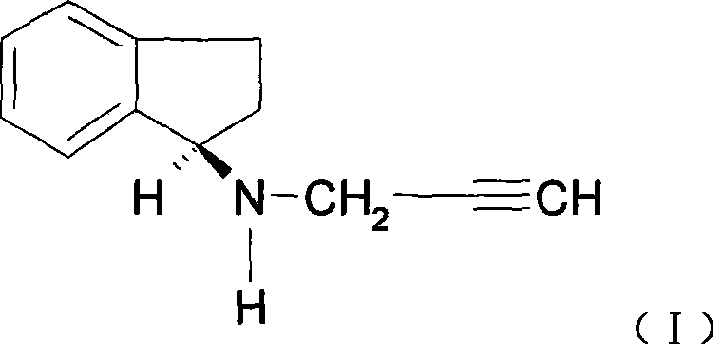
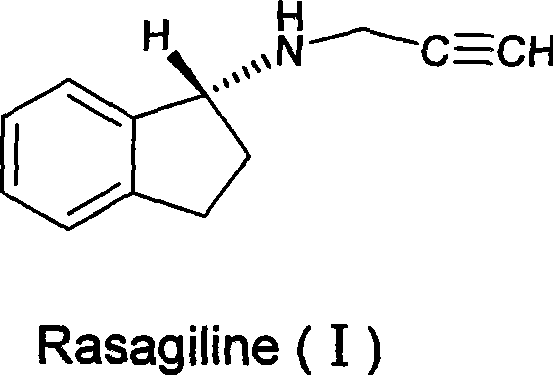
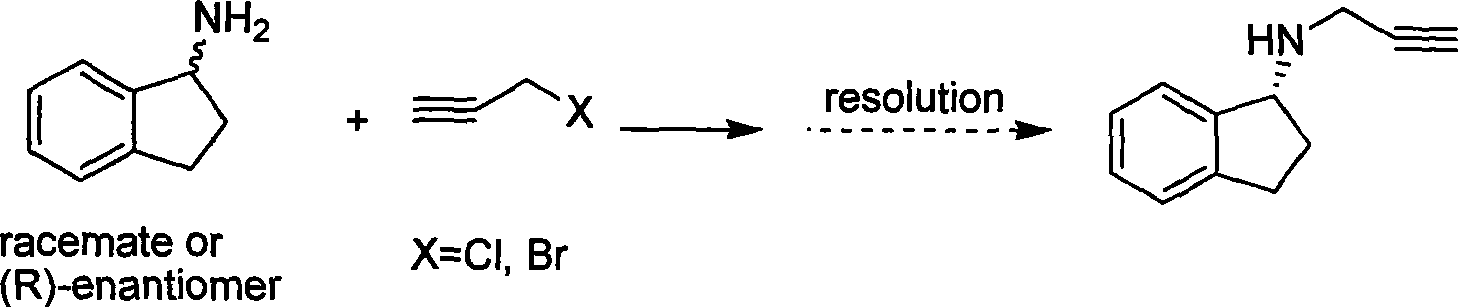
Preparation method of (R)-(+)-N-propargyl-1-indan amines
A technology of propargyl and indene amine, which is applied in the new preparation field of (R)-(+)-N-propargyl-1-indene amine, can solve the problem of different properties of 1-chloroindene or 1-bromoindene. Stability, not easy to industrialize production, serious side reaction of imine, etc
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of compound (R)-N-(2-nitro)benzenesulfonyl-1-indenamine (III):
[0034] (R)-1-Indenamine 0.61g (4.59mmol) was dissolved in 10mL of dichloromethane, triethylamine 0.76mL (5.51mmol) was added, and o-nitrobenzenesulfonyl chloride 1.12g (5.05mmol) was added dropwise under ice cooling Dichloromethane (20mL) solution, reacted at 0°C for 4 hours, added 30mL of water, separated the dichloromethane layer, washed with saturated NaCl 30mL×2, dried over anhydrous sodium sulfate, evaporated the solvent under reduced pressure to obtain a white solid (III) 1.39 g, yield: 95%.
[0035] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 8.25 (m, 1H), 7.90 (m, 1H), 7.78 (m, 2H), 7.24 (m, 2H), 7.17 (m, 2H), 5.58 (d, J=8.4Hz, 1H , NH), 5.03(m, 1H, NCH), 2.95(m, 1H), 2.79(m, 1H), 2.35(m, 1H), 1.85(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ (ppm) 142.8, 141.2, 133.6, 132.9, 130.8, 128.5, 127.0, 125.4, 124.9, 124.0, 59.6, 34.3, 30.0; ESI-MS, m / z: 318.4 [M] + , 336.1[M+H 2 O] + , 450.2[M+MeOH] ...
Embodiment 2
[0037] Preparation of compound (R)-N-propargyl-N-(2-nitro)benzenesulfonyl-1-indenamine (IV):
[0038] Dissolve 318 mg (1 mmol) of the above-mentioned white solid (III) in 10 mL of toluene, add 2.5 mL of aqueous sodium hydroxide solution (1 M) and a catalytic amount of tetrabutylammonium bromide (TBAB), then add 0.18 mL of propargyl bromide dropwise ( 2mmol) diluent of toluene (2mL), reacted at 70°C overnight, added 20mL of water and 20mL of ethyl acetate to the reaction solution, separated the organic layer, washed with saturated NaCl 30mL×2, dried over anhydrous sodium sulfate, evaporated under reduced pressure The solvent was removed, and the obtained oil was recrystallized from petroleum ether / ethyl acetate to obtain 287 mg of light yellow solid (IV), yield: 81%.
[0039] 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 8.24 (d, J = 4.8Hz, 1H), 7.72 (m, 3H), 7.25 (m, 2H), 7.17 (m, 2H), 5.62 (t, J = 8.0Hz, 1H, NCH ), 4.23 (d, J=18.4Hz, 1H, NCH 2 ), 3.62 (d, J=18.8Hz, 1H, NCH 2 ), 3.07...
Embodiment 3
[0041] The preparation of compound (R)-N-propargyl-1-indenamine (rasagiline) (I):
[0042] Dissolve 202 mg (0.57 mmol) of the above light yellow solid in 2 mL N,N-dimethylformamide, add 144 mg (3.43 mmol) of lithium hydroxide monohydrate and 60 μL (0.68 mmol) of mercaptopropionic acid under ice-cooling, and react at 0°C for 3 After 1 hour, 10 mL of water and 20 mL of ethyl acetate were added to the reaction solution, and the organic layer was separated, washed successively with 10 mL of water × 3, and 20 mL of saturated NaCl × 2, dried over anhydrous sodium sulfate, and evaporated to remove the solvent under reduced pressure to obtain a yellow oil (I). 94 mg, yield: 96%.
[0043] 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 7.34 (d, J = 7.2Hz, 1H, ArH), 7.21 (m, 3H, ArH), 4.40 (t, J = 6.4Hz, 1H, NCH), 3.51 (t, J = 2.8Hz , 2H, NCH 2 ), 3.03(m, 1H), 2.82(m, 1H), 2.39(m, 1H), 2.25(t, J=2.4Hz, 1H, C≡C-H), 1.85(m, 1H); 13 C NMR (100MHz, CDCl 3 )δ (ppm) 144.4, 143.7, 127.5, 126.1, 124.8,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com