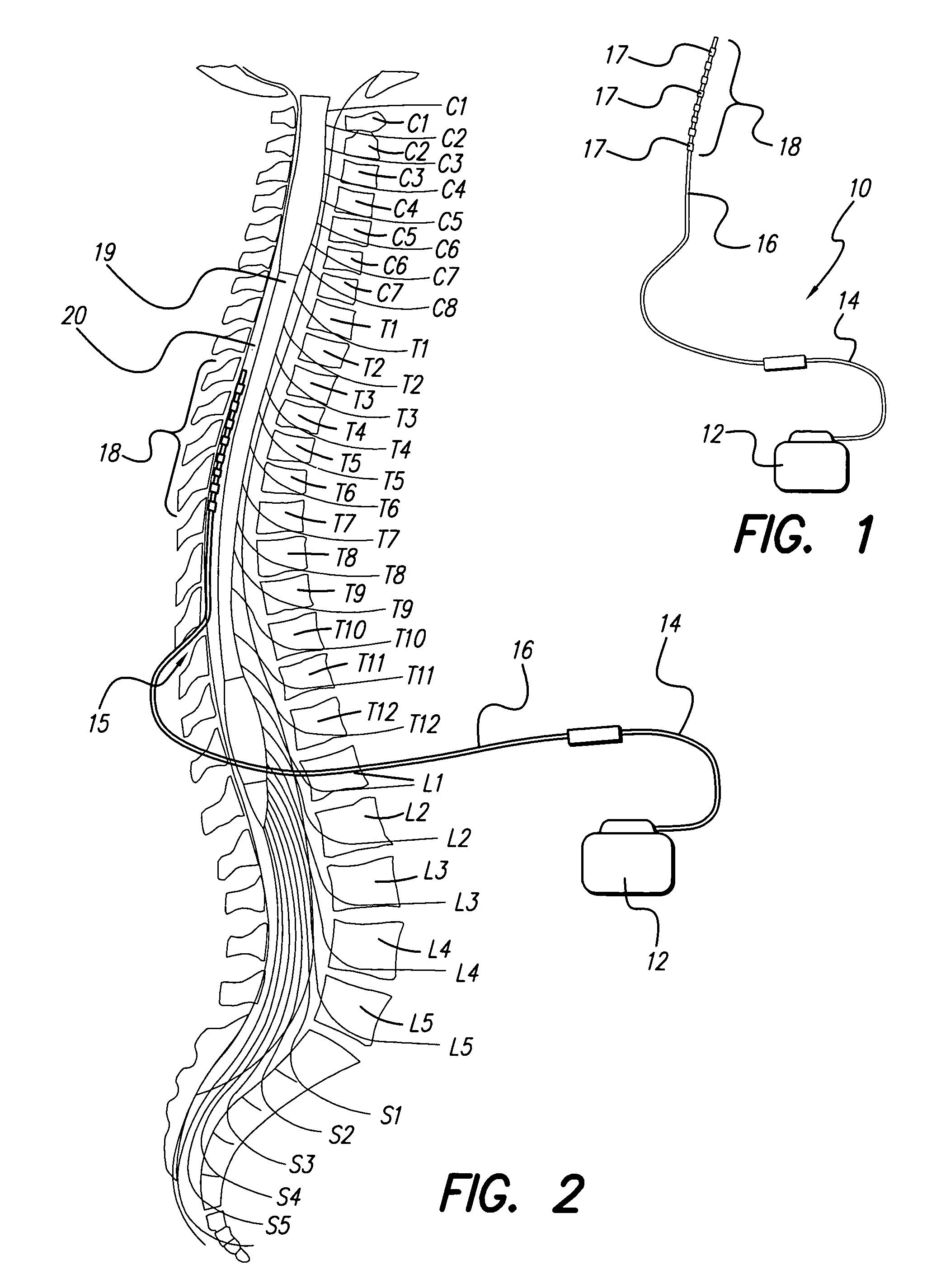
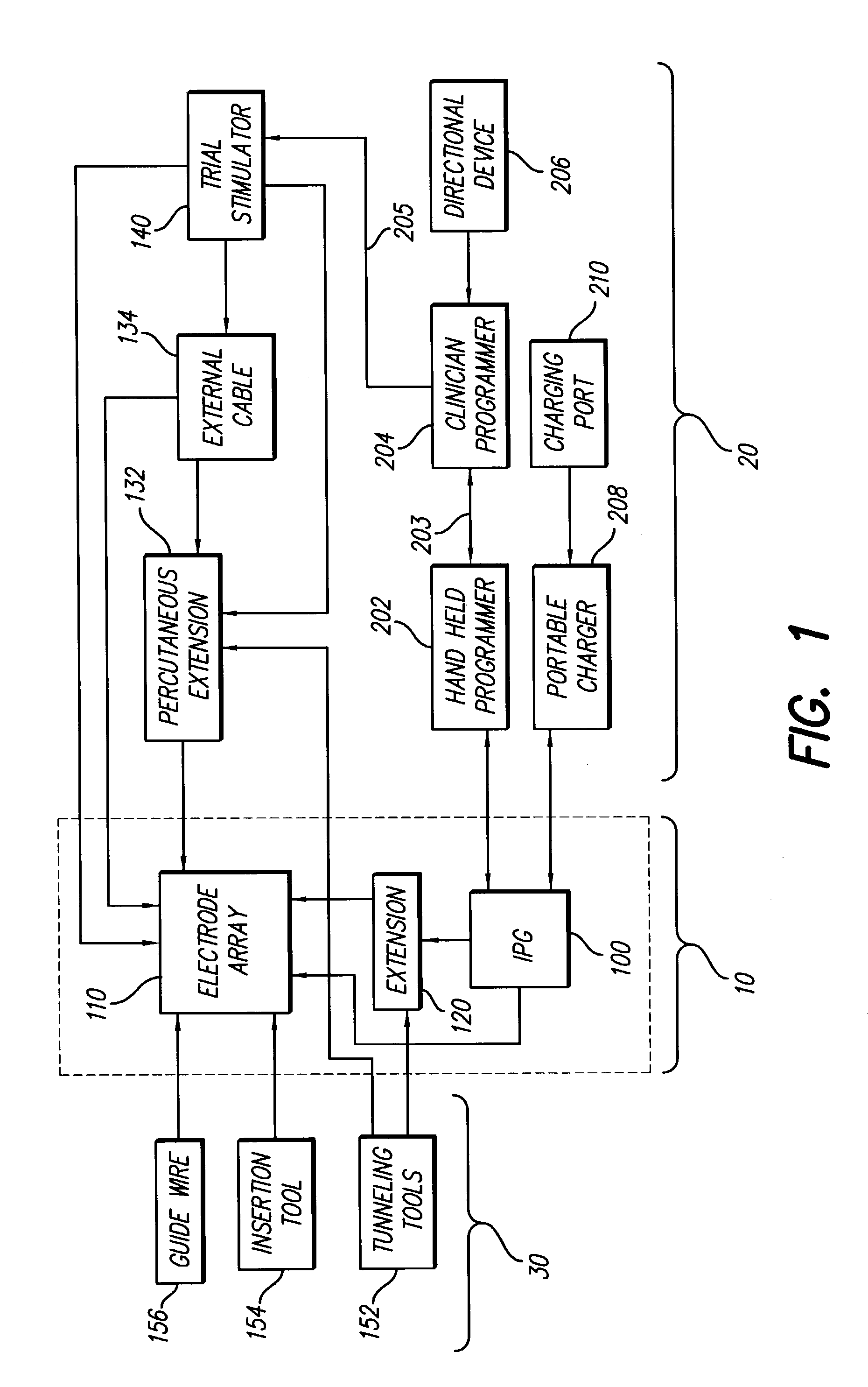
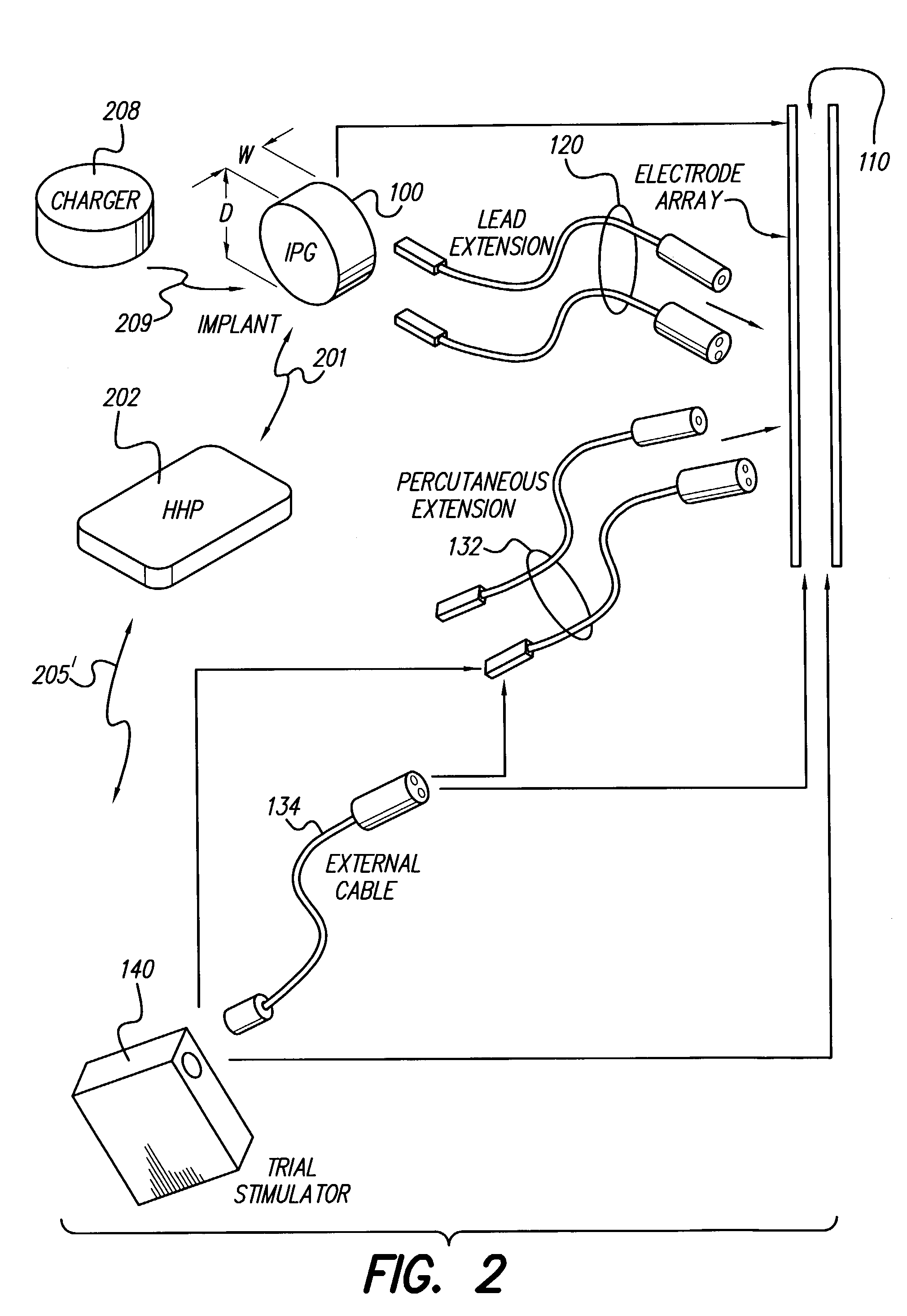
Fully implantable microstimulator for spinal cord stimulation as a therapy for chronic pain
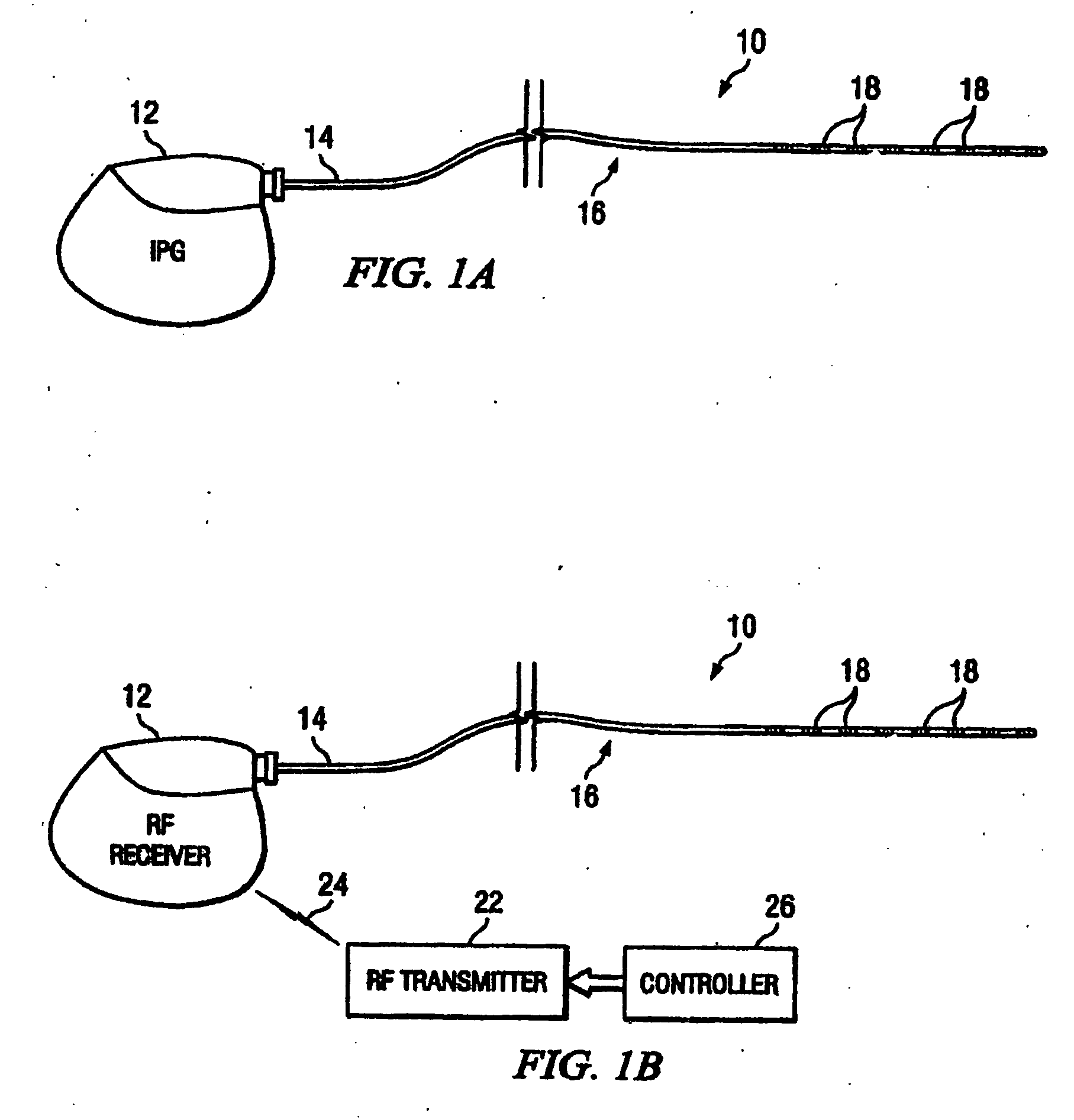
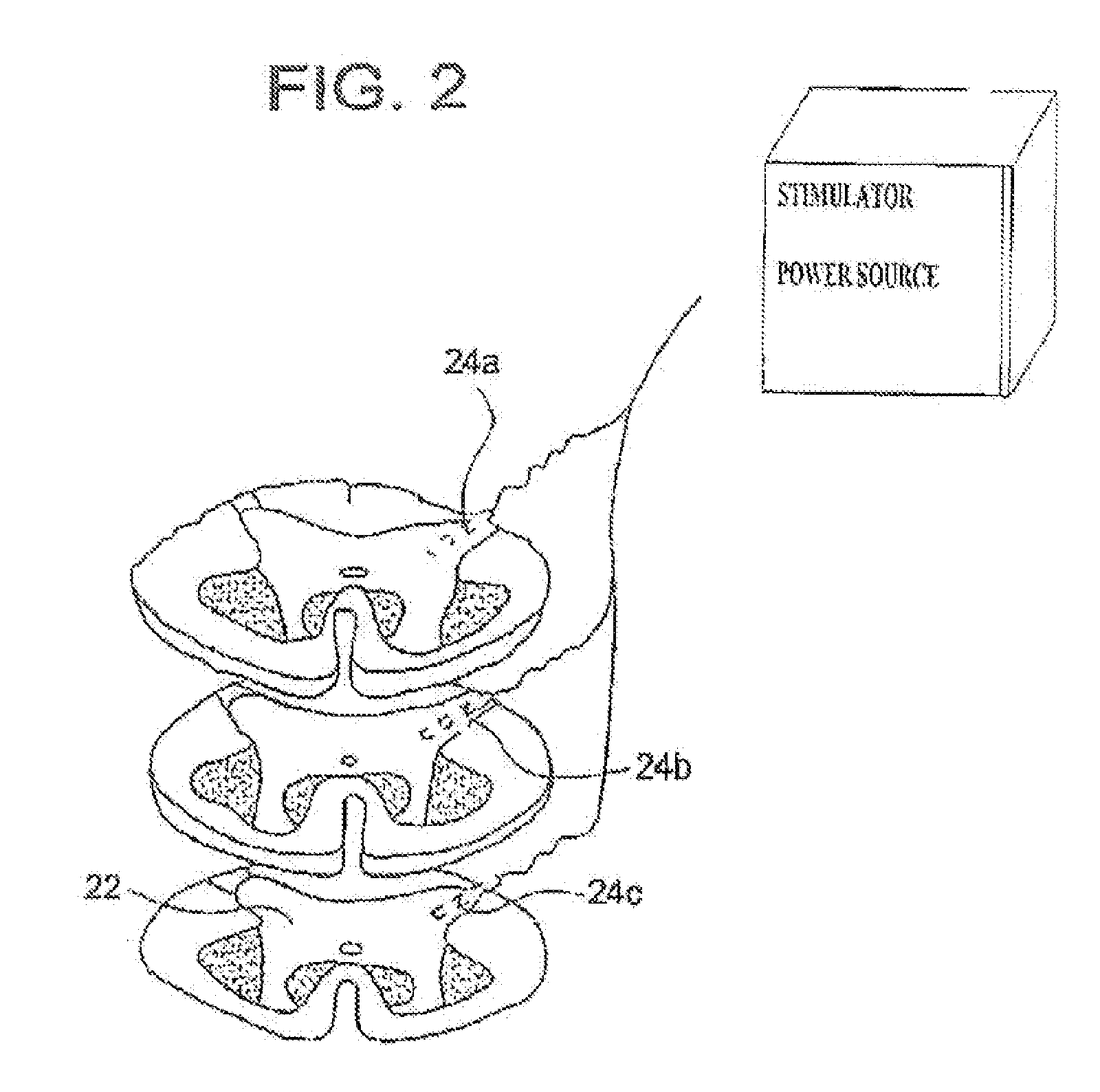
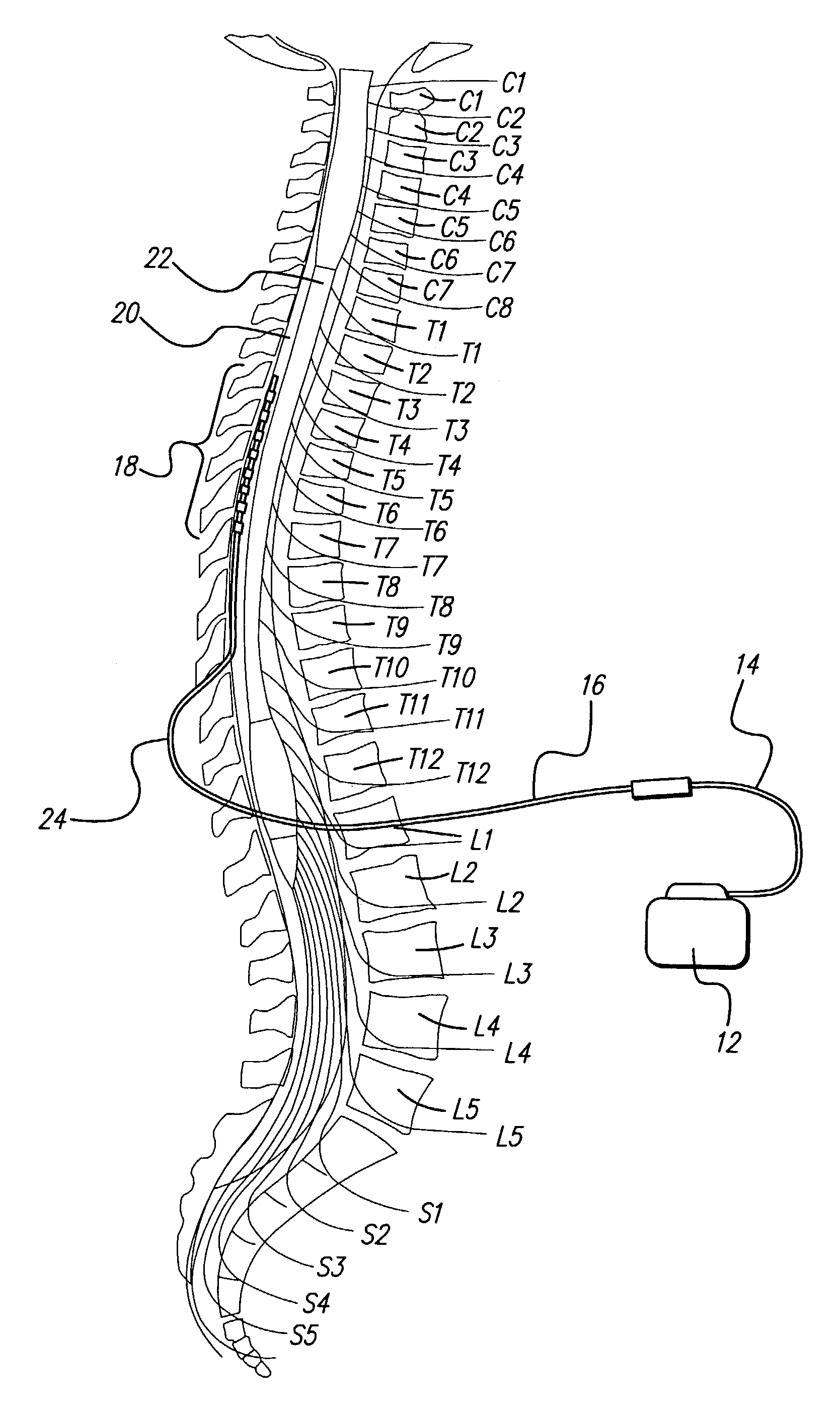
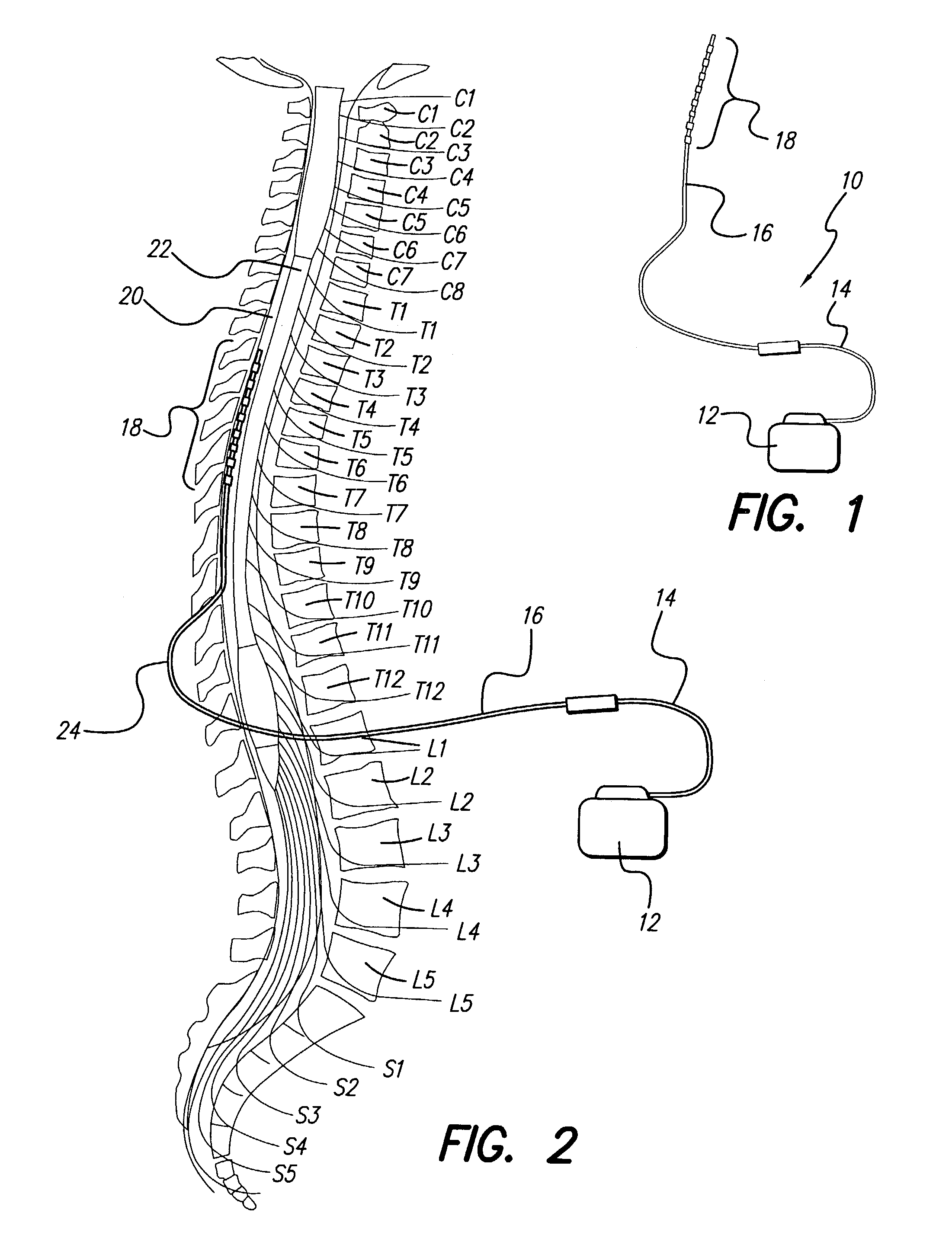
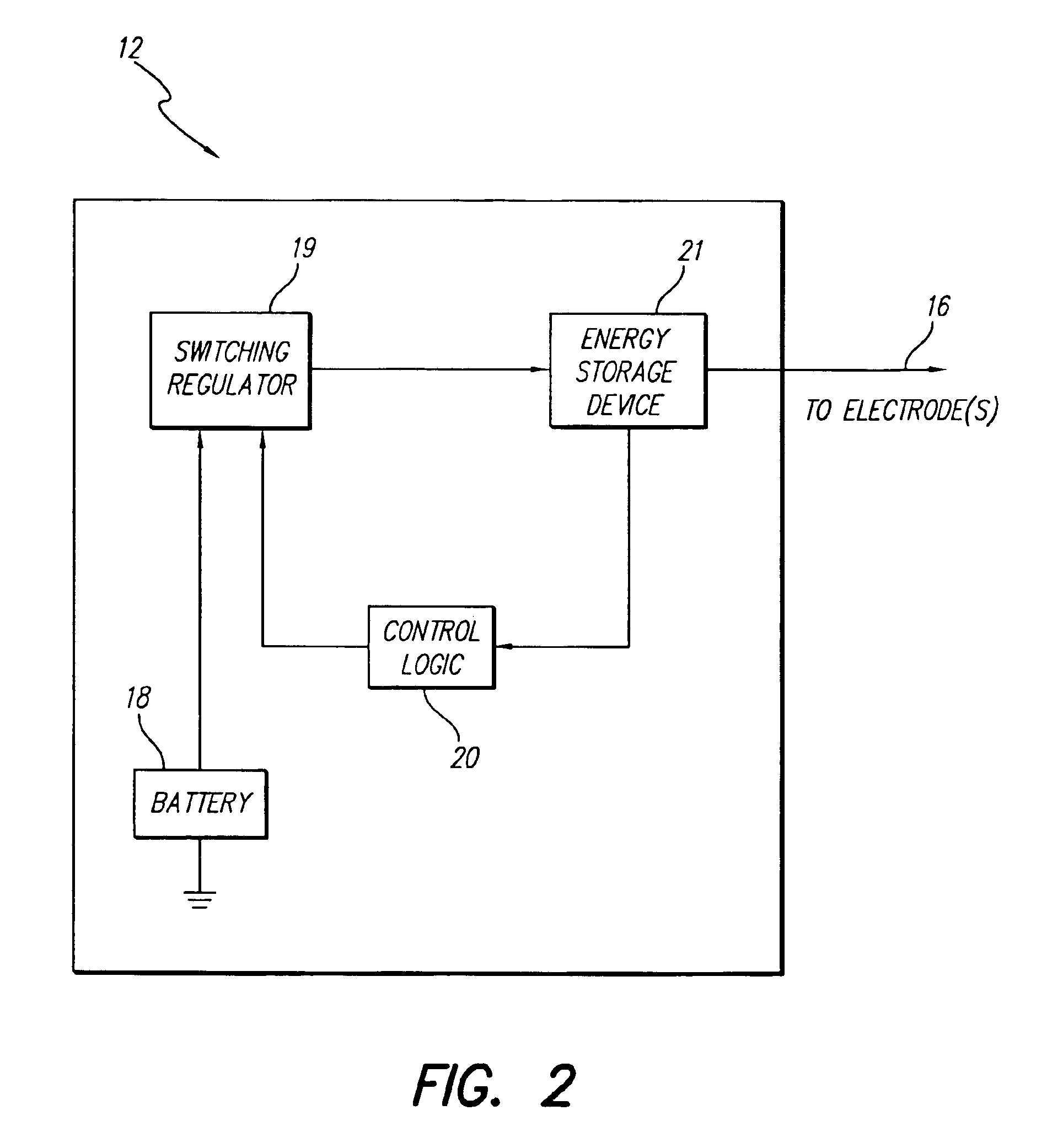
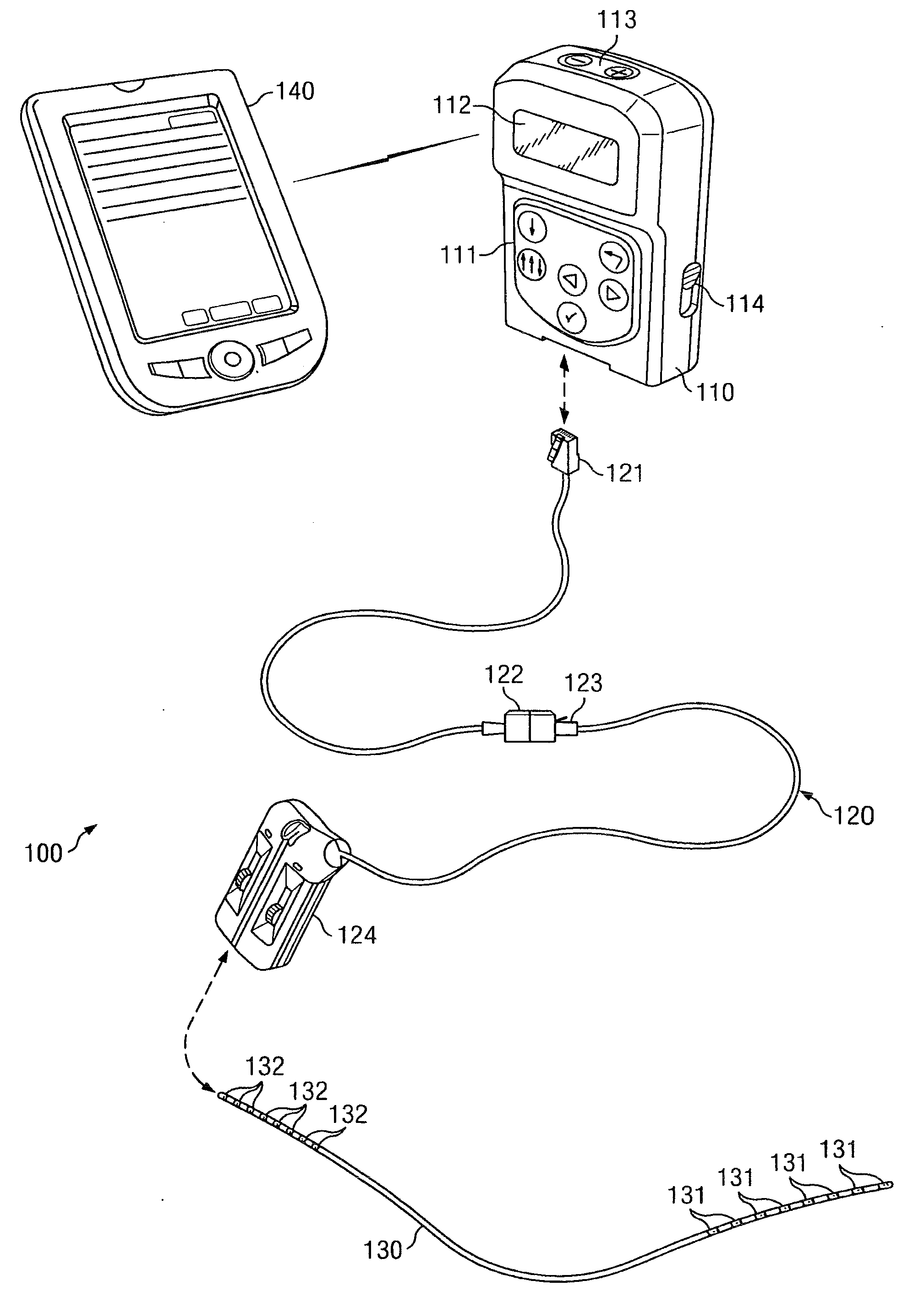
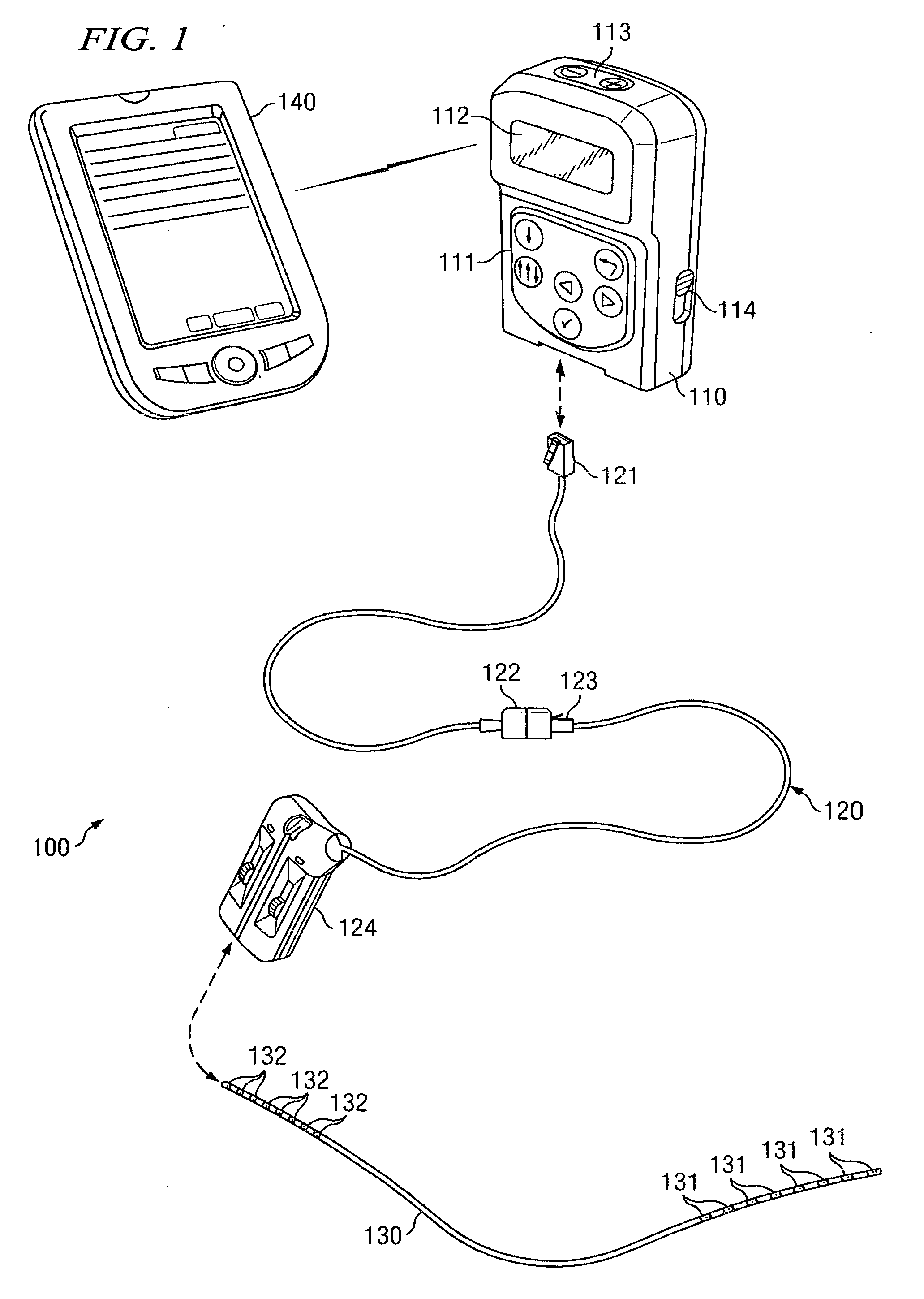
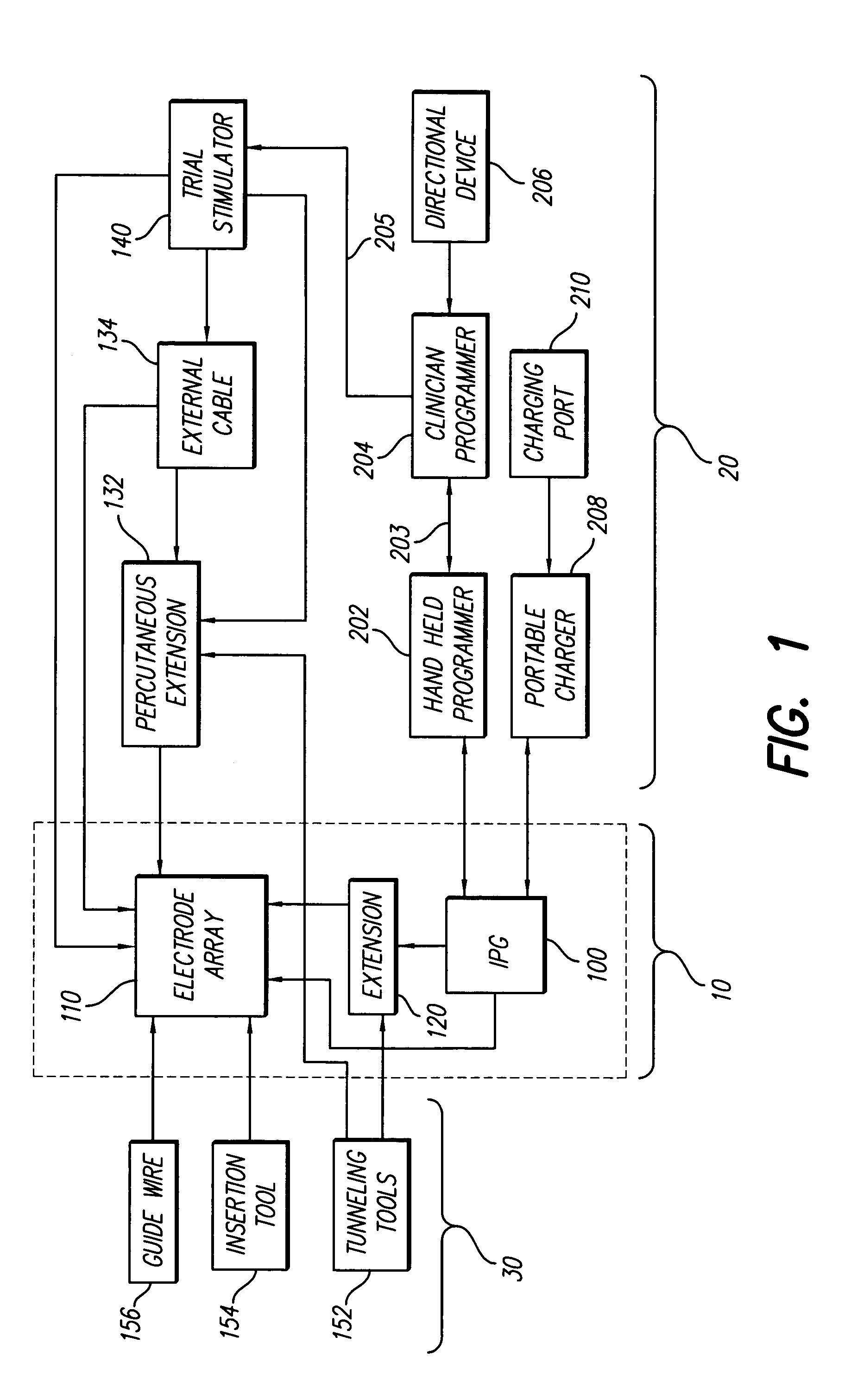
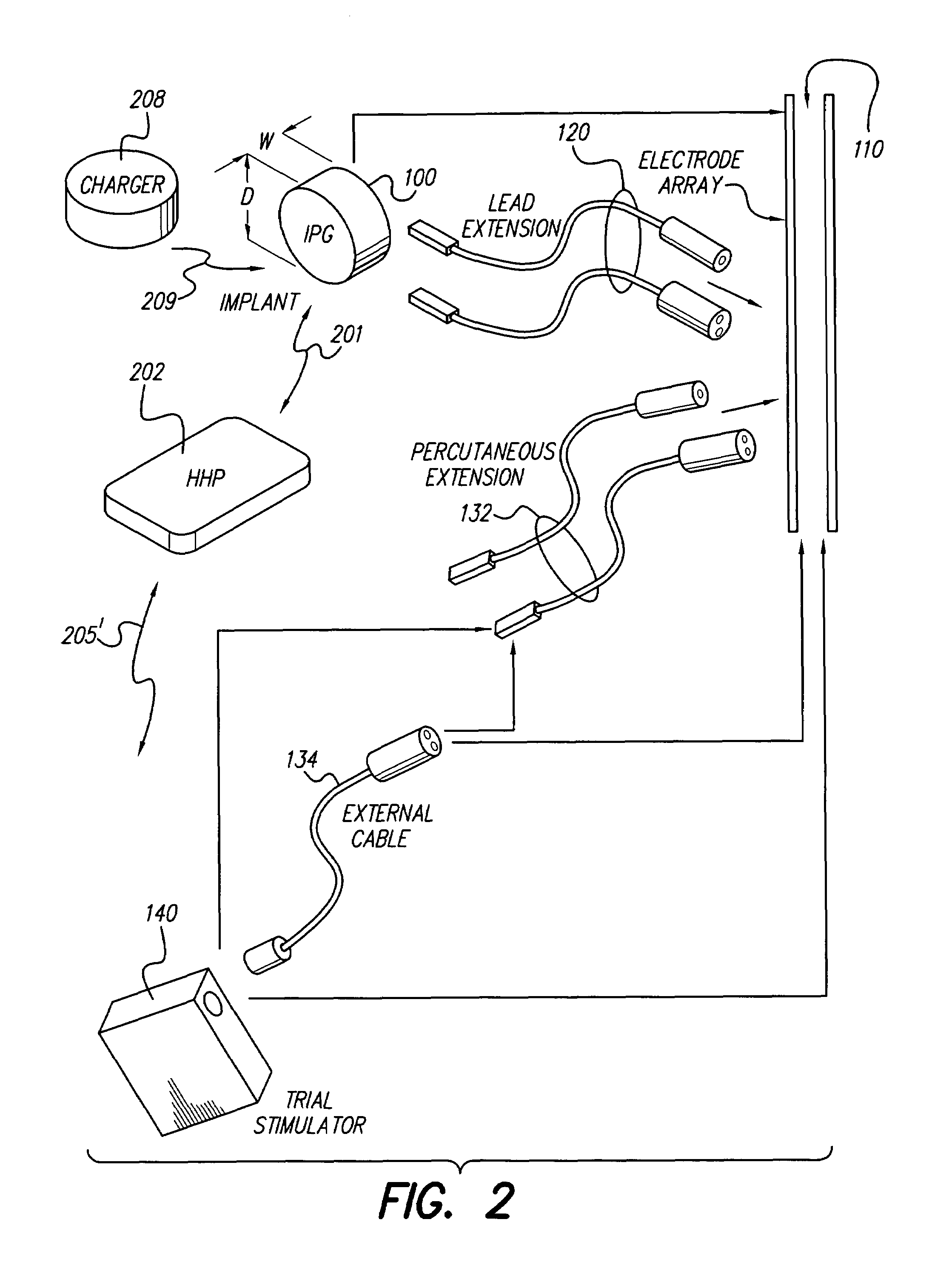
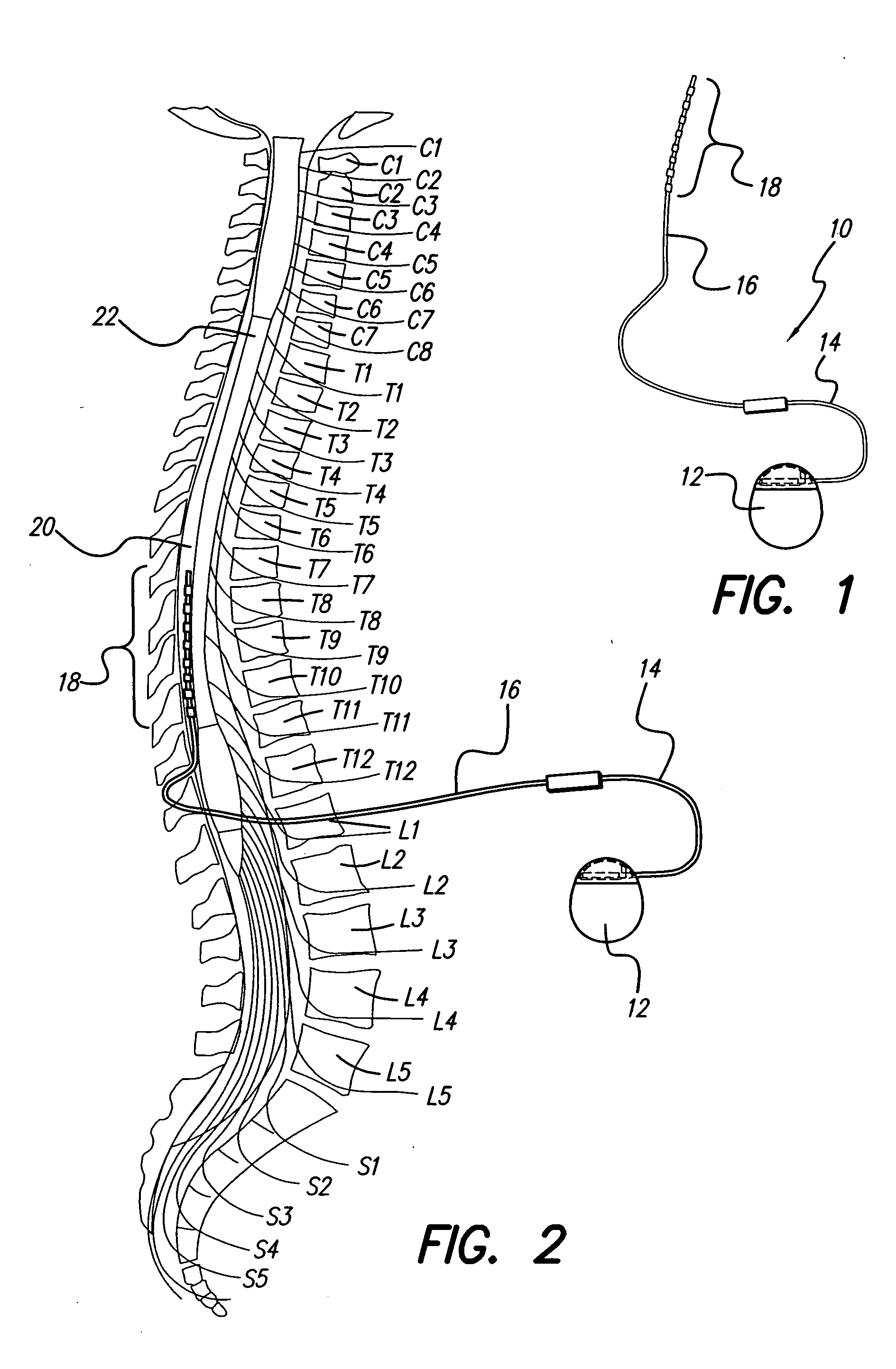
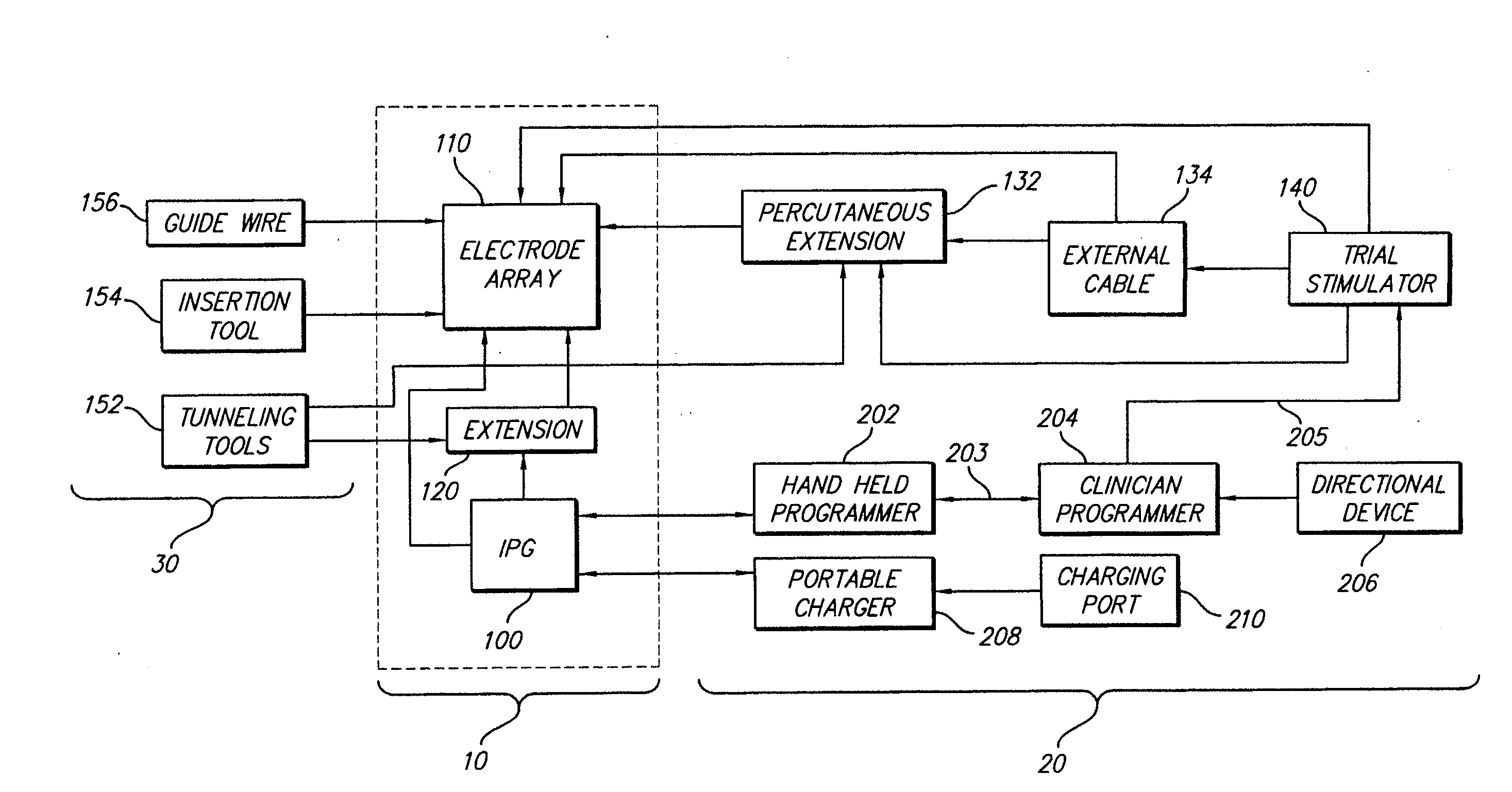
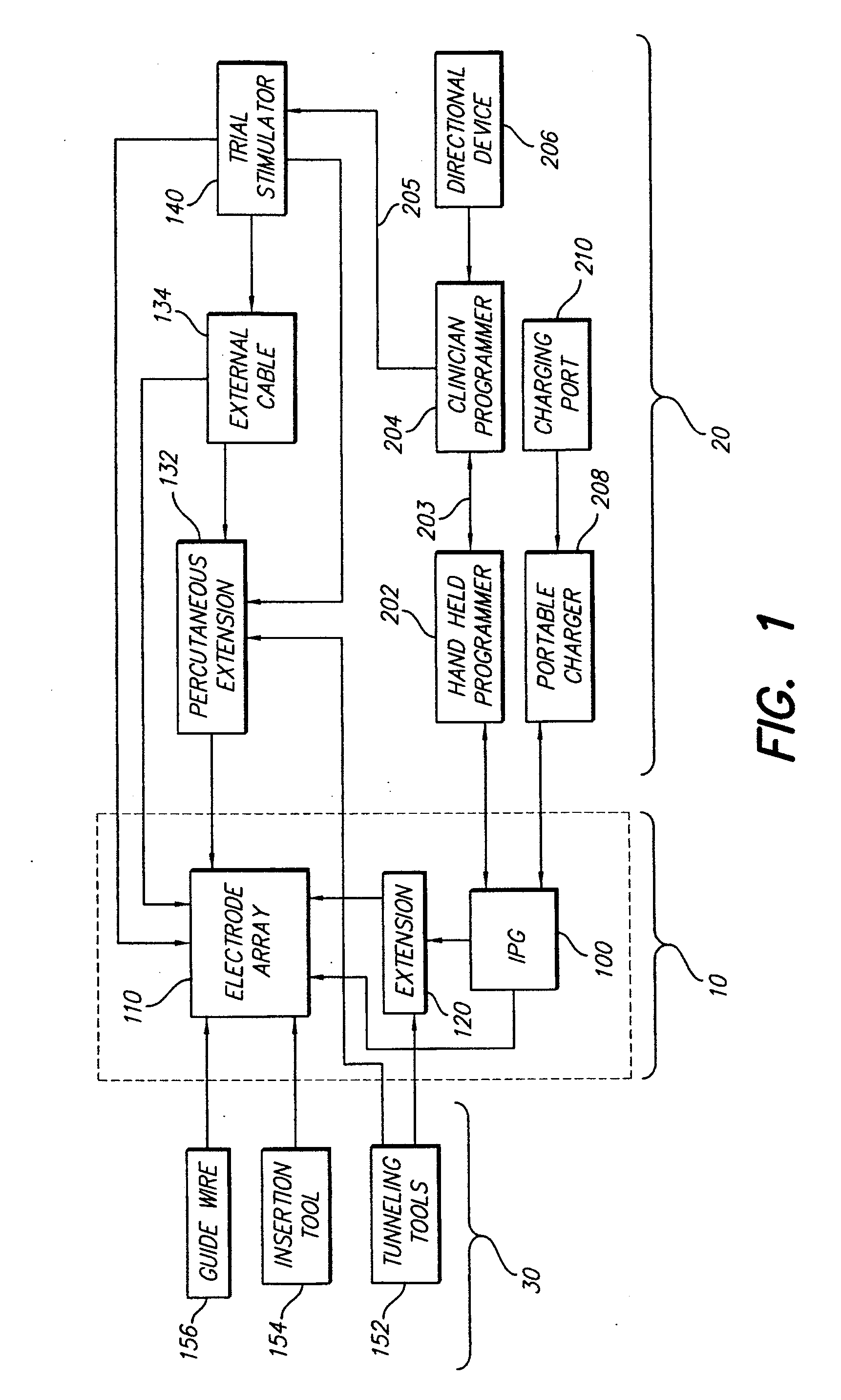
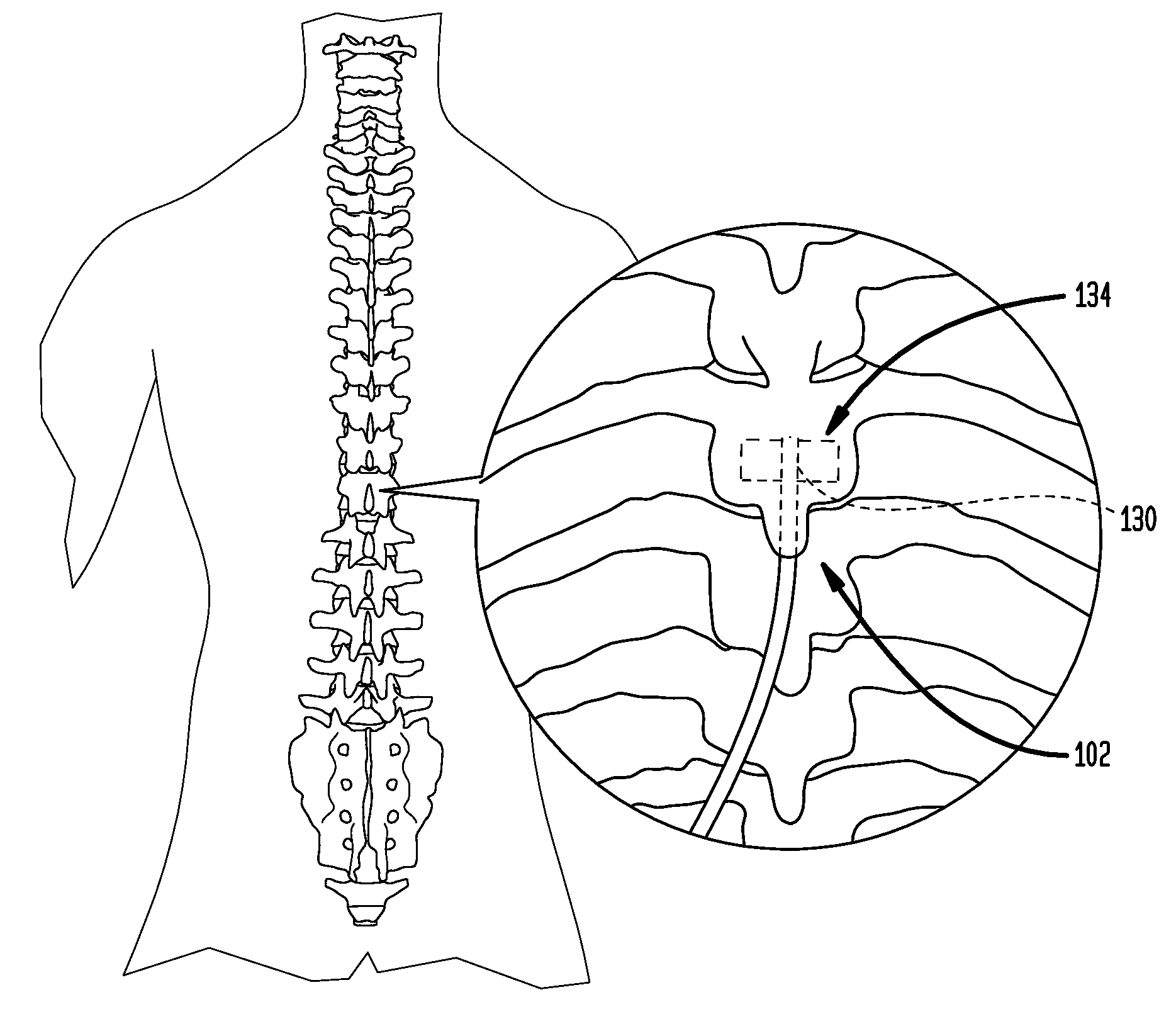
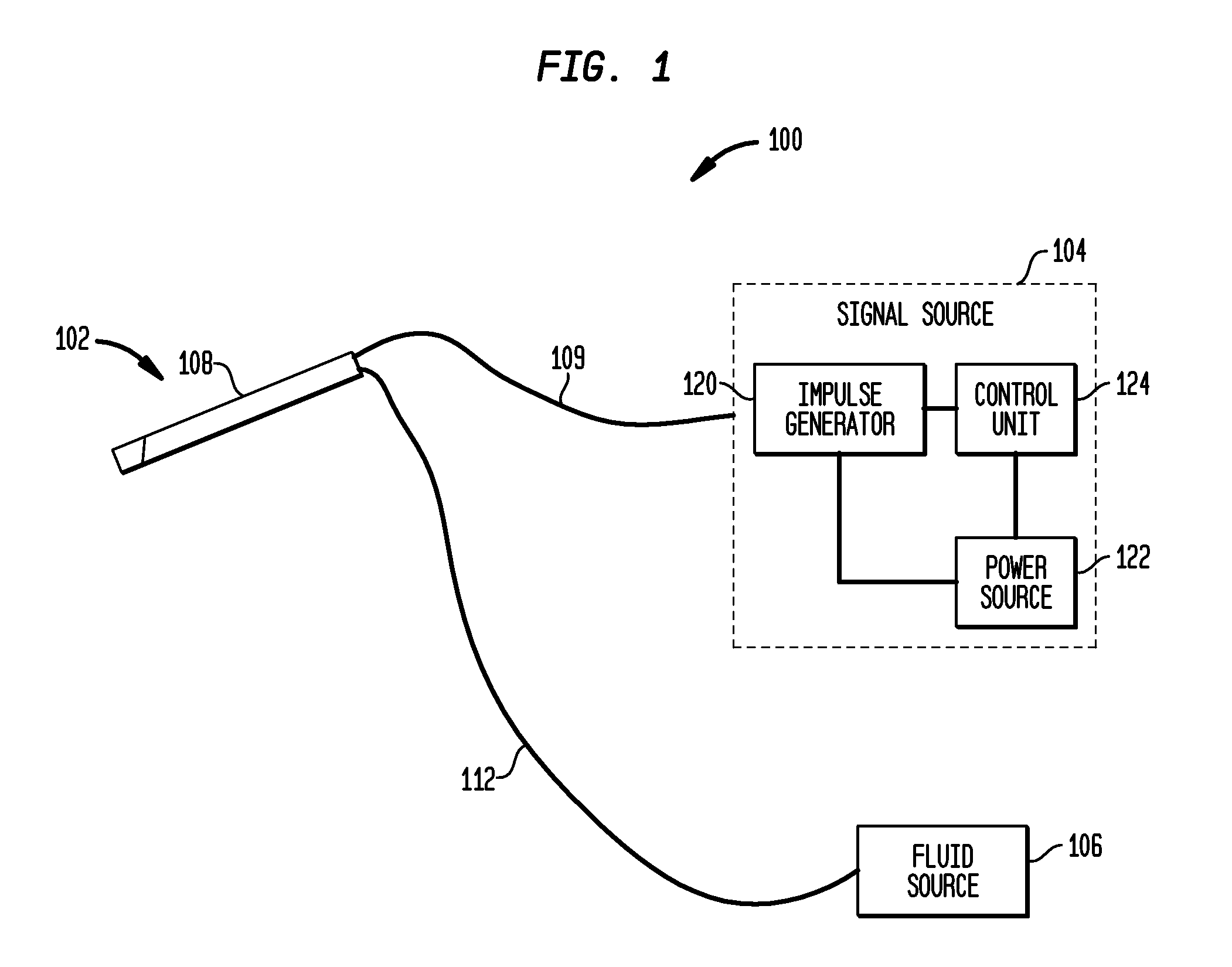
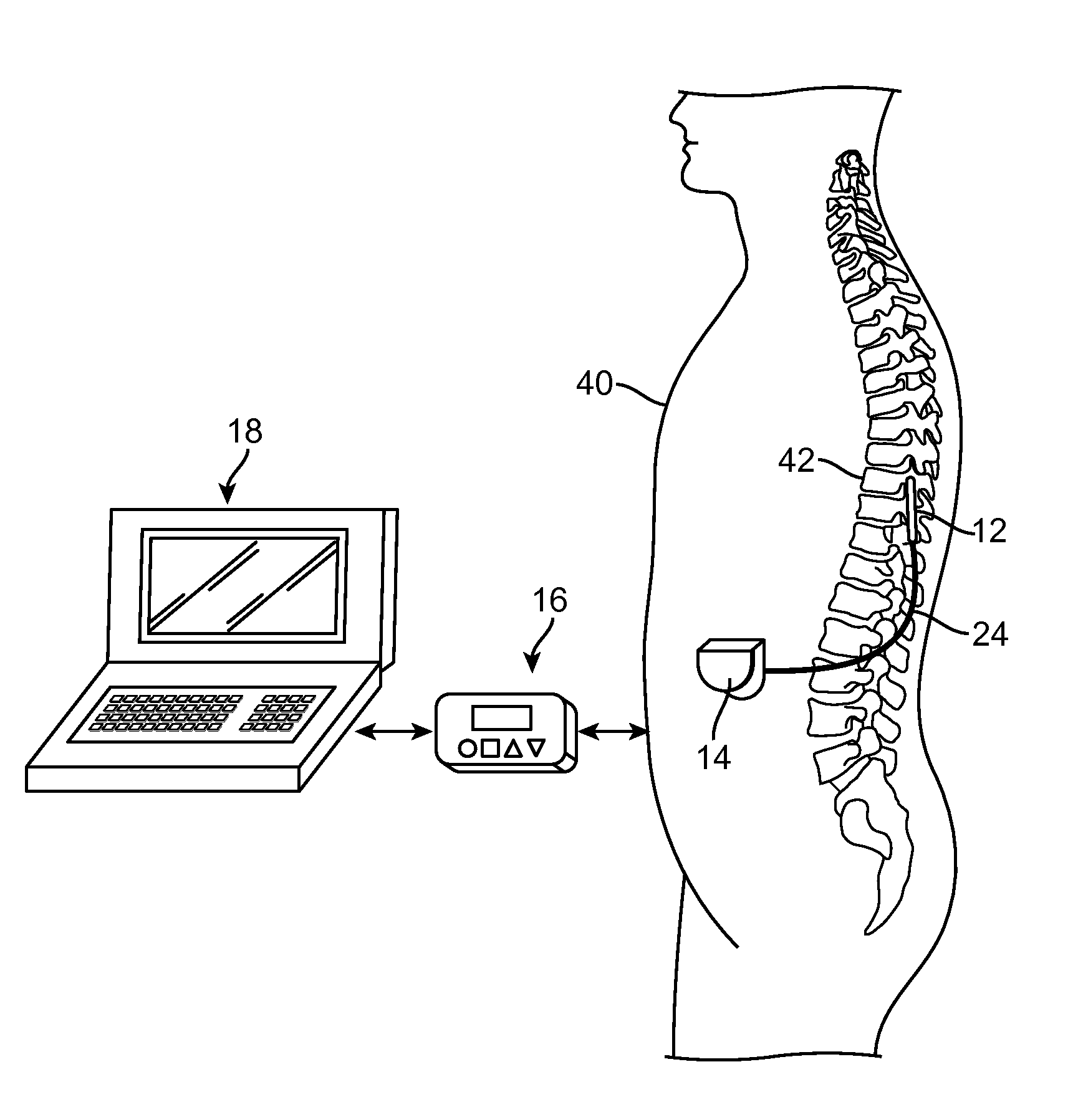
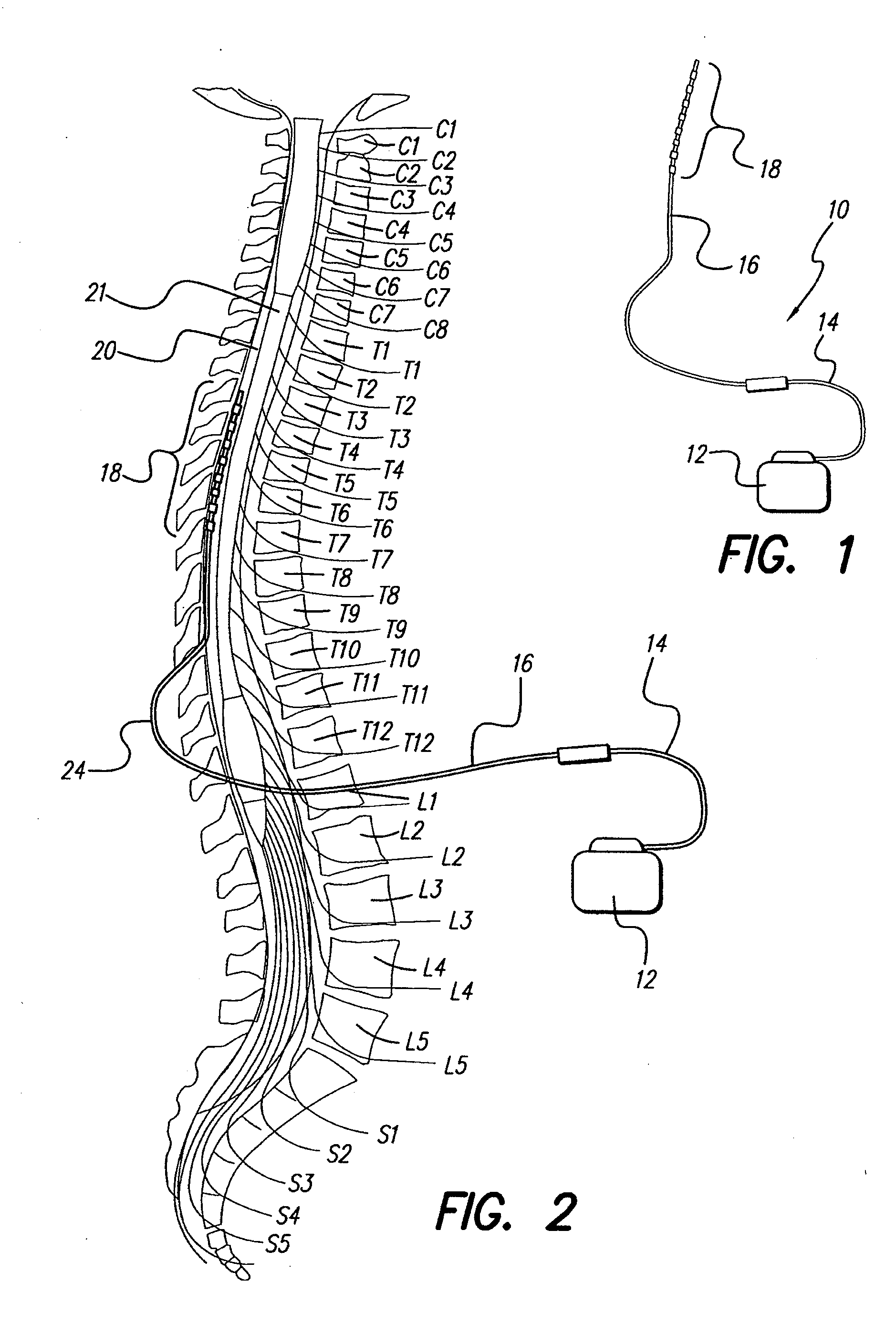
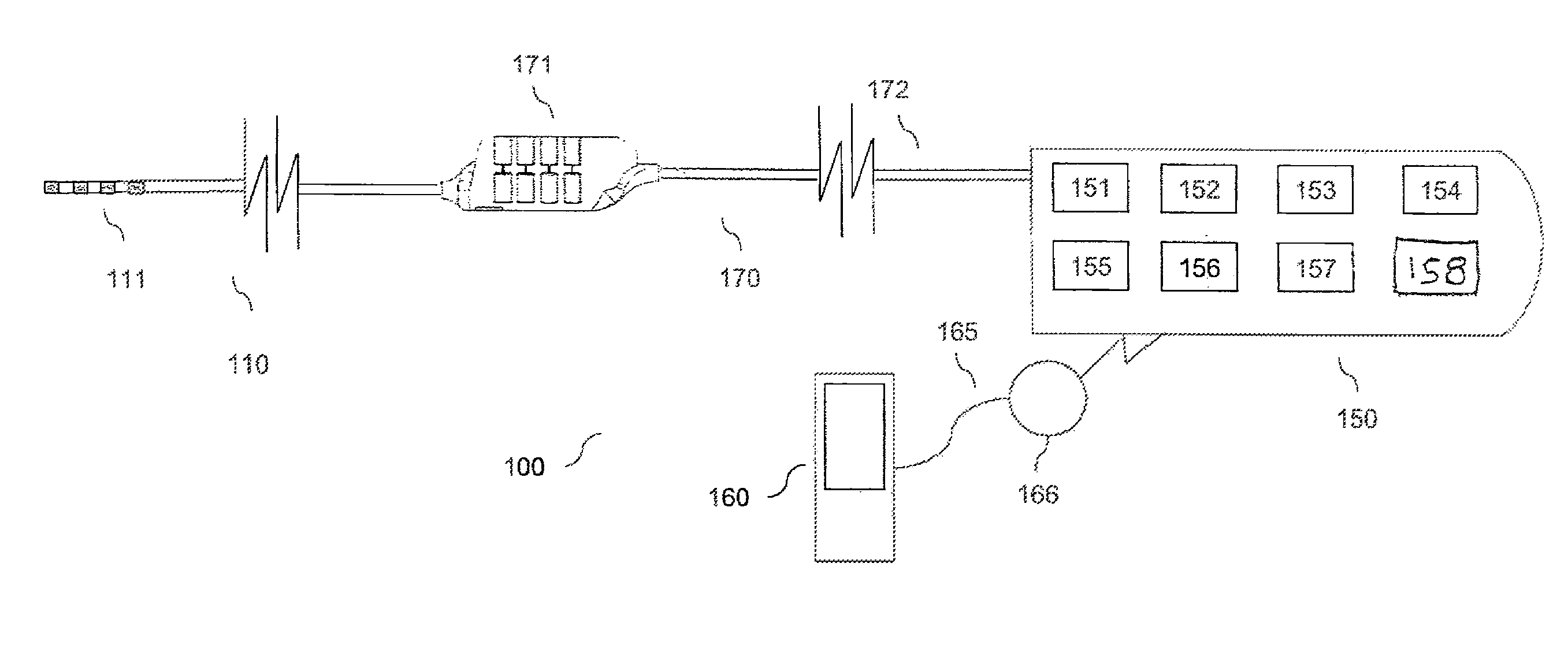
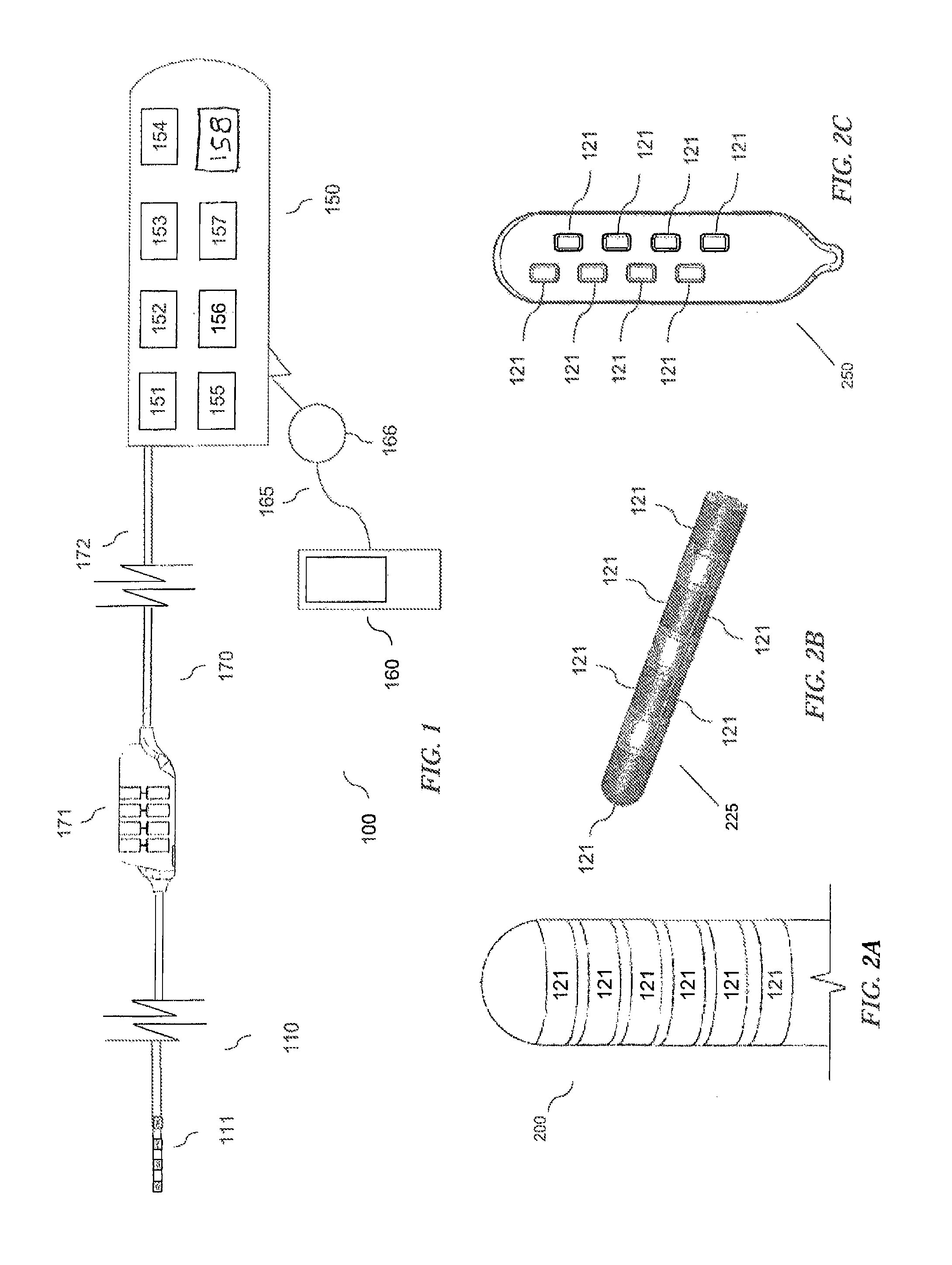
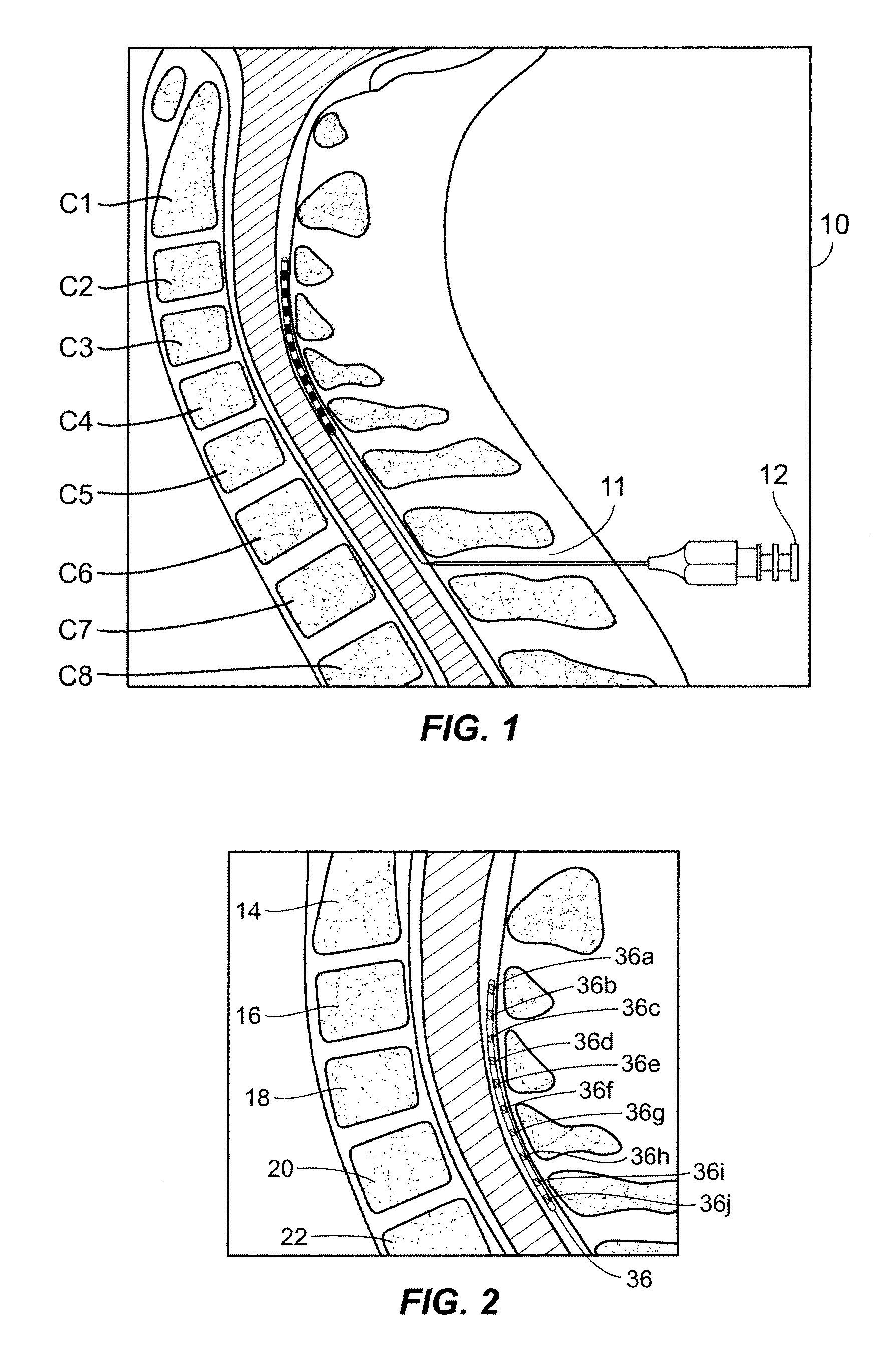
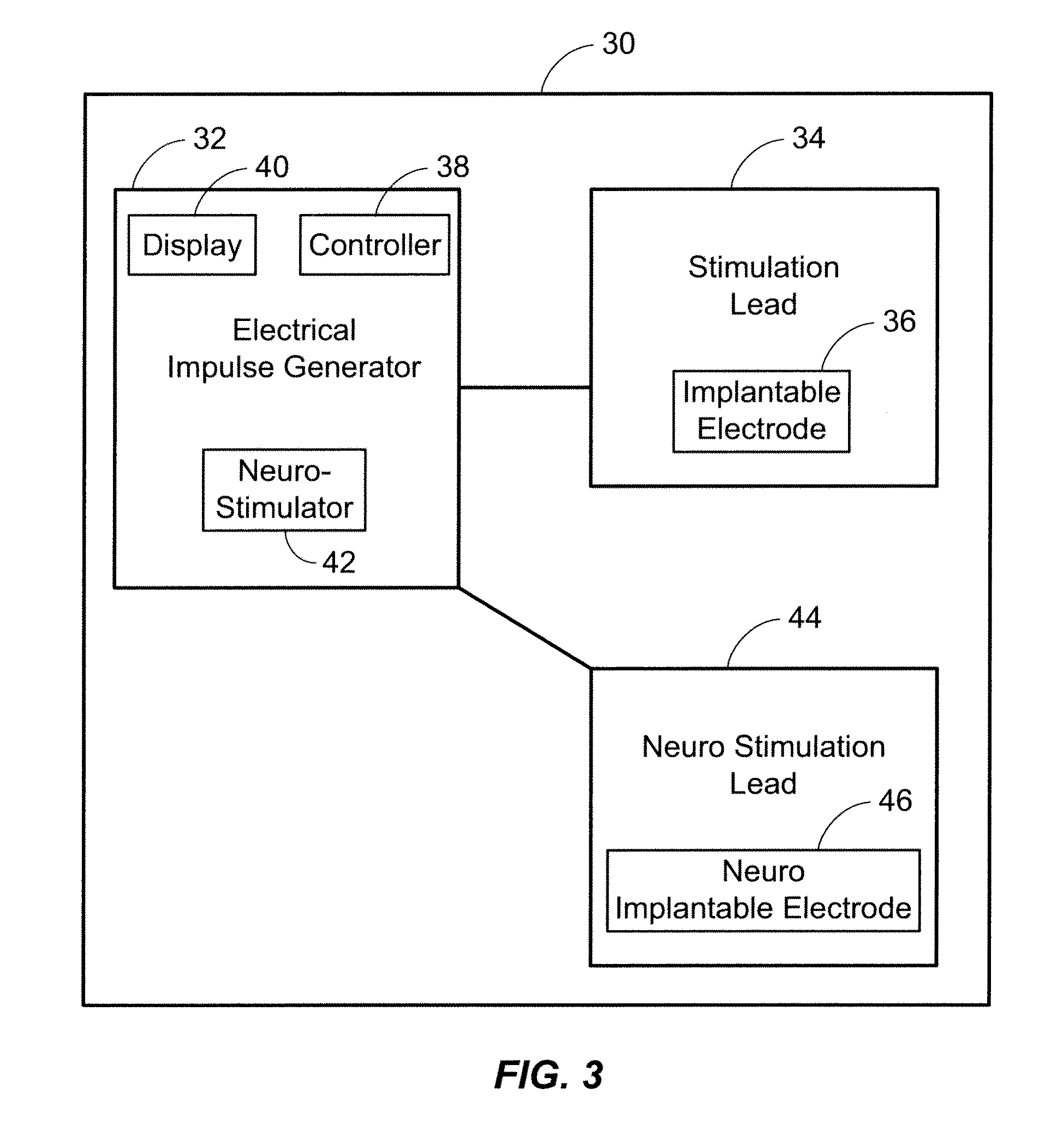
An implantable stimulator(s), small enough to be located near or within an area of the spine responsible for sensations in a region experiencing chronic pain uses a power source / storage device, such as a rechargeable battery. Periodic recharging of such a power source / storage device is accomplished, for example, by inductive coupling with an external appliance. The small stimulator provides a means of stimulating a nerve(s) or other tissue when desired, without the need for external appliances during the stimulation session. When necessary, external appliances are used for the transmission of data to and / or from the stimulator(s) and for the transmission of power, it necessary. In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one implant includes at least one sensor, and the sensed condition is used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
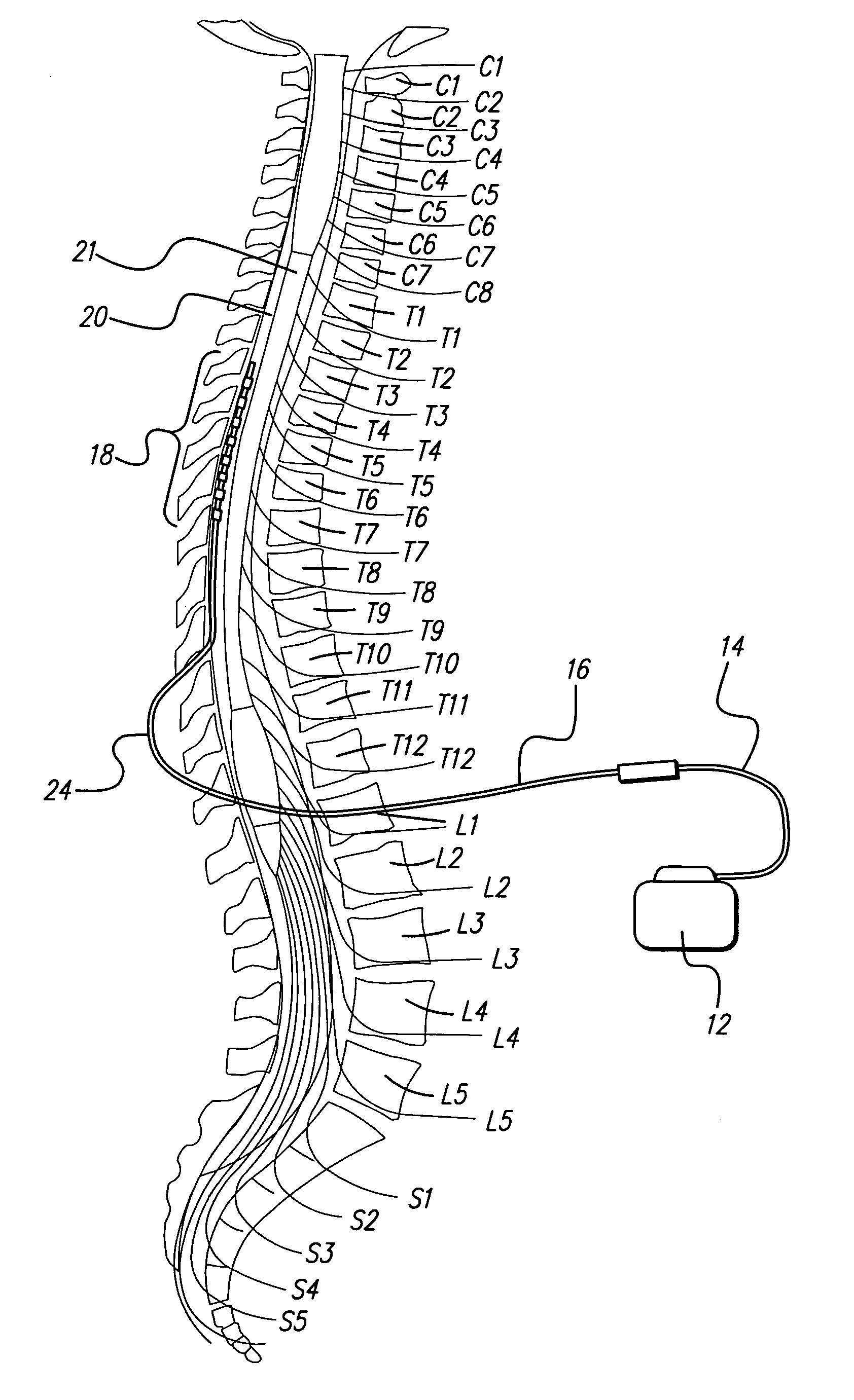
Method of using spinal cord stimulation to treat neurological disorders or conditions
InactiveUS20070060954A1Improve the quality of lifeEffectively treat lack of coordinationSpinal electrodesImplantable neurostimulatorsMedicineElectrical stimulations
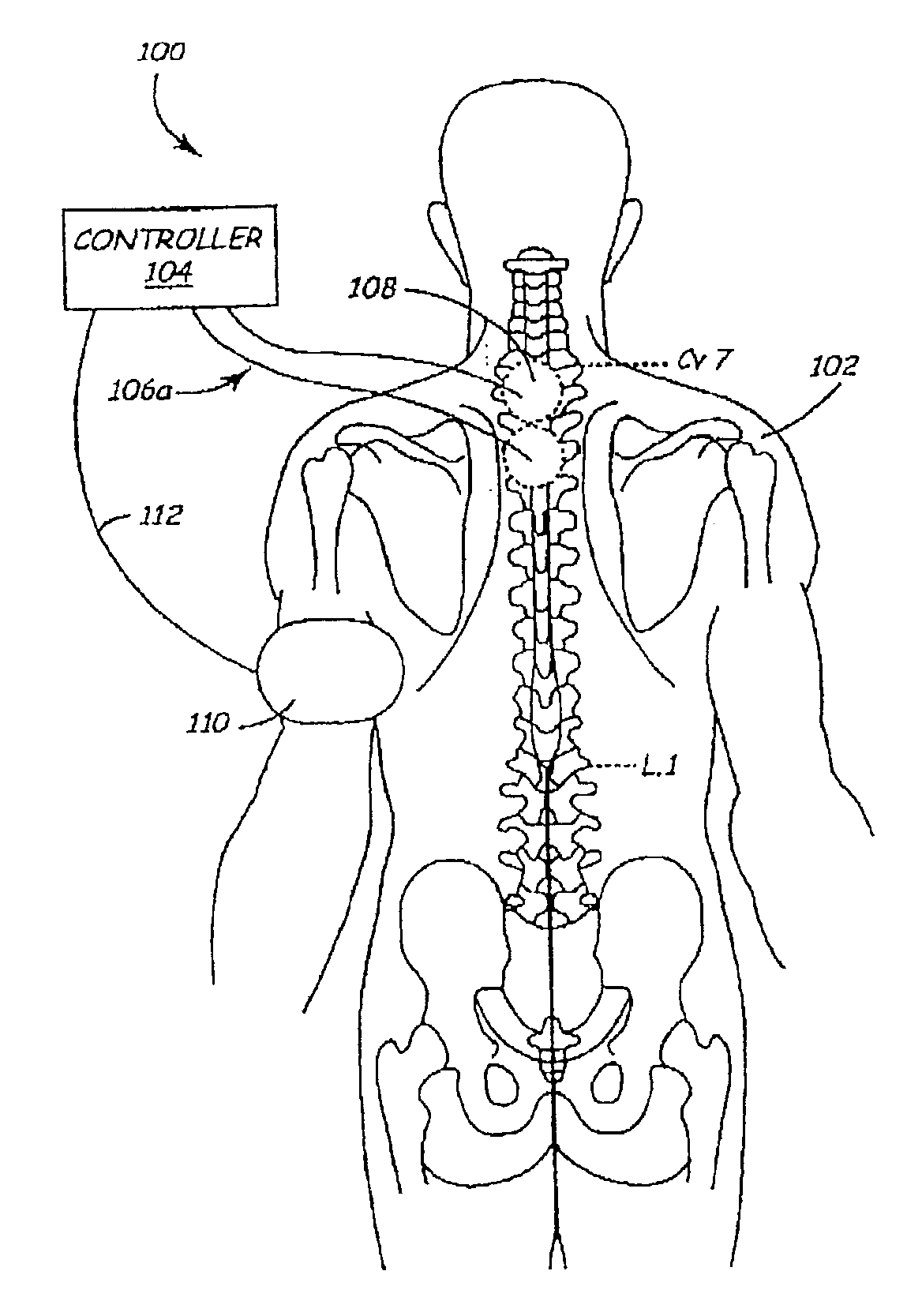
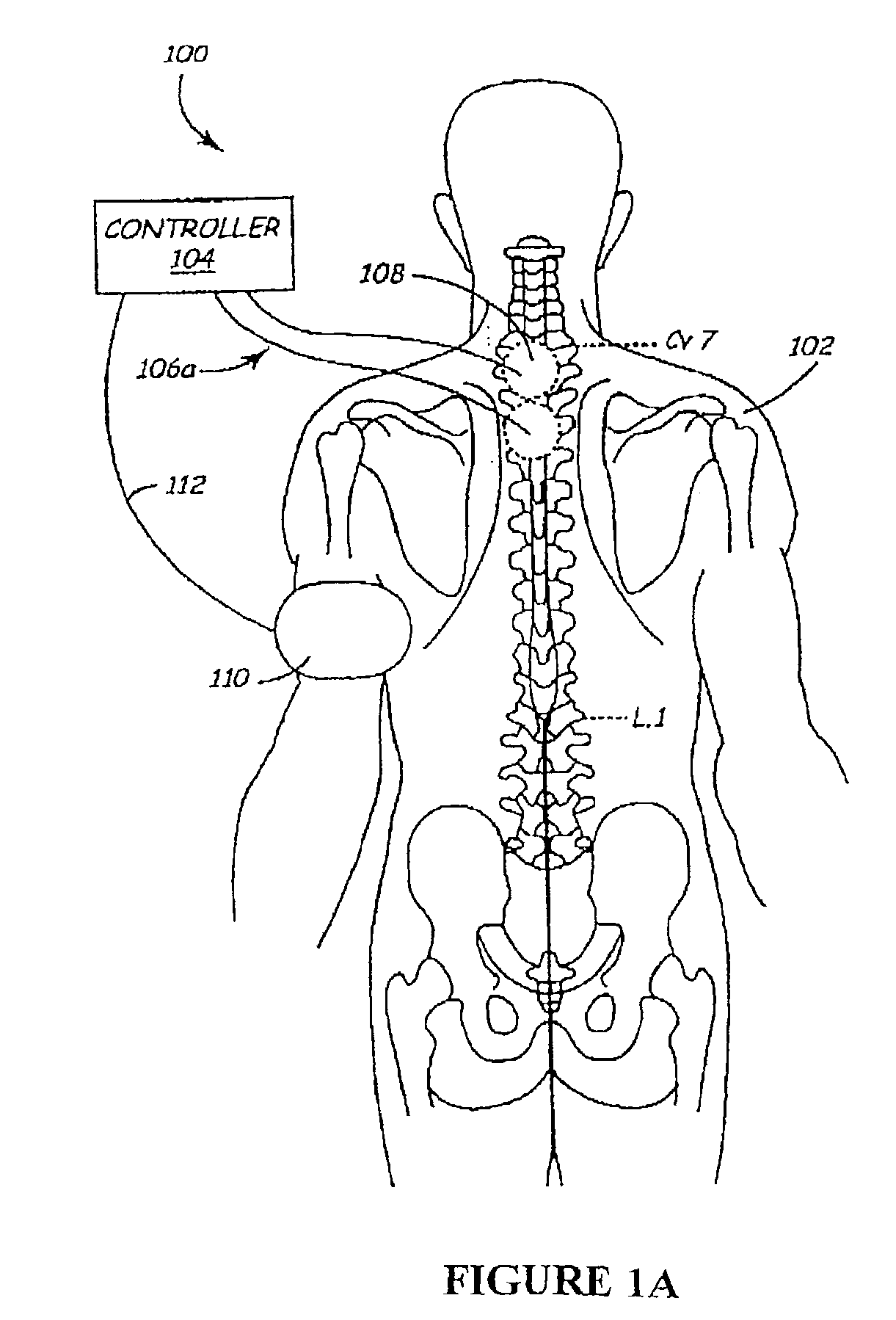
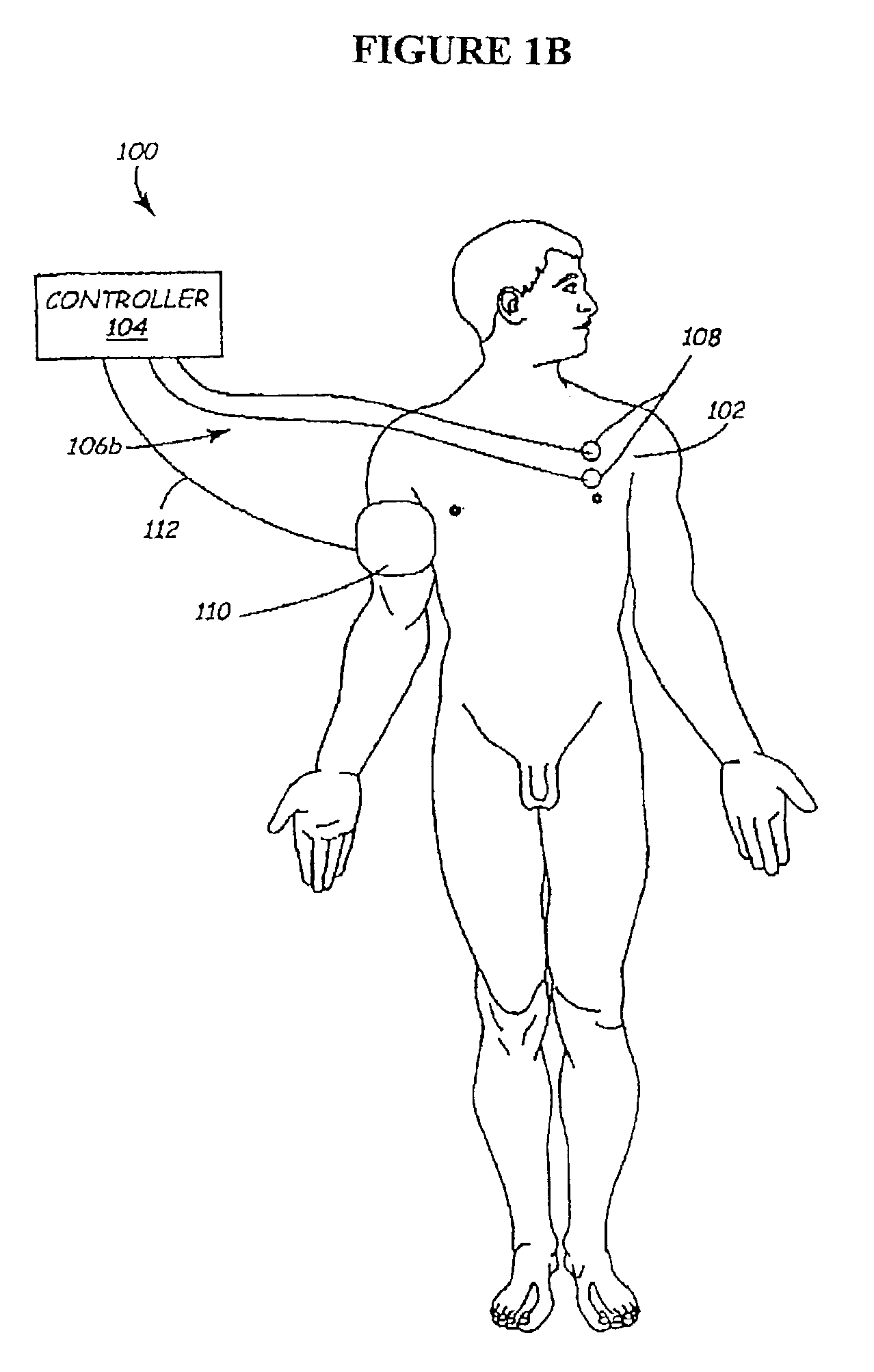
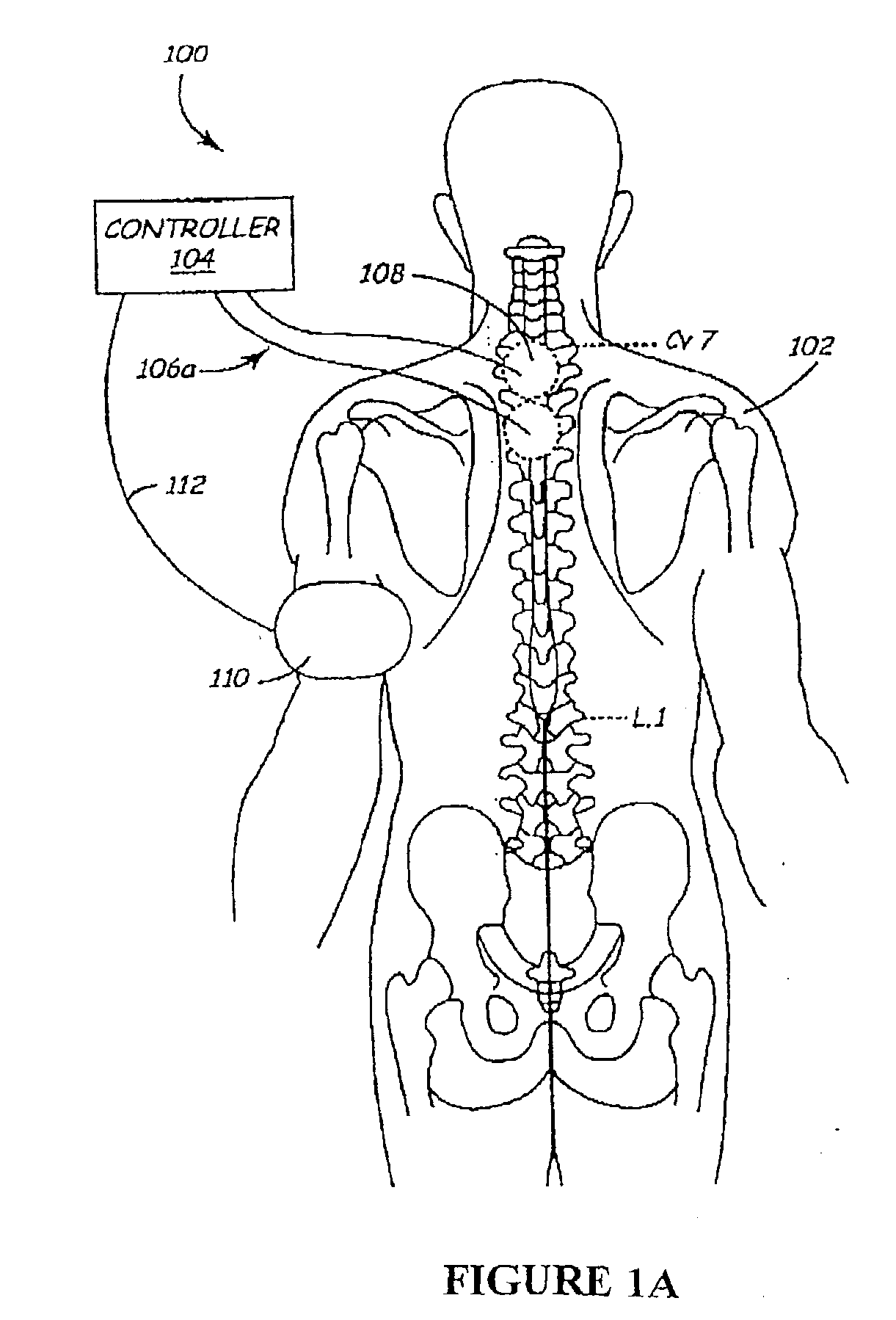
The present invention involves methods and systems for using electrical stimulation to treat neurological disorders. More particularly, the method comprises surgically implanting an electrical stimulation lead that is in communication with spinal nervous tissue associated with a first, second, or third cervical vertebral segment to result in spinal nervous tissue stimulation, thus treating a wide variety of neurological disorders.
Owner:ADVANCED NEUROMODULATION SYST INC
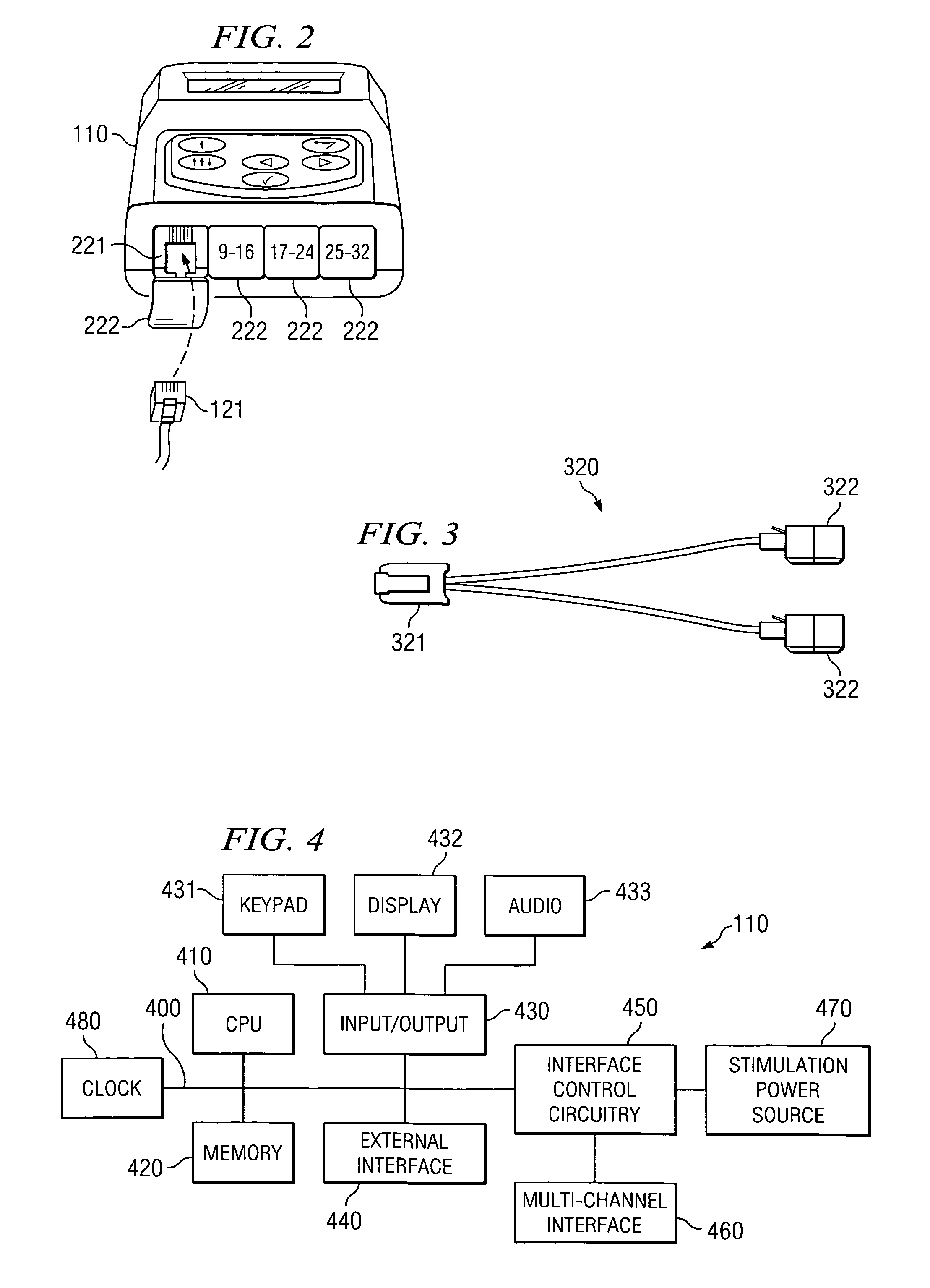
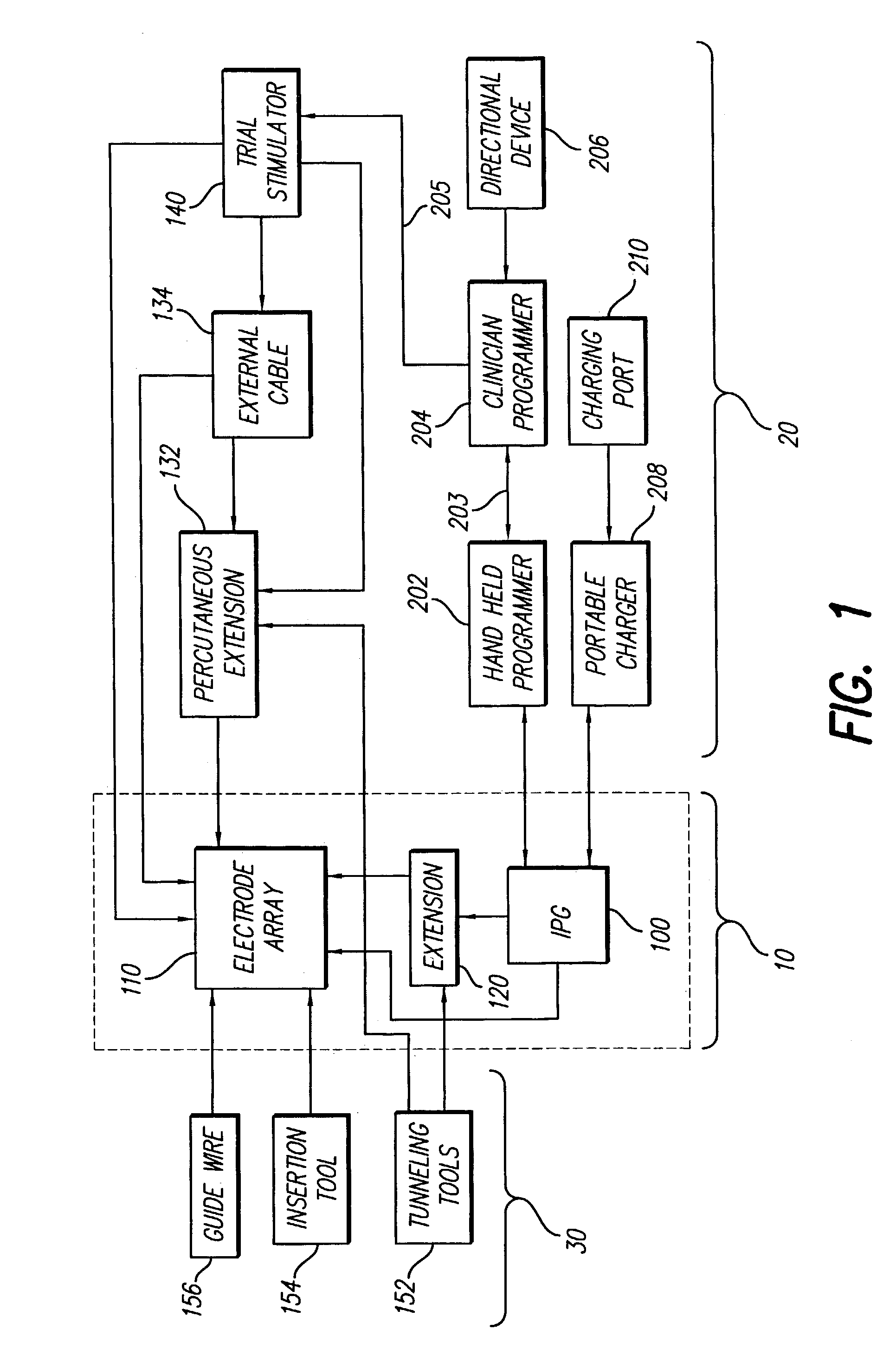
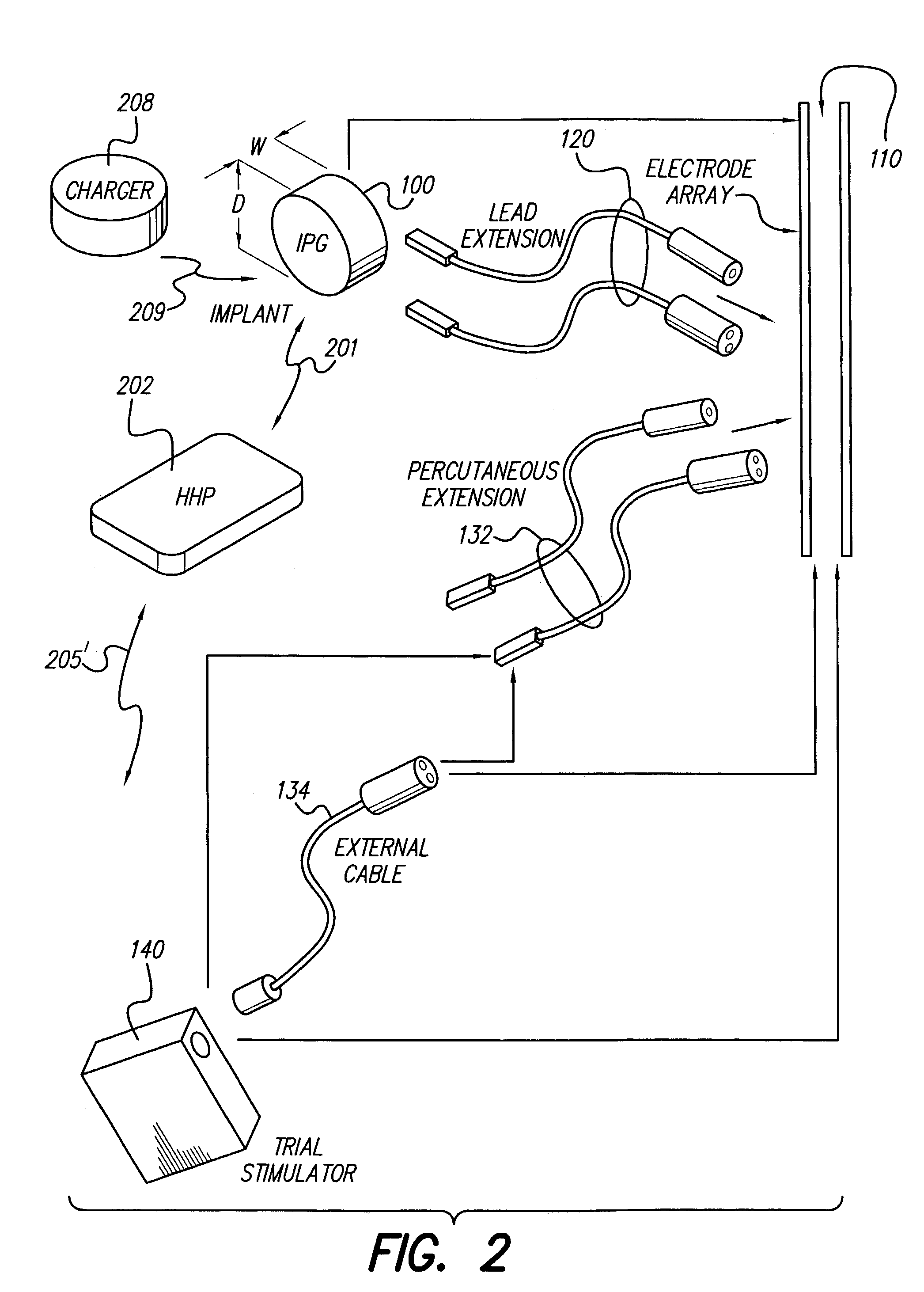
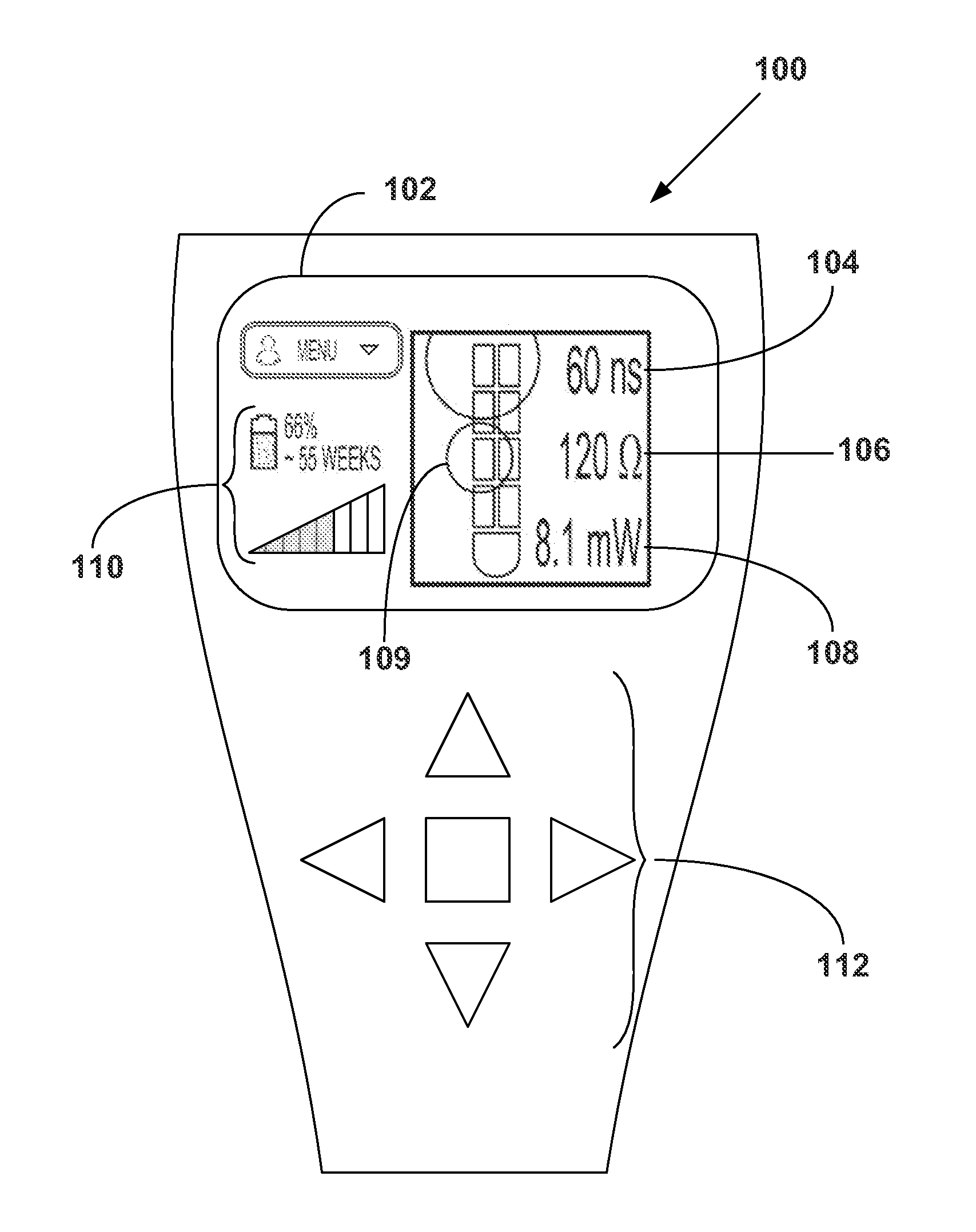
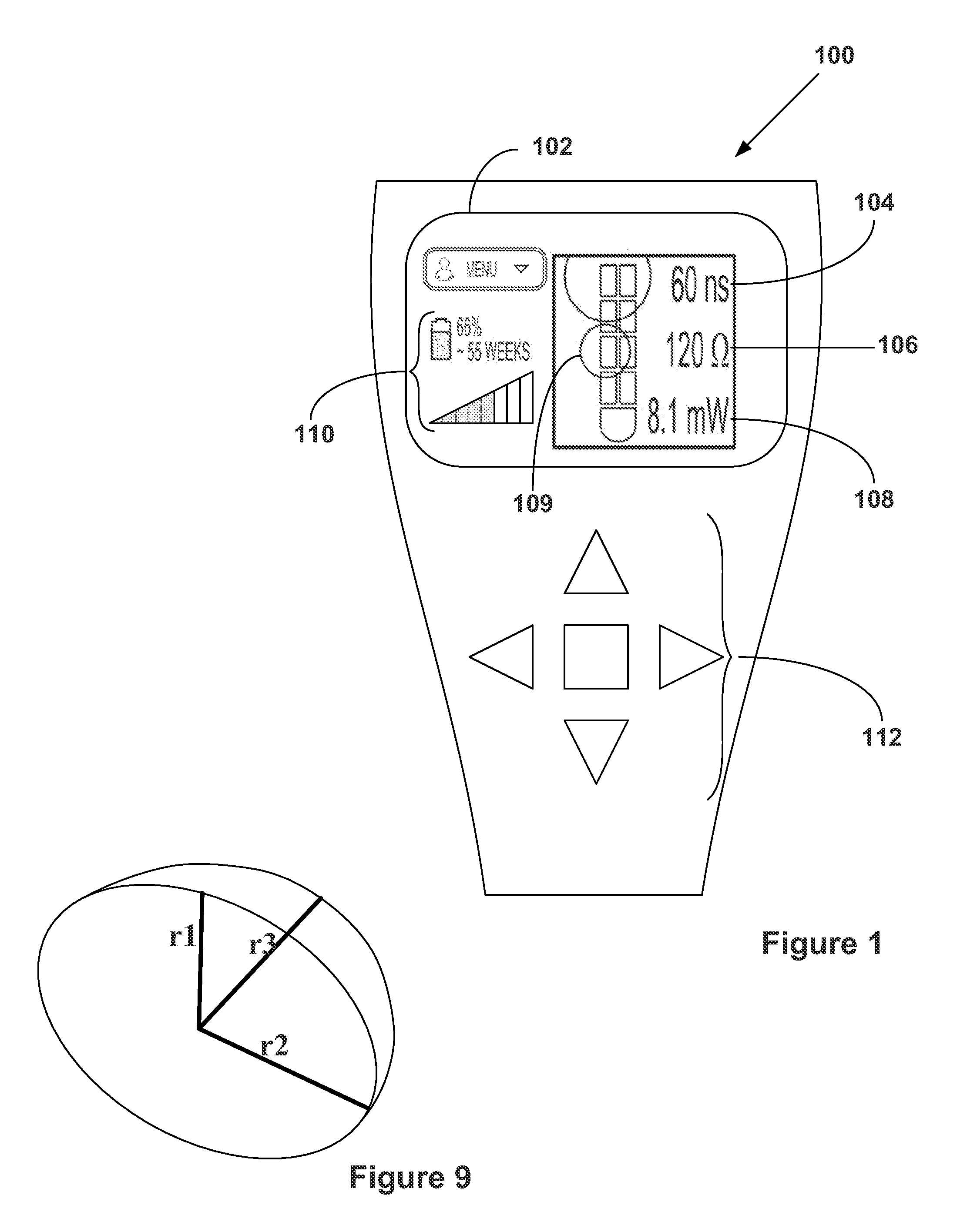
Clinician programmer for use with trial stimulator
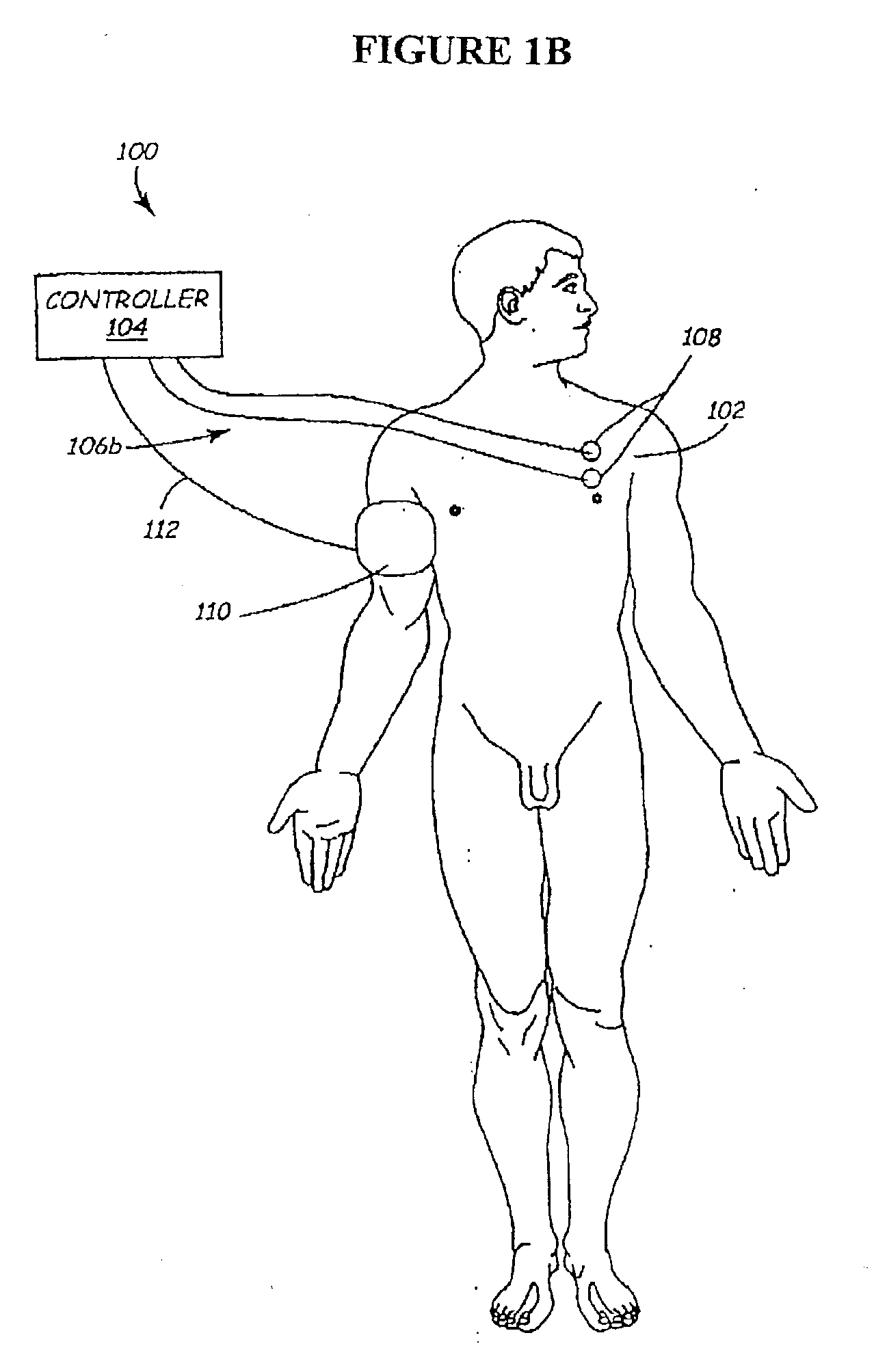
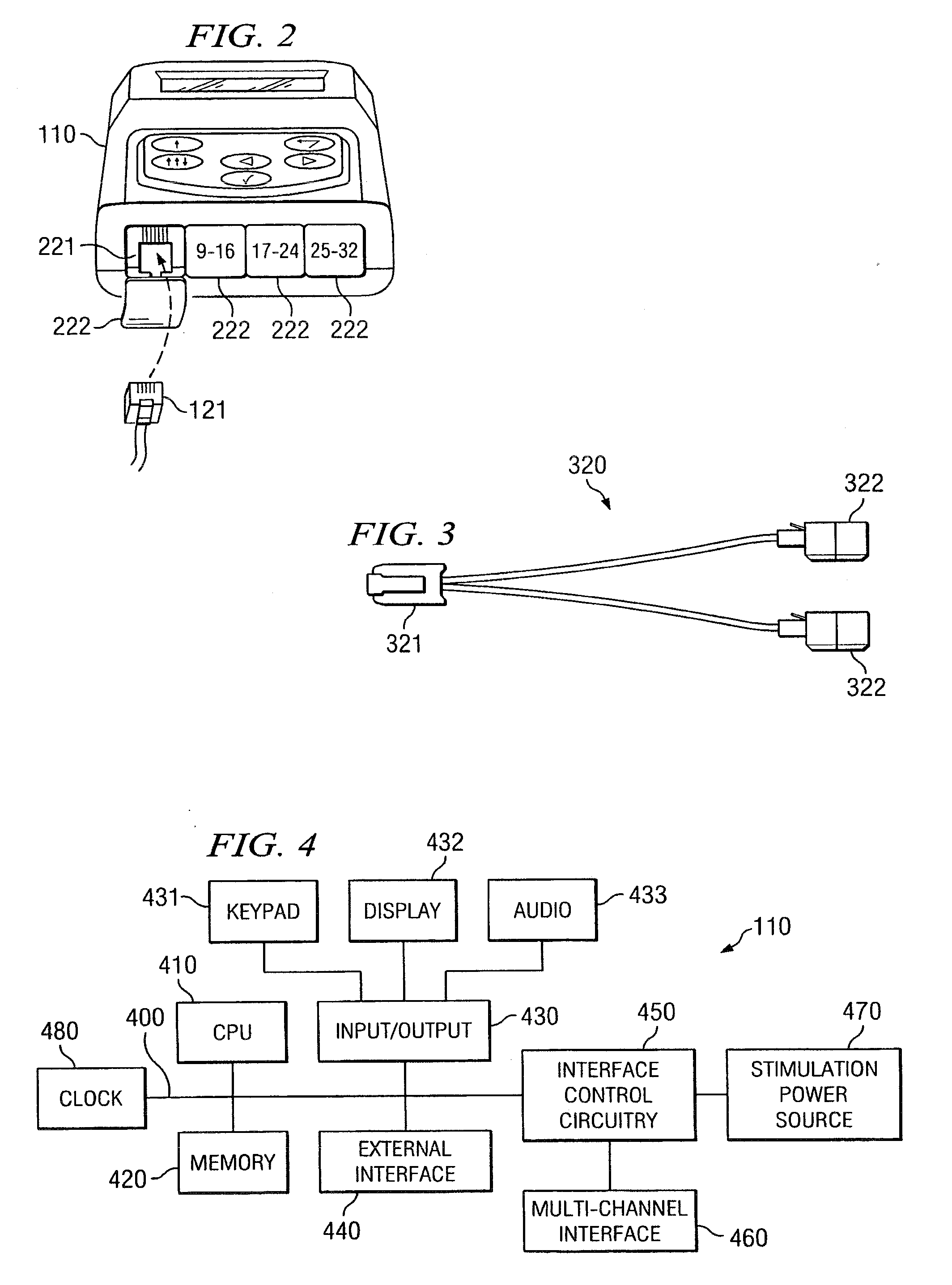
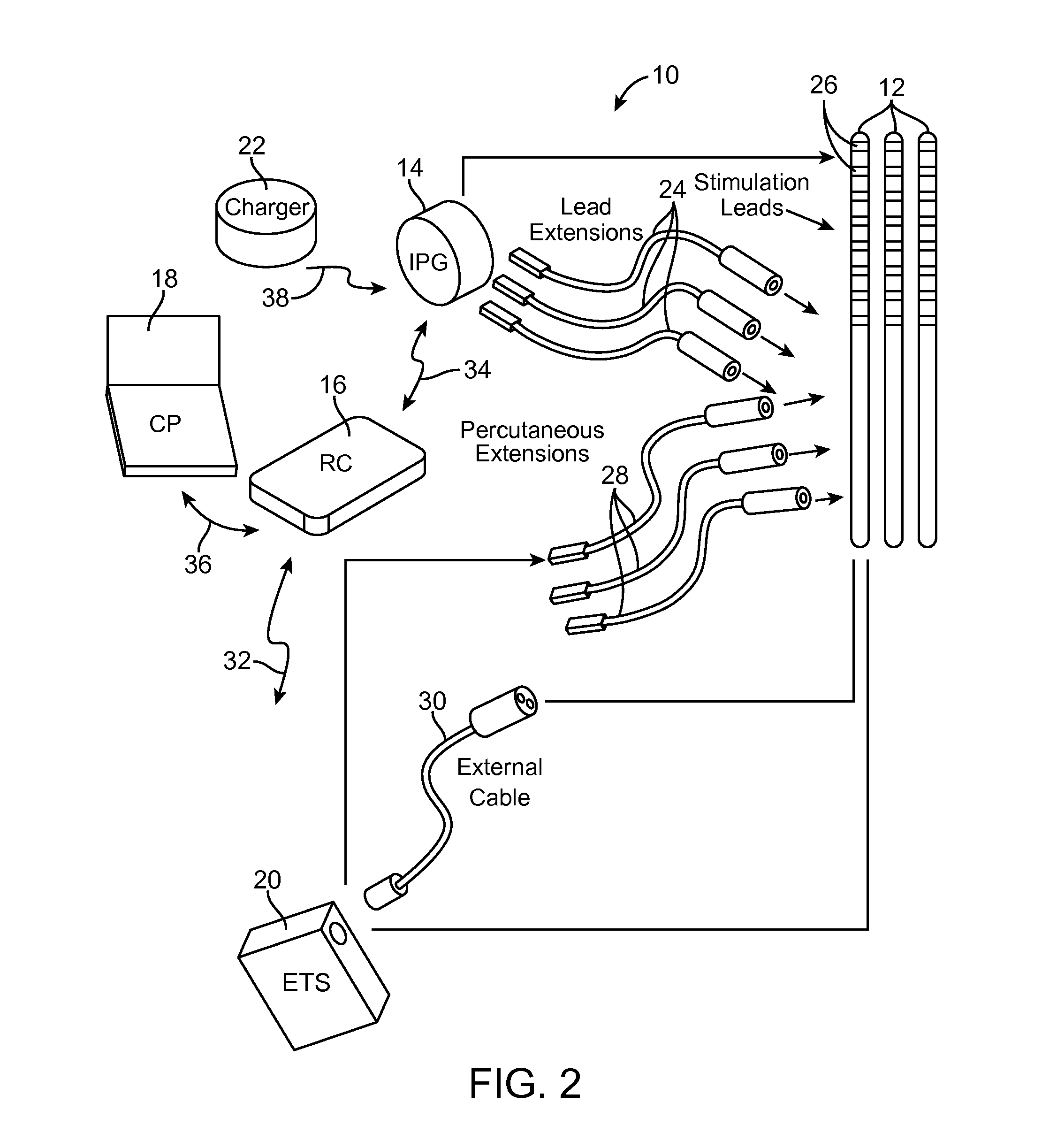
Disclosed are systems and methods which provide an external clinician interface, such as through the use of a laptop computer or a personal digital assistant (PDA). The foregoing clinician interface may be used with trial stimulators well suited for use interoperatively and during patient trial. Stimulators of embodiments are adapted for use in providing stimulation to a plurality of tissues and / or areas of the body, such as spinal cord stimulation, deep brain stimulation, etcetera.
Owner:ADVANCED NEUROMODULATION SYST INC
Methods and apparatus for the regulation of hormone release
ActiveUS7221979B2Modulate hormone levelPrevent hormone imbalancesInternal electrodesExternal electrodesCatecholamineElectrical stimulations
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7–L2 and the associated neural fibers within a region of the T7–L2 dermatomes
Owner:MEDTRONIC INC
System for measuring cardiac rhythm parameters for assessment of spinal cord stimulation
A neuro-stimulation system and method are provided which can monitor EKG signals and provide electrical stimulation. The system comprises a stimulation lead having at least one stimulating electrode on the lead and an IPG having a case and connectors. The connectors can mechanically and electrical connect to the lead and to the at least one stimulating electrode and an EKG electrode can be placed on the stimulating lead. The IPG case may be used variously as an EKG electrode, as well as an indifferent electrode. Alternatively or additionally, a separate, second lead having a second EKG electrode may be connected to the IPG. This second EKG electrode may also double in function as a stimulation electrode.
Owner:BOSTON SCI NEUROMODULATION CORP
Methods and apparatus for the regulation of hormone release
A method and apparatus for delivering corrective therapy through hormone regulation is provided. Inhibition of sympathetic fibers by spinal cord stimulation is used to regulate the levels of hormones such as catecholamines, renin, and calcitonin gene-related peptide. The invention utilizes a closed or open loop feedback system in which physiological parameters such as the concentrations of hormones and sympathetic indicators such as heart rate and urine production are monitored and used to determine the appropriate level of neurostimulation. The site of electrical stimulation includes, but is not limited to, the spinal cord at levels T7-L2 and the associated neural fibers within a region of the T7-L2 dermatomes
Owner:MEDTRONIC INC
Treatment of pain
InactiveUS7333857B2Reduce stimulationAvoid signalingSpinal electrodesExternal electrodesAnatomySpinal tracts
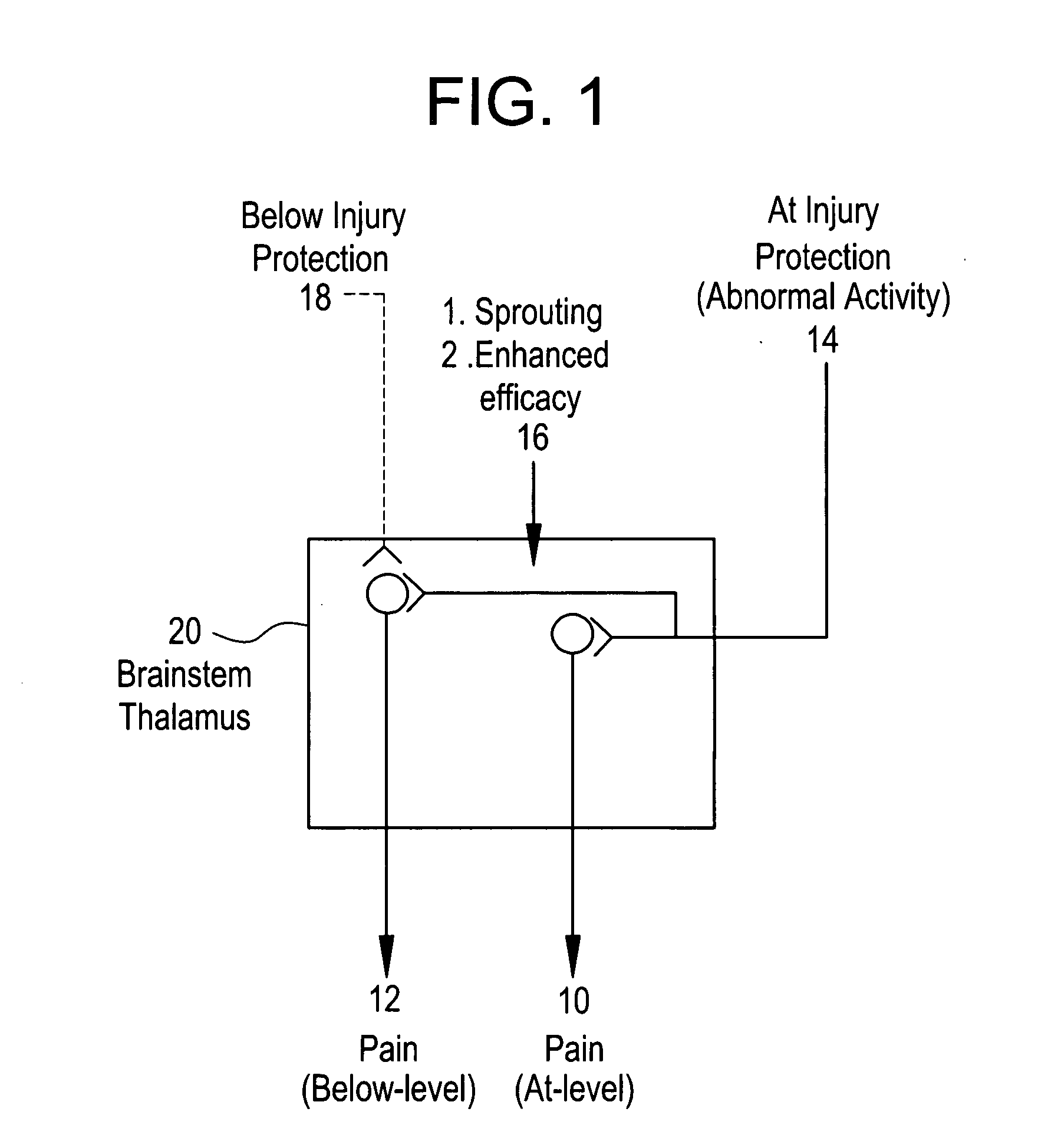
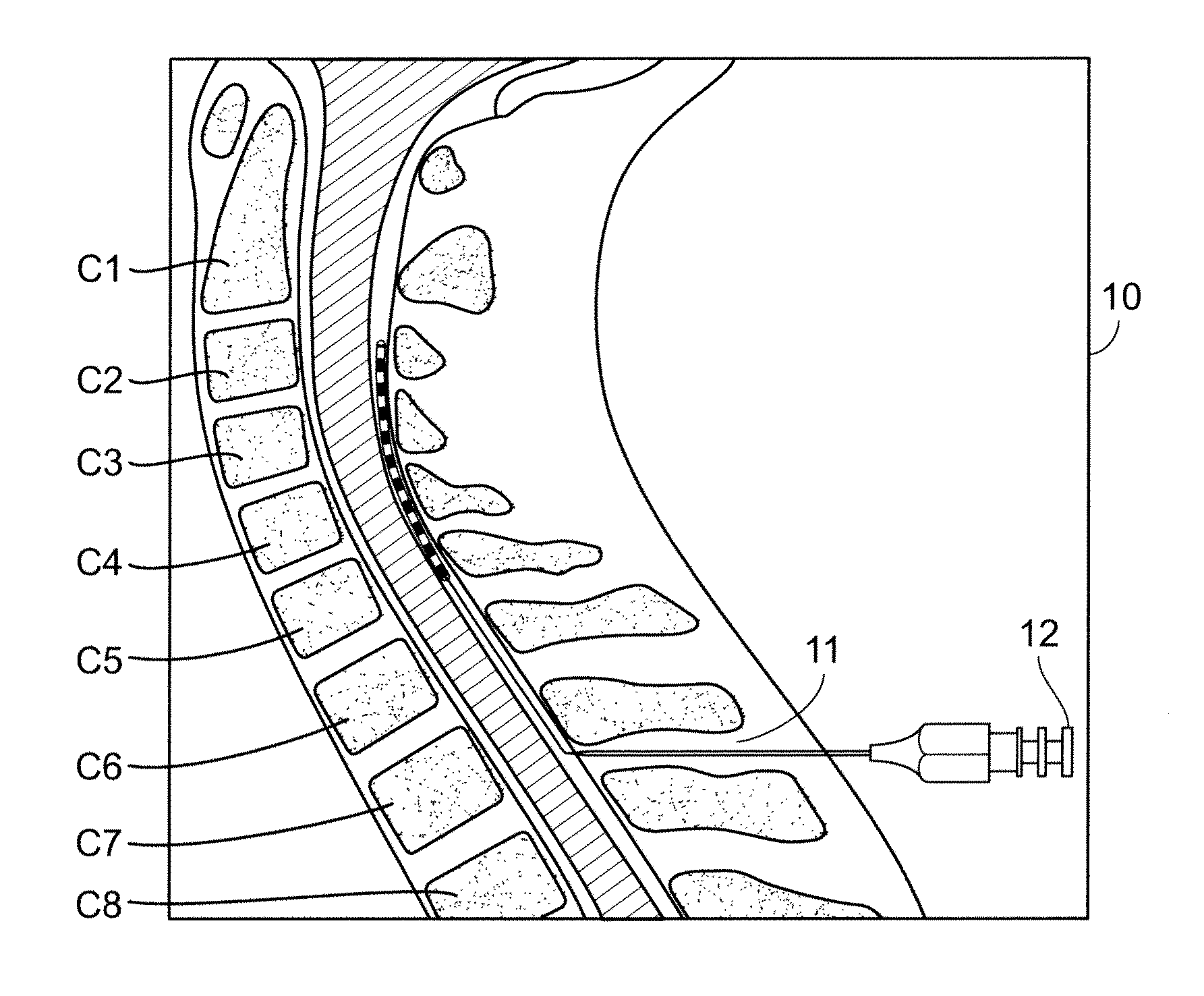
The method disclosed herein entails spinal cord stimulation via electrodes placed directly into the dorsal horn, dorsal column, spinothalamic tract, nucleus cuneatus, nucleus gracilis, spinal tract of V, or spinal nucleus of V (nucleus caudalis) depending on the source of pain. This “intramedullary” stimulation “jams” or otherwise prevents the pain signal from being transmitted. The method provides a means to stimulate the targeted area directly, creating a stable means of stimulating the desired area, and decreasing stimulation of other structures.
Owner:ARC 1
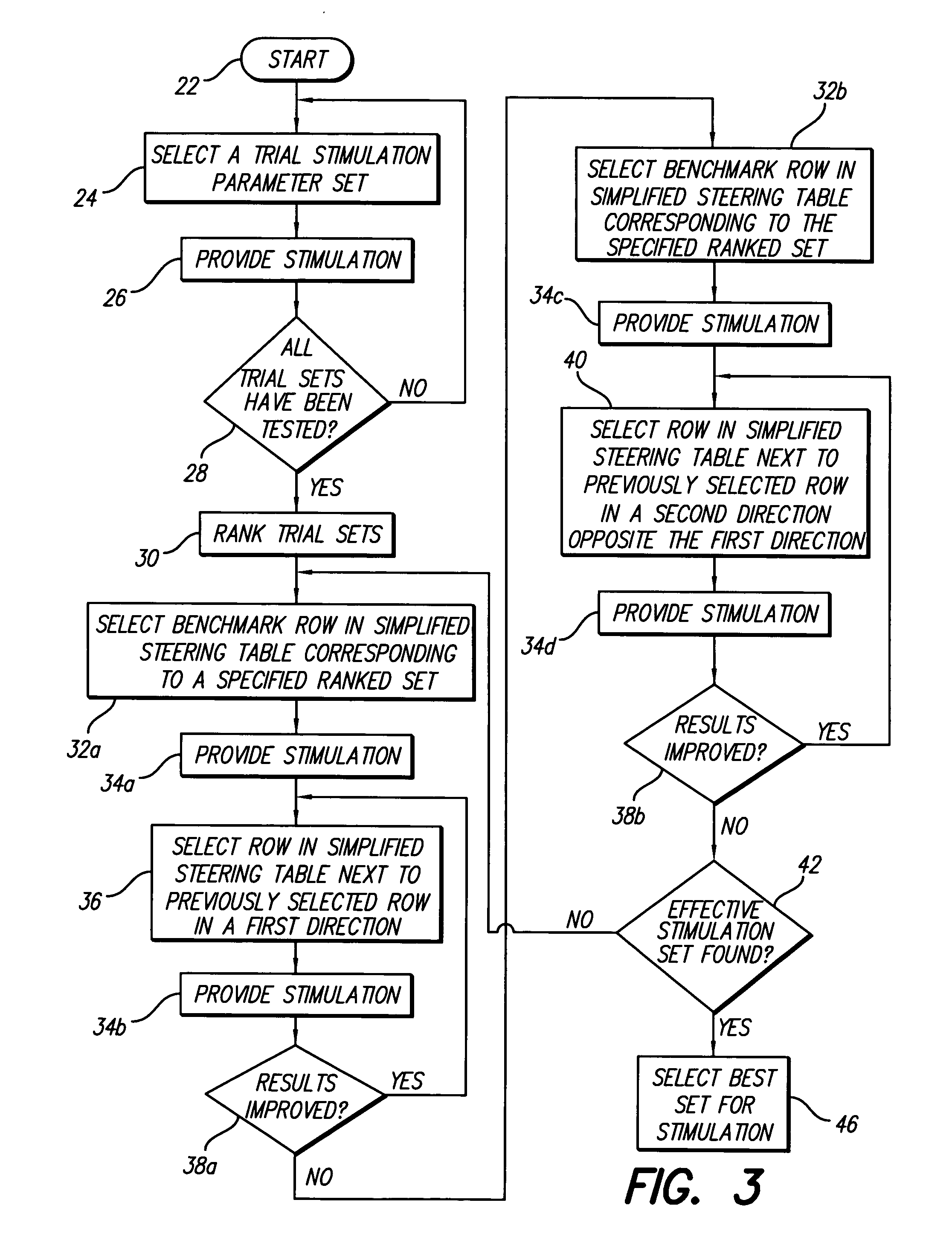
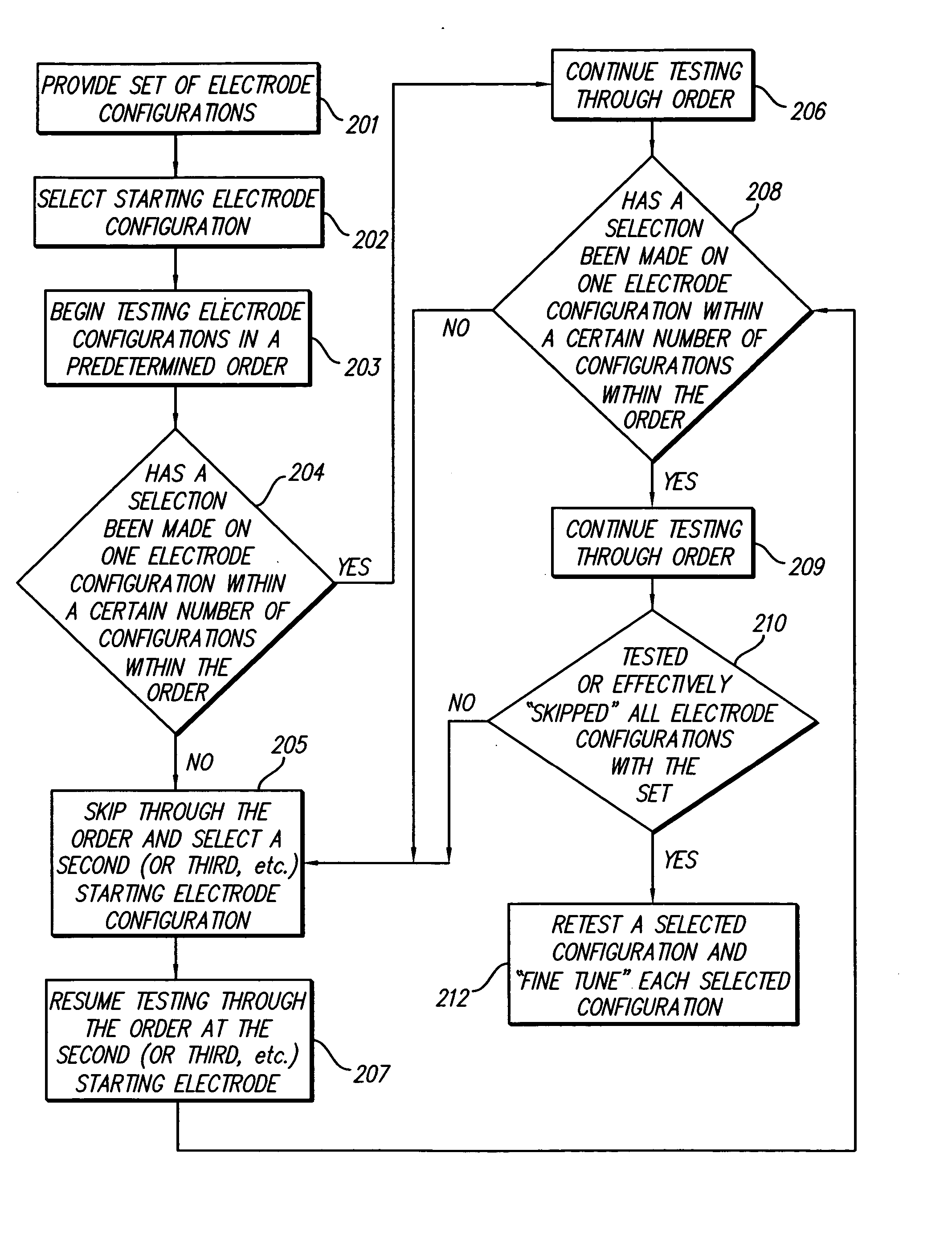
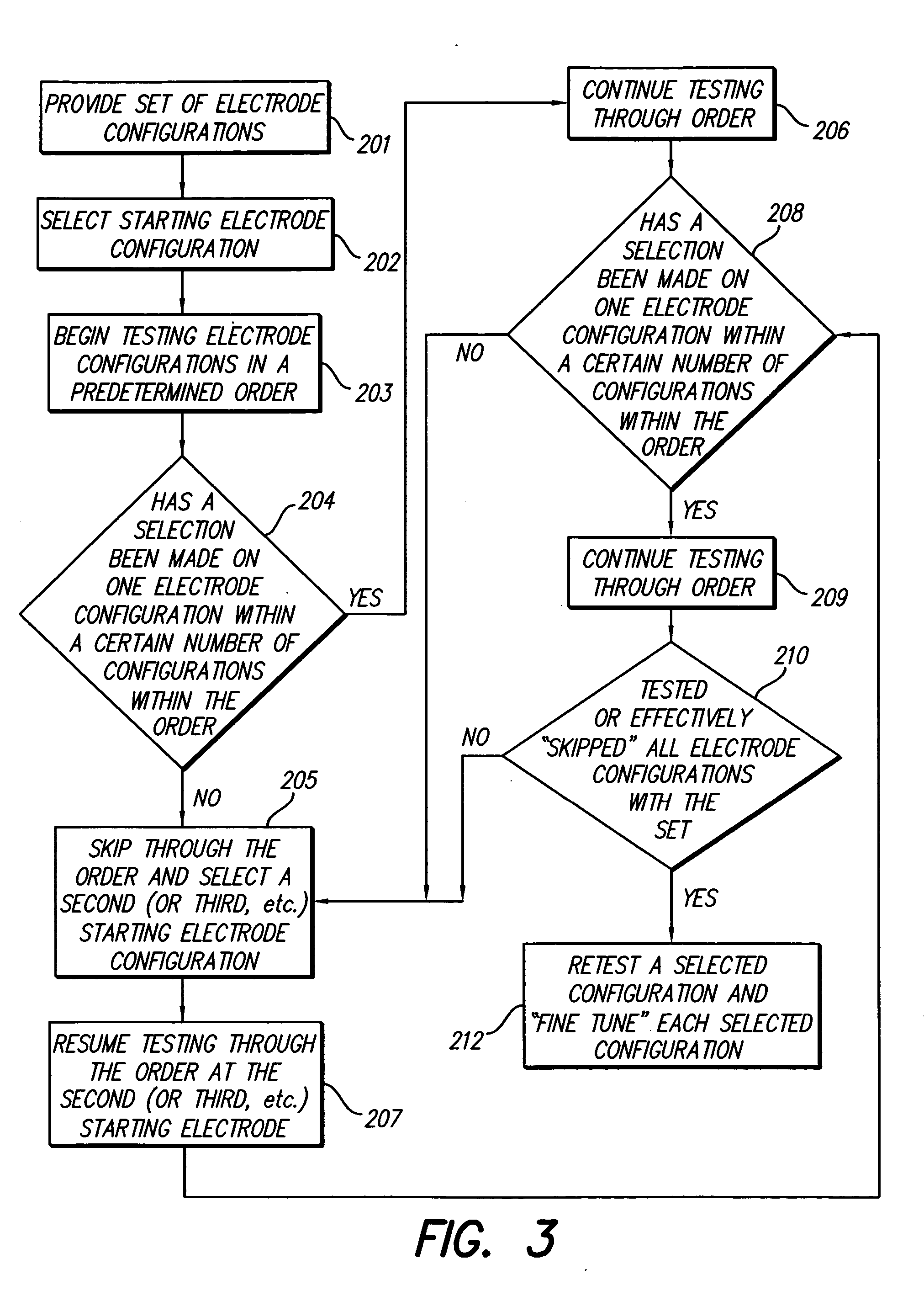
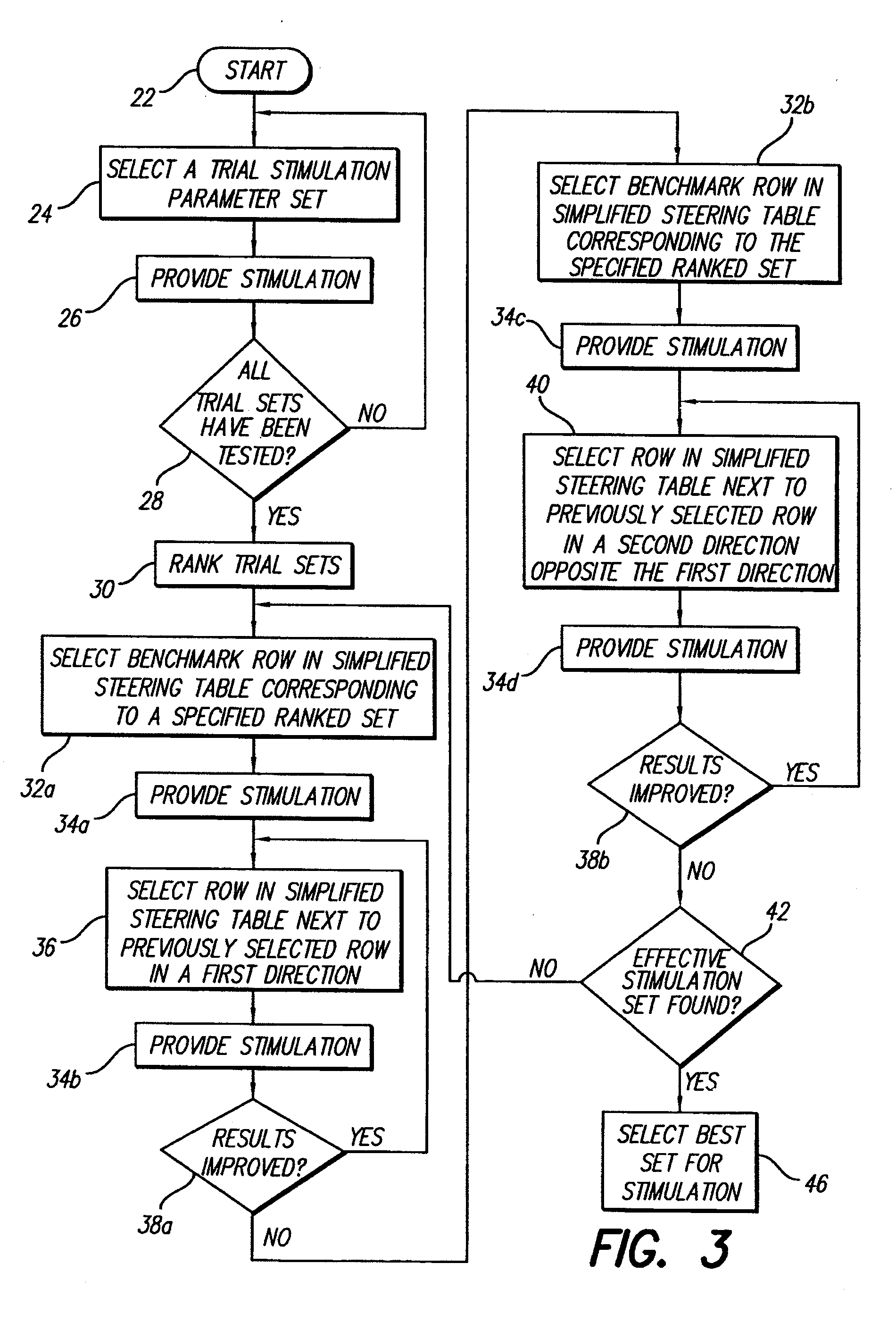
Method for optimizing search for spinal cord stimulation parameter settings
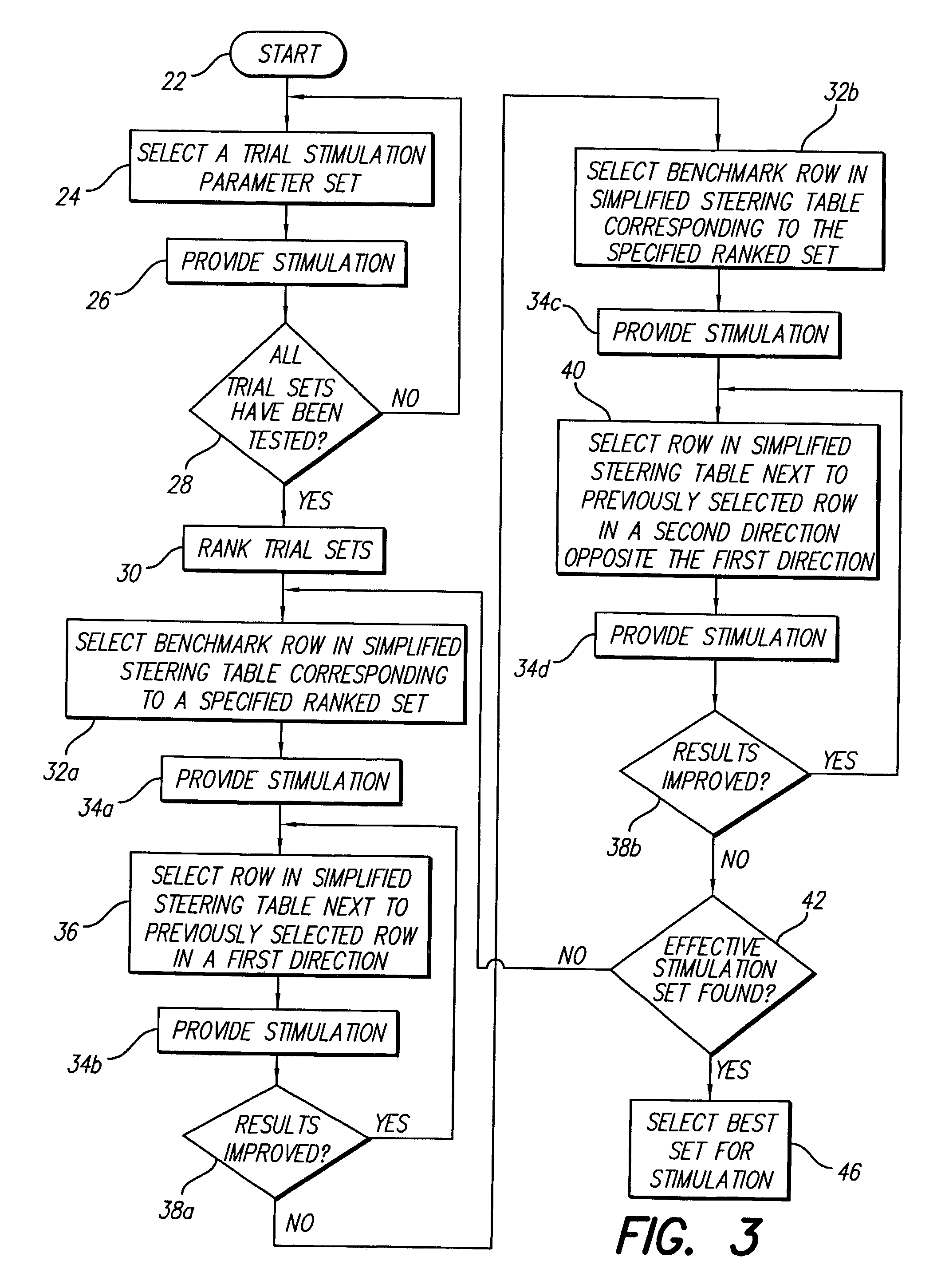
A method for selecting Spinal Cord Stimulation (SCS) stimulation parameter sets guides a clinician towards an effective set of stimulation parameters. The clinician first evaluates the effectiveness of a small number of trial stimulation parameter sets from a Measurement Table. Based on the patient's assessment, the trial stimulation sets are ranked. Then the clinician selects a starting or benchmark row in a Steering Table corresponding to the highest ranked trial stimulation parameter set. The clinician moves either up or down from the starting row, testing consecutive parameter sets. When a local optimum is found, the clinician returns to the benchmark row, and tests in the opposite direction for another local optimum. This process of searching for optimum parameter sets is repeated for a new starting row in the Steering Table that is selected based on the next ranked trial set from the Measurement Table.
Owner:BOSTON SCI NEUROMODULATION CORP
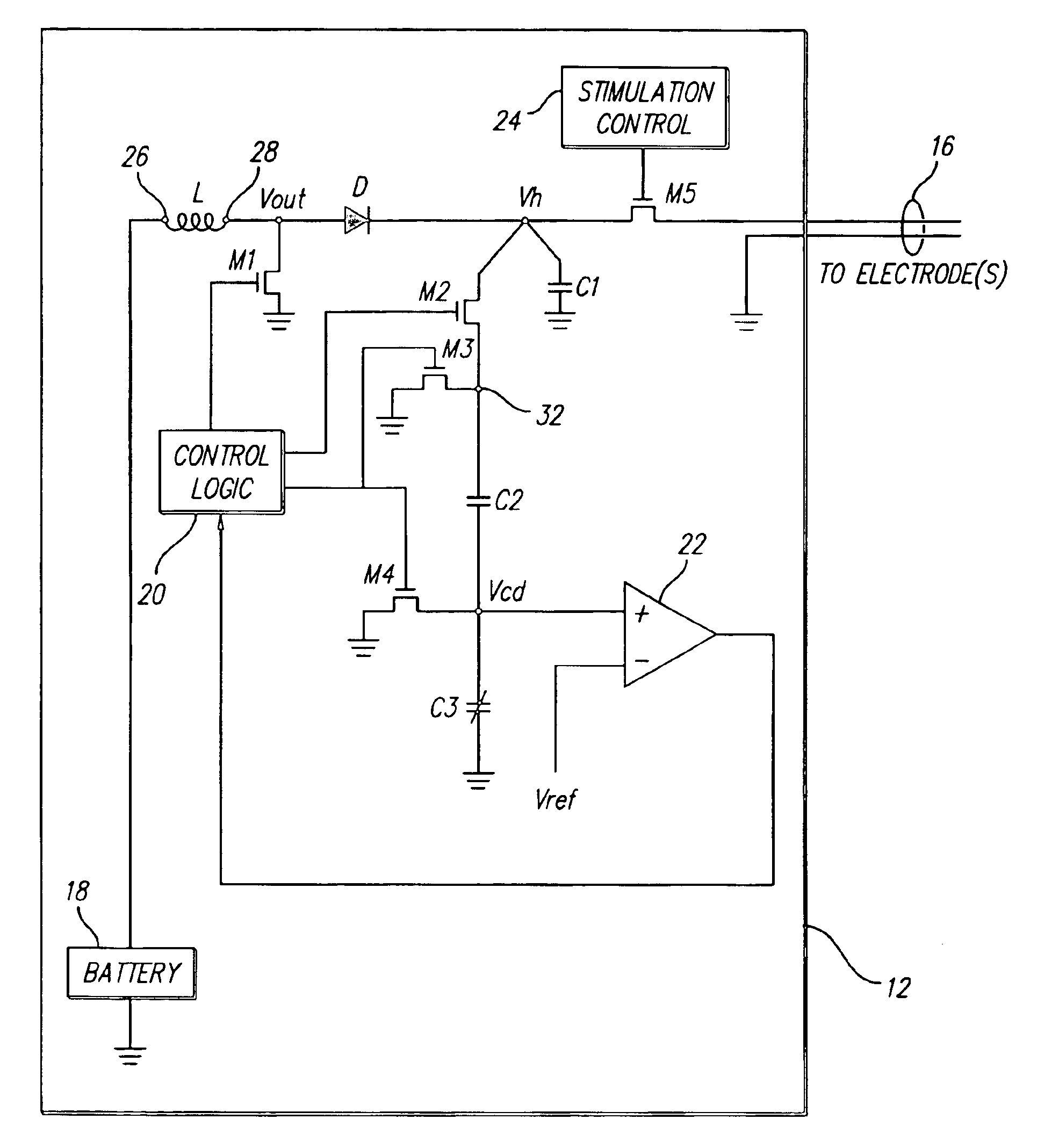
Switching regulator for implantable spinal cord stimulation
An improved switching regulator for implantable medical devices includes a control circuit with a capacitor divider to conserve energy, and selectable duty cycles to efficiently match the duty cycle to the charge level in a holding capacitor. The switching regulator charges the holding capacitor to commanded voltage levels, and the holding capacitor provides current for tissue stimulation. The commanded voltage level is reached by “pumping-up” the holding capacitor with the output of the switching regulator. For control purposes, the high voltage (i.e., the voltage across the holding capacitor) is divided between a fixed capacitor and a variable capacitor, and the voltage between the fixed capacitor and the variable capacitor (i.e., the divided voltage) is compared to a reference voltage. The result of the comparison is used to turn-off the switching regulator once the commanded voltage level is reached. The switching duty cycle is set to one of two values. At start-up, or when the output voltage drops below a determined threshold, a low duty cycle is used. Once the output voltage reaches the threshold, a higher duty cycle is used.
Owner:BOSTON SCI NEUROMODULATION CORP
Neural stimulation lead fixation
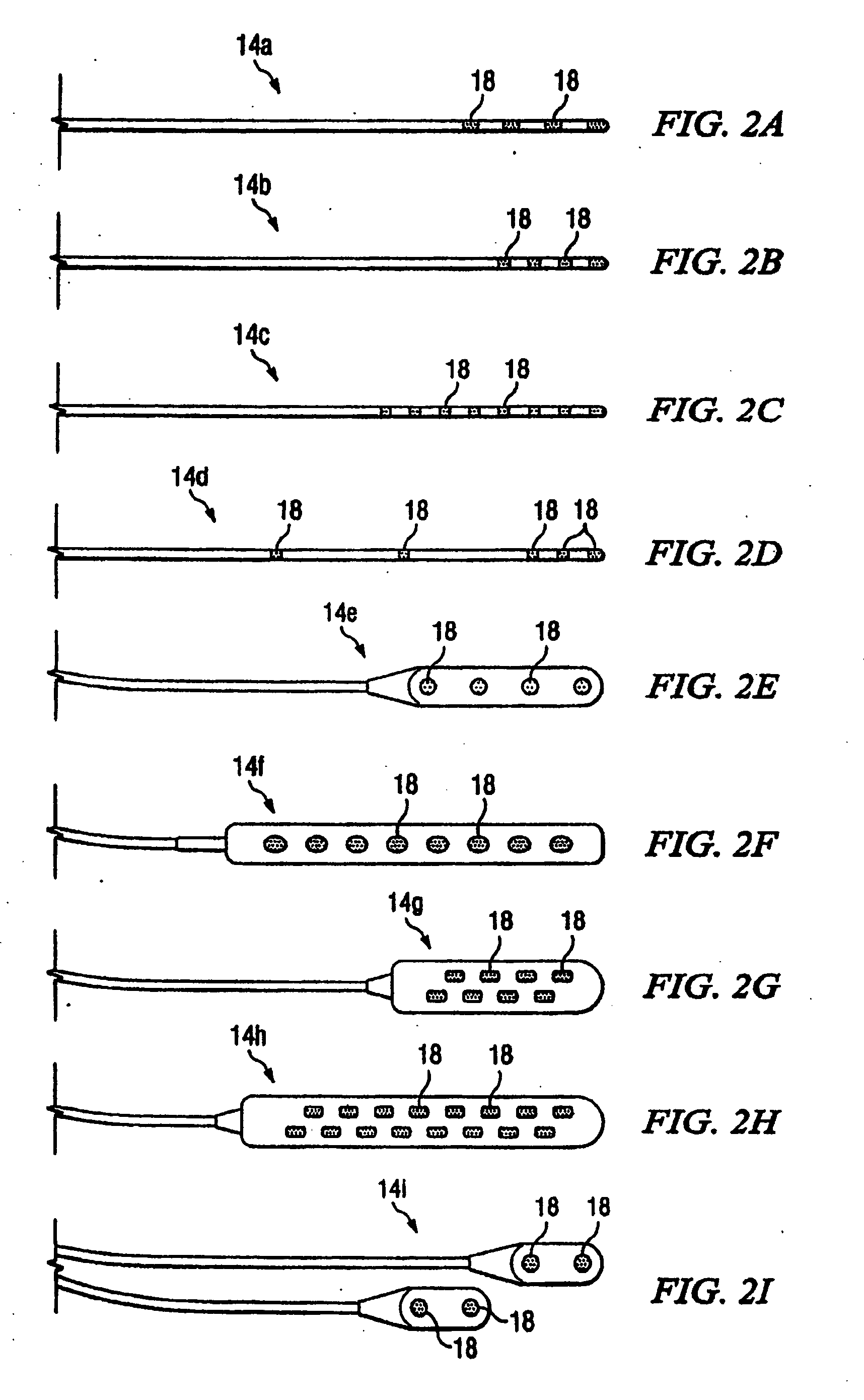
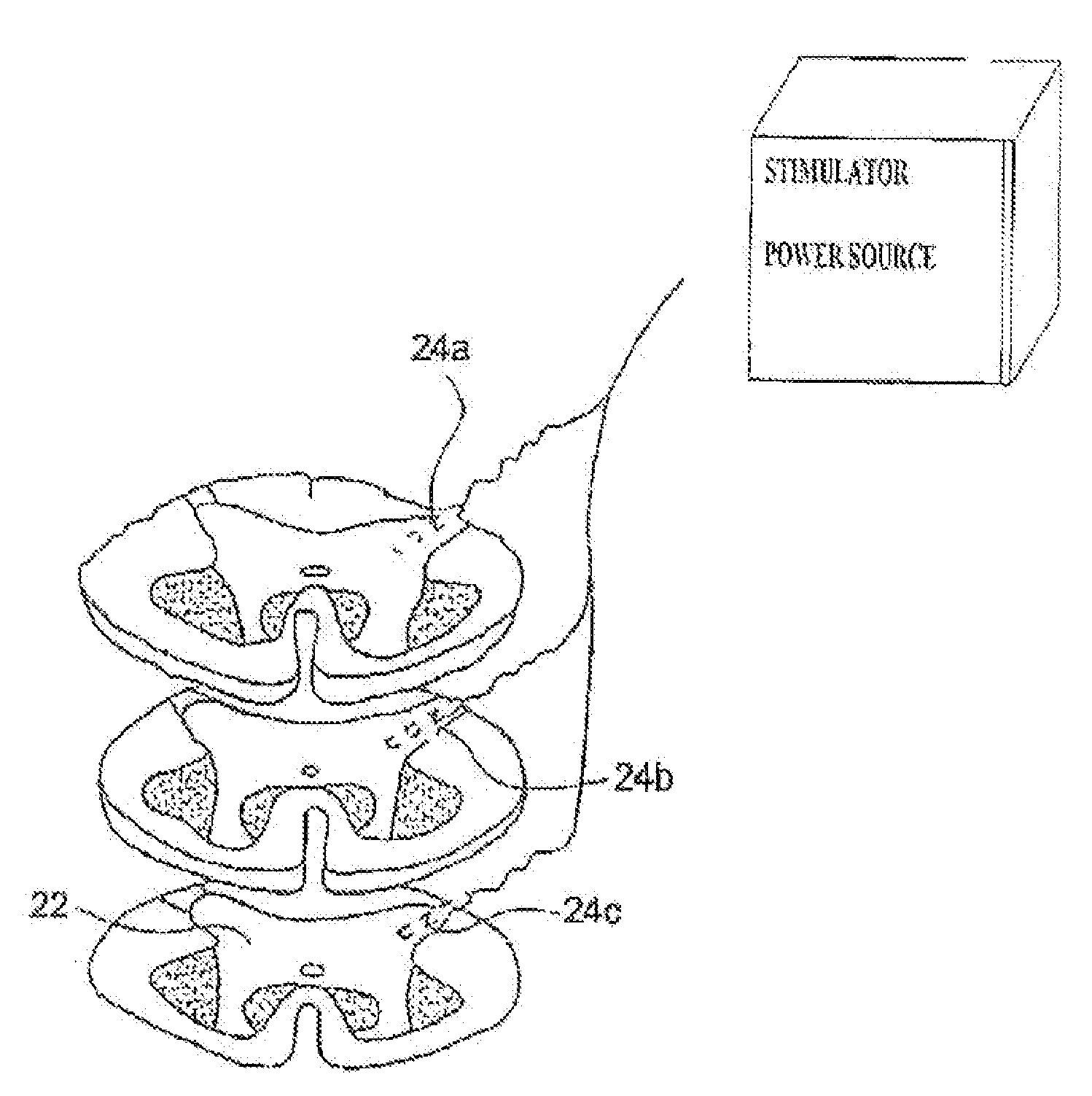
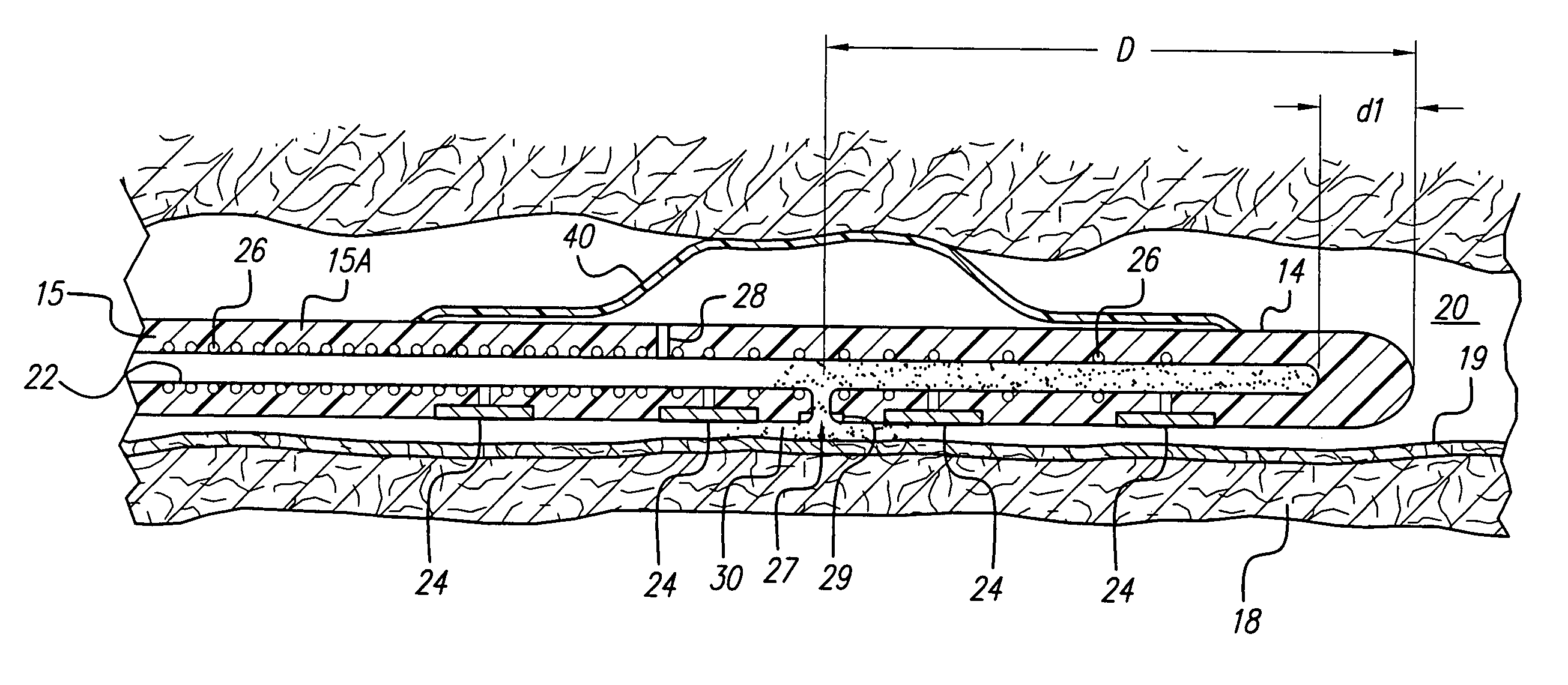
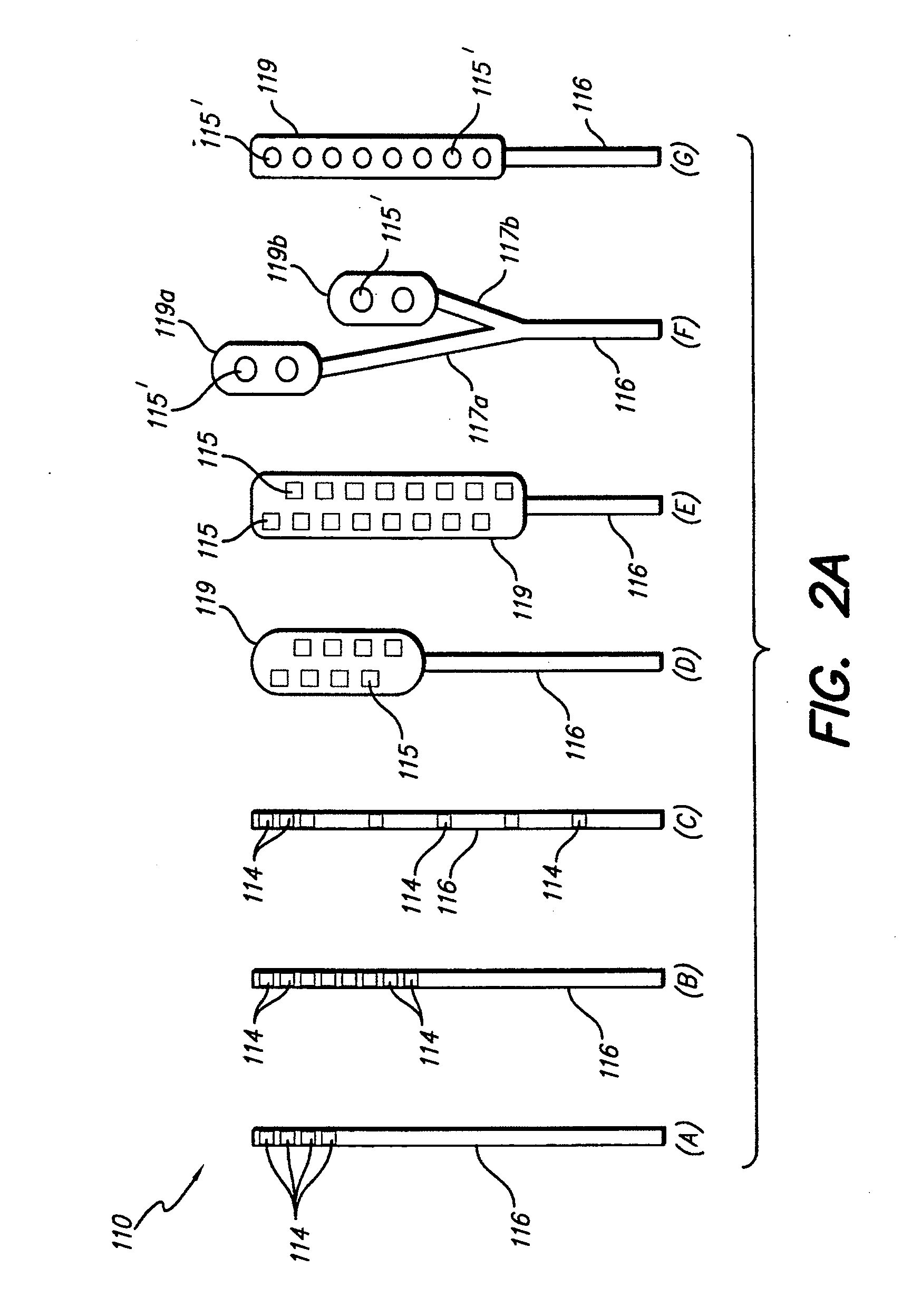
An implantable lead having at least one electrode contact at or near its distal end prevents undesirable movement of the electrode contact from its initial implant location. One embodiment relates to a spinal cord stimulation (SCS) lead. A balloon may be positioned on the electrode lead array. The balloon is filled with air, liquid or a compliant material. When inflated, the balloon stabilizes the lead with respect to the spinal cord and holds the lead in place. The pressure of the balloon is monitored or otherwise controlled during the filling process in order to determine at what point the filling process should be discontinued. An elastic aspect of the balloon serves as a contained relief valve to limit the pressure the balloon may place on the surrounding tissues when the epidural space is constrained.
Owner:BOSTON SCI NEUROMODULATION CORP
Method of using spinal cord stimulation to treat gastrointestinal and/or eating disorders or conditions
ActiveUS20060074456A1Enhance gastric motilityIncreasing) gastric motilityElectrotherapyDiseaseMedicine
The present invention involves a method and a system for using electrical stimulation to treat gastrointestinal and / or eating disorders. More particularly, the method comprises surgically implanting an electrical stimulation lead that is in communication with predetermined thoracic vertebral segments to cause spinal nervous tissue stimulation, thus treating a wide variety of gastrointestinal disorders.
Owner:ADVANCED NEUROMODULATION SYST INC
System and method for treating pain with peripheral and spinal neuromodulation
A system for treating pain uses spinal cord stimulation and peripheral subcutaneous field stimulation separately or in combination. The system includes an implantable device that is configured to deliver several electrical signals. Several electrical leads are connected to the implantable device. The electrical leads are implanted in the patient such that an electrical signal induces a current to flow between a subcutaneous lumbar region of the patient and a spinal cord region of the patient. The system can also include electrical leads that are implanted in the patient such that an electrical signal induces a current to flow across a lumbar region of the patient. A method for treating leg and back pain is also disclosed.
Owner:NAVARRO ROSA M
Mulit-programmable trial stimulator
ActiveUS20090024187A1Simple inputEasy to explainImplantable neurostimulatorsArtificial respirationMedicineElectrode impedance
Disclosed are systems and methods which provide trial stimulators suited for use interoperatively and during patient trial. Trial stimulator embodiments provide a patient interface and / or clinician interface which appears and functions substantially the same as an interface of a pulse generator controller which will be used after a trial period. A compliance monitor feature may be provided to facilitate verifying the proper use of the trial stimulator during a trial period. A diagnostic feature may be provided to facilitate verifying proper operation of various aspects of a trial stimulator, such as electrode impedance analysis. Trial stimulators of embodiments provide stimulation to a plurality of tissues and / or areas of the body, such as spinal cord stimulation, deep brain stimulation, etcetera. Embodiments provide for multi-electrode stimulation and multi-stimulation programs. Embodiments are configured to provide active discharge of stimulation pulses as well as to utilize constant current sources in providing the stimulation pulses.
Owner:ADVANCED NEUROMODULATION SYST INC
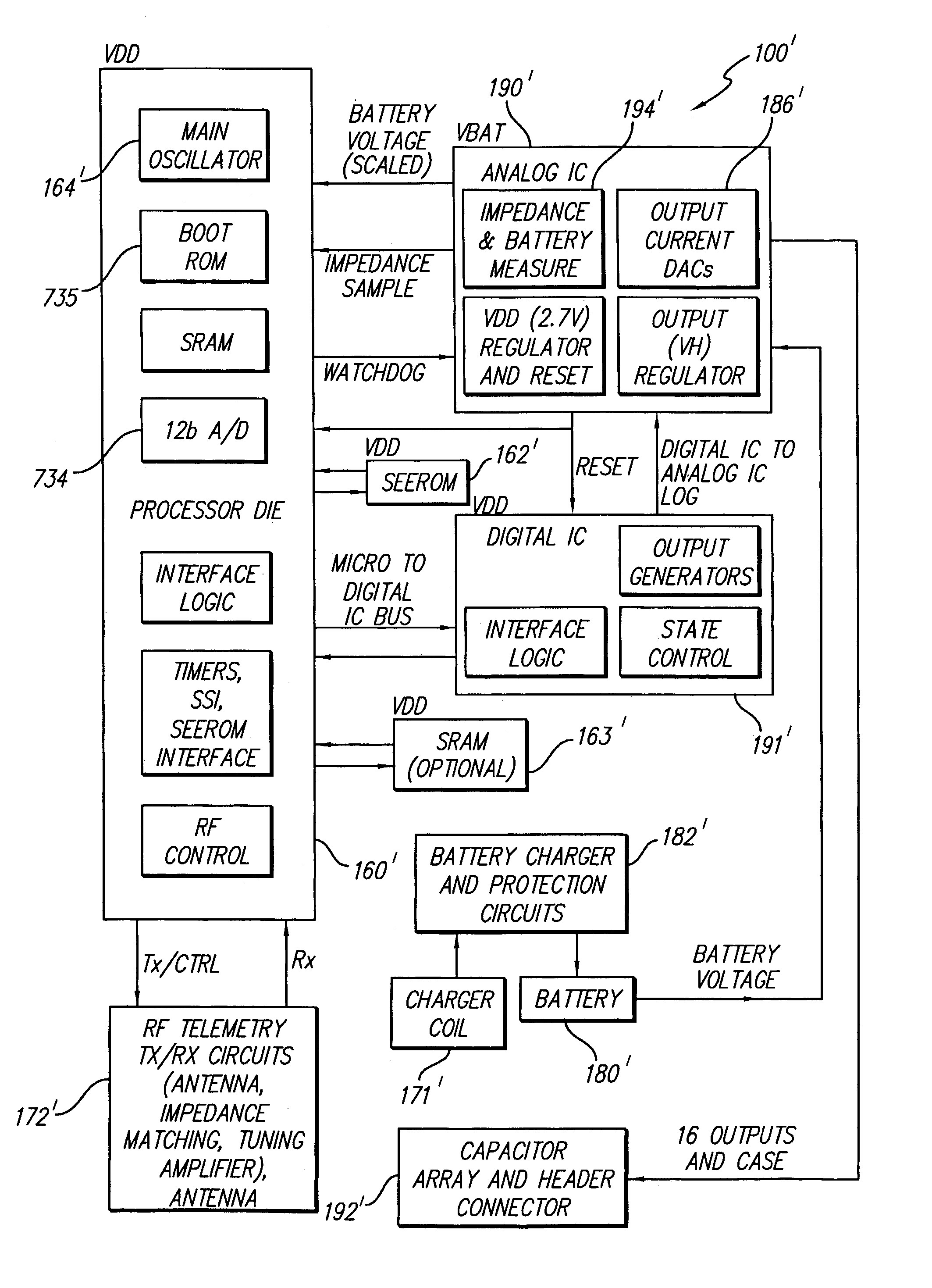
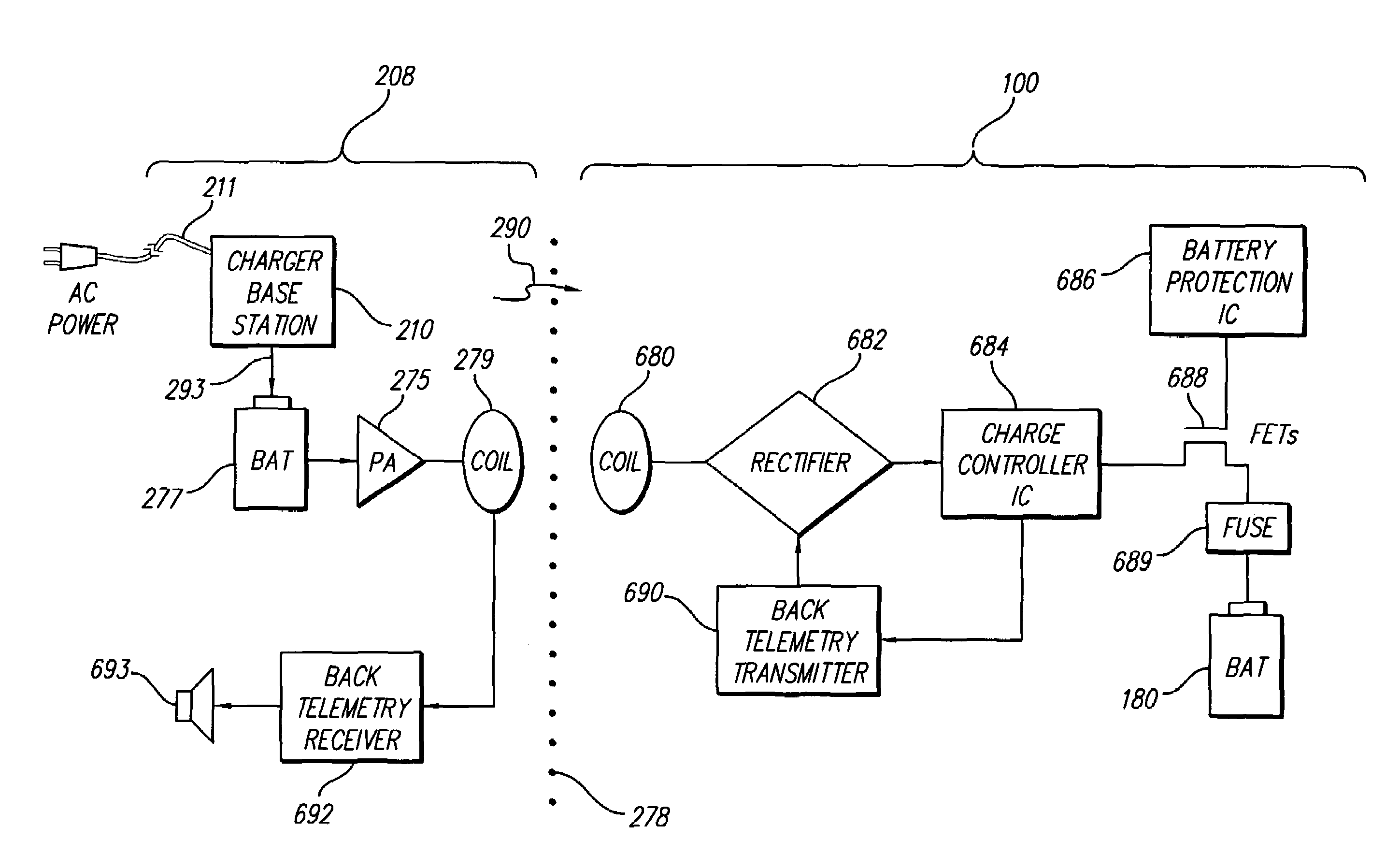
Implantable devices using rechargeable zero-volt technology lithium-ion batteries
InactiveUS7184836B1Assures safe and reliable operation of systemFirmly connectedElectrotherapyLoad circuitLow voltage
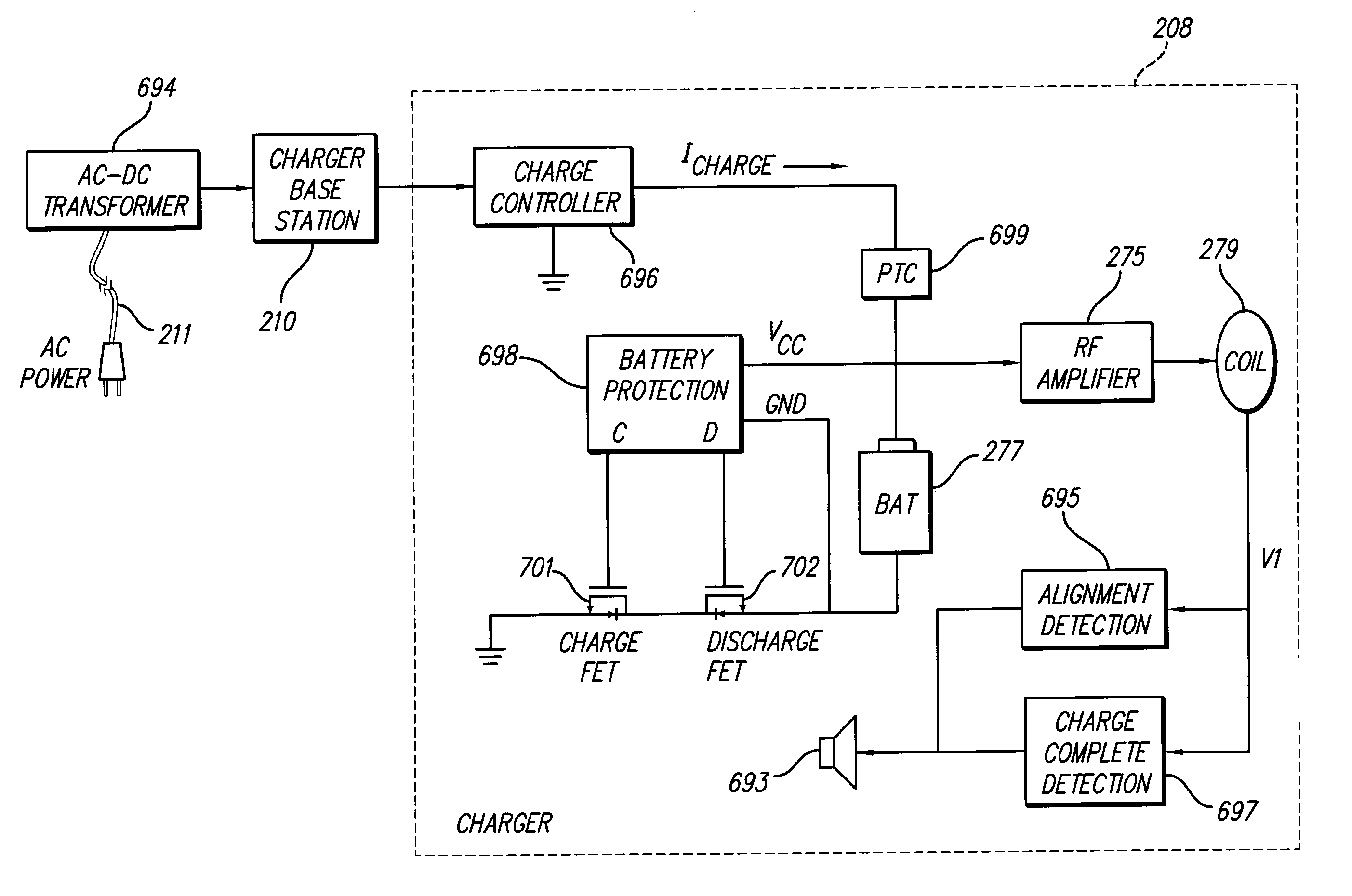
An implantable medical device, such as an implantable pulse generator (IPG) used with a spinal cord stimulation (SCS) system, includes a rechargeable lithium-ion battery having an anode electrode with a substrate made substantially from titanium. Such battery construction allows the rechargeable battery to be discharged down to zero volts without damage to the battery. The implantable medical device includes battery charging and protection circuitry that controls the charging of the battery so as to assure its reliable and safe operation. A multi-rate charge algorithm is employed that minimizes charging time while ensuring the battery cell is safely charged. Fast charging occurs at safer lower battery voltages (e.g., battery voltage above about 2.5 V), and slower charging occurs when the battery nears full charge higher battery voltages (e.g., above about 4.0 V). When potentially less-than-safe very low voltages are encountered (e.g., less than 2.5 V), then very slow (trickle) charging occurs to bring the battery voltage back up to the safer voltage levels where more rapid charging can safely occur. The battery charging and protection circuitry also continuously monitors the battery voltage and current. If the battery operates outside of a predetermined range of voltage or current, the battery protection circuitry disconnects the battery from the particular fault, i.e. charging circuitry or load circuits.
Owner:QUALLION +1
Method for optimizing search for spinal cord stimulation parameter settings
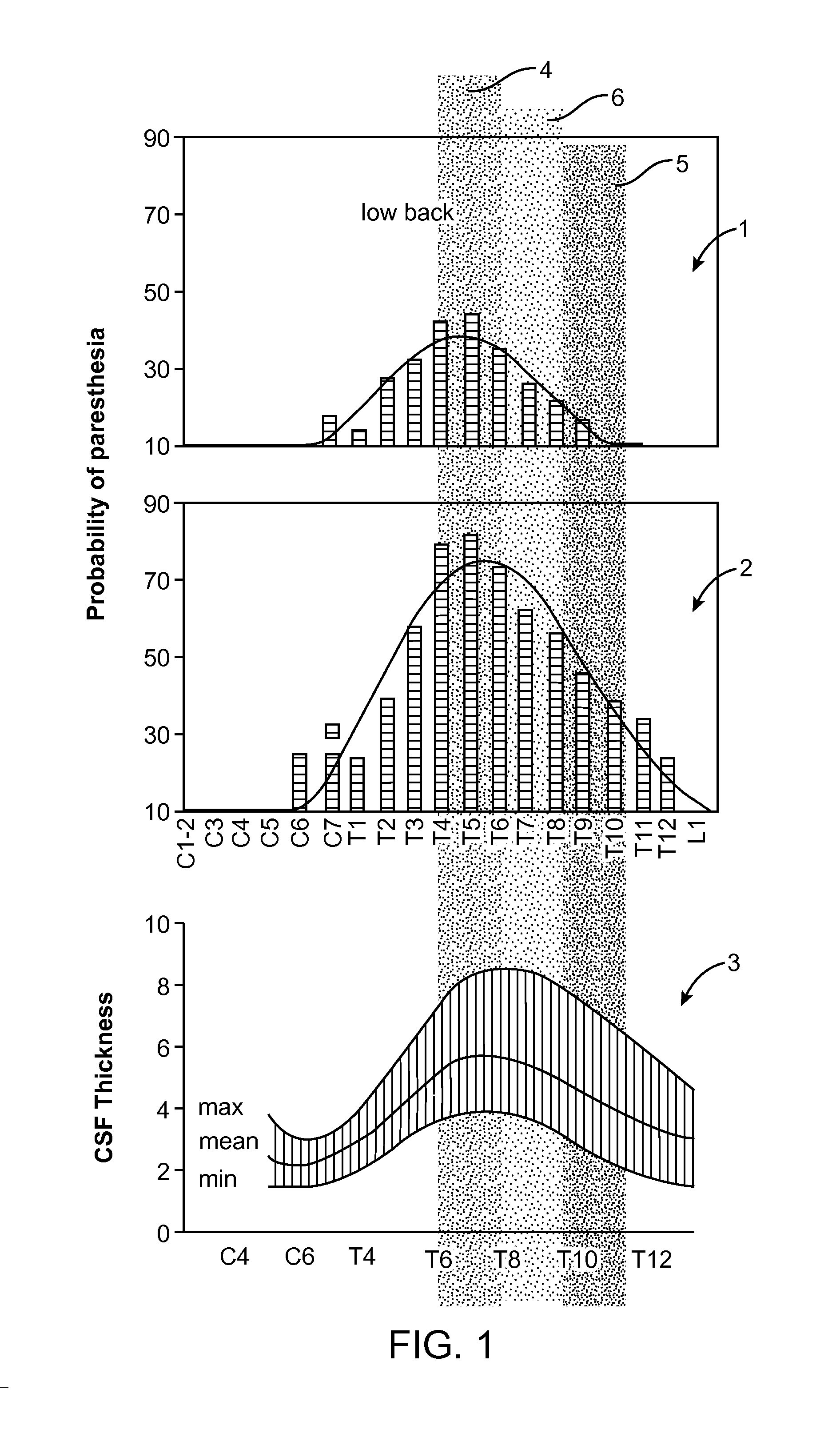
InactiveUS20050209655A1Improve search efficiencyConstant level of paresthesiaSpinal electrodesSurgeryVolumetric Mass DensityPattern perception
Methods for selecting stimulation parameter sets guide a clinician towards an effective set(s) of stimulation parameters. The clinician first evaluates the effectiveness of a small number of trial stimulation parameters sets from a Measurement Table comprising for example, four stimulation parameter sets, measuring the perception threshold and maximum threshold levels. The maximum comfortable step size and desired field shift resolution are determined, and are used along with the measured threshold levels to determine an appropriate step size to use in the current steering process. In order to maintain paresthesia at a relatively constant level during the steering process, a Superposition Equalization (SEQ) algorithm is used to compensate for the physical characteristics of the lead array, i.e., electrode separation and size. A multiplier is determined and a modifying function is used to apply this multiplier to the electrode energy output during transition to maintain a relatively constant current density.
Owner:BOSTON SCI NEUROMODULATION CORP
Implantable pulse generators using rechargeable zero-volt technology lithium-ion batteries
InactiveUS7177691B2System operation is safe and reliableFirmly connectedElectrotherapyLoad circuitSpinal cord stimulation
An implantable medical device, such as an implantable pulse generator (IPG) used with a spinal cord stimulation (SCS) system, includes a rechargeable lithiumion battery having an anode electrode with a substrate made substantially from titanium. Such battery construction allows the rechargeable battery to be discharged down to zero volts without damage to the battery. The implantable medical device includes battery charging and protection circuitry that controls the charging of the battery so as to assure its reliable and safe operation. A multi-rate charge algorithm is employed that minimizes charging time while ensuring the battery cell is safely charged. Fast charging occurs at safer lower battery voltages (e.g., battery voltage above about 2.5 V), and slower charging occurs when the battery nears full charge higher battery voltages (e.g., above about 4.0 V). When potentially less-than-safe very low voltages are encountered (e.g., less than 2.5 V), then very slow (trickle) charging occurs to bring the battery voltage back up to the safer voltage levels where more rapid charging can safely occur. The battery charging and protection circuitry also continuously monitors the battery voltage and current. If the battery operates outside of a predetermined range of voltage or current, the battery protection circuitry disconnects the battery from the particular fault, i.e. charging circuitry or load circuits.
Owner:QUALLION +1
Implantable devices using rechargeable zero-volt technology lithium-ion batteries
InactiveUS7295878B1Assures safe and reliable operation of systemFirmly connectedImplantable neurostimulatorsLoad circuitLow voltage
An implantable medical device, such as an implantable pulse generator (IPG) used with a spinal cord stimulation (SCS) system, includes a rechargeable lithium-ion battery having an anode electrode with a substrate made substantially from titanium. Such battery construction allows the rechargeable battery to be discharged down to zero volts without damage to the battery. The implantable medical device includes battery charging and protection circuitry that controls the charging of the battery so as to assure its reliable and safe operation. A multi-rate charge algorithm is employed that minimizes charging time while ensuring the battery cell is safely charged. Slow charging occurs at lower battery voltages (e.g., battery voltage below about 2.5 V), and fast charging occurs when the battery voltage has reached a safe level (e.g., above about 2.5 V). When potentially less-than-safe very low voltages are encountered (e.g., less than 2.5 V), then very slow (trickle) charging occurs to bring the battery voltage back up to the safer voltage levels where more rapid charging can safely occur. The battery charging and protection circuitry also continuously monitors the battery voltage and current. If the battery operates outside of a predetermined range of voltage or current, the battery protection circuitry disconnects the battery from the particular fault, i.e. charging circuitry or load circuits.
Owner:QUALLION +1
Method for programming implantable device
A method for selecting Spinal Cord Stimulation (SCS) stimulation parameter sets guides a clinician towards an effective set of stimulation parameters. The clinician first evaluates the effectiveness of a small number of trial stimulation parameters sets from a Measurement Table comprising for example, four stimulation parameter sets. Based on the patient's assessment, the trial stimulation parameter sets are ranked. Then the clinician selects a starting or benchmark row in a Steering Table corresponding to the highest ranked trial stimulation parameter set. The clinician moves either up or down form the starting row, testing consecutive parameter sets. The clinician continues as long as the patient indicates that the stimulation results are improving. When a local optimum is found, the clinician returns to the benchmark row, and tests in the opposite direction for another local optimum. If an acceptable set of stimulation parameters is found, the selection process is complete. If an acceptable set is not found, a new starting row in the Steering Table is selected based on the next ranked trial set from the Measurement Table, and the process of searching for local optima is repeated.
Owner:BOSTON SCI NEUROMODULATION CORP
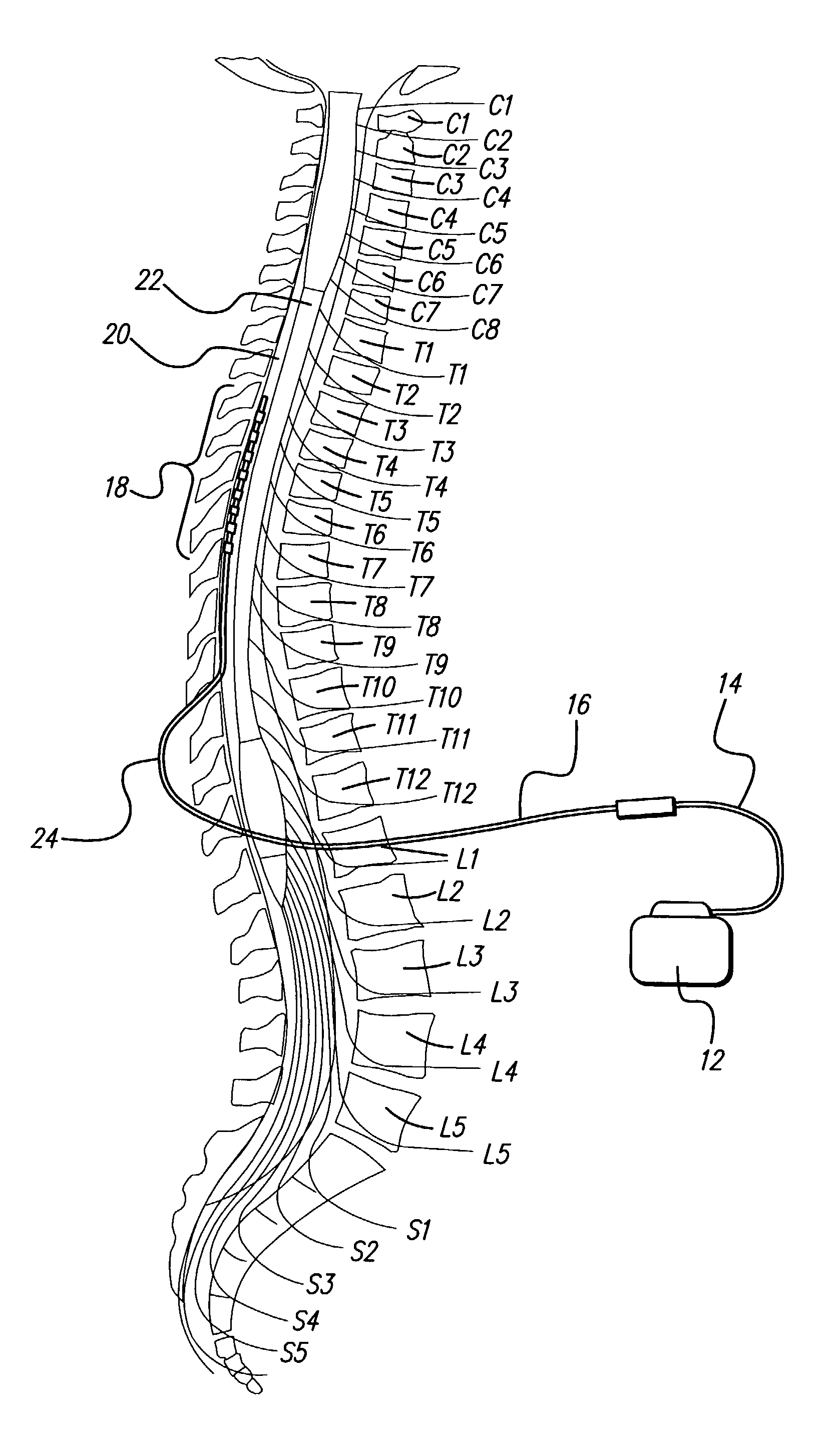
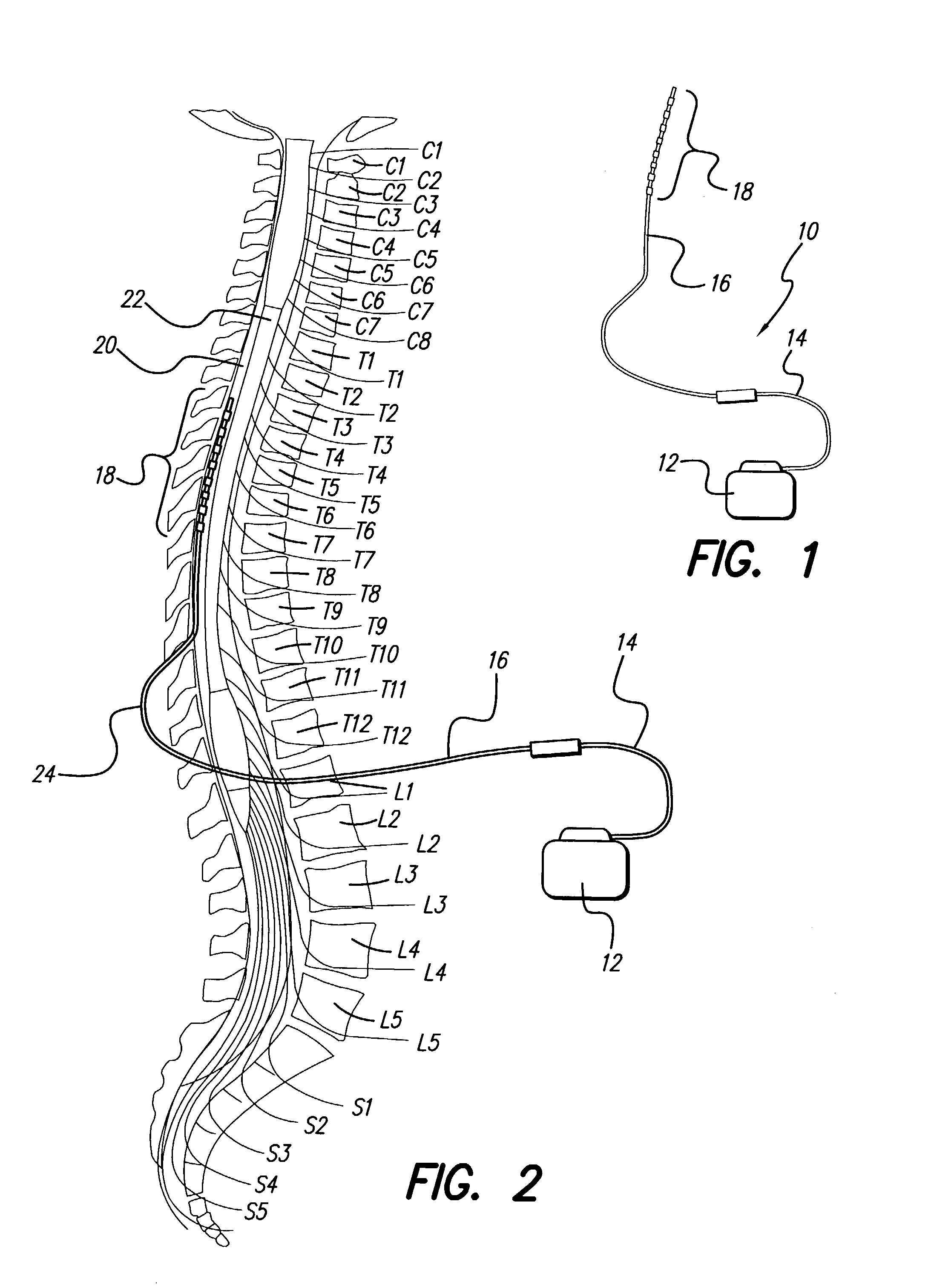
Rechargeable spinal cord stimulation system
InactiveUS20070276450A1Spinal electrodesCircuit arrangements on support structuresElectrical batteryEngineering
A spinal cord stimulation (SCS) system includes multiple electrodes, multiple, independently programmable, stimulation channels within an implantable pulse generator (IPG) which channels can provide concurrent, but unique stimulation fields, permitting virtual electrodes to be realized. The SCS system includes a replenishable power source (e.g., rechargeable battery), that may be recharged using transcutaneous power transmissions between antenna coil pairs. An external charger unit, having its own rechargeable battery can be used to charge the IPG replenishable power source. A real-time clock can provide an auto-run schedule for daily stimulation. An included bi-directional telemetry link in the system informs the patient or clinician the status of the system, including the state of charge of the IPG battery. Other processing circuitry in the IPG allows electrode impedance measurements to be made. Further circuitry in the external battery charger can provide alignment detection for the coil pairs.
Owner:BOSTON SCI NEUROMODULATION CORP
Methods and apparatus for spinal cord stimulation using expandable electrode
InactiveUS20100057178A1Increase the areaLarge treatment areaSpinal electrodesBalloon catheterEffective treatmentBody tissue
The present invention provides systems, apparatus and methods for selectively applying electrical energy to body tissue. More specifically, systems and methods are provided for introducing a spinal cord stimulation electrode device into a patient's epidural space through a small portal, such as a percutaneous penetration, and then expanding the electrode device once inside the epidural space to achieve a larger footprint of contact on the dura. This substantially prevents migration of the electrode within the epidural space and provides for more efficient and effective treatment.
Owner:ELECTROCORE
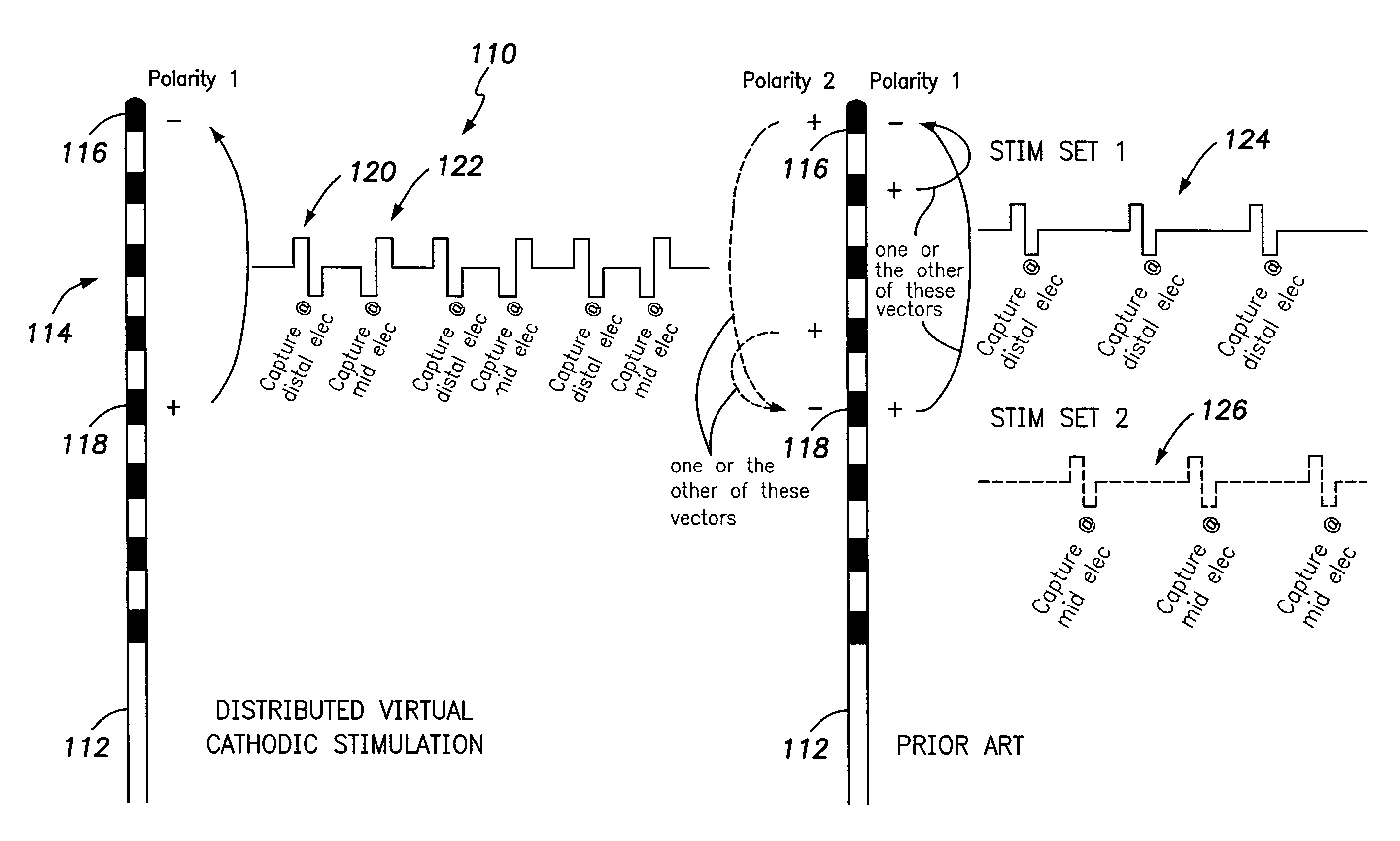
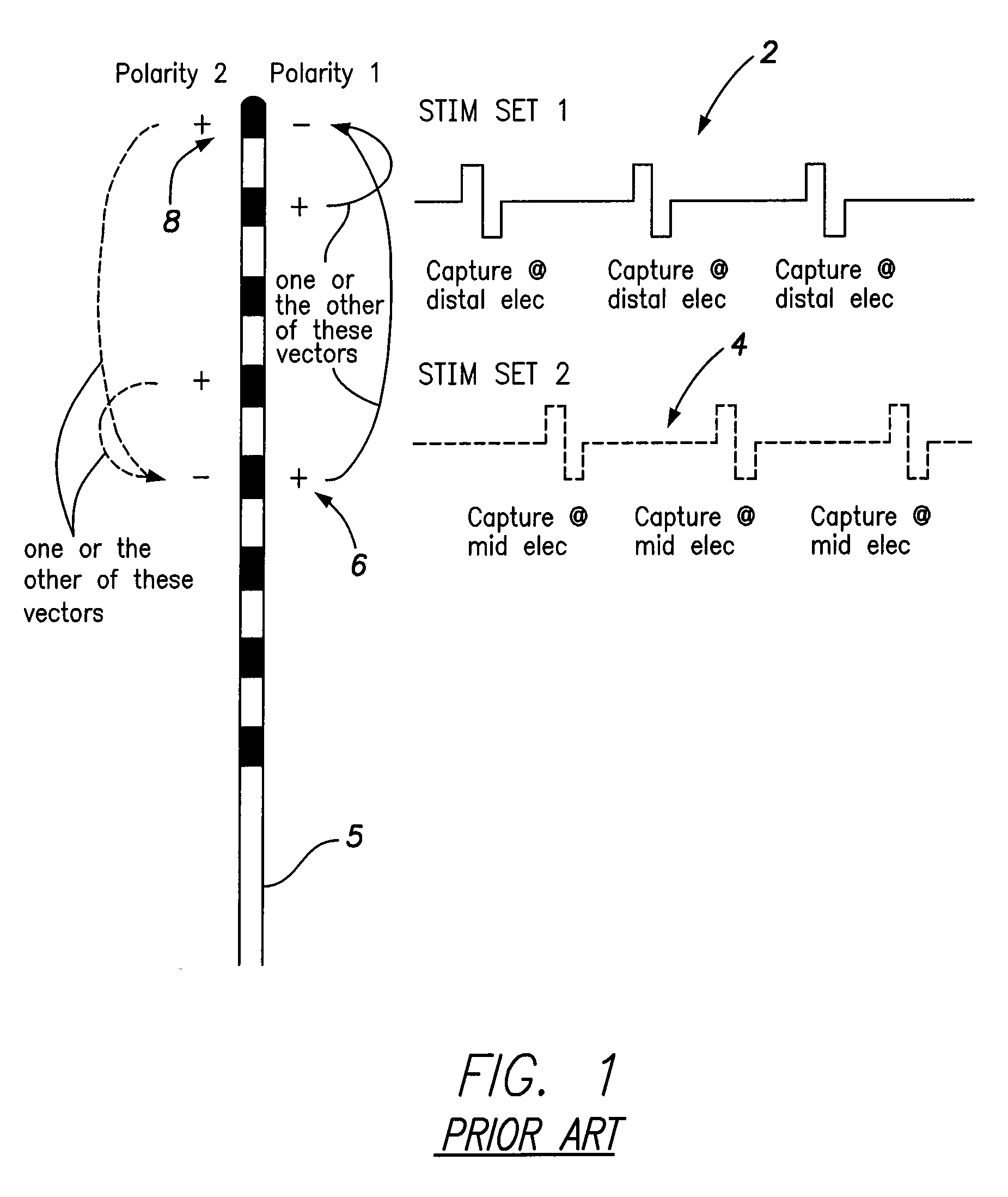
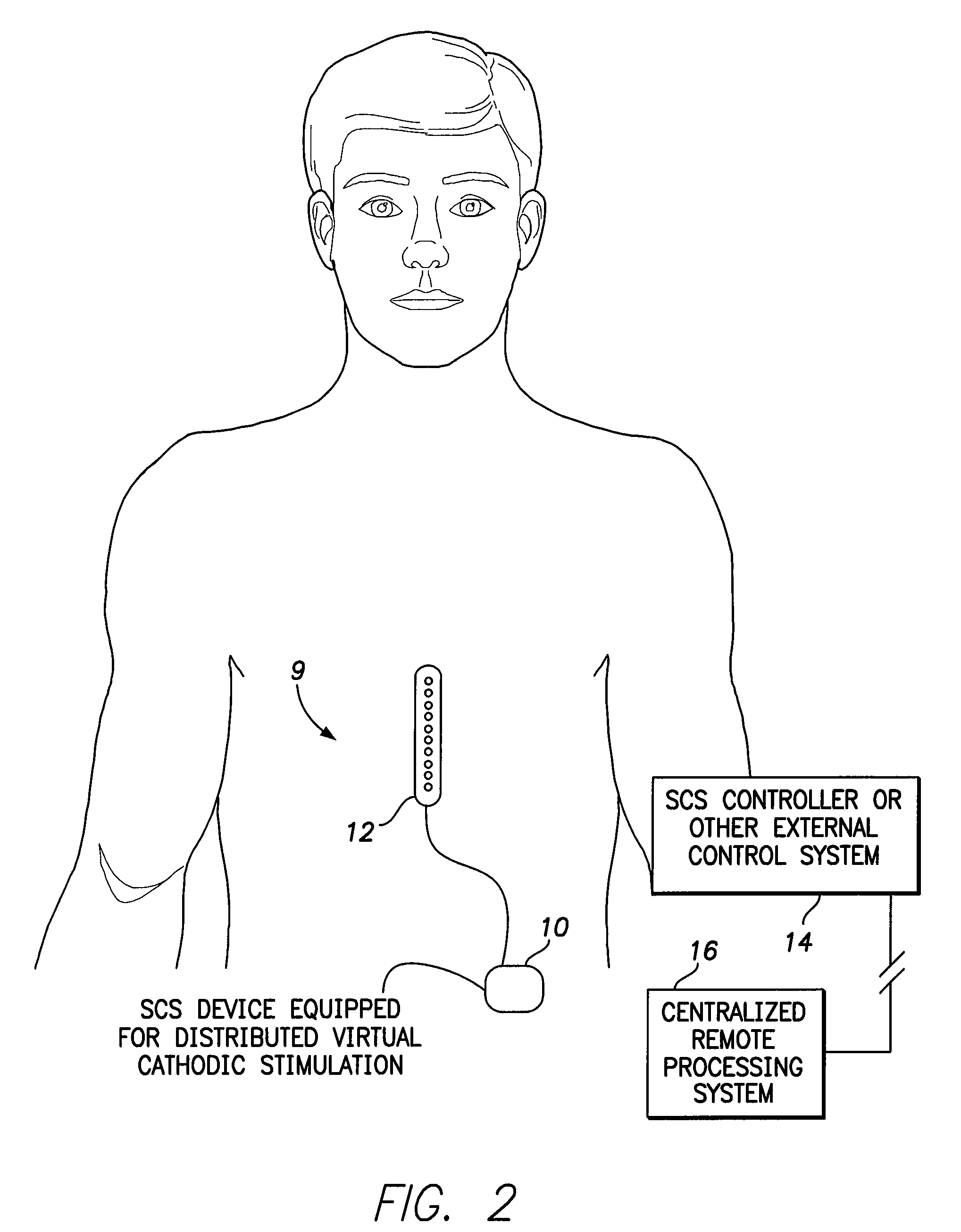
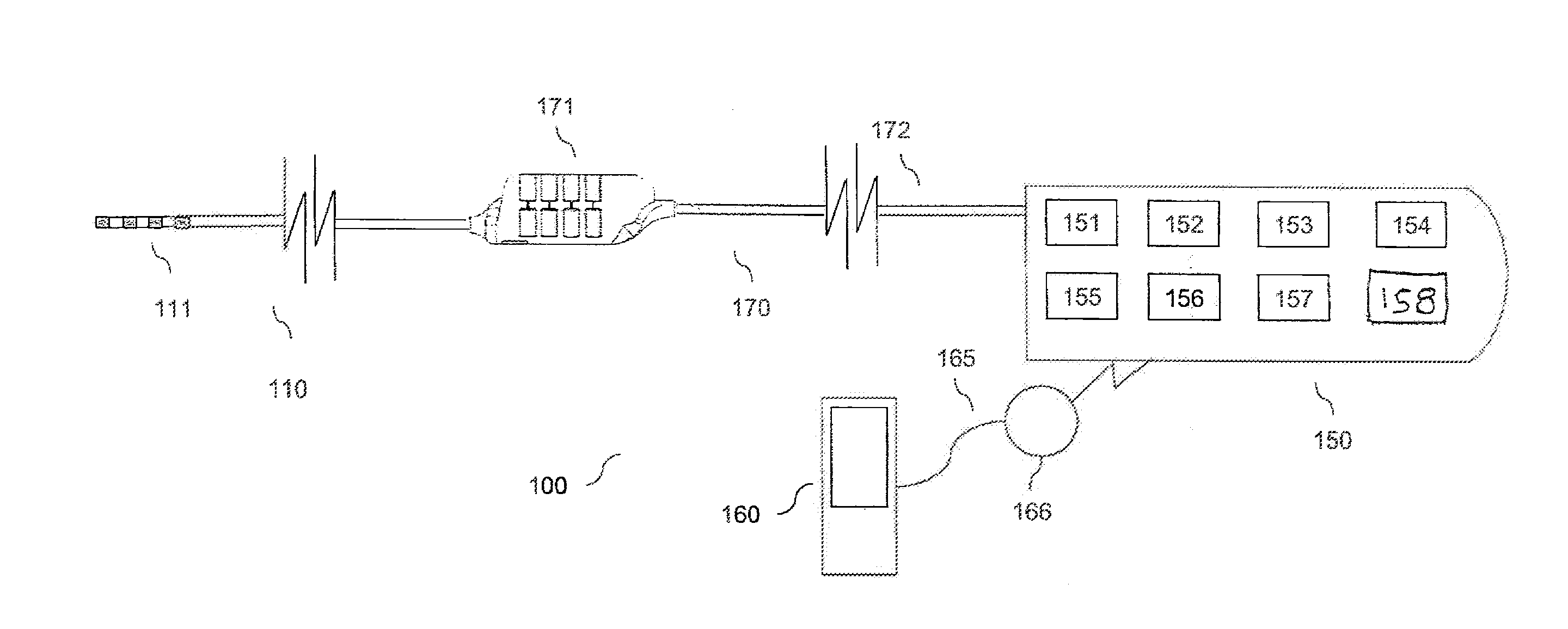
Systems and methods for providing a distributed virtual stimulation cathode for use with an implantable neurostimulation system
ActiveUS9044610B2Increased energy consumption/current drainExpand coverageSpinal electrodesExternal electrodesPulse energyBiomedical engineering
Techniques are provided for controlling and delivering spinal cord stimulation (SCS) or other forms of neurostimulation. In one example, neurostimulation pulses are generated wherein successive pulses alternate in polarity so that a pair of electrodes alternate as cathodes. Each pulse has a cathodic amplitude sufficient to achieve cathodic capture of tissues adjacent the particular electrode used as the cathode for the pulse. The neurostimulation pulses are delivered to patient tissues using the electrodes to alternatingly capture tissues adjacent opposing electrodes via cathodic capture to achieve a distributed virtual stimulation cathode. Various pulse energy savings techniques are also set forth that exploit the distributed virtual stimulation cathode.
Owner:PACESETTER INC
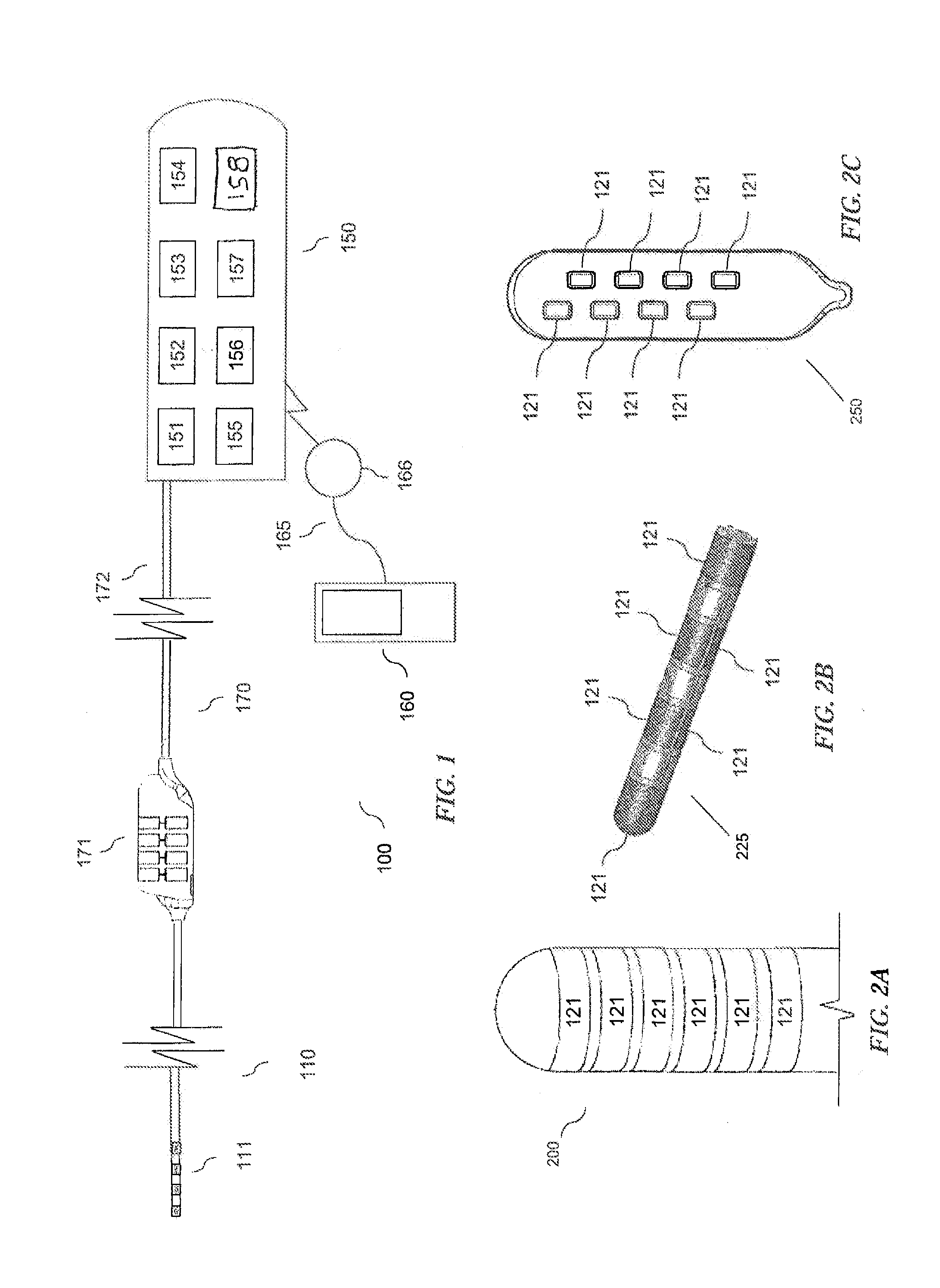
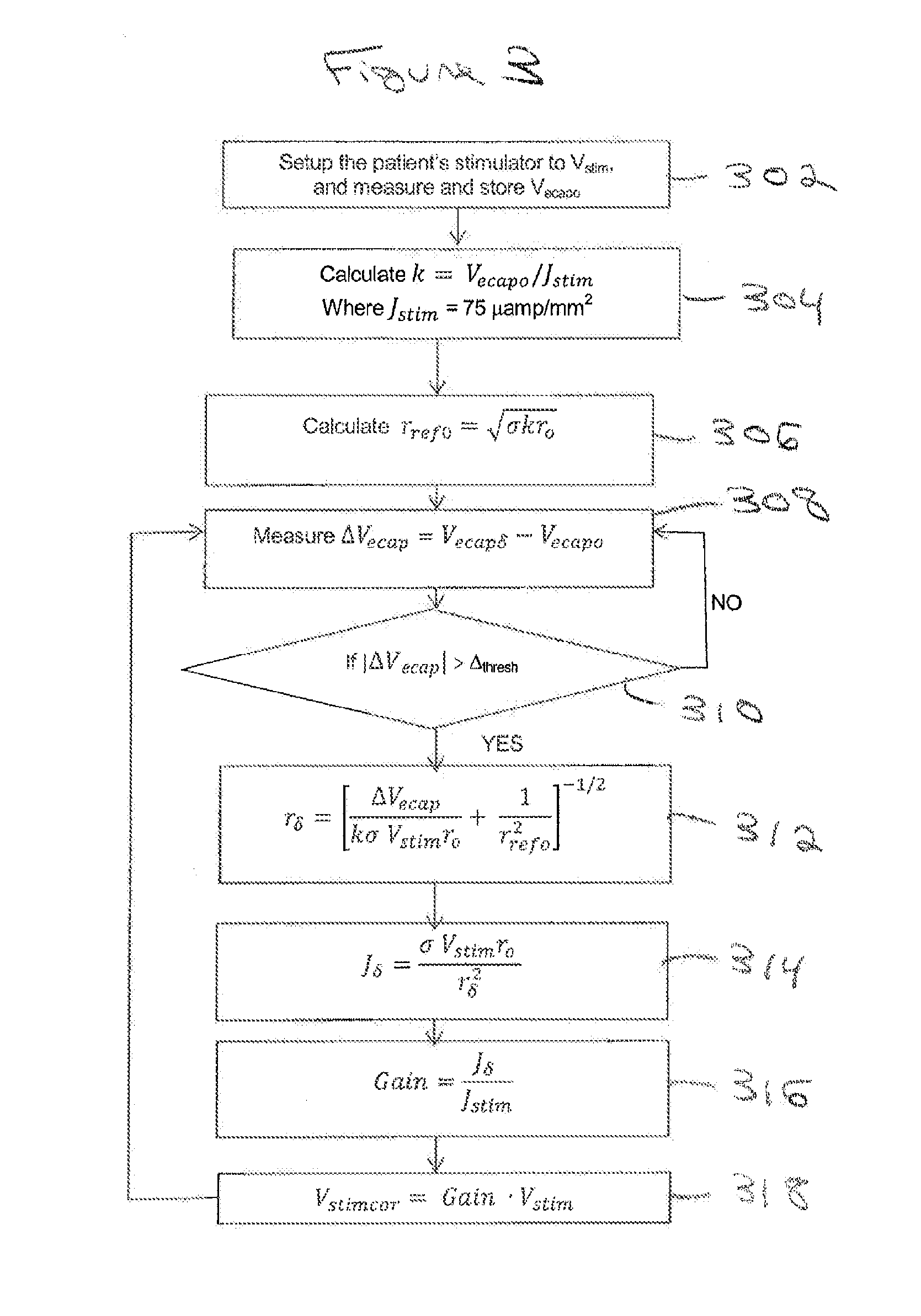
Method and system for non-linear feedback control of spinal cord stimulation
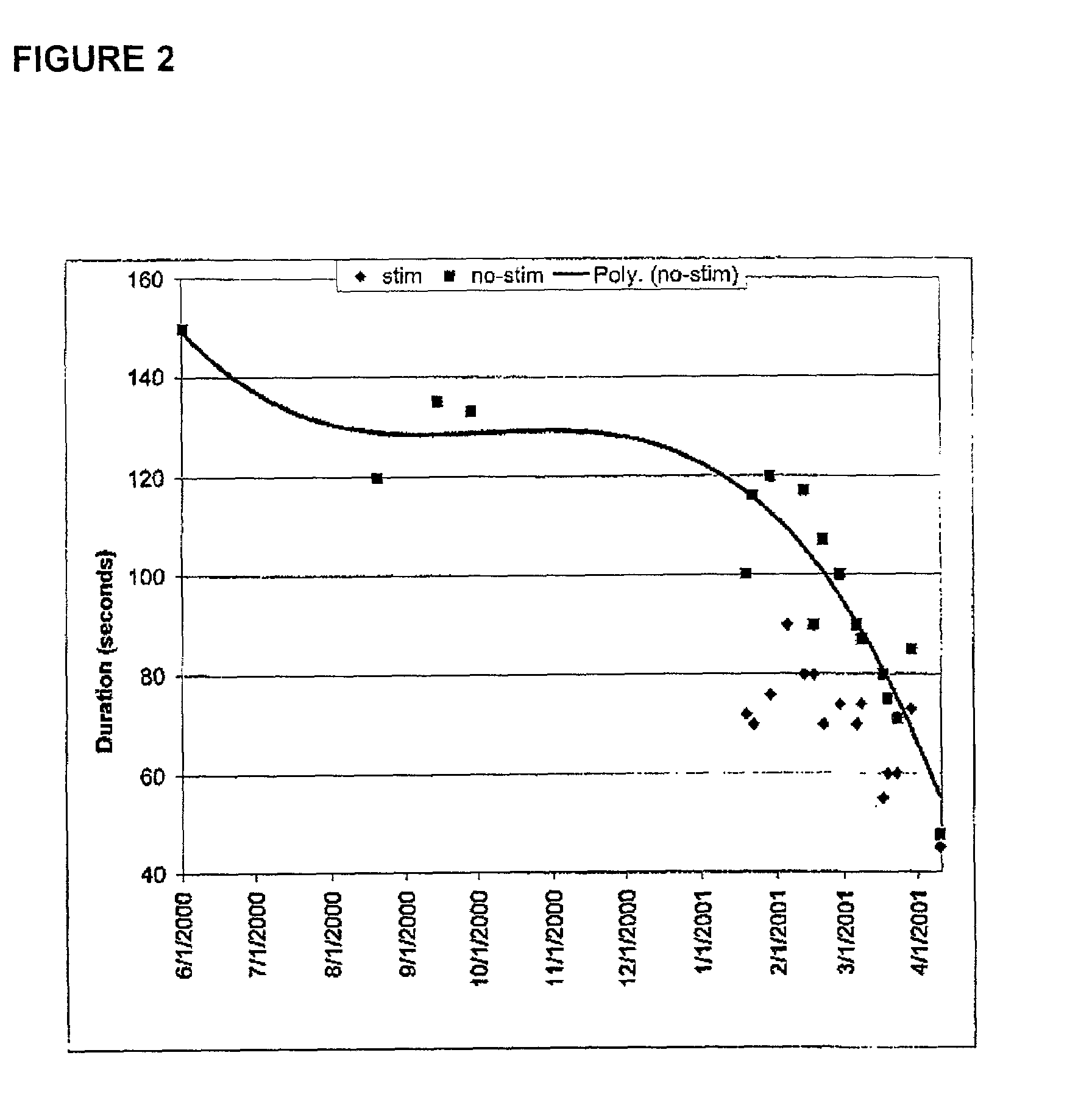
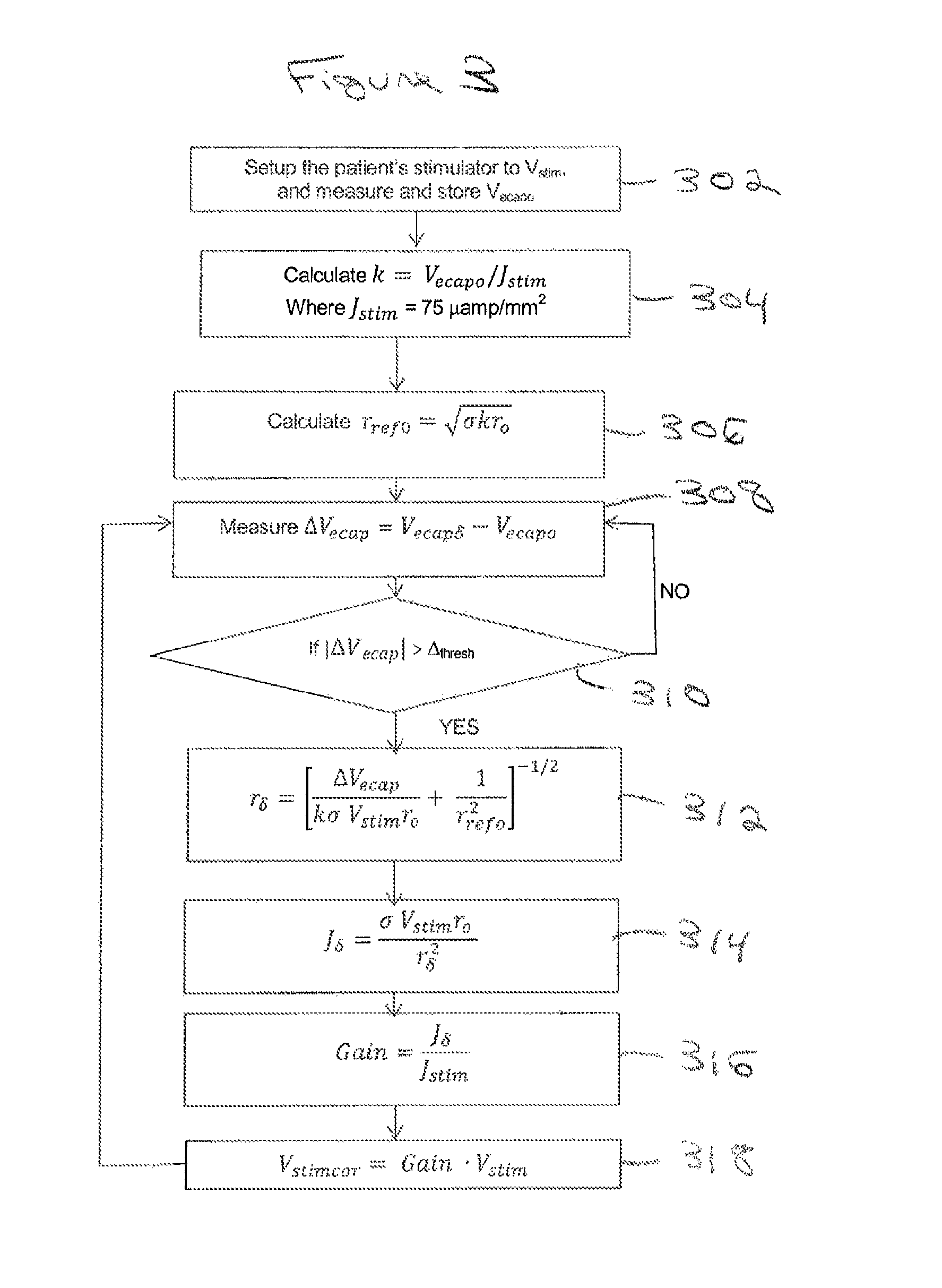
ActiveUS20150360031A1ElectrotherapyArtificial respirationEvoked compound action potentialSpinal column
A system of non-linear feedback control for spinal cord stimulation is provided. The system comprises a lead adapted to be implanted within an epidural space of a dorsal column of a patients spine, and a pulse generator (PG) electrically coupled to the lead. The PG is configured to deliver spinal cord stimulation (SCS) therapy. The system also comprises a sensing circuitry configured to sense an evoked compound action potential (ECAP) response that propagates along the neural pathway. The system also comprises a processor programmed to operation, in response to instructions stored on a non-transient computer-readable medium, to obtain a baseline ECAP response when the lead and spinal cord tissue properties are in baseline states; analyze ECAP responses relative to the baseline ECAP response to obtain an ECAP feedback difference indicative of a change in at least one of the baseline state of the lead and the baseline state of the spinal cord tissue properties. The processor is also programmed to adjust an SCS therapy based on the ECAP feedback.
Owner:PACESETTER INC
Systems and Methods for Treating Essential Tremor or Restless Leg Syndrome Using Spinal Cord Stimulation
InactiveUS20100023103A1Reduce, alleviate, or eliminate at least one adverse effectSpinal electrodesEssential tremorMedicine
A method for treating essential tremor or restless leg syndrome using spinal cord stimulation includes implanting a lead near a spinal cord of a patient. The lead includes a plurality of electrodes disposed on a distal end of the lead and electrically coupled to at least one contact terminal disposed on a proximal end of the lead. Electrical signals are provided from a control module coupled to the lead to stimulate a portion of the spinal cord of the patient using at least one of the electrodes. The electrical signals reduce, alleviate, or eliminate at least one adverse effect of essential tremor or restless leg syndrome.
Owner:BOSTON SCI NEUROMODULATION CORP
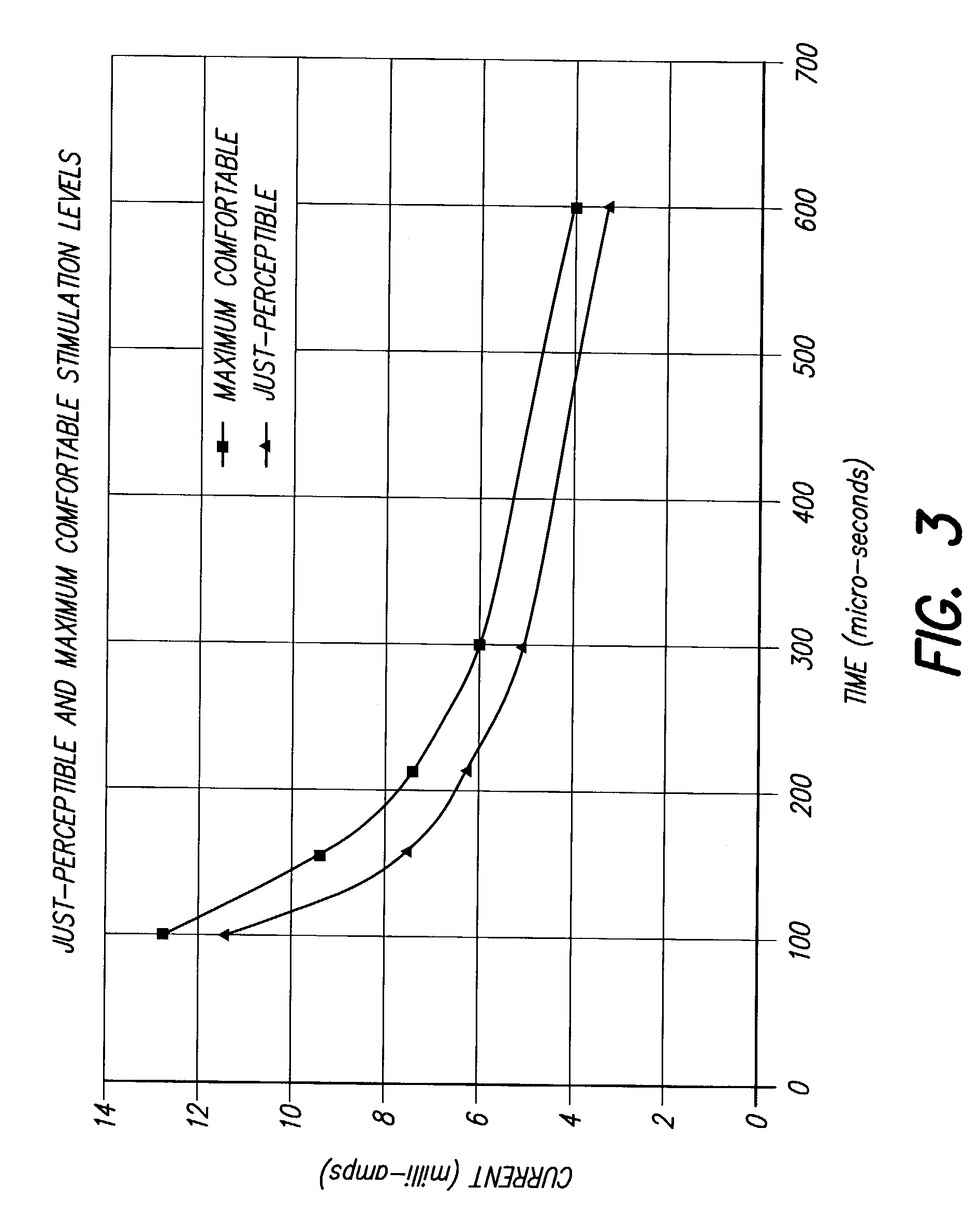
Method for increasing the therapeutic ratio/usage range in a neurostimulator
InactiveUS7127296B2Minimizing sensitivityExpand the scope of treatmentElectrotherapyCurve fittingIntensive care medicine
A system and method for patient control of the stimulation parameters of a Spinal Cord Stimulation (SCS) system, or other neurostimulation system, provides an increased Therapeutic Ratio (TR). Measurements of the just-perceptible stimulation level and the maximum-comfortable stimulation level are made for at least two values of pulse duration and two values of pulse amplitude. A Therapeutic Ratio is determined for stimulation level control strategies based on fixed pulse duration and variable pulse amplitude, and alternatively for fixed pulse amplitude and variable pulse duration. The control strategy providing the greatest therapeutic ratio is then selected for use by the patient. In an alternative embodiment, the pulse durations at the just-perceptible and maximum-comfortable stimulation levels are measured, and the pulse amplitudes at the just-perceptible and maximum-comfortable stimulation levels are determined using a curve-fitting process. Similarly, the pulse amplitudes may first be measured and the pulse widths determined using a curve-fitting process.
Owner:BOSTON SCI NEUROMODULATION CORP
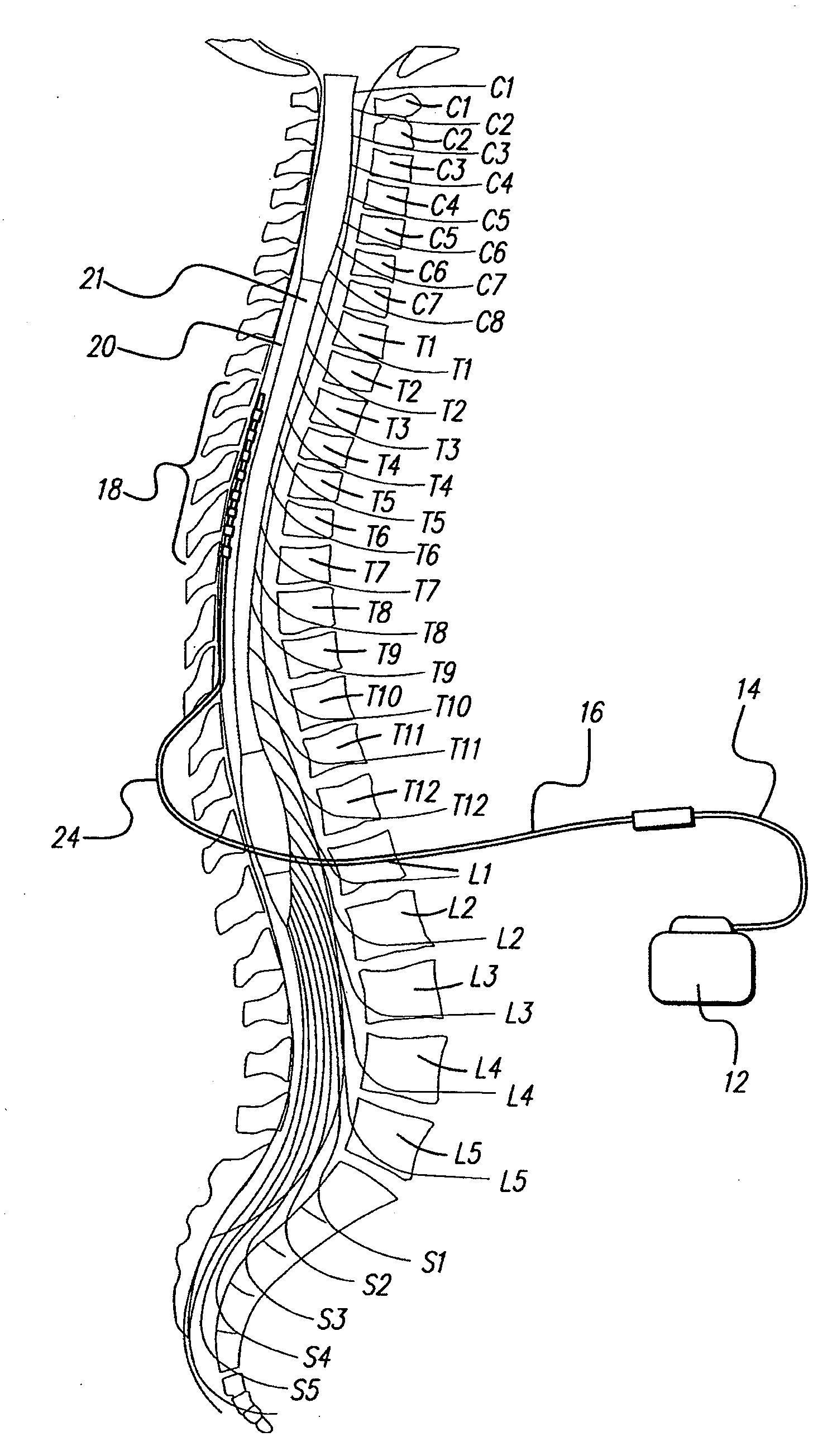
Method for achieving low-back spinal cord stimulation without significant side-effects
ActiveUS20130268021A1Increasing activation thresholdIncreased activationSpinal electrodesArtificial respirationSpinal columnNerve fiber bundle
A method for treating an ailment of a patient using at least one electrode implanted within a spinal column of the patient at a T4-T6 spinal nerve level. The method comprises increasing an activation threshold of a side-effect exhibiting neural structure relative to the activation threshold of a dorsal column (DC) nerve fiber of the patient, and applying electrical stimulation energy to the DC nerve fiber via the at least one electrode while the activation threshold of the neural structure is increased, thereby treating the ailment while minimizing stimulation of the neural structure. Another method comprises applying electrical stimulation energy to the spinal column of the patient via the plurality of electrodes, thereby generating a medio-lateral electrical field relative to the spinal column of the patient and treating the ailment.
Owner:BOSTON SCI NEUROMODULATION CORP
Method for optimizing search for spinal cord stimulation parameter settings
ActiveUS20080071325A1Improve search efficiencyConstant level of paresthesiaElectrotherapyMedicineElectrical current
A method of stimulating tissue of a patient is provided. The method comprises placing an array of electrodes in proximity to the tissue, conveying electrical current between the electrodes of the array to stimulate a tissue site, incrementally shifting the electrical current from at least one cathode to at least another cathode over a first range of fractionalized current values, and incrementally shifting the electrical current from at least one anode to at least another anode over a second range of fractionalized current values. The step sizes for the first and second ranges of fractionalized current values differ.
Owner:BOSTON SCI NEUROMODULATION CORP
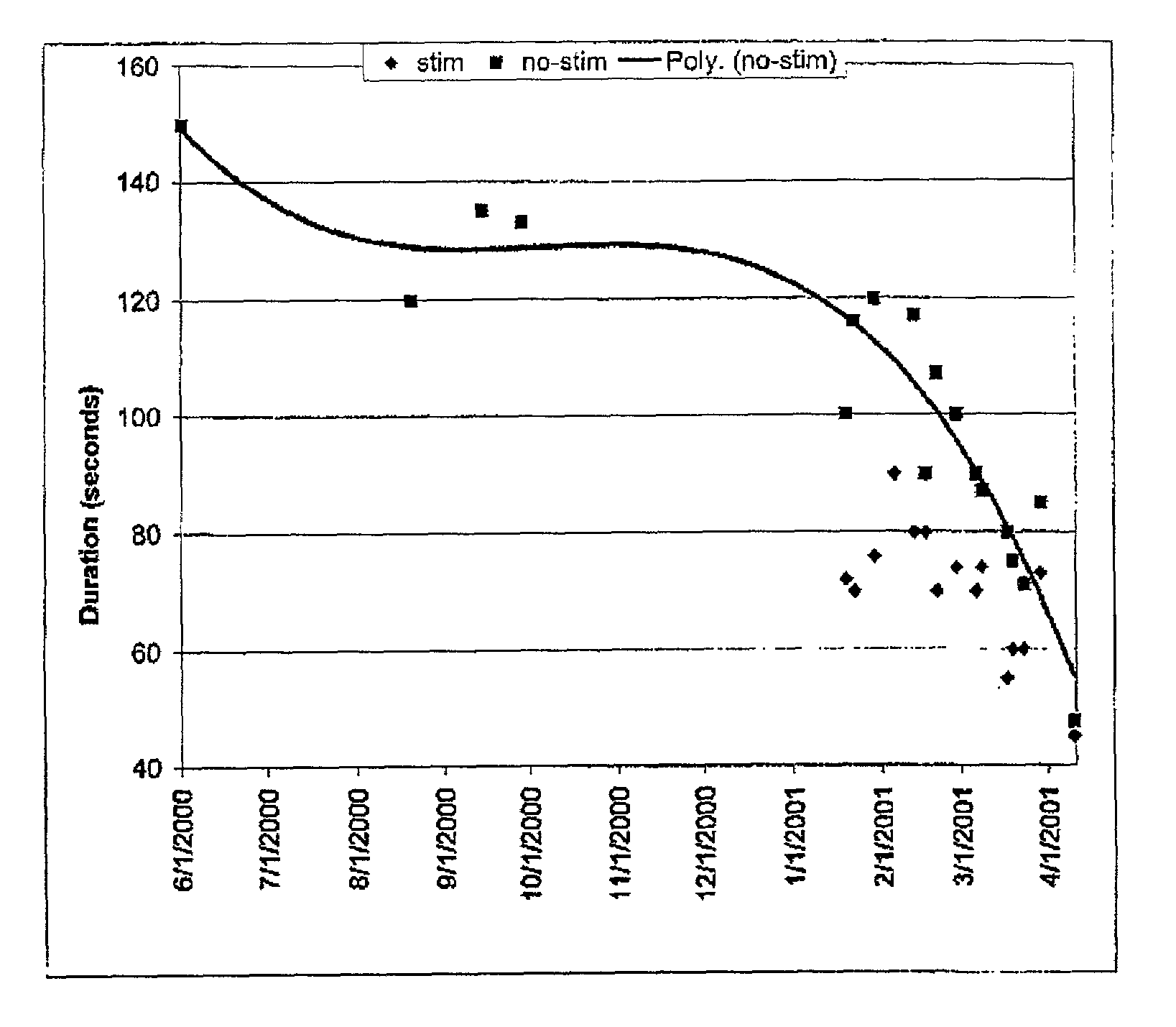
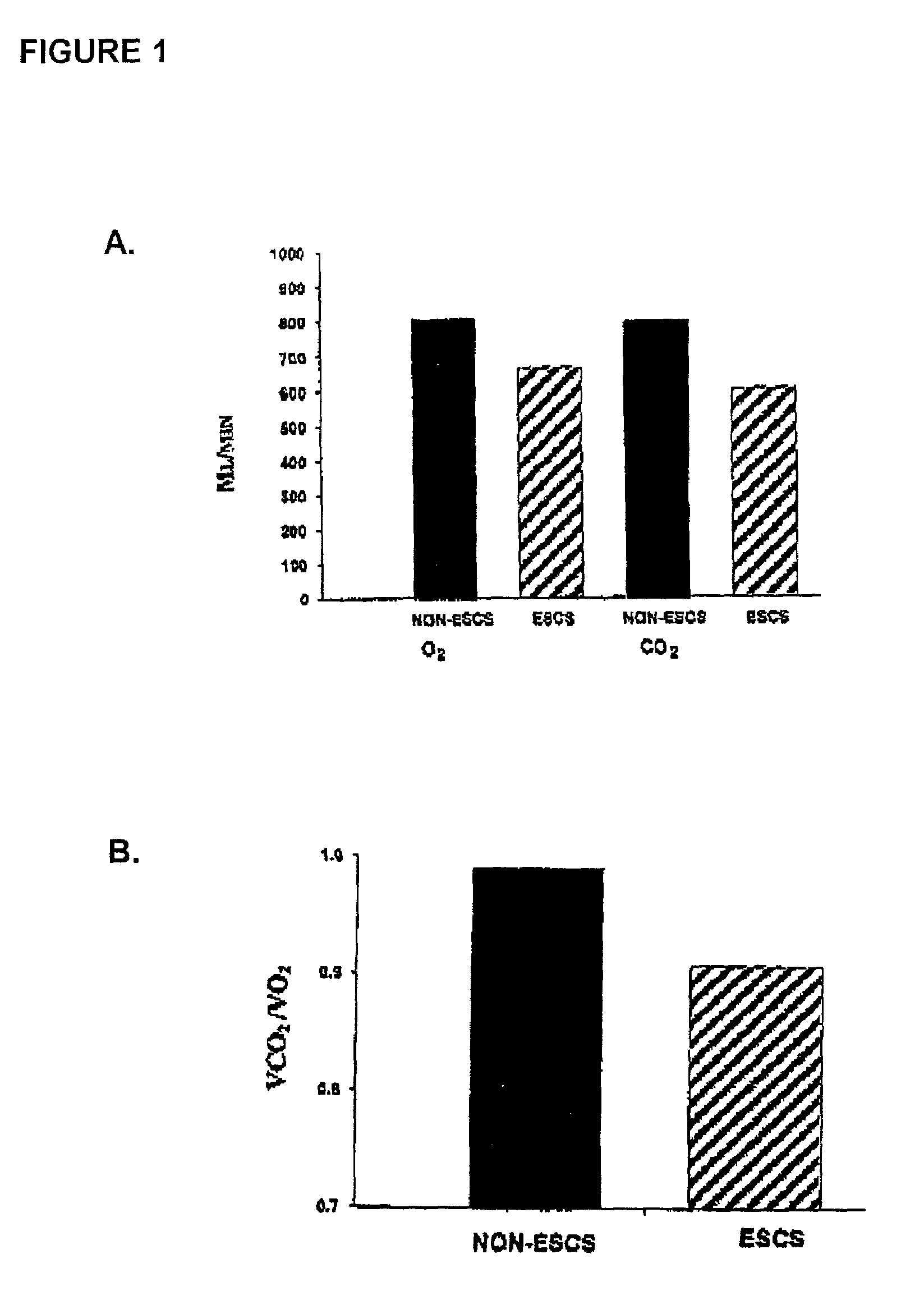
Method for restoring gait in individuals with chronic spinal cord injury
InactiveUS7065408B2Increase chanceElectrotherapyChiropractic devicesGait trainingFull weight bearing
A method for restoring functional ambulation in subjects with incomplete spinal cord injuries which includes partial weight bearing therapy followed by epidural spinal cord stimulation (ESCS) to facilitate partial weight bearing therapy and over-ground walking. Electrical epidural stimulation (EES) is generated by an implanted device during partial weight bearing therapy on a treadmill. The subject is then transitioned to full weight bearing gait training on a treadmill with EES to over-ground gait training with EES and a walker, and finally to full weight bearing independent stepping over-ground with EES with or without an assistive device.
Owner:HERMAN RICHARD M +3
Method and system for non-linear feedback control of spinal cord stimulation
A system of non-linear feedback control for spinal cord stimulation is provided. The system comprises a lead adapted to be implanted within an epidural space of a dorsal column of a patients spine, and a pulse generator (PG) electrically coupled to the lead. The PG is configured to deliver spinal cord stimulation (SCS) therapy. The system also comprises a sensing circuitry configured to sense an evoked compound action potential (ECAP) response that propagates along the neural pathway. The system also comprises a processor programmed to operation, in response to instructions stored on a non-transient computer-readable medium, to obtain a baseline ECAP response when the lead and spinal cord tissue properties are in baseline states; analyze ECAP responses relative to the baseline ECAP response to obtain an ECAP feedback difference indicative of a change in at least one of the baseline state of the lead and the baseline state of the spinal cord tissue properties. The processor is also programmed to adjust an SCS therapy based on the ECAP feedback.
Owner:PACESETTER INC
Systems and methods for stimulation-related volume analysis, creation, and sharing
ActiveUS20130116929A1Quality improvementMaximize usePhysical therapies and activitiesElectrotherapySide effectMedicine
A computer implemented system and method facilitates a cycle of generation, sharing, and refinement of volumes related to stimulation of anatomical tissue, such as brain or spinal cord stimulation. Such volumes can include target stimulation volumes, side effect volumes, and volumes of estimated activation. A computer system and method also facilitates analysis of groups of volumes, including analysis of differences and / or commonalities between different groups of volumes.
Owner:BOSTON SCI NEUROMODULATION CORP
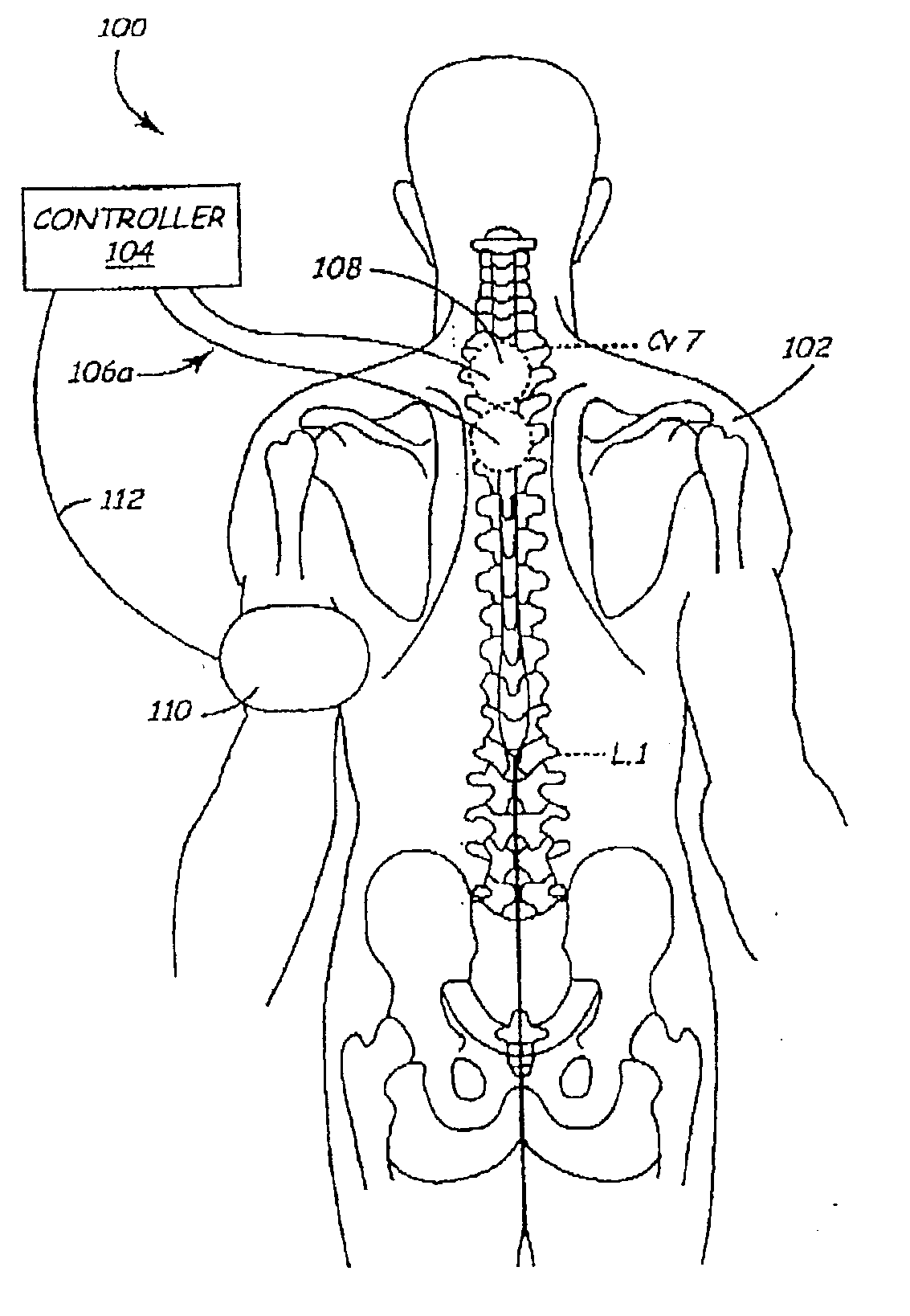
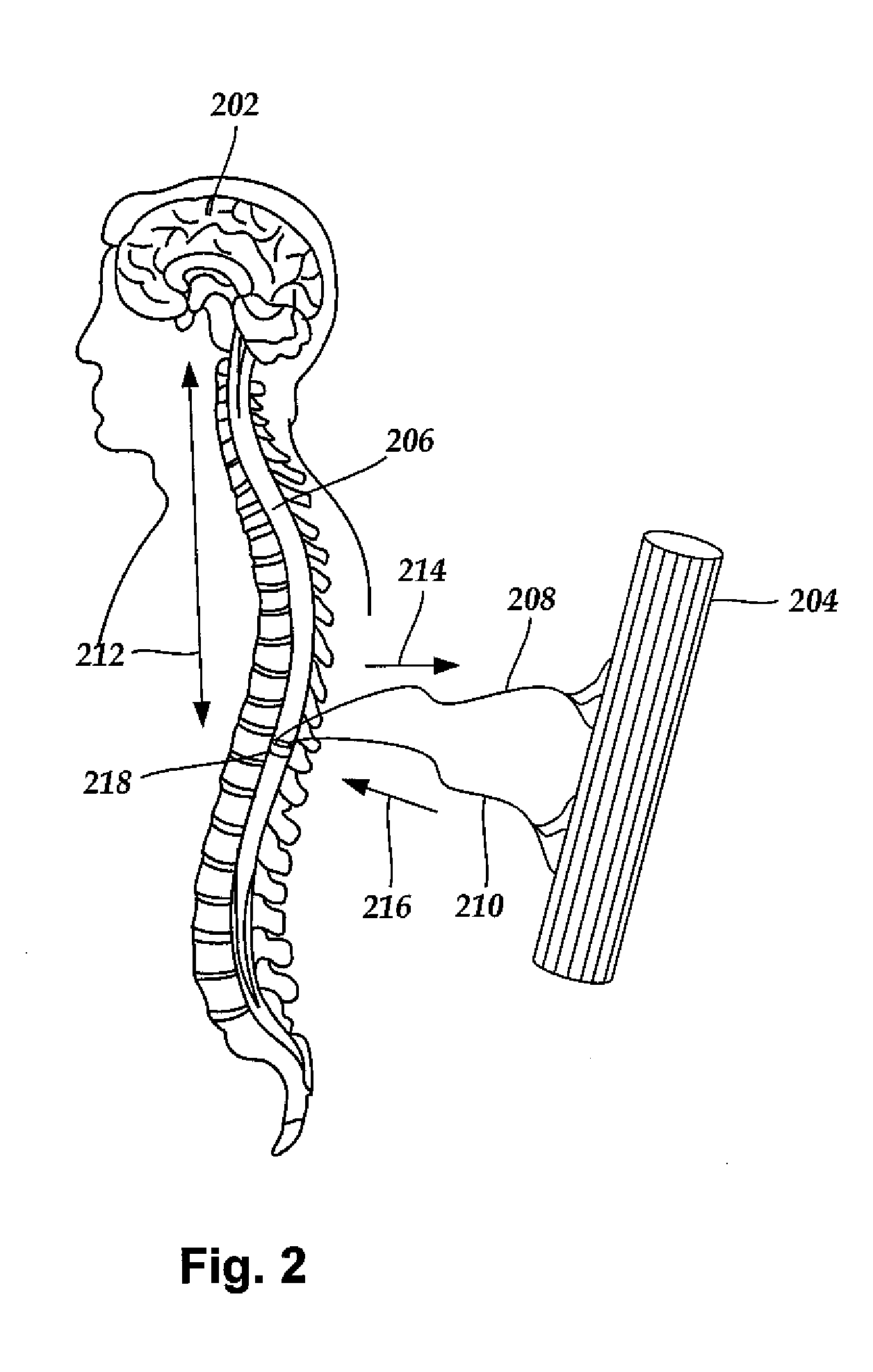
Cervical Spinal Cord Stimulation for the Treatment and Prevention of Cerebral Vasospasm
The present invention relates to a method of prevention and treatment of narrowing of cerebral blood vessels after subarachnoid hemorrhage, and in particular, to a method of applying electrical energy through electrical stimulation electrodes particularly positioned in the cervical region of a patient to affect the sympathetic tone of the blood vessels supplying the brain.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com