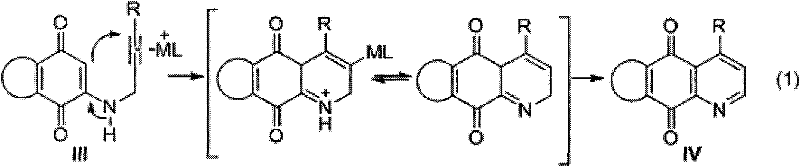
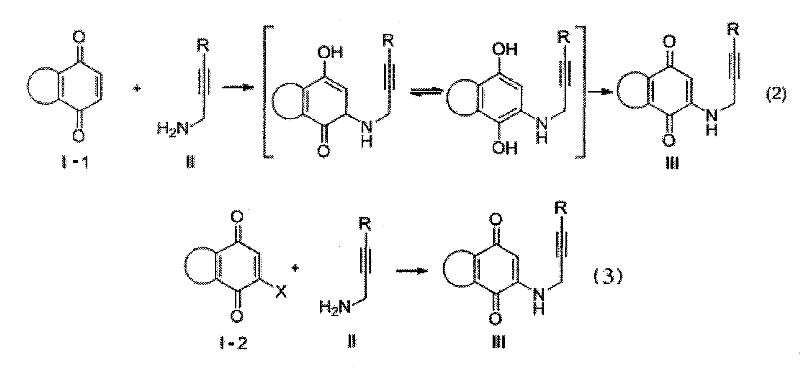
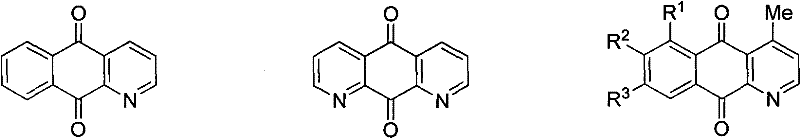
Synthesis method of azepine anthraquinone
A synthetic method and the technology of azaanthrene, which are applied in the field of synthesis of azaanthraquinone, can solve the problems of reduced yield of target product, harsh reaction conditions, difficult separation, etc., and achieve antibacterial activity and cytotoxicity, mild reaction conditions, The effect of simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of 4-(4-methylphenyl)-benzo[g]quinoline-5,10-dione (IV a)
[0029] 1, 2-[3-(4-methylphenyl)-2-propynylamino]-1, the synthesis of 4-naphthoquinone (III a)
[0030] 1,4-naphthoquinone (5.0mmol), 3-(4-methylphenyl)-2-propynylamine (6.0mmol) and 10mL absolute ethanol were contained in a single-necked bottle, and NaAuCl was added under stirring 4 2H 2 O (0.05 mmol). React at room temperature for 4 hours. The reaction mixture was filtered to obtain a reddish brown solid. Then recrystallized from ethanol to obtain 2-[3-(4-methylphenyl)-2-propynylamino]-1,4-naphthoquinone. Product mp 188-190°C; 1 H NMR (300MHz, CDCl 3 )δ8.08(dd, J 1 =16.5Hz,J 2 =7.8Hz, 2H), 7.73(t, J=7.5Hz, 1H), 7.63(t, J=7.5Hz, 1H), 7.32(d, J=8.1Hz, 2H), 7.11(d, J=7.5 Hz, 2H), 6.12 (br, s, 1H), 5.90 (s, 1H), 4.20 (d, J = 5.4 Hz, 2H), 2.34 (s, 3H).
[0031] 2. Synthesis of 4-(4-methylphenyl)-benzo[g]quinoline-5,10-dione (IV a)
[0032] Triphenylphosphogold(I) chloride (14.9mg, 0....
Embodiment 2
[0033] Example 2: Synthesis of 4-(4-methoxyphenyl)-benzo[g]quinoline-5,10-dione (IV b)
[0034] 1, 2-[3-(4-methoxyphenyl)-2-propynylamino]-1, the synthesis of 4-naphthoquinone (III b)
[0035] Obtained from 1,4-naphthoquinone (5.0mmol), 3-(4-methoxyphenyl)-2-propynylamine (6.0mmol) through oxidation-amination reaction, reaction conditions and purification steps in Example 1 2-[3-(4-Methylphenyl)-2-propynylamino]-1,4-naphthoquinone (III a). Product mp172-175℃; 1 H NMR (300MHz, CDCl 3 )δ8.10(d, J=7.5Hz, 1H), 8.05(d, J=7.5Hz, 1H), 7.72(t, J=7.5Hz, 1H), 7.61(t, J=7.2Hz, 1H) , 7.36(d, J=8.7Hz, 2H), 6.82(d, J=9.0Hz, 2H), 6.10(br s, 1H), 5.89(s, 1H), 4.18(d, J=5.7Hz, 2H ), 3.79(s, 3H).
[0036] 2. Synthesis of 4-(4-methoxyphenyl)-benzo[g]quinoline-5,10-dione (IV b)
[0037] The reaction conditions and purification steps are as in Example 1 for 4-(4-methylphenyl)-benzo[g]quinoline-5,10-dione (IV a). Product mp 175-177°C; 1 H NMR (300MHz, CDCl 3 )δ9.00(d, J=4.8Hz, 1H), 8.38-8....
Embodiment 3
[0038] Example 3: Synthesis of 4-(4-nitrophenyl)-benzo[g]quinoline-5,10-dione (IV c)
[0039] 1. Synthesis of 2-[3-(4-nitrophenyl)-2-propynylamino]-1,4-naphthoquinone (III c)
[0040] Obtained from 1,4-naphthoquinone (5.0mmol), 3-(4-nitrophenyl)-2-propynylamine (6.0mmol) through oxidation-amination reaction, the reaction conditions and purification steps are as in Example 1 2-[3-(4-Methylphenyl)-2-propynylamino]-1,4-naphthoquinone (III a). Product mp228-230℃; 1 H NMR (300MHz, CDCl 3 )δ8.21-8.18 (m, 2H), 8.01-7.94 (m, 2H), 7.84 (td, J 1 =7.5Hz,J 2 =1.5Hz, 1H), 7.75(td, J 1 =7.5Hz,J 2 =1.5Hz, 1H), 7.70-7.66(m, 2H), 5.89(s, 1H), 4.38(d, J=5.7Hz, 2H).
[0041] 2. Synthesis of 4-(4-nitrophenyl)-benzo[g]quinoline-5,10-dione (IV c)
[0042] The reaction conditions and purification steps are as in Example 1 for 4-(4-methylphenyl)-benzo[g]quinoline-5,10-dione (IV a). Product mp 270-272°C; 1 H NMR (300MHz, CDCl 3 )δ9.15(d, J=4.8Hz, 1H), 8.42-8.35(m, 3H), 8.11(d, J=6.9Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com