Method for producing 6 alpha-methylprednisolone and its derivatives
A technology for producing methylprednisolone and its production method, which is applied in the direction of chemical instruments and methods, preparation of steroids, steroids, etc., can solve the problems of low yield, complex production process, cumbersome steps, etc., and achieve high yield , good reaction specificity and less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
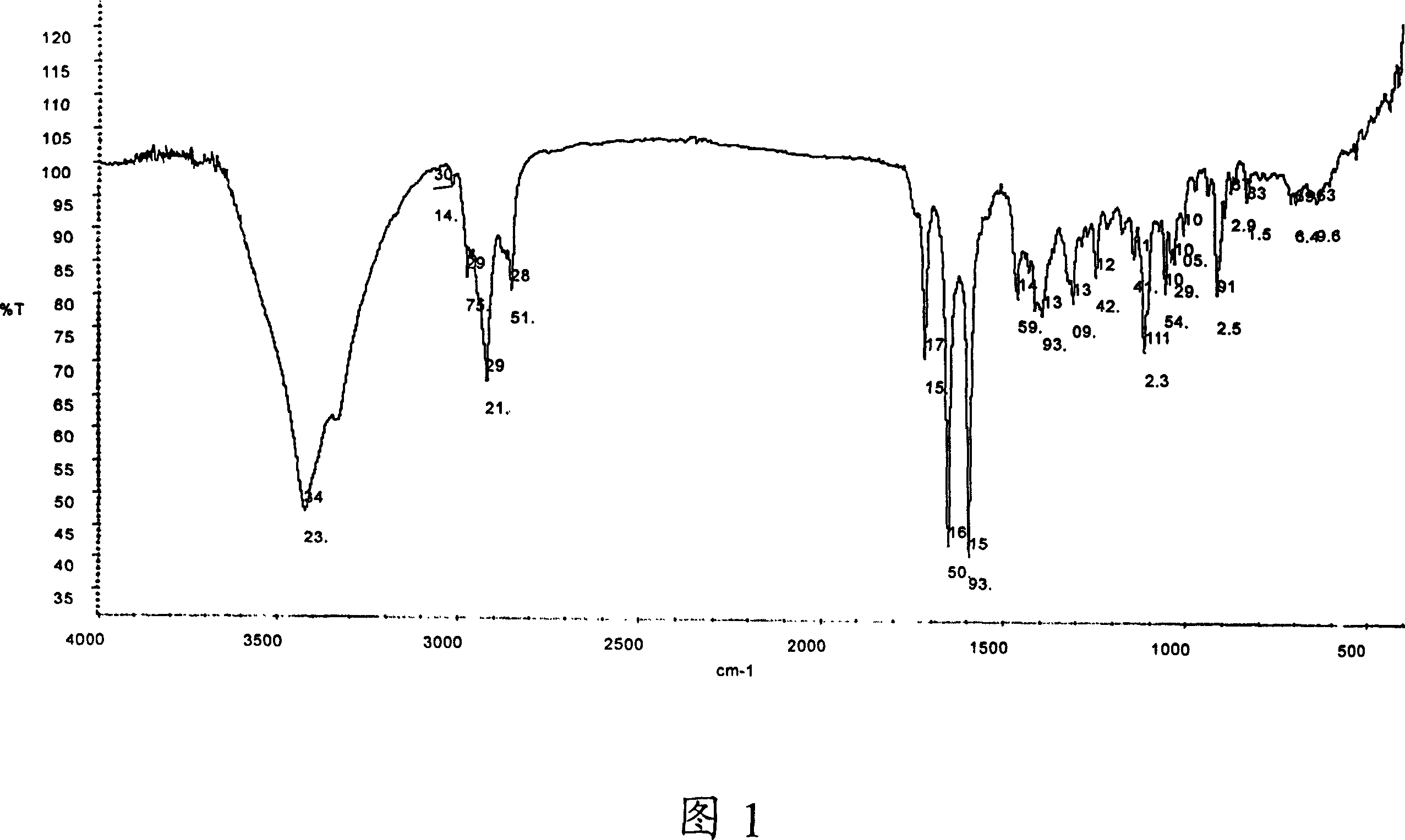
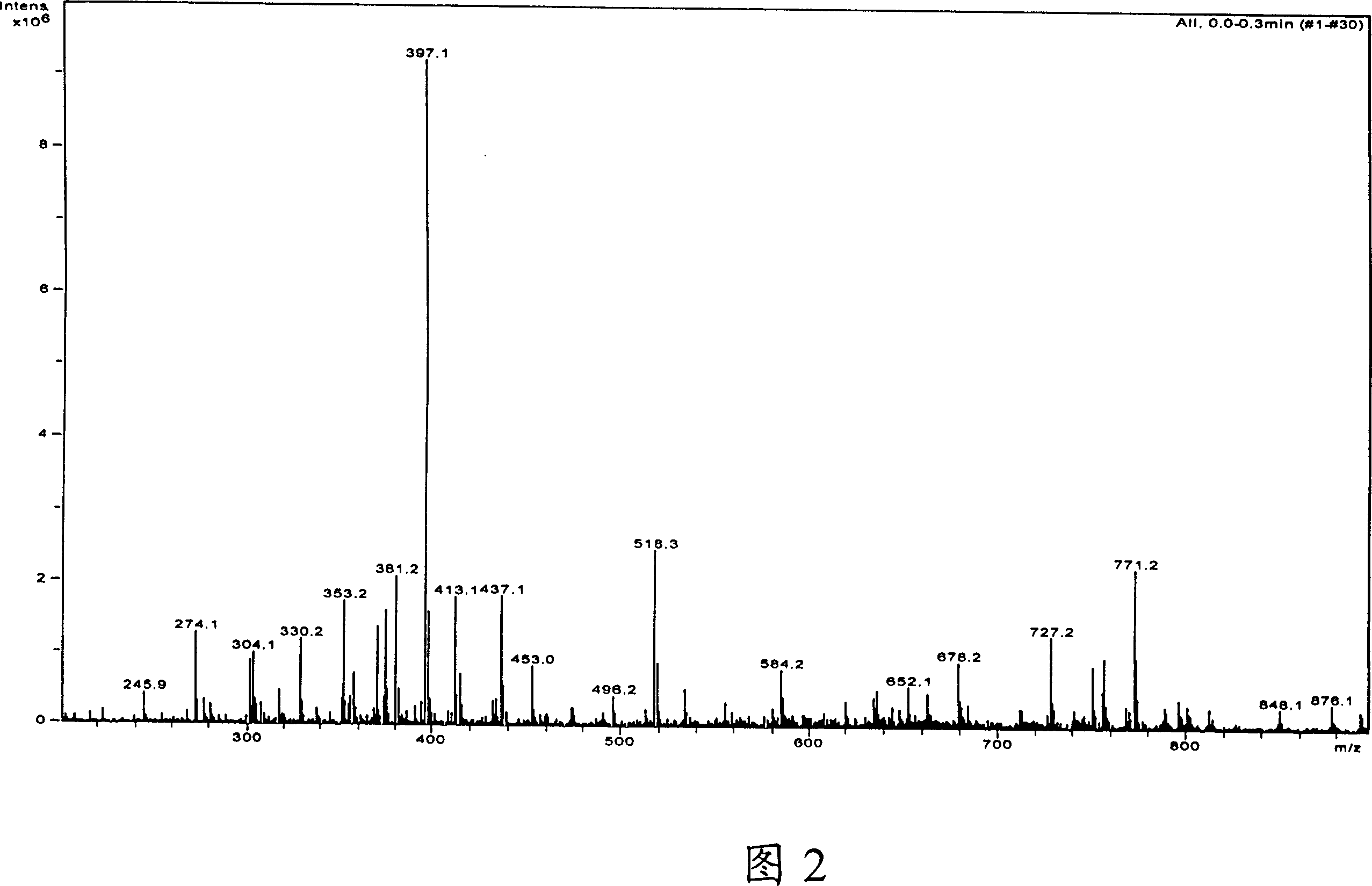
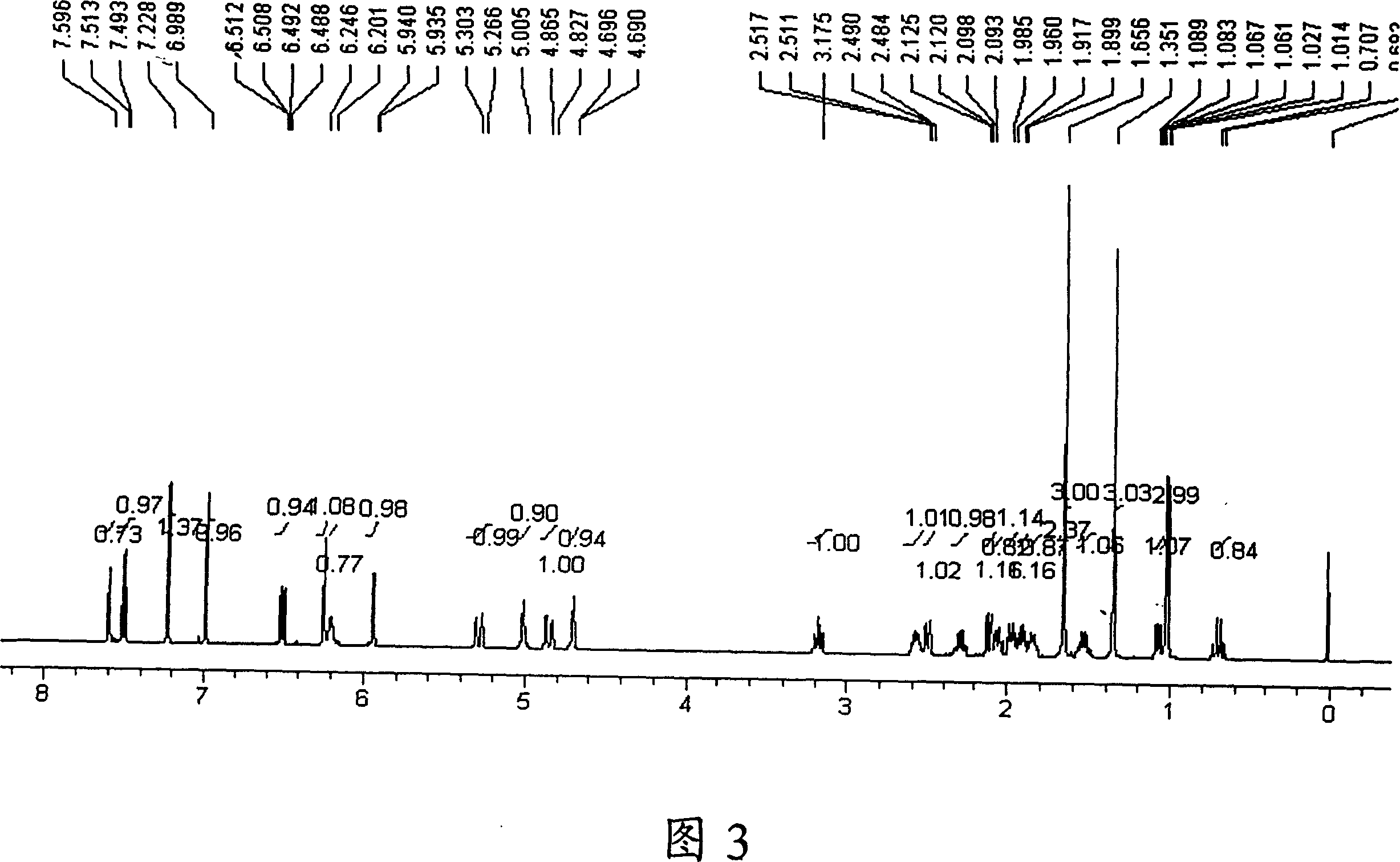
Image
Examples
Embodiment 1-1
[0060] (1) Add 4 g of cortisone acetate shown in formula II to 40 ml of tetrahydrofuran, add 2 g of triethyl orthoformate, 3 ml of absolute ethanol, and 0.04 g of p-toluenesulfonic acid after dissolving, and react at a temperature of 25° C. for 1 hour. The compound represented by formula III can be obtained.
[0061] (2) Add 1ml of N-methylaniline and 1ml of formaldehyde to the above product, react at 20°C for 1 hour, add 400ml of water, react at 20°C for 1 hour, cool to 8°C and keep it for 2 hours, filter and dry to obtain Compound shown in formula VI. The compound shown in formula VI is dissolved with 20ml of dimethyl sulfoxide, and 0.4g of 7.5%Pd / CaCO is added 3 , 4ml of cyclohexene, reacted at 20°C for 2 hours, then concentrated under reduced pressure at a temperature of 0°C and vacuum ≥ 0.085atm to remove the solvent, cooled to 40°C, and added dropwise 12ml of 37% hydrochloric acid under stirring , keep warm for 0.5 hour, slowly adjust the pH to 6, add 10 times of water...
Embodiment 1-2
[0066] (1) Add 4 g of cortisone acetate shown in formula II in 48 ml of ether, add 3.2 g of triethyl orthoformate, 3 ml of absolute ethanol, 0.08 g of sulfuric acid after dissolving, and react at a temperature of 30° C. for 1 hour. The compound represented by formula III can be obtained.
[0067] (2) Add 1.2ml of N-methylaniline and 1.2ml of acetaldehyde to the above product, react at 35°C for 1.5 hours, add 60ml of water, react at 30°C for 1 hour, cool to 9°C for 2 hours, filter and dry , to obtain the compound shown in formula VI. The compound shown in formula VI is dissolved with 28ml of dimethyl sulfoxide, and 0.6g7.5%Pd / CaCO is added 3 , 4ml of cyclohexene, reacted at 100°C for 3 hours, then concentrated under reduced pressure at a temperature of 60°C and vacuum ≥ 0.085atm to remove the solvent, cooled to 45°C, and added dropwise 16ml of 37% hydrochloric acid with stirring , keep warm for 0.75 hours, slowly adjust the pH to 7, add 15 times of water and stir for 2 hours,...
Embodiment 1-3
[0072] (1) Add 4g of cortisone acetate shown in formula II to 60ml of ethyl propyl ether, add 4g of triethyl orthoformate, 3ml of absolute ethanol, 0.12g of perchloric acid after dissolving, and react at a temperature of 40°C for 1 hour . The compound represented by formula III can be obtained.
[0073] (2) Add 1.6ml of N-1-methylaniline and 1.6ml of propionaldehyde to the above product, react at 46°C for 2 hours, add 80ml of water, react at 35°C for 1 hour, cool to 10°C for 2 hours, filter, drying to obtain the compound shown in formula VI. The compound shown in formula VI is dissolved with 40ml of dimethyl sulfoxide, and 0.8g7.5%Pd / CaCO is added 3 , 4ml of cyclohexene, reacted at 180°C for 4 hours, then concentrated under reduced pressure at a temperature of 120°C and vacuum ≥ 0.085atm to remove the solvent, cooled to 50°C, and added dropwise 20ml of 37% hydrochloric acid under stirring , keep warm for 1 hour, slowly adjust the pH to 8, add 20 times of water and stir for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com