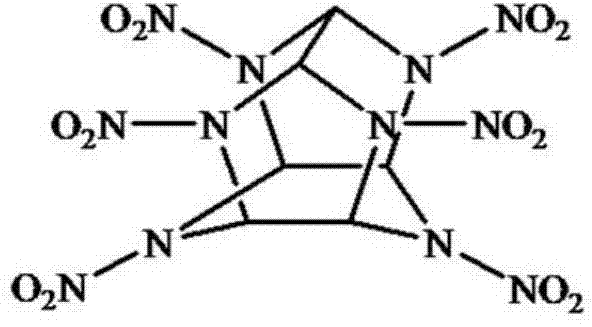
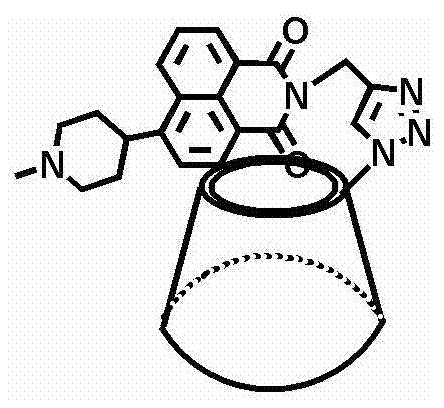
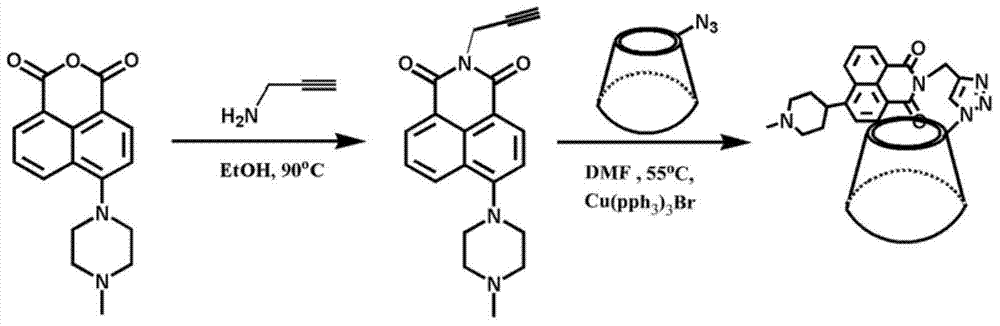
Fluorescent small-molecule probe with CL-20 sensing function as well as preparation method and application method
A small molecule probe, CL-20 technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of infeasible detection of Cl-20, destroying the driving force of Cl-20, etc., and achieve the elimination of terror Threat, high detection limit and sensitivity, effect of increased sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of intermediate p-toluenesulfonyl-γ-cyclodextrin
[0031] Disperse γ-cyclodextrin (12.0 g, 6.4 mmol) in 100 mL of water, and slowly add 1 mL of aqueous sodium hydroxide solution (8.2 mol / L.) in an ice-water bath to completely dissolve the γ-cyclodextrin. At this time, p-toluenesulfonyl chloride (1.748g, 9.2mmol) was dissolved in 4mL of acetonitrile solution and slowly added dropwise to the above-mentioned solution in which γ-cyclodextrin was dissolved, and a white precipitate was formed during the dropwise addition. After the mixture was reacted at room temperature for 4 h, the pH value was adjusted to 5.8-6.2 with 3 mol / L hydrochloric acid solution, filtered, and recrystallized in water to obtain the target product.
[0032] (2) Using the intermediate p-toluenesulfonyl-γ-cyclodextrin to prepare N 3 -γ-cyclodextrin (6γCDN 3 )
[0033] p-toluenesulfonyl-γ-cyclodextrin (1.73g, 1.34mmol), NaN 3 (174mg, 2.68mmol) was dissolved in 10mL DMF, under the prot...
Embodiment 2
[0045] Disperse 12.8 mmol of γ-cyclodextrin in 180 mL of water, and slowly add 2 mL of 8.5 mol / L aqueous sodium hydroxide solution dropwise in an ice-water bath to completely dissolve the γ-cyclodextrin. Dissolve 19.2mmol of p-toluenesulfonyl chloride in 9mL of acetonitrile solution. The concentration of the acetonitrile solution of p-toluenesulfonyl chloride is not strictly controlled here, but the concentration when the p-toluenesulfonyl chloride is just completely dissolved in the acetonitrile solution is appropriate. The acetonitrile solution of p-toluenesulfonyl chloride was slowly added dropwise to the above mixed solution in which γ-cyclodextrin was dissolved, and a white precipitate was produced during the dropwise addition. After the mixture was reacted at room temperature for 3.5 h, the pH value was adjusted to 5.8-6.2 with 3.5 mol / L hydrochloric acid solution, filtered, and recrystallized in water to obtain p-toluenesulfonyl-γ-cyclodextrin.
[0046] 1.32mmol p-tolue...
Embodiment 3
[0052] Using the same preparation method as in Example 1 or Example 2, γ-cyclodextrin is 12.8mmol, 8mol / L sodium hydroxide aqueous solution 2mL, 17.92mmol p-toluenesulfonyl chloride is dissolved in 9mL acetonitrile solution and the mixture is prepared at room temperature After reacting for 4.5 hours, adjust the pH value to 5.8-6.2 with 2.5 mol / L hydrochloric acid solution, filter, and recrystallize in water to obtain p-toluenesulfonyl-γ-cyclodextrin.
[0053] 1.32mmol p-toluenesulfonyl-γ-cyclodextrin, 2mmol NaN 3 , 10 mL of a mixed solution of dimethylformamide was reacted at 108° C. for 3.5 h under the protection of nitrogen. After the reaction was completed, the solution was cooled to room temperature, and settled in acetone, and the solid was collected by filtration, and the collected solid was recrystallized in water to obtain 6γCDN 3 .
[0054] 10.8mmol 4-bromo-1,8-naphthalic anhydride, 25mL pyridine, 13mmol 4-methylpiperazine, 3mL triethylamine (about 0.003mmol). Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com