Patents
Literature
40 results about "N-propargyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
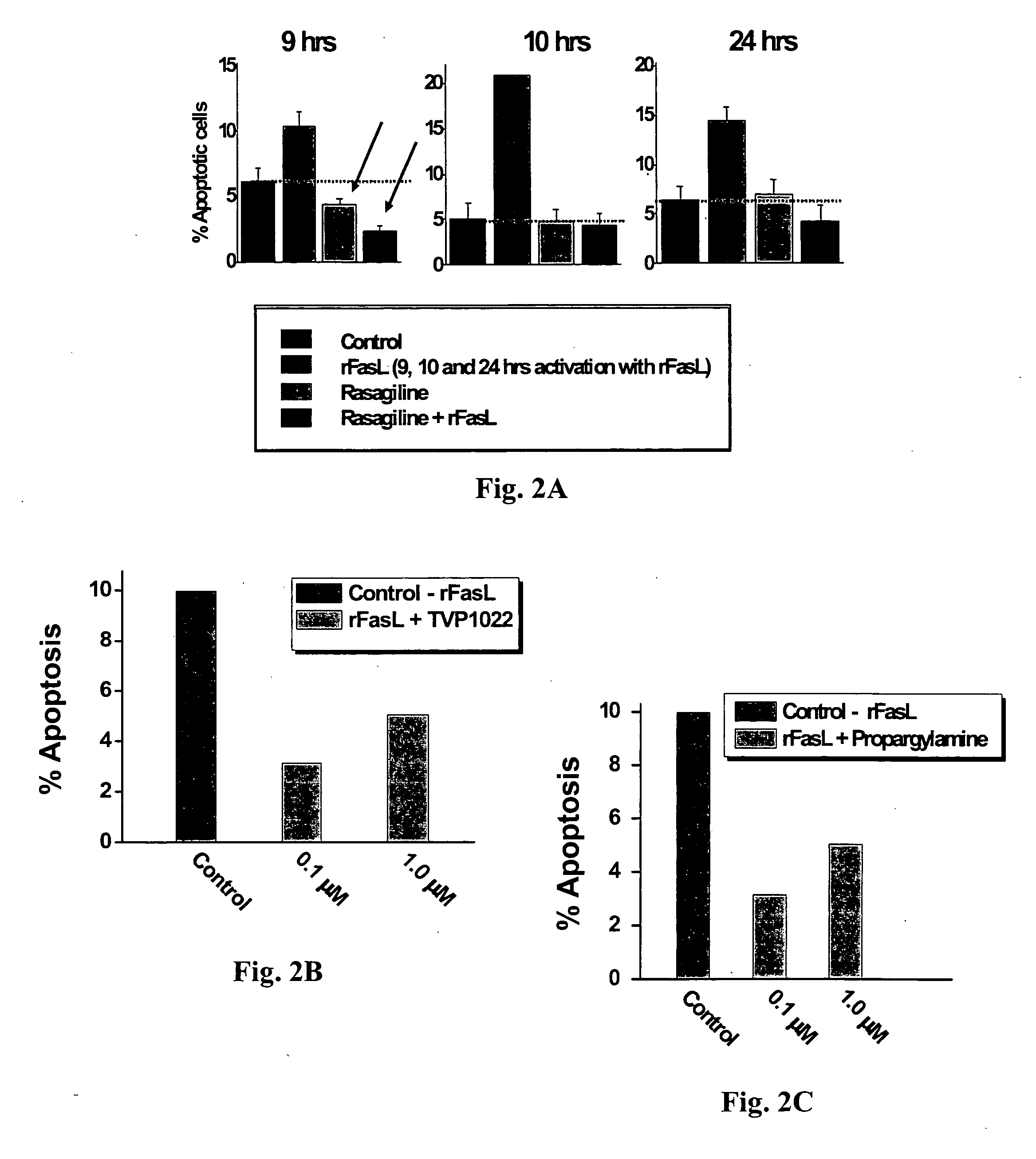
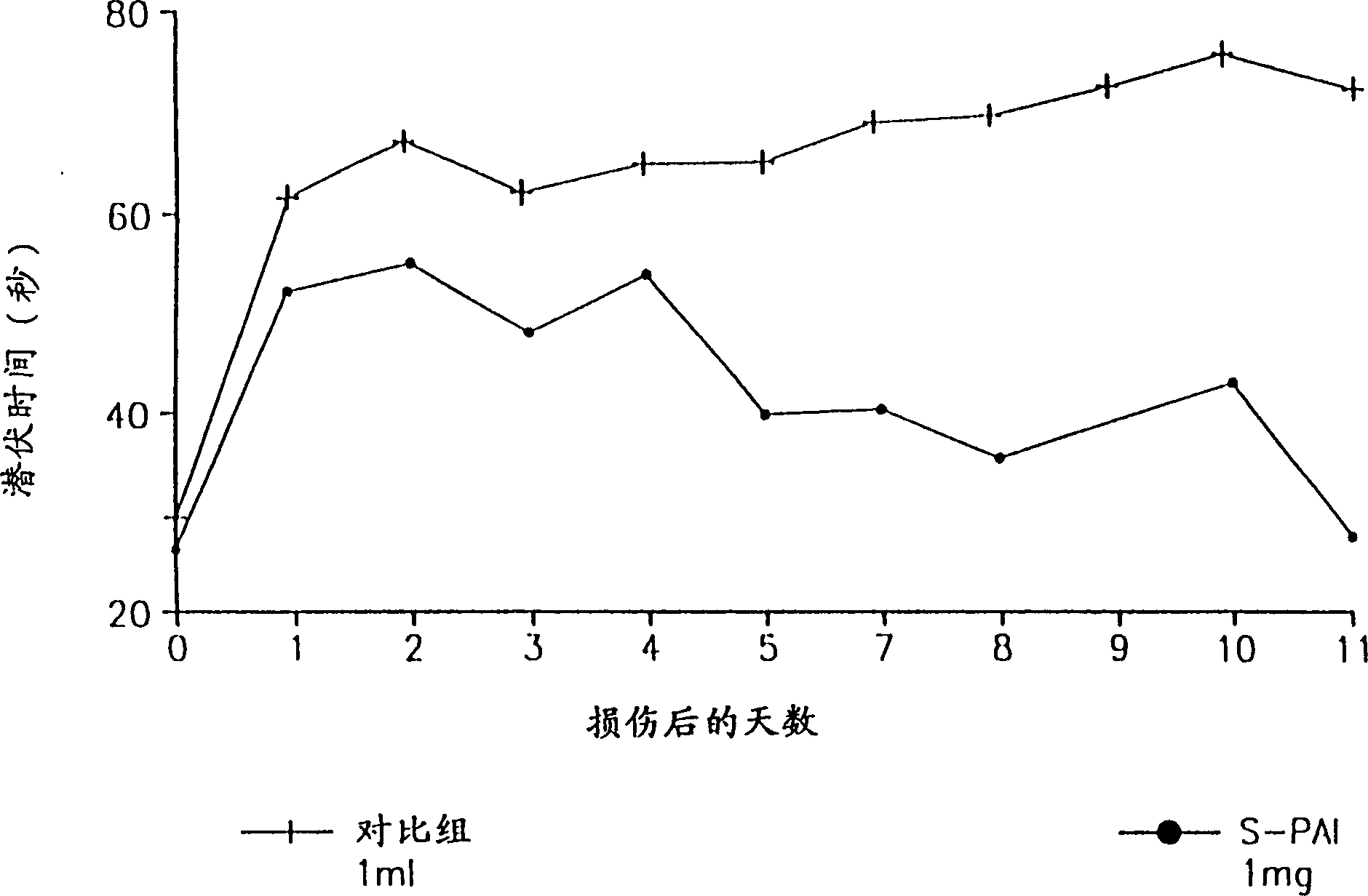
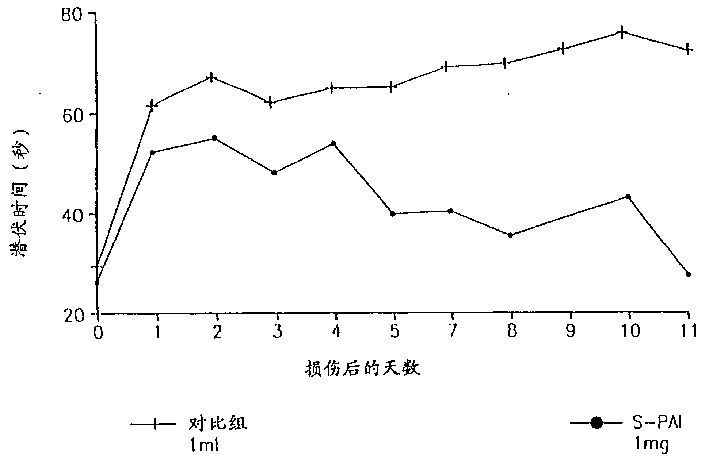
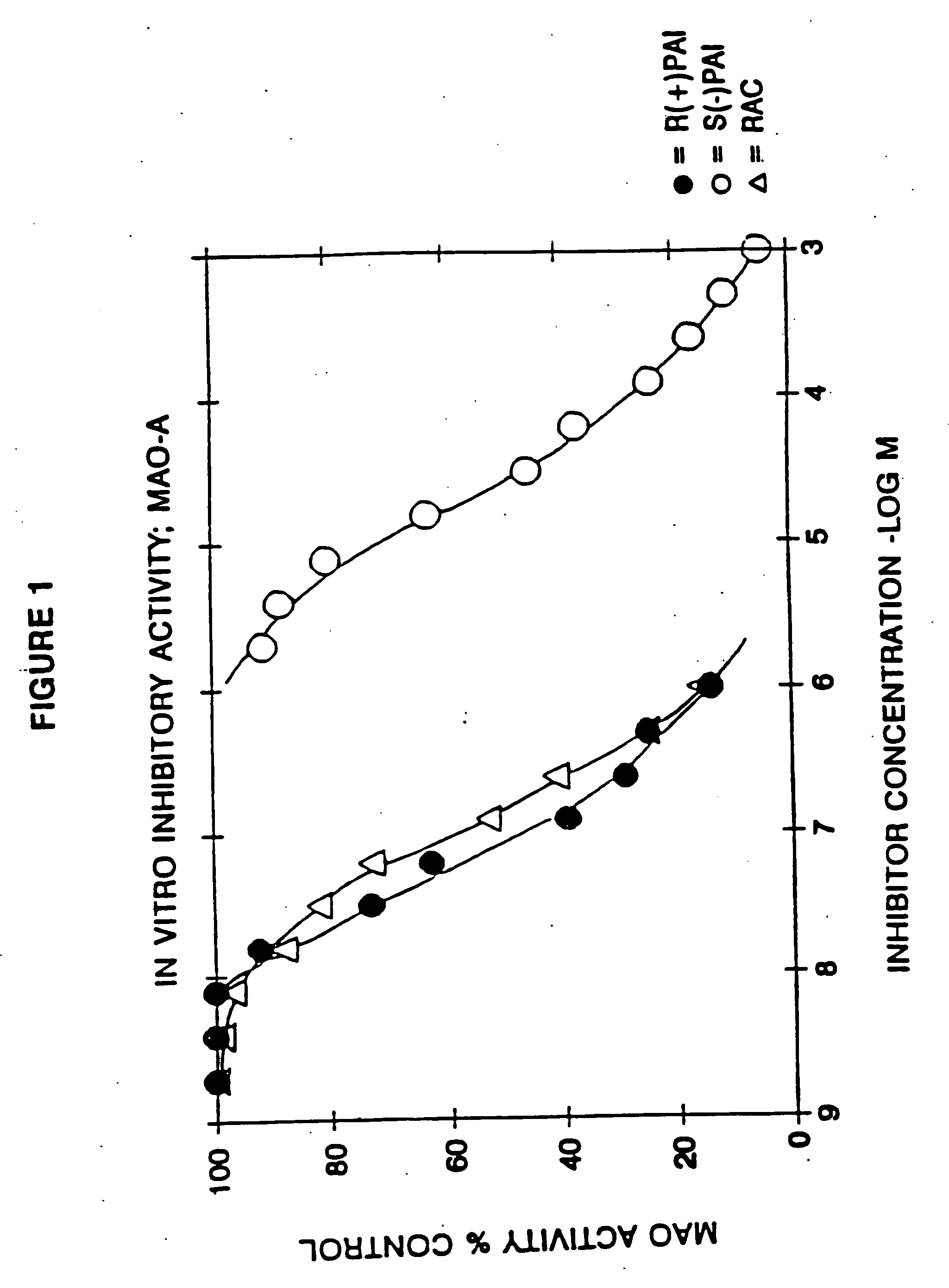
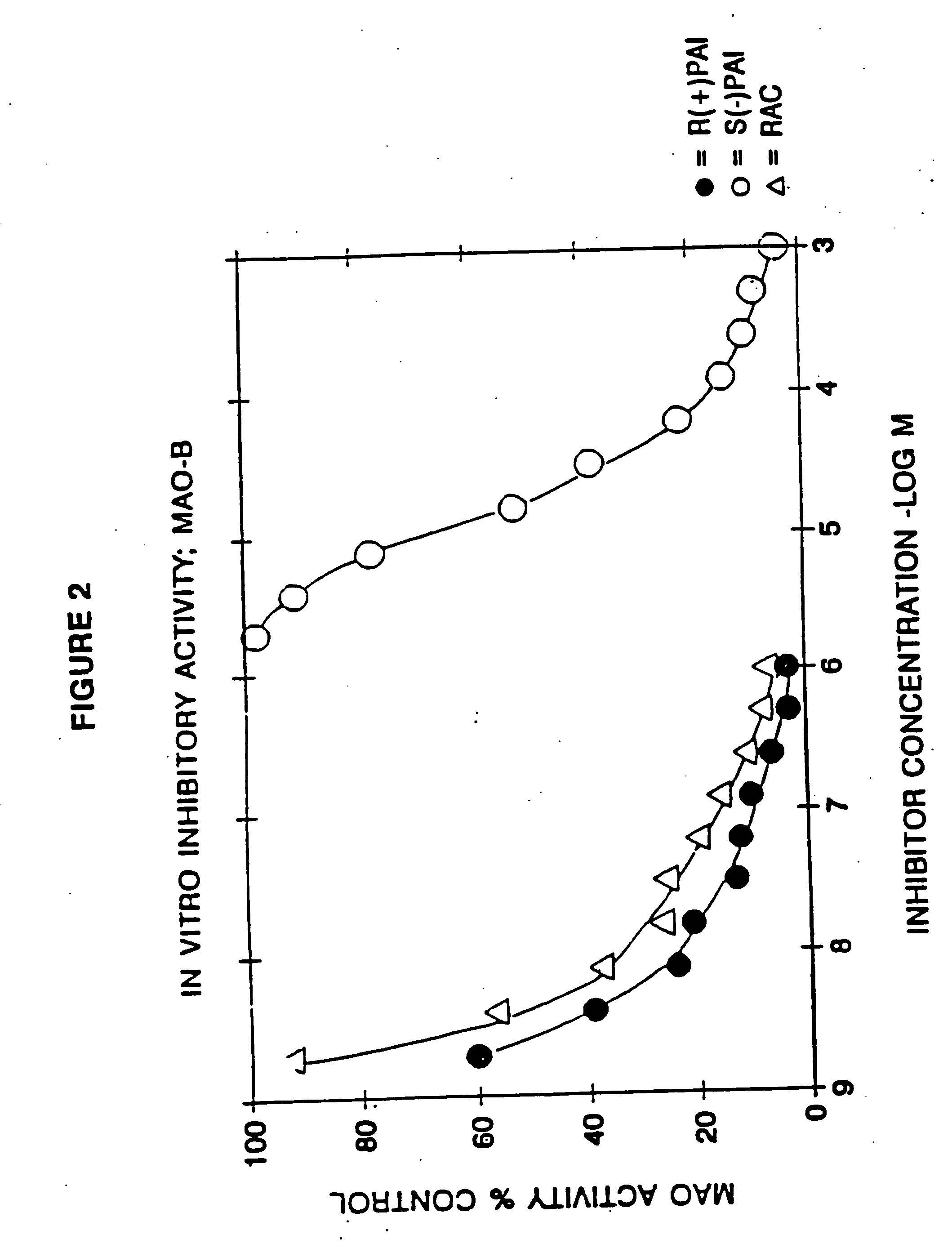
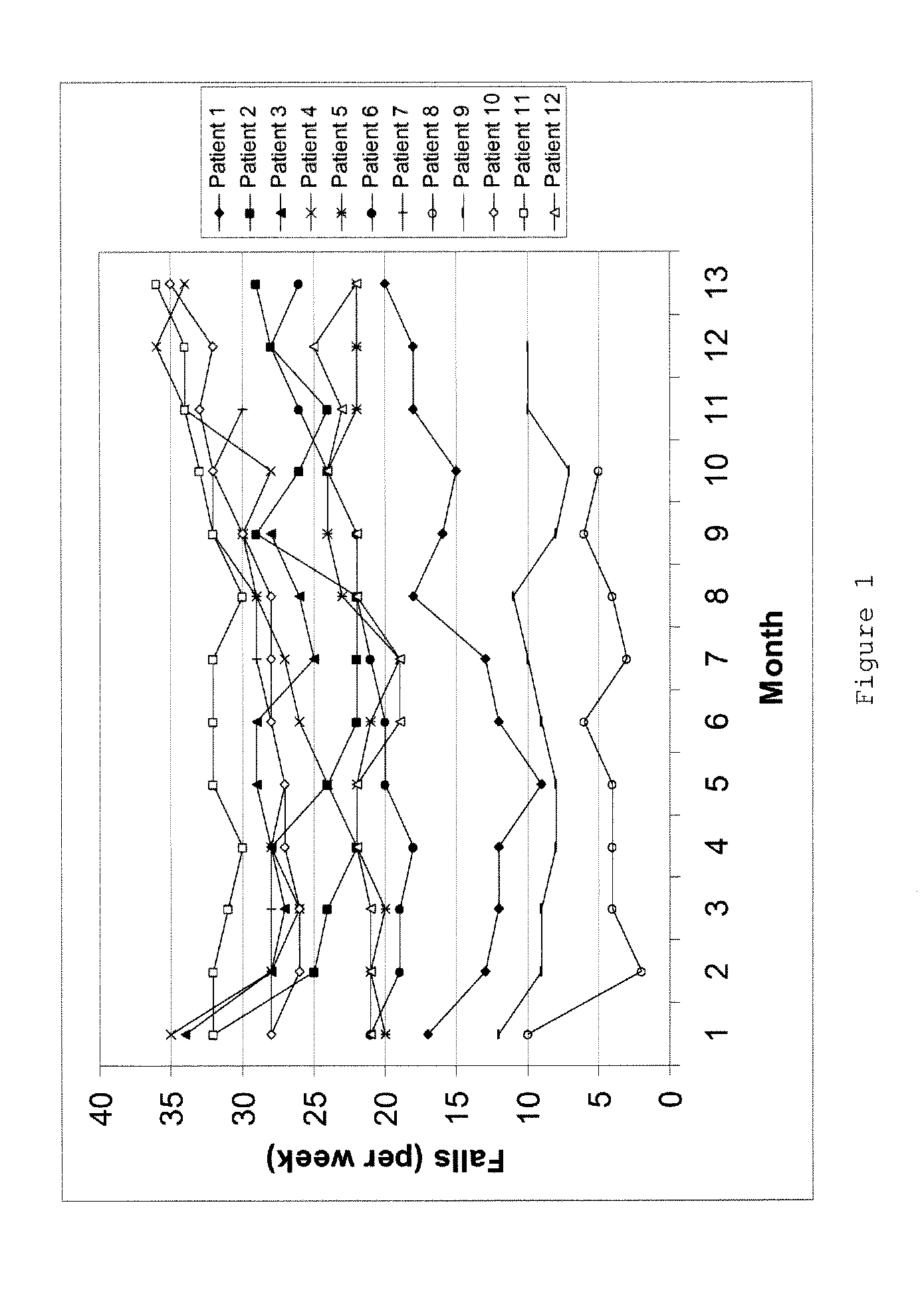
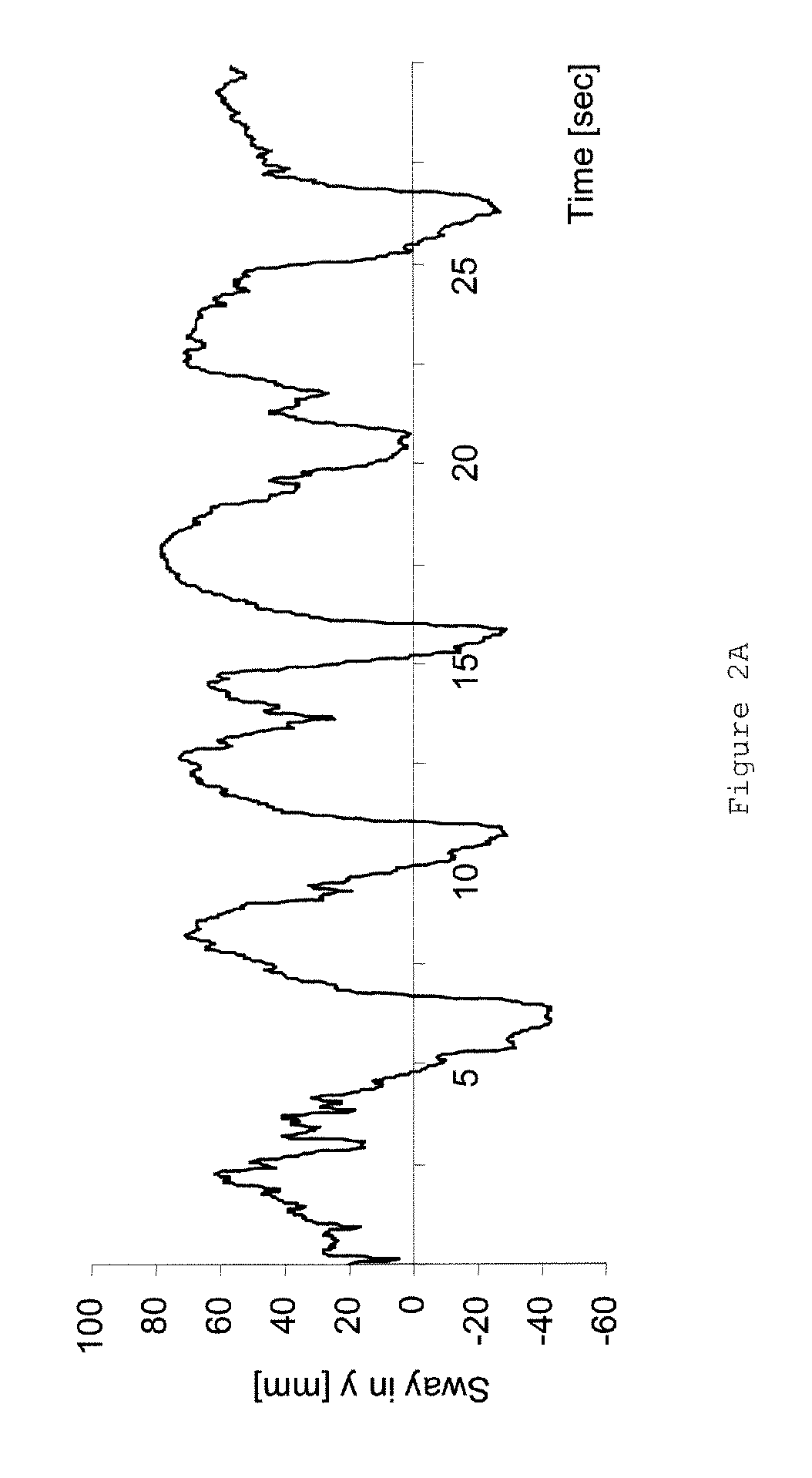
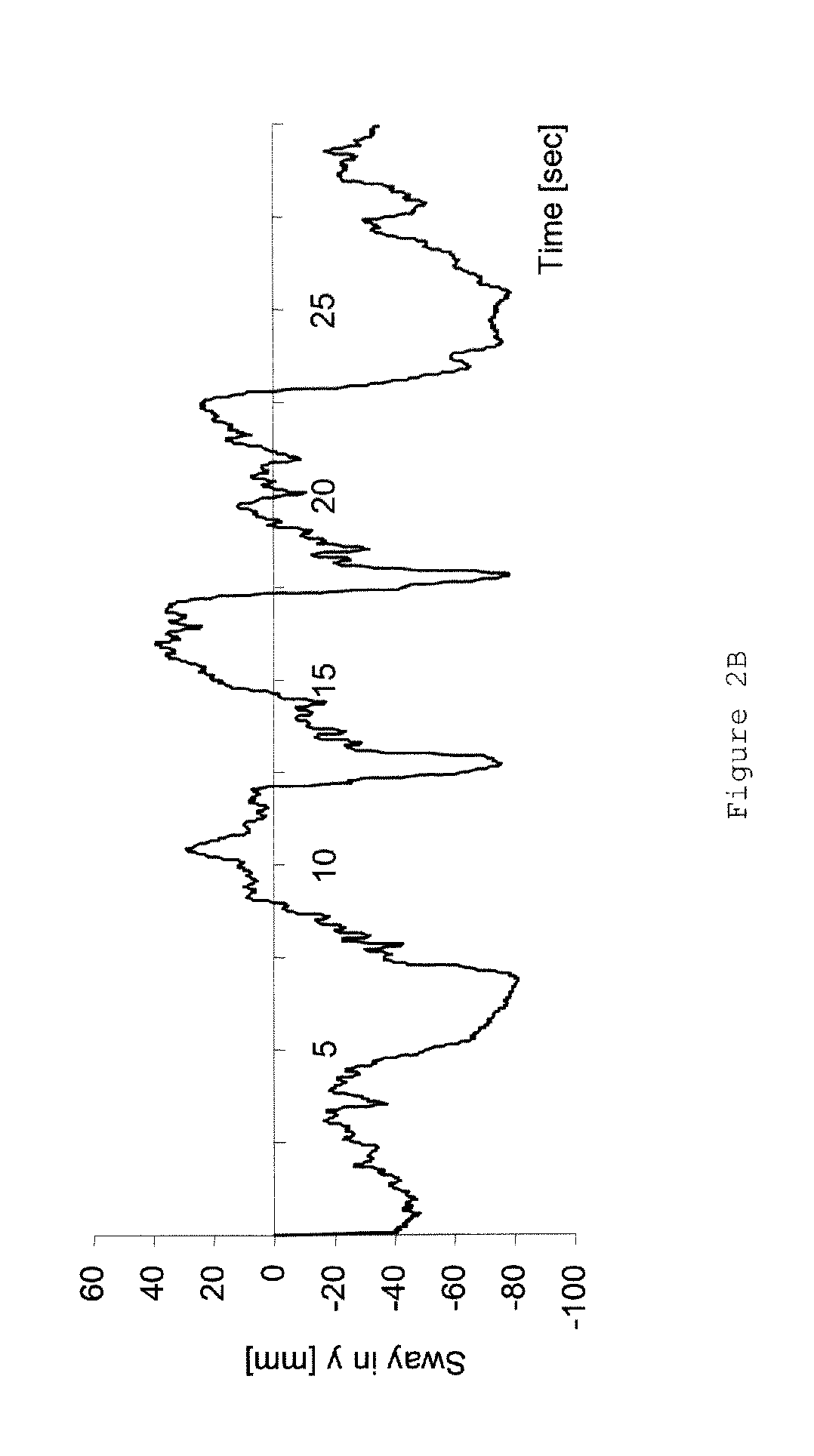
Pharmaceutical compositions comprising S-(-)-N-propargyl-1-aminoindan
InactiveUS6277886B1Enhance memoryBiocideOrganic active ingredientsBULK ACTIVE INGREDIENTNeurological disorder
Pharmaceutical compositions for the treatment of a neurological disorder of neurotrauma or for improving memory in a patient comprising a therapeutically effective amount of S-(-)-N-proparygl-1-aminoindan or a pharmaceutically acceptable salt thereof as active ingredient, and a pharmaceutically active carrier. The pharmaceutical compositions are adapted, in particular for treating a neurological hypoxia or anoxia, neurodegenerative diseases. Parkinson's Disease, Alzheimer's Disease, neurotoxic injury, head trauma injury, spinal trauma injury or any other form of nerve damage.
Owner:TECHNION RES & DEV FOUND LTD +1
Use of R-enantiomer of N-propargyl-1-aminoindan, salts, compositions and uses thereof
InactiveUS20060094783A1Avoid nerve damageBiocideOrganic active ingredientsMemory disorderAttention deficits
The subject invention provides methods of treating a subject afflicted with Parkinson's disease, memory disorder, depression, hyperactive syndrome, Attention Deficit Disorder, dementia, brain ischemia, stroke, head trauma injury, spinal trauma injury, neurotrauma, neurodegenerative disease, neurotoxic injury, multiple sclerosis, nerve damage, affective illness, schizophrenia or symptoms of withdrawal from an addictive substance, using the mesylate salt of R(+)-N-propargyl-1-aminoindan.
Owner:TEVA PHARMA IND LTD +1
Rasagiline formulations of improved content uniformity
ActiveUS20060188581A1Good content uniformityImprove uniformityOrganic active ingredientsBiocideN-propargyl1-aminoindan
Owner:TEVA PHARMA IND LTD
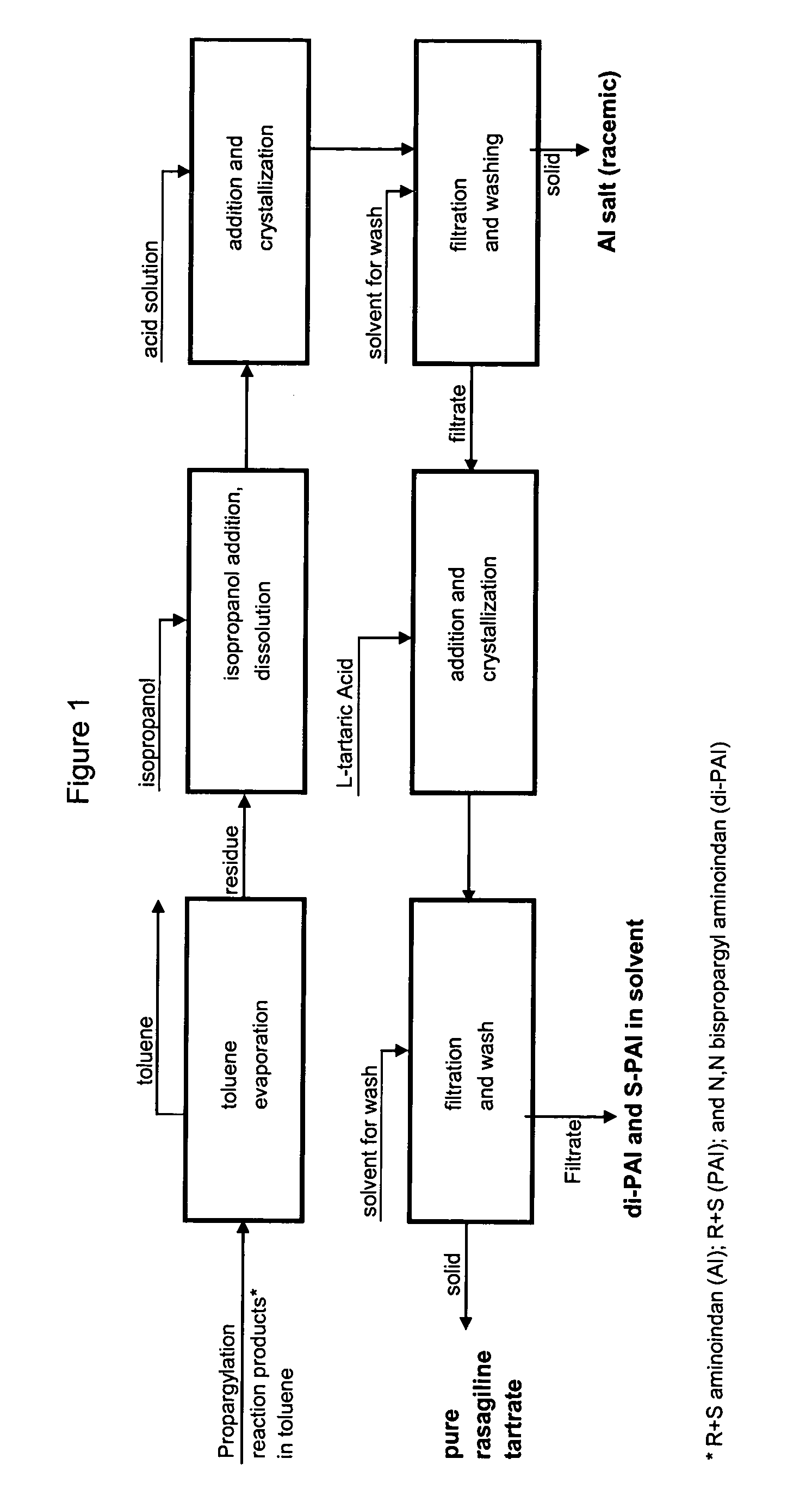
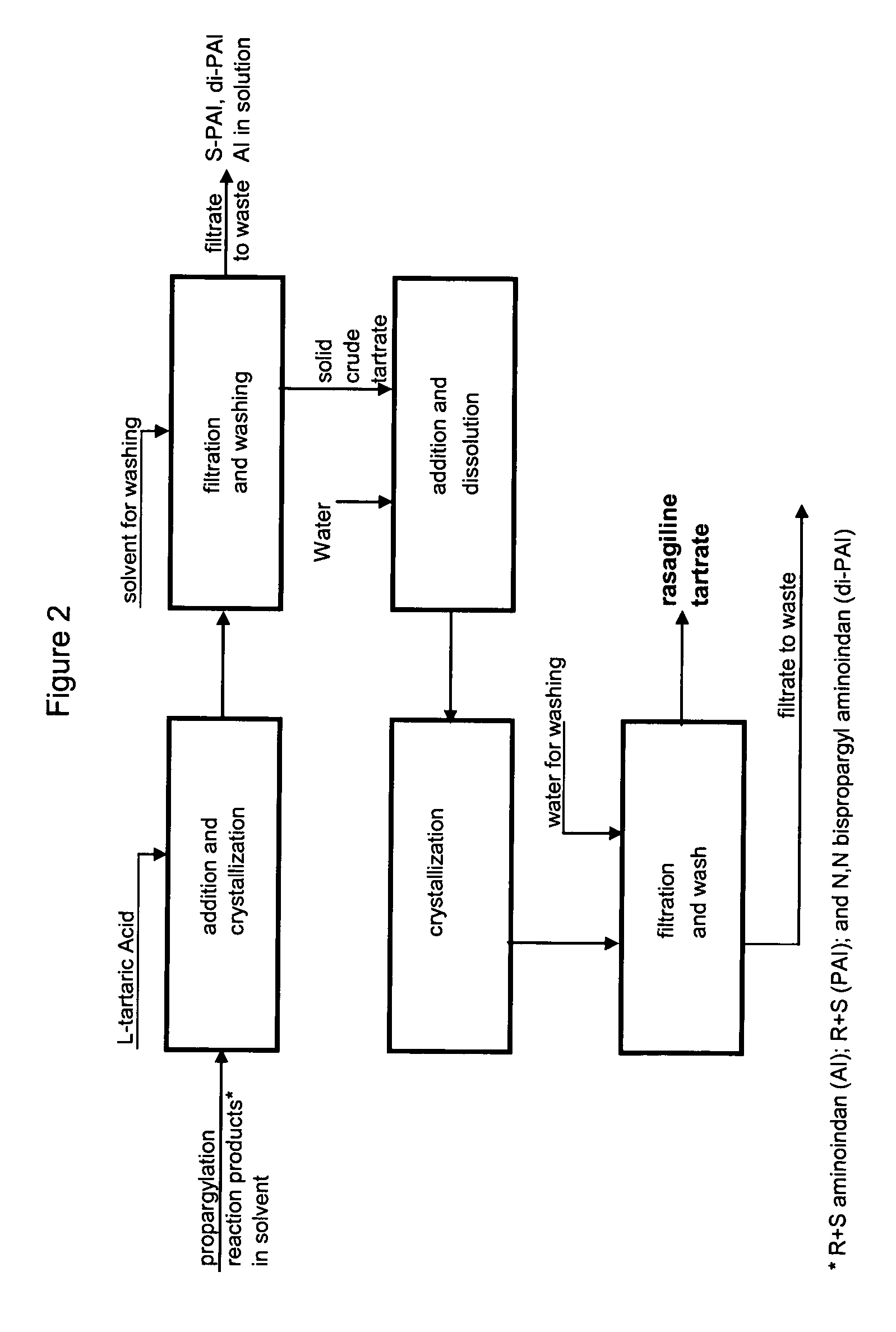
Methods for isolating propargylated aminoindans
A process for isolating from a reaction mixture a salt of a mono-propargylated aminoindan, a process for isolating from a reaction mixture a crystalline diastereomeric salt of a mono-propargylated aminoindan, and a process for isolating from a reaction mixture a salt of enantiomerically pure N-propargyl-1-aminoindan or a salt of enantiomerically pure 6-(N-methyl, N-ethyl-carbamoyloxy)-N′-propargyl-1-aminoindan. The corresponding products are also disclosed.
Owner:TEVA PHARMA IND LTD
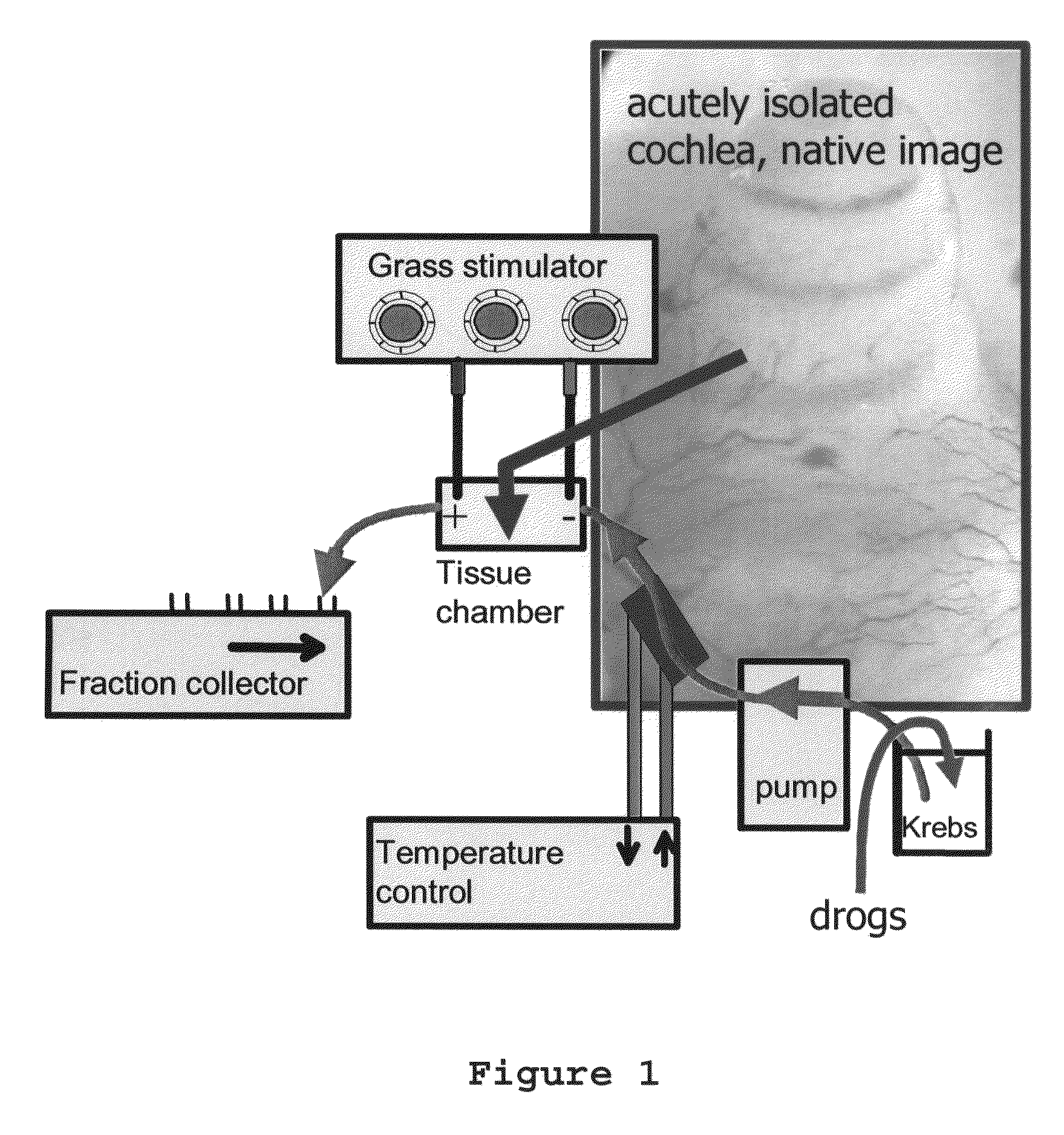
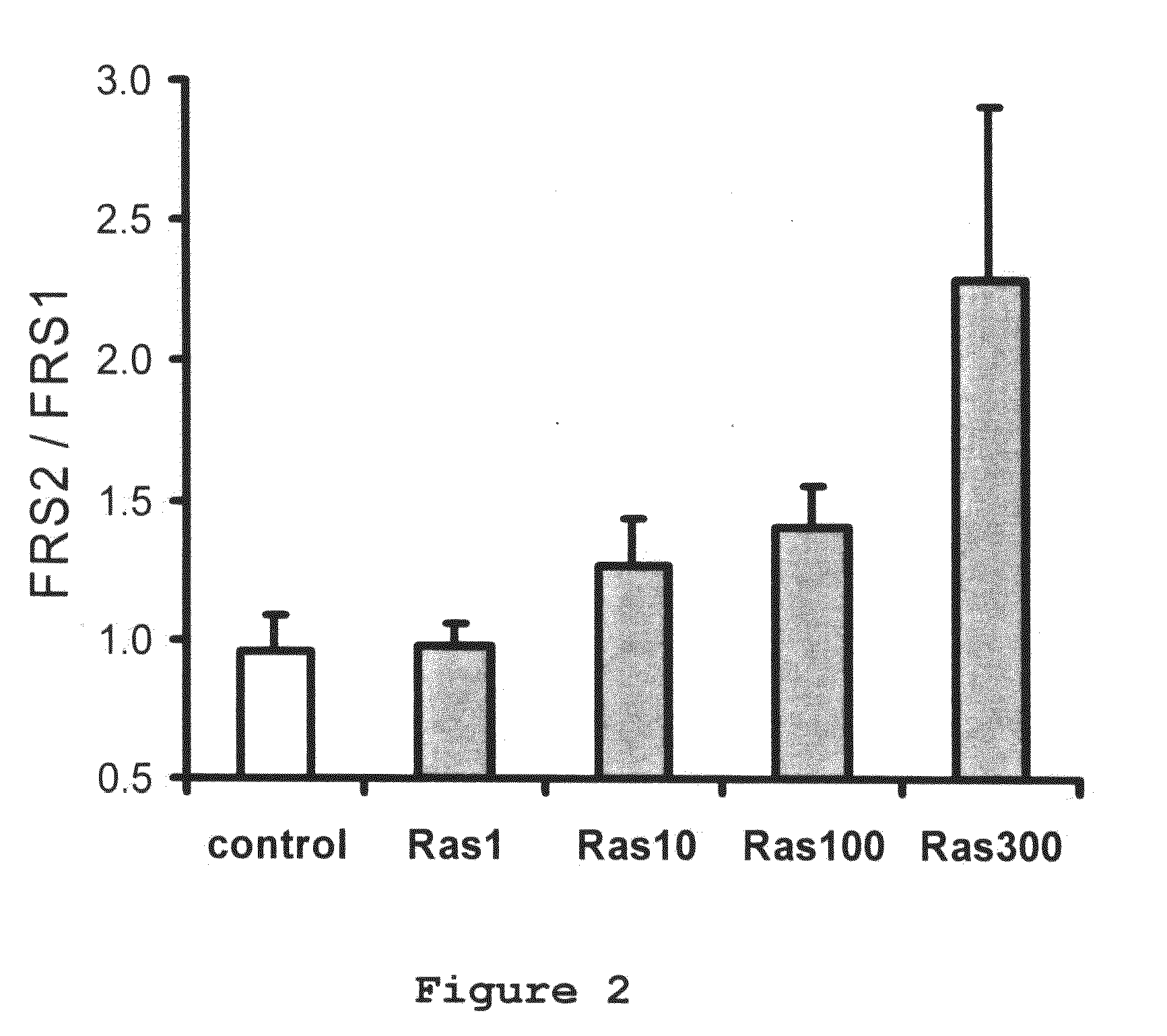
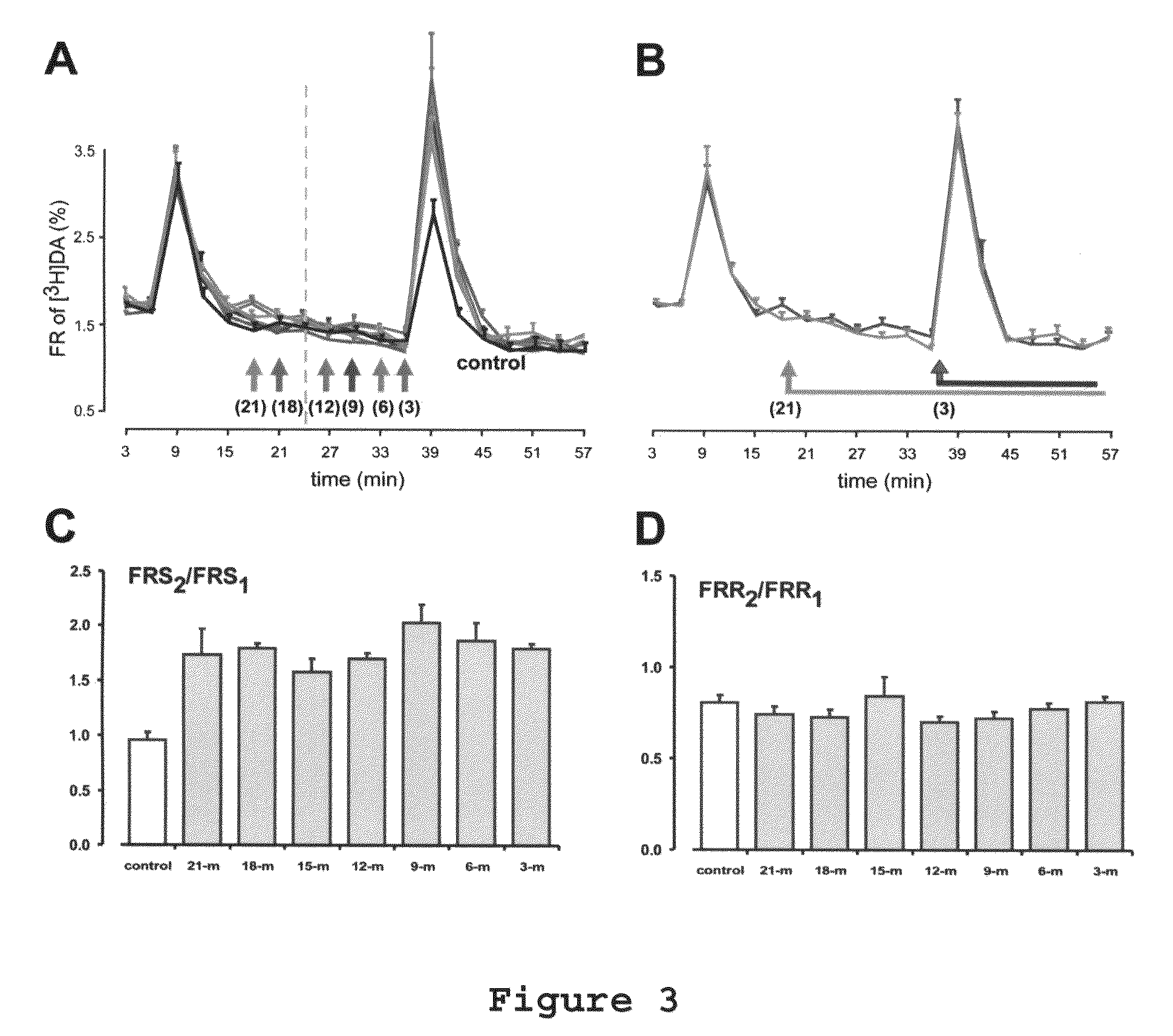
Use of R (+) -N-propargyl-1-aminoindan to treat or prevent hearing loss
A method of treating or inhibiting hearing loss in a mammalian subject, comprising administering to the subject an amount of R(+)-N-propargyl-1-aminoindan or pharmaceutically acceptable salt thereof effective to treat or inhibit the hearing loss in the subject.
Owner:TEVA PHARMA IND LTD
Use of rasagilline for the treatment of restless legs syndrome
ActiveUS20070232700A1Effective treatmentRelieve symptomsBiocideNervous disorderN-propargylPediatrics
Disclosed are methods for the treatment of Restless Legs Syndrome comprising administering an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
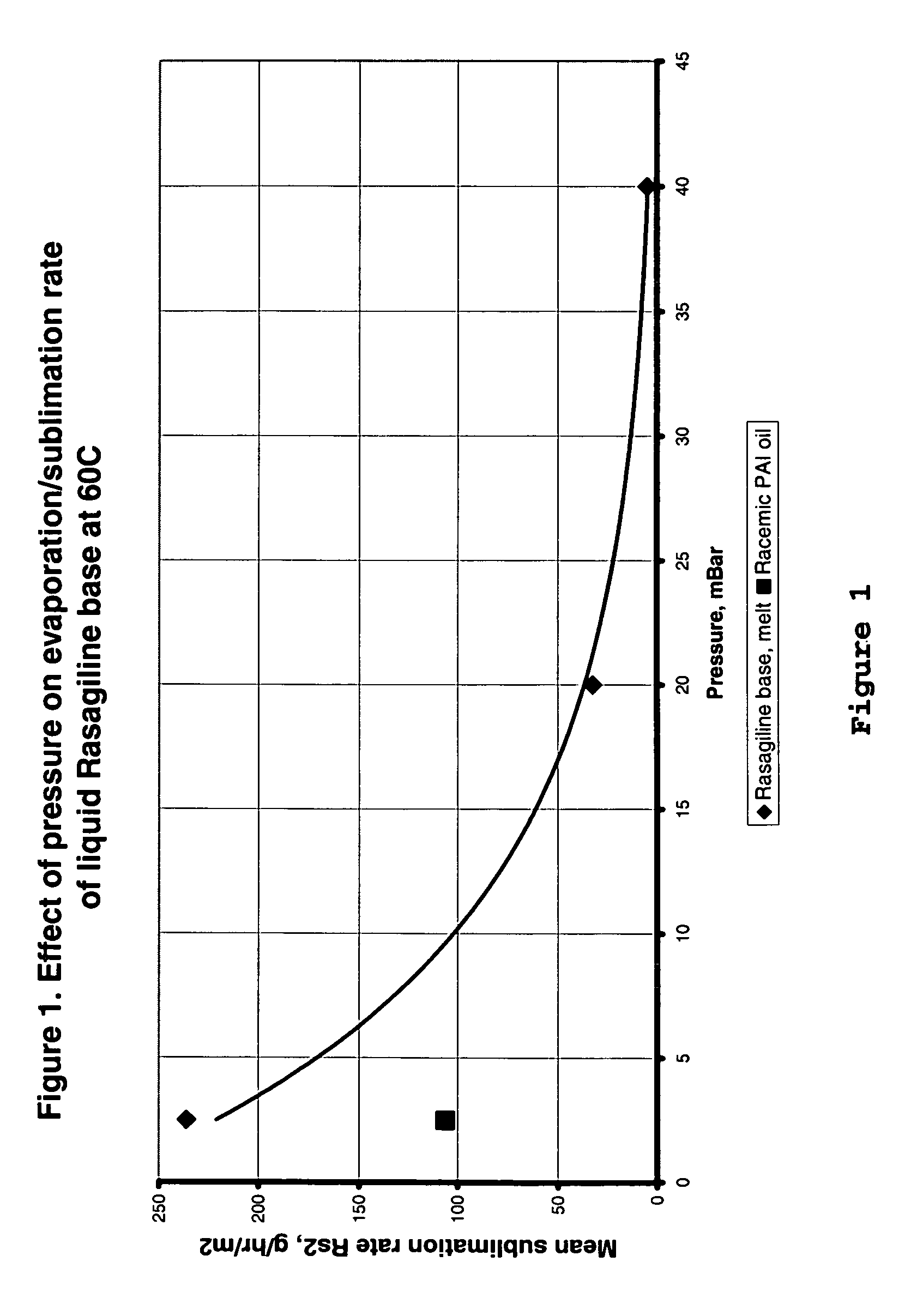
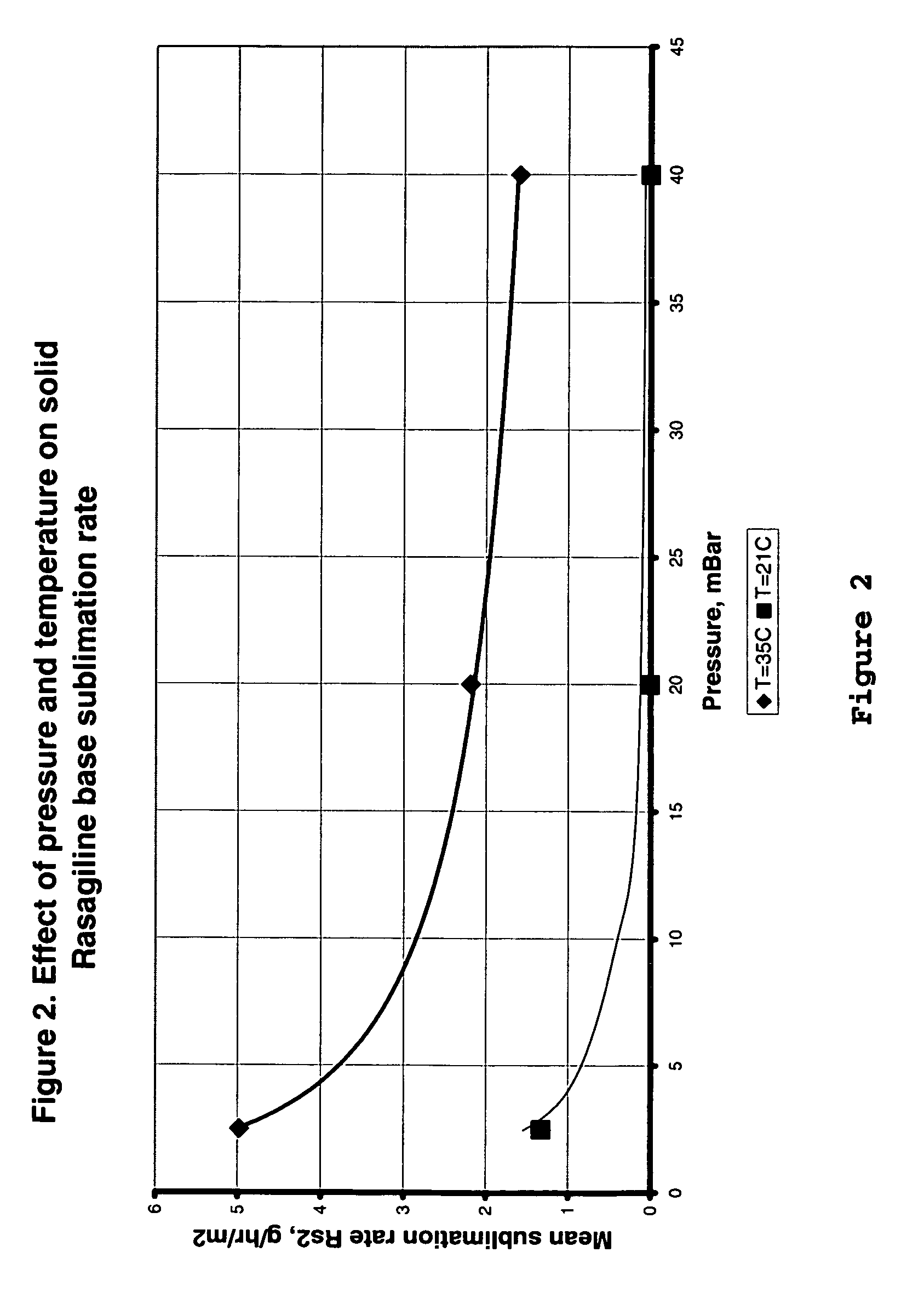
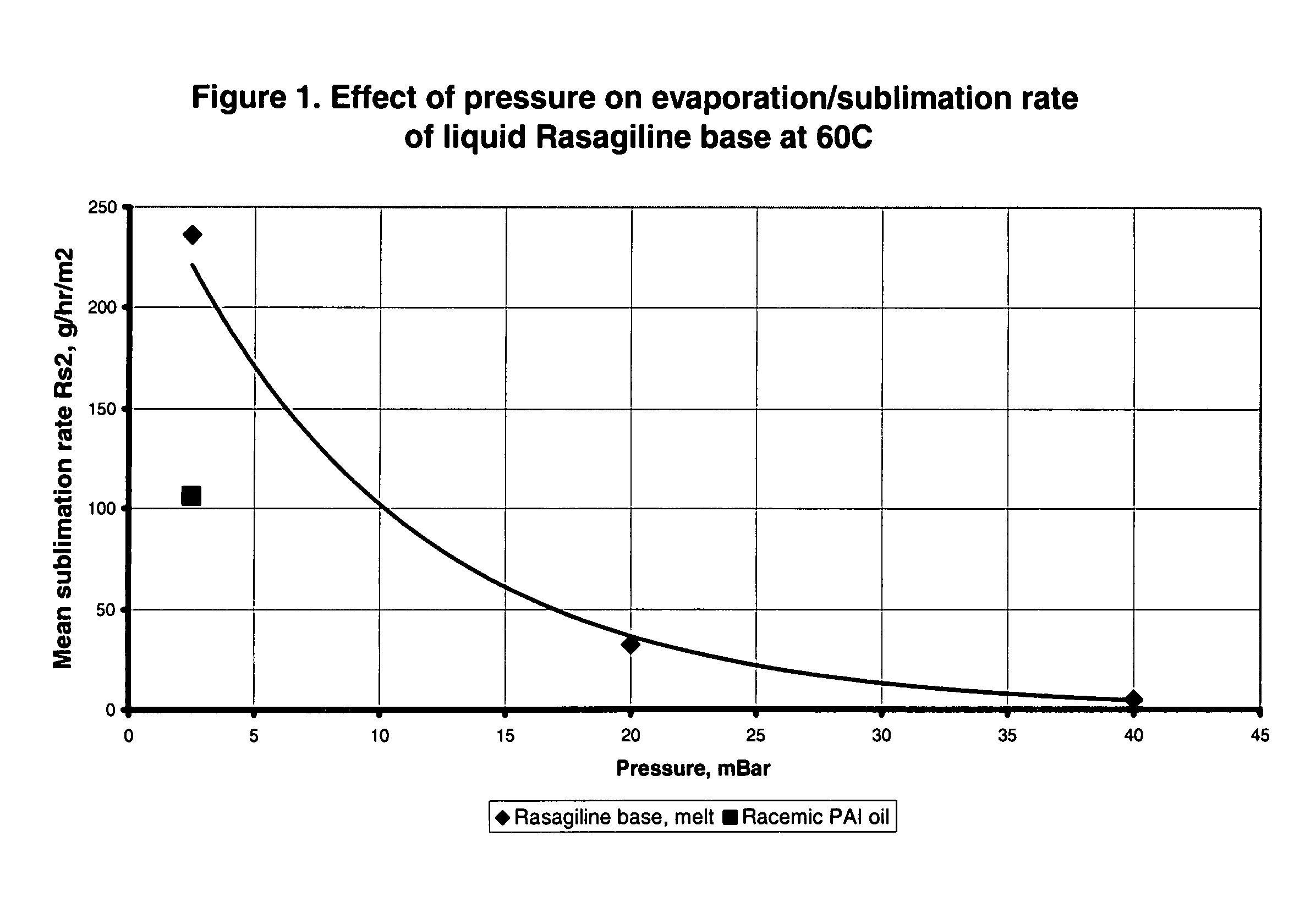
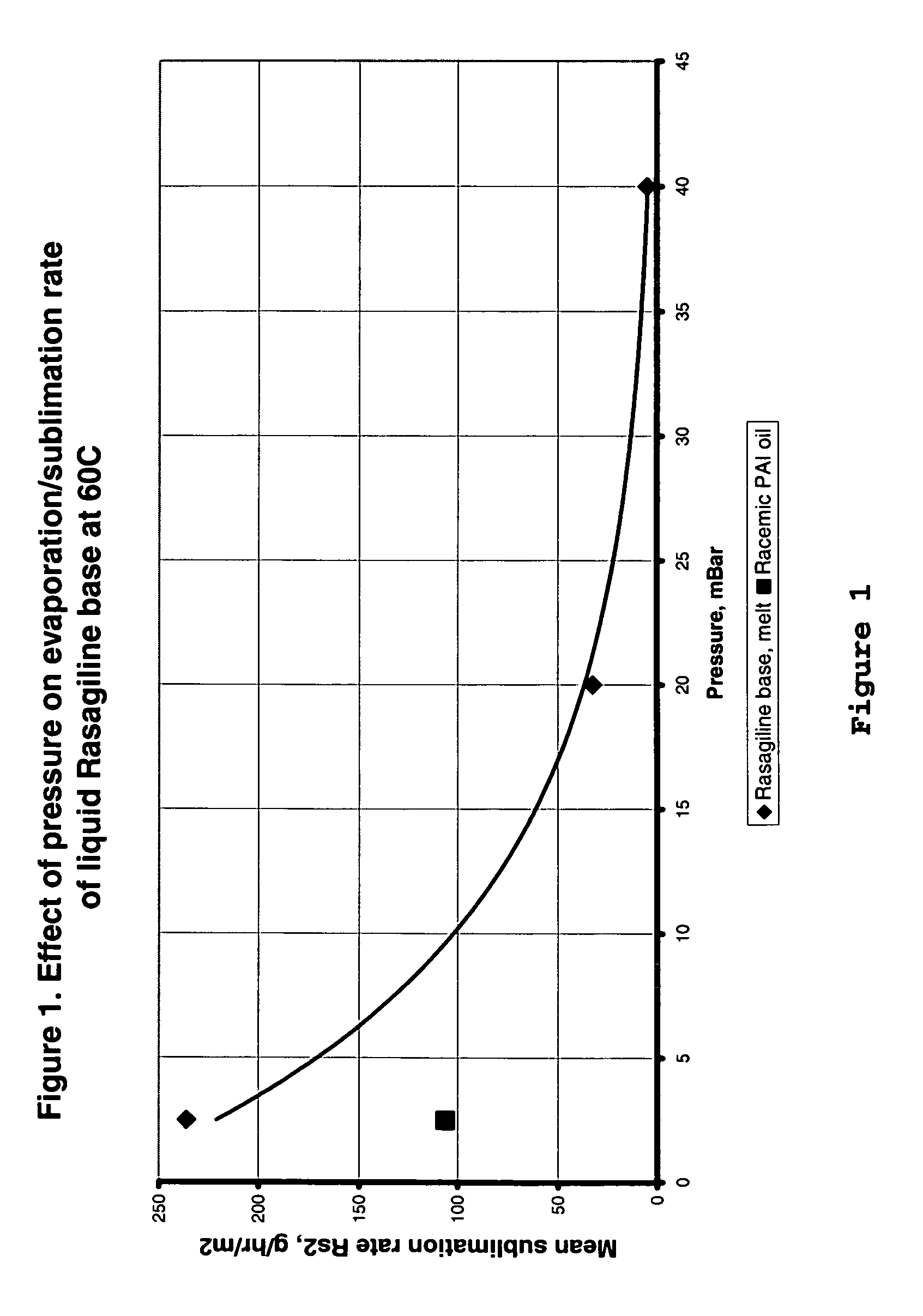
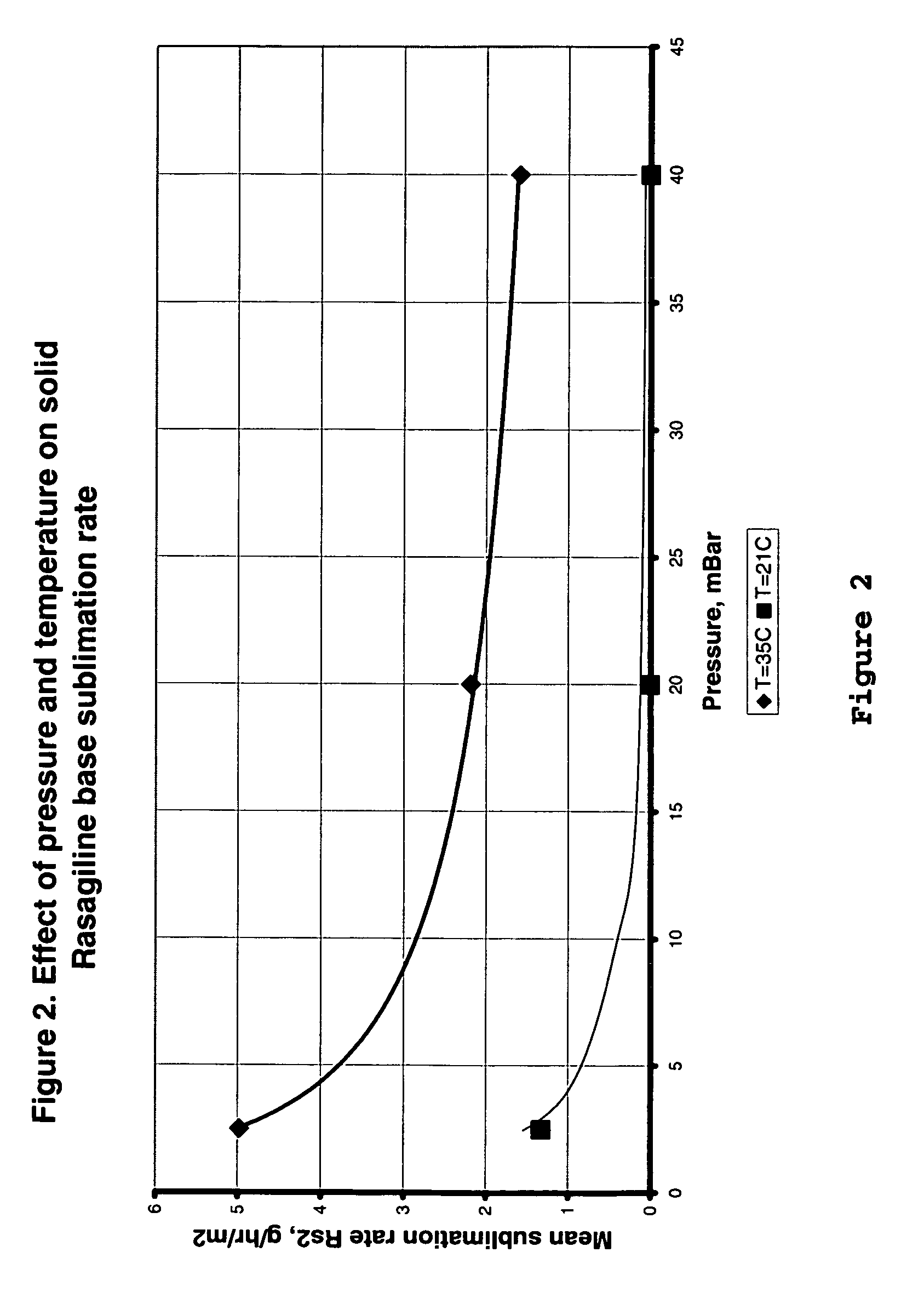
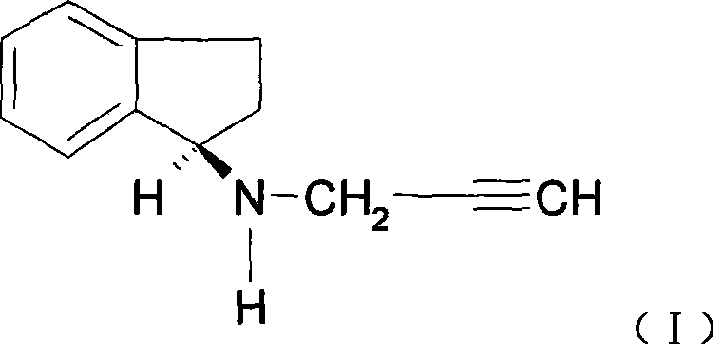
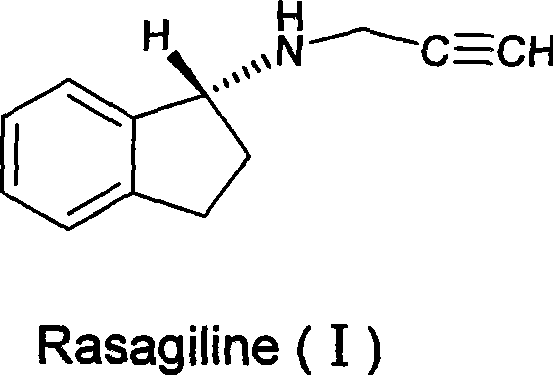
Process for purifying rasagiline base
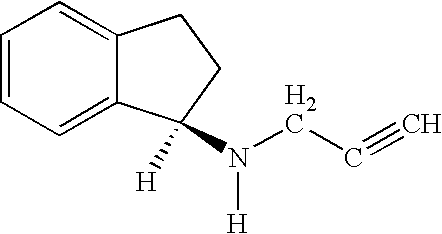
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
Rasagiline formulations of improved content uniformity
Disclosed are pharmaceutical preparations of R(+)-N-propargyl-1-aminoindan salts having enhanced content uniformity, processes for preparation of the compositions, and their uses.
Owner:TEVA PHARMA IND LTD
Crystalline solid rasagiline base
Owner:TEVA PHARMA IND LTD
Crystalline solid rasagiline base
InactiveUS20100145101A1Organic active ingredientsAmino compound purification/separationN-propargyl1-aminoindan
The subject invention provides crystalline R(+)-N-propargyl-1-aminoindan, pharmaceutical compositions and methods of manufacture thereof.
Owner:TEVA PHARMA IND LTD
Use of rasagiline for the treatment of progressive supranuclear palsy
A method for the treatment of Progressive Supranuclear Palsy. Such method includes administering to a subject an amount of R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
Process for purifying rasagiline base
Disclosed is crystalline R(+)-N-propargyl-1-aminoindan and racemic N-propargyl-1-aminoindan characterized by colorless crystals a pharmaceutical composition comprising the same, and the process for the manufacture and the validation thereof. Also disclosed is pure liquid R(+)-N-propargyl-1-aminoindan and a pharmaceutical composition comprising the same, and the process for the manufacture thereof.
Owner:TEVA PHARMA IND LTD
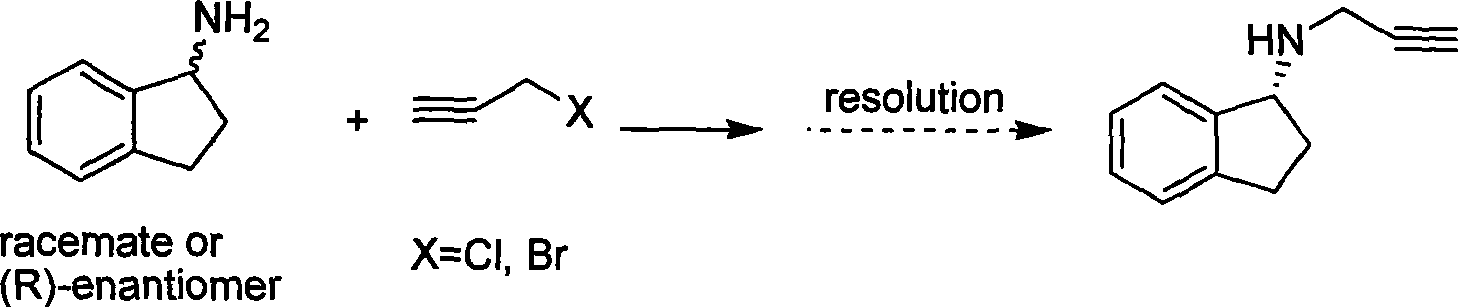
Preparation method of (R)-(+)-N-propargyl-1-indan amines
InactiveCN101381314AStrong response specificityHigh yieldAmino preparation by functional substitutionBulk chemical productionN-propargylSide effect
The invention discloses a method for preparing rasagiline which has simple and convenient operation and is suitable for industrialized production. In the method, primary amine group on 1-indan amine is protected by o-Nos, and the 1-indan amine removes the protecting group after the 1-indan amine is substituted by propargyl chloride (or propargyl bromide); compared with the prior method, two steps of reactions are added in the method, but after the 1-indan amine is protected by the o-Nos, the reaction specificity of the 1-indan amine and the propargyl chloride (or the propargyl bromide) is greatly improved; and the protecting group can be easily removed after reaction without other side effects, so a single product is generated. Besides, raw materials and reaction reagent used in the method is cheap and easily obtained. The method has the advantages of simple and easy operation, mild reaction condition, easy control, good reaction selectivity, total yield improvement and cost reduction, and has excellent industrialized prospect.
Owner:成都和康药业有限责任公司
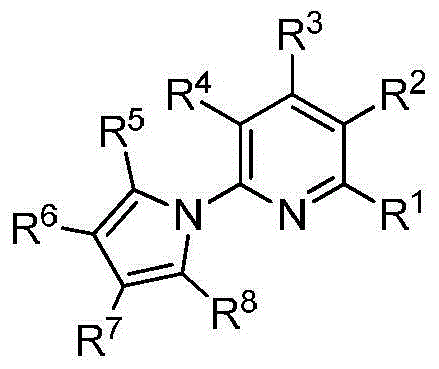
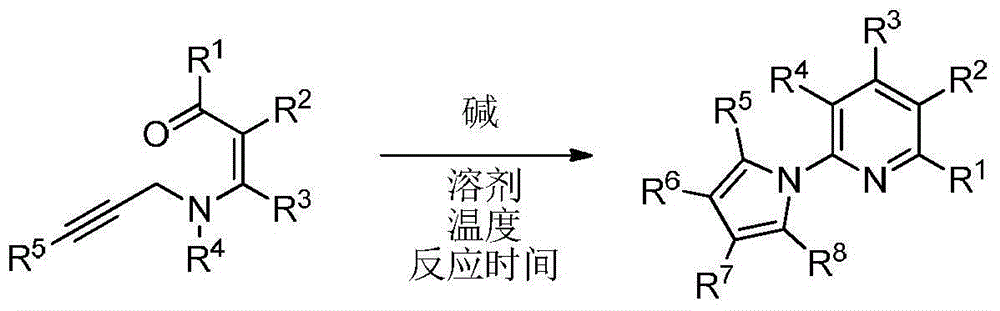
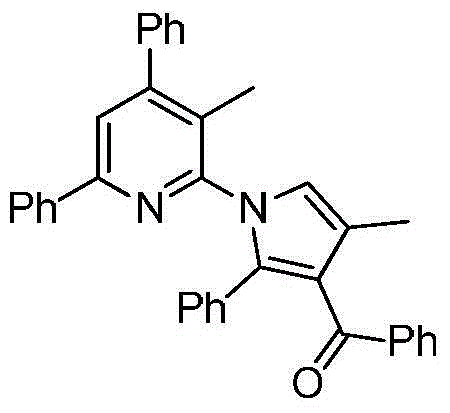
Polysubstituted 2-pyrrolopyridine derivative and preparation method thereof
The invention discloses a polysubstituted 2-pyrrolopyridine derivative. The derivative has a structure shown in a formula in the specification, wherein in the formula, R1, R2, R3, R4, R5, R6, R7 and R8 are selected from any one of hydrogen atoms, halogen atoms, alkyl, aryl, substituted aryl, acyl, amino, nitro and alkoxy. The invention also discloses a preparation method of the polysubstituted 2-pyrrolopyridine derivative. The polysubstituted 2-pyrrolopyridine derivative shown in the formula is obtained at high yield after completing heating reaction of N-propargyl enaminone in a solvent in the presence of appropriate alkali. The polysubstituted 2-pyrrolopyridine derivative and the preparation method have the beneficial effects that the reaction conditions are mild; the reaction time is short; the range of substrates is wide; the reaction specificity is strong; the yield is high; aftertreatment is simple and convenient to carry out.
Owner:HUAQIAO UNIVERSITY
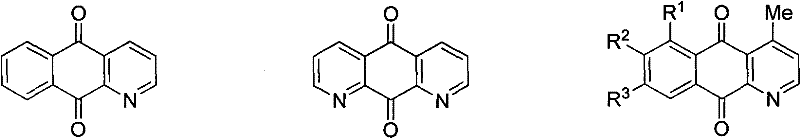
Synthesis method of azepine anthraquinone
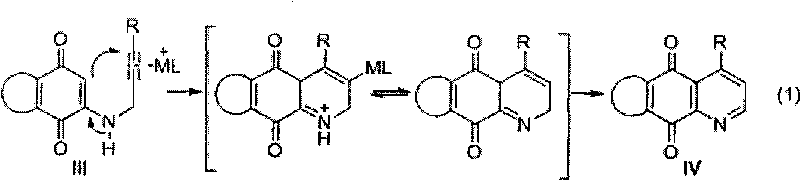
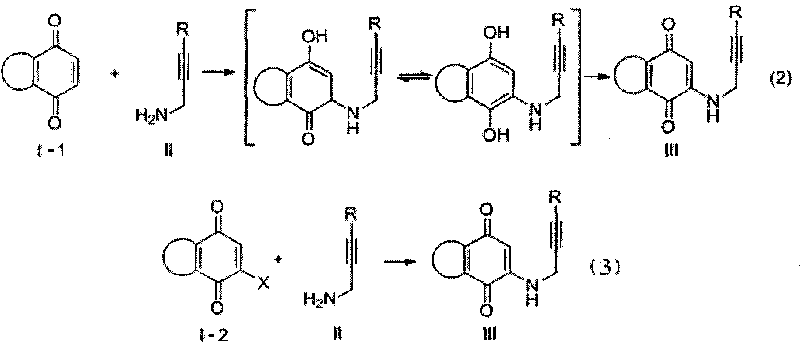
InactiveCN101712648AAchieve synthesisHas antibacterial activityOrganic chemistryPlatinum saltsIsomerization
The invention relates to a synthesis method of azepine anthraquinone. The azepine anthraquinone is obtained by carrying out intramolecular 6-endo-dig cycloisomerisation reaction shown as in formula (1) on N-propargyl quinine with a 1,5-eneyne structure under the action of a metal catalyst, and purifying, wherein the intramolecular 6-endo-dig cycloisomerisation reaction is homogeneous phase metal catalytic reaction, the metal catalyst is gold salt, platinum salt or univalent gold complex; and the use level of the metal catalyst is 0.01-0.5 equivalent weight of the N--propargyl quinine. The gold slat is auri chloridum (AuCl3) or aurous chloride (AuCl); the platinum salt is platinum tetrachloride, platinum bichloride or potassium chloroplatinate; and the univalent gold complex is PPh3AuOTf, PPh3AuSbF6, PPh3AuNTf2 or LAuNTf2, wherein L is nitrogen heterocyclic ring carbene ligand. The invention realizes the synthesis of the azepine anthraquinone by utilizing metal catalytic intramolecular eneyne cyclization reaction, and has the advantages of simple and easy-accessible raw materials and moderate reaction conditions.
Owner:NANJING UNIV
Additive for electro-deposited bright ferro-nickel alloy
The invention relates to an additive for an electro-deposited bright ferro-nickel alloy. The additive comprises a brightener and a complexing agent. The brightener comprises the following components: N-propargyl cyanamide, n-propargyl cyanamide salt, N-propargyl dicyandiamide, N-propargyl dicyandiamide salt, bis-N-propargyl dicyandiamide and bis-N-propargyl dicyandiamide salt; the complexing agent comprises the following components: amino acetic acid, amino acetate, aminopropionic acid, aminopropionate, diphosphonic acid and diphosphonate. By means of a synergistic effect of alkynyl and nitrile functional groups, the propargyl-containing cyanamide compound has very good brightening and leveling effects if being used in a ferronickel plating solution; the ferronickel plating coating can be prevented from flooding and fogging effectively by means of double complexing action of amino acids such as amino acetic acid and aminopropionic acid and diphosphonic acid and salt compositions thereof.
Owner:杭州东方表面技术有限公司
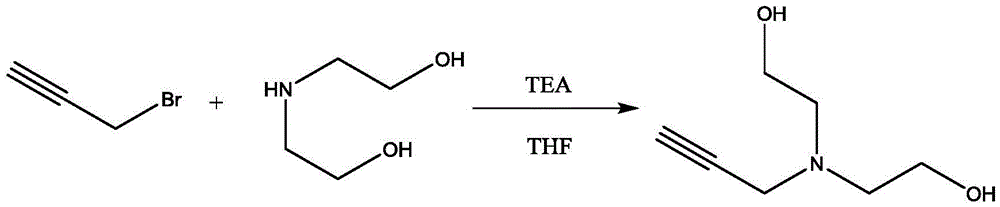
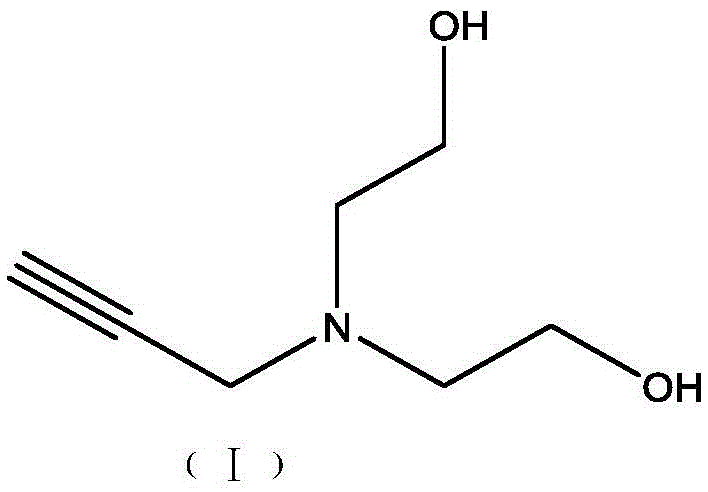
Method for synthesis and purification of bonding agent N-propargyl diethanol amine
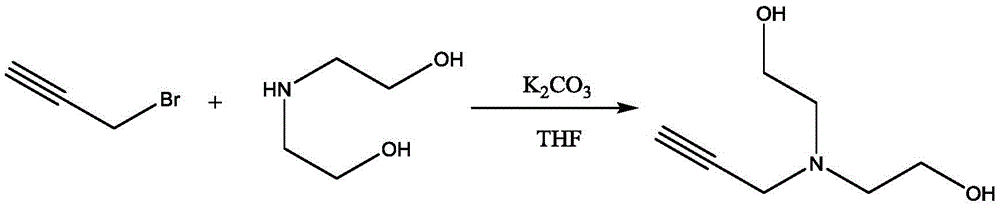
InactiveCN105481704AShort reaction timeHigh yieldOrganic compound preparationAmino-hyroxy compound preparationN-propargylDistillation
The invention discloses a method for synthesis and purification of the bonding agent N-propargyl diethanol amine. The structure of the compound is shown in the description. The bonding agent N-propargyl diethanol amine is expected to be applied to energetic binder type propellants. The method comprises the steps of adding 3-propargyl bromide to a diethanol amine tetrahydrofuran solution dropwise with triethylamine as the acid-binding agent under the ice-bath condition, conducting filtration after three hours of reaction, conducting filtrate concentration and reduced pressure distillation in sequence, and collecting the product. The method has the advantages of being high in yield and environmentally friendly.
Owner:XIAN MODERN CHEM RES INST
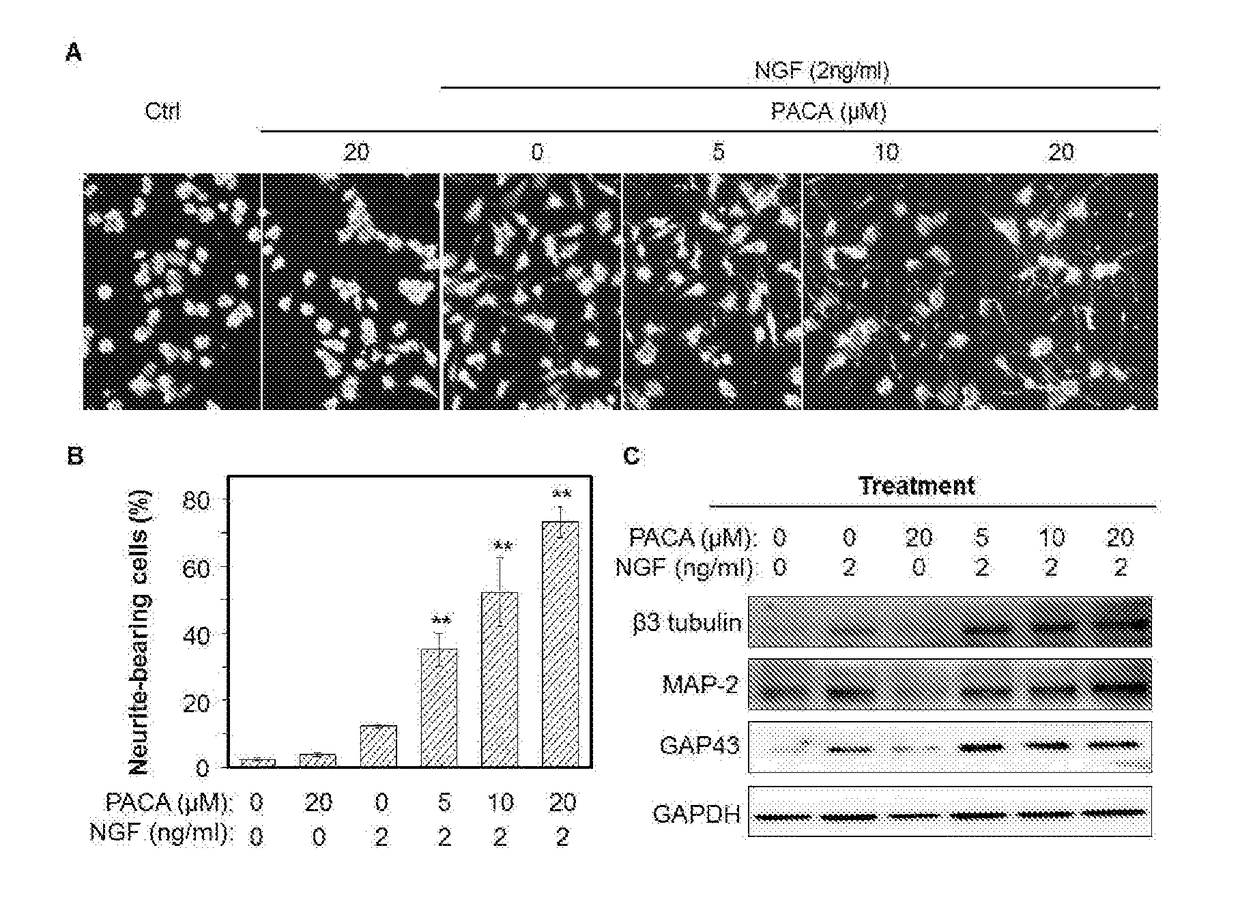
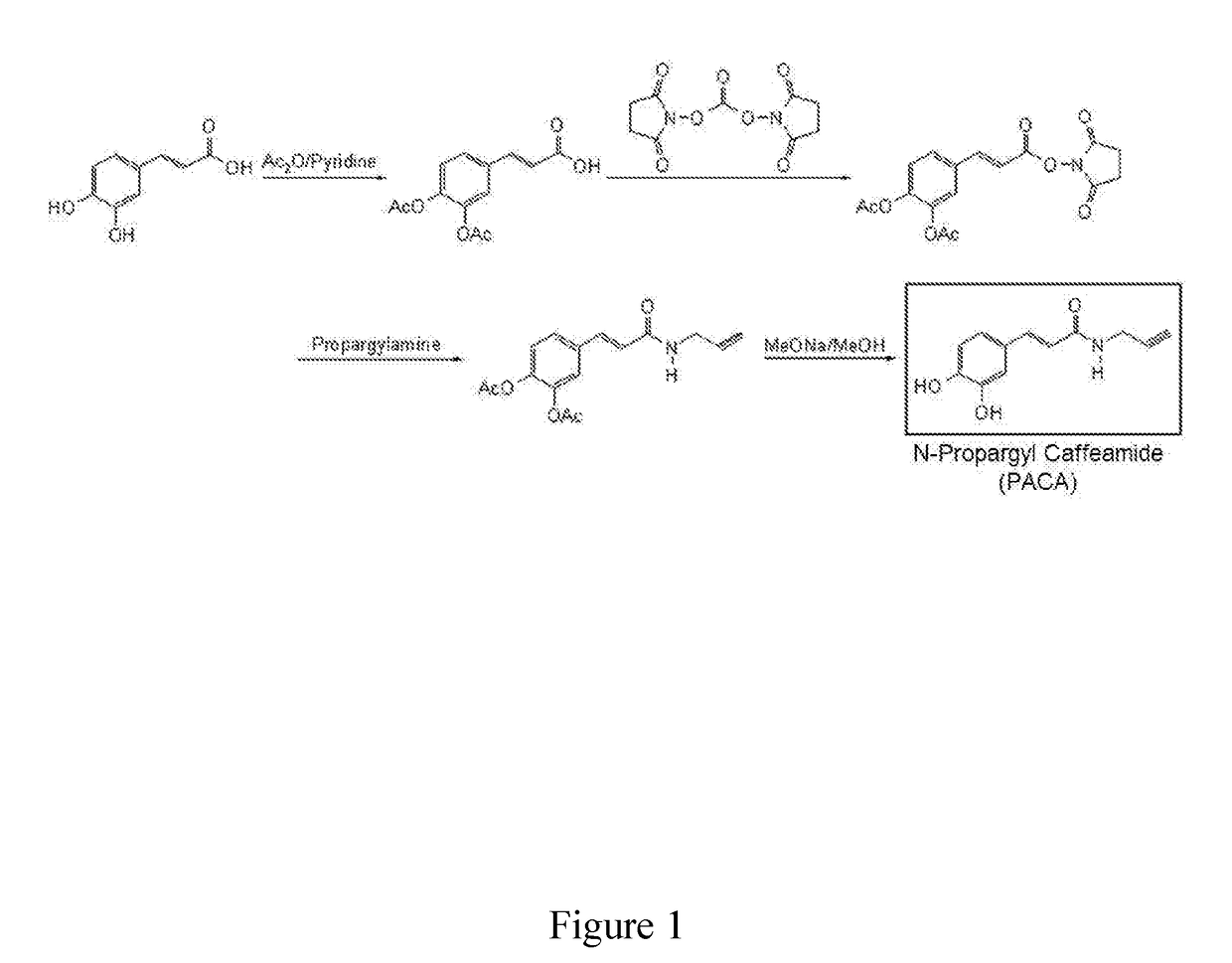
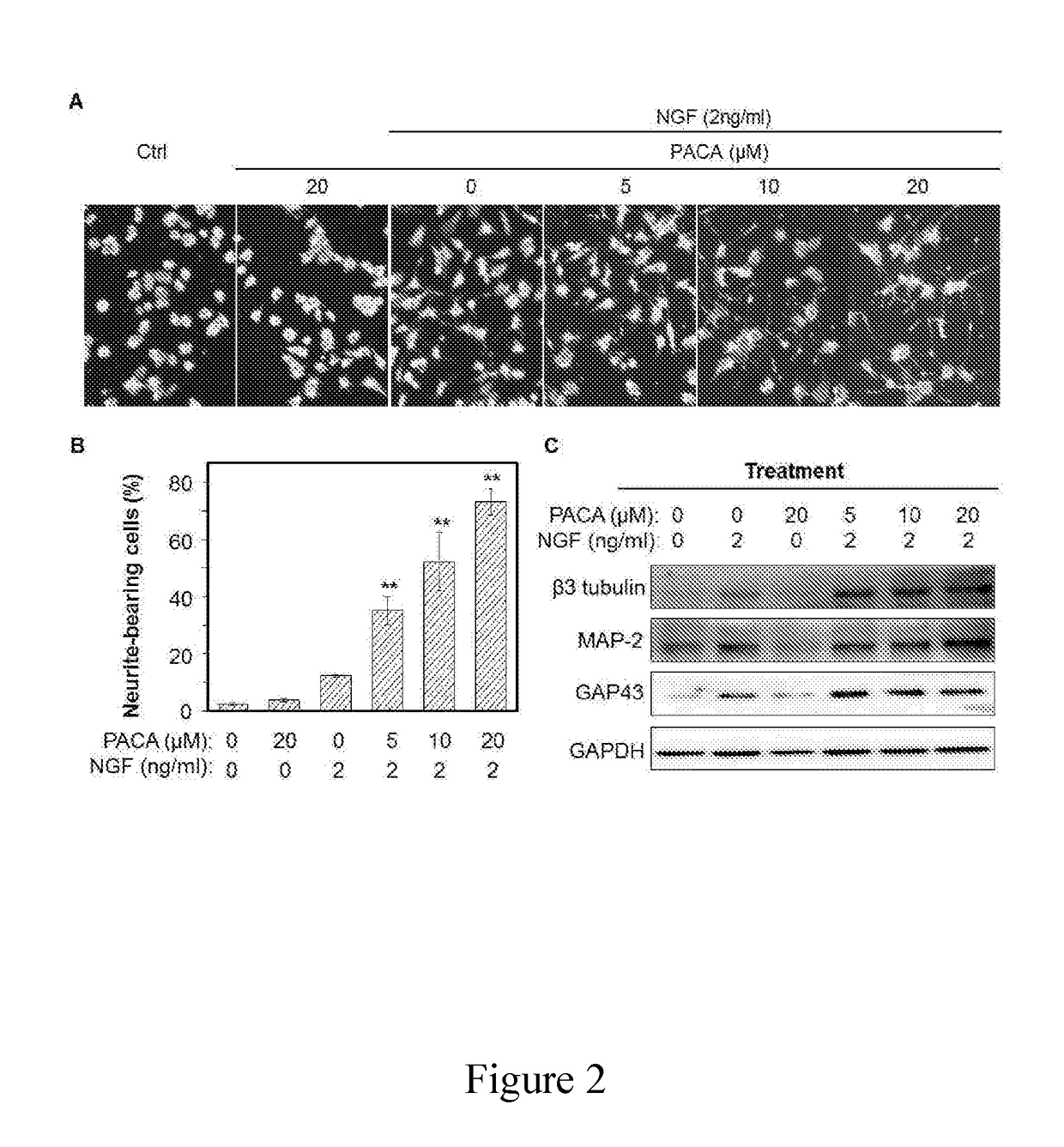
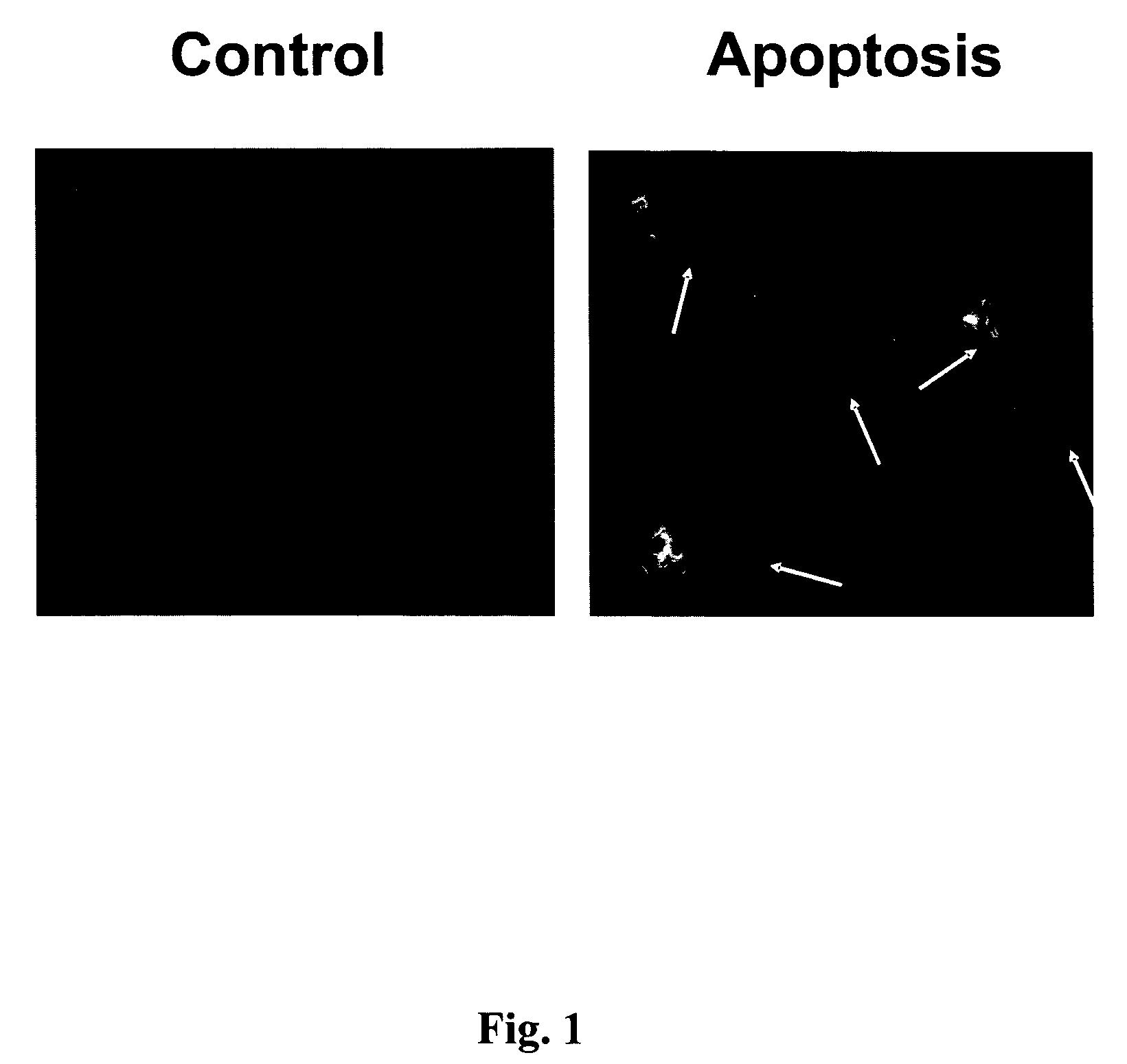
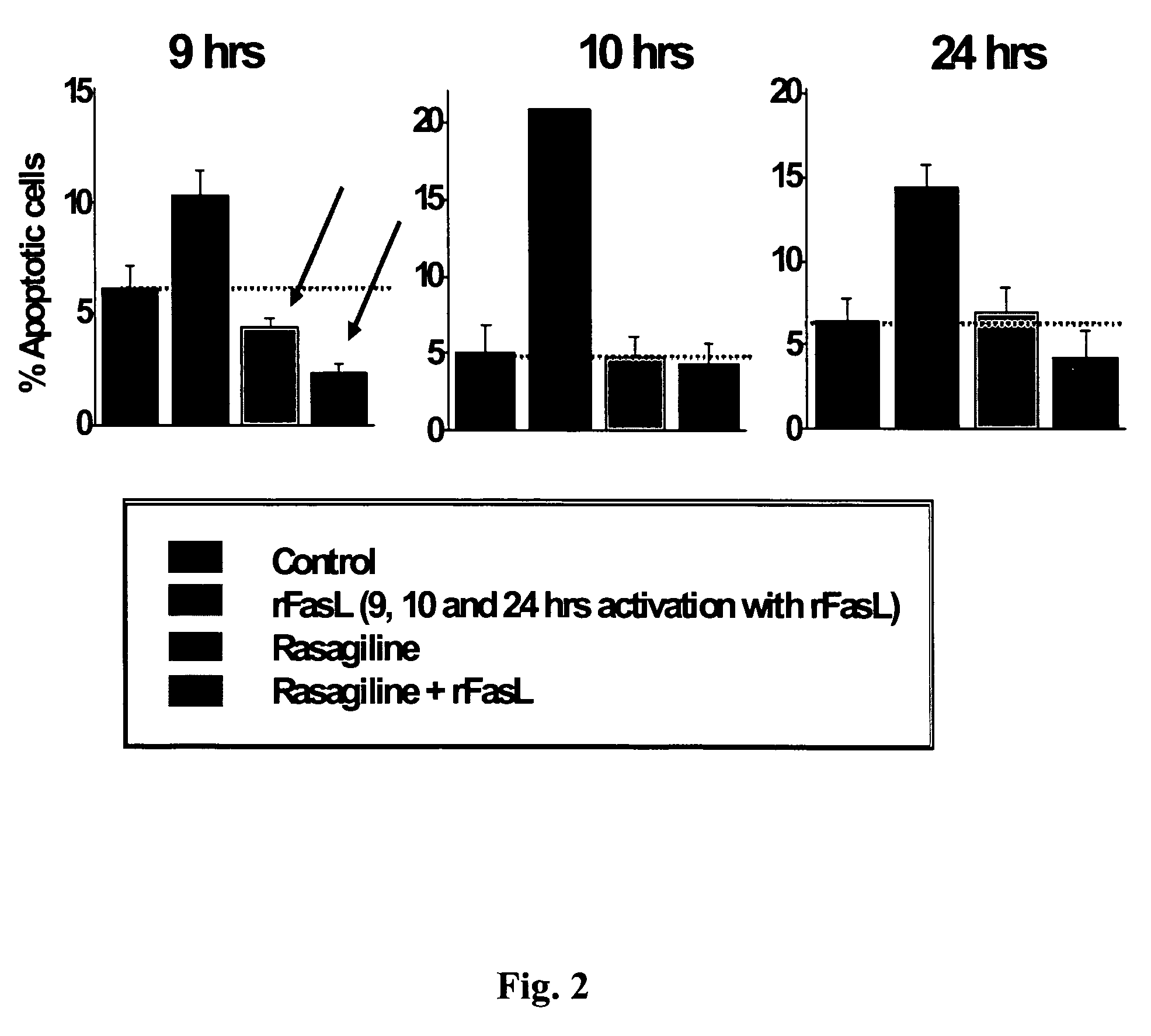
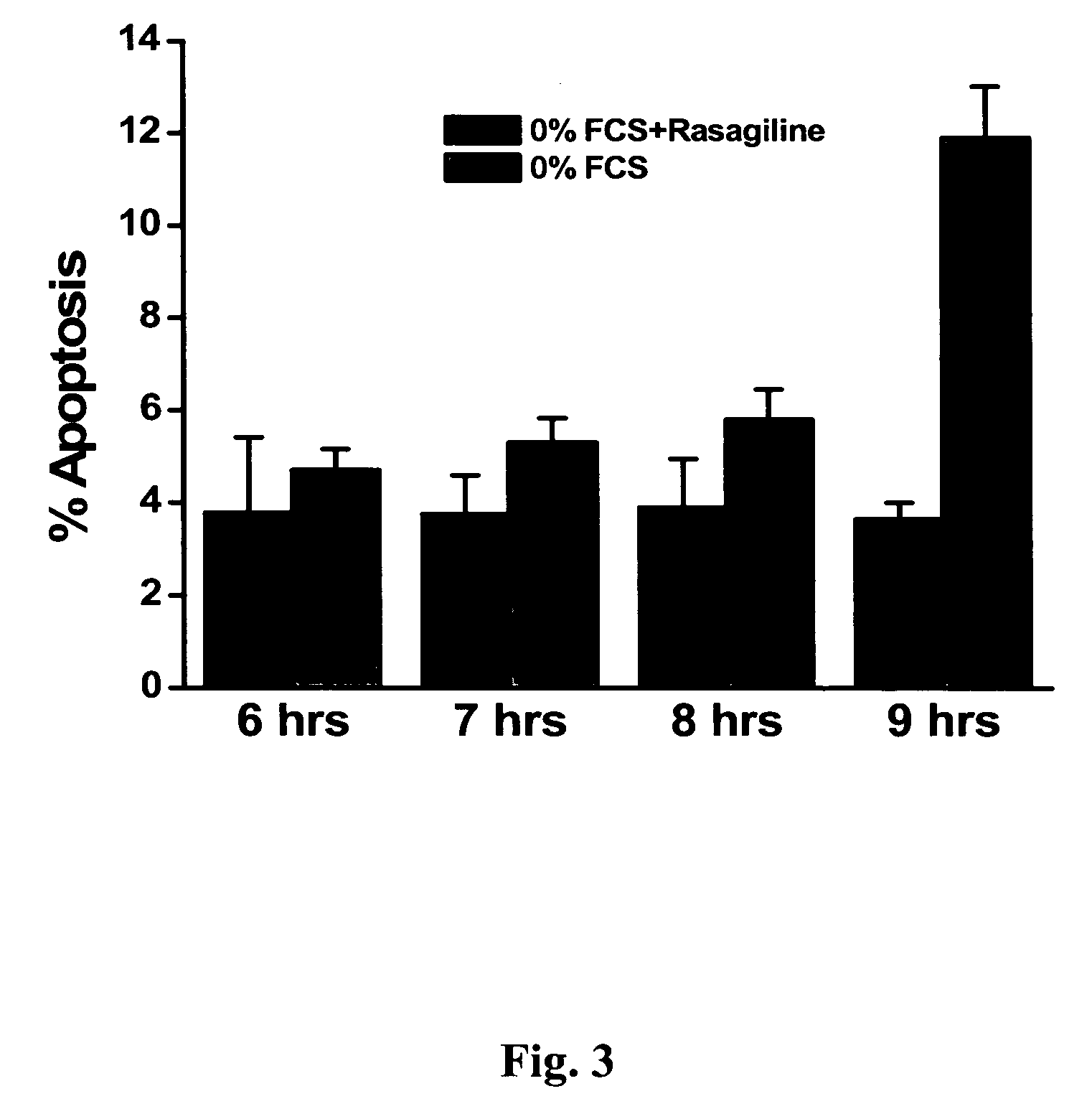
A Novel Neuroprotective and Neurorestorative N-Propargyl Caffeamide (PACA) and Use as A Treatment for Neurodegenerative Diseases
ActiveUS20180155275A1Nervous disorderPeptide/protein ingredientsN-propargylNeuro-degenerative disease
The present invention is directed to compounds, pharmaceutical compositions, and related methods of treatment and / or prevention for neurodegenerative diseases, such as Parkinson's disease.
Owner:THE UNIVERSITY OF HONG KONG
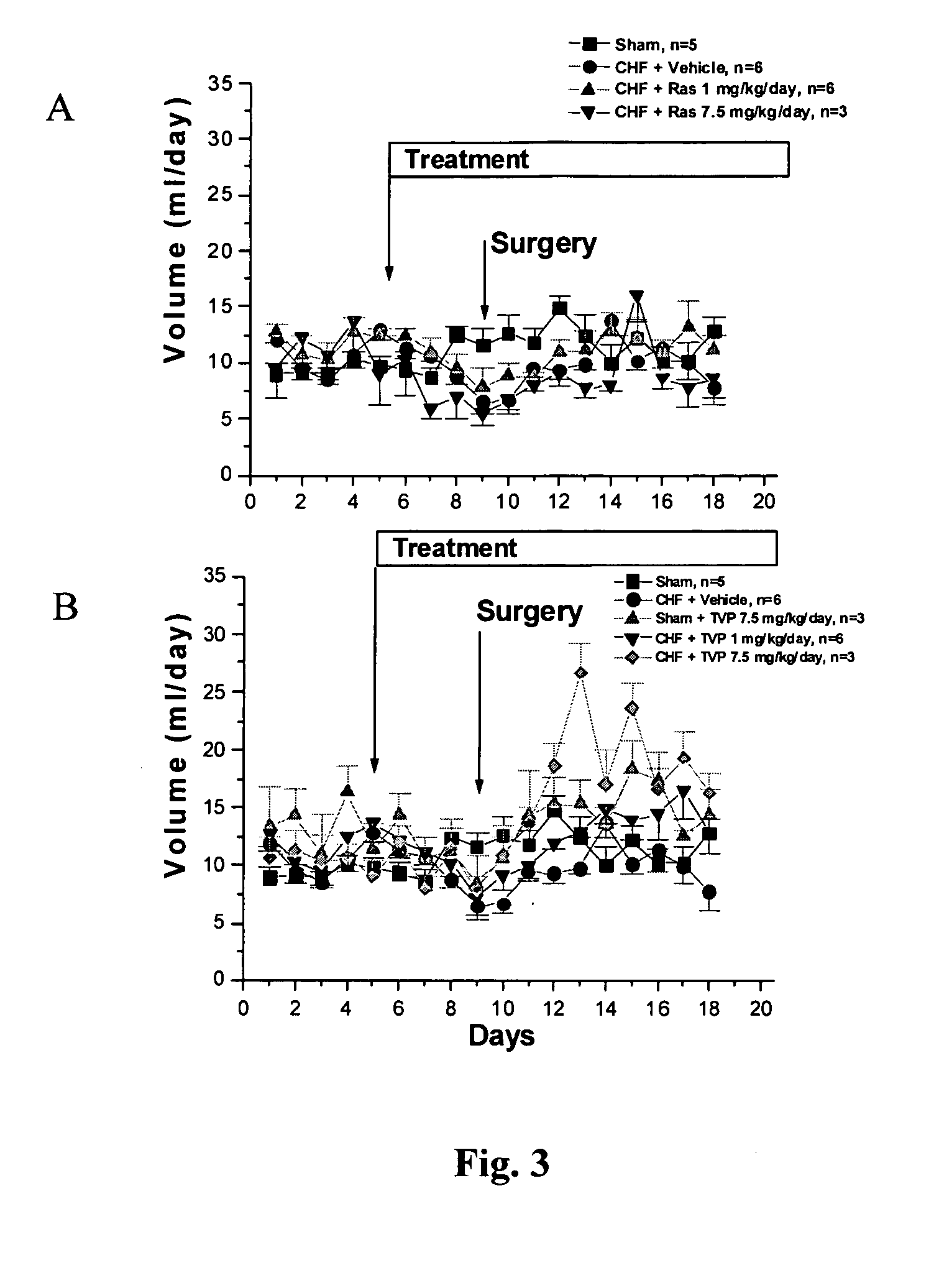
Compositions and methods for treatment of cardiovascular disorders and diseases
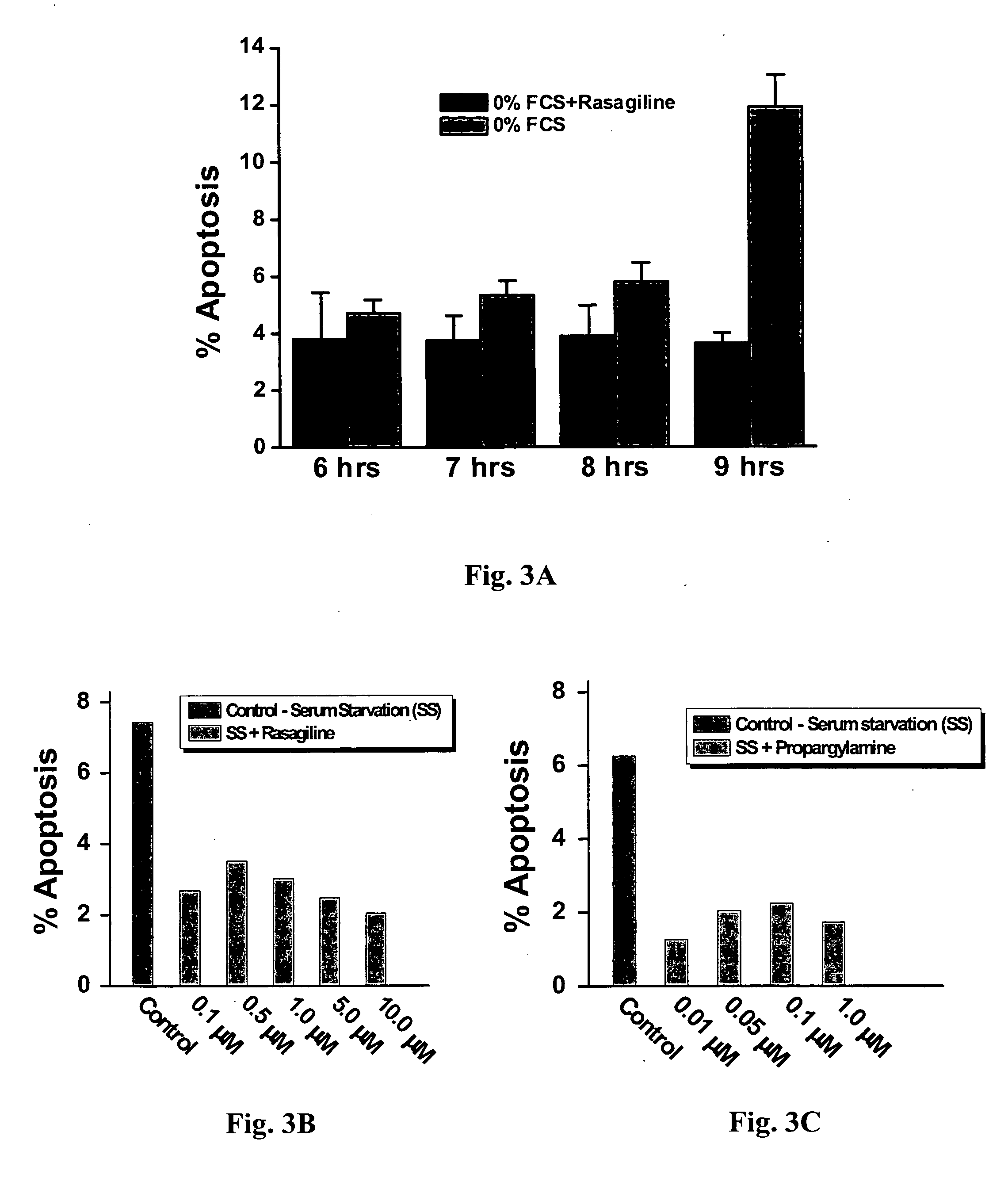
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or treatment of cardiovascular disorders and diseases.
Owner:TECHNION RES & DEV FOUND LTD +1
Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one
InactiveCN109232580AWide variety of sourcesEasy to separateOrganic chemistryAir atmosphereN-propargyl
The invention discloses a method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one. The method comprises the following steps: (1) mixing a N-propargyl indole compound, a gold catalyst, i.e., Me4tBuXPhosAuCl, NaBArF4 and 2,6-dibromo-N-oxypyridine in a solvent in an air atmosphere, and carrying out a reaction in oil bath; (2) cooling the temperature of a reaction mixture to room temperature after the oil-bath reaction in the step (1) is completed, and subjecting the reaction mixture to demineralization, washing, concentrating and purifying, thereby obtaining 1H-pyrrolo[1,2-a]indol-2(3-H)-one. The method disclosed by the invention is simple, and the obtained target compound is high in yield, narrow in melting point range and high in purity.
Owner:NANYANG NORMAL UNIV
Method for preventing or attenuating anthracycline-induced cardiotoxicity
InactiveUS20080090915A1Improve survivalReduce weightBiocideOrganic active ingredientsUltrasound attenuationN-propargyl
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan, enantiomers and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or attenuation of anthracycline-induced cardiotoxicity.
Owner:TECHNION RES & DEV FOUND LTD +1
Methods for treatment of cardiovascular disorders and diseases
ActiveUS20060287401A1Prevents hypertrophic increaseBiocideAmine active ingredientsN-propargyl1-aminoindan
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan, enantiomers and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or treatment of cardiovascular disorders, diseases and conditions.
Owner:TECHNION RES & DEV FOUND LTD +1
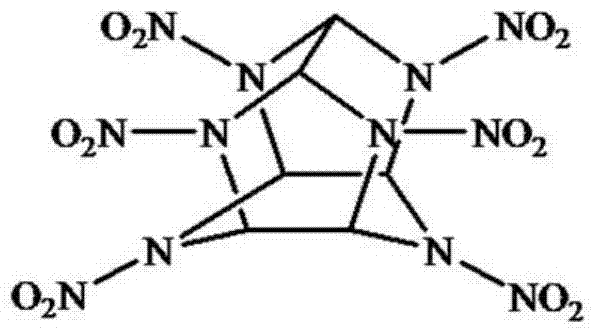
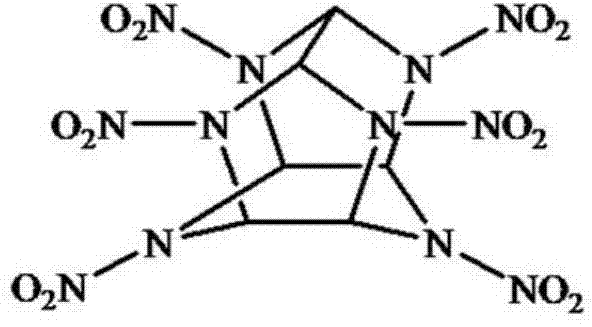
Fluorescent small-molecule probe with CL-20 sensing function as well as preparation method and application method
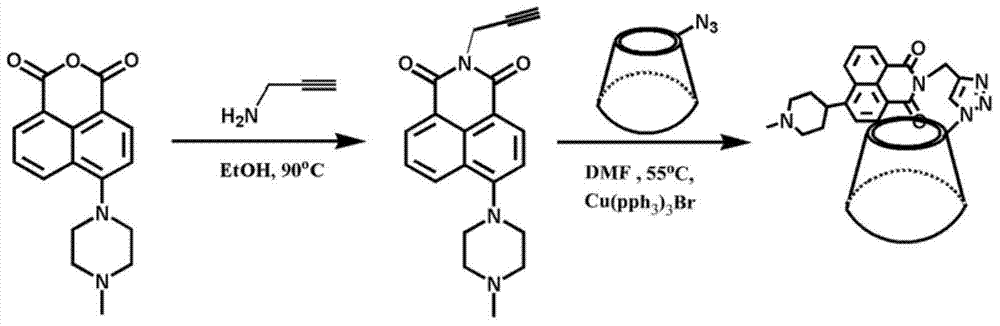
ActiveCN103923636AEasy to synthesizeEasy to detectFluorescence/phosphorescenceLuminescent compositionsQuantum yieldPropargyl
The invention discloses a fluorescent small-molecule probe with a CL-20 sensing function as well as a preparation method and an application method. The fluorescent small-molecule probe is 4-methyl piperazine-1,8-naphthalimide-gamma-cyclodextrin. The fluorescent small-molecule probe is prepared by firstly preparing paratoluensulfonyl-gamma-cyclodextrin with gamma-cyclodextrin and paratoluensulfonyl chloride, then preparing N3-gamma-cyclodextrin with paratoluensulfonyl-gamma-cyclodextrin and NaN3 and then reacting N3-gamma-cyclodextrin with N-propargyl-4-methyl piperazine-1,8-naphthalimide and Cu(PPh3)3Br. The fluorescent small-molecule probe is used for detecting CL-20. The small-molecule probe prepared by utilizing the 1,8-naphthalimide preparation material with higher fluorescence quantum yield has higher limit of detection and sensitivity. Click reaction can ensure the stability of the fluorescent material in water solutions, thus improving the detection sensitivity. The fluorescent small-molecule material provided by the invention has the characteristics of simplicity in synthesis and easiness in synthesis in quantity, thereby providing a technical support for eliminating deep potential terroristic threats.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
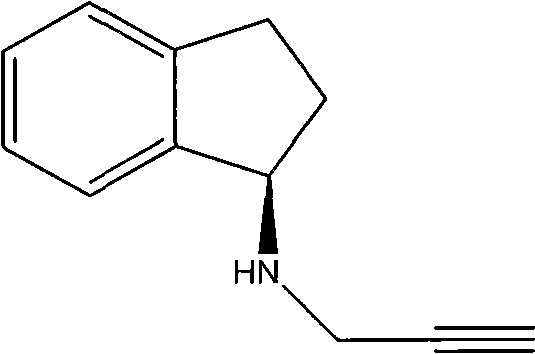
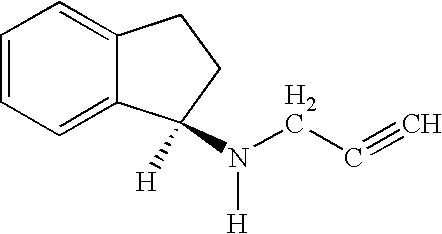
New salt forms of an aminoindan derivative
The present invention relates generally to novel salt forms of R-(+)-N-propargyl-1-aminoindan (i.e. rasagiline base), to a compound of formula Ia, to processes for their preparation and isolation, and to pharmaceutical compositions comprising the same.
Owner:MEDICHEM
Use of rasagilline for the treatment of restless legs syndrome
Methods for the treatment of Restless Legs Syndrome using R(+)-N-propargyl-1-aminoindan or a pharmaceutically acceptable salt thereof.
Owner:TEVA PHARMA IND LTD
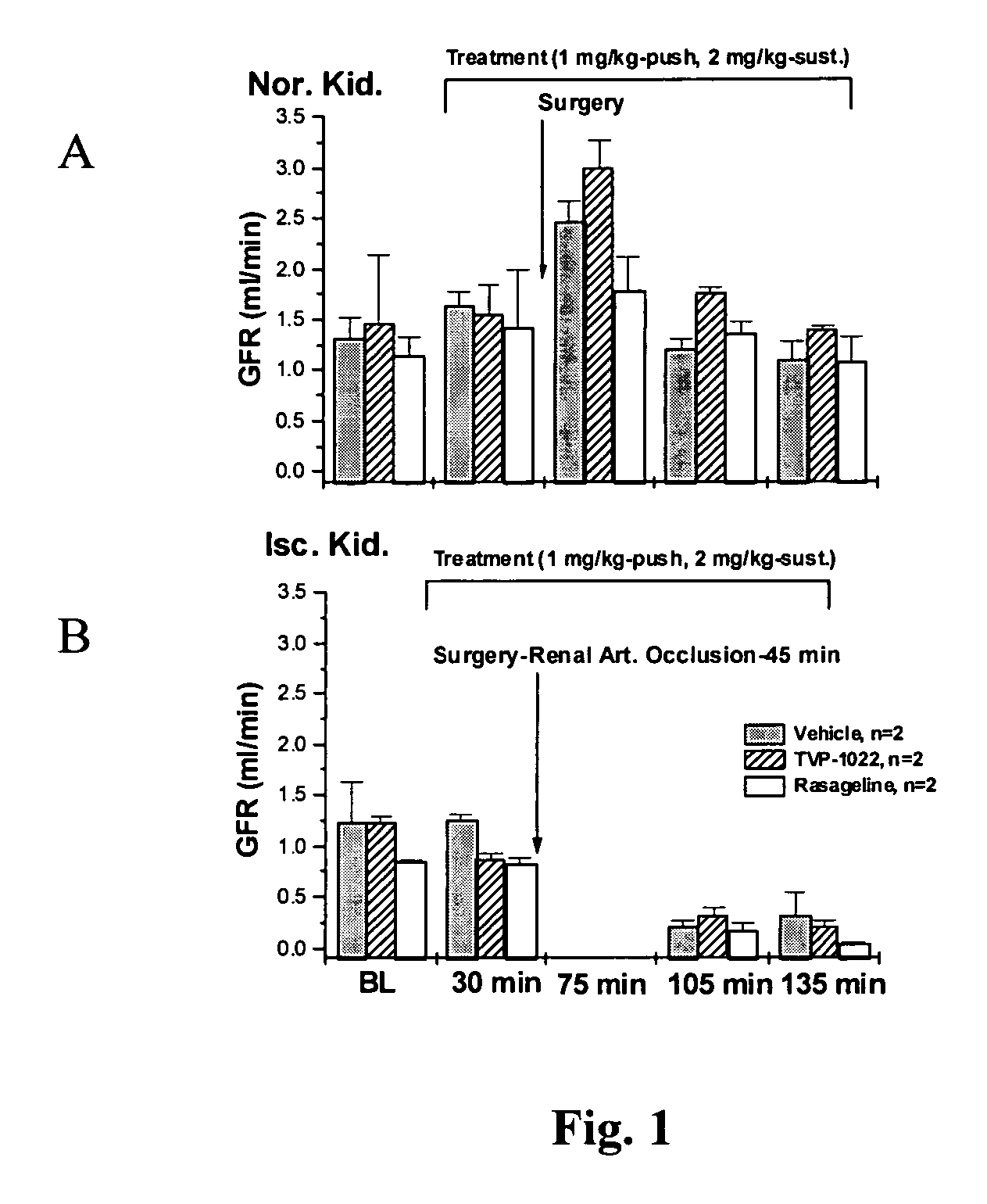
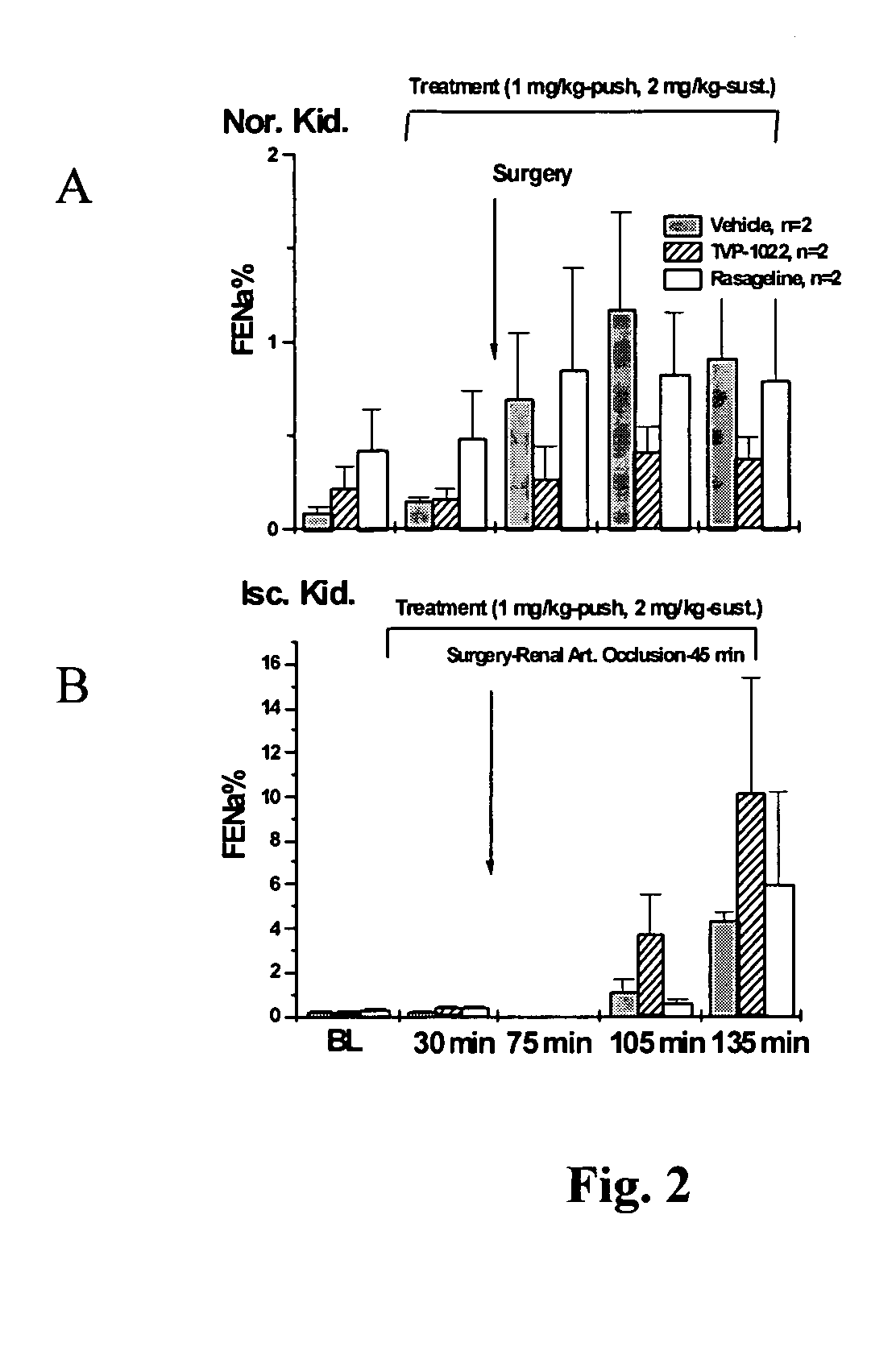
Methods for treatment of renal failure
InactiveUS20070082958A1Promising clinical valueLarge responseBiocideUrinary disorderN-propargylFAILURE KIDNEY
Propargylamine, propargylamine derivatives including N-propargyl-1-aminoindan and analogs thereof, and pharmaceutically acceptable salts thereof, are useful for prevention or treatment of renal failure.
Owner:TECHNION RES & DEV FOUND LTD +1
Method for preparing rasagiline
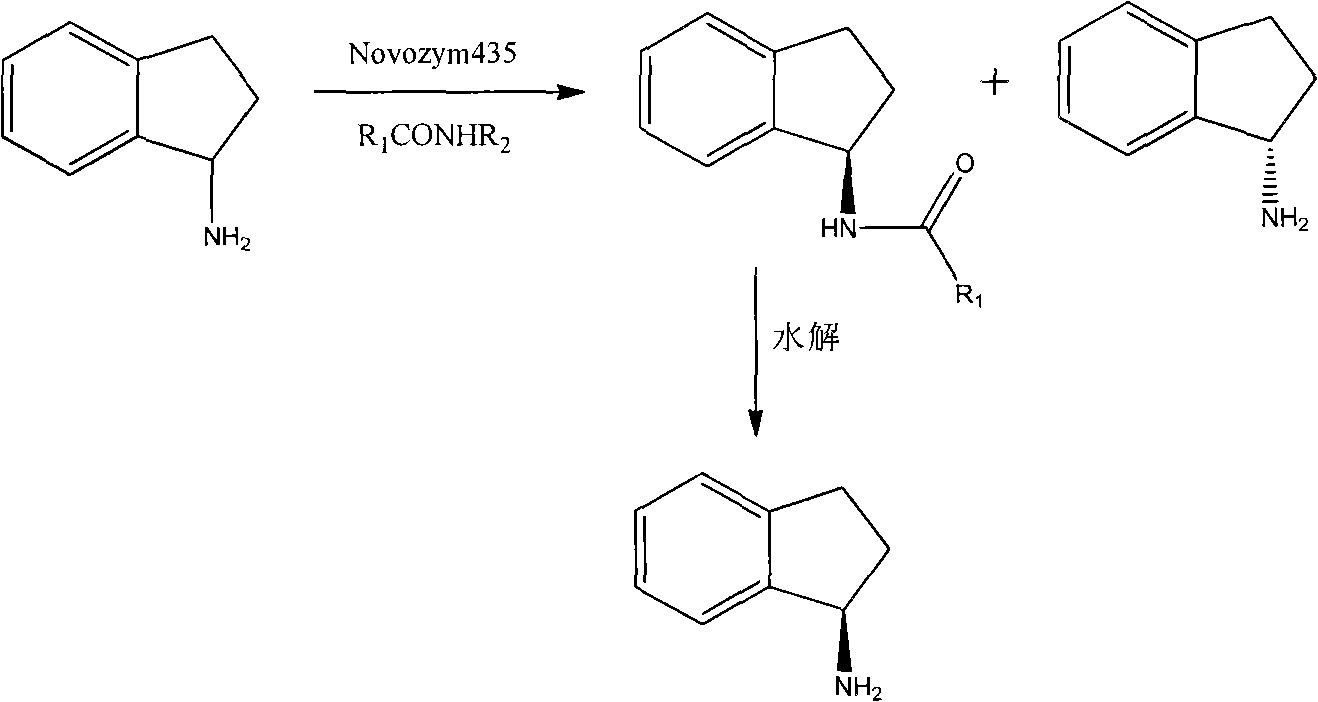
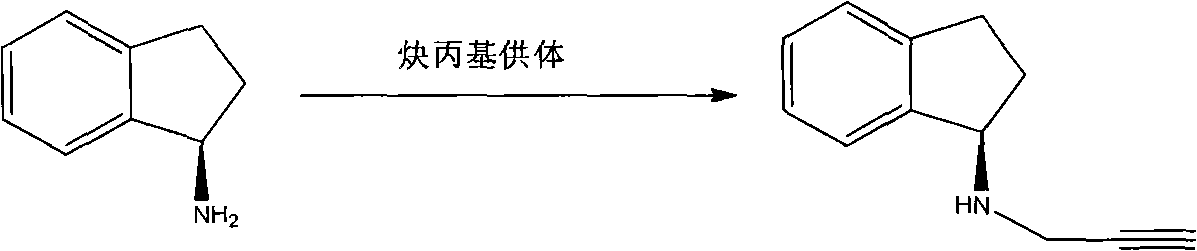
ActiveCN102154432AImprove thermal stabilityEasy to buyFermentationAmino preparation by functional substitutionN-propargylHydrolysis
The invention provides a method for preparing a compound rasagiline [(R)-N-propargyl-1-indanamine]. In the method, the rasagiline is prepared from (R,S)-1-indanamine serving as a raw material through enzyme-catalyzed asymmetric acylation reaction, hydrolysis and N propargylation reaction. The method for preparing the rasagiline provided by the invention has the advantages of readily available reaction raw material, high product yield and high optical purity and is suitable for industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
Synthesis method of azepine anthraquinone
InactiveCN101712648BAchieve synthesisOvercome limitationsOrganic chemistryPlatinum saltsIsomerization
Owner:NANJING UNIV
Fluorescent small-molecule probe with CL-20 sensing function as well as preparation method and application method
ActiveCN103923636BEasy to synthesizeEasy to detectFluorescence/phosphorescenceLuminescent compositionsQuantum yieldPropargyl
The invention discloses a fluorescent small-molecule probe with a CL-20 sensing function as well as a preparation method and an application method. The fluorescent small-molecule probe is 4-methyl piperazine-1,8-naphthalimide-gamma-cyclodextrin. The fluorescent small-molecule probe is prepared by firstly preparing paratoluensulfonyl-gamma-cyclodextrin with gamma-cyclodextrin and paratoluensulfonyl chloride, then preparing N3-gamma-cyclodextrin with paratoluensulfonyl-gamma-cyclodextrin and NaN3 and then reacting N3-gamma-cyclodextrin with N-propargyl-4-methyl piperazine-1,8-naphthalimide and Cu(PPh3)3Br. The fluorescent small-molecule probe is used for detecting CL-20. The small-molecule probe prepared by utilizing the 1,8-naphthalimide preparation material with higher fluorescence quantum yield has higher limit of detection and sensitivity. Click reaction can ensure the stability of the fluorescent material in water solutions, thus improving the detection sensitivity. The fluorescent small-molecule material provided by the invention has the characteristics of simplicity in synthesis and easiness in synthesis in quantity, thereby providing a technical support for eliminating deep potential terroristic threats.
Owner:INST OF CHEM MATERIAL CHINA ACADEMY OF ENG PHYSICS
Pharmaceutical compositions comprising S-(-)-N-Propargyl-1-amino indan
Pharmaceutical compositions for the treatment of a neurological disorder of neurotrauma or for improving memory in a patient comprising a therapeutically effective amount of S-(-)-N-proparygl-1-aminoindan or a pharmaceutically acceptable salt thereof as active ingredient, and a pharmaceutically active carrier. The pharmaceutical compositions are adapted, in particular for treating a neurological hypoxia or anoxia, neurodegenerative diseases. Parkinson's Disease, Alzheimer's Disease, neurotoxic injury, head trauma injury, spinal trauma injury or any other form of nerve damage.
Owner:TEVA PHARMA IND LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



![Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one](https://images-eureka.patsnap.com/patent_img/1a0a5c72-1373-46fb-9f10-6d003e33d7f8/BDA0001865350810000011.png)
![Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one](https://images-eureka.patsnap.com/patent_img/1a0a5c72-1373-46fb-9f10-6d003e33d7f8/BDA0001865350810000012.png)
![Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one Method for synthesizing 1H-pyrrolo[1,2-a]indol-2(3-H)-one](https://images-eureka.patsnap.com/patent_img/1a0a5c72-1373-46fb-9f10-6d003e33d7f8/BDA0001865350810000013.png)